Abstract
Programmatic surveillance of intestinal schistosomiasis during control can typically use four diagnostic tests, either singularly or in combination, but these have yet to be cross-compared directly. Our study assembled a complete diagnostic dataset, inclusive of infection intensities, from 258 children from five Ugandan primary schools. The schools were purposely selected as typical of the endemic landscape near Lake Albert and reflective of high- and low-transmission settings. Overall prevalence was: 44.1% (95% CI 38.0–50.2) by microscopy of duplicate Kato-Katz smears from two consecutive stools, 56.9% (95% CI 50.8–63.0) by urine-circulating cathodic antigen (CCA) dipstick, 67.4% (95% CI 61.6–73.1) by DNA-TaqMan® and 75.1% (95% CI 69.8–80.4) by soluble egg antigen enzyme-linked immunosorbent assay (SEA-ELISA). A cross-comparison of diagnostic sensitivities, specificities, positive and negative predictive values was undertaken, inclusive of a latent class analysis (LCA) with a LCA-model estimate of prevalence by each school. The latter ranged from 9.6% to 100.0%, and prevalence by school for each diagnostic test followed a static ascending order or monotonic series of Kato-Katz, urine-CCA dipstick, DNA-TaqMan® and SEA-ELISA. We confirm that Kato-Katz remains a satisfactory diagnostic standalone in high-transmission settings but in low-transmission settings should be augmented or replaced by urine-CCA dipsticks. DNA-TaqMan® appears suitable in both endemic settings though is only implementable if resources permit. In low-transmission settings, SEA-ELISA remains the method of choice to evidence an absence infection. We discuss the pros and cons of each method concluding that future surveillance of intestinal schistosomiasis would benefit from a flexible, context-specific approach both in choice and application of each diagnostic method, rather than a single one-size fits all approach.
Key words: DNA-TaqMan®, Kato-Katz, latent class analysis, Schistosoma mansoni, SEA-ELISA, urine-CCA
Introduction
Developing appropriate diagnostics tools, methods and protocols to track parasitic diseases before, during and after control is an important component within the multi-disciplinarity of parasitology. It has been previously highlighted (Stothard and Adams, 2014) and with regard to schistosomiasis, intestinal schistosomiasis poses a considerable public health burden in Uganda (Loewenberg, 2014). Since 2003 there has been an active national control programme against it (Kabatereine et al. 2006, 2007; Fenwick et al. 2009; Stanton et al. 2017), as primarily based on preventive chemotherapy campaigns (Montresor et al. 2012; Stothard et al. 2013). Despite much progress in the delivery of praziquantel (PZQ) treatments to school-aged children, infections with Schistosoma mansoni continue to be pervasive, particularly along the immediate shoreline of Lake Albert (Al-Shehri et al. 2016). Moving some 10–20 km inland, however, infection prevalence by school can decline dramatically, at least if measured by faecal egg-patency for if more sensitive diagnostic tools were used, such as urine-antigen dipsticks, such declines are less precipitous (Stothard et al. 2006, 2017b).
The incongruence between ‘estimated’ and ‘true’ prevalence is a well-known diagnostic dilemma in surveillance of intestinal schistosomiasis largely due to an operational compromise between imperfect detection tools and insufficient specimen sampling (Bergquist et al. 2009, 2015; Stothard et al. 2014; Utzinger et al. 2015; Weerakoon et al. 2015). Nonetheless, if control programmes are to be monitored effectively and also permit evidence-based adaptation or revision of control tactics (Tchuente et al. 2017), infection dynamics at an individual level need to be captured alongside any broader changes in the epidemiological landscape amenable to measurement (Hawkins et al. 2016; Stothard et al. 2017a). As the strive towards elimination grows (Hawkins et al. 2016; Colley et al. 2017), previous diagnostic shortcomings are revealed highlighting new diagnostic needs that guide future target product profiles (Utzinger et al. 2015; Weerakoon et al. 2015; Hawkins et al. 2016; Savioli et al. 2017; Tchuente et al. 2017).
At an individual level, often the school-aged child, the diagnostic repertoire for surveillance of intestinal schistosomiasis within national control programmes has remained surprisingly meagre; for many years it has been exclusively founded on parasitological methods alone (Stothard et al. 2014), with only sporadic application of serological methods (Chernet et al. 2017; Hinz et al. 2017). With the growing need for modernization and interest in adoption of more sensitive disease diagnostics in general (Mabey et al. 2004; Solomon et al. 2012; Stothard and Adams, 2014), in recent years there have been two important developments that centre upon scale-up in the use of urine-circulating cathodic antigen (CCA) dipsticks (Colley et al. 2013; Sousa-Figueiredo et al. 2013; Foo et al. 2015; Danso-Appiah et al. 2016; Greter et al. 2016; Kittur et al. 2016) and development of DNA-detection platforms with real-time PCR with parasite-specific TaqMan® hydrolysis probes (ten Hove et al. 2008; Mejia et al. 2013; Easton et al. 2016). Furthermore, recent application of more sophisticated statistical modelling such as latent class analysis (LCA) (Hadgu et al. 2005), has advanced diagnostic tool performance comparisons beyond the direct need of a fixed reference ‘gold’ standard which, for schistosomiasis, is something we currently do not have (Shane et al. 2011; Ibironke et al. 2012; Koukounari et al. 2013; Beltrame et al. 2017).
In this study, we attempt to make a diagnostic comparison for surveillance of intestinal schistosomiasis in school children across five primary schools using four methods namely: microscopy of duplicate Kato-Katz smears from two consecutive stools, urine-CCA dipsticks, real-time PCR of stool with a Schistosoma-specific Taqman® probe and serological analysis of finger-prick blood for antibodies against schistosome soluble egg antigen (SEA). Diagnostic congruence was first assessed by empirical cross-tabulations, assuming a ‘gold standard’, then later by LCA with disease prevalence by school also estimated with a LCA model.
