i. Summary
With the renewed enthusiasm in immuno-oncology, characterization of the tumor immune microenvironment constitutes an essential and unique aspect to the assessment of therapeutics. The isolation of tumor infiltrating lymphocytes (TILs) is a desirable approach towards the understanding of anti-tumor immune response. This chapter provides an effective protocol to mechanically dissociate tumor tissue and generate single-cell suspension from excised tumors. TILs are then isolated by Ficoll-Paque density gradient centrifugation. This protocol is applicable to both human and experimental tumors in immunocompetent murine models.
Keywords: Tumor-infiltrating lymphocytes, tumor microenvironment, immunotherapy, immune escape, mechanical dissociation
1. Introduction
Immune checkpoint receptors (ICRs) play a major role in the regulation of T-cell function and responses, and have been demonstrated to be effective targets in several cancers [1]. However, the response rates to these ICR inhibitors are generally less than 35% among cancer patients, such as those with melanoma or head and neck squamous cell carcinoma [2,3]. In addition to human tumors, the use of immunocompetent mouse models is instrumental in testing new combinatorial therapies to overcome tumor resistance to ICR blockade [4–7]. Therefore, the isolation of tumor-infiltrating lymphocytes (TILs) from human and mouse tumors and downstream analysis, such as flow cytometry and effector function characterization, provide critical insight into strategies that can prime hypoimmunogenic cold cancers.
Ficoll-Paque, which consists of Ficoll PM400 (synthetic polymers of sucrose and epichlorohydrin) and sodium diatrizoate, has been widely used in the clinic to isolate lymphocytes from blood, bone marrow and umbilical cord blood through density gradient centrifugation [8–11]. As the densities of mononuclear cells such as lymphocytes and monocytes are less than that of Ficoll-Paque (eg. 1.077 g/ml), these cells form a layer at the interface during density gradient centrifugation, and thus can be readily collected. Ficoll-Paque has also been used to isolate mononuclear cells from other species including mice.
In this chapter, we describe a protocol that mechanically dissociates tumors and derives single-cell suspensions from human specimens and experimental tumors in immunocompetent mice. TILs and other mononuclear cells are purified using Ficoll-Paque density gradient centrifugation. Tumor dissociation methods mainly comprise enzymatic digestion (e.g. collagenase, trypsin, dispase), mechanical disruption of stroma, or a combination of both. Key consideration during this step is how to preserve viability, function and phenotype of the TILs. Although mechanical dissociation of tumor may be more time-consuming, we found this method to be highly reproducible. The use of enzymatic digestions may result in higher cell yield, but there is a need for careful optimization of enzymatic treatments among different tumor models to retain TIL phenotypes because enzymes may reduce lymphocyte surface markers important for downstream analysis, such as flow cytometry [12–14]. Although there are other centrifugation media available, such as Percoll, this protocol uses Ficoll-Paque which is broadly adaptable for human and mouse samples.
2. Materials
2.1. Tumors
Fresh tumor samples from human or mice are needed for TIL separation. Formalin or paraformaldehyde fixation renders the samples incompatible with this protocol.
Fresh tumor samples need to be hydrated, and placed in either phosphate-buffered saline (PBS) solution or culture media. Dehydration of tumor samples will substantially decrease the TIL yield and viability.
2.2. Reagents
70% (v/v) ethanol in sterile water
1X PBS, pH 7.4 formulated without calcium and magnesium
RPMI-1640
Fetal bovine serum (FBS)
100x Penicillin-streptomycin (10,000 units/ml of penicillin and 10,000μg/ml of streptomycin)
Ficoll-Paque PLUS (see Note 1)
Dimethyl sulfoxide (DMSO)
Complete RPMI media: RPMI-1640 with 10% FBS and 1x penicillin-streptomycin
2.3. Supplies and Equipment
Sterile dissecting forceps and scissors
Single-edge razor blades
Tissue culture hood
Examination gloves
Sterile 50ml conical tubes
5ml, 10ml, 25ml sterile serological pipets
Pipet aid with speed settings
1ml sterile pipette tips
100mm sterile petri dishes
Syringes with rubber plungers
Sterile 70μm cell strainers
Weighing scale
Centrifuge with swing-bucket rotors
2ml screw cap cryovials
Freezing containers
3. Methods
3.1. Preparations before extracting tumors
Media and Ficoll-Paque should be pre-warmed to room temperature of 18ºC-20ºC before use. (see Note 2)
3.2. Mechanical dissociation of tumor to obtain single-cell suspensions (see Note 3)
For mouse tumors, first euthanize mouse according to institution-approved protocol and spray the tumor area with 70% ethanol. Excise the tumor using sterile scissors and forceps.
Using sterile forceps, place the excised human or mouse tumor in a lid of a sterile 100mm petri dish containing 5ml of RPMI-1640 media at room temperature. Once tumor is extracted, keep it in media to prevent dehydration and loss of TILs. (see Note 4)
Mince the tumor into small pieces using two single-edged razor blades. To obtain smaller pieces of 1–2mm, hold two single-edged razor blades in one hand and mince the tumor. Although this may be time consuming, it is critical at this step to ensure that all tissue is minced up.
