Abstract
Dysregulation of the cyclin dependent kinase pathway in luminal breast cancer creates a new therapeutic opportunity for estrogen receptor positive breast cancer. Initial pan-CDK inhibitors were associated with extensive toxicities but in recent years, the development of potent specific CDK inhibitors with favorable tolerability has driven renewed interests in this class of targeted therapies. Palbociclib, ribociclib and abemaciclib are specific CDK4/6 inhibitors that have been approved by the U.S. Food and Drug Administration for use in combination with endocrine therapy for women with advanced hormone receptor positive breast cancer. These three anticancer therapeutics were approved based on progression free survival benefit seen on phase III trials with the most common grade 3 treatment-related side effects being neutropenia, fatigue, nausea and diarrhea. Except for estrogen receptor positivity, no biomarkers predictive of response to CDK4/6 inhibitors have been identified to date. Based on mechanistic insights here described, CDK4/6 inhibitors are currently being explored in combination with other agents, including targeted therapies, immunotherapy and chemotherapy.
Keywords: CDK4, CDK6, Breast cancer, Estrogen receptor, Endocrine resistance
1. Introduction
By the end of 2018, 1,735,350 new cancer cases and 609,640 cancer deaths are projected to occur in the United States. Of these, breast cancer accounts for 15% (268,670) of all new cancer cases and 7% (41,400) of all cancer-related deaths (Siegel, Miller, & Jemal, 2018). Although significant progress has been made in early diagnosis and treatment, molecular heterogeneity and drug resistance have impeded successful treatment of this disease. Each year, the number of breast cancer cases is projected to increase in the United States, and treatment of metastatic breast cancer adds a significant burden to health care and the economy (Montero, Eapen, Gorin, & Adler, 2012).
Most cases of breast cancer are estrogen receptor (ER) positive (Li, Daling, & Malone, 2003) and the ER status can predict response to endocrine therapy. These tumors are usually associated with a better overall survival (OS) (Clahsen et al., 1999) and recurrences occur at a steady rate up to 20 years (Pan et al., 2017), while ER negative breast cancers recur in the first 3 to 5 years. Acquired or adaptive endocrine resistance is common and may occur in 40–50% of ER positive tumors (Osborne & Schiff, 2011).
Gene expression studies in the last two decades have provided valuable insight into the molecular makeup of the heterogeneity that exists across breast cancers. Regulation of cell cycle has emerged as a prominent pathway that promotes overall breast cancer progression and endocrine resistance (aFinn, Aleshin, & Slamon, 2016). The complex mechanism of cell division is controlled by specific phases with unique functions. In the G1-phase, cells divide/grow or enter quiescence, G0, based on external stimuli. Once the decision has been made to divide, the cell enters the S-phase, where DNA replication/synthesis occurs. The next step is the G2-phase where the cells prepare for mitosis followed by the M-phase where the actual division of genetic material and cytoplasm (cytokinesis) takes place resulting in two daughter cells (Clarke & Allan, 2009; King & Cidlowski, 1995). Different cyclin dependent kinases (CDKs) have unique roles at the various phases of the cell cycle. Specifically, CDK4 and 6 form complexes with one of three D-type cyclins (D1, D2, D3) in specific cellular contexts at the G1-phase. The CDK4/6-cyclin D complex hypo-phosphorylates the retinoblastoma 1 (RB1) gene and prepares it for hyper-phosphorylation by the CDK2-cyclinE complex later in the G1-phase prompting the release of the E2F transcription factors that are critical for entry into S phase (Ingham & Schwartz, 2017). Thus, small molecule inhibitors of CDK4/6 inhibit cell cycle progression by inhibiting phosphorylation of RB1 thereby inducing reversible G1-phase cell-cycle arrest in RB1-positive tumors. (Dean, Thangavel, McClendon, Reed, & Knudsen, 2010; Finn et al., 2009; Konecny et al., 2011) (Fig. 1).
Fig. 1.
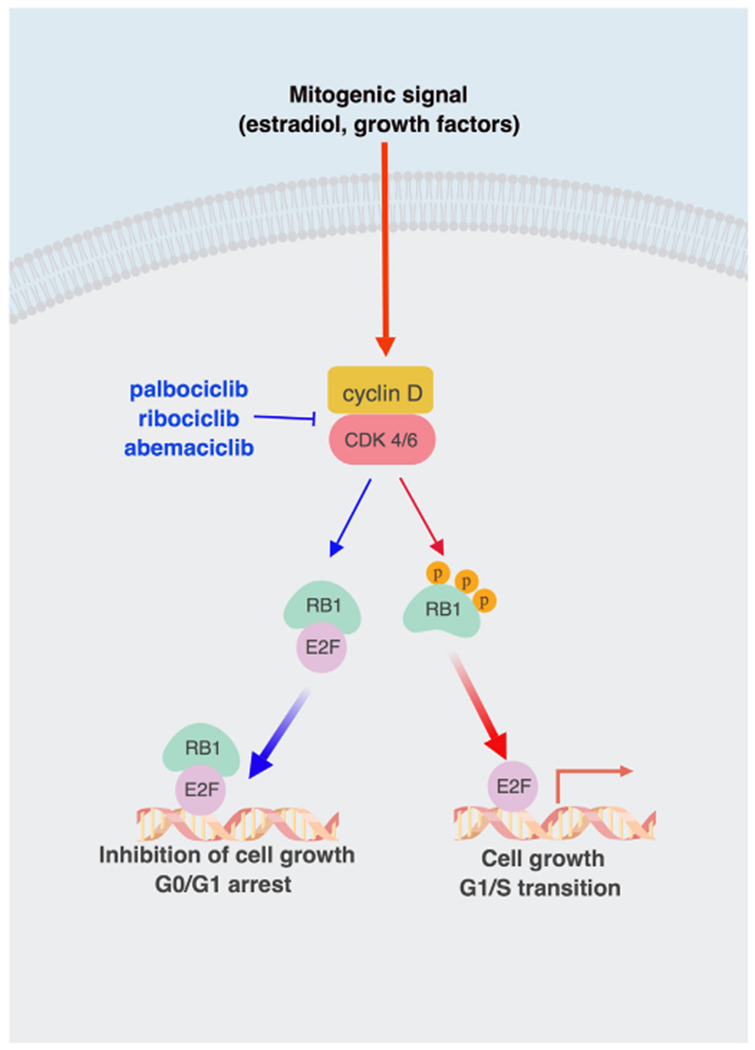
Schematic diagram illustrating the role of CDK4/6 inhibitors in cancer cells. External mitogenic signaling (red arrow) promotes complex formation between CDK4/6 and cyclin D. This CDK4/6-cyclin D complex facilitates the hyper-phosphorylation of RB1, release of E2F transcription factor and transition from G1 into S phase resulting in cell growth. CDK4/6 inhibitors such as palbociclib, ribociclib or abemacicbil (blue arrows) inhibit phosphorylation of RB1 that remains bound to E2F transcription factor and thereby induce cell G1/S cycle arrest arrest resulting in inhibition of cell growth. (Created with BioRender).
Unrestricted cell division in cancer cells is promoted by an intricate signaling mechanism that includes CDKs and cyclins (Landis, Pawlyk, Li, Sicinski, & Hinds, 2006). Increased CDKs, cyclins or decreased levels of endogenous CDK inhibitors such as CDKN2A (INK4/p16) or CDKN1A (Cip/p21) have been reported in various cancers (Ingham & Schwartz, 2017). Data from The Cancer Genome Atlas (TCGA) point to an association between dysregulation of the cyclin D1-CDK 4/6-Rb pathway and luminal B type breast cancer (Cancer Genome Atlas Network, 2012). Luminal B tumors as compared with luminal A tumors are more frequently associated with cyclin D1 gene amplification (58% vs 29% respectively), gain of CDK4 (25% vs 14% respectively) and loss of negative regulators, including p16 and p18. Dysregulation of the CDK pathway in luminal tumors may explain the increased activity of these drugs in ER positive cell lines. Based on these findings, blockage of CDK4/6 is a rational approach to restoring cell cycle control in ER positive breast cancer.
