Abstract
Twenty years ago, we reported from the Collingridge Lab that a single-channel conductance increase through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type ionotropic glutamate receptors (AMPARs) could mediate one form of plasticity associated with long-term potentiation (LTP) in the hippocampus[1]. Revealed through peak-scaled non-stationary fluctuation analysis (PS-NSFA, also known as noise analysis), this component of LTP could be exclusively mediated by direct increases in channel conductance or by increases in the number of high conductance synaptic AMPARs. Re-evaluation of our original data in the light of the molecular details regarding AMPARs, conductance changes and plasticity suggests that insertion of high-conductance GluA1 homomers can account for our initial findings. Any potential cost associated with manufacture or trafficking of new receptors could be mitigated if pre-existing synaptic AMPARs also undergo a modest conductance change. The literature suggests that the presence of high conductance AMPARs and/or GluA1 homomers confers an unstable synaptic state, suggesting state transitions. An experimental paradigm is proposed to differentiate these possibilities. Validation of this state diagram could provide insight into development, disease pathogenesis and treatment.
Keywords: LTP, glutamate, AMPA receptor, conductance, phosphorylation, rectification
Introduction
Several recent reviews have been comprehensive in their evaluation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type ionotropic glutamate receptors (AMPARs)[2-5]. Surprisingly, few have detailed the role of single-channel conductance changes in plasticity. In part, this is likely due to an appreciation of the difficulties in measuring the single-channel conductances that underlie synaptic currents and a full understanding of the limitations introduced by the recording environment[6, 7]. It also reflects the complex regulation of AMPAR conductance, which is influenced by RNA editing, accessory subunits and phosphorylation at multiple different sites. Nevertheless, understanding the role and mechanisms underlying conductance changes has significant importance for several reasons. First, if the conductance change is mediated by changes in existing synaptic receptors, which kinases, phosphatases, scaffolds and/or auxiliary subunit interactions are necessary? Second, if the conductance change is mediated by inserted receptors with a higher conductance, where do they come from, how do they get there, what mediates the higher conductance, is a higher conductance necessary for triggering long-term plasticity, and do the altered ion permeabilities associated with conductance changes have additional consequences for the cell? Finally, what are the factors that reset synapses to a basal state? These questions could potentially impact our understanding of normal physiology and developmental and neurodegenerative disorders. To complement these reviews, we focus here on reported AMPAR conductances, caveats of measured conductances and regulatory factors, with a focus on their potential role in plasticity. Twenty years ago, we reported from the Collingridge Lab that a single-channel conductance increase through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type ionotropic glutamate receptors (AMPARs) could mediate plasticity associated long-term potentiation (LTP) in the hippocampus[1]. We provide a re-interpretation of our published findings, a comprehensive model of synaptic states and suggest an experimental approach relevant to advancing our understanding of plasticity in health, development and disease.
Review of initial motivations, approach and findings.
The initial motivation for our approach was the desire to understand fully the factors that determine the size of the synaptic current. These factors include the number of receptors present (N), the probability that an agonist bound receptor is open (Po), the driving force (V), the conductance of each receptor-channel (γ), from which the synaptic current ISYNAPTIC can be calculated:
| (1) |
We reasoned that determining changes in N, Po and/or γ before and after high frequency stimuli could help us understand the elements controlling post-synaptically mediated plasticity. Furthermore, this information could also provide evidence for pre-synaptic mechanisms of plasticity. While determination of these parameters would be complex, it nevertheless seemed possible given that the changes in synaptic strength that have been measured with synaptic plasticity can be several fold and would be reflected by linear changes in these parameters. The classical techniques used to directly determine γ or N or Po have been to excise outside-out patches from neurons or heterologous expression systems, apply glutamate and directly measure these properties with single-channel analysis. For AMPARs, this is complicated by the relatively low and multiple conductances, brief open times, and desensitization, all of which pushed some unitary currents into the background recording noise. Further, patches and heterologous cells are unlikely to represent native, synaptic receptors. Standard fluctuation analysis (SFA, aka noise analysis), as a means of extracting γ from the noise, was first used at the neuromuscular junction[8, 9]. For time-dependent currents, non-stationary fluctuation analysis (NSFA) was developed[10-12]. Briefly, an ensemble of currents is collected, aligned to their maximal activation and averaged to determine the time-dependent mean time course, or I(t). The average is then subtracted from each individual current in the ensemble, and the resulting difference is squared, summed and divided by the number in the ensemble to determine the time-dependent variance, σ2(t). The variance is then plotted against the mean and fitted to
| (2) |
where i is the single channel current and N is the number of channels in the population; Pomax is I(t)max/(i * N). If a population of channels is present, NSFA will report the bulk average properties[11]. However, application of NSFA to central synapses was not initially feasible, as this analysis requires that the population of channels (N) be the same for each current. At central synapses with low probability of release and activation, this was not possible because the number of receptors activated varied from synapse to synapse and synaptic currents could arise from a different number of active synapses, which could vary with each stimuli[13]. Excised outside-out patches from central neurons would be contaminated by extrasynaptic receptors; it is not feasible to obtain outside out patches from pure synaptic membranes. Nevertheless, measurements of unitary currents from excised patches, either from direct measurements or NSFA, reflect the standard for comparison (Table 1). One alternative approach has been to activate synaptic receptors through focal uncaging of glutamate at identified synapses in cultures, which can activate the same population of receptors, provided there is both constant caged glutamate and consistent stimuli[14]. However, this approach to record from synaptic receptor is also contaminated by the likely activation of extrasynaptic receptors.
Table 1.
