Abstract
Background
Eukaryotic Initiation Factor 4E-Binding Protein (EIF4EBP1, 4EBP1) is overexpressed in many human cancers including breast cancer, yet the role of 4EBP1 in breast cancer remains understudied. Despite the known role of 4EBP1 as a negative regulator of cap-dependent protein translation, 4EBP1 is predicted to be an essential driving oncogene in many cancer cell lines in vitro, and can act as a driver of cancer cell proliferation. EIF4EBP1 is located within the 8p11-p12 genomic locus, which is frequently amplified in breast cancer and is known to predict poor prognosis and resistance to endocrine therapy.
Methods
Here we evaluated the effect of 4EBP1 targeting using shRNA knock-down of expression of 4EBP1, as well as response to the mTORC targeted drug everolimus in cell lines representing different breast cancer subtypes, including breast cancer cells with the 8p11-p12 amplicon, to better define a context and mechanism for oncogenic 4EBP1.
Results
Using a genome-scale shRNA screen on the SUM panel of breast cancer cell lines, we found 4EBP1 to be a strong hit in the 8p11 amplified SUM-44 cells, which have amplification and overexpression of 4EBP1. We then found that knock-down of 4EBP1 resulted in dramatic reductions in cell proliferation in 8p11 amplified breast cancer cells as well as in other luminal breast cancer cell lines, but had little or no effect on the proliferation of immortalized but non-tumorigenic human mammary epithelial cells. Kaplan-Meier analysis of EIF4EBP1 expression in breast cancer patients demonstrated that overexpression of this gene was associated with reduced relapse free patient survival across all breast tumor subtypes.
Conclusions
These results are consistent with an oncogenic role of 4EBP1 in luminal breast cancer and suggests a role for this protein in cell proliferation distinct from its more well-known role as a regulator of cap-dependent translation.
Electronic supplementary material
The online version of this article (10.1186/s12885-019-5667-4) contains supplementary material, which is available to authorized users.
Keywords: EIF4EBP1, 4EBP1, 4E-BP1, PHAS-I, 8p11–12, 8p12–11, 8p11-p12, Chromosomal abnormality, Oncogene, Amplification, Driver, Breast cancer, Estrogen receptor
Background
Estrogen Receptor-positive (ER+) breast cancer accounts for ~ 70% of all breast cancers. Currently, this subtype of breast cancer is treated with endocrine therapy as the standard of care. However, resistance to endocrine therapy is a significant clinical problem and is a leading cause of breast cancer mortality. Amplification of the 8p11-p12 region of the human genome, which occurs in ~ 20–30% of metastatic ER+ breast cancers, is associated with resistance to endocrine therapy and poor prognosis [1].
Our laboratory and others have demonstrated the importance of the 8p11-p12 amplicon and many of its genes in the development and pathogenesis of breast cancer [2–33], including its role in endocrine resistance. The amplicon is composed of four distinct regions, designated A1-A4, each of which contains a number of overexpressed genes [5, 11]. At least 11 genes are associated with the A2 region of the amplicon [5]. The Eukaryotic Initiation Factor 4E-Binding Protein (EIF4EBP1) sequence is located on the short arm of chromosome 8: 38,030,502–38,060,365 (GRCh38.p7; current assembly) and is amplified along with other A1 and A2 region genes. The protein product (herein referred to as 4EBP1) encoded by EIF4EBP1 is canonically regarded as a translational repressor protein that interacts with eukaryotic initiation factor 4E (eIF4E) and represses translation by inhibiting eIF4E from recruiting 40S ribosomal subunits during translation [34–36]. Upon phosphorylation, 4EBP1 dissociates from eIF4E allowing for active cap-dependent translation [37–40].
