Abstract
Reprogramming of cancer metabolism is a newly recognized hallmark of malignancy. The aberrant glucose metabolism is associated with dramatically increased bioenergetics, biosynthetic, and redox demands, which is vital to maintain rapid cell proliferation, tumor progression, and resistance to chemotherapy and radiation. When the glucose metabolism of cancer is rewiring, the characters of cancer will also occur corresponding changes to regulate the chemo- and radio-resistance of cancer. The procedure is involved in the alteration of many activities, such as the aberrant DNA repairing, enhanced autophagy, oxygen-deficient environment, and increasing exosomes secretions, etc. Targeting altered metabolic pathways related with the glucose metabolism has become a promising anti-cancer strategy. This review summarizes recent progress in our understanding of glucose metabolism in chemo- and radio-resistance malignancy, and highlights potential molecular targets and their inhibitors for cancer treatment.
Keywords: Metabolic reprogramming, Chemo-resistance, Radio-resistance, TME
Background
Cancer is a serious public health problem. The incurrence and mortality is increasing year by year [1]. In addition to conventional radiotherapy, chemotherapy, and surgery, there are currently more and more popular neoadjuvant chemotherapy and molecular targeted therapies. These treatment options can cure early and part of the intermediate tumors in certain degrees, but are not ideal for most of cancer in middle and late stages [2]. Among many reasons, the treatment resistance is the one of major drawbacks. Radiotherapy and chemotherapy, as the routine treatment, face substantial challenges of resistance. However, the characters of chemo- and radio-resistance in different kinds of cancers are not exactly the same.
In the early 1920s, German biochemist and physiologist Otto Warburg conducted groundbreaking research and proposed the famous “Warburg effect”: Tumor cells prefer to use glycolysis for glucose metabolism even in oxygen-rich conditions, rather than more efficient mitochondrial oxidative phosphorylation for ATP production [3]. Actually, the entire metabolic network reprograms under the control of oncogenes and tumor suppressor genes, and the flow of nutrient in metabolic networks is also redefined in the process of tumorigenesis. Metabolic reprogramming provides critical information for clinical oncology. The aberrant glucose metabolism is a major kind of metabolic reprogramming in cancer [4], and recent studies have shown that aberrant glucose metabolism regulates cancer proliferation, cell cycle, drug resistance, and DNA repair [5–7]. As the molecular mechanisms underlying chemo- and radio-resistance are still poorly understood, the alteration of glucose metabolism in cancer provides new ideas to explain chemo- and radio-resistance. Herein, this review updates the mechanisms of metabolic reprogramming involved in tumor chemo- and radio-resistance.
Main text
The overview of glucose metabolic reprogramming
Metabolic reprogramming refers to the redefinition of the flow and flux of nutrient in tumor cells in the metabolic network to meet the needs of tumor cells for energy and anabolism [8]. Under oxygen-rich conditions, normal or differentiated cells can metabolize glucose and produce carbon dioxide through a tricarboxylic acid cycle (TCA), which produces 30 or 32 mol of adenosine triphosphate (ATP) per mole of glucose and a small amount of lactate during oxidative phosphorylation [9]. Only under hypoxic conditions, normal or differentiated cells produce large amounts of lactic acid by anaerobic glycolysis. However, German scientist Otto Warburg first proposed that tumor cells rely mainly on glycolysis to provide energy under aerobic conditions [3](Fig. 1). Weinberg characterized “aberrant metabolic phenotype” with “autologous proliferation signaling, apoptosis resistance, evasion of proliferation inhibition, continuous angiogenesis, infiltration and migration, unlimited replication capacity, immune escape” in tumor cells.
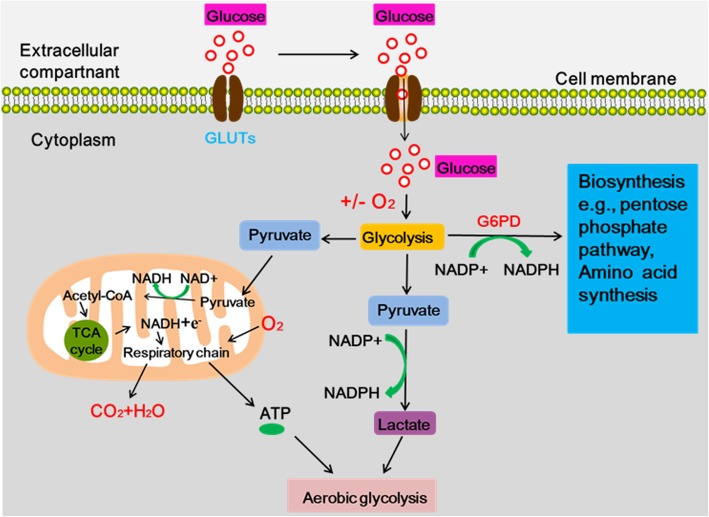
Fig. 1.
The energy metabolism of cancer cells. Under aerobic condition, Most of the glucose is first converted to pyruvate via glycolysis in the cytosol. Most pyruvate are mostly processed to lactate via glycolytic pyruvate even in the presence of oxygen, and only a small portion of pyruvates enters the mitochondria to produce CO2 by undergoing TCA cycle. In addition, small proportion of the glucose is diverted into the upstream of pyruvate production for biosynthesis (e.g., pentose phosphate pathway, and amino acid synthesis)
Glucose metabolic reprogramming between aerobic glycolysis and oxidative phosphorylation, previously speculated as exclusively observable in cancer cells, exists in various types of immune and stromal cells in many different pathological conditions other than cancer [6]. It has been well established that tumor cells have elevated rates of glucose uptake and high lactate production in the presence of oxygen, known as aerobic glycolysis (also termed the Warburg effect) [10]. As a matter of fact, high lactate production also remodels the tumor microenvironment (TME) by contributing to acidosis, acting as a cancer cell metabolic fuel and inducing immunosuppression resulting in aggressive proliferation, invasion, migration and resistance therapy [4]. However, the molecular mechanisms involved in the changes of glucose metabolism are complex. Changes in the tumor microenvironment, activation of oncogenes, and inactivation of tumor suppressor genes all contribute to the disruption of metabolism and steady-state metabolism of cells, ultimately leading to aberrant glucose metabolism [11, 12]. Specific oncogenes activation or tumor suppressor genes deactivation can reprogram the underlying metabolism of tumor tissues. Some genes can act as initiators of glucose consumption, include myc, KRAS, and BRCA1 [13–15]. Despite the progression, we still do not fully know the metabolic pathways that are reprogrammed by oncogenes or suppressor genes.
Glucose metabolic reprogramming and chemo- and radio-resistance
Tumor cell survival under aberrant metabolism of glucose is a vital step not only for the process of tumorigenesis but also in treatment resistance and recurrence, especially for the occurrence of treatment resistance [4]. Chemotherapy in the form of neo-adjuvant or adjuvant therapy is the dominant treatment for most of cancers; the resistance directly affects the survival and prognosis of cancer patients [16]. Theoretically, the tumor mass, made of distinct chemo-resistant cell populations has been recognized as an important mechanism for chemo-resistance [17]. Actually, inhibition of glycolysis not merely inhibited cell proliferation, but alleviated resistance to chemotherapeutic drugs.
Existing evidence indicates that increased glucose uptake and enhanced aerobic glycolysis are able to induce the intrinsic or acquired resistance to DDP in gastric cancer cells [18]. Elevated lactate levels caused by aberrantly activated glycolysis can reinforce DNA repair and promote cisplatin-resistance in cervical carcinoma cells via the inactivation of histone deacetylase [19]. High-precision radiation therapy enables radiation oncologists to decrease delivery of an excessive dose of radiation to normal tissues and also to administer a high and booster dose of radiation, particularly to small target fractions in a malignant tumor [20]. Previous studies have revealed that the Warburg effect or aerobic glycolysis promotes the radio-resistance of various malignant tumors via generating a chemically reduced milieu associated with the development of radio-resistance in laryngeal carcinoma, prostate cancer, head and neck cancer [21–26]. For example, activation of adenosine monophosphate-activated protein kinase (AMPK) mediates metabolic reprogramming in resistant cancer cells through promoting both the Warburg effect and also mitochondrial biogenesis [27–30]. However, both the gene network triggering metabolic reprogramming and the molecular mechanism linking the reprogramming with radio-resistance remain to be determined.
The mechanisms of glucose metabolic reprogramming-mediated chemo- and radio-resistance
Although increasing evidence has confirmed that glucose metabolic reprogramming can induce tumor radiotherapy and chemotherapy resistance, the specific mechanisms are still not clear [31–34]. The previously reported resistance mechanisms include mutations or increases in drug targets, changes in drug metabolism, and alterations in DNA repair, overexpression of anti-apoptotic genes, and inactivation of apoptotic gene products, immunosuppression and the formation of CSCs, etc.
With the increasing research understanding on the resistance of chemo- and radiotherapy, the researchers have pointed out that cancer stem cells, tumor microenvironment, autophagy, and exosomes are all closely related to tumor chemo- and radio-resistance. In fact, recent reports have shown that chemo- and radio-resistance acquisition is coupled to deregulate glucose metabolism and glycolysis [35]. Signaling pathways related to chemo-radiotherapy resistance are abnormally activated or inactivated during metabolic stress, such as Wnt, PI3K/AKT, Notch, NF-κB, MAPK [36–41]. In addition, the metabolic reprogramming mediated by aberrant expression of oncogenes can enhance the pentose phosphate pathway and aerobic glycolysis to promote the DNA repair and apoptosis resistance [42–44]. For example, the glucose metabolic reprogramming of colorectal cancer induced mainly by aberrant MYC expression could activate the pentose phosphate pathway, purine/pyrimidine synthesis pathway, fatty acid oxidation pathway and mitogen-activated protein kinase (MAPK) signaling pathway to prolong the survival of cancer cells under the chemotherapy and radiotherapy [45–47]. In truth, the metabolic reprogramming may induce the DNA repair, the immunosuppression of tumor microenvironment, the anti-apoptosis by enhanced autophagy, and the formation of cancer stem cells mediated by exosomes, which all induce chemo- and radio-resistance. Herein we will introduce mechanisms of glucose metabolic reprogramming in radiotherapy and chemotherapy resistance.