Methods
Study area, participants and ethical approval
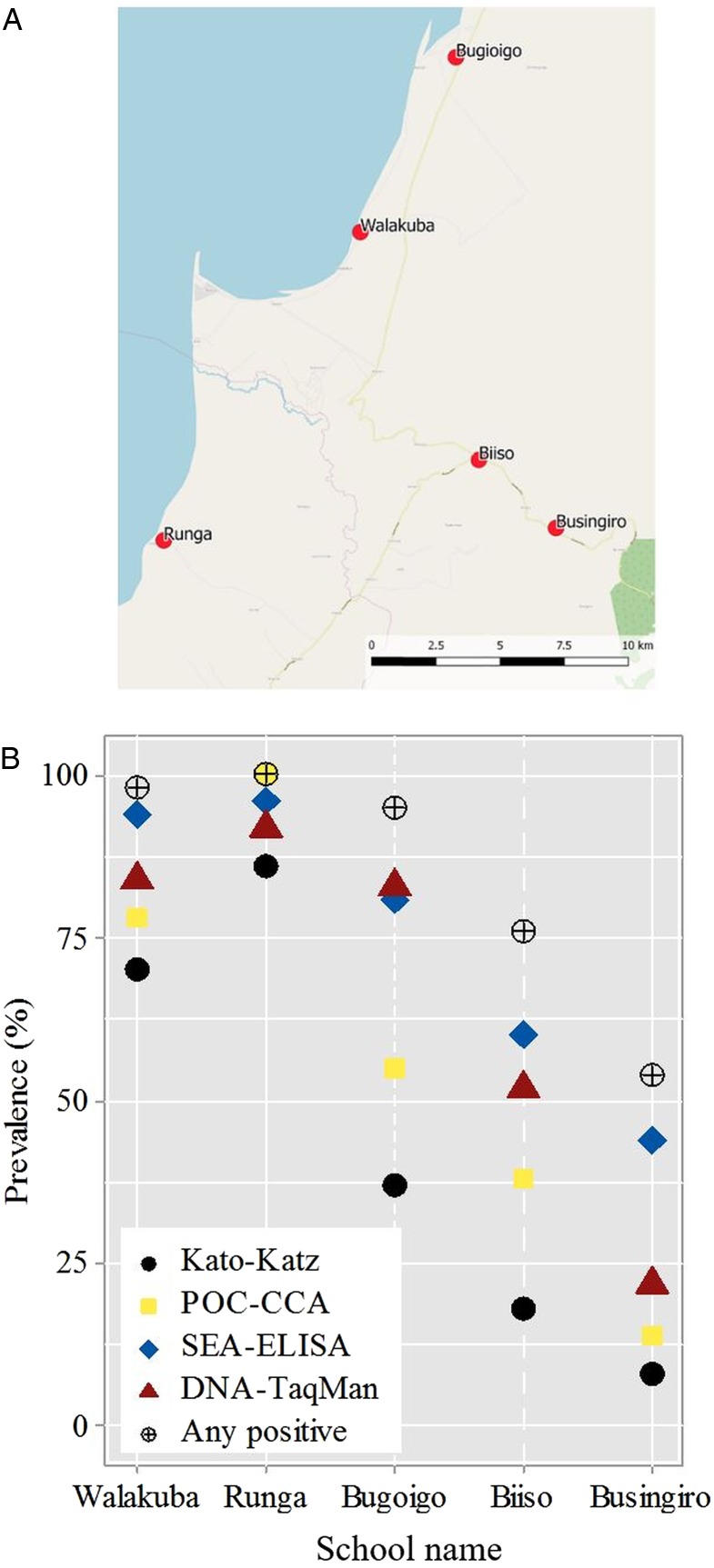
Field sampling and examinations of children took place in May 2015 in five primary schools in Buliisa District located within the Lake Albert region, three of which have been visited previously as sentinel surveillance sites of the national control programme (Kabatereine et al. 2007) and the global positioning system locations (GPS) known (Fig. 1). The schools Walakuba (GPS 01°50.323N, 031°22.740E), Bugoigo (GPS 01°54.004N, 031°24.750E) and Runga (GPS 01°43.828N, 031°18.603E) were located on the immediate shoreline while Biiso (GPS 01°45.516N, 031°25.236E) and Busingiro (GPS 01°44.090N, 031°26.855E) were over 10 km away inland which aimed to represent the current control landscape across high- and low-endemic settings, respectively.
Fig. 1.
(A) Schematic map of the five sampled primary schools in the Lake Albert region, the blue area indicates Lake Albert. (B) Estimated prevalence of Schistosoma mansoni by school for each examined diagnostic test; prevalence by any positive test criterion is also illustrated.
After obtaining written informed consent and verbal assent, a pre-target of 60 children of balanced gender aged between 5 and 10 years of age were enrolled and requested to provide two stool samples on consecutive days, a single urine sample and single finger-prick blood sample. Children were also interviewed with a standardized questionnaire to ascertain recent PZQ treatment history. All participants were provided with a single PZQ (40 mg/kg) treatment by the attending nurse following WHO guidelines (Montresor et al. 1998). The Ugandan Council for Science and Technology and the Liverpool School of Tropical Medicine granted approval for this study.
Diagnostics: faecal microscopy with Kato-Katz
Duplicate Kato-Katz thick smear slides (41.7 mg templates) were prepared from each stool received after first sieving through a 212 µm metal mesh (Montresor et al. 1998). Schistosome eggs were viewed by microscopy (×100 magnification), quantified and expressed as eggs per gram (EPG) of faeces with the intensity of infection classified as: light (1–99 EPG), medium (100–399 EPG) and heavy (⩾400 EPG) following the WHO guidelines (Montresor et al. 1998). For later DNA analysis, a 0.8 g aliquot of sieved stool was each prepared and stored in 95% ethanol before transportation to the UK for processing.
Diagnostics: schistosome urine-antigen CCA dipsticks
The commercially available urine-CCA dipstick was used to test for schistosome antigens in each urine sample received following manufacturer's instructions (Rapid Medical Diagnostics, Pretoria, South Africa). The test result was classified by visual inspection against a colour chart as used previously (Sousa-Figueiredo et al. 2013), by two individuals as negative, trace (±), light positive (+), medium positive (++) and heavy positive (+++). In this setting, all trace reactions were later considered to be positive as justified previously upon biological causality and by prior epidemiological analyses (Standley et al. 2010a, b; Sousa-Figueiredo et al. 2013; Adriko et al. 2014).
Diagnostics: schistosome serology with SEA-enzyme-linked immunosorbent assay (ELISA)
Finger-prick blood was taken from each child and antibodies for SEA were tested using 1 : 40 dilution of harvested sera using a field-based ELISA test following manufacturer's instructions (IVD Inc.; Carlsbad, USA). Upon completion, the micro-titre plate was placed on a white card to view the visual colour of each reaction as graded into pale yellow (light positive), yellow (medium positive) and dark yellow (heavy positive) as recorded previously (Stothard et al. 2009).
DNA diagnostics: TaqMan® real-time PCR
After transfer to the UK, each aliquot of stool was spiked with Phocine Herpes Virus (PhHV-1) to act as an internal control for each DNA extraction and later real-time PCR assay for inhibition following protocols of Meurs et al. (2015) which targetted a 77 base pair segment within the ribosomal internal transcribed spacer (ITS-2) region which can be identified using S. mansoni (GenBank: AF503487) as reference sequence (Obeng et al. 2008; Meurs et al. 2015). Schistosome DNA was detected with the Schistosoma-specific primers of Ssp48F (GGT CTA GAT GAC TTG ATY GAG ATG CT) and Ssp124R (TCC CGA GCG YGT ATA ATG TCA TTA) and TaqMan® probe Ssp78T (ROX – TGG GTT GTG CTC GAG TCG TGGC – Black Hole Quencher 3) as developed by (Obeng et al. 2008; Meurs et al. 2015). DNA-TaqMan® assays were performed in a Chromo-4 with Opticon monitor Version 3.1. (Biorad, Hemel Hempstead, UK) with Biorad iQ™ supermix and thermal cycling conditions of 15 min at 95 °C, followed by 45 cycles, each of 15 s at 95 °C and 60 s at 60 °C. The infection intensity was classified according to Ct values: negative (Ct > 45), light positive (35 > Ct ⩽ 45), medium positive (25 > Ct ⩽ 35), and heavy positive (Ct ⩽ 25).