Interestingly, many human cancers [41, 42], and particularly breast cancers with the 8p11-p12 amplicon overexpress 4EBP1 [43] [44]. Since 4EBP1 inhibits translation, it is expected that overexpression of 4EBP1 would act as a tumor suppressor. However, overexpression of 4EBP1 results in high levels of phosphorylated 4EBP1 which may contribute to breast cancer development [43, 45] [44–47]. Indeed, proteins that can regulate 4EBP1 phosphorylation, like Casein kinase 1ε [48, 49], Glycogen synthase kinase (GSK)-3β [50], G1 To S phase transition 2 (eRF3b) [51, 52], Mammalian target of rapamycin complex 1 (mTORC1) [39, 40, 53–60], Polo like kinase 1 (PLK1) [61–63], Family with sequence similarity 129 member A (Niban) [64], PI3-kinase isoforms [65, 66], Cyclin-dependent kinase 1 (CDK1) [59, 67–70], ATM serine/threonine kinase (ATM) [71, 72], Mitogen activated protein kinase (MAPK) [73, 74], Protein kinase B (AKT) [75], and others [68, 74, 76] have been suggested as therapeutic targets for cancer. Given the relationship between expression of 4EBP1 in the 8p11-p12 amplicon and hyperactivation of mTORC1 observed in endocrine resistant breast cancers, PI3K/AKT/mTORC1 targeted therapies have been suggested for 4EBP1 expressing breast cancers [46, 77–81]. Furthermore, genes within the amplicon as well as mTORC1, which phosphorylates 4EBP1, have been shown to activate ER, potentially contributing to the ability of amplicon bearing breast cancer cells to circumvent endocrine therapy.
Consequently, we set out to evaluate the effect of 4EBP1 targeting in ER+, 8p11-p12 expressing breast cancer cells as well as other breast cancer cell lines, and non-tumorigenic but immortalized human mammary epithelial cells. We first found that 4EBP1 is an essential gene in the SUM-44 cells based on results of a genome-scale shRNA screen, and then found that 4EBP1 targeting reduced proliferation of not only amplicon bearing cells (SUM-44, Cama-1, SUM-52) but also non-amplicon ER+ breast cancer cells as well (MCF7, T47D). This effect was also seen in ER-negative (ER-) 8p11-p12 cells (SUM-52) as well as non-amplicon bearing cells (SUM-229, SUM-149), but to a lesser extent. There was no effect of 4EBP1 targeting on the proliferation of immortalized but non-tumorigenic mammary epithelial cells (MCF10A, H16N2). Consistent with our findings, Kaplan-Meier analysis shows that high levels of 4EBP1 correlates with worsened prognosis in ER+ cohorts (ER+, ER+ Luminal A, and ER+ Luminal B) as well as cohorts that received chemotherapy, tamoxifen, or endocrine therapy. Taken together, our findings suggest that 4EBP1 plays an important role in in breast cancer and may be particularly important in breast cancers with the 8p11-p12 amplicon regardless of ER status.
Methods
Antibodies and inhibitors
The mTOR inhibitor, Everolimus (RAD001), was purchased from Selleckchem (S1120, A112024). The antibodies against 4EBP1 (9644), phospho-4EBP1 Ser65 (9451), phospho-4EBP1 Thr37/46 (2855), phospho-4EBP1 Thr70 (9455) were purchased from Cell Signaling. Antibody against β-actin (A5441) was purchased from Sigma-Aldrich. The CyclinD1 (2978) and p27 Kip1 (3686) antibodies were purchased from Cell Signaling. The ERα antibody (sc-543) was purchased from Santa Cruz Biotechnology.
Cell culture
The SUM-44 (ER+), Cama-1 (ER+), and SUM-52 (ER-) cell lines represent luminal breast cancer and have the 8p11-p12 genomic locus amplified. T47D (ER+), HCC1500 (ER+), and MCF7 (ER+) cells are also luminal but 8p11-p12 is not amplified. SUM-229 and SUM-149 are triple-negative breast cancer cell lines. Normal breast epithelial are represented by immortalized but non-tumorigenic MCF10A and H16N2 cell lines. All cell lines were maintained at 37 °C with 10% CO2. SUM cell lines and culture requirements for maintenance with Hams F12 cell culture medium (Hyclone SH30026FS, Thermo Fisher Scientific) with supplementation have been previously described [82–84] (please refer to the SLKBase (https://sumlineknowledgebase.com/) for additional information about these cell lines). The Cama-1 cell line (obtained from ATCC) and MCF7 cell line (obtained from the Michigan Cancer Foundation) were grown in Dulbecco’s Modified Eagle’s (DMEM) medium (obtained from Thermo Fisher Scientific) containing 10% Fetal Bovine Serum (FBS) purchased from Gemini Bioproducts (900–108) or Atlanta Biologicals (S11050). The T47D cell line (obtained from ATCC) was grown in Roswell Park Memorial Institute (RPMI) medium (Thermo Fisher Scientific) containing 10% FBS. The MCF10A cells were obtained from Dr. Herb Soule at the Michigan Cancer Foundation [85] and were maintained in serum-free Hams F12 supplemented with Bovine serum albumin (BSA) (126,579, Millipore), 5 μg/mL Insulin (700-112P, Gemini Bioproducts), 1 μg/mL Hydrocortisone (H-4001, Sigma-Aldrich), and 10 ng/mL Epidermal Growth Factor (E9644, Sigma-Aldrich) (SFIHE medium). H16N2 cells [86, 87] (immortalized by human papillomavirus (HPV) E6 and E7 oncoproteins) were also grown in SFIHE medium. When trypsinizing cells grown in serum-free medium, 2% FBS was added for the first 24 h. The SUM cell lines were developed in the author’s laboratory and are routinely validated for identity by STR profiling. The remaining cell lines were obtained from ATCC and were used immediately upon arrival. All cell lines are routinely tested for mycoplasma.
