Abstract
Introduction:
Cancer genome sequencing studies have discovered mutations in members of the SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin-remodeling complex in nearly 25% of human cancers. The SWI/SNF complex, first discovered in S. cerevisiae, shows strong conservation from yeast to Drosophila to mammals, contains approximately 10–12 subunits and regulates nucleosome positioning through the energy generated by its ATPase subunits. The unexpected finding of frequent mutations in the complex has fueled studies to identify the mechanisms that drive tumor development and the accompanying therapeutic vulnerabilities.
Areas covered:
In the review, we focus upon the potential roles different SWI/SNF subunit mutations play in human oncogenesis, their common and unique mechanisms of transformation and the potential for translating these mechanisms into targeted therapies for SWI/SNF-mutant tumors.
Expert opinion:
We currently have limited insights into how mutations in different SWI/SNF subunits drive the development of human tumors. Because the SWI/SNF complex participates in a broad range of normal cellular functions, defining specific oncogenic pathways has proved difficult. In addition, therapeutic options for SWI/SNF-mutant cancers have mainly evolved from high-throughput screens of cell lines with mutations in different subunits. Future studies should follow a more coherent plan to pinpoint common vulnerabilities among these tumors.
1. Background/introduction
1.1. The SWI/SNF chromatin remodeling complex- frequently mutated in human cancers.
Numerous studies over the past 40 years have given considerable insights into how mutations in classic oncogenes and tumor suppressor genes (TSGs), such as TP53, RAS, CDKN2A and PIK3CA, drive tumor development. Importantly, recent reports from large-scale cancer genome landscape studies such as the Cancer Genome Atlas (TCGA) and others firmly established the frequent occurrence of these pathogenic mutations across a broad range of human cancers (1). For example, TP53 bears somatic coding mutations in 27% of 148,281 tested tumors (COSMIC v87). However, one unexpected finding from these studies was pathogenic mutations in components of the SWI/SNF (Mating Type Switching/Sucrose Non-Fermentable) chromatin remodeling complex in nearly 25% of all cancers, a rate that approaches the frequency of TP53 mutations.
1.2. The SWI/SNF complex- a key regulator of multiple cellular pathways.
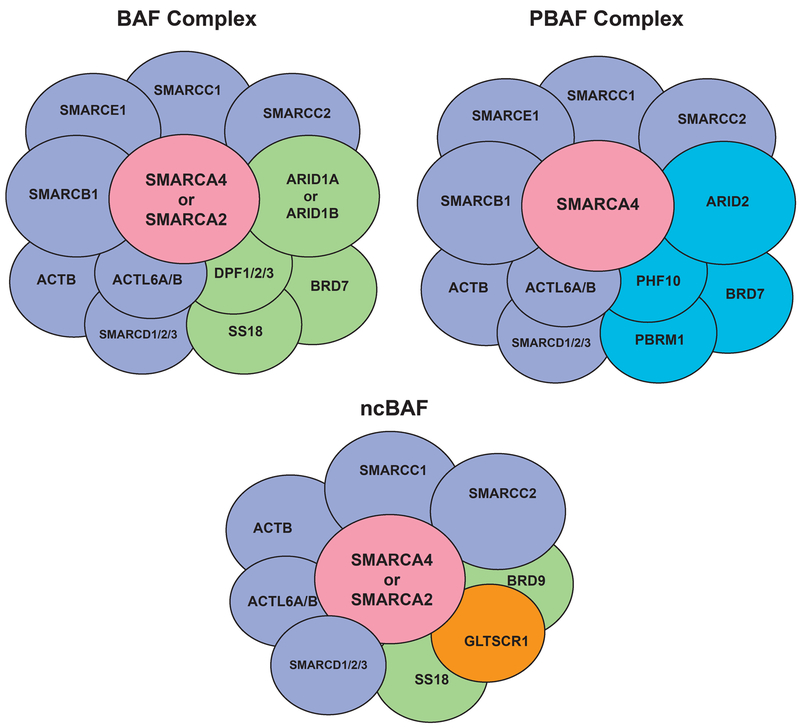
The SWI/SNF complex, first discovered in S. cerevisiae, is an evolutionarily conserved multi-subunit complex that utilizes the energy of ATP hydrolysis to mobilize nucleosomes and remodel chromatin (2, 3) (4–6). Because the complex consists of 12–15 subunits, multiple complex configurations can appear, dependent upon the subunit composition (Figure 1). All complexes include a catalytic ATPase subunit (mutually exclusive SMARCA4/BRG1 or SMARCA2/BRM) and core subunits including BAF155 and SMARCB1(7). Separately, three broad sub-families of SWI/SNF complexes have been identified, BAF (BRG1 associated factors), PBAF (Polybromo-associated BAF) and the recently-described ncBAF, each defined by signature subunits including ARID1A or ARID1B (BAF), PBRM1 and ARID2 (PBAF) and GLTSCR1 (ncBAF) (8–10). SWI/SNF complex subfamilies also contain variant subunits, often encoded by multi-gene families. Multiple reports have shown roles for the complex in the regulation of a broad range of normal functions such as gene transcription, RNA processing, cell cycle, apoptosis, development, differentiation and DNA replication and repair (1). Thus, with its pivotal role in regulating these diverse pathways, one would predict an association of altered SWI/SNF function with disease.
Figure 1.
Major SWI/SNF Complex Configurations.
1.3. Due to its relatively recent linkage to cancer, a paucity of mechanistic studies on SWI/SNF mutations exists.
Compared to the large number of reports detailing the mechanisms by which classic oncogenes/TSGs drive tumorigenesis, only a limited number of mechanistic studies have explored the actions of SWI/SNF mutations in human tumors. For example, in contrast to tumors with mutations in classic oncogenes/TSGs, many SWI/SNF-mutant tumors are genomically simple, possessing only the mutation in a single SWI/SNF subunit. The fact that mutations across members of the SWI/SNF complex appear at one of the highest rates across all human tumors emphasizes the need to accelerate the pace of research in this field to reach the same level of knowledge achieved for other cancer mutations. Whether the recurrently mutated genes that encode SWI/SNF subunits across a wide variety of cancers drive cellular transformation through shared mechanisms and/or share common therapeutic vulnerabilities remains an open question (1). This review will focus upon emerging mechanisms that account for the development of SWI/SNF-mutant tumors and the potential for targeting them for treatment options. We will not cover the range of tumors that possess SWI/SNF subunit mutations or their genetics because of other recent reviews on these topics (1, 11, 12).
2. SWI/SNF complex mutations in human cancers
The first clue linking SWI/SNF complexes to cancer arose from studies identifying biallelic inactivation of SMARCB1 in nearly all rhabdoid tumors, aggressive poorly differentiated pediatric solid tumors (13, 14). Since these seminal reports, the list of other human malignancies showing genetic loss of SMARCB1 has greatly expanded, including epithelioid sarcoma, epithelioid malignant peripheral nerve sheath tumors, extraskeletal myxoid chondrosarcoma, myoepithelial carcinoma, renal medullary carcinoma, and poorly differentiated chordoma (15). Building upon these findings, recent next generation sequencing studies have revealed that mutations in genes encoding other SWI/SNF subunits occur broadly in cancer. For example, SMARCA4 (BRG1), encoding a catalytic ATPase and a core subunit of SWI/SNF complexes, is recurrently mutated in lung cancer (16, 17), medulloblastoma (18), pancreatic cancer (19, 20), and small cell carcinoma of the ovary hypercalcemic type (SCCOHT) (21–23). Inactivating mutations of ARID1A occur in 45% of ovarian clear cell carcinomas, 30% of endometrioid carcinomas, 27% of gastric cancers, 18% of bladder cancers, and approximately 10% of colorectal, pancreatic and liver cancers (24–30). Finally, inactivating mutations of PBRM1 are present in 40% of kidney cancers and 8% of pancreatic cancers (19, 31). Representative examples of tumors with significant frequencies of mutations in these SWI/SNF subunits are shown in Table 1.
Table 1.
Representative Spectrum of Mutations in SWI/SNF Complex Subunits Found in Human Cancers.
| SWI/SNF Subunit | Tumor | Frequency | Reference(s) |
|---|---|---|---|
| SMARCA4 | small cell carcinoma of the ovary- hypercalcemic type(SCCOHT) SMARCA4-deficient thoracic sarcomas(SMARCA4-DTS) undifferentiated uterine sarcoma sinonasal undifferentiated carcinoma (SNUC) lung adenocarcinoma (LUAD) |
>95% ~100% ~100% ~100% 10% |
(21–23) (56–58) (61) (59) (17, 207, 208) |
| SMARCA2 | Adenoid cystic carcinoma(ACC) | <2% | (71) |
| SMARCB1 | rhabdoid tumors epithelioid sarcoma renal medullary carcinomas |
95% 55–80% 55–100% |
(13, 14) (101, 209) (94, 210, 211) |
| ARID1A | ovarian clear cell carcinoma(OCCC) endometrial cancers gastric cancers |
50% 40% 19% |
(24) (24) (124) |
| PBRM1 | clear cell renal cell carcinoma | 29–41% | (31, 128, 129) |
| ARID2 | hepatocellular carcinoma melanoma |
6–18% 7%−14% |
(28, 132) (133–135) |
2.1. Types of SWI/SNF subunit mutations-
The first studies characterizing SMARCB1 loss in rhabdoid tumors showed a complete loss of protein, associated with either nonsense mutations or partial to complete gene deletions (32). Similarly, the preponderance of SMARCA4 mutations in SCCOHTs results in loss of function (21–23). This paradigm has held true for other SWI/SNF subunits- the vast majority of mutations/deletions lead to a lack of protein in tumors (26, 31). Thus, most studies on SWI/SNF complex mutations have focused on genetic inactivation of SWI/SNF subunits.
However, TCGA data also demonstrate focal amplification, over-expression and/or somatic, potentially activating missense mutations in many SWI/SNF subunits including known tumor suppressors SMARCA4 and SMARCB1. For example, approximately 5% of medulloblastomas, a pediatric brain tumor, possess missense mutations in SMARCA4, clustered within the SNF2 and Helicase domains (33–35). Of note, some of the same missense mutations found in human tumors, such as C-terminal K364del and R377H in SMARCB1 and R885H and L921F in SMARCA4, occur in the rare neurodevelopmental disorder, Coffin-Siris syndrome (34–36). Whether these subunit genes with missense mutations produce altered proteins that can impact normal SWI/SNF complex functions and how they might contribute to tumor development remain unknown. A recent report did demonstrate that some SMARCA4 missense mutations found in human tumors had aberrant activities when assessed in mouse embryo fibroblasts (37). However, the authors did not address whether these mutant forms would drive tumor development.
2.2. Mutations in SWI/SNF can act as either tumor suppressors or oncogenes-
Multiple studies strongly support the notion that SWI/SNF loss of function mutations serve as drivers in multiple human tumors. For example, re-expression of SMARCB1 in rhabdoid tumor cell lines or SMARCA4 in SCCOHT cell lines leads to growth arrest followed by replicative senescence (38, 39). Reports using genetically engineered mouse models (GEMMs) have also established the bona fide tumor suppressor activity of SWI/SNF genes. Conditional inactivation of Smarcb1 results in 100% of mice developing cancer at a median of only 11 weeks (40). Inactivation of Arid1a in GEMMs contributes to the development of cancers of the colon, liver and ovary (41). In addition, deletion of Smarca4 in GEMMs results in breast and uterine cancers (42, 43). Germline mutations in SWI/SNF subunits can also lead to familial cancers similar to other well-characterized TSGs such as TP53 and RB1 (11). Importantly, loss of SWI/SNF subunits often correlate with more aggressive tumors [reviewed in (1)]. Many of the pediatric/young adult cancers with a SWI/SNF subunit mutation represent some of the most aggressive tumors with little to no long-term survival (44). In adult malignancies, loss of a single SWI/SNF subunit can result in more aggressive tumor progression with decreased survival (1). Inactivation of SWI/SNF subunits in GEMMs also often leads to highly aggressive tumors. Therefore, the mutations in SWI/SNF subunits found in human cancers significantly impact the course of tumor development.
Several recent reports have implicated that overexpression of SWI/SNF complex subunits, such as ARID1A in liver cancer and SMARCA4 in breast cancer, may drive tumor development, i.e. they can also act as oncogenes (45, 46). The majority of these studies demonstrate overexpression of a SWI/SNF subunit in primary human tumors as well as inhibition of growth after knockdown of the subunit in cell line models. While these studies raise important questions about how cellular context determines the role of aberrant SWI/SNF complex activities in human tumor development, their relevance as therapeutic targets remain unclear. Briefly, the published reports do not address how overexpression of a single SWI/SNF subunit changes the functions of the entire complex. More importantly, loss of expression of individual SWI/SNF subunits often results in significant growth inhibition of normal cells and tissues raising concerns about potential side-effects if used as therapeutic targets (42, 47–50). Therefore, this review will focus upon loss of function/missense mutations in SWI/SNF subunits where therapeutic approaches have recently emerged.
2.3. Overview of individual subunit mutations-
Below, we provide brief overviews of studies of the most commonly mutated SWI/SNF subunits including some potential therapeutic vulnerabilities.
2.3.1. SMARCA2/A4-
SMARCA4 (BRG1) is one of two mutually exclusive ATPases of the SWI/SNF complex. Mutations in SMARCA4 occur more frequently than its sibling ATPase (SMARCA2/BRM) and generally do not appear in a particular tumor type. Mutations in SMARCA4 can be categorized as: (1) a driver event with a simple genetic background (2) a defining characteristic in all tumors, but with a more complex genetic background or (3) not a driver mutation, but adding to the genetic burden and acting as a tumor progression event in the tumor.