Initial pan-CDK inhibitors proved to be unsuccessful in inhibiting cancer cells and were associated with extensive toxicities (Jessen et al., 2007; Shapiro, 2006). However, in recent years, the development of potent specific CDK inhibitors with favorable tolerability has driven renewed interests in this class of targeted therapies (Dukelow, Kishan, Khasraw, & Murphy, 2015). Prolonged treatment with CDK4/6 inhibitors leads to suppression of genes involved in cell cycle, while inducing other genes involved in multiple processes. In fact, CDK4/6 inhibition has also been associated with induction of genes associated with cell growth that are antagonized by endocrine therapy (Knudsen & Witkiewicz, 2016; Knudsen & Witkiewicz, 2017) which further supports the rationale of combining both agents.
Palbociclib (PD0332991), ribociclib (LEE011) and abemaciclib (LY2835219) are three orally active, highly specific inhibitors that bind to the ATP cleft of CDK4 and CDK6 with minimal toxicity (Fig. 1). All three agents have completed evaluation on randomized phase III studies and have received approval by the US Food and Drug Administration (FDA) for use in hormone receptor (HR)-positive breast cancers. Here we review the preclinical and clinical studies with CDK4/6 inhibitors specifically in breast cancer. A comprehensive understanding of the mechanisms of action may provide insight into possible causes of resistance and also the best combination approaches for HR-positive breast cancer.
2. Preclinical studies with CDK 4/6 in breast cancer models
2.1. Palbociclib
Palbociclib (PD0332991, IBRANCE®, Pfizer Inc.) is an orally active potent small molecule inhibitor of CDK4/6 likely to bind to the ATP cleft (Fry et al., 2004). In vitro studies using a panel of breast cancer cell lines show that ER positive breast cancer cells were sensitive to palbociclib with significant decrease in cell cycle progression, through G1 arrest, and blocking hyper-phosphorylation of pRb. Overall, increased Rb and cyclin D1 as well as decreased p16 were associated with sensitivity to the effects of palbociclib on the cell cycle and growth inhibition (Dean et al., 2010; Finn et al., 2009). Least activity was found in non-luminal/basal breast cancer cells except for those with human epidermal growth factor receptor 2 (HER2)- amplification. Palbociclib was synergistic with antiestrogen tamoxifen and anti-HER2 therapy trastuzumab in ER-positive and HER2 amplified cell lines, respectively. Furthermore, palbociclib enhanced sensitivity to tamoxifen in breast cancer cell lines that had acquired resistance to tamoxifen (Finn et al., 2009) and was capable of inducing aspects of cellular senescence in hormone therapy refractory cell lines (Thangavel et al., 2011).
While most preclinical studies with palbociclib have focused on HR-positive breast cancer models, limited preclinical studies have shown some promise with HER2-positive or triple negative breast cancer (TNBC) (Asghar et al., 2017; Witkiewicz, Cox, & Knudsen, 2014). Initial studies in HR-positive, HER2-positive breast cancer cells with luminal features showed increased sensitivity to palbociclib (Finn et al., 2009) and had paved the way to test combination studies in HER2-positive breast cancer models. Studies in HER2-positive breast cancer models and primary human explants showed increased sensitivity to palbociclib and an additive effect in combination with ado-trastuzumab emtansine (T-DM1) (Witkiewicz et al., 2014). In cell models representing luminal androgen receptor (LAR) TNBC subgroup, inhibition of PI3K - phosphoinositide 3-kinase (PI3K) signaling sensitized cells to CDK4/6 inhibition, suggesting that combination of PI3K inhibitors and CDK4/6 inhibitors may be a therapeutic strategy for this subset of TNBC (Asghar et al., 2017).
2.2. Ribociclib
Ribociclib (LEE011; KISQALI; Novartis Pharmaceuticals Corps.) is an orally bioavailable, small molecule inhibitor of both CDK4 and CDK6 that induces complete dephosphorylation of Rb, resulting in sequestration of the E2F transcription factors and G1 cell cycle arrest in Rb-positive cell lines (Rader et al., 2013). In vivo, significant tumor growth inhibition was seen in four ER-positive xenograft models with ribociclib alone and ribociclib with letrozole or fulvestrant (O’Brien et al., 2014). In TNBC models, combination of ribociclib and BYL719 (PI3Kα inhibitor; alpelisib) cooperatively increased cell-cycle arrest, DNA damage, replicative stress, increased tumor antigen presentation as well as immunogenic cell death. Findings in immunocompetent mice revealed that ribociclib and BYL719 increased activation and cytotoxicity of both adaptive and innate immune cell populations as well as decreased frequency of immune-suppressive monocytic myeloid-derived suppressor cells (MDSCs) within the tumor environment (Teo et al., 2017).
2.3. Abemaciclib
Abemaciclib (LY2835219; VERZENIO™, Eli Lilly and Company) binds the ATP cleft and forms a hydrogen bond with a catalytic residue (Lys43) that is conserved among kinases, suggesting it binds with less selectivity than ribociclib and palbociclib (Chen et al., 2016). Abemaciclib inhibits CDK4 and CDK6 and Rb phosphorylation resulting in G1 arrest and inhibition of proliferation, and its activity is specific for Rb-proficient cells. In preclinical models, abemaciclib showed greater affinity for CDK4 in comparison to palbociclib or ribociclib, which may also explain the different toxicity profile. In vivo, equivalent levels of tumor growth inhibition to abemaciclib were observed in ER-positive, HER2-positive and biomarker selected TNBC xenografts (O’Brien et al., 2018). In HER2-positive breast cancer models, cyclin D1-CDK4 pathway mediates resistance to anti-HER2 therapy, and targeting resistant tumor cells with abemaciclib re-sensitizes them to anti-HER2 therapy by increasing their dependence on EGFR-family kinase signaling (Goel et al., 2016). Preclinical studies in rodent models showed that abemaciclib crosses the blood-brain barrier (Raub et al., 2015). Abemaciclib has been shown to enhance the efficacy of several chemotherapeutic agents in multi-drug resistant (MDR) MCF ER-positive breast cancer cells, mediated by inhibition of ABCB1/ABCG2 transport function, leading to an increase of intracellular chemotherapeutic agent accumulation. Interestingly, the reversal of MDR by abemaciclib was independent of the inhibition of CDK4/6 and the blockage of the Rb pathway phosphorylation (Wu et al., 2017).
3. Clinical studies with CDK4/6 inhibitors in breast cancer
3.1. Palbociclib
Palbociclib was the first CDK4/6 inhibitor to be developed in the clinical trial setting. It was initially evaluated as a single agent in two phase I dose escalation trials in patients with advanced malignancies (Flaherty et al., 2012; Schwartz et al., 2011). Overall it was well tolerated with dose-limiting toxicities (DLTs) related primarily to myelosuppresion. The dose and schedule selected to be used in subsequent trials was 125 mg once daily for 14 days followed by 7 days off (2 weeks on/1 week off). The results seen in phase I led to a single arm phase II study of palbociclib given as monotherapy in patients with Rb-positive advanced breast cancer (Demichele et al., 2015). Of 128 patients who consented for tumor Rb expression screening, 115 were Rb-positive, 5 were Rb-negative and 8 had no tissue available. Thirty-seven patients met other eligibility criteria and were enrolled. Of those, 19% had a clinical benefit rate (CBR), defined as partial response (PR) and stable disease (SD) for 24 months or longer. Median progression-free-survival (PFS) was 3.7 months overall but was significantly longer for those with HR-positive disease compared with HR-negative disease (4.5 vs 1.5 months, P = .03).
Subsequent studies evaluated palbociclib in combination with antiestrogen therapy, regardless of the Rb status, based on preclinical data demonstrating synergy of palbociclib with anti-estrogen therapy. The PALOMA-1 trial (Finn et al., 2015) was a multicenter, open-label phase II trial that enrolled 165 postmenopausal women with ER-positive, HER2-negative advanced breast cancer who had not received previous systemic treatment for advanced disease. Patients were randomized in a 1:1 fashion to letrozole 2.5 mg daily or letrozole 2.5 mg daily plus palbociclib. Initially patients were enrolled into two separate cohorts based on the requirement to have CCND1 amplification or loss of p16, or both. After an interim analysis suggesting that patient selection based on CCND1 or p16 loss was unlikely to improve patient outcomes, the design was modified and enrollment continued to one cohort only. Median investigator-assessed PFS was 20.2 months (95% CI 13.8–27.5) in the palbociclib plus letrozole arm and 10.2 months (95% CI 5.7–12.6) in the letrozole alone arm (hazard ratio [HR], 0.488, 95% CI 0.319–0.748). In addition, the ORR in patients with measurable disease was higher in the palbociclib plus letrozole compared with the letrozole alone arm (55.4% vs 39.4%). Palbociclib was the first CDK4/6 inhibitor to receive FDA accelerated approval in advanced breast cancer, on February 2015, based on the clinical benefit seen in PALOMA-1. The results of the PALOMA-2 trial, a phase III randomized double-blind placebo-controlled trial designed to confirm the results of PALOMA-1, provided further evidence of the benefit of using the combination in patients with metastatic breast cancer ER-positive, HER2-negative disease who have not received prior therapy for advanced breast cancer (bFinn et al., 2016).