Generation of EIF4EBP1 knockdown cells
Lentivirus was produced in 293FT cells which were transfected in Opti-MEM with Lipofectamine 2000, pLKO.1-puro gene-targeting plasmid, and Mission packaging mix (Sigma-Aldrich) under optimal conditions. Collected virus was filtered through a 0.2 um filter before storage at − 80 °C. Efficient viral titer production was confirmed by a Lenti-X p24 Rapid Titer Kit (Takara) and 4EBP1 western blot. All BSL-2 safety protocols were performed during production, storage, and continued use. Optimization was performed with listed (Table 1) 4EBP1-targeting plasmids wherein TRCN0000040206 (4EBP sh_1) and TRCN0000298904 (4EBP_sh_2) produced efficient knockdown and were used for subsequent studies. These were obtained from the shRNA Technology Shared Resource (Hollings Cancer Center, the Medical University of South Carolina).
Table 1.
ᅟ.
| Plasmid | Genotype | Region | Sequence |
|---|---|---|---|
| shLACZ | pLKO.1-puro::LACZ | n/a | CGCTAAATACTGGCAGGCGTT |
| Sh4EBP1 #1 |
pLKO.1-puro::EIF4EBP1 TRCN0000040206 |
CDS |
CCGGCGGTGAAGAGTCACAGT TTGACTCGAGTCAAACTGTGA CTCTTCACCGTTTTTG |
| Sh4EBP1 #2 |
pLKO.1-puro::EIF4EBP1 TRCN0000040203 |
3UTR |
CCGGGCCAGGCCTTATGAAA GTGATCTCGAGATCACTTTC ATAAGGCCTGGCTTTTTG |
| Sh4EBP1 #3 |
pLKO.1-puro::EIF4EBP1 TRCN0000310343 |
3UTR |
CCGGGCCAGGCCTTATGAAA GTGATCTCGAGATCACTTTC ATAAGGCCTGGCTTTTTG |
| Sh4EBP1 #4 |
pLKO.1-puro::EIF4EBP1 TRCN0000298904 |
CDS |
CCGGCGGTGAAGAGTCACAG TTTGACTCGAGTCAAACTGT GACTCTTCACCGTTTTTG |
Cells were reverse transfected with lentivirus, with appropriate growth medium, and polybrene. Virus was removed 24 h later and cells were fed with media. Cells began selection with appropriate concentration of antibiotics 48 h following transfection. Antibiotic concentration at 2 μg/ml Puromycin (invivoGen) was sufficient to ensure selection. The SUM-44 cell line requires 3 μg/ml Puromycin selection. Control cells without the addition of lentivirus were plated alongside lentivirus infected cells to ensure the appropriate concentration of antibiotic was used. Cells were continuously maintained in the resistance marker. All further parameters were tested after four days of selection in Puromycin.
Cell proliferation
Cells were plated in 12-well plates at [1E5 cells/well], washed with 1X Phosphate Buffered Saline (PBS), then 0.5 mL HEPES/MgCl2 buffer (Isoton) was added to each dish and agitated for 5 min. Cell swelling was confirmed and 50 uL ZAP (Bretol Solution) was added and incubated for 10 min with agitation. Cells were visualized to confirm bursting and nuclei release, 10 mL NaCl-Formalin Solution was added to prevent deterioration, and read using a Coulter Acuvette. The Coulter Counter was set to count nuclei between 4 and 8 um diameter through a 100 um aperture. Each sample was counted twice and then averaged. The counts were multiplied by 20 to obtain the total number of nuclei, and background counts with NaCl-Formalin were performed with analysis.