Comparison of reported conductance changes of native and recombinant AMPARs. eEPSC = evoked excitatory post-synaptic current. OO = outside-out patch, hp = hippocampus. These are for comparisons to other estimates described in Table 2.
| Reported γ (pS) |
Conditions | Reference |
|---|---|---|
| 4.8 | eEPSCs, CA1 hp, P13-15, PS-NSFA | [1] |
| 11.3 | post-LTP | |
| 10 | eEPSC, CA1 hp, PS-NSFA | [19] |
| 14 | post-LTP | |
| 3.8 | mEPSCs, NSFA, hp cultures | [17] |
| 4.9 | post-CaMKI | |
| 12 | OO patch, recombinant GluA1, HEK293, NSFA, activated by glutamate | [20, 21] and [22, 23] |
| 20 | + CaMKII | [21] |
| 4 | OO patch, hp cultured neuron, SFA, activated by glutamate | [24] |
| 8 | + CaMKII | |
| 3 | OO patch, recombinant Glua1/2/TARP γ8, HEK 293, SFA, activated by glutamate | [24] |
| 6.4 | + CaMKII |
Peak-scaled non-stationary fluctuation analysis (PS-NSFA) was developed[6, 15, 16] as a method to extract γ at central synapses with resultant determination of the average number of channels maximally activated (N*Po, max). There were several additional important caveats to this analysis. First, the recording conditions and the large electrotonic size of some neurons can substantially filter and reduce the estimates of γ and N*Po[6]. Despite these effects, a linear relationship is preserved between variance and open probability for the lowest currents (i.e. the tail of the synaptic current), such that relative changes in γ or N*Po can be reliably detected[7]. Thus, PS-NSFA, while reliable to determine change, may not be a reliable method to determine the values for γ or N*Po in a resting state in electrotonically larger neurons, unless confirmed by other techniques.
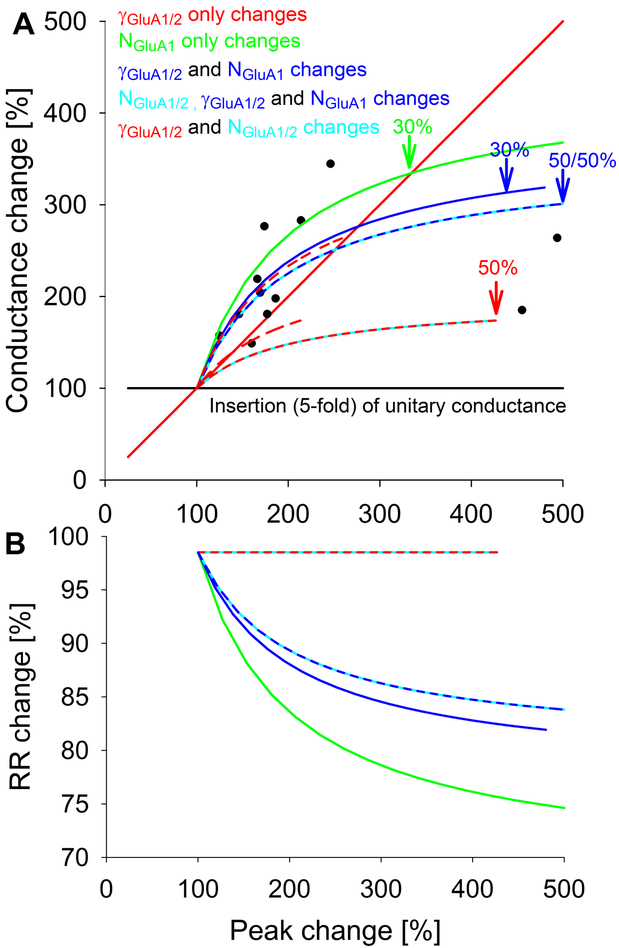
In the Collingridge lab, we applied PS-NSFA to determine changes with LTP[1] and found that LTP was associated with distinct changes in either γ or N*Po (data from[1] (see Fig. 4c), re-plotted in Fig. 1A)(Table 1). When changes in γ were plotted versus the magnitude of LTP, we found that in most cases there was a supra-linear change in γ (above the line of linear slope) but in the largest LTP, this was sublinear (below the line of linear slope). At the time, we thought that this could be due to filtering in the recording environment, but subsequent simulations suggested that this was not the case (see[7], Fig. 10). We assumed that our pre-LTP estimates of γ (~5 pS) represented a filtered estimate of γ. Importantly, changes in γ versus N*Po were detectable by PS-NSFA using manipulations other than LTP by us[1] and others[17, 18].
Figure 1.
A) Increases in AMPAR conductance and LTP. Conductance change (% increase) is plotted versus LTP (% increase in peak synaptic amplitude). Original data from[1] is plotted (circles) versus simulated data for multiple scenarios as described in the text. If conductance gradually increased, it would follow the red solid line (not biophysically plausible); if the number of GluA1/2 heteromers gradually increased, it would follow the black line. If the proportion of high conductance GluA1/2 heteromers increased from 0 to 100%, it would follow the short-dashed red line (supra-linear). If this increased more physiologically from 15% to 100%, it would follow the long-dashed red line (sub-linear). For Scenarios 3 (increased GluA1 homomers only, green) and 4/6 (increased GluA1 homomers and increased GluA1/2 conductance, blue or blue/cyan lines), greater percentage increases in GluA1 homomers are indicated at the points noted by the arrows. For Scenario 5 (increased GluA1/2 conductance and increased GluA1/2 heteromers, red/cyan) increased percentage of GluA1/2 heteromers are noted by the arrow. In scenarios 4 and 5 involving increased GluA1/2 conductance, the initial percentage of high conductance GluA1/2 was 15%. (B) Scenarios involving the insertion of calcium permeable AMPARs can be distinguished based on the rectification observed. Specifically, with increased plasticity, greater rectification will be observed depending on the relative insertion of GluA1 homomers.