Activating DNA damage repair
It’s well known that the essence of chemotherapy and radiotherapy is to cause the disruption of DNA replication, thus leading to cell death or apoptosis and achieving therapeutic purposes [48]. Accumulating evidence suggests that the continuous activation of aerobic glycolysis plays a vital role in tumor development and the expression of many altered genes is accompanied by aerobic glycolysis in tumor development and resistance [49, 50]. Efficient DNA damage repair would depend on anabolic alterations that could provide cancer cells with nucleotide pools for repair of radiation and chemotherapy-induced DNA damage [51]. Recent study has indicated that the chemo-resistant breast cancer cells and mesothelioma cells have high levels of aldehyde dehydrogenase (ALDH) activity. ALDH is an important detoxifying enzyme of glycolysis, which belongs to a class of detoxifying enzymes whose expression is linked to cancer chemo-resistance [52]. Meanwhile, glycolysis can also enable cancer cells to reduce the level of intracellular reactive oxygen species (ROS) by limiting the pyruvate flux into mitochondrial respiration, and thus acquire resistance to apoptosis and DNA impair(Fig. 2) [53–55].
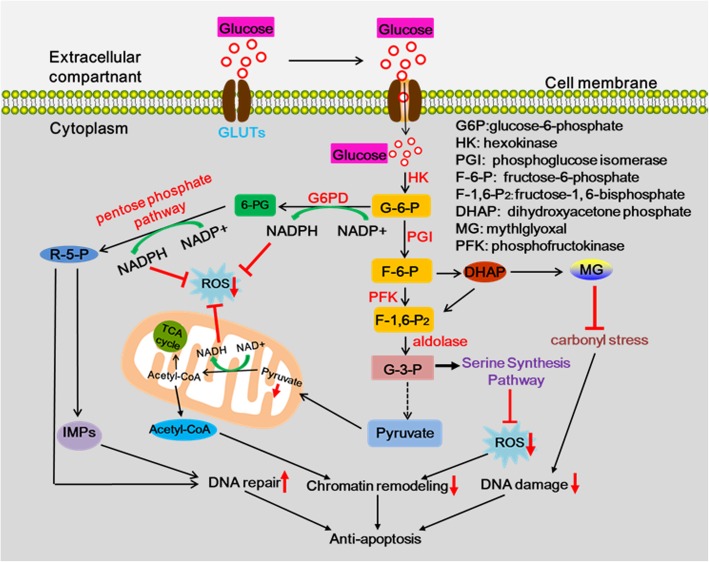
Fig. 2.
Simplified diagram of the main metabolic pathways involved in DNA damage/repair. Continuous activation of aerobic glycolysis can increase the capture of glucose into the cytoplasm by up-regulating the expression of glucose transporters (GLUTs) and substantially enhance the high rate of glucose influx via activating HK, PFK, and aldolase enzyme and promoting their expression, which in turn facilitates the aerobic glycolysis. The glycolytic switch in tumor cells allows the direct or indirect flux of glycolytic intermediates to many biosynthetic pathways (e.g., pentose phosphate pathway, serine synthesis pathway, MG pathway, and nucleotide synthesis), which provides the biomacromolecules and other materials required for prolonging the cancer cell survival via enhancing DNA repair, inhibiting DNA damage and decreasing chromatin remodeling
An elevated endogenous ROS level generated from attacks of mitochondria on nearby mitochondrial DNA (mtDNA) results in an imbalance between production and destruction of ROS, which resulted in oxidative damage to mtDNA under aberrant condition of glucose metabolism [56–59]. ROS, which can increase oxidative DNA damage and hence the load of the DNA-repair machinery, are regulated through different metabolic pathways. High ROS levels affect many aspects of tumor biology like DNA damage and genomic instability. Furthermore, mutations in genes involved in the glucose metabolism rewiring can also block the balance of DDR (DNA damage response)and DNA repair to result in resistance to chemotherapy and radiotherapy. For example, PFKFB3 (6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3), an altered genes significantly accelerates the glycolysis, enhances the ability of DNA repair and its pro-tumor effects during glycolysis through the PFKFB3/Akt/ERCC1 signaling pathway, thus leading to failure of chemotherapy and radiotherapy in HCC [60]. Furthermore, a study indicated that disrupting cancer metabolism had an important role for both glycolysis and glutaminolysis in promoting DNA-DSB (double strand breaks) repair and preventing accelerated senescence after irradiation [61].
The aberrant glycolysis and glutaminolysis could promote DNA repair via targeting the hexosamine biosynthetic pathway (HBP) and tricarboxylic acid (TCA) cycle [62]. The previous researches had indicated that Mucin1 (MUC1), an oncogene overexpressed in multiple solid tumors, can mediate DNA repair in breast cancer cells and facilitate the metabolic reprogramming in pancreatic cancer cells [63]. In MUC1-expressing pancreatic cancer cells, the metabolite levels in glycolysis, PPP and nucleotide biosynthetic pathways increased to enhance the DNA damage repair and inhibit the sensibility of radiation therapy and chemotherapy [64–67]. Furthermore, amplified N-Myc can directly bind with the tetrameric form of p53 at the C-terminal domain in the nucleus to alter p53-dependent transcriptional responses in neuroblastoma patients with wild-type p53, but wild-type p53 negatively regulates G6PD activity, a rate-limiting enzyme of the pentose phosphate pathway that is the most important sources of nucleotides, and then decreases dNTP synthesis, ultimately influencing the DNA repair [46, 68, 69]. Therefore, N-Myc directly suppresses the transcriptional responses of wild-type p53 to inhibit the pentose phosphate pathway and increase the DNA repair.
In essence, the DNA damage repair induced by glucose metabolic reprogramming is a complicated procedure which involves the activation of many oncogenes and activation or silencing of signaling pathways and needs more researches to clarify it.
The apoptosis resistance of enhanced autophagy
Autophagy is an evolutionarily conserved process through which lysosomal degradation of damaged and superfluous cell components are recycled back into basic biomolecules in the cytosol [70, 71]. Low glucose levels could induce autophagy in a wide variety of mammalian cell types, including cancer cells, and this regulation appears to be partially dependent on the activation of AMPK [72]. Enhanced autophagic activity buffers glucose and amino acid starvation most likely by degrading intracellular energy reserves like glycogen, and proteins [73]. E.g. CAFs (cancer-associated fibroblasts) in the tumor stroma exhibit robust activity in terms of aerobic glycolysis and autophagy due to loss of caveolin 1 (Cav-1) expression [74–77]. CAFs with higher levels of aerobic glycolysis and autophagy in the tumor stroma can produce more IL-8 and activate the NF-κB signaling pathway, ultimately leading to resistance to cisplatin in human gastric cancer [75, 76, 78]. In general, enhanced autophagy protects cancer cells during chemotherapy and radiotherapy via supporting the survival of tumor cells, leading to cancer resistance and refractory cancer [75, 79–83]. In addition, increased autophagy regulated by the PI3K/AKT/mTOR pathway prolongs cancer cell survival via resisting to apoptosis under acidic environment stress produced by glycolysis [84].
A new study has found that autophagy is a major way of down-regulating cell metabolism, leading to cancer cell quiescence, survival, and chemo-resistance [85, 86]. The up-regulation of autophagy mediated by metabolic dysfunction could contribute to a common mechanism of resistance to chemotherapy and radiotherapy by suppressing apoptosis, such as rapamycin (Rp) [87–89]. In addition, the induction of autophagy may defend against epirubicin-mediated apoptosis, act as a pro-survival factor, and thus lead to deficient apoptosis in HepG2 and A549 cells [90–92]. Besides, a great deal of evidence suggests that autophagy mostly causes cancer cell survival and resistance to treatment through activation of different autophagy-associated molecules and signaling pathways, such as Wnt, PI3K/AKT, Notch [93–95]. Whereas, autophagy inhibition could promote tumor cell death and enhance the sensibility of radio- and chemotherapies [4, 92, 96–98]. Most studies have suggested that autophagy promotes chemoresistance and targeting autophagy-associated molecules may increase cancer cell chemo-sensitivity [99]. An up-regulation of autophagy may represent a mechanism of resistance to oxidative stress induced by chemotherapeutic drugs and may potentiate the survival to hypoxia and nutrient starvation resulting from the frequently defective tumor vascularization [100]. For example, induction of p53 and transfection of ERK activating RAS mutants but not AKT activating RAS mutant in p53-null ovarian cancer cells promoted autophagy, although the autophagy induced by p53 or ERK activating RAS mutants showed an opposite sensitivity to cisplatin treatment because the activation of RAS/ERK ultimately lead to the increased expression of p-ERK and Bcl-2 and the decreased expression of p-AKT and Bax [101]. Furthermore, a recent study showed that HK-2 (hexokinase-2), a key enzyme of the rate-limiting step in glycolysis up-regulates cisplatin-resistance in ovarian cancer cells by enhancing cisplatin-induced autophagy [102]. Whereas, decreased autophagy induced by Baf A1 treatment, a pharmacological autophagy inhibitor, and knockdown of ATG5 that blocks the non-selective macroautophagy pathway significantly increased apoptotic cell death in chemoresistant breast cancer cells [103]. In the chemo-resistant and radio-resistant cancer cells under periods of glucose metabolic stress, the increased autophagy could prevent cancer cells from apoptosis induced by ER stress (endoplasmic reticulum stress) [104]. As a kind of autophagy, moreover, the enhanced mitochondrial autophagy can prevent apoptosis by reducing mitochondrial outer membrane permeability (MOMP) and reducing the release of mitochondrial pro-apoptotic proteins, such as cytochrome C and SMAC/DIABLO [105].
In spite of a spur in research articles demonstrating the role of autophagy in cancer, the exact role of autophagy induced by metabolic reprogramming on tumor cells is still controversial and remains to be further elucidated [106]. Many of the pathways that control autophagy are deregulated in cancer, and cancer therapeutics targeting these pathways activates autophagy. Taken together, the role of autophagy in tumor initiation and drug resistance is likely context-specific. The functional role of autophagy in these settings needs to be established. A particularly interesting possibility is that autophagy favors tumor cell survival. If this is correct, then inhibition of autophagy might synergize with existing cancer treatments.