Cancers with putative SMARCA4 driver mutations show nearly 100% of tumors with SMARCA4 protein loss, few chromosomal copy number aberrations, and rare mutations in other cancer driver genes. Only 2 tumors, small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) and SMARCA4-deficient/SMARCB1-intact rhabdoid tumors (Rhabdoid tumor predisposition syndrome 2) currently fit these criteria. SCCOHT, a rare and aggressive form of ovarian cancer found in young women, have mutational loss (germline or somatic) of SMARCA4, epigenetic silencing of SMARCA2, and rarely any other genetic abnormalities (21–23, 38, 51). Rhabdoid tumor predisposition syndrome 2 (RTPS2) defines patients with rhabdoid tumors possessing SMARCA4 mutations rather than the hallmark SMARCB1 loss (52–54). However, SMARCA4-loss is relatively rare in childhood rhabdoid tumors in comparison to typical cases of SMARCB1-deficient tumors. The SMARCA4 mutations in RTPS2 were mostly germline, occurred in children less than 12 months old and often associated with a poorer prognosis (52–55).
Recently, there have been tumors described with SMARCA4 loss as a defining characteristic in the context of a complex genetic background, such as SMARCA4-deficient thoracic sarcomas (SMARCA4-DTS) and potentially two newly described tumors. SMARCA4-DTS, a group of aggressive intrathoracic sarcomas, predominantly occur in men with a previous history of smoking (56–58). In addition to SMARCA4 loss in all cases, SMARCA4-DTS tumors have a more complex genetic background with mutations most commonly in TP53 and occasionally alterations in other driver genes such as CDKN2A, KRAS and MYC (56–58). Other tumors with nearly 100% SMARCA4 loss include undifferentiated uterine sarcoma (malignant rhabdoid tumor of the uterus) and sinonasal carcinoma (SNUC). In SMARCA4-deficient undifferentiated uterine sarcoma, a limited oncogene sequencing panel showed TERT promoter mutations, while SMARCA4-deficient SNUC has 3 reported cases with concurrent APC and CDKN2A mutations (59–62).
SMARCA4 mutations are also found in genetically-complex tumors presumably adding to their genetic burden. These mutations, while not found in all cases, can act as progression events in adult cancers and usually correlate with poorer prognosis, as observed with non-small cell lung cancer (17). Interestingly, SMARCA4 mutations and protein loss have also been observed in poorly differentiated tumors, potentially associated with dedifferentiation, another progression event. This phenomenon has been noted in undifferentiated and dedifferentiated endometrial carcinomas (63–66), poorly differentiated endometrioid adenocarcinoma of the uterus (67), undifferentiated rhabdoid carcinomas of the gastrointestinal tract (68), and undifferentiated renal cell carcinoma (69).
2.3.2. SMARCA2-
In stark contrast to SMARCA4, mutations in SMARCA2 seldom occur across any tumor type. Only a few cancers to date have been described with SMARCA2 mutations or deletions in addition to other mutational burdens, including adenoid cystic carcinoma (70–72), non-melanoma skin cancer (73, 74), hepatocellular carcinoma (75) and head and neck squamous cell carcinoma (76). Although not frequently mutated, loss of SMARCA2 expression occurs across numerous tumor types and cancer cell lines through epigenetic silencing (77). The exact mechanism of the epigenetic silencing varies, but can arise through promoter methylation (78), promoter polymorphisms (79–81) or HDAC/EZH2-driven mechanisms (38, 82–84).
The frequency of SMARCA2 loss varies among cancers. In a few cases, nearly 100% of all tumors lose its expression, such as SCCOHT (38, 82–85) and SMARCA4-DTS (56). More commonly, SMARCA2 loss is seen in a subset of cases, e.g. undifferentiated carcinomas of the gastrointestinal tract (68). In SMARCA4-deficient cancers that retain SMARCA2 expression, several reports have shown that SMARCA2 acts a synthetic lethal target, making it a potential therapeutic vulnerability (86, 87). In contrast, tumors with dual SMARCA2/A4 loss do not show this vulnerability including SCCOHT (38, 85), SMARCA-DTS (56) and some cases of dedifferentiated (64) and undifferentiated (66) endometrium carcinomas, undifferentiated renal cell carcinoma (69) and non-small cell lung cancer (88).
2.3.3. SMARCB1-
SMARCB1 (also called BAF47, INI1 and SNF5) regulates the epigenome as a core member of the SWI/SNF ATPase chromatin remodeling complex (89). Mutations in SMARCB1 have previously been identified in 95% of pediatric rhabdoid tumors (RT), poorly differentiated and clinically aggressive tumors that arise primarily in the kidney and central nervous system (89). For many years, diagnosis rested upon the identification of prototypical rhabdoid cells by histopathology, often a difficult analysis for pathologists. They are now defined by biallelic alterations in the SMARCB1 tumor suppressor gene located at chromosome 22q11.2, making loss of expression of SMARCB1 protein a diagnostic hallmark of this cancer (14, 15, 90). Beyond the common SMARCB1 mutation, these tumors appear genetically simple and uniform, bearing no other recurrent driver mutations with primarily diploid genomes (91–93).
SMARCB1 mutations also drive development of other aggressive tumors in children and young adults- epithelioid sarcoma, epithelioid malignant peripheral nerve sheath tumors, extraskeletal myxoid chondrosarcoma, myoepithelial carcinoma, renal medullary carcinoma, and poorly differentiated chordoma (15, 94–96). Chordomas are rare tumors of the skull base and spine thought to arise from remnants of the embryonic notochord that can arise in adults and children (97). Their clinical behavior is marked by slow growth and a predilection to recur despite surgical resection. Most patients with recurrent or metastatic chordomas will eventually die from the disease (97). A subset of pediatric chordomas are described as “poorly differentiated” due to cytological atypia, increased mitotic activity, increased cellularity, and an unstructured growth pattern (95, 97). These tumors show an aggressive clinical course, high mortality, and loss of SMARCB1 expression, contrasting with the retention of SMARCB1 in conventional, less aggressive chordomas (95, 98). However, a discourse continues among the scientific community if poorly differentiated chordomas represent a distinct entity or part of an RT spectrum (95, 98).
Epithelioid sarcoma (ES) are rare and aggressive mesenchymal soft tissue neoplasms occurring in young individuals characterized by consistent loss of SMARCB1 nuclear expression, a feature also shared with RT and chordomas (99–101). A comprehensive investigation into how loss of SMARCB1 function drives ES tumorigenesis remains elusive. However, the results reported by Le Loarer et al. (2014), do corroborate two previous studies suggesting the leading mechanism for SMARCB1 loss of function is biallelic deletion in 22q11 encompassing the SMARCB1 tumor suppressor gene locus (96, 102, 103).
Schwannomatosis, one form of a genetic disorder called neurofibromatosis, consists of multiple cutaneous schwannomas, central nervous system tumors, and other neurological complications (104). A mutation of the SMARCB1 gene has been implicated in the development of schwannomatosis in both sporadic and familial cases (105, 106). SMARCB1 mutations have been found in 45% of familial probands and 9% of sporadic patients (107). The comparison with germline SMARCB1 mutations in patients with RT indicates that nontruncating mutations occur more significantly in schwannomatosis than in RTs (p < 0.0001) (107). Whereas SMARCB1 mutations alone seemingly account for RT development, a substantial heterogeneity of mutations schwannomatosis exists even for familial disease. Schwannomas often possess mutations in the NF2 tumor suppressor, located near the SMARCB1 locus on chromosome 22 (108, 109) and in the LZTR1 gene (110). As reported by Smith et al. (2014), the most common SMARCB1 mutation in schwannomas is found within the 3′UTR- c.*82C>T (107). How this alteration contributes to the development of schwannomatosis remains unknown, but could act by regulating gene expression through alternative splicing within the UTR. Allen et al. (2015) also reported that SMARCB1 mutations in schwannomatosis target an unidentified N-terminal winged helix DNA binding domain also present in BAF45a/PHF10 subunit of the SWI/SNF complex (111).
Several reports have linked SMARCB1 activity to several types of DNA repair mechanisms [reviewed by (112)]. Considering the rapid course of development of SMARCB1-deficient RTs in newborns and young children, SMARCB1 loss could drive tumorigenesis via the rapid accumulation of DNA mutations or chromosome instability. However, multiple studies have failed to detect any major effects of SMARCB1 loss on DNA repair (93, 113, 114) In fact, RTs generally remain diploid and lack gene amplification or deletions detectable at the level of SNP arrays (91, 98). Interestingly, exome sequencing found RTs to have remarkably low rates of mutation with loss of SMARCB1 being essentially the sole recurrent detectable event (91, 112, 113). Given the absence of genomic instability, the mechanisms that drive the onset of SMARCB1-deficient cancers in both humans and mice warrant greater attention.
2.3.4. ARID1A-
ARID1A is an evolutionarily conserved gene that codes for the BAF250 subunit of the SWI/SNF complex (115). The protein product contains a region of amino acids that binds to AT-rich regions of DNA, the ARID region, accounting for its name (116). The subunit, along with its ARID1B paralog, appears in the SWI/SNF complex designated BAF (8, 117, 118) (Figure 1). Functionally, ARID1A plays a role in hormone signaling and development, with increased ARID1A raising the expression of glucocorticoid receptors and other steroid hormone receptors (117, 119). Mutations in ARID1A can also affect regulation of normal development, whereas its absence resulted in poor differentiation of stem cells (120, 121). ARID1A mutations appear in many types of cancers, ranging from nearly 50% in ovarian clear cell carcinoma (OCCC) to 2.5% in breast cancer (26, 122). Other cancers with significant frequencies of ARID1A mutations include gastric, endometrial, lung, medulloblastoma, hepatocellular carcinoma and pancreas (24, 29, 123, 124). In general, most tumors possess truncating mutations of ARID1A although some tumors contain missense mutations (29).
The mechanisms by which ARID1A loss drives tumorigenesis remain under investigation. One mechanism comes from ovarian cancer studies using a GEMM with ARID1A knockdown and PI3K mutations by Kim et al (125). They found that these mutations resulted in the loss of recruitment of an HDAC complex and promotion of an inflammation response (125). The group demonstrated that the loss of ARID1A impairs recruitment of the Sin3A-HDAC complex while the PI3K mutations increased NF-kB signaling (125). ARID1A loss has also been linked individually to either HDAC repression and PI3K pathway mutations. Chandler et al. showed that ARID1A inactivation along with activation of PI3K/AKT pathway upregulated inflammatory pathways resulting in tissue damage and subsequent carcinogenesis (126). Using a novel GEMM, the investigators found that ARID1A inactivation alone was not sufficient for tumor development, but required concurrent activation of PIK3CA (126). They also showed that inactivation of ARID1A and activation of PIK3CA led to an overproduction of IL-6, an inflammatory factor, that drove tumor growth (126). Based upon these results, they proposed that IL-6 could serve as a prognostic marker and therapeutic target (126). Another mechanism proposed by Bitler et al. implicates a dependence upon histone deacetylase 6 (HDAC6) activity for mutant ARID1A-driven tumor development (127). In both cellular and animal models, they found that inactivation of HDAC6 resulted in a reduction in the size of only mutant ARID1A-driven tumors (127). They also showed that ARID1A directly represses transcription of HDAC6 in RMG and TOVG21 ovarian cancer cell lines and in GEMMs. They further showed that HDAC6 inhibition promoted TP53 mediated apoptosis through HDAC6-catalyzed deacetylation of TP53 Lys120 resulting in its inactivation (127). Thus, loss of ARID1A increases HDAC6 expression and inhibits TP53-driven apoptosis implicating HDAC6 inactivation as a potential target for therapeutic intervention (127).
2.3.5. PBRM1-
PBRM1 is the second most commonly mutated gene in clear cell renal cell carcinoma, with mutations occurring in 29–41% of tumors and frequently co-occurring with VHL mutations (31, 128, 129). GEMMs demonstrated that PBRM1 loss in ccRCC is secondary to VHL loss, and the combined disruption of these genes resulted in multifocal tumors (130). PBRM1 contains six bromodomains important for association with acetylated histones. Little is known about the effects of individual bromodomains or the role they play in targeting the PBAF complex to specific genomic loci. However, recent work showed that they are not functionally equivalent in the context of ccRCC cell lines (131).
2.3.6. ARID2-
Mutations in ARID2 are found in a wide spectrum of tumor types including hepatocellular carcinoma (28, 132) and melanoma (133–135). In addition, ARID2 mutations are found at lower levels in some cancers, such as stomach adenocarcinoma (8%) and non-small cell lung cancer (4%), that did not reach significance in reported studies. Nevertheless, cancers with moderate mutation rates in ARID2 may also rely on disruption of the PBAF complex. Whether ARID2 mutations can drive these diseases requires further evaluation. Only a few examples of mechanistic studies into the role of ARID2 in tumor suppression appear in the literature. Studies from cell lines suggest that ARID2 contributes to tumor suppression by modulating the DNA damage response (136) or through control of transcriptional programs associated with oncogenesis and liver development (137). Because no ARID2-dependent mouse models of cancer currently exist, critical experiments monitoring the initiation and progression of ARID2-dependent tumors are lacking.
2.3.7. Other subunits-
Mutations in other SWI/SNF subunits occur less frequently in human tumors. Examples include SMARCE1 mutations in spinal meningiomas, BCL7B/C in gastric cancer and SMARCC2 mutations in gastric and colorectal cancers (11). However, one of the most intriguing mutations involving SWI/SNF subunits are the fusion oncogenes possessing the SS18 subunit found in synovial sarcomas (138). Development of synovial sarcomas, aggressive and rare cancers occurring in the extremities of children and young adults, are driven by an in-frame fusion of the SS18 to the C-terminal end of SSX1 or SSX2 (139, 140). A landmark study by Kadoch and Crabtree demonstrated that the SS18–SSX protein competes with the normal SS18 protein present in SWI/SNF complexes to prevent recruitment of the SMARCB1 subunit into the complexes, resulting in its degradation (138). Therefore, initial models suggested that the fusion protein provided another mechanism for SMARCB1 loss and subsequent tumor development. However, a recent report from McBride et al. demonstrated that SWI/SNF complexes containing the SS18-SSX fusion protein rerouted the activity of the complex to drive tumorigenesis i.e. a gain-of-function event (141). This mechanism contrasts with the majority of studies where SWI/SNF subunits act as tumor suppressors (see above). A different oncogenic fusion protein, EWS-FLI1, also interacts with the SWI/SNF complex and alters its function to drive development of Ewing’s Sarcoma, another pediatric cancer (142). As next-generation sequencing studies identify additional oncogenic fusion genes in human tumors, additional examples of tumorigenesis driven by their interactions with the SWI/SNF complex will likely appear.