Statistical analysis
Growth results were analyzed using a two-way ANOVA model with an interaction effect between day and condition. Each cell line was analyzed individually and all analyses were done on the log scale. Differences between conditions were exponentiated to obtain fold change estimates. Significance testing was completed using Tukey’s honestly significant difference method to maintain a family-wise alpha of 0.05 within each cell line.
Immunoblotting
Cells were continuously maintained on ice and harvested using Radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich) supplemented with a protease inhibitor cocktail (Millipore, #539131) and PhosSTOP (Roche). Bradford Protein Assay was used to fit samples to a standard curve and determine protein concentrations prior to SDS-PAGE. After transfer, PVDF membrane was blocked 1 h with 5% skim milk in 1X TBST at room temperature and incubated overnight with antibody per the manufacturer’s instructions. The membrane was visualized with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). The membrane was developed using the Li-COR Odyssey Fc.
Flow cytometry of live cells
Cells were trypsinized, counted, and analyzed at [1E6 cells/mL]. Vybrant DyeCycle Orange (V35005, Thermo Scientific) was used according to the manufacturer’s protocol for live cell-cycle analysis. Conditions were optimized to a final stain concentration of 5 μM in 1X PBS in all cell lines tested. Cells were promptly analyzed using a BSL2 FACSAria Cell Sorter. Verity ModFit LT 4.1 was used to analyze and visualize the generated data.
KM plotter database analysis
The KM plotter for breast cancer (http://kmplot.com) [88] was used on all releases available from the database accessed spring 2018. Restricted analyses of different populations are indicated and altered the number of breast cancer patients with available survival data as shown by the number at risk. The determined and represented prognostic values by relapse free survival (RFS) of EIF4EBP1 in all analyses were more than 500 samples, indicating highly reliable analysis using all parameters presented. The JetSet best probe set for EIF4EBP1 (probe ID: 221539_at) was used for all analyses. Patients were divided into a high and low expression group by median mRNA expression values, all possible cutoff values between the lower and upper quartiles were computed and the best performing threshold was determined by using auto select the best cutoff. RFS was plotted using suggested quality controls. This excluded biased arrays, removed redundant samples, and checked proportional hazards assumptions. The cutoff values, probe expression range, false discovery rate (FDR), and p-value were extracted from the KM plotter webpage and each analysis is represented.
cBioPortal database analysis
The cBioPortal(http://www.cbioportal.org/) [89, 90] was used to generate the overall survival curve (shown in Fig. 1b) for breast tumors with and without A2 8p11-p12 region alterations using The Cancer Genome Atlas (TCGA) provisional data. The Amplification frequency of 4EBP1 in the TCGA or the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) breast data cohorts were also determined using the cBioPortal. Data was accessed spring 2018.
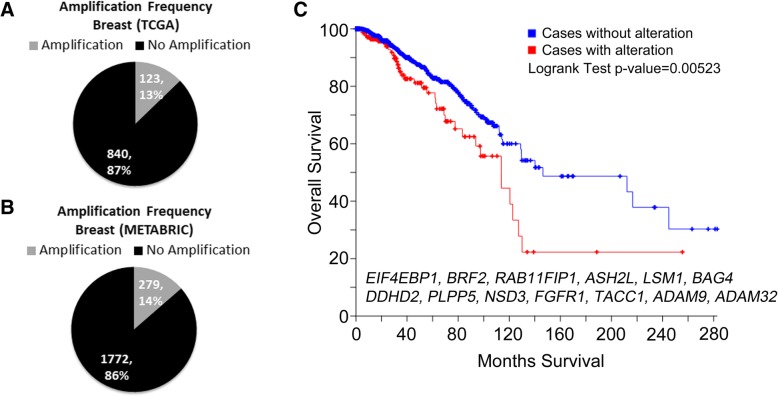
Fig. 1.