The immunosuppressive effect of tumor microenvironment
Hitherto, as to metabolic reprogramming, tumor cells finely regulate ATP synthesis by regulating substrate uptake, as well as enzymes related to glycolysis, which enables them adapt to the nutrient microenvironment [107–112]. Metabolic changes occur not only in tumor cells, but also in immune cells infiltrated in the tumor tissues that undergo metabolic reprogramming to accommodate functional changes [113]. In fact, the altered tumor microenvironment (TME) can induce the tumor cells secretion of immunosuppressive cytokines to inhibit the immune effector cells or induction of suppressive immune cells to exert immunosuppressive effects, then inducing the immune escaping of cancer cells and ultimately contributing to chemotherapy and radiation resistance [114, 115]. During recent years, the interaction between immunosuppression and treatment resistance in different subsets of tumor cells within the TME was increasingly valued by cancer researchers [116–118] (Fig. 3).
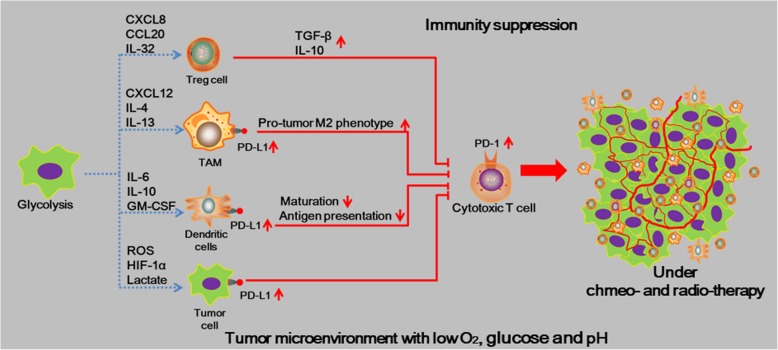
Fig. 3.
The immunosuppressive effect of the tumor microenvironment. The hypoxia and acidosis of the tumor microenvironment (TME) contribute to immunosuppression via several mechanisms. These mechanisms include increased accumulation, activation, and expansion of immunosuppressive regulatory T (Treg) cells; recruitment of inflammatory monocytes and tumor-associated macrophages (TAMs) and reprogramming of TAMs towards the pro-tumor M2 phenotype; suppression of dendritic cell (DC) maturation, which results in inhibiting activation of tumour-specific cytotoxic T lymphocytes (CTLs). Importantly, the programmed cell death protein 1 (PD-1)–programmed cell death 1 ligand 1 (PD-L1) pathway is often activated in the TME as a mechanism to evade anticancer immune responses, with up-regulation of PD-L1 expression on TAMs, DCs, and tumor cells. In addition, tumor-infiltrating CTLs typically up-regulate PD-1, limiting their cytotoxic potential against tumor cells. CCL20, C-C-motif chemokine ligand 20; CXCL, C-X-C-motif chemokine ligand; GM-CSF, granulocyte–macrophage colony-stimulating factor; TGFβ, transforming growth factor β; IL, Interleukin
Tumor cells have to adapt their metabolism to survive and proliferate in this harsh microenvironment. Changes in the tumor microenvironment can affect the levels of infiltrating cell-associated chemokines in tumor cells. These chemokines, in turn, recruit Tregs to tumor tissues to exert immunosuppressive effects [119]. For example, under an inflamed microenvironment, the TLR (Toll-like receptor) can increase glucose uptake and lactate production in Treg cells through up-regulating the expression of key enzymes Glut1 (a glucose transporters), which is beneficial to the proliferation of Treg cells [102, 120]. Tregs exert immunosuppressive effects by inhibiting effector T cells and dendritic cells to enhance the effect of anti-apoptosis and the survival of cancer cells [121]. Because of the TME comprising of stromal and various components of the immune system where reprogramming of the metabolism manifests Warburg phenotype (enhanced aerobic glycolysis), it can play a significant role in suppressing the immune attack on the tumor cells leading to cancer cell survival, proliferation and resistance to therapies [122]. Moreover, Verduzco and others widely accept that the alterations in tumor microenvironment during chemo−/radiotherapy lead to the expression of TME-related factors, which significantly contributes to chemo−/radio-resistance [123–125]. E.g. Genetic ablation of AMPK activates mammalian target of rapamycin (mTOR) signal with enhanced expression of hypoxia-inducible factor-1 alpha (HIF-1α), resulting in rapid cellular proliferation accompanied by activation of aerobic glycolysis [29, 30, 126]. HIF-1α, a biomarker of the hypoxia microenvironment, demonstrates an emerging role in increasing resistance to current cancer therapies, including chemo−/radio-resistance [125]. Moreover, HIF-1α stabilized by hypoxia microenvironment is also able to activate the expression of PD-L1 by binding of HIF to a specific hypoxic response element in the promoter of PD-L1 in cancer cells [127, 128]. PD-L1 expression in cancer cells enables them to deliver an inhibitory signal to PD-1-positive T-cells, suppressing T-cell function. This may be responsible for the accumulation and the activation of immunosuppressive cells [129–131]. In addition, the under hypoxic condition, the tumor cells tend to be anaerobic with glucose and secrete IL-10 that thriggers STAT3 phosphorylation and activation of the PD-1/PD-L1 pathway [132]. In multiple myeloma (MM), increased glucose metabolism of cancer cells can increase the expression of HK-2 and lactate dehydrogenase A (LDHA) to reduce the therapeutic effects of standard care drugs, such as bortezomib and melphalan [133] via inhibiting T cell immunity and promoting cancer stem-like properties. Moreover, tumor LDHA affects MDSCs (myeloid-derived suppressor cells) to control tumor immunity [134]. Human MDSCs induced by granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) inhibit T cell immunity in the tumor microenvironment in patients with cancer [135]. This strongly suggests the importance of cancer metabolic reprogramming in maintaining the interaction between the tumor microenvironment and the immunosuppression.
Regardless of the role of complexity components of TME in chemo−/radio-resistance of cancer cell, the concrete mechanisms of immunosuppression regulated by TME are still not verified and need lots of studies to confirm.
The formation of cancer stem cells mediated by exosomes
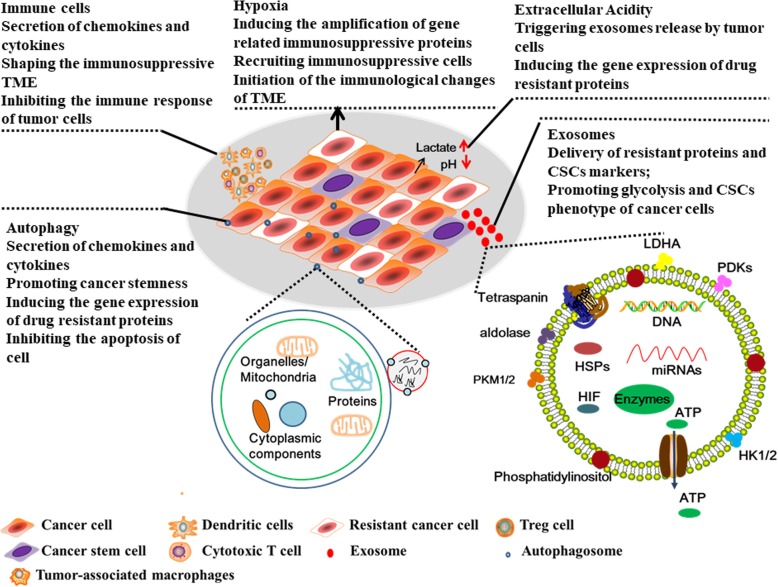
Exosomes are 30–150 nm in diameter microvesicles derived from the multi-vesicular endosome pathway [136]. Cancer cells that utilize aerobic glycolysis as the main energy generating pathway can enhance the exosome secretion [137–140]. The increased secretion of endogenous exosomes from the resistant cancer cells can be taken up by recipient cells and leads to the modulation of aerobic glycolysis and chemotherapy and radiotherapy sensitivity [141–144]. For example, PC-derived exosomes (isolated from murine pancreatic cancer cells) could inhibit glucose intake and promote lipidosis, developing an eventual state of insulin resistance in skeletal muscle cells [142]. The newest documents have found that the exosomes can induce the formation of cancer stem cells (CSCs) to decrease the effect of chemo- and radio-therapy [145–147] (Fig. 4).
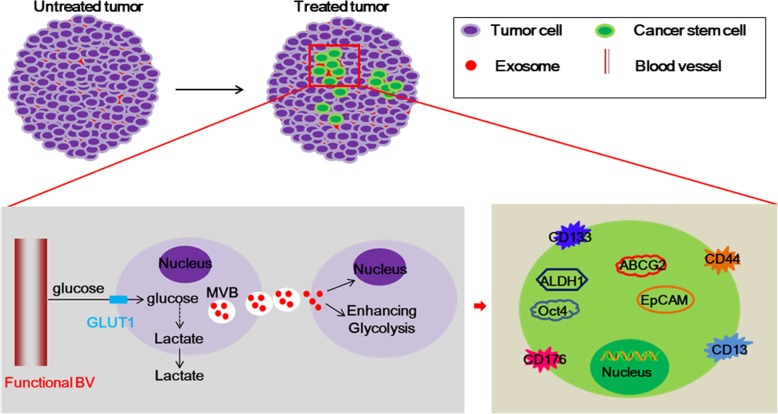
Fig. 4.