3. Shared mechanisms of mutant SWI/SNF subunits that drive tumor development
3.1. Chromatin organization-
A consistent theme emerging from recent studies is mutations in key members of the SWI/SNF complex affect nucleosome positioning. In the absence of a normal complex, cells display genome-wide disruptions of normal patterns of nucleosome placement (5, 6, 143). While the chromatin remodeling activity of SWI/SNF predicted these findings, the non-random nature of the nucleosome positioning changes was unexpected. Recent studies from several laboratories have established that aberrant SWI/SNF complexes preferentially affect enhancers and/or super-enhancers (37, 144–146). Some of these studies have further implicated these changes as drivers of altered differentiation in SWI/SNF mutant cancers (6, 37, 144, 147). The mechanisms that determine SWI/SNF interactions with enhancers/super-enhancers and whether mutations in different subunits affect the same and/or different sites remains unknown. However, the recent development of CRISPR technologies, allowing the targeting of specific regions of the genome for deletion, silencing or activation, opens the possibilities of targeting therapeutic vulnerabilities caused by changes in enhancer/super-enhancer accessibilities.
3.2. Histone interactions-
Studies have shown that SWI/SNF complex interactions with histone modifying enzymes provides another mechanism of tumor development. The first described interaction demonstrated an antagonistic relationship between polycomb repressive complex 2 (PRC2) and SWI/SNF (148). Loss of the SMARCB1 subunit led to upregulation of the PRC2 catalytic methyltransferase subunit, EZH2, resulting in an increase in H3K27me3 marks (148). This antagonistic relationship led to uncovering the therapeutic vulnerability in SWI/SNF-mutant cancers to EZH2 inhibitors in (82, 149–151). Consistent with the antagonism with PRC2, restoration of SWI/SNF activity in deficient tumors will directly evict polycomb repressive complex 1 (PRC1) and PRC2 from chromatin, dependent upon the ATPase activity of the complex (152, 153). The chromatin independent interactions of SWI/SNF and PRC1 also depend on the ATPase activity of SMARCA4 because point mutations inactivating the its activity disrupt these interactions (152).
In addition to PRC1 and PRC2, the SWI/SNF complex also interacts with histone demethylases (HDMs), histone deacetylases (HDACs), and histone acetyltransferases (HATs). SMARCA4 interacts with KDM3A to determine the specificity of AP-1 target genes in hepatocellular carcinoma (154). In synovial sarcoma, the SS18-SSX fusion oncogene interacts with KDM2B histone demethylase, a component of the non-conical PRC1.1, to promote transformation (155). SWI/SNF also interacts with the histone acetyltransferases p300/CBP at enhancer sites, which are reduced in SMARCB1-deficient rhabdoid tumor cell lines (144). However, in SMARCB1-deficient AML, a residual SWI/SNF complex is recruited to oncogenic target genes with p300/CBP to promote H3K27Ac and gene expression (156).
3.3. Changes in SWI/SNF complex composition-
Recent studies have suggested that inactivation or loss of one subunit of the SWI/SNF complex may impact its composition, resulting in significant functional changes (145, 146, 157, 158). Co-inactivation of SMARCB1 and SMARCA2 expression occurs in cell lines and primary tumors, including RTs (84, 159, 160). The concomitant loss of SMARCB1, SMARCA2, and PBRM1 expression was detected in two epithelioid sarcomas with pure rhabdoid features (159, 161). These results suggest loss of SMARCB1 works in tandem with other SWI/SNF core subunits to drive tumorigenesis. Several recent reports underscore the impact of changes in the subunit composition of the SWI/SNF complex upon its normal activities. Mashtalir et al. have described the assembly of subcomplexes that ultimately unite to form the full SWI/SNF complex (162). Importantly, they also show that many of the subunit mutations found in human cancers alter the normal assembly of SWI/SNF complexes resulting in aberrant forms (162). Another recent report demonstrated that inhibition of BRD9, a SWI/SNF subunit that can appear in abnormal complexes induced by mutations in other subunits, can reactive epigenetically-silenced genes in tumors (163). They also demonstrated that BRD9 could regulate the activity of SWI/SNF through phosphorylation of the SMARCA4 ATPase subunit (163). Overall, these findings emphasize the critical need for further studies on the composition and activities of the SWI/SNF complex in cancers drive by mutations in different subunits, especially for identifying novel avenues for therapeutic interventions.
3.4. Interactions with established oncogenic pathways-
One of the first studies on proteins that interact with SWI/SNF complex members showed a direct and functional interaction between SMARCB1 and the CMYC transcription factor (164). This report provided a logical mechanism for how loss of SMARCB1 in tumors could fuel tumor development through increased expression of CMYC, a known oncogene. Since 1999, a growing number of reports have appeared showing direct interactions between SWI/SNF subunits and a broad array of oncogenes and TSGs such as LKB1/STK11, TP53, CDKN2A, BRCA1, CCNDE and NRF2 (165–171). However, many of these reports appear once, without validation from other laboratories. In addition, the functional consequences of these putative interactions remained unclear due to the lack of appropriate biological models. However, the role of several key oncogenic pathways to the SWI/SNF-mutant driven tumorigenesis appear clear. We discuss their interactions with the SWI/SNF complex below.
3.4.1. TP53-
TP53, the most commonly mutated tumor suppressor in human cancers, regulates a diverse series of cellular functions including apoptosis, genomic stability, and cell cycle control (172). Nearly half of human cancers possess a TP53 mutation and anti-cancer drugs often initiate TP53 activation to force cancer cell death (173). Several reports suggest that an interaction between the SWI/SNF complex and TP53 facilitates the latter’s ability to mediate gene expression and perform its tumor suppressor functions. Lee et al. showed that SWI/SNF subunits bind to TP53 and were recruited to promoters of TP53 mediated genes (174). Inactivating mutations of SMARCB1 and SMARCA4 inhibited the cell growth suppression and apoptosis controlled by TP53 (174). Oh et al. later showed that the SMARCD1 (BAF60a) subunit directly interacted with TP53 through immunoprecipitation (171). They also found that TP53 could not exert its anti-cancer functions without direct interaction with the SWI/SNF complex and suggested that CDKN1A (p21) was the protein most dependent upon this interaction (171). Pfsiter et al. showed that expression of VEGFR2, a tyrosine kinase commonly mutated in breast cancer that regulates endothelial vascularization, depended upon a TP53-SWI/SNF (175). They found that mutant TP53 interacts with the SWI/SNF complex to regulate the VEGFR2 promoter through chromatin remodeling (175). He et al. further demonstrated that SMARCE1 (BAF57), SMARCD1 (BAF60A), and SMARCB1 regulated replicative senescence by directly binding to TP53 (176). Mouse models have also established functional relationships between TP53 function and SWI/SNF complex activity (48, 177). Whether the recent efforts to therapeutically target TP53 in human cancers will extend to the treatment of SWI/SNF-mutant tumors remains an exciting possibility.
3.4.2. CDKN2A (p16INK4A)/RB1-
Reports have also established the interaction of the SWI/SNF complex with the RB tumor suppressor pathway that regulates the progression of the cell from G1 to S phase of the cell cycle (178). Reports have shown that SMARCA4-deficient cell lines do not respond to RB-mediated cell cycle arrest (179, 180). Upon re-expression of SMARCA4, the RB growth arrest pathway was restored (179, 180). Of interest, re-expression of either SMARCA2 or SMARCA4 could restore RB meditated cell cycle arrest, suggesting that each ATPase subunit could compensate for one other in mediating RB pathways (160, 179, 180). Other groups later showed that the tumor suppressor role of SMARCB1 also depended on the CDKN2A/RB pathway (48, 181). However, while cell culture models have established the interplay between SWI/SNF complex activities and the RB signaling pathway, mouse model studies remain ambiguous. Mice heterozygous for both the Rb1 and Smarcb1 null alleles develop mutually-exclusive RB1-deficient or SMARCB1-deficient pituitary tumors (182). Similarly, Bultman et al did not observe an effect of Rb1 loss on mammary gland tumor incidence in a mouse heterozygous for Smarca4 null alleles (183). Furthermore, inactivation of CDKN2A or RB1 did not accelerate tumor formation in a SMARCB1-conditional mouse model, despite aberrant upregulation of genes regulated by the target of RB pathway regulation- the E2F transcription factor (48). While these finding suggest that therapies targeting the RB pathway will prove ineffective for SWI/SNF-mutant tumors, a recent report demonstrates efficacy of CDK4/6 inhibitors for SCCOHTs and SMARCA4-deficient lung tumors (184, 185).
3.4.3. Long non-coding RNAs (lncRNAs)-
One other family of regulatory molecules solidly linked to the SWI/SNF complex in cancer are long non-coding RNAs (lncRNA). Prensner et al. reported that the lncRNA, SChLAP1, antagonizes the SWI/SNF complex by binding and impairing the tumor suppressor functions of the complex in prostate cancer (186). In bladder cancers, UCA1 was reported to bind to SMARCA4 and reduce the expression of the RB/CDKN2A pathway to inhibit cell cycle arrest (187). Some lncRNA, rather than binding to the complex, recruit it, such as lncTCF7 recruiting the SWI/SNF complex to the promoter of the TCF7 gene to activate the Wnt signaling pathway (188). Other reports implicate the SWI/SNF complex in the regulation of macrophage functions and assembly of nuclear bodies through direct interactions with lncRNAs (189, 190). Because effective treatment options based on targeting lncRNAs remain in their infancy, their application as therapeutic options for SWI/SNF-mutant cancers await further developments.
4. Therapeutic vulnerabilities
Evidence for clinical activity of approved or investigational agents in SWI/SNF-mutant tumors is limited with no drugs approved specifically for treatment of SWI/SNF-mutant cancers. In pediatric SWI/SNF-mutant tumors, such as RTs and SCCOHT, the standard of care remains non-specific – surgery, high dose chemotherapy with stem cell rescue, and radiation (191, 192). SWI/SNF therapeutic targets and developing opportunities have been extensively reviewed and readers are directed to recent ones (193, 194). Therefore, this review of SWI/SNF therapeutic vulnerabilities will focus on current studies connecting SWI/SNF subunits with neoplasms to associated vulnerability and clinical trials.
Targeted drug development in SWI/SNF-mutant cancers has focused on synthetic lethal relationships with loss of SWI/SNF subunits because no gene replacement strategies have yet proven clinically effective in cancer (Table 2). For example, elucidation of antagonism between the SWI/SNF complex and PRC2 that regulates transcriptional silencing through H3K27 methylation has identified the PRC2 catalytic subunit, EZH2, as synthetic lethal with SMARCB1 loss. Thus, EZH2, a promising therapeutic target in rhabdoid tumors and other SWI/SNF-mutant cancers, is currently in numerous clinical trials (195). For example, Tazemetostat (EPZ-6438), a selective small molecule inhibitor of the histone-lysine methyltransferase EZH2 gene, is in clinical trial, NCT02601937, a phase I open-label, dose escalation and dose expansion study for patients with rhabdoid tumors, SMARCB1-negative tumors, synovial sarcoma, and SCCOHT (196). Tazemetostat is also in phase II clinical trial (NCT02601950), a multicenter study for adults with SMARCB1-negative tumors or relapsed/refractory synovial sarcoma (197). Although both clinical trials are on-going, Italiano et al. (2018) reported the first-in-human results of Tazemetostat showing a favorable safety profile and activity in patients with refractory B-cell non-Hodgkin lymphoma and advanced solid tumors, including epithelioid sarcoma (198). However, additional clinical investigation of tazemetostat monotherapy is ongoing in phase 2 studies in adults and a phase 1 study for children, who have B-cell non-Hodgkin lymphoma and SMARCB1-negative or SMARCA4-negative tumors. Although loss of SMARCA2 and SMARCA4 appears in numerous cancers, only one clinical trial is investigating the effectiveness of EZH2 inhibitors in other SWI/SNF-mutant tumors. The phase II Pediatric MATCH trial (NCT0321366) is investigating how well tazemetostat works in patients with metastatic, recurrent or non-responsive solid tumors, non-hodgkin lymphoma, or histiocytic disorders that have EZH2, SMARCB1, or SMARCA4 gene mutations (199).
Table 2.
Synthetic Lethality Targets For The Most Commonly Mutated SWI/SNF Complex Subunits.
| TUMOR | SUBUNIT | TARGET | DRUG | REFERENCE |
|---|---|---|---|---|
| LUAD SCCOHT |  |
CDK4/6 BRD4 HDAC FGFR EZH2 |
Palbociclib OTX015 Quisinostat Ponatinib Tazemetostat |
(184, 185) (212) (83) (201) (82, 151) |
| Gastric Endometrial Ovarian Clear Cell Colorectal |  |
AKT Protein Kinases ATR BRD2 PI3K, PARP AURKA EZH2 HDAC6 |
MK-2206 Dasatinib VX-970 JQ1, iBET762 BKM120, Olaparib AURKAi GSK126 Rocilinostat |
(213) (214) (215) (216) (217) (218) (150, 219) (127) |
| Renal Clear Cell |  |
EZH2 PD1 PDL1 |
GSK126 Nivolumab Atezolizumab |
(150) (204, 205) (204, 205) |
| Epithelioid Sarcoma AT/RT RTK |  |
MDM2/4 Proteosome FGFR EZH2 |
Idasanutlin, ATSP-7041 Ixazomib NVP-BGJ398 Tazemetostat |
(220) (221) (200) (82, 151) |
| Synovial Sarcomas AT/RT RTK |  |
BRD9 | dBRD9 | (10) |
Similar to PRC2 and SWI/SNF antagonism promoting EZH2 inhibitor therapies, SWI/SNF interactions with HDACs may also provide therapeutic vulnerabilities for SWI/SNF-mutant cancers. Specifically pan-HDAC inhibitors have therapeutic potential in SCCOHT (83). Additionally, ARID1A-mutant ovarian clear cell carcinomas that depend on HDAC6 expression appear sensitive to HDAC6 inhibitors (127). Several recent reports have also discovered synergy between EZH2 and HDAC inhibition, laying the rationale for combination therapy (83, 151).