Investigations of LTP by others in the hippocampus using PS-NSFA either found similar changes (initial γ of 10 pS, post LTP 14 pS)[19] or only changes in N*Po (initial γ of 20 pS)[18]. Subsequent studies suggested that higher initial conductance estimates are potentially artefactual due to the presence of other sources of synaptic variability (misalignment associated with variabilities in synaptic release across multiple synapses, inaccurate peak scaling, variable filtering of events due to differences in dendritic location and baseline drift) and that recordings where this has been detected are not reliable for PS-NSFA[17] (Benke and Traynelis, unpublished). Conductance changes were not found in extrasynaptic AMPARs[25] measured in outside-out dendritic patches with NSFA after LTP (initial γ of 8 pS), consistent with the idea that they were synapse-specific. The Collingridge lab found that conductance changes associated with LTP could be reversed by depotentiation[26] but no changes in γ were detectable with stable long-term depression (LTD) (initial γ of 6 pS)[27]. Changes in conductance were not found with LTP early in development (initial γ of ~5 pS). In fact, reductions in γ were found in association with a pre-synaptically mediated form of LTP at post-natal day (P) 6 and earlier[28]. This suggested that either the conductance of existing AMPARs was reduced, or they were replaced by AMPARs with a lower conductance. Robust conductance changes appear developmentally after P12 (initial γ of ~3 pS) [28].
Plasticity at synapses outside of the hippocampus has also been investigated. No conductance change was found at mossy fiber CA3 synapses associated with LTD (initial γ of ~30 pS)[29]. In immature granule cell to Purkinje cell synapses, cerebellar LTP involves a conductance change (initial γ of 23 pS, post LTP 32 pS)[30], while LTD does not (initial γ of ~22 pS)[31]. Synapse development in the nucleus tractus solitarius proceeds, remarkably, without the involvement of N-methyl-D-aspartate-type glutamate receptors (NMDARs), calcium permeable AMPARs, or a conductance change of AMPARS (γ of 12 pS)[32].
These findings highlight several points. First, developmental age must always be considered as a factor mediating the mechanisms of plasticity. In addition, the mechanism of plasticity invoked are dependent on the protocol (details of pairing and stimulation) and likely specific to the synaptic circuit. Finally, the initial estimates of γ, are not representative of the actual conductance due to filtering; thus estimates that exceed what would be expected under basal conditions (Tables 1 and 2) should be considered to be potentially contaminated by artefact.
Table 2.
Comparisons of reported conductances of native AMPARs. OO = outside-out patch, hp = hippocampus. uEPSCs = uncaged EPSC.
| Reported γ (pS) |
Conditions | Reference |
|---|---|---|
| 8 and 15 pS | Cerebellar granule cells, OO patch, single channels activated by kainate | [48] and [49] |
| 8.5 pS | CA3, OO somatic patch, NSFA, activated by glutamate | [43] |
| 12 pS | OO somatic patch, hp culture +/− PKA, NSFA, activated by glutamate | [44] |
| 5.7-11.4 pS | uEPSCs, NSFA, hp cultures, MNI-glutamate (uncaged on spines) | [14] |
| 3 pS | OO patch, GluA1/2, HEK 293, NSFA, activated by glutamate | [21] |
| 6.9 and 11.2 | CA1, OO somatic patch, single channels activated by glutamate | [42] and [50] |
| 7 pS | Climbing fiber-Purkinje cell, P4, synaptic, PS-NSFA (corrected for filtering) | [51] |
| 11 pS | Climbing fiber-Purkinje cell, extrasynaptic, PS-NSFA (corrected for filtering) | [51] |
| 11 pS | Climbing fiber-Purkinje cell, P4, NSFA, uEPSC (MNI-glutamate uncaged at hotspots) | [52] |
Multiple factors regulate AMPAR conductance in hippocampus
AMPARs in hippocampus primarily exist as either GluA1/2 (>80%) or GluA2/3 heteromers[33, 34]. GluA1 homomeric receptors appear to be restricted to immature (less than post-natal day (P) 7) CA1 synapses[35, 36] or synapses in culture[37, 38] (but see [39] which suggested a maximum of 8-10%). GluA2/3 heteromeric receptors are not thought to participate in plasticity[34, 40] (but see[41]). At mature hippocampal neurons, recordings of extrasynaptic AMPARs in outside-out patch recordings (considered as a standard for comparison), revealed conductances of 6.9 and 11.2 pS [42] with NSFA measurements reflecting the bulk average varying from 4[24], 8.5[43] to 12 pS[44] (See Table 2). In order to determine how subunit composition controls conductance, recombinant subunits have been expressed in heterologous systems so that determination of conductance can be compared to that determined for native receptors. This approach has determined that subunit composition regulates single channel currents, with GluA1 homomers conducting, on average via NSFA 12 pS[20, 24] while GluA1/2 heteromers conduct much less (3 pS[21, 24]). These studies have employed saturating glutamate concentration, as occurs in the synapse[45]. Recombinant GluA receptors exhibit multiple conductance substates, with the highest states seen with saturating glutamate concentration[46, 47]. This property is a function of the coupling efficiency and the number of agonist-bound subunits, such that there is a greater probability that all four subunits will contribute to gating and produce a larger conductance channel opening in the presence of saturating glutamate[24].