Place a 70μm cell strainer in the other half of the 100mm dish.
Cut a 1ml pipet tip using a razor blade. Using this cut tip, transfer the tumor tissue to the cell strainer. If there are pieces of tissue left on the lid, add another 5ml of RPMI-1640 media and transfer them to cell strainer.
Use the rubber plunger of a syringe to mesh the tissue through the cell strainer. The media in the dish will be cloudy due to dissociation of cells. Small pieces of tissue will remain on the strainer.
Place a new 70μm cell strainer onto a sterile labeled 50ml conical tube.
Pass the cell suspension through the strainer. Add more RPMI-1640 media to the plate, and pipet up and down several times to resuspend all remaining cells. Pass the media from the dish through the cell strainer.
Top up the media to 30ml with RPMI-1640 media at room temperature. Keep samples at room temperature of 18ºC-20ºC. (see Note 5)
3.3. Isolation of mononuclear cells
Immediately before the addition of Ficoll-Paque media, use a 25ml pipet to mix the single-cell suspension. This ensures that cells do not pellet at the bottom or form clumps.
Thoroughly mix the Ficoll-Paque media by inverting the container. (see Note 6)
Set the speed of pipet aid to slow release. Pipette 10ml of Ficoll-Paque media, and carefully insert the pipet to the bottom of the 50ml conical tube containing 30ml of single-cell suspensions (Figure 1A).
Slowly release the Ficoll-Paque media to form a layer of Ficoll-Paque below the cell suspensions. This reduces mixing of the two solutions. A clear distinct separation between Ficoll-Paque and cell solution is seen (Figure 1B).
Weigh samples to ensure proper balance of the rotors. (see Note 7)
Centrifuge at 1025 g for 20 min at 20ºC with slow acceleration and brakes turned off.
Carefully remove the tube from the rotor to avoid any disturbance to the layers formed during centrifugation (Figure 1C). Pipet 20ml of the upper layer of media to a waste bottle.
Transfer the layer of mononuclear cells to a sterile labeled 50ml conical tube using a sterile pipette, along with the remainder of the media above the Ficoll-Paque. Minimize transfer of the Ficoll-Paque where possible.
Figure 1: Separation of mononuclear cells using Ficoll-Paque media and density gradient centrifugation.
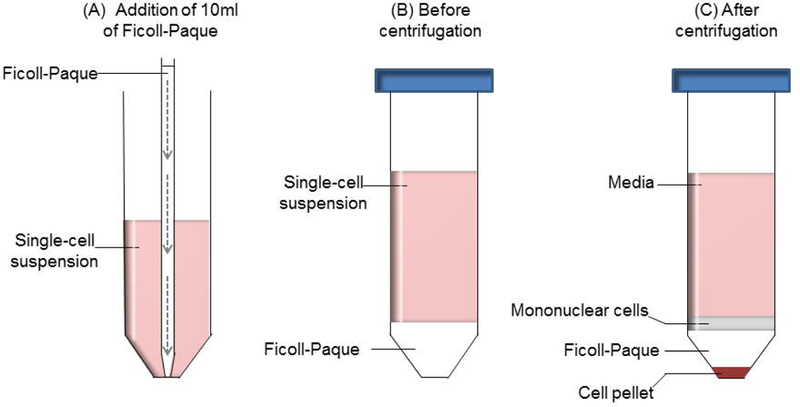
(A) 10ml of Ficoll-Paque is added to 30ml of single-cell suspension using a 10ml serological pipet and pipet aid. Slow and gentle release (indicated by the dashed arrows) of the Ficoll-Paque will result in a distinct separation between the two layers. (B) Before the density gradient centrifugation, the layer of single-cell suspension is above the Ficoll-Paque media. (C) After centrifugation, TILs and monocytes are found in the interface between the media and Ficoll-Paque, while the pellet consists of tumor cells and the erythrocytes.
3.4. Washing the isolated tumor infiltrating lymphocytes
Fill the 50ml conical tubes to 40ml with complete RPMI media and gently mix using a 25ml pipette. (see Note 8)
Centrifuge at 650 g for 10 min at 20ºC.
Remove the supernatant, and gently resuspend cells in 30ml of complete RPMI media.
Centrifuge at 650 g for 10 min at 20ºC.
Remove the supernatant and depending on downstream application resuspend the cells accordingly. (see Note 9)
4. Notes
There are several Ficoll-Paque media available, and users should decide which to use depending on the species and the cells needed. Ficoll-Plaque PLUS (1.077 g/ml) is widely used for the isolation of human mononuclear cells, and we have had success with it to isolate TILs from immunocompetent mice bearing syngeneic mouse tumors. However, lymphocytes from mouse are denser than that of human [8], and thus the use of Ficoll-Paque PREMIUM 1.084 is recommended if TILs are to be isolated from mouse tumors.