Data management and statistical analysis
All data collected in the field and processed in the laboratory were recorded on proforma data sheets. These were then double entered in Microsoft Excel prior to the generation of summary tables for prevalence and intensity of infection (Tables 1 and 2). Empirical estimates of sensitivity, specificity, negative predictive value and positive predictive value was calculated in R statistical package v 2·10·1 (The R Foundation for Statistical Computing, Vienna, Austria) and SPSS software (v 24.0, SPSS Inc., IBM, USA) assuming the urine-CCA as the ‘gold’ standard against the remaining three diagnostic tests (Table 3). For percentage values, 95% confidence intervals (95% CI) were estimated using the exact method (Armitage and Berry, 1994). We have decided to assume the urine-CCA as the gold standard for our descriptive analyses (i.e. empirical estimates of diagnostic performance), since there have been extensive evaluations of urine-CCA dipsticks (Colley et al. 2013) and WHO recommendation of its use in surveillance mapping (Danso-Appiah et al. 2016).
Table 1.
Prevalence (%) of Schistosoma mansoni according to each diagnostic test across five primary schools with 95% confidence intervals
| School name | ||||||
|---|---|---|---|---|---|---|
| Walakuba (n = 50) | Runga (n = 50) | Bugoigo (n = 58) | Biiso (n = 50) | Busingiro (n = 50) | Total (N = 258) | |
| Diagnostic method | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| Kato-Katz | 70.0 (56.8–83.1) | 86.0 (76.0–95.9) | 37.9 (25.0–50.8) | 20.0 (8.5–31.4) | 8.0 (0.2–15.7) | 44.1 (38.0–50.2) |
| Urine-CCA dipstick | 78.0 (66.1–89.8) | 100 (NA) | 55.1 (41.9–68.3) | 38.0 (24.0–51.9) | 14.0 (4.0–23.9) | 56.7 (50.8–63.0) |
| SEA-ELISA | 94.0 (87.1–100.8) | 96.0 (90.3–101.6) | 81.0 (70.6–91.4) | 60.0 (45.9–74.0) | 44.0 (29.7–58.2) | 75.1 (69.8–80.4) |
| DNA-TaqMan | 84.0 (73.4–94.5) | 92.0 (84.2–99.7) | 82.7 (72.7–92.7) | 52.0 (37.6–66.3) | 24.0 (10.1–33.8) | 67.4 (61.6–73.1) |
| Positive by any test | 98.0 (93.9–102.0) | 100 (NA) | 94.8 (88.9–100.0) | 76.0 (63.7–88.2) | 54.0 (39.6–68.3) | 84.8 (80.4–89.2) |
Table 2.
Intensity of infection categories for Schistosoma mansoni by each examined diagnostic test across the five primary schools
| Diagnostic test and intensity category | School name | |||||
|---|---|---|---|---|---|---|
| Walakuba (n = 50) | Runga (n = 50) | Bugoigo (n = 58) | Biiso (n = 50) | Busingiro (n = 50) | Total (N = 258) | |
| (%) n | (%) n | (%) n | (%) n | (%) n | N (%) | |
| Kato-Katza | ||||||
| Negative | 15 (30.0) | 7 (14.0) | 36 (60.1) | 40 (80.0) | 46 (92.0) | 144 (55.8) |
| Light (<100 EPG) | 5 (10.0) | 7 (14.0) | 16 (27.6) | 3 (6.0) | 1 (2.0) | 32 (12.4) |
| Medium (100–399 EPG) | 10 (20.0) | 5 (10.0) | 3 (5.2) | 5 (10.0) | 2 (4.0) | 25 (9.7) |
| Heavy (≥400 EPG) | 20 (40.0) | 31 (62.0) | 3 (5.2) | 2 (4.0) | 1 (2.0) | 57 (22.1) |
| Urine-CCA | ||||||
| Negative | 11 (22.0) | 0 (0.0) | 26 (44.8) | 31 (62.0) | 43 (86.0) | 111 (43.0) |
| Light (+, incl. trace) | 5 (10.0) | 10 (22.0) | 19 (32.8) | 7 (14.0) | 4 (8.0) | 45 (17.4) |
| Medium (++) | 6 (12.0) | 9 (18.0) | 7 (12.1) | 6 (12.0) | 1 (2.0) | 29 (11.2) |
| Heavy (+++) | 28 (56.0) | 31 (62.0) | 6 (10.3) | 6 (12.0) | 2 (4.0) | 73 (28.3) |
| SEA-ELISA | ||||||
| Negative | 3 (6.0) | 2 (4.0) | 11 (19.0) | 20 (40.0) | 28 (56.0) | 64 (24.8) |
| Light (+, incl. trace) | 4 (8.0) | 3 (6.0) | 14 (24.1) | 16 (32.0) | 13 (26.0) | 50 (19.3) |
| Medium (++) | 33 (66.0) | 19 (38.0) | 27 (46.5) | 11 (22.0) | 8 (16.0) | 98 (37.9) |
| Heavy (+++) | 10 (20.0) | 26 (52.0) | 6 (10.3) | 3 (6.0) | 1 (2.0) | 46 (17.8) |
| DNA TaqMan® | ||||||
| Negative (Ct > 45) | 8 (16.0) | 4 (8.0) | 10 (17.2) | 24 (48.0) | 38 (76.0) | 84 (32.5) |
| Light (35 > Ct ⩽ 45) | 4 (8.0) | 2 (4.0) | 20 (34.5 | 18 (36.0) | 8 (16.0) | 52 (20.1) |
| Medium (25 > Ct ⩽ 35) | 19 (38.0) | 9 (18.0) | 19 (32.8) | 5 (10.0) | 2 (4.0) | 54 (20.9) |
| Heavy (Ct ⩽ 25) | 19 (38.0) | 35 (70.0) | 9 (15.5) | 3 (6.0) | 2 (4.0) | 68 (26.3) |
aDuplicate faecal smears from two consecutive stools.
Table 3.