Palbociclib has also been investigated in the second-line setting. In PALOMA-3 study, a phase III randomized (2:1) double blind study, 521 patients with HR-positive, HER2-negative breast cancer that relapsed or progressed on previous endocrine therapy were assigned to fulvestrant with palbociclib or placebo (Turner et al., 2015). Premenopausal or perimenopausal women were eligible if also treated with goserelin. The median age was 57 years and 20.7% of the patients were premenopausal. Women on the experimental arm (347 patients) had more than double median PFS (primary endpoint) compared with those in the placebo arm (9.2 months vs 3.8 months, HR 0.42, P < .001). The relative difference in primary outcome between the placebo and palbociclib groups was consistent regardless of menopausal status of the patients. Although the efficacy benefit was not as large as seen in the first-line setting, these results led to the expansion of the currently approved label of palbociclib in breast cancer to include combination with fulvestrant, in addition to aromatase inhibitors.
Palbociclib is overall well tolerated, either as monotherapy or in combination with anti-estrogen therapies. However, dosage reductions or treatment delays are often required primarily due to hematologic toxicity (dosage reduction was required in 40% of patients in PALOMA-1, 36% in PALOMA-2 and 34% in PALOMA-3). Surprisingly, although grade 3/4 neutropenia was reported in the majority of patients in the experimental arm, very few cases of febrile neutropenia or neutropenia-related infections were seen. Among the nonhematologic all-grade adverse events (AEs), the most common were fatigue (40%), nausea (25%), arthralgia (23%), and diarrhea (21%). Dose modifications are recommended for management of grade ≥ 3 AEs, with a first reduction to 100 mg and a second to 75 mg (FDA palbociclib label, accessed on March 17, 2018). Although grade ¾ neutropenia is frequent with palbociclib, dose modifications for grade ¾ neutropenia had no effect on PFS in the PALOMA 2 and 3 trials (Rugo et al., 2017; Verma et al., 2016).
3.2. Ribociclib
Ribociclib was the second CDK4/6 inhibitor to receive FDA approval for treatment of HR-positive, HER2-negative advanced breast cancer. In breast cancer cell lines and xenograft models, ribociclib showed inhibition of growth and synergistic antitumor activity when used with endocrine therapies (O’Brien et al., 2014). In the first-in-human phase I trial of adult patients with advanced solid tumors or lymphoma, 132 patients were enrolled. The maximum tolerated dose (MTD) and recommended dose for expansion (RDE) were established as 900 and 600 mg/day 3-weeks-on/1-week-off, respectively. Similar to palbociclib, common treatment-related AE were (all-grade; grade ¾) neutropenia (46%; 27%), leukopenia (43%; 17%), fatigue (45%; 2%), and nausea (42%; 2%). Asymptomatic Fridericia’s corrected QT prolongation was seen with ribociclib but specific to doses ≥ 600 mg/day (9% of patients at 600 mg/day; 33% at doses > 600 mg/day). There were 3 PR and 43 patients achieved a best response of SD (Infante et al., 2016).
Based on phase I data indicating the absence of drug-to-drug interaction between ribociclib and letrozole (Munster et al., 2014), the phase III trial MONALEESA-2 trial was launched. 668 postmenopausal women with ER-positive, HER2-negative advanced breast cancer without prior systemic treatment were randomized to ribociclib (600 mg/day, 3 weeks on/1 week off) plus letrozole (2.5 mg/day, continuous), or letrozole plus placebo. Median PFS was 16.0 months in the placebo arm and 25.3 months for the ribociclib arm (HR 0.568; 95% CI, 0.457–0.704, p < .001) (Hortobagyi et al., 2016; Hortobagyi et al., 2017). Adverse events were similar to those seen with palbociclib, with 59.3% of neutropenia in the ribociclib group vs. 0.9% in the placebo group, and leukopenia in 21% in the ribociclib group vs. 0.6% in the control group.
In March 2017, ribociclib in combination with an aromatase inhibitor as initial endocrine-based therapy for the treatment of postmenopausal women with HR-positive, HER2 — negative advanced breast cancer was approved by the FDA. Given increased risk of QT prolongation, the FDA label recommends to monitor electrocardiogram (ECG) and electrolytes prior to initiation of the treatment, and at the beginning of each cycle for 6 cycles. In addition to an ECG on day 14 of first cycle. The results of MONALEESA-7, the first phase III trial to look at the role of a CDK4/6 inhibitor with hormonal therapy and goserelin exclusively in premenopausal women, aromatase inhibitor with goserelin as initial hormonal therapy for advanced breast cancer significantly prolonged PFS compared to ET alone: 23.8 months (95% CI: 19.2 months-not reached) vs. 13.0 months (95% CI: 11.0–16.4 months); HR = 0.553; 95% CI: 0.441–0.694; p < .0001 (Tripathy et al., 2017). MONALEESA-3, a phase III trial comparing ribociclib plus fulvestrant vs. placebo with fulvestrant in women with ER-positive, HER2-negative advanced breast cancer (Germa et al., 2017) has completed accrual and results are expected in 2020.
3.3. Abemaciclib
Abemaciclib is the latest CDK4/6 inhibitor approved by the FDA in view of the results of the MONARCH 1 and 2 trials. In comparison to palbociclib and ribociclib, abemaciclib is 14 times more potent against CDK4 than against CDK6, which may explain the different toxicity profile. In the phase I studies, abemaciclib alone and in combination with antihormonal therapies, showed favorable toxicity profiles in patients with HR-positive metastatic breast cancer, with most common grade 3 treatment-related side effects being diarrhea, neutropenia, nausea and fatigue. No febrile neutropenia or grade 4 events were reported. The results were very encouraging with DCR of 81% and ORR 26% in heavily pretreated HR-positive patients (Patnaik et al., 2014; Tolaney et al., 2015).
These results led to the phase II trial MONARCH 1 that evaluated the single-agent activity and safety of abemaciclib in patients with refractory metastatic breast cancer whose disease had progressed following multiple prior treatments, including chemotherapy (Dickler et al., 2017). With a median of three lines of prior therapy for advanced disease and at the 8-month interim analysis, the confirmed odds risk ratio (ORR) was 17.4%, the CBR was 42.4%, and median PFS was 5.7 months. Of note 90.2% of patients had visceral disease and 50.8% had more than three sites of metastases. The early evidence of response and favorable toxicity profile prompted the launch of a phase III trial, the MONARCH 2, that compared abemaciclib plus fulvestrant vs. placebo with fulvestrant in women with ER-positive, HER2-negative advanced breast cancer (Sledge et al., 2017). Patients had experienced disease progression on or within 12 months of receiving endocrine treatment in the neoadjuvant or adjuvant setting or while receiving first-line endocrine therapy for metastatic disease. The combination arm was superior to fulvestrant alone in this group of patients: median PFS in the combination group was 16.4 months in comparison to 9.3 months of the fulvestrant alone group (HR 0.553; 95% CI: 0449–0.681; p < .001). In patients with measurable disease, abemaciclib plus fulvestrant achieved an ORR of 48.1% (95% CI, 42.6% to 53.6%) compared with 21.3% (95% CI, 15.1% to 27.6%) in the control arm. The most common AEs in the abemaciclib arm were diarrhea (86.4% vs. 24.7%), neutropenia (46.0% vs. 4.0%), nausea (45.1% vs. 22.9%), and fatigue (39.9% vs. 26.9%) when compared to placebo.
Based on these results, the FDA granted approval for abemaciclib on September 2017, in combination with fulvestrant in patients with HR-positive, HER2-negative advanced breast cancer who progressed following endocrine therapy. In addition, abemaciclib also received FDA approval as a single-agent therapy in a similar population to the one enrolled on MONARCH-1.