The role of the exosomes in the formation of CSCs. The cancer cells with enhanced glycolysis could release a large amount of exosomes contained several of glycolytic enzymes and CSCs markers. These exosomes can be taken up by the recipient cancer cells, and then promote the glycolysis and induce the dedifferentiation of cancer cells to acquire stemness phenotype through transfer their stemness-related molecules
The aberrant glycolytic reaction of CSCs contributes to therapy resistance via preserving stemness and tumorigenic properties of CSCs [148–150]. Exosomal LMP1 activates the PI3K/AKT pathway, and then up-regulates the expression of the surface marker CD44+/High, ultimately increasing the populations of CD44+/High cells, which are the putative stem cell in nasopharyngeal carcinoma cells [150–152]. Besides, exosomal LMP1 could reduce the phosphorylation of AMPK and changed its subcellular location after irradiation, which appears to occur through a disruption of the physical interaction between AMPK and DNA-PK, and then causes decreasing in AMPK activity which is associated with LMP1-mediated glycolysis and resistance to apoptosis induced by irradiation [126, 153, 154]. Similarity, the resistant cancer cells with enhancing glycolysis can secrete a large amount of exosomes containing EpCAM protein, an epithelial cancer stem-like cell markers and glycolysis enzymes [126, 155–159]. The neighboring non-resistant cells can take up these exosomes and positively regulate mTOR and epithelial growth factor receptor (EGFR) signaling pathways to enhance the glycolysis and promote EpCAM+ tumor cells to ovarian cancer stem cells (CD133+ and CD117+CD44+) and putative drug-resistant tumor cell phenotype (EpCAM+ CD45+) transformation [152, 155, 159–162]. Besides, the exosomes secreted from resistant tumor cells can be taken up by non-resistant cells and induce the production of ROS via enhancing metabolic reprogramming [163]. The increased level of ROS can activate the Wnt signaling pathway to accumulate the cancer stem-like cells with CD44v8-10high/Fbw7high/c-Myclow or CD44v8-10high/Fbw7low/c-Mychigh, leading to the formation of resistant sites [147, 149, 152, 164].
Transport of exosomal components can contribute to the chemo- and radio-resistance of cancer cells [165–167]. Among them, transfer of miR-100, miR-222 and miR-30a from the exosomes derived from adriamycin- and docetaxel-resistant MCF-7 breast cancer cells to drug sensitive MCF-7 cells increased the drug resistance of the sensitive cell line through increasing CSCs proportion in cancer cell populations and promoting the phenotypic transition of non-CSCs toward the CSCs phenotype [168–170]. Actually, exosomal HSPs could be involved in the occurrence of EMT and ECM remodeling which were closely associated with the formation of stem cells to mediate the resistance of cancer cells [171]. E.g. exosomal HspDNAJB8, an Hsp40 family member, has a role in maintenance of renal cell carcinoma CSCs/CICs (called cancer stem–like cells/cancer-initiating cells), resistance to chemotherapy and radiotherapy [172, 173]. Similarly, the exosomal lncRNA UCA1 is demonstrated to possibly activate the Wnt signaling pathway and facilitate the malignant transformation of stem cell through the modification of the gene network by tail modification of histone to increase chemo-resistance of cancer cells [174, 175].
Exosomes are speculated as a novel target for solving the radio- and chemo-resistance because they can promote CSCs phenotype. However, the research about the role of exosomes in the treatment resistance of cancer is not much more; it isn’t a good explanation to verify the concrete effect of exosomes and need to more studies to confirm.
Perspectives of metabolic inhibitors
Up to date, the metabolic inhibitors aim to inhibiting the enzymes about tumor metabolism, and then decrease the level of cancer glucose consumption to decrease amount of ATP, attenuating amino acids and nucleotides synthesis, and generate reactive oxygen species (ROS) [126, 176–182]. Metabolic inhibitors reduce the metabolite levels in glycolysis, PPP and nucleotide biosynthetic pathways to down-regulate the resistant effect of cancer cells via preventing DNA damage repair and enhancing chemotherapy and radiation responsiveness [47, 183]. For example, 3-BrPA (3-bromopyruvate), a special inhibitor of HK-2 kinase, can induce the imbalance of intracellular redox via inhibiting the glycolysis and strengthening the tricarboxylic acid cycle in cancer cells, during which a large amount of ROS is produced and accumulated in the cancer cells, destroying the normal structure inside the cell and causing the cell to gradually die [184]. Therefore, 3-BrPA can sensitize first-line anti-tumor drugs in the resistant cancer cells, such as 5-fluorouracil, doxorubicin, mycin, mitoxantrone and platinum drugs (e.g. cisplatin, oxaliplatin) [185]. In addition, the covalent inhibitor JX06 targeting PDK via structural modification hinders access of ATP to its binding pocket and in turn impairs PDK1 enzymatic activity, which increases the sensitivity of chemotherapy and radiotherapy by promoting cellular oxidative stress and apoptosis [186]. FX11, an LDHA inhibitor, can be capable of blocking aerobic glycolysis via inactivating the CK2/PKM2/LDHA axis to induce oxidative stress, and suppress drug resistance in various cancers [187]. 3PO, a glycolysis inhibitor targeting PFKFB3, can inhibit the glycolysis of nintedanib- and sunitinib-resistant tumor cells via inducing cell-cycle arrest and apoptosis, and thus promote the therapeutic efficacy of chemo- and radio-therapy [188].
Even though some metabolic inhibitors have been approved for clinical treatment, the efficacy is not ideal and rigorous evidence-based medical evidence lacks. There are inextricable links between cell metabolism, tumor immunity, and tumor epigenetics. Metabolic inhibitors can only achieve maximum biological efficacy when combined with targeted inhibitors of macromolecule synthesis, cellular immune-agonists, and agonists or inhibitors associated with metabolic pathways. Furthermore, most metabolic inhibitors lack specificity and cannot target tumor cells and have a killing effect on normal cells. Therefore, the researches on metabolic inhibitors have promising development prospects.
Conclusions
Extensive studies have provided strong evidence for reprogramming of cancer metabolism in chemo- and radio-resistant cancer. Aberrant glucose metabolism could alter many physiological activities(Fig. 5), e.g. inducing DNA damage repair, enhancing autophagy, changing tumor microenvironment and increasing the secretion of exosomes, etc. However, these alterations are not a simple relationship between chemo- and radio-resistance and glucose metabolism. Additional studies are needed to better understand the molecular mechanisms linking resistance to cell metabolism. Additionally, it will be important to understand whether the effects of metabolic inhibitors are cell type-specific. Because changes in treatment resistance can directly or indirectly impact multiple processes--including metabolism, ROS signaling, and calcium signals. The outcome may be critically dependent on cell types. Finally, once the interconnections between the glucose metabolism of cancer cells and resistance to treatments are better understood, we will hopefully be able to harness this information to devise therapies for cancer resistance.
Fig. 5.
The overview of acquired chemoradiotherapy resistance mediated by metabolic reprogramming in cancer cells
Acknowledgments
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81872281, 81472595), the Natural Science Foundation of Hunan Province (2018JJ1013, 2017JJ3190, 2016JJ4059), the Research Project of the Health and Family Planning Commission of Hunan Province (B20180400, B20180582, B2016056), and the Changsha Science and Technology Board (kq1706045, kq1706043).
Availability of data and materials
Not applicable.
Abbreviations
- 6PGD
6-phosphogluconate dehydrogenase
- ALDH
Aldehyde dehydrogenase
- AMPK
AMP-activated protein kinase
- ATP
Adenosine triphosphate
- CSCs
Cancer stem cells
- DDR
DNA damage response
- DNA-DSB
DNA-double strand breaks
- EOC
Epithelial ovarian cancer
- ETC
Electron transport chain
- G6PD
Glucose-6-phosphate dehydrogenase
- Glut1
Glucose transporter-1
- HBP
Hexosamine biosynthetic pathway
- HK-2
Hexokinase-2
- LDH
Lactate dehydrogenase
- LDHA
Lactate dehydrogenase A
- mtDNA
mitochondrial DNA
- MUC1
Mucin1
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NSCLC
Non-small cell lung cancer
- PDK1
Pyruvate dehydrogenase kinase 1
- PFK
Phosphofructokinase
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
- PGAM
Phosphoglyceric acidmutase
- PKM2
Pyruvate kinase-2
- PPARδ
Peroxisome-proliferator-activated receptor δ
- PPP
Pentose phosphate pathway
- ROS
Reactive Oxygen Species
- SLC1-A5
Solute carrier family 1 member 5
- TCA
Tricarboxylic acid cycle
- TME
Tumor microenvironment
Authors’ contributions
LJG and XLZ contributed to drafting and editing of the manuscript, shared the first authorship. LQJ and ZYJ designed, revised and finalized the manuscript. LJX, HYQ, WHR, OYLD, and TSM participated in the drafting and editing manuscript. TYT, RS participated in the revision and coordination. CXY, TYY, LX contributed to literature search. SM, WY, WH participated in the conception and coordination. All authors contributed toward data analysis, drafting and revising the paper and agreed to be accountable for all aspects of the work.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jinguan Lin, Email: linjingguan@hnszlyy.com.
Longzheng Xia, Email: 179915320@qq.com.
Jiaxin Liang, Email: jane_leung@163.com.
Yaqian Han, Email: hanyaqian@hnszlyy.com.
Heran Wang, Email: wangheran11111@hotmail.com.
Linda Oyang, Email: 3397620@qq.com.
Shiming Tan, Email: 527936389@qq.com.
Yutong Tian, Email: 15211004520@163.com.
Shan Rao, Email: raoshan3333@126.com.
Xiaoyan Chen, Email: 479488462@qq.com.
Yanyan Tang, Email: tangyanyan@hnszlyy.com.
Min Su, Email: sumin@hnszlyy.com.
Xia Luo, Email: 28579277@qq.com.
Ying Wang, Email: wangyin@hnszlyy.com.
Hui Wang, Email: wanghui710327@163.com.
Yujuan Zhou, Phone: 86-731-88651681, Email: yujany_zhou@163.com.
Qianjin Liao, Phone: 86-731-88651681, Email: march-on@126.com.