Empirical estimates of sensitivity (SS), specificity (SP), negative predictive value (NPV) and positive predictive value (PPV), Cohen's kappa for each diagnostic test against urine-CCA dipstick as ‘gold standard’
| Evaluating diagnostic test | Urine-CCA as reference ‘gold standard’ | Cohen's kappa (95% Cls) | |||||
|---|---|---|---|---|---|---|---|
| Negative (%) | Positive (%) | Measurement estimate % (95% Cls) | Diagnostic accuracy % (95% Cls) | ||||
| DNA-TaqMan® | Total (%) | ||||||
| Negative | 62 (24.0) | 22 (8.5) | 84 (32.6) | Sensitivity | 85.0 (78.4–89.9) | 72.5 (66.7–77.6) | 0.4 (0.3–0.5) |
| Positive | 49 (18.9) | 125 (48.5) | 174 (67.4) | Specificity | 55.9 (46.6–64.7) | ||
| Total (%) | 111 (43.0) | 147 (57.0) | 258 (100.0) | PPV | 71.8 (64.7–77.9) | ||
| NPV | 73.8 (63.5–82.0) | ||||||
| SEA-ELISA | |||||||
| Negative | 59 (22.9) | 5 (1.9) | 64 (24.8) | Sensitivity | 96.6 (92.3–98.5) | 77.9 (72.5–82.5) | 0.5 (0.4–0.6) |
| Positive | 52 (20.2) | 142 (55.0) | 194 (75.2) | Specificity | 53.2 (43.9–62.2) | ||
| Total (%) | 111 (43.0) | 147 (57.0) | 258 (100.0) | PPV | 73.2 (66.6–78.9) | ||
| NPV | 92.2 (82.9–96.6) | ||||||
| Kato-Katz | |||||||
| Negative | 110 (42.6) | 34 (13.2) | 144 (55.8) | Sensitivity | 76.9 (69.2–82.9) | 86.4 (81.7–90.1) | 0.73 (0.6–0.9) |
| Positive | 1 (0.4) | 113 (43.8) | 144 (44.2) | Specificity | 99.1 (95.1–99.8) | ||
| Total (%) | 111 (43.0) | 147 (56.9) | 258 (100.0) | PPV | 99.1 (95.2–99.8) | ||
| NPV | 76.4 (68.8–82.6) | ||||||
Subsequently, to tackle the inherent problems with diagnostic measurement error, we employed a LCA and full information maximum likelihood estimation (Table 4). LCA allows grouping of categorical data (in the current study not infected and infected from the diagnostic tests under examination) into latent classes indicating S. mansoni infection via a probability model. Given the well-known epidemiological landscape of Lake Albert region, such a model was designed to allow LCA estimated prevalence of S. mansoni to vary by school (Table 4). Through this approach, model-based estimates of sensitivity and specificity across diagnostic tests without assuming a gold standard were also obtained.
Table 4.
Latent class analysis (LCA) estimates of sensitivity and specificity and LCA model of prevalence of Schistosoma mansoni by school with 95% CIs for each diagnostic method
| Diagnostic method | Sensitivity | Specificity |
|---|---|---|
| Kato-Katz | 84.4% (76.0–92.9) | 100% (NA) |
| Urine-CCA | 99.1% (97.3–100) | 89.3% (80.9–97.6) |
| SEA-ELISA | 97.7% (95.1–100) | 49.5% (39.4–59.6) |
| DNA-TaqMan® | 90.2% (84.2–96.2) | 57.5% (48.6–66.5) |
| School name | Prevalence by LCA model | |
| Walakuba | 75.7% (62.9–88.5) | |
| Runga | 100.0% (NA) | |
| Bugoigo | 49.7% (35.0–64.5) | |
| Biiso | 27.0% (12.2–41.9) | |
| Busingiro | 9.6% (9.0–18.4) | |
The classification certainty of this model was evaluated through entropy; values of entropy near one indicate high certainty in classification while values near zero indicate low certainty (Celeux and Soromenho, 1996). LCA assumes the relationships between the observed variables (i.e. diagnostic tests in the current study) are accounted for by their class membership and thus conditioning on class membership (i.e. the disease status in the current study) such that if the model estimated disease status is misclassified by one test, the probability that it will be misclassified by another test will not be affected. We assessed this assumption by speculating the standardized residuals for each response pattern from the diagnostic tests as estimated from the LCA model. Further technical details of these models in the context of schistosomiasis have been described elsewhere and thus they are not repeated here (Ibironke et al. 2012). The LCA model was fitted using MPlus version 7.3 (Muthén and Muthén, 1998–2012).
Results
Prevalence of intestinal schistosomiasis
A total data set was assembled from 258 children with a prevalence of intestinal schistosomiasis by each diagnostic test presented, see Table 1. Overall prevalence of intestinal schistosomiasis was: 44.1% (95% CI 38.0–50.2) by microscopy of duplicate Kato-Katz smears from two consecutive stools, 56.9% (95% CI 50.8–63.0) by urine-CCA dipstick, 67.4% (95% CI 61.6–73.1) by DNA-TaqMan® and 75.1% (95% CI 69.8–80.4) by SEA-ELISA.
The prevalence of infection at Runga and Walakuba was observed to be highest, exceeding 50% in all diagnostic tests, whereas prevalence of infection at Busingiro was lowest falling well short of 50% by any test, although pooling infection status upon being positive by any test revealed that just under half of the children attending this school could be considered ‘free’ from infection. For the total dataset, just over a quarter of children (n = 69) could be considered to have no evidence of intestinal schistosomiasis.
The geographical proximity of each of the five schools to Lake Albert shoreline is depicted in schematic in Fig. 1A; on-the-ground shortest distance to the lake shoreline can be ranked in the following order of Walakuba (0.2 km), Runga (0.4 km), Bugoigo (0.9 km), Biiso (9.4 km) and Busingiro (13.2 km). Notably, both Runga and Bugoigo schools are located for safety and convenience on slightly higher ground behind each village so as not to flood, which during wetter periods has detrimentally affected Walakuba in the past (J.R.S., personal observation). Whilst diagnostic comparisons are made on the basis of binary data, it is worth noting that infection intensity also varied by school setting, in that ‘heavy intensity’ infections or ‘strong positive’ by any test were particularly common at Runga but were rare at Busingiro, Table 2. As shown in Fig. 1B the changing prevalence by school for each method is clearly visible in that the prevalence of inferred from each diagnostic test typically followed a static ascending order or monotonic series of Kato-Katz, urine-CCA dipstick, DNA-TaqMan® and SEA-ELISA, although the relative position of the estimated prevalence by urine-CCA at Runga slightly exceeds SEA-ELISA and DNA-TaqMan®.
Empirical and LCA modelling of estimates of diagnostic performance
Assuming the urine-CCA as an arbitrary gold standard, the diagnostic performance for the three remaining tests is shown along with diagnostic accuracy and Cohen's kappa statistic, Table 3. The sensitivity of SEA-ELISA is the highest (96.6%) but also has the lowest specificity (53.2%), with the highest negative predictive value of all methods. By contrast, the sensitivity of Kato-Katz is the lowest (76.9%) but also has the highest specificity (99.1%), with the highest positive predictive value of all methods.
On the basis of LCA analysis the sensitivity and specificity of each method can be estimated on the basis of their latent class assignment which highlights the trade-off between diagnostic specificity (i.e. false positive) and sensitivity (i.e. false negative). In this analysis, sensitivity and specificity of SEA-ELISA and urine-CCA are broadly equivalent with DNA-TaqMan® appearing to have slightly lower sensitivity and specificity. Estimating the prevalence of infection by school with LCA, Table 4, reveals a lower prevalence than that on the basis of positivity by any test but follows the same static ascending order or monotonic series (Fig. 1B). It is evident that at Runga intestinal schistosomiasis is universal whereas at Busingiro around 9.6% of children are suspected of harbouring infections.