Given the relative abundance of approved receptor tyrosine kinase (RTK) inhibitors in oncology, several groups have explored the efficacy of such agents in RT and SCCOHT for rapid repurposing. These studies have led to identification of SWI/SNF-mutation-dependent sensitivities to RTK inhibitors such as ponatinib and NVP-BGJ398 that likely result from altered activity of mutant SWI/SNF complexes at RTK promoters (200, 201). Currently, the clinical activity of a protein kinase inhibitor, dasatinib, is being evaluated in phase II clinical trial (NCT02059265) (202). This ongoing trial focuses on patients with recurrent or persistent ovarian, fallopian tube, primary peritoneal and endometrial clear cell carcinoma, with or without the loss of ARID1A expression, using objective tumor response (complete and partial) (203). The clinical trial is ongoing and results are not yet available.
Because many SWI/SNF-mutant cancers, such as pediatric RT and SCCOHT, have low mutation-burden, they were considered unlikely to respond to immunologic checkpoint blockade. Excitingly, recent preclinical evidence suggests that SWI/SNF mutations may underlie inflammatory phenotypes that can drive response to checkpoint blockade (204, 205). These reports identified a relationship between expression of PBAF subunits and response to checkpoint inhibitors and T-cell mediated killing (204, 205). Using CRISPR screens and cohorts of responsive patients, the investigators uncovered a relationship between loss of PBAF expression and response to therapies. This work conflicts with the simple model that loss of PBAF and SWI/SNF subunits merely drives transformation. These exciting studies illuminate a novel therapeutic window for immunotherapies specific to the expression of the PBAF complex and highlight their importance, considering the full repertoire of SWI/SNF activity in a tumor. Indeed, recent case reports and correlative analyses of the immune microenvironment in SCCOHT suggest that immunologic checkpoint blockade may be effective in relapsed/refractory SCCOHT (206).
Despite these emerging lines of preclinical and clinical inquiry into SWI/SNF-mutation dependent therapeutic vulnerabilities, whether SWI/SNF-mutant cancers may share these vulnerabilities and how they depend on mutant SWI/SNF subunit and/or tissue type remain underexplored questions. Key differences also exist between adult and pediatric SWI/SNF-mutant tumors. For example, SMARCA2 may comprise a therapeutic target in SMARCA4-mutant adult lung cancers, but most pediatric rhabdoid tumors and SCCOHTs lack SMARCA2 expression through epigenetic silencing.
5. Conclusions
The finding of frequent mutations of subunits of the SWI/SNF chromatin remodeling complex by the TCGA and other tumor sequencing projects across a broad range of cancers has sparked renewed interest in targeting the epigenome with new treatment options. Because many of these mutations result in loss of function, efforts at identifying therapeutic vulnerabilities have suffered the same hurdles encountered for tumor suppressor genes such as BRCA1, RB1 or TP53. While recent studies have implicated the underlying mechanisms by which mutant SWI/SNF subunits can drive tumorigenesis, this knowledge has translated into limited novel treatment options. The only options that have led to drugs in clinical trials for these cancers have come from characterizing downstream signaling pathways altered by these mutations or through synthetic lethality screens of tumor cell lines. Therefore, accelerating the pace of research for understanding how mutations in SWI/SNF subunits drive development of nearly 25% of human cancers should become a high priority.
6. Expert opinion
To identify therapeutic vulnerabilities in SWI/SNF-mutant cancers, most studies have focused on finding downstream dependencies i.e. a synthetic lethality, created by the specific subunit mutation. This approach has primarily relied upon high-throughput methods including RNAi, CRISPR and drug screens. As discussed above, several successful drug targets have come from these studies including EZH2 and tyrosine receptor kinase inhibitors. However, a major caveat to these studies comes from the use of tumor cell lines, generally grown on plastic dishes, that may not accurately represent the original tumor from the patient.
Another concern from the screening studies relates to the focus of each screen on one type of SWI/SNF-mutant tumor. For example, the discovery of the dependence of cells with SMARCB1-deficiency upon EZH2-catalyzed H3K27 methylation came from studies with rhabdoid tumors. Once EZH2 inhibitors became available, multiple groups subsequently assessed its effects on tumors with mutations in different SWI/SNF subunits. Similar stories have emerged for HDAC, DNMT and EGFR inhibitors. These efficacy studies continue to expand as cell lines become available from tumors with newly-discovered mutations in SWI/SNF subunits. While this scheme works, it does not appear like the most efficient manner to find new treatment avenues for SWI/SNF-mutant tumors.
We advocate for a more unified approach to the study of SWI/SNF-mutant cancers that looks for the common mechanisms that drive tumorigenesis. We believe this approach will lead to novel insights into the roles of mutations in epigenetic regulators in human tumor development and to the identification of new targets for therapeutic intervention for aggressive cancers that often possess mutations in SWI/SNF subunits. As discussed in this review, shared mechanisms have already been identified, such as altered activities in enhancers and super-enhancers, disruption of the normal functions of the RB1 and TP53 tumor suppressors, changes in SWI/SNF complex interactions with histones, sensitivity to immune checkpoint inhibitors and aberrant activities of residual SWI/SNF complexes after subunit loss. High-throughput screening efforts using cell lines with mutations in different SWI/SNF subunits should lead to faster identification of potential therapeutic vulnerabilities and allow for their prioritization for drug discovery efforts.
Another emerging issue lies in the potential for development of drug resistance. As is the case for many single therapeutic agents, cases of resistance to some of these agents have begun to appear in clinical trials. This problem may be particularly relevant to SWI/SNF-mutant tumors where increased epigenetic fluidity appears more frequently than genomic instability. The plasticity in the epigenome of these tumors may allow for rapid acquisition of drug resistance. However, this feature could also prove beneficial when considering two agent therapies. For example, resistance to tyrosine kinase inhibitors can arise through the reactivation or increased expression of a related tyrosine kinase, generally through an epigenetic change. Therefore, treatment with an epigenetic inhibitor, such as tazemetostat, in combination with a tyrosine kinase inhibitor, such as dasatinib, could be synergic and effective for SWI/SNF-mutant tumors. Again, rather than 2 agent screens on one type of SWI/SNF-mutant tumor, a combined approach on representative tumors may prove more expedient.
One last area that remains understudied is the potential role of missense mutation in SWI/SNF subunits. As discussed in the review, missense mutations appear at “hotspots” in SMARCA4 and SMARCB1 in SCCOHTs and rhabdoid tumors, respectively. Missense mutations also occur at specific sites in some of the other subunits including SMARCA2, ARID1B and SMARCC1. Of import, many of the same missense mutations appear to drive the development of several human developmental disorders. The few studies that have assessed the effects of these missense mutations on SWI/SNF activities report significant functional consequences. Considering that the SS18-SSX and EWS-FLI1 fusion proteins imbue the SWI/SNF complex with de novo oncogenic features, these missense mutations could exert similar effects. Therapeutic vulnerabilities may also arise in tumors with missense mutations that do appear in those with loss of function, similar to differences seen between TP53 deletions versus missense mutations upon tumorigenesis. Further characterization of these SWI/SNF missense mutations may also prove efficacious for adult malignancies which often have missense mutations in SWI/SNF subunits rather than loss of protein expression.
Studies that identify common mechanisms of tumor development among different epigenetic regulators such as the SWI/SNF complex, histone modification and substitutions and DNA methyltransferases will yield new drugs for more effective treatments of a broad range of human cancers. With the further development of CRISPR technologies, treatments for SWI/SNF-mutant tumors will appear that disrupt the key enhancers and/or super enhancers required for tumor development. The completion of clinical trials for targeting of epigenetic vulnerabilities in SWI/SNF-mutant tumors such as EZH2, HDACs and BRD9 will establish the efficacy of these treatment approaches.
Article highlights.
Mutations in subunits of the SWI/SNF complex appear in nearly 25% of all human cancers. Many of these tumors behave aggressively with little to no long-term survival. Importantly, some of these tumors possess only a mutation in one SWI/SNF subunit along with little evidence of genomic instability. Therefore, these tumors may represent true “epigenetically-driven” cancers.
The SWI/SNF complex regulates nucleosome positioning throughout the human epigenome through an energy-driven mechanism. Because of this central role in controlling chromatin organization, mutations in the complex can disrupt the regulation of many cellular processes such as proliferation, programmed cell death and differentiation as well as normal development.
Because of its broad regulatory roles, studies have identified multiple mechanisms underlying the development of SWI/SNF-mutant tumors including changes in enhancer and super-enhancer sites, altered interactions with key histone modifications, aberrant regulation of the RB and TP53 tumor suppressor pathways and changes in interactions with non-coding RNAs.
Identification of targeted therapies for SWI/SNF-mutant cancers is in its infancy. However, a notable new class of drugs inhibits the EZH2 histone methyltransferase, a synthetic lethality in a subset of cancers with loss of expression of specific SWI/SNF subunits. Other studies have implicated existing tyrosine kinase inhibitors and immune checkpoint inhibitors as effective on SWI/SNF-mutant tumors. However, the efforts to identify new and effective treatments for these tumors would benefit from a unified approach of seeking common therapeutic vulnerabilities among the mutations in different subunits.
Funding
This paper was funded by the National Institute of Health
Grant to BE Weissman: CA195670
Grant to T Walhart: CA217824
Grant to V Nguyen: ES007126
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Articles or patents of special interest have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Savas S, Skardasi G. The SWI/SNF complex subunit genes: Their functions, variations, and links to risk and survival outcomes in human cancers. Crit Rev Oncol Hematol. 2018;123:114–31. Epub 2018/02/28. doi: 10.1016/j.critrevonc.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Kassabov SR, Zhang B, Persinger J, Bartholomew B. SWI/SNF unwraps, slides, and rewraps the nucleosome. Mol Cell. 2003;11(2):391–403. [DOI] [PubMed] [Google Scholar]
- 3.Phelan ML, Sif S, Narlikar GJ, Kingston RE. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol Cell. 1999;3(2):247–53. [DOI] [PubMed] [Google Scholar]
- 4.Hu G, Schones DE, Cui K, Ybarra R, Northrup D, Tang Q, et al. Regulation of nucleosome landscape and transcription factor targeting at tissue-specific enhancers by BRG1. Genome Res. 2011;21(10):1650–8. Epub 2011/07/29. doi: gr.121145.111 [pii] 10.1101/gr.121145.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolstorukov MY, Sansam CG, Lu P, Koellhoffer EC, Helming KC, Alver BH, et al. Swi/Snf chromatin remodeling/tumor suppressor complex establishes nucleosome occupancy at target promoters. Proc Natl Acad Sci U S A. 2013;110(25):10165–70. Epub 2013/06/01. doi: 10.1073/pnas.13022091101302209110 [pii].* Tolstorukov et al. is the first report to show that loss of the SWI/SNF complex subunits SMARCA4 and SMARCB1 caused dramatic effects on nucleosome positioning in mammalian cells, establishing a model for how their inactivation might drive human tumorigenesis.
- 6.You JS, De Carvalho DD, Dai C, Liu M, Pandiyan K, Zhou XJ, et al. SNF5 is an essential executor of epigenetic regulation during differentiation. PLoS Genet. 2013;9(4):e1003459 Epub 2013/05/03. doi: 10.1371/journal.pgen.1003459PGENETICS-D-12-02468 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reisman DN, Sciarrotta J, Bouldin TW, Weissman BE, Funkhouser WK. The expression of the SWI/SNF ATPase subunits BRG1 and BRM in normal human tissues. Appl Immunohistochem Mol Morphol. 2005;13(1):66–74. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Cote J, Xue Y, Zhou S, Khavari PA, Biggar SR, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. Embo J. 1996;15(19):5370–82. [PMC free article] [PubMed] [Google Scholar]
- 9.Wu JI, Lessard J, Crabtree GR. Understanding the words of chromatin regulation. Cell. 2009;136(2):200–6. Epub 2009/01/27. doi: 10.1016/j.cell.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel BC, D’Avino AR, Cassel SH, Mashtalir N, McKenzie ZM, McBride MJ, et al. A non-canonical SWI/SNF complex is a synthetic lethal target in cancers driven by BAF complex perturbation. Nat Cell Biol. 2018;20(12):1410–20. Epub 2018/11/07. doi: 10.1038/s41556-018-0221-1.** Michel et al. identified a new non-canonical form of the SWI/SNF complex containing a new subunit, GLTSCR1. The dependence of this complex upon the BRD9 subunit offers a new therapeutic target for SWI/SNF-mutant tumors.
- 11.Biegel JA, Busse TM, Weissman BE. SWI/SNF chromatin remodeling complexes and cancer. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2014;166:350–66. doi: 10.1002/ajmg.c.31410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valencia AM, Kadoch C. Chromatin regulatory mechanisms and therapeutic opportunities in cancer. Nat Cell Biol. 2019;21(2):152–61. Epub 2019/01/04. doi: 10.1038/s41556-018-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59(1):74–9. [PubMed] [Google Scholar]
- 14.Versteege I, Sevenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–6.** Versteege et al. are the first to describe a mutation in a SWI/SNF complex member in cancer. This study provided the evidence that the SWI/SNF complex may act as a tumor suppressor and be mutated in other cancers.