References
- 1.Kang YP, Ward NP, DeNicola GM. Recent advances in cancer metabolism: a technological perspective. Exp Mol Med. 2018;50(4):31. doi: 10.1038/s12276-018-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marinova M, Huxold HC, Henseler J, Mucke M, Conrad R, Rolke R, et al. Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic Cancer. Ultraschall Med. 2018. [DOI] [PubMed]
- 3.Kato Y, Maeda T, Suzuki A, Baba Y. Cancer metabolism: new insights into classic characteristics. Jpn Dent Sci Rev. 2018;54(1):8–21. doi: 10.1016/j.jdsr.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Gang, Wang Jun-Jie, Yin Pei-Hao, Xu Ke, Wang Yu-Zhu, Shi Feng, Gao Jing, Fu Xing-Li. New strategies for targeting glucose metabolism-mediated acidosis for colorectal cancer therapy. Journal of Cellular Physiology. 2018;234(1):348–368. doi: 10.1002/jcp.26917. [DOI] [PubMed] [Google Scholar]
- 5.Vallee A, Lecarpentier Y, Guillevin R, Vallee JN. Demyelination in multiple sclerosis: reprogramming energy metabolism and potential PPARgamma agonist treatment approaches. Int J Mol Sci. 2018;19(4). [DOI] [PMC free article] [PubMed]
- 6.Kim J. Regulation of immune cell functions by metabolic reprogramming. J Immunol Res. 2018;2018:8605471. doi: 10.1155/2018/8605471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su B, Su J, Zeng Y, Ding E, Liu F, Tan T, et al. Diallyl disulfide inhibits TGFbeta1induced upregulation of Rac1 and betacatenin in epithelialmesenchymal transition and tumor growth of gastric cancer. Oncol Rep. 2018;39(6):2797-806. [DOI] [PubMed]
- 8.Singh Amar M., Dalton Stephen. What Can ‘Brown-ing’ Do For You? Trends in Endocrinology & Metabolism. 2018;29(5):349–359. doi: 10.1016/j.tem.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dar S, Chhina J, Mert I, Chitale D, Buekers T, Kaur H, et al. Bioenergetic adaptations in Chemoresistant ovarian Cancer cells. Sci Rep. 2017;7(1):8760. doi: 10.1038/s41598-017-09206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam SO, Yotsumoto F, Miyata K, Fukagawa S, Yamada H, Kuroki M, et al. Warburg effect regulated by amphiregulin in the development of colorectal cancer. Cancer Med. 2015;4(4):575–587. doi: 10.1002/cam4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Duran A, Reina-Campos M, Valencia T, Castilla EA, Muller TD, et al. Adipocyte p62/SQSTM1 suppresses tumorigenesis through opposite regulations of metabolism in adipose tissue and tumor. Cancer Cell. 2018;33(4):770–84 e6. doi: 10.1016/j.ccell.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai C, Arceo J, Arnold J, Sreekumar A, Dovichi NJ, Li J, et al. Metabolomics of oncogene-specific metabolic reprogramming during breast cancer. Cancer Metab. 2018;6:5. doi: 10.1186/s40170-018-0175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV. MYC, metabolism, and Cancer. Cancer Discov. 2015;5(10):1024–1039. doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terunuma A, Putluri N, Mishra P, Mathe EA, Dorsey TH, Yi M, et al. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J Clin Invest. 2014;124(1):398–412. doi: 10.1172/JCI71180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Privat M, Radosevic-Robin N, Aubel C, Cayre A, Penault-Llorca F, Marceau G, et al. BRCA1 induces major energetic metabolism reprogramming in breast cancer cells. PLoS One. 2014;9(7):e102438. doi: 10.1371/journal.pone.0102438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herranz D, Ambesi-Impiombato A, Sudderth J, Sanchez-Martin M, Belver L, Tosello V, et al. Metabolic reprogramming induces resistance to anti-NOTCH1 therapies in T cell acute lymphoblastic leukemia. Nat Med. 2015;21(10):1182–1189. doi: 10.1038/nm.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afonso J, Santos LL, Morais A, Amaro T, Longatto-Filho A, Baltazar F. Metabolic coupling in urothelial bladder cancer compartments and its correlation to tumor aggressiveness. Cell Cycle. 2016;15(3):368–380. doi: 10.1080/15384101.2015.1121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu W, Wang S, Chen Q, Zhang Y, Ni P, Wu X, et al. TXNL1-XRCC1 pathway regulates cisplatin-induced cell death and contributes to resistance in human gastric cancer. Cell Death Dis. 2014;5:e1055. doi: 10.1038/cddis.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner W, Ciszewski WM, Kania KD. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun Signal. 2015;13:36. doi: 10.1186/s12964-015-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Intensity Modulated Radiation Therapy Collaborative Working G Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51(4):880–914. doi: 10.1016/S0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 21.Milosevic M, Warde P, Menard C, Chung P, Toi A, Ishkanian A, et al. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin Cancer Res. 2012;18(7):2108–2114. doi: 10.1158/1078-0432.CCR-11-2711. [DOI] [PubMed] [Google Scholar]
- 22.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(11):1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 23.Zhao F, Ming J, Zhou Y, Fan L. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother Pharmacol. 2016;77(5):963–972. doi: 10.1007/s00280-016-3007-9. [DOI] [PubMed] [Google Scholar]
- 24.Harada H. Hypoxia-inducible factor 1-mediated characteristic features of cancer cells for tumor radioresistance. J Radiat Res. 2016;57(Suppl 1):i99–i105. doi: 10.1093/jrr/rrw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimura T, Noma N, Sano Y, Ochiai Y, Oikawa T, Fukumoto M, et al. AKT-mediated enhanced aerobic glycolysis causes acquired radioresistance by human tumor cells. Radiother Oncol. 2014;112(2):302–307. doi: 10.1016/j.radonc.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Cao K, Li J, Chen J, Qian L, Wang A, Chen X, et al. microRNA-33a-5p increases radiosensitivity by inhibiting glycolysis in melanoma. Oncotarget. 2017;8(48):83660–83672. doi: 10.18632/oncotarget.19014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan W, Zhong Z, Carney RP, Men Y, Li J, Pan T, et al. Deciphering the metabolic role of AMPK in cancer multi-drug resistance. Semin Cancer Biol. 2018. [DOI] [PubMed]
- 28.Jin L, Chun J, Pan C, Kumar A, Zhang G, Ha Y, et al. The PLAG1-GDH1 Axis promotes Anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung Cancer. Mol Cell. 2018;69(1):87–99 e7. doi: 10.1016/j.molcel.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan M, Gingras MC, Dunlop EA, Nouet Y, Dupuy F, Jalali Z, et al. The tumor suppressor folliculin regulates AMPK-dependent metabolic transformation. J Clin Invest. 2014;124(6):2640–2650. doi: 10.1172/JCI71749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17(1):113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mari A, D'Andrea D, Abufaraj M, Foerster B, Kimura S, Shariat SF. Genetic determinants for chemo- and radiotherapy resistance in bladder cancer. Transl Androl Urol. 2017;6(6):1081–1089. doi: 10.21037/tau.2017.08.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, et al. Extracellular metabolic energetics can promote cancer progression. Cell. 2015;160(3):393–406. doi: 10.1016/j.cell.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Yu H, Huang X, Tan H, Li S, Luo Y, et al. A novel engineered VEGF blocker with an excellent pharmacokinetic profile and robust anti-tumor activity. BMC Cancer. 2015;15:170. doi: 10.1186/s12885-015-1140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung MF, Liu HY, Lin KJ, Chia WT, Sung HW. A pH-responsive carrier system that generates NO bubbles to trigger drug release and reverse P-glycoprotein-mediated multidrug resistance. Angew Chem Int Ed Engl. 2015;54(34):9890–9893. doi: 10.1002/anie.201504444. [DOI] [PubMed] [Google Scholar]
- 35.Vidal RS, Quarti J, Rumjanek FD, Rumjanek VM. Metabolic reprogramming during multidrug resistance in Leukemias. Front Oncol. 2018;8:90. doi: 10.3389/fonc.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paredes F, Sheldon K, Lassegue B, Williams HC, Faidley EA, Benavides GA, et al. Poldip2 is an oxygen-sensitive protein that controls PDH and alphaKGDH lipoylation and activation to support metabolic adaptation in hypoxia and cancer. Proc Natl Acad Sci U S A. 2018;115(8):1789–1794. doi: 10.1073/pnas.1720693115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao F, Xiao C, Evans KS, Theivanthiran T, DeVito N, Holtzhausen A, et al. Paracrine Wnt5a-beta-catenin signaling triggers a metabolic program that drives dendritic cell Tolerization. Immunity. 2018;48(1):147–60 e7. doi: 10.1016/j.immuni.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi Mei, Cai Jing, Li Junjun, Chen Shengnan, Zeng Zhaoyang, Peng Qian, Ban Yuanyuan, Zhou Ying, Li Xiaoling, Xiong Wei, Li Guiyuan, Xiang Bo. Rediscovery of NF-κB signaling in nasopharyngeal carcinoma: How genetic defects of NF-κB pathway interplay with EBV in driving oncogenesis? Journal of Cellular Physiology. 2018;233(8):5537–5549. doi: 10.1002/jcp.26410. [DOI] [PubMed] [Google Scholar]
- 39.Pan Y, Cao M, Liu J, Yang Q, Miao X, Go VLW, et al. Metabolic regulation in mitochondria and drug resistance. Adv Exp Med Biol. 2017;1038:149–171. doi: 10.1007/978-981-10-6674-0_11. [DOI] [PubMed] [Google Scholar]
- 40.Knoechel B, Aster JC. Metabolic mechanisms of drug resistance in leukemia. Cell Metab. 2015;22(5):759–760. doi: 10.1016/j.cmet.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Best SA, De Souza DP, Kersbergen A, Policheni AN, Dayalan S, Tull D, et al. Synergy between the KEAP1/NRF2 and PI3K pathways drives non-small-cell lung Cancer with an altered immune microenvironment. Cell Metab. 2018;27(4):935–43 e4. doi: 10.1016/j.cmet.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Ippolito L, Morandi A, Taddei ML, Parri M, Comito G, Iscaro A, et al. Cancer-associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene. 2019. [DOI] [PubMed]
- 43.Phillips RM, Lam C, Wang H, Tran PT. Bittersweet tumor development and progression: emerging roles of epithelial plasticity glycosylations. Adv Cancer Res. 2019;142:23–62. doi: 10.1016/bs.acr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Yang H, Zhu R, Zhao X, Liu L, Zhou Z, Zhao L, et al. Sirtuin-mediated deacetylation of hnRNP A1 suppresses glycolysis and growth in hepatocellular carcinoma. Oncogene. 2019. [DOI] [PubMed]