The LCA model generated similar sensitivity for SEA-ELISA and urine-CCA but was slightly lower for DNA-TaqMan®, Table 4. Kato-Katz was again shown through LCA to have the highest specificity among all the four tests. The specificity of 89.3% (95% CI 80.9–97.6) for the urine-CCA test was acceptable but for the SEA-ELISA and the DNA-TaqMan, specificities were less so and estimated to be 49.5% (95% CI 39.4–59.6) and 57.5% (95% CI 48.6–66.5), trending as with empirical calculations, see Table 3. Furthermore, LCA estimated infection prevalence of S. mansoni by the school to be lower than that on the basis of positivity by any test (for the latter see Fig. 1B). Nevertheless, both of these approaches suggested that intestinal schistosomiasis was universal at Runga, however, at Busingiro LCA estimated a prevalence of S. mansoni infection to be 9.6% (95% CI 9.0−18.4), much lower than that revealed by positivity upon any test. Finally, the entropy of the LCA model was estimated to be 0.921. This indicated a clear delineation of classes in the fitted model standardized residuals for each response pattern from the four diagnostic tests from this model were between −2 and 2, evidencing that local independence of the four diagnostic tests is not obviously violated.
Discussion
Owing to the complicated developmental and population biology of the schistosome within the mammalian host, it is well known that accurate detection of intestinal schistosomiasis by any biomarker can be problematic and has been the topic of at-length discussions previously (Bergquist et al. 2009, 2015; Stothard et al. 2014; Utzinger et al. 2015). Foremost, the insensitivity of the Kato-Katz, especially in the detection of light egg-patent infections or in patients with a recent history of PZQ treatment, is perhaps the most obvious obstacle to overcome (Kongs et al. 2001; Koukounari et al. 2013; Leuenberger et al. 2016).
Indeed, how we debate and assess the significance of egg-negative infections is changing alongside measuring morbidity associated with intestinal schistosomiasis which goes beyond what Kato-Katz assessments can offer (King, 2015). Nevertheless, Kato-Katz can still be promoted as a field-applicable standalone and appropriate in high-endemic settings, as seen here in both Runga and Walakuba, where prevalence and intensity of infection were high. Nonetheless, Kato-Katz has several deficits when applied to lower transmission settings, as exemplified by the other schools sampled here and is more misleading perhaps than informative. To compensate, de Vlas et al. (1993) developed a useful corrective prevalence chart which took into account infection intensity; however, its uptake was not as good as anticipated (de Vlas et al. 1993). It is also outside the scope of the present paper to discuss economic cost–benefit of faecal microscopy (Meheus et al. 2015) other than that mobile microscopy with handheld devices offers some attractive cost-saving solutions for surveillance of intestinal schistosomiasis in high-endemic areas (Stothard et al. 2005; Bogoch et al. 2014). However, as control programmes move forward towards elimination, the Kato-Katz methodology will be inappropriate and will be unable to provide sufficient quality epidemiological information for precision mapping of disease foci (Tchuente et al. 2017). The latter is pivotal in the local intensification of delivery of treatments and surveillance interventions to confirm interruption of transmission (Rollinson et al. 2013; Stothard et al. 2017a). Indeed from the information reported here, we would suggest that control efforts in locations such as Busingiro should be intensified rather than reduced.
Of the remaining diagnostic methods, the diagnostic pros and cons of each method have been discussed elsewhere often using the ASSURED framework (Bergquist et al. 2009; Stothard et al. 2014; Utzinger et al. 2015). DNA-TaqMan® methods are, however, increasingly gaining favour and offer a multiplex DNA-platform for co-detection of several neglected tropical diseases as well as many other infectious agents (Verweij and Stensvold, 2014); much more so than other any other current biomarker method can provide (ten Hove et al. 2008; Solomon et al. 2012; Mejia et al. 2013; Easton et al. 2016). There also is the suggestion that DNA-TaqMan® could become an acceptable ‘gold’ standard (Meurs et al. 2015), and whilst we ultimately share some enthusiasm in this there are some impediments to discuss. Foremost, DNA-TaqMan® requires specialist equipment and is not currently amenable to point-of-contact settings although there is growing interest in the use of more field-friendly methods (Minetti et al. 2016), such as loop-mediated iso-thermal amplification (LAMP) (He et al. 2016) and recombinase polymerase amplification (RPA) (Rosser et al. 2015). Nonetheless, from our results here the DNA-TaqMan® has been somewhat outperformed upon consideration of Table 4. In our opinion, perhaps the most significant advantage of DNA-based platform is that DNA-TaqMan® assays can cross-over into environmental monitoring through detection of environmental (e)DNA and therefore broaden the vision of schistosomiasis control in general potentially uniting clinical and environmental surveillance (Rollinson et al. 2013; Stothard et al. 2017a).
In the absence of a ‘gold’ standard diagnostic test and complexity of the changing epidemiological landscape in which tests are being applied in Uganda (Standley et al. 2010a, b; Adriko et al. 2014; Al-Shehri et al. 2016), our analysis presented in Table 3 postulated that urine-CCA dipsticks could be an ‘error-free’ standard which, in Table 4, was further explored by LCA. Here the probabilistic statistical model applied does not assume any ‘gold’ standard and therefore points towards the urine-CCA as having near-optimal diagnostic scores of sensitivity (99.1%) and specificity of (89.3%). Moreover, these scores are significantly better than those reported previously by empirical comparisons (Stothard et al. 2006) and illustrate how advances in statistical modelling developed elsewhere on urine-CCA dipsticks (Knopp et al. 2015; Koukounari et al. 2013) can provide a deeper insight into diagnostic score evaluations over and above simple empirical calculations (Colley et al. 2013; Danso-Appiah et al. 2016). Nonetheless a theoretical issue of adopting LCA-models exclusively is an assumption of independence of tests which, given the biological biomarkers employed here could be somewhat confounded; Kato-Katz detects eggs directly, DNA-TaqMan® measures Schistosoma-DNA in stool (presumably from excreted eggs) and SEA-ELISA detects antibodies to secreted egg-antigens, thus these three methods are somewhat interrelated to similar biomarkers of the egg itself although will have each have differing physical, biochemical and physiological components. However, the urine-CCA dipstick is less directly connected to egg-biomarkers for it utilizes carbohydrate-antigens released from feeding worms of either sex and hence offers an alternative biomarker appraisal. Since violations of the conditional independence assumption can lead to biased LCA estimates of accuracy and prevalence, performing and reporting checks of whether assumptions are met is essential which was why we compared LCA estimates of diagnostic performance with empirical ones, drawing conclusions for each of the diagnostic tests used. In addition, speculation of standardized residuals from the LCA model indicated that the assumption of local independence of the four diagnostic tests under examination was not obviously violated.