- 15.Margol AS, Judkins AR. Pathology and diagnosis of SMARCB1-deficient tumors. Cancer Genetics. 2014;207(9):358–64. doi: 10.1016/j.cancergen.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Medina PP, Romero OA, Kohno T, Montuenga LM, Pio R, Yokota J, et al. Frequent BRG1/SMARCA4-inactivating mutations in human lung cancer cell lines. Human Mutation. 2008;29(5):617–22. doi: 10.1002/humu.20730. [DOI] [PubMed] [Google Scholar]
- 17.Reisman DN, Sciarrotta J, Wang W, Funkhouser WK, Weissman BE. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: correlation with poor prognosis. Cancer research. 2003;63(3):560–6. [PubMed] [Google Scholar]
- 18.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331(6016):435–9. Epub 2010/12/18. doi: 10.1126/science.1198056science.1198056 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shain AH, Giacomini CP, Matsukuma K, Karikari CA, Bashyam MD, Hidalgo M, et al. Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer. Proceedings of the National Academy of Sciences. 2012;109(5):E252–9. doi: 10.1073/pnas.1114817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong AK, Shanahan F, Chen Y, Lian L, Ha P, Hendricks K, et al. BRG1, a component of the SWI-SNF complex, is mutated in multiple human tumor cell lines. Cancer Res. 2000;60(21):6171–7. [PubMed] [Google Scholar]
- 21.Jelinic P, Mueller JJ, Olvera N, Dao F, Scott SN, Shah R, et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nature genetics. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos P, Karnezis AN, Craig DW, Sekulic A, Russell ML, Hendricks WP, et al. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat Genet. 2014;46(5):427–9. doi: 10.1038/ng.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witkowski L, Carrot-Zhang J, Albrecht S, Fahiminiya S, Hamel N, Tomiak E, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nature genetics. 2014. [DOI] [PubMed] [Google Scholar]
- 24.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1AMutations in Endometriosis-Associated Ovarian Carcinomas. New England Journal of Medicine. 2010;363(16):1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nature Genetics. 2011;43(9):875–8. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones S, Wang T-L, Shih I-M, Mao T-L, Nakayama K, Roden R, et al. Frequent Mutations of Chromatin Remodeling Gene ARID1A in Ovarian Clear Cell Carcinoma. Science. 2010;330(6001):228–31. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shain AH, Pollack JR. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PloS one. 2013;8(1):e55119. doi: 10.1371/journal.pone.0055119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44(7):760–4. Epub 2012/05/29. doi: 10.1038/ng.2291ng.2291 [pii]. [DOI] [PubMed] [Google Scholar]
- 29.Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33(1):100–3. Epub 2011/10/20. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43(12):1219–23. Epub 2011/11/01. doi: ng.982 [pii] 10.1038/ng.982. [DOI] [PubMed] [Google Scholar]
- 31.Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469(7331):539–42. Epub 2011/01/21. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biegel JA. Molecular genetics of atypical teratoid/rhabdoid tumor. Neurosurg Focus. 2006;20(1):E11 Epub 2006/02/08. [DOI] [PubMed] [Google Scholar]
- 33.Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–34. Epub 2012/11/24. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. Epub 2012/05/17. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1 Epub 2013/04/04. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsurusaki Y, Okamoto N, Ohashi H, Kosho T, Imai Y, Hibi-Ko Y, et al. Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nature Genetics. 2012. doi: 10.1038/ng.2219. [DOI] [PubMed] [Google Scholar]
- 37.Hodges HC, Stanton BZ, Cermakova K, Chang C-Y, Miller EL, Kirkland JG, et al. Dominant-negative SMARCA4 mutants alter the accessibility landscape of tissue-unrestricted enhancers. Nature Structural & Molecular Biology . 2018;25(1):61–72. doi: 10.1038/s41594-017-0007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karnezis AN, Wang Y, Ramos P, Hendricks WP, Oliva E, D’Angelo E, et al. Dual loss of the SWI/SNF complex ATPases SMARCA4/BRG1 and SMARCA2/BRM is highly sensitive and specific for small cell carcinoma of the ovary, hypercalcaemic type. J Pathol. 2016;238(3):389–400. doi: 10.1002/path.4633.* SCCOHT is the first cancer described to have dual loss of both ATPases in a simple genetic background. Karnezis et al. show that dual loss of both ATPases by IHC is sensitive and specific for the diagnosis of SCCOHT and distinguish it from other gynecological mimics.
- 39.Betz BL, Strobeck MW, Reisman DN, Knudsen ES, Weissman BE. Re-expression of hSNF5/INI1/BAF47 in pediatric tumor cells leads to G1 arrest associated with induction of p16ink4a and activation of RB. Oncogene. 2002;21(34):5193–203. doi: 10.1038/sj.onc.1205706.* Betz et al. is the first study to link SMARCB1 loss with the CDKN2A/RB signaling pathway.
- 40.Roberts CW, Leroux MM, Fleming MD, Orkin SH. Highly penetrant, rapid tumorigenesis through conditional inversion of the tumor suppressor gene Snf5. Cancer Cell. 2002;2(5):415–25. [DOI] [PubMed] [Google Scholar]
- 41.Mathur R ARID1A loss in cancer: Towards a mechanistic understanding. Pharmacol Ther. 2018;190:15–23. Epub 2018/05/08. doi: 10.1016/j.pharmthera.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, et al. A Brg1 Null Mutation in the Mouse Reveals Functional Differences among Mammalian SWI/SNF Complexes. Molecular Cell. 2000;6(6):9-. doi: 10.1016/S1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 43.Serber DW, Rogala A, Makarem M, Rosson GB, Simin K, Godfrey V, et al. The BRG1 Chromatin Remodeler Protects Against Ovarian Cysts, Uterine Tumors, and Mammary Tumors in a Lineage-Specific Manner. PloS one. 2012;7(2):e31346. doi: 10.1371/journal.pone.0031346.s005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St. Pierre R, Kadoch C. Mammalian SWI/SNF complexes in cancer: emerging therapeutic opportunities. Current Opinion in Genetics & Development. 2017;42:56–67. doi: 10.1016/j.gde.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roy N, Malik S, Villanueva KE, Urano A, Lu X, Von Figura G, et al. Brg1 promotes both tumor-suppressive and oncogenic activities at distinct stages of pancreatic cancer formation. Genes Dev. 2015;29(6):658–71. Epub 2015/03/21. doi: 10.1101/gad.256628.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, Wang SC, Wei Y, Luo X, Jia Y, Li L, et al. Arid1a Has Context-Dependent Oncogenic and Tumor Suppressor Functions in Liver Cancer. Cancer Cell. 2017;32(5):574–89 e6. Epub 2017/11/15. doi: 10.1016/j.ccell.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bourgo RJ, Siddiqui H, Fox S, Solomon D, Sansam CG, Yaniv M, et al. SWI/SNF deficiency results in aberrant chromatin organization, mitotic failure, and diminished proliferative capacity. Molecular biology of the cell. 2009;20(14):3192–9. doi: 10.1091/mbc.E08-12-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isakoff MS, Sansam CG, Tamayo P, Subramanian A, Evans JA, Fillmore CM, et al. Inactivation of the Snf5 tumor suppressor stimulates cell cycle progression and cooperates with p53 loss in oncogenic transformation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17745–50. doi: 10.1073/pnas.0509014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandler RL, Magnuson T. The SWI/SNF BAF-A complex is essential for neural crest development. Developmental Biology. 2016;411(1):15–24. doi: 10.1016/j.ydbio.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto S, Banine F, Struve J, Xing R, Adams C, Liu Y, et al. Brg1 is required for murine neural stem cell maintenance and gliogenesis. Developmental Biology. 2006;289(2):372–83. doi: 10.1016/j.ydbio.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 51.Kupryjańczyk J, Dansonka-Mieszkowska A, Moes-Sosnowska J, Plisiecka-Hałasa J, Szafron Ł, Podgórska A, et al. Ovarian small cell carcinoma of hypercalcemic type – evidence of germline origin and smarca4 gene inactivation. a pilot study. Polish Journal of Pathology. 2013;4:238–46. doi: 10.5114/pjp.2013.39331. [DOI] [PubMed] [Google Scholar]
- 52.Hasselblatt M, Gesk S, Oyen F, Rossi S, Viscardi E, Giangaspero F, et al. Nonsense Mutation and Inactivation of SMARCA4 (BRG1) in an Atypical Teratoid/Rhabdoid Tumor Showing Retained SMARCB1 (INI1) Expression. The American Journal of Surgical Pathology. 2011;35(6):933–5. doi: 10.1097/pas.0b013e3182196a39. [DOI] [PubMed] [Google Scholar]
- 53.Schneppenheim R, Frühwald MC, Gesk S, Hasselblatt M, Jeibmann A, Kordes U, et al. Germline Nonsense Mutation and Somatic Inactivation of SMARCA4/BRG1 in a Family with Rhabdoid Tumor Predisposition Syndrome. The American Journal of Human Genetics. 2010;86(2):279–84. doi: 10.1016/j.ajhg.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witkowski L, Lalonde E, Zhang J, Albrecht S, Hamel N, Cavallone L, et al. Familial rhabdoid tumour ‘avant la lettre’-from pathology review to exome sequencing and back again. The Journal of Pathology. 2013;231(1):35–43. doi: 10.1002/path.4225. [DOI] [PubMed] [Google Scholar]
- 55.Hasselblatt M, Nagel I, Oyen F, Bartelheim K, Russell RB, Schuller U, et al. SMARCA4-mutated atypical teratoid/rhabdoid tumors are associated with inherited germline alterations and poor prognosis. Acta Neuropathol. 2014;128(3):453–6. Epub 2014/07/26. doi: 10.1007/s00401-014-1323-x. [DOI] [PubMed] [Google Scholar]
- 56.Le Loarer F, Watson S, Pierron G, de Montpreville VT, Ballet S, Firmin N, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nature Genetics. 2015;47(10):1200–5. doi: 10.1038/ng.3399. [DOI] [PubMed] [Google Scholar]
- 57.Perret R, Chalabreysse L, Watson S, Serre I, Garcia S, Forest F, et al. SMARCA4-deficient Thoracic Sarcomas. The American Journal of Surgical Pathology. 2018:1-. doi: 10.1097/pas.0000000000001188. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida A, Kobayashi E, Kubo T, Kodaira M, Motoi T, Motoi N, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2017;30(6):797–809. doi: 10.1038/modpathol.2017.11. [DOI] [PubMed] [Google Scholar]
- 59.Agaimy A, Weichert W. SMARCA4-deficient Sinonasal Carcinoma. Head and neck pathology. 2017;11(4):541–5. doi: 10.1007/s12105-017-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jo VY, Chau NG, Hornick JL, Krane JF, Sholl LM. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Modern Pathology. 2017;30(5):650–9. doi: 10.1038/modpathol.2016.239. [DOI] [PubMed] [Google Scholar]
- 61.Kolin DL, Dong F, Baltay M, Lindeman N, MacConaill L, Nucci MR, et al. SMARCA4-deficient undifferentiated uterine sarcoma (malignant rhabdoid tumor of the uterus): a clinicopathologic entity distinct from undifferentiated carcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2018;31(9):1442–56. doi: 10.1038/s41379-018-0049-z. [DOI] [PubMed] [Google Scholar]
- 62.Mito JK, Bishop JA, Sadow PM, Stelow EB, Faquin WC, Mills SE, et al. Immunohistochemical Detection and Molecular Characterization of IDH-mutant Sinonasal Undifferentiated Carcinomas. The American Journal of Surgical Pathology. 2018;42(8):1067–75. doi: 10.1097/pas.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 63.Al-Hussaini M, Lataifeh I, Jaradat I, Abdeen G, Otay L, Badran O, et al. Undifferentiated Endometrial Carcinoma, an Immunohistochemical Study Including PD-L1 Testing of a Series of Cases From a Single Cancer Center. International Journal of Gynecological Pathology. 2018:1-. doi: 10.1097/PGP.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 64.Karnezis AN, Hoang LN, Coatham M, Ravn S, Almadani N, Tessier-Cloutier B, et al. Loss of switch/sucrose non-fermenting complex protein expression is associated with dedifferentiation in endometrial carcinomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2016;29(3):302–14. doi: 10.1038/modpathol.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Köbel M, Hoang LN, Tessier-Cloutier B, Meng B, Soslow RA, Stewart CJR, et al. Undifferentiated Endometrial Carcinomas Show Frequent Loss of Core Switch/Sucrose Nonfermentable Complex Proteins. The American Journal of Surgical Pathology. 2017:1-. doi: 10.1097/pas.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramalingam P, Croce S, McCluggage WG. Loss of expression of SMARCA4 (BRG1), SMARCA2 (BRM) and SMARCB1 (INI1) in undifferentiated carcinoma of the endometrium is not uncommon and is not always associated with rhabdoid morphology. Histopathology. 2016;70(3):359–66. doi: 10.1111/his.13091. [DOI] [PubMed] [Google Scholar]
- 67.Strehl JD, Wachter DL, Fiedler J, Heimerl E, Beckmann MW, Hartmann A, et al. Pattern of SMARCB1 (INI1) and SMARCA4 (BRG1) in poorly differentiated endometrioid adenocarcinoma of the uterus: analysis of a series with emphasis on a novel SMARCA4-deficient dedifferentiated rhabdoid variant. Annals of Diagnostic Pathology. 2015;19(4):198–202. doi: 10.1016/j.anndiagpath.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 68.Agaimy A, Daum O, Märkl B, Lichtmannegger I, Michal M, Hartmann A. SWI/SNF Complex–deficient Undifferentiated/Rhabdoid Carcinomas of the Gastrointestinal Tract. The American Journal of Surgical Pathology. 2016;40(4):544–53. doi: 10.1097/pas.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 69.Agaimy A, Cheng L, Egevad L, Feyerabend B, Hes O, Keck B, et al. Rhabdoid and Undifferentiated Phenotype in Renal Cell Carcinoma. The American Journal of Surgical Pathology. 2017;41(2):253–62. doi: 10.1097/pas.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 70.Chae YK, Chung SY, Davis AA, Carneiro BA, Chandra S, Kaplan J, et al. Adenoid cystic carcinoma: current therapy and potential therapeutic advances based on genomic profiling. Oncotarget. 2015;6(35). doi: 10.18632/oncotarget.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet. 2013;45(7):791–8. Epub 2013/05/21. doi: 10.1038/ng.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stephens PJ, Davies HR, Mitani Y, Van Loo P, Shlien A, Tarpey PS, et al. Whole exome sequencing of adenoid cystic carcinoma. Journal of Clinical Investigation. 2013;123(7):2965–8. doi: 10.1172/jci67201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bock VL, Lyons JG, Huang XXJ, Jones AM, McDonald LA, Scolyer RA, et al. BRM and BRG1 subunits of the SWI/SNF chromatin remodelling complex are downregulated upon progression of benign skin lesions into invasive tumours. The British journal of dermatology. 2011;164(6):1221–7. doi: 10.1111/j.1365-2133.2011.10267.x. [DOI] [PubMed] [Google Scholar]
- 74.Moloney FJ, Lyons JG, Bock VL, Huang XX, Bugeja MJ, Halliday GM. Hotspot mutation of Brahma in non-melanoma skin cancer. The Journal of investigative dermatology. 2009;129(4):1012–5. doi: 10.1038/jid.2008.319. [DOI] [PubMed] [Google Scholar]
- 75.Endo M, Yasui K, Zen Y, Gen Y, Zen K, Tsuji K, et al. Alterations of the SWI/SNF chromatin remodelling subunit-BRG1 and BRM in hepatocellular carcinoma. Liver International. 2012;33(1):105–17. doi: 10.1111/liv.12005. [DOI] [PubMed] [Google Scholar]
- 76.Gunduz E, Gunduz M, Ali MAS, Beder L, Tamamura R, Katase N, et al. Loss of heterozygosity at the 9p21–24 region and identification of BRM as a candidate tumor suppressor gene in head and neck squamous cell carcinoma. Cancer investigation. 2009;27(6):661–8. doi: 10.1080/07357900802563010. [DOI] [PubMed] [Google Scholar]
- 77.Glaros S, Cirrincione GM, Muchardt C, Kleer CG, Michael CW, Reisman D. The reversible epigenetic silencing of BRM: implications for clinical targeted therapy. Oncogene. 2007;26(49):7058–66. doi: 10.1038/sj.onc.1210514. [DOI] [PubMed] [Google Scholar]
- 78.Wu J, He K, Zhang Y, Song J, Shi Z, Chen W, et al. Inactivation of SMARCA2 by promoter hypermethylation drives lung cancer development. Gene. 2019;687:193–9. doi: 10.1016/j.gene.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 79.Liu G, Cuffe S, Liang S, Azad AK, Cheng L, Brhane Y, et al. BRM Promoter Polymorphisms and Survival of Advanced Non–Small Cell Lung Cancer Patients in the Princess Margaret Cohort and CCTG BR.24 Trial. Clinical Cancer Research. 2016;23(10):2460–70. doi: 10.1158/1078-0432.ccr-16-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu G, Gramling S, Munoz D, Cheng D, Azad AK, Mirshams M, et al. Two novel BRM insertion promoter sequence variants are associated with loss of BRM expression and lung cancer risk. Oncogene. 2011;30(29):3295–304. doi: 10.1038/onc.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang JR, Gramling SJB, Goldstein DP, Cheng D, Chen D, Azad AK, et al. Association of two BRM promoter polymorphisms with head and neck squamous cell carcinoma risk. Carcinogenesis. 2013;34(5):1012–7. doi: 10.1093/carcin/bgt008. [DOI] [PubMed] [Google Scholar]
- 82.Chan-Penebre E, Armstrong K, Drew A, Grassian AR, Feldman I, Knutson SK, et al. Selective Killing of SMARCA2- and SMARCA4-deficient Small Cell Carcinoma of the Ovary, Hypercalcemic Type Cells by Inhibition of EZH2: In Vitro and In Vivo Preclinical Models. Molecular cancer therapeutics. 2017;16(5):850–60. doi: 10.1158/1535-7163.MCT-16-0678. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Chen SY, Colborne S, Lambert G, Shin CY, Santos ND, et al. Histone Deacetylase Inhibitors Synergize with Catalytic Inhibitors of EZH2 to Exhibit Antitumor Activity in Small Cell Carcinoma of the Ovary, Hypercalcemic Type. Molecular cancer therapeutics. 2018;17(12):2767–79. doi: 10.1158/1535-7163.MCT-18-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamamichi N, Yamamichi-Nishina M, Mizutani T, Watanabe H, Minoguchi S, Kobayashi N, et al. The Brm gene suppressed at the post-transcriptional level in various human cell lines is inducible by transient HDAC inhibitor treatment, which exhibits antioncogenic potential. Oncogene. 2005;24(35):5471–81. doi: 10.1038/sj.onc.1208716.* Yamamichi et al. describe that SMARCA2/BRM expression can be epigenetically regulated by HDACs in cancer.