AMPAR subunits have multiple phosphorylation sites on their C-terminal tails, thought to regulate channel function[53] and especially plasticity[54]. GluA1 subunit phosphorylation at S831, S818 and T849 may play a role in regulating channel conductance[20, 21, 24, 55], while S845 may play a role in Po[44] and regulating receptor trafficking in the membrane[53]. CaMKII and PKC are likely kinases at S831, S818 and T840[24, 55], while PKA is the likely kinase at S845[53]. All sites have been implicated in receptor trafficking in the membrane[53]. At rest, S831 and S845 are phosphorylated in ~15% of receptors (on average, across all synapses, with unknown inter-synaptic variability)[56]. With LTP, phosphorylation of each can increase 2-fold[56], while LTD can result in loss of phosphorylation at S845[57-60] with no changes at S831[60]. Nitrosylation of GluA1 may also regulate channel conductance[61]. Phosphorylation of GluA1 by CaMKII or phosphomimicry of S831 increases the channel conductance from 12 pS to 20 pS[24]. Initial studies of recombinant GluA1/2 heteromers suggested that CaMKII was unable to regulate channel conductance[21, 24], however more recent studies found that inclusion of the accessory protein TARP-γ8 resulted in CaMKII mediated 2.5-fold increase in channel conductance[24]. This conductance change is also seen with phosphomimicry of S831, indicating that phosphorylation of this site is sufficient to account for the conductance change[24]. This role of TARP-γ8 is not surprising, as TARPs can mediate critical subunit-subunit interactions that influence rectification[2] and thus likely the coupling efficiency that mediates channel conductance[24]. Importantly, enhanced coupling efficiency due to S831 phosphorylation does not increase maximal channel conductance, rather it increases the likelihood that maximal channel conductance will be achieved[24].
Review of CP-AMPAR role In plasticity; implications for conductance changes
Synaptic plasticity in the hippocampus has long been associated with post-synaptic calcium accumulations in neurons[62]. NMDARs have been implicated as the primary source of calcium entry until recently when it was found that calcium-permeable AMPARs (CP-AMPARs) can also mediate LTP[63]. Calcium permeable AMPARs are detectable by their property of inward rectification (reduced current passage at positive holding potentials), which reflects channel block by internal polyamines at depolarized potentials. This property renders CP-AMPARs sensitive to externally-applied channel blocking antagonists such as IEM-1460, PhTx-433 or NASPM[53, 64]. CP-AMPARs are assemblies of either unedited (Q/R) GluA2 subunits in GluA1/2 heteromers or homomeric GluA1 AMPARs. Since GluA2 is fully edited at the Q/R site postnatally in cortex and hippocampus[53], any CP-AMPARs detected are likely GluA1 homomers. The dependence of LTP on the insertion of CP-AMPARs displays a biphasic developmental profile, being present around P14[59, 63], diminishing soon after, and then reappearing after P30[65, 66]. The etiology of this intriguing developmental profile is unknown. LTD also requires the insertion of CP-AMPARs[59]. In both LTP and LTD, the presence of CP-AMPARs after the induction of plasticity has been demonstrated or inferred to be transient, especially since CP-AMPARs are not observed basally after P7[35]. In hippocampal cultures, adding only 5% homomeric GluA1s to the pool of AMPARs can account for a conductance change and plasticity associated with CaMKI[17]. This is because the mean conductance determined from noise analysis is weighted by the conductance amplitude, meaning that larger conductances disproportionally influence the weighted mean conductance[13]. Thus, our initial observation of a conductance change in hippocampal slices[1] could be accounted for by insertion or trafficking of a relatively small number of homomeric GluA1s to synaptic sites. Recent data suggests that spines may be resistant to large shifts in NGluA1[67].
Modeling conductance changes and receptor insertion with hippocampal LTP
We replotted our changes in conductance versus the magnitude of LTP (see Fig. 4c in[1]). We then modelled the parameters that could result in the conductance changes we observed associated with LTP[1] to determine the relative contributions of homomeric GluA1 insertion, insertion of GluA1/2 heteromers and increases in conductance of GluA1/2 heteromers (Fig. 1A) according to:
| (3) |
| (4) |
[24] where V is assumed to be constant and thus left out of the calculations. At the basal, initial state, NGluA1 is assumed to be 0. NGluA1/2 is assigned a value of 0.85 and NGluA1/2_HIGH is assigned a value of 0.15 (total kept constant at 1), assuming that GluA1/2 is 15% phosphorylated and in a higher conductance state. γGluA1/2 is assigned 3 pS and γGluA1/2_HIGH is assigned 8 pS. The open probabilities for GluA1 homomers and GluA1/2 heteromers, PoGluA1 and PoGluA1/2, are assumed to be equal[24, 44] (but see considerations in[17]) and are assigned a value of 1. These initial starting conditions, based on literature cited, will affect the initial slope of the relationship of γavg versus Isynpatic, total (see Figure 1A).
We considered multiple possible ways that GluA receptors with different conductances might combine to produce both an increase in synaptic amplitude (i.e. LTP) and the observed increase in weighted mean conductance. In the first scenario, only conductance changes of GluA1/2 heteromers were considered by allowing the percentage of unphosphorylated GluA1/2 to change, that is the total number of GluA1/2 is unchanged but the proportion of unphosphorylated NGluA1/2 (γGluA1/2 of 3 pS) and phosphorylated NGluA1/2_HIGH (γGluA1/2_HIGH of 8 pS) does change. The shape of this curve depends on the initial starting conditions. When starting from NGluA1/2 (γGluA1/2 of 3 pS) of 100% (Fig. 1A, red short-dashed curve) to 100% NGluA1/2_HIGH (γGluA1/2_HIGH of 8 pS), the curve is supralinear. In comparison, when starting from a likely more physiological 85% NGluA1/2 (γGluA1/2 of 3 pS) to 100% NGluA1/2_HIGH (γGluA1/2_HIGH of 8 pS) (red long-dashed curve) the curve is sublinear, and not consistent with prior data. The solid red unity line was not considered, as a continuous change in γGluA1/2 was not considered biophysically plausible. Further, based on the known conductances for GluA1/2 heteromers, at most a 2.5 fold change might be expected with phosphorylation[24]. In the second scenario, only the number of GluA1/2 heteromers were considered by allowing NGluA1/2 to change 5-fold (black line). Individual synapses may not be capable of increasing the number of receptors 5-fold to account for a purely post-synaptically mediated LTP. Since receptor numbers and response amplitudes correlate with synaptic size[52, 68], changes in synaptic size much larger than previously measured[69-72] would need to be considered. Alternatively, previous silent synapses could become activated[73]. Thus, the range of observed plasticity changes are unlikely to be accounted for by these two scenarios.