Since then the results of MONARCH 3 have been presented (Goetz et al., 2017), a phase III randomized trial comparing abemaciclib or placebo in combination with a nonsteroidal aromatase inhibitor (NSAI) in postmenopausal patients with advanced HR-positive, HER2 — negative advanced breast cancer who had no previous systemic therapies. The trial enrolled 493 patients. Addition of abemaciclib to NSAI significantly prolonged PFS with an observed HR of 0.54 (95% CI: 0.41–0.72; p < .000021), while the median PFS was not reached in the abemaciclib arm at interim analysis against 14.7 months in the placebo arm. ORR was 48.2% (95% CI: 42.8%–53.6%) in patients receiving abemaciclib versus 34.5% (95% CI: 27.3%–41.8%) in the placebo-assigned patients (p < .002). Consistent with MONARCH 1 and 2 results, the most frequent grade 3 or 4 AEs with abemaciclib compared to placebo were neutropenia (21.1% vs. 1.2%), diarrhea (9.5% vs. 1.2%), and leukopenia (7.6% vs. 0.6%). In February 2018, the FDA also approved abemaciclib in combination with an aromatase inhibitor as initial therapy for postmenopausal patients with advanced HR-positive, HER2-negative breast cancer.
3.4. Future development and clinical guidelines
Other ongoing phase II and III trials are currently looking at the combination of palbociclib, ribociclib or abemaciclib with various endocrine therapies in patients with early stage high risk disease after undergoing surgery or in the neoadjuvant setting. Current National Comprehensive Cancer Network (NCCN) guidelines (v 4.2017) recommend palbociclib or ribociclib in combination with an aromatase inhibitor for first line therapy of postmenopausal women who have not received endocrine therapy within the previous year. For premenopausal women and/or women who had prior endocrine therapy within 1 year, recommendation is to consider endocrine therapy with or without CDK4/6 inhibitor or mTOR inhibitor. The American Society of Clinical Oncology (ASCO) guidelines for the management of HR-positive metastatic breast cancer were last updated in 2016 (Rugo et al., 2016) and state that the preferred first-line endocrine therapy for postmenopausal women should be aromatase inhibitors with or without palbociclib. As second line therapy, fulvestrant is recommended with or without palbociclib, with addition of palbociclib limited to those without prior exposure to CDK4/6 inhibitors.
4. Biomarkers of sensitivity and resistance to CDK4/6 inhibitors
In vitro studies have shown that breast cancers with increased ER, cyclin D1 and Rb and low p16 levels show increased sensitivity to CDK4/6 inhibitors (Dean et al., 2010; Finn et al., 2009). Although ER status is useful to identify patients who will benefit from CDK4/6 inhibitors, the use of ER protein level as a biomarker for increased drug efficacy has not been confirmed in clinical trials (Cristofanilli et al., 2015). Moreover, IHC staining with cyclin D1 or p16 levels did not show differential benefit from palbociclib in patients with tumors with different degrees of expression for either of these proteins (Finn et al., 2015).
Validating levels of functional Rb protein in human tumor has been challenging due to multiple factors including the establishment of threshold that separates Rb-high from Rb-low tumors and the fact that total Rb protein levels do not reflect functionality (Garrido-Castro & Goel, 2017). However, since CDK4/6 inhibitors are approved for ER-positive breast cancer treatment, determination of Rb levels could potentially be helpful in personalizing treatment of patients with ER-positive breast cancer (Malorni et al., 2016). Furthermore, Rb levels, irrespective of ER status may be a more useful biomarker for CDK4/6 sensitivity since both Rb high ER-positive and TNBC cells have shown increased sensitivity to these drugs (Lehmann et al., 2011). Comparison of genotyping of tissue and peripheral blood from pre- and post-treatment (with palbociclib or ribociclib) samples show emergence of somatic RB1 mutations in patients with metastatic breast cancer (Condorelli et al., 2018).
Even though CDK4/6 inhibitors have shown promising results in the clinic, there are cases of de novo resistance, and eventually acquired resistance emerges months to years after initiation (Guarducci et al., 2017) In ER-positive breast cancer cell models, resistant clones show amplification of CDK6 and decreased expression of ER and PR and subsequent diminished response to antiestrogens (Yang et al., 2017). Wang et al. recently showed that human cancers expressing high levels of cyclin D3 and CDK6 respond to CDK4/6-inhibition by undergoing tumor cell death, while cancers that express other types of cyclin D-CDK4/6 complexes undergo cell cycle arrest. Thus, determination of cyclin D3-CDK6 levels in primary tumors may allow identification of cancers that are particularly amenable to anti-CDK4/6 therapy (Wang et al., 2017). In human colorectal carcinoma cells, upregulation of MYC-driven metabolic shifts has been reported following CDK4/6 inhibition suggesting possible synergistic effects with agents targeting glutaminolysis or PI3K/mTOR signaling (Tarrado-Castellarnau et al., 2017). Aberrant PI3K/mTOR signaling pathway is common in ER-positive breast cancers (Mayer & Arteaga, 2014) and PI3K inhibitors emerge as synergistic partners of CDK4/6 inhibitors (Franco, Witkiewicz, & Knudsen, 2014; Vora et al., 2014). Triple combination of endocrine therapy, CDK4/6, and PI3K inhibition was more effective than paired combinations, provoking rapid tumor regressions in a ER + breast cancer patient derived tumor xenograft (PDX) (Cortes et al., 2017; Herrera-Abreu et al., 2016).
Our understanding of the biological mechanisms of CDK4/6 inhibitors in cancer is incomplete and has only recently expanded beyond cell cycle regulation (Anders et al., 2011; Franco, Balaji, Frienkman, Witkiewicz, & Knuden, 2016). Studies in murine models of breast carcinoma showed that treatment with CDK4/6 inhibitors not only induces tumor cell cycle arrest, but also promotes anti-tumor immunity. In this study (Goel et al., 2017), treatment with CDK4/6 inhibitors increased tumor cells’ functional capacity to present antigens and reduced the immunosuppressive Treg population by suppressing their proliferation through suppression of the Rb-E2F axis, leading to reduced DNMT1 expression. Moreover, CDK4/6 inhibition was shown to enhance IL2 secretion and activate effector T cells (Deng et al., 2017). Thus, CDK4/6 inhibitors might enhance the susceptibility of such tumors to immune checkpoint blockade. More recently, early changes in circulating tumor DNA (ctDNA) level were explored as a predictor of response (O’Leary et al., 2018).
Despite the significant interest in determining predictors of responsiveness, the identification of specific biomarkers remains unclear. Significant efforts have been made to identify a biomarker predictive of response to CDK4/6 inhibitors without success to date (Andre, Stemmer, & Campone, 2017; cFinn et al., 2016; Cristofanilli et al., 2016; Finn et al., 2009; Finn et al., 2015; Turner et al., 2016). Strategies such as focusing biomarker analysis on the “extremes” (those who do not benefit from CDK4/6 inhibitors and “exceptional responders” on endocrine therapy alone) have been suggested. It is crucial that the effort to identify biomarkers of response continues as effective patient selection will maximise efficacy while minimizing toxicity and costs.
5. Combination therapies with CDK4/6 inhibitors
Based on the mechanistic insights mentioned above, CDK4/6 inhibitors are being explored in combination with other agents, including targeted therapies, immunotherapy and chemotherapy, and there are several ongoing clinical trials listed on clinicaltrials.gov (Table 1).
Table 1.