- 85.Jelinic P, Schlappe BA, Conlon N, Tseng J, Olvera N, Dao F, et al. Concomitant loss of SMARCA2 and SMARCA4 expression in small cell carcinoma of the ovary, hypercalcemic type. Modern Pathology. 2015;29(1):60–6. doi: 10.1038/modpathol.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffman GR, Rahal R, Buxton F, Xiang K, McAllister G, Frias E, et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proceedings of the National Academy of Sciences. 2014;111(8):3128–33. doi: 10.1073/PNAS.1316793111.** In SMARCA4 deficient cancers with complex genetic backgrounds, SMARCA2 is a synthetic lethal target.
- 87.Oike T, Ogiwara H, Tominaga Y, Ito K, Ando O, Tsuta K, et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer research. 2013;73(17):5508–18. doi: 10.1158/0008-5472.CAN-12-4593. [DOI] [PubMed] [Google Scholar]
- 88.Herpel E, Rieker RJ, Dienemann H, Muley T, Meister M, Hartmann A, et al. SMARCA4 and SMARCA2 deficiency in non–small cell lung cancer: immunohistochemical survey of 316 consecutive specimens. Annals of Diagnostic Pathology. 2017;26:47–51. doi: 10.1016/j.anndiagpath.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Wei D, Weissman BE. Genetics and Genomics of Malignant Rhabdoid Tumors. eLS 2014 [Internet]. 2014. Available from: http://www.els.net.
- 90.Sévenet N, Lellouch-Tubiana A, Schofield D, Hoang-Xuan K, Gessler M, Birnbaum D, et al. Spectrum of hSNF5/INI1 somatic mutations in human cancer and genotype-phenotype correlations. Human molecular genetics. 1999;8:2359–68. [DOI] [PubMed] [Google Scholar]
- 91.Lee RS, Stewart C, Carter SL, Ambrogio L, Cibulskis K, Sougnez C, et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest. 2012;122(8):2983–8. Epub 2012/07/17. doi: 10.1172/JCI64400.* Lee et al establish that that only recurrent mutation in rhabdoid tumors is in SMARCB1.
- 92.Chun H-JE, Lim EL, Heravi-Moussavi A, Saberi S, Mungall KL, Bilenky M, et al. Genome-Wide Profiles of Extra-cranial Malignant Rhabdoid Tumors Reveal Heterogeneity and Dysregulated Developmental Pathways. Cancer Cell. 2016;29(3):394–406. doi: 10.1016/j.ccell.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johann PD, Erkek S, Zapatka M, Kerl K, Buchhalter I, Hovestadt V, et al. Atypical Teratoid/Rhabdoid Tumors Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes. Cancer Cell. 2016;29(3):379–93. doi: 10.1016/j.ccell.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 94.Calderaro J, Moroch J, Pierron G, Pedeutour F, Grison C, Maille P, et al. SMARCB1/INI1 inactivation in renal medullary carcinoma. Histopathology. 2012;61(3):428–35. Epub 2012/06/13. doi: 10.1111/j.1365-2559.2012.04228.x. [DOI] [PubMed] [Google Scholar]
- 95.Mobley BC, McKenney JK, Bangs CD, Callahan K, Yeom KW, Schneppenheim R, et al. Loss of SMARCB1/INI1 expression in poorly differentiated chordomas. Acta Neuropathologica. 2010;120:745–53. doi: 10.1007/s00401-010-0767-x. [DOI] [PubMed] [Google Scholar]
- 96.Sullivan LM, Folpe AL, Pawel BR, Judkins AR, Biegel JA. Epithelioid sarcoma is associated with a high percentage of SMARCB1 deletions. Modern Pathology. 2013;26:385–92. doi: 10.1038/modpathol.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gulluoglu S, Turksoy O, Kuskucu A, Ture U, Bayrak OF. The molecular aspects of chordoma. Neurosurgical Review. 2016;39:185–96. doi: 10.1007/s10143-015-0663-x. [DOI] [PubMed] [Google Scholar]
- 98.Hasselblatt M, Thomas C, Hovestadt V, Schrimpf D, Johann P, Bens S, et al. Poorly differentiated chordoma with SMARCB1/INI1 loss: a distinct molecular entity with dismal prognosis. Acta Neuropathologica. 2016;132:149–51. doi: 10.1007/s00401-016-1574-9. [DOI] [PubMed] [Google Scholar]
- 99.Arnaud O, Le Loarer F, Tirode F. BAFfling pathologies: Alterations of BAF complexes in cancer. Cancer Letters. 2018;419:266–79. doi: 10.1016/j.canlet.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 100.Kohashi K, Yamamoto H, Yamada Y, Kinoshita I, Taguchi T, Iwamoto Y, et al. SWI/SNF Chromatin-remodeling Complex Status in SMARCB1/INI1-preserved Epithelioid Sarcoma. The American Journal of Surgical Pathology. 2018;42:312–8. doi: 10.1097/PAS.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 101.Modena P, Lualdi E, Facchinetti F, Galli L, Teixeira MR, Pilotti S, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65(10):4012–9. [DOI] [PubMed] [Google Scholar]
- 102.Gasparini P, Facchinetti F, Boeri M, Lorenzetto E, Livio A, Gronchi A, et al. Prognostic determinants in epithelioid sarcoma. European journal of cancer (Oxford, England : 1990). 2011;47:287–95. doi: 10.1016/j.ejca.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Le Loarer F, Zhang L, Fletcher CD, Ribeiro A, Singer S, Italiano A, et al. Consistent SMARCB1 homozygous deletions in epithelioid sarcoma and in a subset of myoepithelial carcinomas can be reliably detected by FISH in archival material. Genes, Chromosomes and Cancer. 2014;53:475–86. doi: 10.1002/gcc.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.MacCollin M, Woodfin W, Kronn D, Short MP. Schwannomatosis: a clinical and pathologic study. Neurology. 1996;46(4):1072–9. Epub 1996/04/01. [DOI] [PubMed] [Google Scholar]
- 105.Boyd C, Smith MJ, Kluwe L, Balogh A, Maccollin M, Plotkin SR. Alterations in the SMARCB1 (INI1) tumor suppressor gene in familial schwannomatosis. Clin Genet. 2008;74(4):358–66. Epub 2008/07/24. doi: 10.1111/j.1399-0004.2008.01060.xCGE1060 [pii]. [DOI] [PubMed] [Google Scholar]
- 106.Hulsebos TJ, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet. 2007;80(4):805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smith MJ, Wallace AJ, Bowers NL, Eaton H, Evans DGR. SMARCB1 mutations in schwannomatosis and genotype correlations with rhabdoid tumors. Cancer Genetics. 2014;207:373–8. doi: 10.1016/j.cancergen.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 108.Sestini R, Bacci C, Provenzano A, Genuardi M, Papi L. Evidence of a four-hit mechanism involving SMARCB1 and NF2 in schwannomatosis-associated schwannomas. Human mutation. 2008;29:227–31. doi: 10.1002/humu.20679. [DOI] [PubMed] [Google Scholar]
- 109.Hadfield KD, Newman WG, Bowers NL, Wallace A, Bolger CM, Colley A, et al. Molecular characterisation of SMARCB1 and NF2 in familial and sporadic schwannomatosis. J Med Genet. 2008. [DOI] [PubMed] [Google Scholar]
- 110.Paganini I, Chang VY, Capone GL, Vitte J, Benelli M, Barbetti L, et al. Expanding the mutational spectrum of LZTR1 in schwannomatosis. European journal of human genetics : EJHG. 2015;23:963–8. doi: 10.1038/ejhg.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Allen MD, Freund SMV, Zinzalla G, Bycroft M. The SWI/SNF Subunit INI1 Contains an N-Terminal Winged Helix DNA Binding Domain that Is a Target for Mutations in Schwannomatosis. Structure. 2015;23:1344–9. doi: 10.1016/j.str.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim KH, Roberts CWM. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genetics. 2014;207:365–72. doi: 10.1016/j.cancergen.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hasselblatt M, Isken S, Linge A, Eikmeier K, Jeibmann A, Oyen F, et al. High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes, chromosomes & cancer. 2013;52:185–90. doi: 10.1002/gcc.22018. [DOI] [PubMed] [Google Scholar]
- 114.McKenna ES, Sansam CG, Cho Y-J, Greulich H, Evans JA, Thom CS, et al. Loss of the Epigenetic Tumor Suppressor SNF5 Leads to Cancer without Genomic Instability. Molecular and Cellular Biology. 2008;28:6223–33. doi: 10.1128/MCB.00658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Collins RT, Treisman JE. Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes Dev. 2000;14(24):3140–52. doi: 10.1101/gad.854300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilsker D, Patsialou A, Zumbrun SD, Kim S, Chen Y, Dallas PB, et al. The DNA-binding properties of the ARID-containing subunits of yeast and mammalian SWI/SNF complexes. Nucleic Acids Res. 2004;32(4):1345–53. doi: 10.1093/nar/gkh277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nie Z, Xue Y, Yang D, Zhou S, Deroo BJ, Archer TK, et al. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol Cell Biol. 2000;20(23):8879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xue Y, Canman JC, Lee CS, Nie Z, Yang D, Moreno GT, et al. The human SWI/SNF-B chromatin-remodeling complex is related to yeast rsc and localizes at kinetochores of mitotic chromosomes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(24):13015–20. doi: 10.1073/pnas.240208597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fryer CJ, Archer TK. Chromatin remodelling by the glucocorticoid receptor requires the BRG1 complex. Nature. 1998;393(6680):88–91. doi: 10.1038/30032. [DOI] [PubMed] [Google Scholar]
- 120.Flores-Alcantar A, Gonzalez-Sandoval A, Escalante-Alcalde D, Lomelí H. Dynamics of expression of ARID1A and ARID1B subunits in mouse embryos and in cells during the cell cycle. Cell and Tissue Research. 2011;345(1):137–48. doi: 10.1007/s00441-011-1182-x. [DOI] [PubMed] [Google Scholar]
- 121.Sun X, Chuang J-C, Kanchwala M, Wu L, Celen C, Li L, et al. Suppression of the SWI/SNF Component Arid1a Promotes Mammalian Regeneration. Cell Stem Cell. 2016;18(4):456–66. doi: 10.1016/j.stem.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cornen S, Adelaide J, Bertucci F, Finetti P, Guille A, Birnbaum DJ, et al. Mutations and deletions of ARID1A in breast tumors. Oncogene. 2012;31(38):4255–6. doi: 10.1038/onc.2011.598. [DOI] [PubMed] [Google Scholar]
- 123.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44(6):694–8. Epub 2012/05/09. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang K, Yuen ST, Xu J, Lee SP, Yan HHN, Shi ST, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nature Genetics. 2014;46(6):573–82. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 125.Kim M, Lu F, Zhang Y. Loss of HDAC-Mediated Repression and Gain of NF-κB Activation Underlie Cytokine Induction in ARID1A- and PIK3CA-Mutation-Driven Ovarian Cancer. Cell Reports. 2016;17(1):275–88. doi: 10.1016/j.celrep.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, et al. Coexistent ARID1A–PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nature Communications. 2015;6(1). doi: 10.1038/ncomms7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bitler BG, Wu S, Park PH, Hai Y, Aird KM, Wang Y, et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nature Cell Biology. 2017;19(8):962–73. doi: 10.1038/ncb3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cancer Genome Atlas Research N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499(7456):43–9. Epub 2013/06/25. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45(8):860–7. Epub 2013/06/26. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 130.Nargund AM, Pham CG, Dong Y, Wang PI, Osmangeyoglu HU, Xie Y, et al. The SWI/SNF Protein PBRM1 Restrains VHL-Loss-Driven Clear Cell Renal Cell Carcinoma. Cell Rep. 2017;18(12):2893–906. Epub 2017/03/23. doi: 10.1016/j.celrep.2017.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Porter EG, Dykhuizen EC. Individual Bromodomains of Polybromo-1 Contribute to Chromatin Association and Tumor Suppression in Clear Cell Renal Carcinoma. J Biol Chem. 2017;292(7):2601–10. Epub 2017/01/06. doi: 10.1074/jbc.M116.746875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li M, Zhao H, Zhang X, Wood LD, Anders RA, Choti MA, et al. Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma. Nat Genet. 2011;43(9):828–9. Epub 2011/08/09. doi: 10.1038/ng.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–63. Epub 2012/07/24. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cancer Genome Atlas N Genomic Classification of Cutaneous Melanoma. Cell. 2015;161(7):1681–96. Epub 2015/06/20. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Garman B, Anastopoulos IN, Krepler C, Brafford P, Sproesser K, Jiang Y, et al. Genetic and Genomic Characterization of 462 Melanoma Patient-Derived Xenografts, Tumor Biopsies, and Cell Lines. Cell Rep. 2017;21(7):1936–52. Epub 2017/11/16. doi: 10.1016/j.celrep.2017.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oba A, Shimada S, Akiyama Y, Nishikawaji T, Mogushi K, Ito H, et al. ARID2 modulates DNA damage response in human hepatocellular carcinoma cells. J Hepatol. 2017;66(5):942–51. Epub 2017/02/28. doi: 10.1016/j.jhep.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 137.Raab JR, Resnick S, Magnuson T. Genome-Wide Transcriptional Regulation Mediated by Biochemically Distinct SWI/SNF Complexes. PLoS Genet. 2015;11(12):e1005748 Epub 2015/12/31. doi: 10.1371/journal.pgen.1005748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kadoch C, Crabtree GR. Reversible Disruption of mSWI/SNF (BAF) Complexes by the SS18-SSX Oncogenic Fusion in Synovial Sarcoma. Cell. 2013;153:71–85. doi: 10.1016/j.cell.2013.02.036.** Kadoch et al. describe the SS18-SSX oncogenic fusion protein contains part of a SWI/SNF complex member and drives development of synovial sarcomas by disruption of SWI/SNF complex formation.