In the third scenario, NGluA1 alone was allowed to increase by 0.4-fold. Including the insertion of GluA1 homomers of 20 pS conductance, nearly 7-fold greater than baseline (ie 20 pS for phosphorylated GluA1 homomers versus 3 pS for GluA1/2 heteromers), now modeled a supra-linear to sub-linear change in conductance with plasticity (green curve). Therefore, to achieve this supralinearity, insertion of GluA1 homomers are sufficient. Since this does not closely model the extreme shifts with sublinearity in plasticity (two data points on far right) and this would require a substantial shift of GluA1s into the synaptic membrane, mixed scenarios were considered. In scenario 4, if the phosphorylation-mediated conductance change of GluA1/2 heteromers was allowed (from 85% with 3 ps to 100% with 8pS) with a smaller 0.3-fold change of NGluA1, then a closer approximation to the original data is achieved with minimal cost and realistic changes in conductance (blue curve). In scenario 5, if a 0.5-fold change in NGluA1/2_HIGH is allowed in addition to the shift from 15% to 100% phosphorylation of GluA1/2, this (red/cyan curve) comes close to the extreme limits of the data, but does not mimic the original data due to sublinearity and requires a substantial shift of receptors. In scenario 6, if a 0.5-fold change in NGluA1 and NGluA1/2_HIGH is allowed in addition to the shift from 15% to 100% phosphorylation of GluA1/2, this (blue/cyan curve) closely mimics the original data, but again requires substantial shifts of receptors. In summary, there are a myriad of ways to increase amplitude given synapses can hold two different receptors (GluA1 and GluA1/2) that each can exist in multiple phosphorylation states. Our original data can be modeled best by simultaneous increases in NGluA1 and phosphorylation mediated changes in γGluA1/2. A larger data set would be helpful to support the proposed nature of the curve in order to better distinguish among these scenarios, especially the extreme shifts. It would be important to understand how often and under what conditions the range of LTP is observed. The extreme shifts may represent unsilencing of synapses, which are noted here to happen concurrently with conductance changes. Further, there is a critical assumption that basal phosphorylation of GluA1/2 has increased the initial γavg which affects the initial slope of these curves. In addition, we have not included basal NGluA1 or its phosphorylation state (12 pS versus 20 pS for unphosphorylated and phosphorylated, respectively) which would also affect the initial γavg.
Proposal of state diagram: Conclusions
In addition to conductance, we can measure additional properties of mixed receptor populations to infer their underlying content. Specifically, measurement of rectification and comparisons to shifts in plasticity will indicate whether or not large shifts in NGluA1 have occurred. Relative rectification [RR] versus plasticity is determined according to
| (5) |
[24]
| (6) |
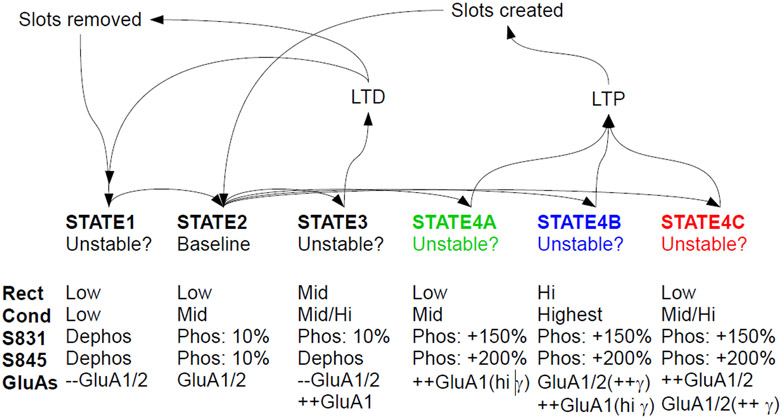
[24] where RRGluA1 = 0.43 and RRGluA1/2 = 0.66 with 0.67 assumed to be a linear channel without rectification[24]. Thus, greater rectification versus plasticity changes (Fig. 1B) would suggest larger insertions of NGluA1. Based on this we can propose different initial states and final states that consider the possible scenarios supported by the data. We propose that hippocampal synapses have a stable baseline state. This state is comprised primarily of GluA1/2 heteromers that are phosphorylated ~15% at S831 and S845. This state (Fig. 2, State 2) has minimal rectification and a low conductance state. When plasticity is induced, either LTP or LTD, distinct unstable states are transiently formed. We assume that these are unstable and transient since they are not observed at rest, as synapses with either a large number of receptors (quantal size), high rectification or high conductance are not seen basally. However, alterations of kinases, phosphatases or scaffolds such as AKAP79/150 could stabilize these abnormal states, which then causes abnormal induction and expression of LTP and LTD [59, 74]. The transient LTD state (State 3), characterized by GluA1 homomers of mid-high conductance (only 15% S831 phosphorylation), has moderate rectification and dephosphorylation of S845[59, 74]. This unstable state results in a subsequent loss of recently added GluA1 homomers and pre-existing GluA1/2 heteromers and then settles back into the stable state, possibly via State 1 with a gross loss of receptor slots and eventually synapses. Three transient LTP states (States 4abc) are considered, based on the model; all states have maximal phosphorylation of S831 and S845. State 4a would reflect scenario 3, State 4b would reflect scenario 4/6 and State 4c would reflect scenario 5. Each of these states could potentially be distinguished by simultaneous determination of their core properties: conductance, rectification and phosphorylation status. The transient LTP states eventually evolve into a greater number of receptor slots, either via larger spines or eventually new synapses, which settle into the baseline, stable state (State 2). While the states were modelled as a continuum of increasing N or shifts in γ in Fig. 1A, the opportunity for different states to exist along this continuum is entirely possible, depending on the plasticity-inducing stimulus. It is also possible that states transition sequentially, that is State 4a could transition to 4c, etc. In other words, at one particular stage in development or with one particular LTP-triggering stimulus, State 4a would be favored, while State4c would be favored under different conditions. Another permutation is that in immature neurons, all states are possible. This would also be consistent with different kinases and phosphatases uniquely involved with LTP versus LTD and modulation by development.