Ficoll-Paque densities are inversely correlated to temperature. Factors which contribute to the temperature include the temperature of single-cell suspension media, Ficoll-Paque media and temperature of the centrifuge, and this may affect the yield of mononuclear cells obtained from density gradient separations. Therefore, it is important to ensure all media used are pre-warmed to room temperature of 18ºC-20ºC and that the centrifugation is done at 18ºC-20ºC.
Upon obtaining the tumor, all subsequent steps are performed in a tissue culture hood to minimize contamination.
When mincing the tumors, the media used is RPMI-1640 without any FBS. This is to prevent any clumping of cells due to FBS. After density gradient centrifugation, complete RPMI media is used to wash the cells.
Cell viability may be affected if temperature is too high. If needed, place tubes containing cell suspension briefly on a rack on ice.
Protect the Ficoll-Paque media from light as sodium diatrizoate, which helps to maintain optimal density, is light-sensitive. It is also important to use aseptic techniques to maintain the sterility of the media.
Ensure that the rotors are properly balanced before starting the centrifuge as vibrations due to imbalance may lead to mixing of two layers and reduce yield and purity. Balance any differences in weight using RPMI-1640 media by gently adding the media to the top of the single-cell suspension. If a balance tube is needed for centrifugation, use a tube with similar weight, not volume.
The washing steps help to remove any contaminating Ficoll-Paque media, resulting in a highly purified and viable population of lymphocytes for downstream analyses.
If cells are to be frozen for future use, resuspend cells in freshly made freezing media of 90% FBS and 10% DMSO, and add 1ml of freezing media to each sample. Transfer to sterile labeled cryovials. Cryovials are kept in a freezing container (i.e. Mr. Frosty containing fresh isopropyl alcohol) in −80ºC freezer overnight prior to being stored in liquid nitrogen.
Acknowledgements:
This work is supported by NIH grants R00 DE024173 (YLL) and R01 DE026728 (YLL), Rogel Cancer Center Fund for Discovery (YLL).
References:
- 1.Zou W, Wolchok JD, Chen L (2016) PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 8 (328):328rv324. doi: 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A, Hamid O, Daud A, et al. (2016) Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA 315 (15):1600–1609. doi: 10.1001/jama.2016.4059 [DOI] [PubMed] [Google Scholar]
- 3.Ferris RL, Blumenschein G, Fayette J Jr., et al. (2016) Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. The New England journal of medicine 375 (19):1856–1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach DG, Dharmaraj N, Piotrowski SL, et al. (2018) STINGel: Controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials 163:67–75. doi: 10.1016/j.biomaterials.2018.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Judd NP, Allen CT, Winkler AE, Uppaluri R (2012) Comparative analysis of tumor-infiltrating lymphocytes in a syngeneic mouse model of oral cancer. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 147 (3):493–500. doi: 10.1177/0194599812442037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun ZJ, Zhang L, Hall B, et al. (2012) Chemopreventive and Chemotherapeutic Actions of mTOR Inhibitor in Genetically Defined Head and Neck Squamous Cell Carcinoma Mouse Model. Clin Cancer Res 18 (19):5304–5313. doi: 10.1158/1078-0432.CCR-12-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei Y, Xie Y, Tan YS, et al. (2016) Telltale tumor infiltrating lymphocytes (TIL) in oral, head & neck cancer. Oral oncology 61:159–165. doi: 10.1016/j.oraloncology.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyum A, Lovhaug D, Tresland L, Nordlie EM (1991) Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality. Scand J Immunol 34 (6):697–712 [DOI] [PubMed] [Google Scholar]
- 9.Li J, Jie HB, Lei Y, et al. (2015) PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer research 75 (3):508–518. doi: 10.1158/0008-5472.CAN-14-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kansy BA, Concha-Benavente F, Srivastava RM, et al. (2017) PD-1 Status in CD8(+) T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer research 77 (22):6353–6364. doi: 10.1158/0008-5472.CAN-16-3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyum A (1977) Separation of lymphocytes, lymphocyte subgroups and monocytes: a review. Lymphology 10 (2):71–76 [PubMed] [Google Scholar]
- 12.Abuzakouk M, Feighery C, O’Farrelly C (1996) Collagenase and Dispase enzymes disrupt lymphocyte surface molecules. J Immunol Methods 194 (2):211–216 [DOI] [PubMed] [Google Scholar]
- 13.Mulder WM, Koenen H, van de Muysenberg AJ, et al. (1994) Reduced expression of distinct T-cell CD molecules by collagenase/DNase treatment. Cancer immunology, immunotherapy : CII 38 (4):253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Autengruber A, Gereke M, Hansen G, et al. (2012) Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur J Microbiol Immunol (Bp) 2 (2):112–120. doi: 10.1556/EuJMI.2.2012.2.3 [DOI] [PMC free article] [PubMed] [Google Scholar]