- 139.Clark J, Rocques PJ, Crew AJ, Gill S, Shipley J, Chan AM, et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7(4):502–8. Epub 1994/08/01. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- 140.de Leeuw B, Balemans M, Olde Weghuis D, Geurts van Kessel A. Identification of two alternative fusion genes, SYT-SSX1 and SYT-SSX2, in t(X;18)(p11.2;q11.2)-positive synovial sarcomas. Hum Mol Genet. 1995;4(6):1097–9. Epub 1995/06/01. [DOI] [PubMed] [Google Scholar]
- 141.McBride MJ, Pulice JL, Beird HC, Ingram DR, D’Avino AR, Shern JF, et al. The SS18-SSX Fusion Oncoprotein Hijacks BAF Complex Targeting and Function to Drive Synovial Sarcoma. Cancer Cell. 2018;33(6):1128–41 e7. Epub 2018/06/05. doi: 10.1016/j.ccell.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boulay G, Sandoval GJ, Riggi N, Iyer S, Buisson R, Naigles B, et al. Cancer-Specific Retargeting of BAF Complexes by a Prion-like Domain. Cell. 2017;171(1):163–78 e19. Epub 2017/08/29. doi: 10.1016/j.cell.2017.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Orvis T, Hepperla A, Walter V, Song S, Simon J, Parker J, et al. BRG1/SMARCA4 inactivation promotes non-small cell lung cancer aggressiveness by altering chromatin organization. Cancer research. 2014;74(22):6486–98. doi: 10.1158/0008-5472.CAN-14-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alver BH, Kim KH, Lu P, Wang X, Manchester HE, Wang W, et al. The SWI/SNF chromatin remodelling complex is required for maintenance of lineage specific enhancers. Nature Communications. 2017;8:14648-. doi: 10.1038/ncomms14648.* Alver et al. show that SWI/SNF complex interacts with the p300 histone acetylase to modulate H3K27Ac at distal enhancers and plays an important role in controlling cell fate.
- 145.Nakayama RT, Pulice JL, Valencia AM, McBride MJ, McKenzie ZM, Gillespie MA, et al. SMARCB1 is required for widespread BAF complex-mediated activation of enhancers and bivalent promoters. Nature Genetics. 2017. doi: 10.1038/ng.3958.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X, Lee RS, Alver BH, Haswell JR, Wang S, Mieczkowski J, et al. SMARCB1-mediated SWI/SNF complex function is essential for enhancer regulation. Nature Genetics. 2017;49:289–95. doi: 10.1038/ng.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vierbuchen T, Ling E, Cowley CJ, Couch CH, Wang X, Harmin DA, et al. AP-1 Transcription Factors and the BAF Complex Mediate Signal-Dependent Enhancer Selection. Molecular cell. 2017;68(6):1067–82.e12. doi: 10.1016/j.molcel.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wilson BG, Wang X, Shen X, McKenna ES, Lemieux ME, Cho Y-J, et al. Epigenetic Antagonism between Polycomb and SWI/SNF Complexes during Oncogenic Transformation. Cancer Cell. 2010;18(4):316–28. doi: 10.1016/j.ccr.2010.09.006.** Wilson et al. describe the antagonistic relationship between PRC2 and SWI/SNF, providing the evidence for targeting PRC2 for treatment of SWI/SNF-deficient cancers.
- 149.Januario T, Ye X, Bainer R, Alicke B, Smith T, Haley B, et al. PRC2-mediated repression of SMARCA2 predicts EZH2 inhibitor activity in SWI/SNF mutant tumors. Proceedings of the National Academy of Sciences. 2017(15):201703966-. doi: 10.1073/pnas.1703966114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim KH, Kim W, Howard TP, Vazquez F, Tsherniak A, Wu JN, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nature Medicine. 2015;21(12):1491–6. doi: 10.1038/nm.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y, Chen SY, Karnezis AN, Colborne S, Santos ND, Lang JD, et al. The histone methyltransferase EZH2 is a therapeutic target in small cell carcinoma of the ovary, hypercalcaemic type. The Journal of pathology. 2017;242(3):371–83. doi: 10.1002/path.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kadoch C, Williams RT, Calarco JP, Miller EL, Weber CM, Braun SMG, et al. Dynamics of BAF–Polycomb complex opposition on heterochromatin in normal and oncogenic states. Nature Genetics. 2016;49(2):213–22. doi: 10.1038/ng.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stanton BZ, Hodges C, Calarco JP, Braun SMG, Ku WL, Kadoch C, et al. Smarca4 ATPase mutations disrupt direct eviction of PRC1 from chromatin. Nature Genetics. 2016;49(2):282–8. doi: 10.1038/ng.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakatsuka T, Tateishi K, Kudo Y, Yamamoto K, Nakagawa H, Fujiwara H, et al. Impact of histone demethylase KDM3A-dependent AP-1 transactivity on hepatotumorigenesis induced by PI3K activation. Oncogene. 2017. doi: 10.1038/onc.2017.222. [DOI] [PubMed] [Google Scholar]
- 155.Banito A, Li X, Laporte AN, Roe J-S, Sanchez-Vega F, Huang C-H, et al. The SS18-SSX Oncoprotein Hijacks KDM2B-PRC1.1 to Drive Synovial Sarcoma. Cancer Cell. 2018;34(2):346–8. doi: 10.1016/j.ccell.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chatterjee SS, Biswas M, Boila LD, Banerjee D, Sengupta A. SMARCB1 Deficiency Integrates Epigenetic Signals to Oncogenic Gene Expression Program Maintenance in Human Acute Myeloid Leukemia. Molecular Cancer Research. 2018;16(5):791–804. doi: 10.1158/1541-7786.mcr-17-0493. [DOI] [PubMed] [Google Scholar]
- 157.Wang X, Sansam CG, Thom CS, Metzger D, Evans JA, Nguyen PTL, et al. Oncogenesis Caused by Loss of the SNF5 Tumor Suppressor Is Dependent on Activity of BRG1, the ATPase of the SWI/SNF Chromatin Remodeling Complex. Cancer Research. 2009;69:8094–101. doi: 10.1158/0008-5472.CAN-09-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wei D, Goldfarb D, Song S, Cannon C, Yan F, Sakellariou-Thompson D, et al. SNF5/INI1 deficiency redefines chromatin remodeling complex composition during tumor development. Molecular Cancer Research. 2014;12(11):1574–85. doi: 10.1158/1541-7786.MCR-14-0005.* This study provides the first description of how SWI/SNF complex composition changes in MRTs and shows how SMARCB1 loss is a necessary component for complex formation in MRTs.