Figure 2.
Proposed state diagram for hippocampal excitatory synapses. Each synapse may exist in one of these states at a given time; State 2 is the stable state and all states return to this state after plasticity inducing stimuli. States 1, 3 and 4 are potentially unstable, intermediate states that form after plasticity inducing stimuli. LTP stimuli eventually result in larger or more numerous synapses, while LTD stimuli eventually result in smaller or fewer synapses.
Due to the non-linear nature of conductance versus plasticity, scenario 1 seems unlikely unless a complete shift from 0 to 100% of low-conductance (3 pS) to high conductances (8pS) GluA1/2 is considered. This would suggest that GluA1 phosphorylated at S831 may be peri-synaptic. Scenario 2 has been demonstrated, but may have a limited range of plasticity depending on the available space for insertion of GluA1/2 heteromers[71, 72, 75]. We postulate that to conserve energy, synapses should probably favor inserting the minimal NGluA1 since they must eventually be removed and recent data does not support large shifts in NGluA1[67]. Scenarios three, four/six and five can be distinguished by comparing relative rectification [RR] versus plasticity. Thus, greater rectification versus plasticity changes (Fig. 1B) would suggest larger insertions of NGluA1 in Scenarios 3 (State4a) or 4/6 (State 4b) (green or blue curves). Further, measurement of conductance after induction of LTP in the presence of GluA1 specific antagonists would distinguish between States 4b and 4c.
This state diagram raises additional questions. First, do unstable states dictate the stability and direction (potentiation versus depression) of long-term plasticity? Recent experiments suggest that this is the case[59, 65, 74, 76]. Second, does higher or lower conductance of GluA1 homomers and dependence on S831 phosphorylation influence plasticity? Third, does the presence of different states change with development? In other words, could the presence or absence of states explain the period during development when hippocampal LTP does not depend on CP-AMPARs? LTP is more robust in early development[77]. Is this due to an imbalance, presence or absence of states that favors LTP over LTD? Importantly, are these states impacted by neurodevelopmental, degenerative and neuropsychiatric disorders, many of which have alterations in plasticity as a defining characteristic? Determining these states will suggest multiple alternative therapeutic approaches that are potentially disease and developmentally specific.
Acknowledgements
The author appreciates the input provided by Drs. Dell’Acqua, Aoto, Bayer and Kennedy, their laboratories and Dr. Caballes and Ms. Castano in the Benke laboratory. Supported by NIH NS101288 and the Children’s Hospital Colorado Foundation (Ponzio Family Chair in Neurology Research) (Benke) and NIH NS036654 (Traynelis).
Contributor Information
Tim Benke, Departments of Pediatrics, Pharmacology, Neurology and Otolaryngology, University of Colorado, School of Medicine, Anschutz Medical Campus, Aurora, CO.
Stephen F Traynelis, Department of Pharmacology, Emory University School of Medicine, Atlanta, GA.