- 45.Satoh K, Yachida S, Sugimoto M, Oshima M, Nakagawa T, Akamoto S, et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proc Natl Acad Sci U S A. 2017;114(37):E7697–EE706. doi: 10.1073/pnas.1710366114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida GJ. Emerging roles of Myc in stem cell biology and novel tumor therapies. J Exp Clin Cancer Res. 2018;37(1):173. doi: 10.1186/s13046-018-0835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furukawa T, Tabata S, Yamamoto M, Kawahara K, Shinsato Y, Minami K, et al. Thymidine phosphorylase in cancer aggressiveness and chemoresistance. Pharmacol Res. 2018;132:15–20. doi: 10.1016/j.phrs.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Matheson Christopher J., Casalvieri Kimberly A., Backos Donald S., Reigan Philip. Development of Potent Pyrazolopyrimidinone-Based WEE1 Inhibitors with Limited Single-Agent Cytotoxicity for Cancer Therapy. ChemMedChem. 2018;13(16):1681–1694. doi: 10.1002/cmdc.201800188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 50.Myint K, Li Y, Paxton J, McKeage M. Multidrug resistance-associated protein 2 (MRP2) mediated transport of Oxaliplatin-derived platinum in membrane vesicles. PLoS One. 2015;10(7):e0130727. doi: 10.1371/journal.pone.0130727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isebaert SF, Swinnen JV, McBride WH, Begg AC, Haustermans KM. 5-aminoimidazole-4-carboxamide riboside enhances effect of ionizing radiation in PC3 prostate cancer cells. Int J Radiat Oncol Biol Phys. 2011;81(5):1515–1523. doi: 10.1016/j.ijrobp.2011.06.1964. [DOI] [PubMed] [Google Scholar]
- 52.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2(1):3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar B, Kowluru A, Kowluru RA. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56(5):2985–2992. doi: 10.1167/iovs.15-16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32(10):1893–1907. doi: 10.1128/MCB.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11(6):1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castilho A, Aveleira CA, Leal EC, Simoes NF, Fernandes CR, Meirinhos RI, et al. Heme oxygenase-1 protects retinal endothelial cells against high glucose- and oxidative/nitrosative stress-induced toxicity. PLoS One. 2012;7(8):e42428. doi: 10.1371/journal.pone.0042428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: implications for diabetic retinopathy. Am J Pathol. 2010;177(1):447–455. doi: 10.2353/ajpath.2010.091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trudeau K, Muto T, Roy S. Downregulation of mitochondrial connexin 43 by high glucose triggers mitochondrial shape change and cytochrome C release in retinal endothelial cells. Invest Ophthalmol Vis Sci. 2012;53(10):6675–6681. doi: 10.1167/iovs.12-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi WK, Zhu XD, Wang CH, Zhang YY, Cai H, Li XL, et al. PFKFB3 blockade inhibits hepatocellular carcinoma growth by impairing DNA repair through AKT. Cell Death Dis. 2018;9(4):428. doi: 10.1038/s41419-018-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Efimova EV, Takahashi S, Shamsi NA, Wu D, Labay E, Ulanovskaya OA, et al. Linking Cancer metabolism to DNA repair and accelerated senescence. Mol Cancer Res. 2016;14(2):173–184. doi: 10.1158/1541-7786.MCR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saha Arka, Roy Souvick, Kar Madhabananda, Roy Shomereeta, Thakur Shweta, Padhi SwatiShree, Akhter Yusuf, Banerjee Birendranath. Role of Telomeric TRF2 in Orosphere Formation and CSC Phenotype Maintenance Through Efficient DNA Repair Pathway and its Correlation with Recurrence in OSCC. Stem Cell Reviews and Reports. 2018;14(6):871–887. doi: 10.1007/s12015-018-9823-z. [DOI] [PubMed] [Google Scholar]
- 63.Mehla K, Singh PK. MUC1: a novel metabolic master regulator. Biochim Biophys Acta. 2014;1845(2):126–135. doi: 10.1016/j.bbcan.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gunda V, Souchek J, Abrego J, Shukla SK, Goode GD, Vernucci E, et al. MUC1-mediated metabolic alterations regulate response to radiotherapy in pancreatic Cancer. Clin Cancer Res. 2017;23(19):5881–5891. doi: 10.1158/1078-0432.CCR-17-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohnken R, Kodigepalli KM, Wu L. Regulation of deoxynucleotide metabolism in cancer: novel mechanisms and therapeutic implications. Mol Cancer. 2015;14:176. doi: 10.1186/s12943-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chabes A, Georgieva B, Domkin V, Zhao X, Rothstein R, Thelander L. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell. 2003;112(3):391–401. doi: 10.1016/S0092-8674(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 67.Le TM, Poddar S, Capri JR, Abt ER, Kim W, Wei L, et al. ATR inhibition facilitates targeting of leukemia dependence on convergent nucleotide biosynthetic pathways. Nat Commun. 2017;8(1):241. doi: 10.1038/s41467-017-00221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agarwal S, Milazzo G, Rajapakshe K, Bernardi R, Chen Z, Eveline B, et al. Correction: MYCN acts as a direct co-regulator of p53 in MYCN amplified neuroblastoma. Oncotarget. 2018;9(52):30024. doi: 10.18632/oncotarget.25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agarwal S, Milazzo G, Rajapakshe K, Bernardi R, Chen Z, Barberi E, et al. MYCN acts as a direct co-regulator of p53 in MYCN amplified neuroblastoma. Oncotarget. 2018;9(29):20323–20338. doi: 10.18632/oncotarget.24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan Xueyu, Weng Yuanyuan, Bai Yongfeng, Wang Zongpan, Wang Siwei, Zhu Jin, Zhang Feng. Lipin-1 determines lung cancer cell survival and chemotherapy sensitivity by regulation of endoplasmic reticulum homeostasis and autophagy. Cancer Medicine. 2018;7(6):2541–2554. doi: 10.1002/cam4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X, Yin H, Zhang Y, Li X, Tong H, Zeng Y, et al. Hypoxia-induced autophagy promotes gemcitabine resistance in human bladder cancer cells through hypoxia-inducible factor 1alpha activation. Int J Oncol. 2018;53(1):215–224. doi: 10.3892/ijo.2018.4376. [DOI] [PubMed] [Google Scholar]
- 72.Williams T, Forsberg LJ, Viollet B, Brenman JE. Basal autophagy induction without AMP-activated protein kinase under low glucose conditions. Autophagy. 2009;5(8):1155–1165. doi: 10.4161/auto.5.8.10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8(23):3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 75.Yoshida GJ. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: from pathophysiology to treatment. J Hematol Oncol. 2017;10(1):67. doi: 10.1186/s13045-017-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang WB, Yao M, Cheng N. Priming cancer cells for drug resistance: role of the fibroblast niche. Front Biol (Beijing) 2014;9(2):114–126. doi: 10.1007/s11515-014-1300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, et al. Caveolin-1−/− null mammary stromal fibroblasts share characteristics with human breast cancer-associated fibroblasts. Am J Pathol. 2009;174(3):746–761. doi: 10.2353/ajpath.2009.080658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhai J, Shen J, Xie G, Wu J, He M, Gao L, et al. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37–43. doi: 10.1016/j.canlet.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5(9):726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 80.Levine B. Unraveling the role of autophagy in cancer. Autophagy. 2006;2(2):65–66. doi: 10.4161/auto.2.2.2457. [DOI] [PubMed] [Google Scholar]
- 81.Buchser WJ, Laskow TC, Pavlik PJ, Lin HM, Lotze MT. Cell-mediated autophagy promotes cancer cell survival. Cancer Res. 2012;72(12):2970–2979. doi: 10.1158/0008-5472.CAN-11-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 84.Wojtkowiak JW, Rothberg JM, Kumar V, Schramm KJ, Haller E, Proemsey JB, et al. Chronic autophagy is a cellular adaptation to tumor acidic pH microenvironments. Cancer Res. 2012;72(16):3938–3947. doi: 10.1158/0008-5472.CAN-11-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dash S, Sarashetti PM, Rajashekar B, Chowdhury R, Mukherjee S. TGF-beta2-induced EMT is dampened by inhibition of autophagy and TNF-alpha treatment. Oncotarget. 2018;9(5):6433–6449. doi: 10.18632/oncotarget.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017;25(5):1037–1043. doi: 10.1016/j.cmet.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu S, Feng N, Lin G, Tong Y, Jiang X, Yang Q, et al. Metabolic shift induced by omega −3 PUFAs and rapamycin Lead to Cancer cell death. Cell Physiol Biochem. 2018;48(6):2318–2336. doi: 10.1159/000492648. [DOI] [PubMed] [Google Scholar]
- 88.Meng MB, Wang HH, Guo WH, Wu ZQ, Zeng XL, Zaorsky NG, et al. Targeting pyruvate kinase M2 contributes to radiosensitivity of non-small cell lung cancer cells in vitro and in vivo. Cancer Lett. 2015;356(2 Pt B):985–993. doi: 10.1016/j.canlet.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 89.Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu P, et al. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J Exp Clin Cancer Res. 2019;38(1):71. doi: 10.1186/s13046-019-1093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie JM, Li B, Yu HP, Gao QG, Li W, Wu HR, et al. TIGAR has a dual role in cancer cell survival through regulating apoptosis and autophagy. Cancer Res. 2014;74(18):5127–5138. doi: 10.1158/0008-5472.CAN-13-3517. [DOI] [PubMed] [Google Scholar]
- 91.Alayev A, Berger SM, Kramer MY, Schwartz NS, Holz MK. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J Cell Biochem. 2015;116(3):450–457. doi: 10.1002/jcb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16(7):487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gawriluk TR, Ko C, Hong X, Christenson LK, Rucker EB., 3rd Beclin-1 deficiency in the murine ovary results in the reduction of progesterone production to promote preterm labor. Proc Natl Acad Sci U S A. 2014;111(40):E4194–E4203. doi: 10.1073/pnas.1409323111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katheder NS, Khezri R, O'Farrell F, Schultz SW, Jain A, Rahman MM, et al. Microenvironmental autophagy promotes tumour growth. Nature. 2017;541(7637):417–420. doi: 10.1038/nature20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shin JH, Park CW, Yoon G, Hong SM, Choi KY. NNMT depletion contributes to liver cancer cell survival by enhancing autophagy under nutrient starvation. Oncogenesis. 2018;7(8):58. doi: 10.1038/s41389-018-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim M, Jung JY, Choi S, Lee H, Morales LD, Koh JT, et al. GFRA1 promotes cisplatin-induced chemoresistance in osteosarcoma by inducing autophagy. Autophagy. 2017;13(1):149–168. doi: 10.1080/15548627.2016.1239676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lamoureux F, Thomas C, Crafter C, Kumano M, Zhang F, Davies BR, et al. Blocked autophagy using lysosomotropic agents sensitizes resistant prostate tumor cells to the novel Akt inhibitor AZD5363. Clin Cancer Res. 2013;19(4):833–844. doi: 10.1158/1078-0432.CCR-12-3114. [DOI] [PubMed] [Google Scholar]