- 159.Rao Q, Xia Q-y, Wang Z-y, Li L, Shen Q, Shi S-s, et al. Frequent co-inactivation of the SWI/SNF subunits SMARCB1, SMARCA2 and PBRM1 in malignant rhabdoid tumours. Histopathology. 2015;67:121–9. doi: 10.1111/his.12632. [DOI] [PubMed] [Google Scholar]
- 160.Reisman DN, Strobeck MW, Betz BL, Sciariotta J, Funkhouser W Jr, Murchardt C, et al. Concomitant down-regulation of BRM and BRG1 in human tumor cell lines: differential effects on RB-mediated growth arrest vs CD44 expression. Oncogene. 2002;21(8):1196–207. doi: 10.1038/sj.onc.1205188. [DOI] [PubMed] [Google Scholar]
- 161.Li L, Fan X-S, Xia Q-Y, Rao Q, Liu B, Yu B, et al. Concurrent loss of INI1, PBRM1, and BRM expression in epithelioid sarcoma: implications for the cocontributions of multiple SWI/SNF complex members to pathogenesis. Human Pathology. 2014;45:2247–54. doi: 10.1016/j.humpath.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 162.Mashtalir N, D’Avino AR, Michel BC, Luo J, Pan J, Otto JE, et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell. 2018;175(5):1272–88 e20. Epub 2018/10/23. doi: 10.1016/j.cell.2018.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang H, Pandey S, Travers M, Sun H, Morton G, Madzo J, et al. Targeting CDK9 Reactivates Epigenetically Silenced Genes in Cancer. Cell. 2018;175(5):1244–58 e26. Epub 2018/11/21. doi: 10.1016/j.cell.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cheng SWG, Davies KP, Yung E, Beltran RJ, Yu J, Kalpana GV. c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function. Nature Genetics. 1999;22:102–5. [DOI] [PubMed] [Google Scholar]
- 165.Shanahan F, Seghezzi W, Parry D, Mahony D, Lees E. Cyclin E associates with BAF155 and BRG1, components of the mammalian SWI-SNF complex, and alters the ability of BRG1 to induce growth arrest. Molecular and cellular biology. 1999;19(2):1460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marignani PA, Kanai F, Carpenter CL. LKB1 associates with Brg1 and is necessary for Brg1-induced growth arrest. The Journal of biological chemistry. 2001;276(35):32415–8. doi: 10.1074/jbc.C100207200. [DOI] [PubMed] [Google Scholar]
- 167.Zhang J, Ohta T, Maruyama A, Hosoya T, Nishikawa K, Maher JM, et al. BRG1 Interacts with Nrf2 To Selectively Mediate HO-1 Induction in Response to Oxidative Stress. Molecular and cellular biology. 2006;26(21):7942–52. doi: 10.1128/MCB.00700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Becker TM, Haferkamp S, Dijkstra MK, Scurr LL, Frausto M, Diefenbach E, et al. The chromatin remodelling factor BRG1 is a novel binding partner of the tumor suppressor p16INK4a. Molecular Cancer. 2009;8(1):4. doi: 10.1186/1476-4598-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bochar DA, Wang L, Beniya H, Kinev A, Xue Y, Lane WS, et al. BRCA1 is associated with a human SWI/SNF-related complex: linking chromatin remodeling to breast cancer. Cell. 2000;102(2):257–65. [DOI] [PubMed] [Google Scholar]
- 170.Wang M, Gu C, Qi T, Tang W, Wang L, Wang S, et al. BAF53 interacts with p53 and functions in p53-mediated p21-gene transcription. J Biochem. 2007;142(5):613–20. Epub 2007/09/20. doi: 10.1093/jb/mvm176. [DOI] [PubMed] [Google Scholar]
- 171.Oh J, Sohn DH, Ko M, Chung H, Jeon SH, Seong RH. BAF60a interacts with p53 to recruit the SWI/SNF complex. J Biol Chem. 2008;283(18):11924–34. Epub 2008/02/28. doi: 10.1074/jbc.M705401200. [DOI] [PubMed] [Google Scholar]
- 172.Farnebo M, Bykov VJN, Wiman KG. The p53 tumor suppressor: A master regulator of diverse cellular processes and therapeutic target in cancer. Biochemical and Biophysical Research Communications. 2010;396(1):85–9. doi: 10.1016/j.bbrc.2010.02.152. [DOI] [PubMed] [Google Scholar]
- 173.Marcel V, Nguyen Van Long F, Diaz JJ. 40 Years of Research Put p53 in Translation. Cancers (Basel). 2018;10(5). Epub 2018/06/09. doi: 10.3390/cancers10050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee D, Kim JW, Seo T, Hwang SG, Choi E-J, Choe J. SWI/SNF Complex Interacts with Tumor Suppressor p53 and Is Necessary for the Activation of p53-mediated Transcription. Journal of Biological Chemistry. 2002;277(25):22330–7. doi: 10.1074/jbc.m111987200. [DOI] [PubMed] [Google Scholar]
- 175.Pfister NT, Fomin V, Regunath K, Zhou JY, Zhou W, Silwal-Pandit L, et al. Mutant p53 cooperates with the SWI/SNF chromatin remodeling complex to regulateVEGFR2in breast cancer cells. Genes & Development. 2015;29(12):1298–315. doi: 10.1101/gad.263202.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.He L, Chen Y, Feng J, Sun W, Li S, Ou M, et al. Cellular senescence regulated by SWI/SNF complex subunits through p53/p21 and p16/pRB pathway. The International Journal of Biochemistry & Cell Biology. 2017;90:29–37. doi: 10.1016/j.biocel.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 177.Klochendler-Yeivin A, Picarsky E, Yaniv M. Increased DNA damage sensitivity and apoptosis in cells lacking the Snf5/Ini1 subunit of the SWI/SNF chromatin remodeling complex. Molecular and cellular biology. 2006;26(7):2661–74. doi: 10.1128/MCB.26.7.2661-2674.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dyson NJ. RB1: a prototype tumor suppressor and an enigma. Genes Dev. 2016;30(13):1492–502. Epub 2016/07/13. doi: 10.1101/gad.282145.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Strobeck MW, Knudsen KE, Fribourg AF, DeCristofaro MF, Weissman BE, Imbalzano AN, et al. BRG-1 is required for RB-mediated cell cycle arrest. Proceedings of the National Academy of Sciences. 2000;97(14):7748–53. doi: 10.1073/pnas.97.14.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang HS, Gavin M, Dahiya A, Postigo AA, Ma D, Luo RX, et al. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell. 2000;101:79–89. [DOI] [PubMed] [Google Scholar]
- 181.Chai J, Charboneau AL, Betz BL, Weissman BE. Loss of the hSNF5 gene concomitantly inactivates p21CIP/WAF1 and p16INK4a activity associated with replicative senescence in A204 rhabdoid tumor cells. Cancer research. 2005;65(22):10192–8. doi: 10.1158/0008-5472.CAN-05-1896. [DOI] [PubMed] [Google Scholar]
- 182.Guidi CJ, Mudhasani R, Hoover K, Koff A, Leav I, Imbalzano AN, et al. Functional interaction of the retinoblastoma and Ini1/Snf5 tumor suppressors in cell growth and pituitary tumorigenesis. Cancer research. 2006;66(16):8076–82. doi: 10.1158/0008-5472.CAN-06-1451. [DOI] [PubMed] [Google Scholar]
- 183.Bultman SJ, Herschkowitz JI, Godfrey V, Gebuhr TC, Yaniv M, Perou CM, et al. Characterization of mammary tumors from Brg1 heterozygous mice. Oncogene. 2008;27(4):460–8.* SMARCA4 promotes mammary tumors in mouse models via a haplosufficient mechanism.
- 184.Xue Y, Meehan B, Fu Z, Wang XQD, Fiset PO, Rieker R, et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat Commun. 2019;10(1):557 Epub 2019/02/06. doi: 10.1038/s41467-019-08380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xue Y, Meehan B, Macdonald E, Venneti S, Wang XQD, Witkowski L, et al. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat Commun. 2019;10(1):558 Epub 2019/02/06. doi: 10.1038/s41467-018-06958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Prensner JR, Iyer MK, Sahu A, Asangani IA, Cao Q, Patel L, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nature Genetics. 2013;45(11):1392–8. doi: 10.1038/ng.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang X, Gong Y, Jin BO, Wu C, Yang J, Wang LE, et al. Long non-coding RNA urothelial carcinoma associated 1 induces cell replication by inhibiting BRG1 in 5637 cells. Oncology Reports. 2014;32(3):1281–90. doi: 10.3892/or.2014.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang Y, He L, Du Y, Zhu P, Huang G, Luo J, et al. The Long Noncoding RNA lncTCF7 Promotes Self-Renewal of Human Liver Cancer Stem Cells through Activation of Wnt Signaling. Cell Stem Cell. 2015;16(4):413–25. doi: 10.1016/j.stem.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 189.Hu G, Gong A-Y, Wang Y, Ma S, Chen X, Chen J, et al. LincRNA-Cox2 Promotes Late Inflammatory Gene Transcription in Macrophages through Modulating SWI/SNF-Mediated Chromatin Remodeling. Journal of immunology (Baltimore, Md : 1950). 2016;196(6):2799–808. doi: 10.4049/jimmunol.1502146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kawaguchi T, Tanigawa A, Naganuma T, Ohkawa Y, Souquere S, Pierron G, et al. SWI/SNF chromatin-remodeling complexes function in noncoding RNA-dependent assembly of nuclear bodies. Proceedings of the National Academy of Sciences. 2015;112(14):4304–9. doi: 10.1073/pnas.1423819112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Frühwald MC, Biegel JA, Bourdeaut F, Roberts CW, Chi SN. Atypical teratoid/rhabdoid tumors—current concepts, advances in biology, and potential future therapies. Neuro-oncology. 2016;18(6):764–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Witkowski L, Goudie C, Ramos P, Boshari T, Brunet J-S, Karnezis AN, et al. The influence of clinical and genetic factors on patient outcome in small cell carcinoma of the ovary, hypercalcemic type. Gynecologic oncology. 2016;141(3):454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McBride MJ, Kadoch C. Disruption of mammalian SWI/SNF and polycomb complexes in human sarcomas: mechanisms and therapeutic opportunities. J Pathol. 2018;244(5):638–49. Epub 2018/01/24. doi: 10.1002/path.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nemes K, Fruhwald MC. Emerging therapeutic targets for the treatment of malignant rhabdoid tumors. Expert Opin Ther Targets. 2018;22(4):365–79. Epub 2018/03/13. doi: 10.1080/14728222.2018.1451839. [DOI] [PubMed] [Google Scholar]
- 195.Kim KH, Roberts CW. Targeting EZH2 in cancer. Nature medicine. 2016;22(2):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Epizyme. A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma (NCT02601937)- ClinicalTrials.gov. doi: https://clinicaltrials.gov/ct2/show/NCT02601937.
- 197.Epizyme. A Phase II, Multicenter Study of the EZH2 Inhibitor Tazemetostat in Adult Subjects With INI1-Negative Tumors or Relapsed/Refractory Synovial Sarcoma (NCT02601950) - ClinicalTrials.gov. doi: https://clinicaltrials.gov/ct2/show/NCT02601950?cond=NCT02601950&rank=1.
- 198.Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, Varga A, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018;19(5):649–59. Epub 2018/04/14. doi: 10.1016/S1470-2045(18)30145-1. [DOI] [PubMed] [Google Scholar]
- 199.Chi S Tazemetostat in Treating Patients With Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphoma, or Histiocytic Disorders With EZH2, SMARCB1, or SMARCA4 Gene Mutations (A Pediatric MATCH Treatment Trial) - (NCT03213665) - ClinicalTrials.gov. 2018. doi: https://clinicaltrials.gov/ct2/show/NCT03213665?cond=BRG1&rank=2.
- 200.Wöhrle S, Weiss A, Ito M, Kauffmann A, Murakami M, Jagani Z, et al. Fibroblast growth factor receptors as novel therapeutic targets in SNF5-deleted malignant rhabdoid tumors. PloS one. 2013;8(10):e77652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lang JD, Hendricks WP, Orlando KA, Yin H, Kiefer J, Ramos P, et al. Ponatinib shows potent antitumor activity in small cell carcinoma of the ovary hypercalcemic type (SCCOHT) through multi-kinase inhibition. Clinical Cancer Research. 2018:clincanres. 1928.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Roy S, Quiñones R, Matzger AJ. Structural and Physicochemical Aspects of Dasatinib Hydrate and Anhydrate Phases. Crystal Growth & Design. 2012;12:2122–6. doi: 10.1021/cg300152p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hyman D Dasatinib in Treating Patients With Recurrent or Persistent Ovarian, Fallopian Tube, Endometrial or Peritoneal Cancer - (NCT02059265)- ClinicalTrials.gov. doi: https://clinicaltrials.gov/ct2/show/NCT02059265.
- 204.Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359(6377):801–6. Epub 2018/01/06. doi: 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pan D, Kobayashi A, Jiang P, Ferrari de Andrade L, Tay RE, Luoma AM, et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science. 2018;359(6377):770–5. Epub 2018/01/06. doi: 10.1126/science.aao1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jelinic P, Ricca J, Van Oudenhove E, Olvera N, Merghoub T, Levine DA, et al. Immune-active microenvironment in small cell carcinoma of the ovary, hypercalcemic type: rationale for immune checkpoint blockade. JNCI: Journal of the National Cancer Institute. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fukuoka J, Fujii T, Shih JH, Dracheva T, Meerzaman D, Player A, et al. Chromatin remodeling factors and BRM/BRG1 expression as prognostic indicators in non-small cell lung cancer. Clin Cancer Res. 2004;10(13):4314–24. [DOI] [PubMed] [Google Scholar]
- 208.Rodriguez-Nieto S, Cañada A, Pros E, Pinto AI, Torres-Lanzas J, Lopez-Rios F, et al. Massive parallel DNA pyrosequencing analysis of the tumor suppressor BRG1/SMARCA4 in lung primary tumors. Human Mutation. 2010;32(2):E1999–E2017. doi: 10.1002/humu.21415. [DOI] [PubMed] [Google Scholar]
- 209.Jamshidi F, Bashashati A, Shumansky K, Dickson B, Gokgoz N, Wunder JS, et al. The genomic landscape of epithelioid sarcoma cell lines and tumours. The Journal of Pathology. 2016;238(1):63–73. doi: 10.1002/path.4636. [DOI] [PubMed] [Google Scholar]
- 210.Cheng JX, Tretiakova M, Gong C, Mandal S, Krausz T, Taxy JB. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Modern Pathology. 2008;21(6):647–52. doi: 10.1038/modpathol.2008.44. [DOI] [PubMed] [Google Scholar]
- 211.Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol. 2013;37(3):368–74. Epub 2013/01/26. doi: 10.1097/PAS.0b013e3182770406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shorstova T, Marques M, Su J, Johnston J, Kleinman CL, Hamel N, et al. SWI/SNF-compromised cancers are susceptible to bromodomain inhibitors. Cancer Res. 2019. Epub 2019/03/17. doi: 10.1158/0008-5472.CAN-18-1545. [DOI] [PubMed] [Google Scholar]
- 213.Samartzis EP, Gutsche K, Dedes KJ, Fink D, Stucki M, Imesch P. Loss of ARID1A expression sensitizes cancer cells to PI3K- and AKT-inhibition. Oncotarget. 2014;5(14):5295–303. Epub 2014/07/01. doi: 10.18632/oncotarget.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Miller RE, Brough R, Bajrami I, Williamson CT, McDade S, Campbell J, et al. Synthetic Lethal Targeting of ARID1A-Mutant Ovarian Clear Cell Tumors with Dasatinib. Mol Cancer Ther. 2016;15(7):1472–84. Epub 2016/07/02. doi: 10.1158/1535-7163.MCT-15-0554. [DOI] [PubMed] [Google Scholar]
- 215.Williamson CT, Miller R, Pemberton HN, Jones SE, Campbell J, Konde A, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun. 2016;7:13837 Epub 2016/12/14. doi: 10.1038/ncomms13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Berns K, Caumanns JJ, Hijmans EM, Gennissen AMC, Severson TM, Evers B, et al. ARID1A mutation sensitizes most ovarian clear cell carcinomas to BET inhibitors. Oncogene. 2018;37(33):4611–25. Epub 2018/05/16. doi: 10.1038/s41388-018-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang L, Yang G, Ding Y, Huang Y, Liu S, Zhou L, et al. Combined treatment with PI3K inhibitor BKM120 and PARP inhibitor olaparib is effective in inhibiting the gastric cancer cells with ARID1A deficiency. Oncol Rep. 2018;40(1):479–87. Epub 2018/05/17. doi: 10.3892/or.2018.6445. [DOI] [PubMed] [Google Scholar]
- 218.Wu C, Lyu J, Yang EJ, Liu Y, Zhang B, Shim JS. Targeting AURKA-CDC25C axis to induce synthetic lethality in ARID1A-deficient colorectal cancer cells. Nat Commun. 2018;9(1):3212 Epub 2018/08/12. doi: 10.1038/s41467-018-05694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21(3):231–8. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Howard TP, Arnoff TE, Song MR, Giacomelli AO, Wang X, Hong AL, et al. MDM2 and MDM4 are Therapeutic Vulnerabilities in Malignant Rhabdoid Tumors. Cancer Res. 2019. Epub 2019/02/14. doi: 10.1158/0008-5472.CAN-18-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Carugo A, Minelli R, Sapio L, Soeung M, Carbone F, Robinson FS, et al. p53 Is a Master Regulator of Proteostasis in SMARCB1-Deficient Malignant Rhabdoid Tumors. Cancer Cell. 2019;35(2):204–20 e9. Epub 2019/02/13. doi: 10.1016/j.ccell.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]