EIF4EBP1 is amplified in human breast cancer and correlates with reduced overall survival. (a) Amplification data from The Cancer Genome Atlas (TCGA) and (b) the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC). (c) Expression analysis using TCGA provisional data shows that high expression of A2 genes: BRF2, RAB11FIP1, EIF4EBP1, ASH2L, LSM1, BAG4, DDHD2, PLPP5, NSD3, FGFR1, TACC1, ADAM9, and ADAM32 correlates with reduced overall survival
Results
Frequency and prognostic significance of 4EBP1 amplification in breast cancer
EIF4EBP1, the gene that encodes the 4EBP1 protein, resides within the 8p11-p12 genomic locus. It is frequently amplified in endocrine resistant luminal breast cancers, rarely coincides with PIK3CA mutations, and is associated with poor prognosis. The frequency of EIF4EBP1 amplification across all breast cancer subtypes is approximately 13% according to data from The Cancer Genome Atlas (TCGA) and 14% according to data from the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) [91] (Fig. 1a & b). Furthermore, we found that expression analysis of the TCGA provisional data shows that high expression of the genes in the A2 region of the 8p11-p12 amplicon, which includes EIF4EBP1, BRF2, RAB11FIP1, ASH2L, LSM1, BAG4, DDHD2, PLPP5, NSD3, FGFR1, TACC1, ADAM9, and ADAM32, correlates with reduced overall survival (Fig. 1c).
4EBP1 is highly expressed and phosphorylated in 8p11-p12 breast cancer cells
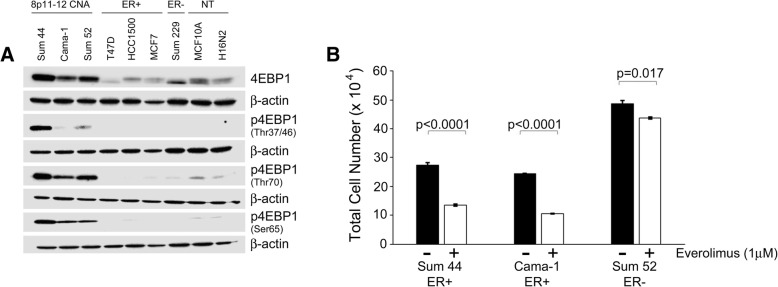
To investigate the significance of 4EBP1 overexpression in breast cancer, we employed a set of human breast cancer cell lines representing ER+ and ER- samples, including SUM-44, SUM-52, and Cama-1 (ER+, amplicon bearing), MCF-7, T47D, and HCC1500 (ER+, non-amplicon bearing), and SUM-229, as well as two non-tumorigenic but immortalized mammary epithelial cell lines, MCF10A, and H16N2 cells. As expected, SUM-44, Cama-1, and SUM-52 expressed high levels of 4EBP1 due to the amplification of the EIF4EBP1 gene, whereas MCF10A and H16N2 did not express any more or less 4EBP1 protein than the T47D, HCC1500, MCF7, or SUM-229 cell lines (Fig. 2a). High levels of phosphorylated 4EBP1 were also readily detected in the SUM-44, Cama-1 and SUM-52 cells compared to the other cell lines tested (Fig. 2a).
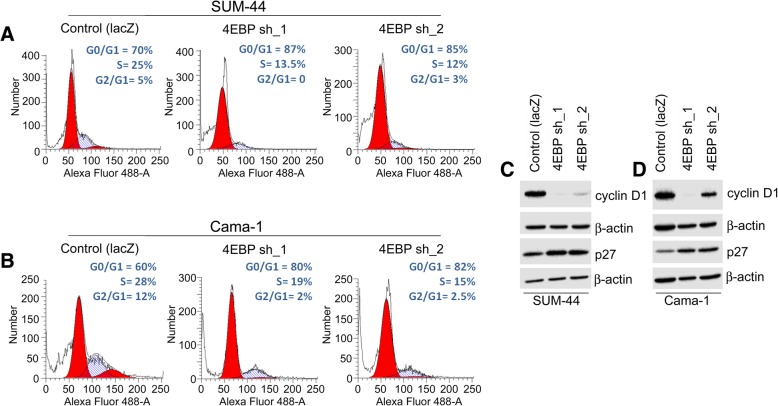
Fig. 2.