- 98.Wang L, Shang Z, Zhou Y, Hu X, Chen Y, Fan Y, et al. Autophagy mediates glucose starvation-induced glioblastoma cell quiescence and chemoresistance through coordinating cell metabolism, cell cycle, and survival. Cell Death Dis. 2018;9(2):213. doi: 10.1038/s41419-017-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Z, Zhou L, Chen Z, Nice EC, Huang C. Stress management by autophagy: implications for chemoresistance. Int J Cancer. 2016;139(1):23–32. doi: 10.1002/ijc.29990. [DOI] [PubMed] [Google Scholar]
- 100.Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Du GS, Qiu Y, Wang WS, Peng K, Zhang ZC, Li XS, et al. Knockdown on aPKC-iota inhibits epithelial-mesenchymal transition, migration and invasion of colorectal cancer cells through Rac1-JNK pathway. Exp Mol Pathol. 2019;107:57–67. doi: 10.1016/j.yexmp.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Zhang X, Zhao H, Li Y, Xia D, Yang L, Ma Y, et al. The role of YAP/TAZ activity in cancer metabolic reprogramming. Mol Cancer. 2018;17(1):134. doi: 10.1186/s12943-018-0882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Das CK, Linder B, Bonn F, Rothweiler F, Dikic I, Michaelis M, et al. BAG3 overexpression and Cytoprotective autophagy mediate apoptosis resistance in Chemoresistant breast Cancer cells. Neoplasia. 2018;20(3):263–279. doi: 10.1016/j.neo.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goldsmith J, Levine B, Debnath J. Autophagy and cancer metabolism. Methods Enzymol. 2014;542:25–57. doi: 10.1016/B978-0-12-416618-9.00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saita S, Shirane M, Nakayama KI. Selective escape of proteins from the mitochondria during mitophagy. Nat Commun. 2013;4:1410. doi: 10.1038/ncomms2400. [DOI] [PubMed] [Google Scholar]
- 106.Bilanges B, Alliouachene S, Pearce W, Morelli D, Szabadkai G, Chung YL, et al. Vps34 PI 3-kinase inactivation enhances insulin sensitivity through reprogramming of mitochondrial metabolism. Nat Commun. 2017;8(1):1804. doi: 10.1038/s41467-017-01969-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Semenza GL. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36(3):252–259. doi: 10.15252/embj.201695204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu L, Lu Y, Martinez J, Bi Y, Lian G, Wang T, et al. Proinflammatory signal suppresses proliferation and shifts macrophage metabolism from Myc-dependent to HIF1alpha-dependent. Proc Natl Acad Sci U S A. 2016;113(6):1564–1569. doi: 10.1073/pnas.1518000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kouidhi S, Ben Ayed F, Benammar Elgaaied A. Targeting tumor metabolism: a new challenge to improve immunotherapy. Front Immunol. 2018;9:353. doi: 10.3389/fimmu.2018.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohamed Eslam, Al-Khami Amir A., Rodriguez Paulo C. The cellular metabolic landscape in the tumor milieu regulates the activity of myeloid infiltrates. Cellular & Molecular Immunology. 2018;15(5):421–427. doi: 10.1038/s41423-018-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang Lan, Xu Huaxi, Peng Guangyong. TLR-mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cellular & Molecular Immunology. 2018;15(5):428–437. doi: 10.1038/cmi.2018.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakashima R, Goto Y, Koyasu S, Kobayashi M, Morinibu A, Yoshimura M, et al. UCHL1-HIF-1 axis-mediated antioxidant property of cancer cells as a therapeutic target for radiosensitization. Sci Rep. 2017;7(1):6879. doi: 10.1038/s41598-017-06605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chambers AM, Lupo KB, Matosevic S. Tumor microenvironment-induced Immunometabolic reprogramming of natural killer cells. Front Immunol. 2018;9:2517. doi: 10.3389/fimmu.2018.02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhatia A, Kumar Y. Cellular and molecular mechanisms in cancer immune escape: a comprehensive review. Expert Rev Clin Immunol. 2014;10(1):41–62. doi: 10.1586/1744666X.2014.865519. [DOI] [PubMed] [Google Scholar]
- 115.Wang Hairui, Tang Yisi, Fang Yuefei, Zhang Meng, Wang Huiyuan, He Zhidi, Wang Bing, Xu Qin, Huang Yongzhuo. Reprogramming Tumor Immune Microenvironment (TIME) and Metabolism via Biomimetic Targeting Codelivery of Shikonin/JQ1. Nano Letters. 2019;19(5):2935–2944. doi: 10.1021/acs.nanolett.9b00021. [DOI] [PubMed] [Google Scholar]
- 116.Fu Y, Liu S, Zeng S, Shen H. The critical roles of activated stellate cells-mediated paracrine signaling, metabolism and onco-immunology in pancreatic ductal adenocarcinoma. Mol Cancer. 2018;17(1):62. doi: 10.1186/s12943-018-0815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Belli C, Trapani D, Viale G, D'Amico P, Duso BA, Della Vigna P, et al. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018;65:22–32. doi: 10.1016/j.ctrv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 118.Sun Yeqi, Wang Ruifen, Qiao Meng, Xu Yanchun, Guan Wenbin, Wang Lifeng. Cancer associated fibroblasts tailored tumor microenvironment of therapy resistance in gastrointestinal cancers. Journal of Cellular Physiology. 2018;233(9):6359–6369. doi: 10.1002/jcp.26433. [DOI] [PubMed] [Google Scholar]
- 119.Braza MS, van Leent MMT, Lameijer M, Sanchez-Gaytan BL, Arts RJW, Perez-Medina C, et al. Inhibiting inflammation with myeloid cell-specific Nanobiologics promotes organ transplant acceptance. Immunity. 2018;49(5):819–28 e6. doi: 10.1016/j.immuni.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, et al. Foxp3 and toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol. 2016;17(12):1459–1466. doi: 10.1038/ni.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 122.Gupta S, Dwarakanath B. Modulation of Immuno-biome during radio-sensitization of tumors by glycolytic inhibitors. Curr Med Chem. 2018. [DOI] [PubMed]
- 123.Verduzco D, Lloyd M, Xu L, Ibrahim-Hashim A, Balagurunathan Y, Gatenby RA, et al. Intermittent hypoxia selects for genotypes and phenotypes that increase survival, invasion, and therapy resistance. PLoS One. 2015;10(3):e0120958. doi: 10.1371/journal.pone.0120958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl) 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang G, Wang JJ, Fu XL, Guang R, To ST Advances in the targeting of HIF-1alpha and future therapeutic strategies for glioblastoma multiforme (Review) Oncol Rep. 2017;37(2):657–670. doi: 10.3892/or.2016.5309. [DOI] [PubMed] [Google Scholar]
- 126.Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. doi: 10.1186/s13046-015-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74(3):665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 129.Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, et al. Association of Patient Characteristics and Tumor Genomics with Clinical Outcomes among Patients with non-Small Cell Lung Cancer Using a Clinicogenomic database. JAMA. 2019;321(14):1391–1399. doi: 10.1001/jama.2019.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Makker Vicky, Rasco Drew, Vogelzang Nicholas J, Brose Marcia S, Cohn Allen L, Mier James, Di Simone Christopher, Hyman David M, Stepan Daniel E, Dutcus Corina E, Schmidt Emmett V, Guo Matthew, Sachdev Pallavi, Shumaker Robert, Aghajanian Carol, Taylor Matthew. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. The Lancet Oncology. 2019;20(5):711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Toffalori C, Zito L, Gambacorta V, Riba M, Oliveira G, Bucci G, et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med. 2019;25(4):603–611. doi: 10.1038/s41591-019-0400-z. [DOI] [PubMed] [Google Scholar]
- 132.Kondo K, Shaim H, Thompson PA, Burger JA, Keating M, Estrov Z, et al. Ibrutinib modulates the immunosuppressive CLL microenvironment through STAT3-mediated suppression of regulatory B-cell function and inhibition of the PD-1/PD-L1 pathway. Leukemia. 2018;32(4):960–970. doi: 10.1038/leu.2017.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.El Arfani C, De Veirman K, Maes K, De Bruyne E, Menu E. Metabolic features of multiple myeloma. Int J Mol Sci. 2018;19(4). [DOI] [PMC free article] [PubMed]
- 134.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wylie, Macri, Mintern, Waithman Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers. 2019;11(4):521. doi: 10.3390/cancers11040521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016;531(7595):513–517. doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu Y, Du H, Wei S, Feng L, Li J, Yao F, et al. Adipocyte-derived Exosomal MiR-27a induces insulin resistance in skeletal muscle through repression of PPARgamma. Theranostics. 2018;8(8):2171–2188. doi: 10.7150/thno.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garcia NA, Ontoria-Oviedo I, Gonzalez-King H, Diez-Juan A, Sepulveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One. 2015;10(9):e0138849. doi: 10.1371/journal.pone.0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chiarugi P, Cirri P. Metabolic exchanges within tumor microenvironment. Cancer Lett. 2016;380(1):272–280. doi: 10.1016/j.canlet.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 141.Hesari A, Golrokh Moghadam SA, Siasi A, Rahmani M, Behboodi N, Rastgar-Moghadam A, et al. Tumor-derived exosomes: potential biomarker or therapeutic target in breast cancer? J Cell Biochem. 2018;119(6):4236–4240. doi: 10.1002/jcb.26364. [DOI] [PubMed] [Google Scholar]