Ongoing combination trials (recruiting or not yet recruiting) with CDK4/6 inhibitors in breast cancer (www.clinicaltrials.gov, updated on 3/7/18).
| Drug | Phase | Arms | Planned n |
Trial status | NCT | Remarks |
|---|---|---|---|---|---|---|
| PALBOCICLIB | ||||||
| HR+/HER2− | ||||||
| CPI | ||||||
| Pembrolizumab | II | Palbociclib + letrozole + pembrolizumab | 22 | Recruiting | NCT02778685 | |
| Pan-PI3K inhibitor | ||||||
| Copanlisib | II | Palbociclib + fulvestrant ± copanlisib | 178 | Not yet recruiting | NCT03377101 | |
| Copanlisib | I/II | Palbociclib + letrozole + copanlisib | 102 | Recruiting | NCT03128619 | |
| α-specific PI3K inh | ||||||
| GDC-0077 | I | Palbociclib + letrozole + GDC-0077 | 156 | Recruiting | NCT03006172 | |
| mTOR inhibitors | ||||||
| Everolimus | I/II | Palbociclib + everolimus + exemestane | 32 | Recruiting | NCT02871791 | |
| Gedatolisib | Ib | Palbociclib + gedatolisib + letrozole or fulvestrant | 120 | Recruiting | NCT02684032 | |
| SERD/SERM | ||||||
| GDC-9545 | I | Palbociclib + GDC-9545 + LHRH | 130 | Recruiting | NCT03332797 | |
| SAR439859 | I/II | SAR439859 ± palbociclib | 156 | Recruiting | NCT03284957 | |
| Bazedoxifene | I/II | Palbociclib + Bazedoxifene | 37 | Recruiting | NCT02448771 | |
| FGFR inhibitor | ||||||
| Erdafitinib | I | Palbociclib + fulvestrant + erdafitinib | 32 | Recruiting | NCT03238196 | ER+/HER2-FGFR amplified |
| HER2+ | ||||||
| Trastuzumab + pertuzumab | I/II | Anastrozole + palbociclib + trastuzumab + pertuzumab | 36 | Recruiting | NCT03304080 | ER+ only |
| Trastuzumab | II | Letrozole + palbociclib + trastuzumab | 48 | Recruiting | NCT02907918 | Neoadjuvant ER+ only |
| Trastuzumab | II | Palbociclib + trastuzumab ± letrozole | 138 | Recruiting | NCT02448420 | |
| Anti-HER2 therapy | III | Anti-HER2 Therapy + ET ± palbociclib | 496 | Recruiting | NCT02947685 | |
| TDM1 | Ib | Palbociclib + TDM1 | 17 | Recruiting | NCT01976169 | |
| Tucatinib | Ib/II | Palbociclib + letrozole + tucatinib | 40 | Not yet recruiting | NCT03054363 | ER+ only |
| AR+ | ||||||
| AR antagonists | ||||||
| Bicalutamide | I/II | Palbociclib + bicalutamide | 51 | Recruiting | NCT02605486 | |
| RIBOCICLIB | ||||||
| HR+ HER2− | ||||||
| CPI | ||||||
| PDR001 | I | Ribociclib + PDR001 + fulvestrant | 60 | Recruiting | NCT03294694 | |
| SERD | ||||||
| LSZ102 | I/Ib | LSZ102 | 312 | Recruiting | NCT02734615 | |
| LSZ102 + ribociclib | ||||||
| LSZ102 + alpelisib | ||||||
| α-specific PI3K inh | ||||||
| Alpelisib | Ib/II | Ribociclib + alpelisib + letrozole | 253 | Recruiting | NCT01872260 | |
| mTOR inhibitors | ||||||
| Everolimus | I/II | Ribociclib + everolimus + exemestane | 51 | Recruiting | NCT02732119 | |
| HER2− | ||||||
| Capecitabine | I | Ribociclib + capecitabine | 52 | Recruiting | NCT02754011 | |
| Any HR/HER2 | ||||||
| Paclitaxel | I | Riboclicib + paclitaxel | 28 | Recruiting | NCT02599363 | |
| HER2+ | ||||||
| Trastuzumab or TDM1 | Ib/II | Ribociclib + TDM1 | 86 | Recruiting | NCT02657343 | |
| Ribociclib + trastuzumab ± fulvestrant | ||||||
| AR+ | ||||||
| AR antagonists | ||||||
| Bicalutamide | I/II | Ribociclib + bicalutamide | 58 | Recruiting | NCT03090165 | |
| ABEMACICLIB | ||||||
| HR+ HER− | ||||||
| CPI | ||||||
| LY3300054 | I | Abemaciclib + LY3300054 | 115 | Recruiting | NCT02791334 | |
| mTOR and PI3K inhibitos | ||||||
| Everolimus | I | Abemaciclib + everolimus + exemestane | 123 | Recruiting | NCT02057133 | |
| LY3023414 | Abemaciclib + LY3023414 + fulvestrant | |||||
| LY3023414 | I | Abemaciclib + LY3023414 + Letrozole | 130 | Recruiting | NCT01655225 | |
| IGF antibody | ||||||
| Xentuzumab | I | Abemaciclib + xentuzumab ± ET | 88 | Recruiting | NCT03099174 | |
| HER2+ | ||||||
| Trastuzumab | I | Abemaciclib + trastuzumab | 123 | Recruiting | NCT02057133 | |
| Trastuzumab | II | Abemaciclib + trastuzumab ± fulvestrant vs chemotherapy | 225 | Not yet recruiting | NCT02675231 |
Legend: ET – endocrine therapy; IGF - insulin like growth factor; HR – hormone receptor; HER2 – human epidermal receptor2; AR – androgen receptor; TDM1 – ado trastuzumab; FGFR – fibroblast growth factor receptor; SERD – selective estrogen receptor degrader; SERM – selective estrogen receptor modulator; mTOR – mammalian target of rapamycin; CPI – checkpoint inhibitor; PI3K - Phosphatidylinositol-3 kinase.
5.1. Combination with agents targeting the PI3K/mTOR pathway
As mentioned above, preclinical data suggests that there may be a synergistic effect when agents targeting the PI3K/mTOR pathway are added to CDK4/6 inhibition (Augereau et al., 2017; Cortes et al., 2017; Herrera-Abreu et al., 2016). Preliminary data on small numbers of patients have been reported with the use of mTOR inhibitors (everolimus) and α-specific PI3K inhibitor (alpelisib) with ribociclib and abemaciclib. A phase Ib trial of ribociclib, everolimus and exemestane with 83 heavily pretreated patients with HR + HER2 advanced breast cancer reported an ORR 13%. Twenty three percent of the patients had received prior PI3K/AKT/mTOR inhibitors (Oliveira, Chavez-MacGregor, & Modi, 2016). The triple therapy was overall well tolerated and the safety profile was broadly consistent with everolimus and exemestane combination. Abemaciclib was tested in combination with everolimus and exemestane in 19 patients with HR+ HER2− metastatic breast cancer. Of 15 patients evaluable for response, the ORR was 33% and the clinical benefit rate at 6 months was 73%. The most common treatment related AEs included fatigue, gastrointestinal and haematological toxicities, stomatitis, and rash. A phase Ib trial of letrozole, ribociclib and alpelisib showed some preliminary evidence of response. Among 27 evaluable patients, 2 (7%) had a PR, 4 (15%) had unconfirmed partial response and 6 (22%) had SD. 33% of patients had previously received PI3K/AKT/mTOR inhibitors. A total of 8 (22%) patients discontinued treatment due to toxicity, being the most frequent nausea (all grades, 44%; G¾, 6%), hyperglycemia (44%; 17%), neutropenia (42%; 22%), and fatigue (36%; 11%) (Juric et al., 2016).
Currently there are several ongoing trials exploring triplet combinations in patients with HR+ HER2− advanced breast cancer with palbociclib, ribociclib and abemaciclib (Table 1). Different agents targeting the PBK/mTOR pathway are part of the triplet approach including pan-PI3k inhibitors (copanlisib), α-specific PI3K inhibitors (alpelisib, GDC-0077), PI3K/mTOR dual inhibitor (LY3023414) and mTOR inhibitors (everolimus).
5.2. Combination with checkpoint inhibitors
The possible role of CDK4/6 inhibition in promoting anti-tumor immunity in animal models of breast cancer was further confirmed in the NeoPalAna trial (Ma et al., 2017). As part of this neoadjuvant study, patients with primary ER-positive breast cancer underwent serial tumor biopsies and gene expression data were obtained. When biopsies taken before initiation of palbociclib were compared to those after 12 weeks of treatment in terms of upregulated genes, the most common signatures were “allograft rejection”, “inflammatory response” and “interferon gamma response”. Interestingly, these gene sets were already significantly upregulated after 2 weeks of palbociclib treatment. These signatures have also been described as being upregulated in xenograft models. Currently there are ongoing trials looking at the potential benefit of adding checkpoint inhibitors to CDK4/6 inhibitors (LY3300054, PDR001 and pembrolizumab) with the expectation that it may cause more prolonged responses or even cures (Table 1).