4EBP1 is highly expressed and phosphorylated in 8p11-p12 breast cancer cells. (a) Western blot of 4EBP1 and phospho-4EBP1 on residues Thr 37/37, Thr 70, and Ser 65 in SUM-44 (ER+), Cama-1 (ER+), and SUM-52 (ER-) cells with amplification of the 8p11-p12 genomic locus (8p11-12 CNA) as well as T47D (ER+), HCC1500 (ER+), MCF7 (ER+), and SUM-229 (ER-) cells without amplification of the 8p11-p12 genomic locus. Immortalized but non-tumorigenic breast epithelial cells are represented by MCF10A and H16N2 cells. (b) Cell proliferation was assessed in SUM-44, Cama-1, and SUM-52 cells in the presence or absence of 1 μM everolimus treatment for 72 h. Error bars represent standard deviation among replicates and p values are for the difference in cell proliferation in control versus treated cells. The p-value for the difference between the effect in SUM-52 cells and the other cell lines is <0.0001
4EBP1 is thought to be phosphorylated by mTORC1 in a hierarchical fashion [39, 40]. Our findings that 4EBP1 expression and phosphorylation levels are high on multiple residues in SUM-44, Cama-1, and SUM-52 cells, as well as our observation of high levels of phospho-S6 (not shown) suggest active mTORC1 signaling in these 8p11-p12 models. Therefore, we tested the effect of mTOR pathway inhibition on cell proliferation of the 8p11-p12 cell lines. Cells were plated in equal number and on day 1 after plating, cells were exposed to 1 uM of the inhibitor everolimus (Affinitor). To assess proliferation, the total number of cells was quantitated for each group at day 1, prior to treatment, and on day 4, 72 h after exposure to everolimus. Treatment with everolimus significantly reduced the proliferation of all three cell lines, however the fold-change observed for the SUM-44 cells and the Cama-1 cells (0.49 and 0.43 respectively) were significantly greater than the fold change observed in the SUM-52 cells (0.9). The difference in response to everolimus between the SUM-52 cells and the other two cell lines was significant with a p-value of < 0.0001. (Fig. 2b). This result suggests that ER expression plays a role in the responsiveness of breast cancer cells to everolimus.
4EBP1 is essential to breast cancer cell lines
Our laboratory recently completed a genomic scale shRNA screen for the entire panel of SUM breast cancer cell lines, and some of the results from these screens have been reported elsewhere [92] and can be found at The SUM Breast Cancer Cell Line Knowledge Base (SLKBase) (https://sumlineknowledgebase.com/) [93]. Interestingly, despite the fact that the SUM-44 cells have been shown to overexpress several genes from the 8p11-p12 amplicon that can behave as transforming oncogenes in vitro, EIF4EBP1 was the strongest hit among all 8p11 amplified genes in this RNA interference screen. The DepMap [111, 112, 113] crispr (Avana) gene essentiality screens also predict 4EBP1 as a driver of cancer cell lines including all of the breast cancer cell line models currently represented within the portal (https://depmap.org/portal/) [94] Therefore, we performed experiments to validate the importance of 4EBP1 knockdown in SUM-44 cells and extended that to other breast cancer cell lines. To gain a broader understanding of 4EBP1 in different settings, we performed experiments to assess the effect of 4EBP1 knock-down on proliferation of cell lines that represent different subtypes of breast cancer.