- 142.Wang L, Zhang B, Zheng W, Kang M, Chen Q, Qin W, et al. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci Rep. 2017;7(1):5384. doi: 10.1038/s41598-017-05541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kosaka N, Yoshioka Y, Fujita Y, Ochiya T. Versatile roles of extracellular vesicles in cancer. J Clin Invest. 2016;126(4):1163–1172. doi: 10.1172/JCI81130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sousa D, Lima RT, Vasconcelos MH. Intercellular transfer of Cancer drug resistance traits by extracellular vesicles. Trends Mol Med. 2015;21(10):595–608. doi: 10.1016/j.molmed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 145.Rodrigues CFD, Serrano E, Patricio MI, Val MM, Albuquerque P, Fonseca J, et al. Stroma-derived IL-6, G-CSF and Activin-a mediated dedifferentiation of lung carcinoma cells into cancer stem cells. Sci Rep. 2018;8(1):11573. doi: 10.1038/s41598-018-29947-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Santos JC, Lima NDS, Sarian LO, Matheu A, Ribeiro ML, Derchain SFM. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci Rep. 2018;8(1):829. doi: 10.1038/s41598-018-19339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016;107(1):5–11. doi: 10.1111/cas.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yoshida GJ. Correction to: emerging roles of Myc in stem cell biology and novel tumor therapies. J Exp Clin Cancer Res. 2018;37(1):285. doi: 10.1186/s13046-018-0964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yoshida GJ. The heterogeneity of cancer stem-like cells at the invasive front. Cancer Cell Int. 2017;17:23. doi: 10.1186/s12935-017-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen YA, Wang CY, Hsieh YT, Chen YJ, Wei YH. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle. 2015;14(1):86–98. doi: 10.4161/15384101.2014.974419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang CF, Yang GD, Huang TJ, Li R, Chu QQ, Xu L, et al. EB-virus latent membrane protein 1 potentiates the stemness of nasopharyngeal carcinoma via preferential activation of PI3K/AKT pathway by a positive feedback loop. Oncogene. 2016;35(26):3419–3431. doi: 10.1038/onc.2015.402. [DOI] [PubMed] [Google Scholar]
- 152.Yoshida GJ, Saya H. Inversed relationship between CD44 variant and c-Myc due to oxidative stress-induced canonical Wnt activation. Biochem Biophys Res Commun. 2014;443(2):622–627. doi: 10.1016/j.bbrc.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 153.Lu J, Tang M, Li H, Xu Z, Weng X, Li J, et al. EBV-LMP1 suppresses the DNA damage response through DNA-PK/AMPK signaling to promote radioresistance in nasopharyngeal carcinoma. Cancer Lett. 2016;380(1):191–200. doi: 10.1016/j.canlet.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 154.Polakovicova I, Jerez S, Wichmann IA, Sandoval-Borquez A, Carrasco-Veliz N, Corvalan AH. Role of microRNAs and exosomes in helicobacter pylori and Epstein-Barr virus associated gastric cancers. Front Microbiol. 2018;9:636. doi: 10.3389/fmicb.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Castillo J, Bernard V, San Lucas FA, Allenson K, Capello M, Kim DU, et al. Surfaceome profiling enables isolation of cancer-specific exosomal cargo in liquid biopsies from pancreatic cancer patients. Ann Oncol. 2018;29(1):223–229. doi: 10.1093/annonc/mdx542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Goran Ronquist K. Extracellular vesicles and energy metabolism. Clin Chim Acta. 2019;488:116–121. doi: 10.1016/j.cca.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 158.Heiler S, Wang Z, Zoller M. Pancreatic cancer stem cell markers and exosomes - the incentive push. World J Gastroenterol. 2016;22(26):5971–6007. doi: 10.3748/wjg.v22.i26.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yoshida GJ, Saya H. EpCAM expression in the prostate cancer makes the difference in the response to growth factors. Biochem Biophys Res Commun. 2014;443(1):239–245. doi: 10.1016/j.bbrc.2013.11.093. [DOI] [PubMed] [Google Scholar]
- 160.Zhang H, Wang Z, Zhang Q, Wang F, Liu Y. Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosens Bioelectron. 2019;124-125:184–190. doi: 10.1016/j.bios.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 161.Akhter MZ, Sharawat SK, Kumar V, Kochat V, Equbal Z, Ramakrishnan M, et al. Aggressive serous epithelial ovarian cancer is potentially propagated by EpCAM(+)CD45(+) phenotype. Oncogene. 2018;37(16):2089–2103. doi: 10.1038/s41388-017-0106-y. [DOI] [PubMed] [Google Scholar]
- 162.Chen J, Cao S, Situ B, Zhong J, Hu Y, Li S, et al. Metabolic reprogramming-based characterization of circulating tumor cells in prostate cancer. J Exp Clin Cancer Res. 2018;37(1):127. doi: 10.1186/s13046-018-0789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cianflone E, Torella M, Chimenti C, De Angelis A, Beltrami AP, Urbanek K, et al. Adult cardiac stem cell aging: a reversible stochastic phenomenon? Oxidative Med Cell Longev. 2019;2019:5813147. doi: 10.1155/2019/5813147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li H, Li F. Exosomes from BM-MSCs increase the population of CSCs via transfer of miR-142-3p. Br J Cancer. 2018;119(6):744–755. doi: 10.1038/s41416-018-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Campanella C, Bucchieri F, Merendino AM, Fucarino A, Burgio G, Corona DF, et al. The odyssey of Hsp60 from tumor cells to other destinations includes plasma membrane-associated stages and Golgi and exosomal protein-trafficking modalities. PLoS One. 2012;7(7):e42008. doi: 10.1371/journal.pone.0042008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280(24):23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 168.Paquet-Fifield S, Koh SL, Cheng L, Beyit LM, Shembrey C, Molck C, et al. Tight junction protein Claudin-2 promotes self-renewal of human colorectal Cancer stem-like cells. Cancer Res. 2018;78(11):2925–2938. doi: 10.1158/0008-5472.CAN-17-1869. [DOI] [PubMed] [Google Scholar]
- 169.Sanchez CA, Andahur EI, Valenzuela R, Castellon EA, Fulla JA, Ramos CG, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7(4):3993–4008. doi: 10.18632/oncotarget.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Boominathan L. The guardians of the genome (p53, TA-p73, and TA-p63) are regulators of tumor suppressor miRNAs network. Cancer Metastasis Rev. 2010;29(4):613–639. doi: 10.1007/s10555-010-9257-9. [DOI] [PubMed] [Google Scholar]
- 171.Rajesh Y, Biswas A, Mandal M. Glioma progression through the prism of heat shock protein mediated extracellular matrix remodeling and epithelial to mesenchymal transition. Exp Cell Res. 2017;359(2):299–311. doi: 10.1016/j.yexcr.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 172.Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, et al. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc Natl Acad Sci U S A. 2015;112(19):E2497–E2506. doi: 10.1073/pnas.1412651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nishizawa S, Hirohashi Y, Torigoe T, Takahashi A, Tamura Y, Mori T, et al. HSP DNAJB8 controls tumor-initiating ability in renal cancer stem-like cells. Cancer Res. 2012;72(11):2844–2854. doi: 10.1158/0008-5472.CAN-11-3062. [DOI] [PubMed] [Google Scholar]
- 174.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, et al. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014;281(7):1750–1758. doi: 10.1111/febs.12737. [DOI] [PubMed] [Google Scholar]
- 175.Gui X, Li H, Li T, Pu H, Lu D. Long noncoding RNA CUDR regulates HULC and beta-catenin to govern human liver stem cell malignant differentiation. Mol Ther. 2015;23(12):1843–1853. doi: 10.1038/mt.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Andronesi OC, Arrillaga-Romany IC, Ly KI, Bogner W, Ratai EM, Reitz K, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun. 2018;9(1):1474. doi: 10.1038/s41467-018-03905-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Han J, Goldstein LA, Hou W, Chatterjee S, Burns TF, Rabinowich H. HSP90 inhibition targets autophagy and induces a CASP9-dependent resistance mechanism in NSCLC. Autophagy. 2018;14(6):958-71. [DOI] [PMC free article] [PubMed]
- 178.Stack JP, Moslehi J, Sayed N, Wu JC. Cancer therapy-induced cardiomyopathy: can human induced pluripotent stem cell modelling help prevent it? Eur Heart J. 2018. [DOI] [PMC free article] [PubMed]
- 179.Vila IK, Yao Y, Kim G, Xia W, Kim H, Kim SJ, et al. A UBE2O-AMPKalpha2 Axis that promotes tumor initiation and progression offers opportunities for therapy. Cancer Cell. 2017;31(2):208–224. doi: 10.1016/j.ccell.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E, et al. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic Cancer. Cancer Cell. 2017;32(1):71–87 e7. doi: 10.1016/j.ccell.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70(2):348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 182.Garcia-Bermudez J, Baudrier L, La K, Zhu XG, Fidelin J, Sviderskiy VO, et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat Cell Biol. 2018;20(7):775–781. doi: 10.1038/s41556-018-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang Y, Zhu G, Dong B, Piao J, Chen L, Lin Z. The NQO1/PKLR axis promotes lymph node metastasis and breast cancer progression by modulating glycolytic reprogramming. Cancer Lett. 2019;453:170–183. doi: 10.1016/j.canlet.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 184.Fan Tengjiao, Sun Guohui, Sun Xiaodong, Zhao Lijiao, Zhong Rugang, Peng Yongzhen. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers. 2019;11(3):317. doi: 10.3390/cancers11030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jones AT, Narov K, Yang J, Sampson JR, Shen MH. Efficacy of dual inhibition of glycolysis and Glutaminolysis for therapy of renal lesions in Tsc2(+/−) mice. Neoplasia. 2019;21(2):230–238. doi: 10.1016/j.neo.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun W, Xie Z, Liu Y, Zhao D, Wu Z, Zhang D, et al. JX06 selectively inhibits pyruvate dehydrogenase kinase PDK1 by a covalent cysteine modification. Cancer Res. 2015;75(22):4923–4936. doi: 10.1158/0008-5472.CAN-15-1023. [DOI] [PubMed] [Google Scholar]
- 187.He Y, Chen X, Yu Y, Li J, Hu Q, Xue C, et al. LDHA is a direct target of miR-30d-5p and contributes to aggressive progression of gallbladder carcinoma. Mol Carcinog. 2018;57(6):772–783. doi: 10.1002/mc.22799. [DOI] [PubMed] [Google Scholar]
- 188.Pisarsky L, Bill R, Fagiani E, Dimeloe S, Goosen RW, Hagmann J, et al. Targeting metabolic Symbiosis to overcome resistance to anti-angiogenic therapy. Cell Rep. 2016;15(6):1161–1174. doi: 10.1016/j.celrep.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.