References
- 1.Benke TA, Luthi A, Isaac JTR, Collingridge GL (1998) Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 395:793–797 [DOI] [PubMed] [Google Scholar]
- 2.Greger IH, Watson JF, Cull-Candy SG (2017) Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 94:713–730 [DOI] [PubMed] [Google Scholar]
- 3.Lisman J (2017) Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Philos Trans R Soc Lond B Biol Sci 372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hell JW (2016) How Ca2+-permeable AMPA receptors, the kinase PKA, and the phosphatase PP2B are intertwined in synaptic LTP and LTD. Sci Signal 9:pe2. [DOI] [PubMed] [Google Scholar]
- 5.Herring BE, Nicoll RA (2016) Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol 78:351–365 [DOI] [PubMed] [Google Scholar]
- 6.Traynelis SF, Silver RA, Cull-Candy SG (1993) Estimated conductance of glutamate receptor channels activated during EPSCs at the cerebellar mossy fiber-granule cell synapse. Neuron 11:279–289 [DOI] [PubMed] [Google Scholar]
- 7.Benke TA, Luthi A, Palmer MJ, Wikstrom M, Anderson WW, Isaac JTR, Collingridge GL (2001) Mathematical modeling of non-stationary fluctuation analysis for studying channel properties of synaptic AMPA receptors. J Physiol 537.2:407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz B, Miledi R (1972) The statistical nature of the acetycholine potential and its molecular components. J Physiol 224:665–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson CR, Stevens CF (1973) Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol 235:655–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigworth FJ (1980) The variance of sodium current fluctuations at the node of Ranvier. J Physiol 307:97–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallanti A, Tonelli A, Cardin V, Bussone G, Bresolin N, Bassi MT (2008) A novel de novo nonsense mutation in ATP1A2 associated with sporadic hemiplegic migraine and epileptic seizures. J Neurol Sci 273:123–126 [DOI] [PubMed] [Google Scholar]
- 12.Heinemann SH, Conti F (1992) Nonstationary noise analysis and application to patch clamp recordings. Methods Enzymol 207:131–148 [DOI] [PubMed] [Google Scholar]
- 13.Traynelis SF, Jaramillo F (1998) Getting the most out of noise in the central nervous system. Trends in Neuroscience 21:137–145 [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H (2001) Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci 4:1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson CL, Tate WP, Mason SE, Lawlor PA, Dragunow M, Abraham WC (1992) Correlation Between the Induction of an Immediate Early Gene, zif/268, and Long-Term Potentiation in the Dentate Gyrus. Brain Res 580:147–154 [DOI] [PubMed] [Google Scholar]
- 16.De Koninck Y, Mody I (1994) Noise analysis of miniature IPSCs in adult rat brain slices: Properties and modulation of synaptic GABA A receptor channels. Journal of Neurophysiology 71:1318–1335 [DOI] [PubMed] [Google Scholar]
- 17.Guire ES, Oh MC, Soderling TR, Derkach VA (2008) Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci 28:6000–6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling DS, Benardo LS, Sacktor TC (2006) Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus 16:443–452 [DOI] [PubMed] [Google Scholar]
- 19.Poncer JC, Esteban JA, Malinowm R (2002) Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci 22:4406–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derkach V, Barria A, Soderling TR (1999) Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA 96:3269–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh MC, Derkach VA (2005) Dominant role of the GluR2 subunit in regulation of AMPA receptors by CAMKII. Nat Neurosci 8:853–854 [DOI] [PubMed] [Google Scholar]
- 22.Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG (1996) Effect of RNA editing and subunit co-assembly on single- channel properties of recombinant kainate receptors. J Physiol 492:129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson GT, Kamboj SK, Cull-Candy SG (1997) Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci 17:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF (2011) Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci 14:727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrasfalvy BK, Magee JC (2004) Changes in AMPA receptor currents following LTP induction on rat CA1 pyramidal neurones. J Physiol 559.2:543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luthi A, Palmer MJ, Anderson WW, Benke TA, Isaac JTR, Collingridge GL (2004) Bi-directional modulation of AMPA receptor unitary conductance by synaptic activity. BMC Neuroscience 5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JTR, Collingridge GL (1999) Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron 24:389–399 [DOI] [PubMed] [Google Scholar]
- 28.Palmer MJ, Isaac JTR, Collinridge GL (2004) Multiple, developmentally regulated expression mechanisms of long-term potentiation at CA1 synapses. J Neurosci 24:4903–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei S, Pelkey KA, Topolnik L, Congar P, Lacaille JC, McBain CJ (2003) Depolarization-induced long-term depression at hippocampal mossy fiber-CA3 pyramidal neuron synapses. J Neurosci 23:9786–9795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosman LW, Takechi H, Hartmann J, Eilers J, Konnerth A (2008) Homosynaptic long-term synaptic potentiation of the "winner" climbing fiber synapse in developing Purkinje cells. J Neurosci 28:798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linden DJ (2001) The expression of cerebellar LTD in culture is not associated with changes in AMPA-receptor kinetics, agonist affinity, or unitary conductance. Proc Natl Acad Sci U S A 98:14066–14071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balland B, Lachamp P, Strube C, Kessler JP, Tell F (2006) Glutamatergic synapses in the rat nucleus tractus solitarii develop by direct insertion of calcium-impermeable AMPA receptors and without activation of NMDA receptors. J Physiol 574:245–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenthold RJ, Petralia RS, Blahos J II, Niedzielski AS (1996) Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci 16:1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA (2009) Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62:254–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stubblefield EA, Benke TA (2010) Distinct AMPA-type glutamatergic synapses in developing rat CA1 hippocampus. J Neurophysiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adesnik H, Nicoll RA (2007) Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci 27:4598–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson HR, Gibson ES, Benke TA, Dell'Acqua ML (2009) Regulation of postsynaptic structure and function by an A-kinase anchoring protein-membrane-associated guanylate kinase scaffolding complex. JNeurosci 29:7929–7943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattison HA, Bagal AA, Mohammadi M, Pulimood NS, Reich CG, Alger BE, Kao JP, Thompson SM (2014) Evidence of calcium-permeable AMPA receptors in dendritic spines of CA1 pyramidal neurons. J Neurophysiol 112:263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rozov A, Sprengel R, Seeburg PH (2012) GluA2-lacking AMPA receptors in hippocampal CA1 cell synapses: evidence from gene-targeted mice. Front Mol Neurosci 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA (2013) LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renner MC, Albers EH, Gutierrez-Castellanos N, Reinders NR, van Huijstee AN, Xiong H, Lodder TR, Kessels HW (2017) Synaptic plasticity through activation of GluA3-containing AMPA-receptors. Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gebhardt C, Cull-Candy SG (2006) Influence of agonist concentration on AMPA- and kainate channels in CA1 pyramidal cells in rat hippocampal slices. J Physiol 573:371–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonas P, Major G, Sakmann B (1993) Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol 472:615–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banke TG, Bowie D, Lee HK, Huganir RL, Schousboe A, Traynelis SF (2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20:89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clements JD, Lester RAJ, Tong G, Jahr CE, Westbrook Gl (1992) The time course of glutamate in the synaptic cleft. Science 258:1498–1501 [DOI] [PubMed] [Google Scholar]
- 46.Smith TC, Howe JR (2000) Concentration-dependent substate behavior of native AMPA receptors. Nat Neurosci 3:992–996 [DOI] [PubMed] [Google Scholar]
- 47.Smith TC, Wang LW, Howe JR (2000) Heterogeneous conductance levels of native AMPA receptors. J Neurosci 20:2073–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cull-Candy SG, Howe JR, Ogden DC (1988) Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol 400:189–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cull-Candy SG, Usowicz MM (1987) Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature 325:525–528 [DOI] [PubMed] [Google Scholar]
- 50.Jahr CE, Stevens CF (1987) Glutamate activates multiple single channel conductances in hippocampal neurons. Nature 325:522–525 [DOI] [PubMed] [Google Scholar]
- 51.Momiyama A, Silver RA, Hausser M, Notomi T, Wu Y, Shigemoto R, Cull-Candy SG (2003) The density of AMPA receptors activated by a transmitter quantum at the climbing fibre-Purkinje cell synapse in immature rats. J Physiol 549:75–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka J, Matsuzaki M, Tarusawa E, Momiyama A, Molnar E, Kasai H, Shigemoto R (2005) Number and density of AMPA receptors in single synapses in immature cerebellum. J Neurosci 25:799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacological Reviews 62:405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Z, Liu A, Xia S, Leung C, Qi J, Meng Y, Xie W, Park P, Collingridge GL, Jia Z (2018) The C-terminal tails of endogenous GluA1 and GluA2 differentially contribute to hippocampal synaptic plasticity and learning. Nat Neurosci 21:50–62 [DOI] [PubMed] [Google Scholar]
- 55.Jenkins MA, Wells G, Bachman J, Snyder JP, Jenkins A, Huganir RL, Oswald RE, Traynelis SF (2014) Regulation of GluA1 alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor function by protein kinase C at serine-818 and threonine-840. Mol Pharmacol 85:618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diering GH, Heo S, Hussain NK, Liu B, Huganir RL (2016) Extensive phosphorylation of AMPA receptors in neurons. Proc Natl Acad Sci U S A 113:E4920–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee HK, Kameyama K, Huganir RL, Bear MF (1998) NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21:1151–1162 [DOI] [PubMed] [Google Scholar]
- 58.Kameyama K, Lee HK, Bear MF, Huganir RL (1998) Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron 21:1163–1175 [DOI] [PubMed] [Google Scholar]
- 59.Sanderson JL, Gorski JA, Dell'Acqua ML (2016) NMDA Receptor-Dependent LTD Requires Transient Synaptic Incorporation of Ca(2)(+)-Permeable AMPARs Mediated by AKAP150-Anchored PKA and Calcineurin. Neuron 89:1000–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coultrap SJ, Freund RK, O'Leary H, Sanderson JL, Roche KW, Dell'Acqua ML, Bayer KU (2014) Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Rep 6:431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selvakumar B, Jenkins MA, Hussain NK, Huganir RL, Traynelis SF, Snyder SH (2013) S-nitrosylation of AMPA receptor GluA1 regulates phosphorylation, single-channel conductance, and endocytosis. Proc Natl Acad Sci U S A 110:1077–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39. [DOI] [PubMed] [Google Scholar]
- 63.Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JTR (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9:602–604 [DOI] [PubMed] [Google Scholar]
- 64.Isaac JT, Ashby MC, McBain CJ (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54:859–871 [DOI] [PubMed] [Google Scholar]
- 65.Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW (2007) Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J 26:4879–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park P, Sanderson TM, Amici M, Choi SL, Bortolotto ZA, Zhuo M, Kaang BK, Collingridge GL (2016) Calcium-Permeable AMPA Receptors Mediate the Induction of the Protein Kinase A-Dependent Component of Long-Term Potentiation in the Hippocampus. J Neurosci 36:622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinnen BL, Bowen AB, Forte JS, Hiester BG, Crosby KC, Gibson ES, Dell'Acqua ML, Kennedy MJ (2017) Optogenetic Control of Synaptic Composition and Function. Neuron 93:646–660 e645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GC, Kitamura K, Kano M, Matsuzaki M, Kasai H (2011) In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J Physiol 589:2447–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hosokawa T, Rusakov DA, Bliss TVP, Fine A (1995) Repeated confocal imaging of individual dendritic spines in the living hippocampal slice: Evidence for changes in length and orientation associated with chemically induced LTP. J Neurosci 15:5560–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsuzaki M, Ellis-Davies GCR, Nemoto T, Miyashita Y, Iino M, Kasai H (2001) Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci 4:1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429:761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pi HJ, Otmakhov N, El Gaamouch F, Lemelin D, De Koninck P, Lisman J (2010) CaMKII control of spine size and synaptic strength: role of phosphorylation states and nonenzymatic action. Proc Natl Acad Sci U S A 107:14437–14442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Isaac JTR, Nicoll RA, Malenka RC (1995) Evidence for silent synapses: Implications for the expression of LTP. Neuron 15:427–434 [DOI] [PubMed] [Google Scholar]
- 74.Sanderson JL, Gorski JA, Gibson ES, Lam P, Freund RK, Chick WS, Dell'Acqua ML (2012) AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J Neurosci 32:15036–15052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hohne-Zell B, Ecker A, Weller U, Gratzl M (1994) Synaptobrevin cleavage by the tetanus toxin light chain is linked to the inhibition of exocytosis in chromaffin cells. FEBS Letters 355:131–134 [DOI] [PubMed] [Google Scholar]
- 76.Lu Y, Zhang M, Lim IA, Hall DD, Allen M, Medvedeva Y, McKnight GS, Usachev YM, Hell JW (2008) AKAP150-anchored PKA activity is important for LTD during its induction phase. J Physiol 586:4155–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 12:2685–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]