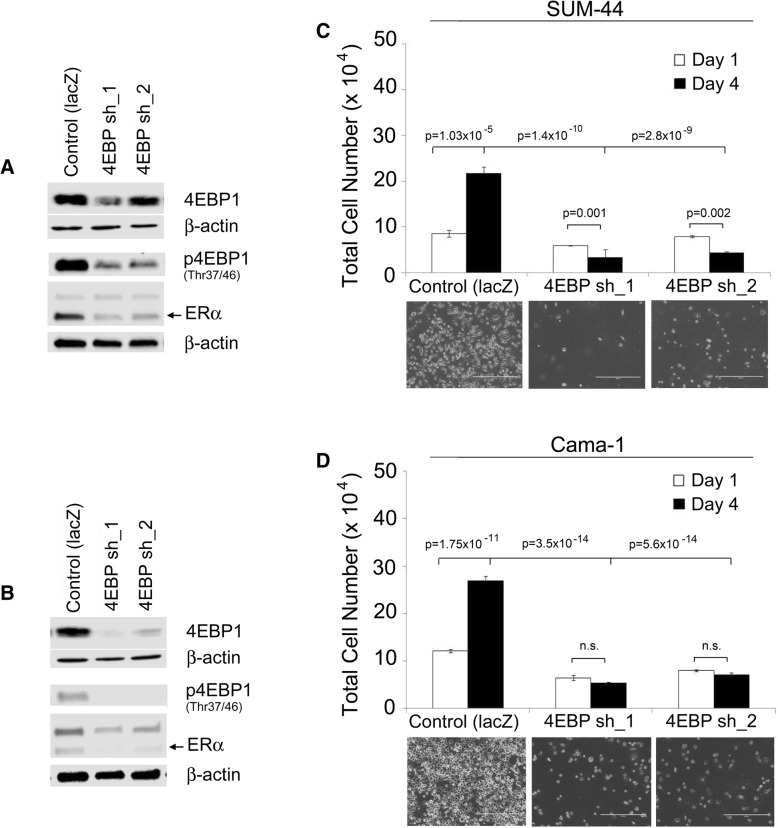
To determine the effect of directly targeting 4EBP1 in breast cancer cells, we first tested the two ER+ 8p11-p12 cell lines, SUM-44 and Cama-1, and used lentiviral vectors for two different shRNAs against EIF4EBP1. shRNA targeting lacZ was used as a control. Fig. 3 shows that both shRNAs were effective at reducing levels of 4EBP1 protein, and there was a concomitant decrease in the levels of phosphorylated 4EBP1 (Fig. 3 a & b). We then measured proliferation of cells expressing EIF4EBP1 shRNA compared to control cells. Cells were evaluated by counting the number of nuclei at day 1 and day 4 after plating. The data shown in Fig. 3 c and d show that there as a significant increase in cell number in the LacZ control cells over the 4-day culture period, there was little or no proliferation in the sh4EBP1 groups in either cell line. Indeed, there was a significant reduction in cell number over the 4 day period in the SUM-44 cells (fold change = 0.5, p < 0.001, 0.002), whereas in the Cama-1 cells, there was a smaller (approximately 0.8 fold) but still significant difference in cell number over the same period (p < 0.002, and 0.07). The largest and most statistically significant difference was detected in the day 4 cell counts between control LacZ cells and the sh4EBP1 cells in both cell lines, with fold-differences of approximately 4 and 6-fold, and p-values ranging from 10− 9 to 10–14 The full ANOVA analysis of the data for all groups and all time points are shown in Additional file 3: Table S1.
Fig. 3.
4EBP1 knockdown inhibits proliferation of ER+ 8p11-p12 breast cancer cells. (a) Western blot of 4EBP1, phospho-4EBP1 on residues Thr 37/46, and ERα in SUM-44 cells engineered with either control shRNA to lacZ or two individual shRNAs to EIF4EBP1 (4EBP sh_1 or sh_2). (b) Western blot of 4EBP1, phospho-4EBP1 on residues Thr 37/46, and ERα in Cama-1 cells engineered with either control shRNA to lacZ or two individual shRNAs to EIF4EBP1 (4EBP sh_1 or sh_2). (c) Cell proliferation was assessed in SUM-44 and (d) Cama-1 control and EIF4EBP1 knockdown cells on day 1 and day 4 in culture following selection in puromycin containing media. Error bars represent standard deviation among replicates and p-values represent the statistical comparison between each corresponding group
Prior studies from our lab and others have demonstrated the effects of genes associated with the 8p11-p12 amplicon on ERα expression [1, 28–31, 100]. Therefore, we next evaluated ERα expression in the SUM-44 and Cama-1 EIF4EBP1 knockdown cell lines and found that ERα levels were reduced (Fig. 3 a & b) compared to control cells expressing lacZ shRNA. These findings show that reducing 4EBP1 levels impairs proliferation of the ER+ 8p11-p12 breast cancer cell models and results in downregulation of ERα.