5.3. Combination with chemotherapy
Preclinical data suggest that CDK4/6 inhibitors should not be combined with DNA damaging therapies with the risk of decreasing antitumor activity (Roberts et al., 2012). However, the concept of combining pharmacological quiescence induced by the CDK4/6 inhibitors with effective cytotoxic chemotherapy seems very attractive (Knudsen & Witkiewicz, 2017). Therefore, strategies looking at intermittent dosing of CDK4/6 inhibitors have been explored to allow successful combination of CDK4/6 inhibitors and chemotherapy have been pursued.
Based on mouse xenografts showing that CDK4/6 inhibition is synergistic with 5FU in suppressing tumor growth, a phase I study with this combination enrolled 29 heavily pretreated patients with solid tumors. RP2D was palbociclib 100 mg daily 7 days on 7 days off with 5FU 2400 mg/m2 continuous infusion administered on days 8–10. Of the 26 evaluable patients, one experienced a confirmed PR (breast) and 6 SD for a DCR of 27% (Pishvaian et al., 2016). Most patients had colorectal (19) or breast (6) cancer. The combination was overall well tolerated.
Palbociclib was also investigated in combination with weekly pacli-taxel in patients with metastatic breast cancer. Palbociclib was taken on days 2–6, 9–14, 16–20 of each 28-day cycle. Among 15 patients enrolled, 11 experienced a PR or SD, and of those 8 continued on treatment for >6 months (Clark et al., 2014). Because grade ¾ neutropenia was common and frequently led to dose reduction or dose interruption, an additional phase 1 expansion was started to examine palbociclib on a 3-day schedule (day 2–4, 9–11 and 16–18). Currently there are ongoing trials looking at the combination of ribociclib with capecitabine and ribociclib with paclitaxel in patients with advanced breast cancer.
5.4. Combination with HER2 targeted therapies
Finn et al. tested the growth inhibitory effects of palbociclib in a large panel of breast cancer cell lines and identified potent activity in two therapeutic groups: those that were ER-positive and HER2-amplified, while nonluminal/basal cell types were most resistant (Finn et al., 2009). They also demonstrated synergistic anti-tumor effect when combining tamoxifen and palbociclib in ER-positive cell lines and trastuzumab and palbociclib in HER2-amplified cell lines, respectively. CDK4/6 inhibition has been shown to overcome resistance to targeted therapy in HER2-positive breast cancer by increasing tumor cell dependence on EGFR family kinase signaling. In PDX models, CDK4/6 inhibitors were able to resensitize tumors to HER2 targeted therapies (Goel et al., 2016). This combination has been tested in NA-PHER, a neoadjuvant study that enrolled 36 patients with HR-positive HER2-positive breast cancer. Patients were treated every 3 weeks with trastuzumab and pertuzumab for six cycles plus oral palbociclib 125 mg once a day for 21 days in a 4-week cycle and fulvestrant 500 mg every 4 weeks for five cycles. At baseline, geometric mean Ki67 expression was 31.9% (SD 15.7), versus 4.3% (15.0) at week 2 (n = 25; p < 0·0001) and 12.1% (20.0) at time of surgery (n = 22; p = .013). At surgery, eight (27%) patients had a pathological complete response (pCR) in breast and axillary nodes (Gianni et al., 2018). Currently all three CDK4/6 inhibitors are being tested with antiHER2 targeted therapies including trastuzumab, pertuzumab, TDM1 and tucatinib.
5.5. Combination with radiation therapy
Preclinical studies of palbociclib in combination with radiation therapy for the treatment of glioblastoma have shown this to be an effective treatment modality (Whittaker et al., 2017). Currently there are two ongoing studies of palbociclib with radiation in patients with head and neck carcinomas (NCT03389477 and NCT03024489). There is lack of data on the efficacy and safety of the combination of radiation therapy and CDK4/6 inhibitors in breast cancer. A small study reported results of 5 patients who received palliative radiation therapy concurrently with palbociclib and concluded that on this small group of patients there was no increased toxicity (Hans, Cottu, & Kirova, 2018).
6. Conclusion and outlook
CDK4/6 inhibitors have dramatically changed the landscape of treatment for patients with HR-positive advanced breast cancer. All the three FDA approved drugs (palbociclib, ribociclib and abemaciclib) have been associated with improved outcomes and acceptable toxicities when compared to endocrine therapy alone. If ongoing trials show evidence of clinical benefit, it is possible that in the near future CDK4/6 inhibitors will have a role in the neoadjuvant or adjuvant setting. Unfortunately, resistance eventually emerges and new strategies to delay or overcome resistance are currently being investigated. Studies focused on identification of patients who can be treated initially with endocrine therapy alone are currently being planned and are of paramount importance.
Acknowledgements
Filipa Lynce is the recipient of an ASPIRE award for Breast Cancer Research from Pfizer. Ayesha N. Shajahan-Haq is the recipient of a Public Health Service award R01CA201092.
Abbreviations:
- AEs
adverse events
- ASCO
American Society of Clinical Oncology
- CBR
clinical benefit rate
- CCND1
cyclin D1
- CDKs
cyclin dependent kinases
- CDK4/6
cyclin dependent kinase 4 and 6
- DLTs
dose-limiting toxicities
- ECG
electrocardiogram
- ER
estrogen receptor
- FDA
US Food and Drug Administration
- HER2
human epidermal receptor 2
- HR
hormone receptor
- HR
hazard ratio
- IHC
immunohistochemistry
- LAR
luminal androgen receptor
- MDR
multidrug resistance
- MDSCs
monocytic myeloid-derived suppressor cells
- MTD
maximum tolerated dose
- mTOR
mechanistic target of rapamyrin
- NCCN
National Comprehensive Cancer Network
- NSAI
nonsteroidal aromatase inhibitor
- ORR
odds risk ratio
- OS
overall survival
- pCR
pathological complete response
- PDX
patient derived tumor xenograft
- PFS
progression free survival
- PI3K
phosphoinositide 3-kinase
- PR
progesterone receptor
- PR
partial response
- RB1
retinoblastoma 1 (gene)
- pRB
retinoblastoma (protein)
- RDE
recommended dose for expansion
- RP2D
recommended phase 2 dose
- SD
stable disease
- TCGA
The Cancer Genome Atlas
- TDM1
ado-trastuzumab emtansine
- TIL
tumor infiltrating lymphocytes
- TNBC
triple negative breast cancer
Footnotes
Conflict of interest statement
FL: Bristol-Myers-Squibb, Calithera, Chugai, Pfizer, Regeneron, Tesaro (Research to Institution). ASH has no conflicts of interest to declare. SMS: Novartis, Pfizer, and Roche (Honoraria); AstraZeneca, Genentech/Roche, Inivata, Lilly, and Pieris Pharmaceuticals (Consulting/Advisory); Merrimack Pharmaceuticals, Genentech/Roche, Lilly, Pfizer, Puma Biotechnology, and Roche (Research to Institution); Caris Life Sciences, Genentech/Roche, and Inivata (Travel).