Over and above the routine diagnostic scores of sensitivity, specificity, negative and positive predictive values with or without LCA models, however, it is also necessary to further consider each diagnostic tool against the ASSURED criteria. This seeks to understand whether a diagnostic test can be used at scale and is ultimately useful in several clinical and epidemiological surveillance settings (Mabey et al. 2004; Peeling et al. 2006; Stothard and Adams, 2014). The roll-out of the urine-CCA test has been discussed previously (Stothard, 2009) and it is pleasing to see it become further endorsed at the policy level (Danso-Appiah et al. 2016). The most desirable features of this test are its affordability, stable commercial production, the use of urine-sampling, the speed of test and a short time to obtain results which has a very pragmatic consideration for the end-user in this emphasis. All of the above potentially make dissemination of epidemiological results back to the local community obtained by the urine-CCA dipstick much quicker, which is vital to increase local ownership of preventive chemotherapy campaigns in future (Tchuente et al. 2017).
Concluding remarks
The study has shown that intestinal schistosomiasis continues to be a public health challenge on the shoreline of Lake Albert which now presents as a heterogenous epidemiological landscape of high- and low-transmission settings. A total of four diagnostic tests were each assessed regarding contemporary surveillance for intestinal schistosomiasis finding that Kato-Katz sampling is a satisfactory diagnostic standalone in high-transmission settings but in low-transmission settings should be augmented or replaced by urine-CCA dipsticks. DNA-TaqMan® appears suitable in both endemic settings though is only implementable if resources permit. In low-transmission settings, SEA-ELISA remains the method of choice to evidence an absence of infection. In the dearth of a diagnostic ‘gold’ standard for intestinal schistosomiasis, LCA offered useful computations of diagnostic performance between tests.
Acknowledgements
We thank the children and teachers who took part in this survey; to the VCD field team stationed at Bugoigo Camp for their help during fieldwork and assistance of John Archer and Francesca Poole. JRS and HA-S would like to thank Jaco Verweij and Lisette van Lieshout for sharing their technical skill and advice on helminth diagnostics. We thank David Lambillotte of IVD-diagnostics for a donation of the SEA-ELISA tests used here. We are also grateful to the British Society for Parasitology for helping to support the work of HA-S and support the Autumn Symposium on multi-disciplinarity of parasitology. JRS is Director of COUNTDOWN, a DFID supported implementation consortium.
Financial support
The research was funded by Ministry of Health Uganda, Liverpool School of Tropical Medicine and PhD training scholarship awarded to HA-S by Kingdom of Saudi Arabia. MCS holds an MRC-population health fellowship. This study also benefited directly from prior support from The Wellcome Trust as part of the Schistosomiasis in Mothers and Infants study (project 085440).
References
- Adriko M, Standley CJ, Tinkitina B, Tukahebwa EM, Fenwick A, Fleming FM, Sousa-Figueiredo JC, Stothard JR and Kabatereine NB (2014) Evaluation of circulating cathodic antigen (CCA) urine-cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda. Acta Tropica 136, 50–57. [DOI] [PubMed] [Google Scholar]
- Al-Shehri H, Stanton MC, LaCourse JE, Atuhaire A, Arinaitwe M, Wamboko A, Adriko M, Kabatereine NB and Stothard JR (2016) An extensive burden of giardiasis associated with intestinal schistosomiasis and anaemia in school children on the shoreline of Lake Albert, Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 110, 597–603. [DOI] [PubMed] [Google Scholar]
- Armitage P and Berry G. (1994) Statistical Methods in Medical Research. Oxford: Blackwell Scientific Publications. [Google Scholar]
- Beltrame A, Guerriero M, Angheben A, Gobbi F, Requena-Mendez A, Zammarchi L, Formenti F, Perandin F, Buonfrate D and Bisoffi Z (2017) Accuracy of parasitological and immunological tests for the screening of human schistosomiasis in immigrants and refugees from African countries: an approach with Latent Class Analysis. PLoS Neglected Tropical Diseases, 11, e0005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist R, Johansen MV and Utzinger J (2009) Diagnostic dilemmas in helminthology: what tools to use and when? Trends in Parasitology 25, 151–156. [DOI] [PubMed] [Google Scholar]
- Bergquist R, Yang GJ, Knopp S, Utzinger J and Tanner M (2015) Surveillance and response: tools and approaches for the elimination stage of neglected tropical diseases. Acta Tropica 141, 229–234. [DOI] [PubMed] [Google Scholar]
- Bogoch II, Coulibaly JT, Andrews JR, Speich B, Keiser J, Stothard JR, N'Goran EK and Utzinger J (2014) Evaluation of portable microscopic devices for the diagnosis of Schistosoma and soil-transmitted helminth infection. Parasitology 141, 1811–1818. [DOI] [PubMed] [Google Scholar]
- Celeux G and Soromenho G (1996) An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification 13, 195–212. [Google Scholar]
- Chernet A, Kling K, Sydow V, Kuenzli E, Hatz C, Utzinger J, van Lieshout L, Marti H, Nickel B, Labhardt ND and Neumayr A (2017) Accuracy of diagnostic tests for Schistosoma mansoni infection in asymptomatic Eritrean refugees: serology and point-of-care Circulating Cathodic Antigen against stool microscopy. Clinical Infectious Diseases 65, 568–574. [DOI] [PubMed] [Google Scholar]
- Colley DG, Binder S, Campbell C, King CH, Tchuente LAT, N'Goran EK, Erko B, Karanja DMS, Kabatereine NB, van Lieshout L and Rathbun S (2013) A five-country evaluation of a point-of-care Circulating Cathodic Antigen urine assay for the prevalence of Schistosoma mansoni. American Journal of Tropical Medicine and Hygiene 88, 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley DG, Andros TS and Campbell CH (2017) Schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infectious Diseases of Poverty 6. doi: 10.1186/s40249-017-0275-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso-Appiah A, Minton J, Boamah D, Otchere J, Asmah RH, Rodgers M, Bosompem KM, Eusebi P and De Vlas SJ (2016) Accuracy of point-of-care testing for circulatory cathodic antigen in the detection of schistosome infection: systematic review and meta-analysis. Bulletin of the World Health Organization 94, 522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vlas SJ, Gryseels B, Vanoortmarssen GJ, Polderman AM and Habbema JDF (1993) A pocket chart to estimate the true Schistosoma mansoni prevalences. Parasitology Today 9, 305–307. [PubMed] [Google Scholar]
- Easton AV, Oliveira RG, O'Connell EM, Kepha S, Mwandawiro CS, Njenga SM, Kihara JH, Mwatele C, Odiere MR, Brooker SJ, Webster JP, Anderson RM and Nutman TB (2016) Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming. Parasites & Vectors 9. doi: 10.1186/s13071-016-1314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, Zhang Y, Garba A, Stothard JR, Gabrielli AF, Clements ACA, Kabatereine NB, Toure S, Dembele R, Nyandindi U, Mwansa J and Koukounari A (2009) The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology 136, 1719–1730. [DOI] [PubMed] [Google Scholar]