References
- aFinn RS, Aleshin A, & Slamon DJ (2016). Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Research 18 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, … Sicinski P (2011). A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescense suppression in cancer cells. Cancer Cell 20(5), 620–634 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre F, Stemmer S, & Campone M (2017). Ribociclib+ letrozole for first-line treatment of HR+, HER2− advanced breast cancer: Efficacy by baseline tumor markers. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics: Abstract CT045. [Google Scholar]
- Asghar US, Barr AR, Cutts R, Beaney M, Babina I, Sampath D, … Turner NC (2017). Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clinical Cancer Research 23(18), 5561–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augereau P, Patsouris A, Bourbouloux E, Gourmelon C, Abadie Lacourtoisie S, Rigaud B, … Campone M (2017). Hormonoresistance in advanced breast cancer: A new revolution in endocrine therapy. Therapeutic Advances in Medical Oncology 9(5), 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- bFinn R, Martin M, Rugo HS, Jones SE, Im SA, Gelmon K, … Slamon DJ (2016). Palbociclib and Letrozole in advanced breast Cancer. The New England Journal of Medicine 375, 1925–1936. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network (2012). Comprehensive molecular portraits of human breast tumours. Nature 490(7418), 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- cFinn R, Jiang Y, Rugo H, Moulder SL, Im S, Gelmon KA, … Slamon D (2016). Biomarker analyses from the phase 3 PALOMA-2 trial of palbociclib (P) with letrozole (L) compared with placebo (PLB) plus L in postmenopausal women. Annals of Oncology 27(6), 1–36. [Google Scholar]
- Chen P, Lee NV, Hu W, Xu M, Ferre RA, Lam H, … Murray BW (2016). Spectrum and degree of CDK drug interactions predicts clinical performance. Molecular Cancer Therapeutics 15(10), 2273–2281. [DOI] [PubMed] [Google Scholar]
- Clahsen PC, van de Velde CJ, Duval C, Pallud C, Mandard AM, Delobelle-Deroide A, van de Vijver MJ (1999). The utility of mitotic index, oestrogen receptor and Ki-67 measurements in the creation of novel prognostic indices for node-negative breast cancer. European Journal of Surgical Oncology 25(4), 356–363. [DOI] [PubMed] [Google Scholar]
- Clark AS, O’Dwyer PJ, Heitjan D, Lal P, Feldman MD, Gallagher M, … DeMichele A (2014). A phase I trial of palbociclib and paclitaxel in metastatic breast cancer. Journal of Clinical Oncology 32(15_suppl). 10.1200/jco.2014.32.15_suppl.527 527–52724419114 [DOI] [Google Scholar]
- Clarke PR, & Allan LA (2009). Cell-cycle control in face of damage—A matter of life or death. Trends in Cell Biology 19(3), 89–98. [DOI] [PubMed] [Google Scholar]
- Condorelli R, Spring L, O’Shaughnessy J, Lacroix L, Bailleux C, Scott V, Bardia A (2018). Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Annals of Oncology 29(3), 640–645. 10.1093/annonc/mdx784. [DOI] [PubMed] [Google Scholar]
- Cortes J, Im SA, Holgado E, Perez-Garcia JM, Schmid P, & Chavez-MacGregor M (2017). The next era of treatment for hormone receptor-positive HER2-negative advanced breast cancer: Triplet combination-based endocrine therapies. Cancer Treatment Reviews 61, 53–60. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, & Slamon D (2016). Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. The Lancet Oncology 17(4), 425–439. [DOI] [PubMed] [Google Scholar]
- Dean JL, Thangavel C, McClendon AK, Reed CA, & Knudsen ES (2010). Therapeutic CDK4/6 inhibition in breast cancer: Key mechanisms of response and failure. Oncogene 29(28), 4018–4032. [DOI] [PubMed] [Google Scholar]
- DeMichele A, Clark AS, Tan KS, Heitian DF, Gramlich K, Gallagher M, … O’Dwyer (2015). CDK4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: Phase II activity, safety, and predictive biomarker assessment. Clinical Cancer Research 21(5), 995–1001. [DOI] [PubMed] [Google Scholar]
- Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Wong K-K (2017). CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discovery 8(2), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, Patt D, … Baselga J (2017). MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clinical Cancer Research 23(17), 5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukelow T, Kishan D, Khasraw M, & Murphy CG (2015). CDK4/6 inhibitors in breast cancer. Anti-Cancer Drugs 26(8), 797–806. [DOI] [PubMed] [Google Scholar]
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, … Slamon DJ (2015). The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. The Lancet Oncology 16(1), 25–35. [DOI] [PubMed] [Google Scholar]
- Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, … Slamon DJ. (2009). PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Research 11(5), R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Lorusso PM, Demichele A, Abramson VG, Courtney R, Randolph SS, & Schwartz GK (2012). Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD0332991, administered using a 21-day schedule in patients with advanced cancer. Clinical Cancer Research 18(2), 568–576. [DOI] [PubMed] [Google Scholar]
- Franco J, Balaji U, Frienkman E, Witkiewicz AK, & Knuden ES (2016). Metabolic reprogramming of pancreatic Cancer mediated by CDK4/6 inhibition elicits unique vulnerabilities. Cell Reports 14(5), 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco J, Witkiewicz AK, & Knudsen ES (2014). CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget 5(15), 6512–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, & Trachet E (2004). Specific inhibition of cyclin-dependent kinase 4/6 by PD0332991 and associated antitumor activity in human tumor xenografts. Molecular Cancer Therapeutics 3(11), 1427–1438. [PubMed] [Google Scholar]
- Garrido-Castro AC, & Goel S (2017). CDK4/6 inhibition in breast cancer: Mechanisms of response and treatment failure. Current Breast Cancer Reports 9(1), 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germa C, Miller M, Mukhopadhyay P, Hewes B, Caponigro G, Scherer SJ, & Hirawat S (2017). Discovery and development of novel therapies in advanced breast cancer: Rapid development of ribociclib. Annals of Oncology 28(8), 2021–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni L, Bisagni G, Colleoni M, Del Mastro L, Zamagni C, Mansutti M, Viale G. (2018). Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): An exploratory, open-label, phase 2 study. The Lancet Oncology 19(2), 249–256. [DOI] [PubMed] [Google Scholar]
- Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Zhao JJ (2017). CDK4/6 inhibition triggers anti-tumour immunity. Nature 548(7668), 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Wang Q, Watt AC, Tolaney SM, Dillon DA, Li W, Zhao JJ (2016). Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 29(3), 255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Di Leo A (2017). MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. Journal of Clinical Oncology 35(32), 3638–3646. [DOI] [PubMed] [Google Scholar]
- Guarducci C, Bonechi M, Boccalini G, Benelli M, Risi E, Di Leo A, … Migliaccio I (2017). Mechanisms of resistance to CDK4/6 inhibitors in breast cancer and potential biomarkers of response. Breast Care (Basel) 12(5), 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S, Cottu P, & Kirova YM (2018). Preliminary results of the association of palbociclib and radiotherapy in metastatic breast cancer patients. Radiotherapy and Oncology 126(1), 181. [DOI] [PubMed] [Google Scholar]
- Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, … Serra V (2016). Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Research 76(8), 2301–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, … O’Shaughnessy J (2016). Ribociclib as first-line therapy for HR-positive, advanced breast cancer. The New England Journal of Medicine 375(18), 1738–1748. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, … O’Shaughnessy J (2017). Updated results from MONALEESA-2, a phase 3 trial of first-line ribociclib + letrozole in hormone receptor-positive (HR+), HER2-negative (HER2−), advanced breast cancer (ABC). Journal of Clinical Oncology 35 (15_suppl), 1038. [Google Scholar]
- Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, … Shapiro GI (2016). A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clinical Cancer Research 22(23), 5696–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham M, & Schwartz GK (2017). Cell-cycle therapeutics come of age. Journal of Clinical Oncology 35(25), 2949–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen BA, Lee L, Koudriakova T, Haines M, Lundgren K, Price S, & Stevens GJ (2007). Peripheral white blood cell toxicity induced by broad spectrum cyclin-dependent kinase inhibitors. Journal of Applied Toxicology 27(2), 133–142. [DOI] [PubMed] [Google Scholar]
- Juric D, Ismail-Khan R, Campone M, Garaa-Estevez L, Becerra C, De Boer R, … Pagani O (2016). Phase Ib/II study of ribociclib and alpelisib and letrozole in ER+, HER2− breast cancer: Safety, preliminary efficacy and molecular analysis. Cancer Research 76(4 suppl) abstr P3–14-01. [Google Scholar]
- King KL, & Cidlowski JA (1995). Cell cycle and apoptosis: Common pathways to life and death. Journal of Cellular Biochemistry 58(2), 175–180. [DOI] [PubMed] [Google Scholar]