- Foo KT, Blackstock AJ, Ochola EA, Matete DO, Mwinzi PNM, Montgomery SP, Karanja DMS and Secor WE (2015) Evaluation of point-of-contact Circulating Cathodic Antigen assays for the detection of Schistosoma mansoni infection in low-, moderate-, and high-prevalence schools in Western Kenya. American Journal of Tropical Medicine and Hygiene 92, 1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter H, Krauth SJ, Ngandolo BNR, Alfaroukh IO, Zinsstag J and Utzinger J (2016) Validation of a point-of-care Circulating Cathodic Antigen urine cassette test for Schistosoma mansoni diagnosis in the Sahel, and potential cross-reaction in pregnancy. American Journal of Tropical Medicine and Hygiene 94, 36r–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadgu A, Dendukuri N and Hilden J (2005) Evaluation of nucleic acid amplification tests in the absence of a perfect gold-standard test – a review of the statistical and epidemiologic issues. Epidemiology 16, 604–612. [DOI] [PubMed] [Google Scholar]
- Hawkins KR, Cantera JL, Storey HL, Leader BT and de los Santos T (2016) Diagnostic tests to support late-stage control programs for schistosomiasis and soil-transmitted helminthiases. PLoS Neglected Tropical Diseases 10. doi: 10.1371/journal.pntd.0004985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Song LG, Xie H, Liang JY, Yuan DY, Wu ZD and Lv ZY (2016) Nucleic acid detection in the diagnosis and prevention of schistosomiasis. Infectious Diseases of Poverty 5. doi: 10.1186/s40249-016-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz R, Schwarz NG, Hahn A and Frickmann H (2017) Serological approaches for the diagnosis of schistosomiasis – a review. Molecular and Cellular Probes 31, 2–21. [DOI] [PubMed] [Google Scholar]
- Ibironke O, Koukounari A, Asaolu S, Moustaki I and Shiff C (2012) Validation of a new test for Schistosoma haematobium based on detection of Dra1 DNA fragments in urine: evaluation through latent class analysis. PLoS Neglected Tropical Diseases 6. doi: 10.1371/journal.pntd.0001464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabatereine NB, Tukahebwa E, Kazibwe F, Namwangye H, Zaramba S, Brooker S, Stothard JR, Kamenka C, Whawell S, Webster JP and Fenwick A (2006) Progress towards countrywide control of schistosomiasis and soil-transmitted helminthiasis in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 100, 208–215. [DOI] [PubMed] [Google Scholar]
- Kabatereine NB, Brooker S, Koukounari A, Kazibwe F, Tukahebwa EM, Fleming FM, Zhang Y, Webster JP, Stothard JR and Fenwick A (2007) Impact of a national helminth control programme on infection and morbidity in Ugandan schoolchildren. Bulletin of the World Health Organization 85, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CH (2015) It's time to dispel the myth of “asymptomatic” Schistosomiasis. PLoS Neglected Tropical Diseases 9. doi: 10.1371/journal.pntd.0003504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittur N, Castleman JD, Campbell CH, King CH and Colley DG (2016) Comparison of Schistosoma mansoni prevalence and intensity of infection, as determined by the circulating Cathodic antigen urine assay or by the Kato-Katz fecal assay: a systematic review. American Journal of Tropical Medicine and Hygiene 94, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp S, Corstjens P, Koukounari A, Cercamondi CI, Ame SM, Ali SM, de Dood CJ, Mohammed KA, Utzinger J, Rollinson D and van Dam GJ (2015) Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of Schistosoma haematobium in low endemic settings. PLoS Neglected Tropical Diseases 9. doi: 10.1371/journal.pntd.0003752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongs A, Marks G, Verle P and Van der Stuyft P (2001) The unreliability of the Kato-Katz technique limits its usefulness for evaluating Schistosoma mansoni infections. Tropical Medicine & International Health 6, 163–169. [DOI] [PubMed] [Google Scholar]
- Koukounari A, Donnelly CA, Moustaki I, Tukahebwa EM, Kabatereine NB, Wilson S, Webster JP, Deelder AM, Vennervald BJ and van Dam GJ (2013) A latent Markov modelling approach to the evaluation of circulating Cathodic antigen strips for schistosomiasis diagnosis pre- and post-praziquantel treatment in Uganda. PLoS Computational Biology 9. doi: 10.1371/journal.pcbi.1003402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger A, Nassoro T, Said K, Fenner L, Sikalengo G, Letang E, Montresor A, Zhou XN, Steinmann P, Marti H, Utzinger J and Knopp S (2016) Assessing stool quantities generated by three specific Kato-Katz thick smear templates employed in different settings. Infectious Diseases of Poverty 5. doi: 10.1186/s40249-016-0150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenberg S (2014) Uganda's struggle with schistosomiasis. Lancet 383, 1707–1708. [DOI] [PubMed] [Google Scholar]
- Mabey D, Peeling RW, Ustianowski A and Perkins MD (2004) Diagnostics for the developing world. Nature Reviews Microbiology 2, 231–240. [DOI] [PubMed] [Google Scholar]
- Meheus F, Burza S, Becker S, N'Goran EK, Sacko M, Polman K, Chappuis F, Boelaert M and Utzinger J (2015) Modelling the cost-effectiveness of diagnosis of Schistosoma mansoni infection: a comparison of Kato-Katz and urine-circulating cathodic antigen cassette test. Tropical Medicine & International Health 20, 103–103. [Google Scholar]
- Mejia R, Vicuna Y, Broncano N, Sandoval C, Vaca M, Chico M, Cooper PJ and Nutman TB (2013) A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. American Journal of Tropical Medicine and Hygiene 88, 1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs L, Brienen E, Mbow M, Ochola EA, Mboup S, Karanja DMS, Secor WE, Polman K and van Lieshout L (2015) Is PCR the next reference standard for the diagnosis of Schistosoma in stool? A comparison with microscopy in Senegal and Kenya. PLoS Neglected Tropical Diseases 9. doi: 10.1371/journal.pntd.0003959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C, LaCourse EJ, Reimer L and Stothard JR (2016) Focusing nucleic acid-based molecular diagnostics and xenomonitoring approaches for human helminthiases amenable to preventive chemotherapy. Parasitology Open 2, doi: 10.1017/pao.2016.1013 [DOI] [Google Scholar]
- Montresor A, Crompton D, Hall A, Bundy D and Savioli L (1998). Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level. Geneva: WHO. [Google Scholar]
- Montresor A, Gabrielli AF, Chitsulo L, Ichimori K, Mariotti S, Engels D and Savioli L (2012) Preventive chemotherapy and the fight against neglected tropical diseases. Expert Review of Anti-Infective Therapy 10, 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L and Muthén B (1998. –2012). MPlus Version 7.3 User's Guide. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, Yazdanbakhsh M, Hartgers FC, Boakye DA, Verweij JJ, van Dam GJ and van Lieshout L (2008). Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Annals of Tropical Medicine and Parasitology 102, 625–633. [DOI] [PubMed] [Google Scholar]
- Peeling RW, Mabey D, Herring A and Hook EW (2006) Why do we need quality-assured diagnostic tests for sexually transmitted infections? Nature Reviews Microbiology 4, 909–921. [DOI] [PubMed] [Google Scholar]
- Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuente LAT, Garba A, Mohammed KA, Schur N, Person B, Colley DG and Utzinger J (2013) Time to set the agenda for schistosomiasis elimination. Acta Tropica 128, 423–440. [DOI] [PubMed] [Google Scholar]
- Rosser A, Rollinson D, Forrest M and Webster BL (2015) Isothermal recombinase polymerase amplification (RPA) of Schistosoma haematobium DNA and oligochromatographic lateral flow detection. Parasites & Vectors 8. doi: 10.1186/s13071-015-1055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savioli L, Albonico M, Colley DG, Correa-Oliveira R, Fenwick A, Green W, Kabatereine N, Kabore A, Katz N, Klohe K, Loverde PT, Rollinson D, Stothard JR, Tchuente LAT, Waltz J and Zhou XN (2017) Building a global schistosomiasis alliance: an opportunity to join forces to fight inequality and rural poverty. Infectious Diseases of Poverty 6. doi: 10.1186/s40249-017-0280-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PNM, Butler SE, Karanja DMS and Secor WE (2011) Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in Western Kenya. PLoS Neglected Tropical Diseases 5. doi: 10.1371/journal.pntd.0000951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon AW, Engels D, Bailey RL, Blake IM, Brooker S, Chen JX, Chen JH, Churcher TS, Drakeley CJ, Edwards T, Fenwick A, French M, Gabrielli AF, Grassly NC, Harding-Esch EM, Holland MJ, Koukounari A, Lammie PJ, Leslie J, Mabey DC, Rhajaoui M, Secor WE, Stothard JR, Wei H, Willingham AL, Zhou XN and Peeling RW (2012) A diagnostics platform for the integrated mapping, monitoring, and surveillance of neglected tropical diseases: rationale and target product profiles. PLoS Neglected Tropical Diseases 6. doi: 10.1371/journal.pntd.0001746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Figueiredo JC, Betson M, Kabatereine NB and Stothard JR (2013) The urine circulating cathodic antigen (CCA) dipstick: a valid substitute for microscopy for mapping and point-of-care diagnosis of intestinal schistosomiasis. PLoS Neglected Tropical Diseases 7. doi: 10.1371/journal.pntd.0002008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley CJ, Adriko M, Arinaitwe M, Atuhaire A, Kazibwe F, Fenwick A, Kabatereine NB and Stothard JR (2010a) Epidemiology and control of intestinal schistosomiasis on the Sesse Islands, Uganda: integrating malacology and parasitology to tailor local treatment recommendations. Parasites & Vectors 3. doi: 10.1186/1756-3305-3-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley CJ, Lwambo NJS, Lange CN, Kariuki HC, Adriko M and Stothard JR (2010b) Performance of circulating cathodic antigen (CCA) urine-dipsticks for rapid detection of intestinal schistosomiasis in schoolchildren from shoreline communities of Lake Victoria. Parasites & Vectors 3. doi: 10.1186/1756-3305-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton MC, Adriko M, Arinaitwe M, Howell A, Davies J, Allison G, LaCourse EJ, Muheki E, Kabatereine NB and Stothard JR (2017) Intestinal schistosomiasis in Uganda at high altitude (>1400 m): malacological and epidemiological surveys on Mount Elgon and in Fort Portal crater lakes reveal extra preventive chemotherapy needs. Infectious Diseases of Poverty 6. doi: 10.1186/s40249-017-0248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard JR (2009) Improving control of African schistosomiasis: towards effective use of rapid diagnostic tests within an appropriate disease surveillance model. Transactions of the Royal Society of Tropical Medicine and Hygiene 103, 325–332. [DOI] [PubMed] [Google Scholar]
- Stothard JR and Adams E (2014) A preface on advances in diagnostics for infectious and parasitic diseases: detecting parasites of medical and veterinary importance. Parasitology 141, 1781–1788. [DOI] [PubMed] [Google Scholar]
- Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Mathieson W, Webster JP and Fenwick A (2005) Field evaluation of the Meade Readiview handheld microscope for diagnosis of intestinal schistosomiasis in Ugandan school children. American Journal of Tropical Medicine and Hygiene 73, 949–955. [PubMed] [Google Scholar]
- Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, Mathieson W, Webster JP and Fenwick A (2006) Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta Tropica 97, 219–228. [DOI] [PubMed] [Google Scholar]
- Stothard JR, Sousa-Figueiredo JC, Standley C, Van Dam GJ, Knopp S, Utzinger J, Ameri H, Khamis AN, Khamis IS, Deelder AM, Mohammed KA and Rollinson D (2009) An evaluation of urine-CCA strip test and fingerprick blood SEA-ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar. Acta Tropica 111, 64–70. [DOI] [PubMed] [Google Scholar]
- Stothard JR, Sousa-Figueiredo JC and Navaratnam AMD (2013) Advocacy, policies and practicalities of preventive chemotherapy campaigns for African children with schistosomiasis. Expert Review of Anti-Infective Therapy 11, 733–752. [DOI] [PubMed] [Google Scholar]
- Stothard JR, Stanton MC, Bustinduy AL, Sousa-Figueiredo JC, Van Dam GJ, Betson M, Waterhouse D, Ward S, Allan F, Hassan AA, Al-Helal MA, Memish ZA and Rollinson D (2014) Diagnostics for schistosomiasis in Africa and Arabia: a review of present options in control and future needs for elimination. Parasitology 141, 1947–1961. [DOI] [PubMed] [Google Scholar]
- Stothard JR, Campbell SJ, Osei-Atweneboana MY, Durant T, Stanton MC, Biritwum NK, Rollinson D, Ombede DRE and Tchuem-Tchuente LA (2017a) Towards interruption of schistosomiasis transmission in sub-Saharan Africa: developing an appropriate environmental surveillance framework to guide and to support ‘end game’ interventions. Infectious Diseases of Poverty 6. doi: 10.1186/s40249-016-0215-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard JR, Kabatereine NB, Archer J, Al-Shehri H, Tchuem-Tchuente LA, Gyapong M and Bustinduy AL (2017b) A centenary of Robert T. Leiper's lasting legacy on schistosomiasis and a COUNTDOWN on control of neglected tropical diseases. Parasitology 144, 1602–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuente LAT, Rollinson D, Stothard JR and Molyneux D (2017) Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infectious Diseases of Poverty 6. doi: 10.1186/s40249-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Hove RJ, Verweij JJ, Vereecken K, Polman K, Dieye L and van Lieshout L (2008) Multiplex real-time PCR for the detection and quantification of Schistosoma mansoni and S-haematobium infection in stool samples collected in northern Senegal. Transactions of the Royal Society of Tropical Medicine and Hygiene 102, 179–185. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Becker SL, van Lieshout L, van Dam GJ and Knopp S (2015) New diagnostic tools in schistosomiasis. Clinical Microbiology and Infection 21, 529–542. [DOI] [PubMed] [Google Scholar]
- Verweij JJ and Stensvold CR (2014) Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clinical Microbiology Reviews 27, 371–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakoon K, Gobert GN, Cai PF and McManus DP (2015) Advances in the diagnosis of human schistosomiasis. Clinical Microbiology Reviews 28, 939–967. [DOI] [PMC free article] [PubMed] [Google Scholar]