- Knudsen ES, & Witkiewicz AK (2016). Defining the transcriptional and biological response to CDK4/6 inhibition in relation to ER+/HER2− breast cancer. Oncotarget 7 (43), 69111–69123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen ES, & Witkiewicz AK (2017). The strange case of CDK4/6 inhibitors: Mechanisms, resistance, and combination strategies. Trends Cancer 3(1), 39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecny GE, Winterhoff B, Kolarova T, Qi J, Manivong K, Dering J, … Slamon DJ. (2011). Expression of p16 and retinoblastoma determines response to CDK4/6 inhibiion in ovarian cancer. Clinical Cancer Research 17(6), 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis MV, Pawlyk BS, Li T, Sicinski P, & Hinds PW (2006). Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 9 (1), 13–22. [DOI] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, & Pietenpol JA (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of Clinical Investigation 121(7), 2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CI, Daling JR, & Malone KE (2003). Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. Journal of Clinical Oncology 21 (1), 28–34. [DOI] [PubMed] [Google Scholar]
- Ma CX, Gao F, Luo J, Northfelt DW, Goetz M, Forero A, … Ellis MJ (2017). NeoPalAna: Noeadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clinical Cancer Research 23(15), 4055–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malorni L, Piazza S, Ciani Y, Guarducci C, Bonechi M, Biagioni C, … Migliaccio I. (2016). A gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget 7(42), 68012–68022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer IA, & Arteaga CL (2014). PIK3CA activating mutations: A discordant role in early versus advanced hormone-dependent estrogen receptor-positive breast cancer? Journal of Clinical Oncology 232(27), 2932–2934. [DOI] [PubMed] [Google Scholar]
- Montero AJ, Eapen S, Gorin B, & Adler P (2012). The economic burden of metastatic breast cancer: A U.S. managed care perspective. Breast Cancer Research and Treatment 134(2), 815–822. [DOI] [PubMed] [Google Scholar]
- Munster P, Hamilton E, Franklin C, Bhansali S, Wan K, Hewes B, & Juric D (2014). Phase Ib study of LEE011 and BYL719 in combination with letrozole in estrogen receptor-positive, HER2-negative breast cancer (ER+, HER2− BC). Journal of Clinical Oncology 32(15_suppl), 533. [Google Scholar]
- O’Brien N, Conklin D, Beckmann R, Luo T, Chau K, Thomas J, … Slamon DJ (2018). Preclinical activity of abemaciclib alone or in combination with anti-mitotic and targeted therapies in breast cancer. Molecular Cancer Therapeutics 17(5), 897–907. 10.1158/1535-7163.MCT-17-0290 (Epub 2018 Feb 26). [DOI] [PubMed] [Google Scholar]
- O’Brien N, Di Tomaso E, Ayala R, Tong L, Issakhanian S, Linnartz R, … Slamon DJ (2014). In vivo efficacy of combined targeting of CDK4/6, ER and PI3K signaling in ER+ breast cancer. Cancer Research 74(19 Suppl) Abstract nr 4756. [Google Scholar]
- O’Leary B, Hrebien S, Morden JP, Beaney M, Fribbens C, Huang X, … Turner NC (2018). Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nature Communications 9(1), 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M, Chavez-MacGregor M, & Modi S (2016). Adding ribociclib to everolimus and exemestane in ER+/HER2− advanced breast cancer: Feasibility and possible benefits. CoBrCa abstract 07. [Google Scholar]
- Osborne CK, & Schiff R (2011). Mechanisms of endocrine resistance in breast cancer. Annual Review of Medicine 62, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, … EBCTCG (2017). 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. The New England Journal of Medicine 377(19), 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, … Shapiro G (2014). LY2835219, a novel cell cycle inhibitor selective for CDK4/6, in combination with fulvestrant for patients with hormone receptor positive (HR+) metastatic breast cancer. Journal of Clinical Oncology 32(15_Suppl), 534. [Google Scholar]
- Pishvaian MJ, Wang H, Smaglo BG, Gao J, Macke L, Redmond E, … Pohlmann PR (2016). A phase I study of the CDK4/6 inhibitor, palbociclib plus 5-fluorouracil (5FU) in patients with advanced solid tumor malignancies (NCT01522989). Journal of Clinical Oncology 34(15_suppl) 2589–2589. [Google Scholar]
- Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, … Maris JM (2013). Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clinical Cancer Research 19(22), 6173–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raub TJ, Wishart GN, Kulanthaivel P, Staton BA, Ajamie RT, Sawada GA, … De Dios A (2015). Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metabolism and Disposition 43 (9), 1360–1371. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, … Sharpless NE (2012). Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. Journal of the National Cancer Institute 104(6), 476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo HS, Finn RS, Dieras V, Ettl J, Lipatov O, Joy A, … Gauthier ER (2017). Palbociclib (PAL) plus letrozole (LET) as first-line therapy in estrogen receptor-positive (ER plus)/human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC): Efficacy and safety updates with longer follow-up across patient subgroups. Cancer Research 78(4 Supplement) P5-21-03. [Google Scholar]
- Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, … Burstein HJ (2016). Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. Journal of Clinical Oncology 34(25), 3069–3103. [DOI] [PubMed] [Google Scholar]
- Schwartz GK, LoRusso PM, Dickson MA, Randolph SS, Shaik MN, & Wilner KD (2011). Phase I study of PD0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (schedule 2/1). British Journal of Cancer 104(12), 1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GI (2006). Cyclin-dependent kinase pathways as targets for cancer treatment. Journal of Clinical Oncology 24(11), 1770–1783. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, &Jemal A (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68, 7–30. [DOI] [PubMed] [Google Scholar]
- Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, … Llombart-Cussac A (2017). MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. Journal of Clinical Oncology 35(25), 2875–2884. [DOI] [PubMed] [Google Scholar]
- Tarrado-Castellarnau M, de Atauri P, Tarrago-Celada J, Perarnau J, Yuneva M, Thomson TM, & Cascante M (2017). De novo MYC addiction as an adaptive response of cancer cells to CDK4/6 inhibition. Molecular Systems Biology 13(10), 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo ZL, Versaci S, Dushvanthen S, Caramia F, Savas P, Mintoff CP, … Loi S (2017). Combined CDK4/6 and PI3Kα inhibition is synergistic and immunogenic in triple-negative breast cancer. Cancer Research 77(22), 6340–6352. [DOI] [PubMed] [Google Scholar]
- Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, … Knudsen ES (2011). Therapeutically activating RB: Reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocrine-Related Cancer 18(3), 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolaney SM, Beeram M, Beck JT, Colin AK, Dees C, Dickler MN, … Goetz MP (2015). A phase Ib study of abemaciclib with therapies for metastatic breast cancer. Journal of Clinical Oncology 33(15_Suppl), 522.25584003 [Google Scholar]
- Tripathy D, Sohn J, Im S-A, Colleoni M, Franke F, Bardia A, … Lu Y-S (2017). First-line ribociclib vs placebo with goserelin and tamoxifen or a non-steroidal aromatase inhibitor in premenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer: Results from the randomized phase III MONALEESA-7 trial. Cancer Research 78(4 Supplement), GS2–05. [Google Scholar]
- Turner NC, Jiang Y, O’Leary B, Hrebien S, Cristofanilli M, Andre F, … Loi S (2016). Efficacy of palbociclib plus fulvestrant (P+F) in patients (pts) with metastatic breast cancer (MBC) and ESR1 mutations (mus) in circulating tumor DNA (ctDNA). Journal of Clinical Oncology 34(suppl) abstr 512. [Google Scholar]
- Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, … PALOMA3 Study Group (2015). Palbociclib in hormone-receptor-positive advanced breast cancer. The New England Journal of Medicine 373(3), 209–219. [DOI] [PubMed] [Google Scholar]
- Verma S, Bartlett CH, Schnell P, DeMichele AM, Loi S, Ro J, … Rugo HS (2016). Palbociclib in combination with fulvestrant in women with hormone recepot-positive/HER2-negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). The Oncologist 21(10), 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, … Engelman JA (2014). CDK4/6 inhibitors sensitize PIK3CA mutant breast cancer to PIK3 inhibitors. Cancer Cell 26(1), 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Nicolay BN, Chick JM, Gao X, Geng Y, Ren H, … Sicinski P (2017). The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival. Nature 546 (7658), 426–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker S, Madani D, Joshi S, Chung SA, Johns T, Day B, … McDonald KL (2017). Combination of palbociclib and radiotherapy for glioblastoma. Cell Death Discovery 3, 17033 eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewicz AK, Cox D, & Knudsen ES (2014). CDK4/6 inhibition provides a potent adjunct to Her2 targeted therapies in preclinical breast cancer models. Genes & Cancer 5(7–8), 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Chen Z, To KKW, Fang X, Wang F, Cheng B, & Fu L (2017). Effect of abemaciclib (LY2835219) on enhancement of chemotherapeutic agents in ABCB1 and ABCG2 overexpressing cells in vitro and in vivo. Biochemical Pharmacology 124, 29–42. [DOI] [PubMed] [Google Scholar]
- Yang C, Li Z, Bhatt T, Dickler M, Giri D, Scaltriti M, … Chandarlapaty S (2017). Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36(16), 2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]