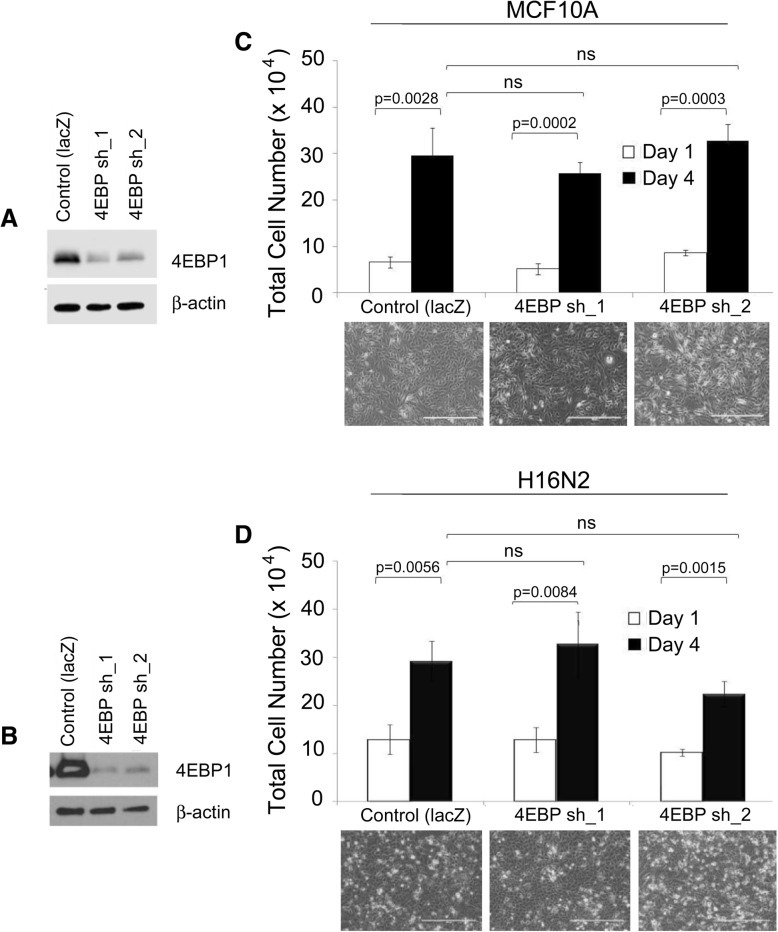
We next wanted to evaluate the potential effects of 4EBP1 targeting in non-tumorigenic human breast epithelial cells. 4EBP1 was knocked down in MCF10A (Fig. 4a) and H16N2 cells (Fig. 4b). Cell proliferation was then measured by counting the total number of cell nuclei present at day 1 and day 4 after plating. All populations increased in number significantly over four days and no statistically significant differences were observed between control and EIF4EBP1 knockdown in MCF10A cells (Fig. 4c) or H16N2 cells (Fig. 4d). These results indicate that downregulation of 4EBP1 in non-tumorigenic breast epithelial cell lines, at least to the same levels as was achieved in the breast cancer cell lines does not affect the proliferative capacity of these cells.
Fig. 4.
4EBP1 knockdown does not affect proliferation of MCF10A and H16N2 non-transformed breast epithelial cells. (a) Western blot of 4EBP1 in MCF10A cells and (b) H16N2 cells engineered with either control shRNA to lacZ or two individual shRNAs to EIF4EBP1 (4EBP sh_1 or sh_2). (c) Cell proliferation was assessed in MCF10A and (d) H16N2 control and EIF4EBP1 knockdown cells on day 1 and day 4 in culture following selection in puromycin-containing medium. Error bars represent standard deviation among replicates and p-values represent significance between each corresponding group
Downregulation of 4EBP1 in ER+ 8p11-p12 breast cancer cells causes cell cycle arrest
Previous studies suggest that 4EBP1 regulates cell cycle progression [59, 61, 68, 101–104]. To better understand the cellular effects of 4EBP1 knockdown, SUM-44 and Cama-1 cells were assessed by flow cytometry to evaluate cell cycle progression. An increase in the number of cells in G1 cell-cycle in both SUM-44 (Fig. 5a) and Cama-1 cells (Fig. 5 b) was observed with EIF4EBP1 knockdown when compared to control cells. These results show that knockdown of 4EBP1 promotes G1 cell cycle arrest.
Fig. 5.
4EBP1 knockdown leads to G0/G1 arrest in ER+ 8p11-p12 breast cancer cells. (a) Cell cycle analysis of SUM-44 and (b) Cama-1 cells shows that 4EBP1 knockdown results in an accumulation of cells in G0/G1 with an associated decrease in cells in S-phase. (c) Western blot of cyclin D1 and p27 in SUM-44 and (d) Cama-1 cells engineered with either control shRNA to lacZ or two individual shRNAs to EIF4EBP1 (4EBP sh_1 or sh_2)
To study the cell cycle arrest induced by 4EBP1 knock-down further, we evaluated the protein expression levels of key cell cycle regulators. We found that Cyclin D1 protein levels were decreased in SUM-44 and Cama-1 cells following EIF4EBP1 knockdown (Fig. 5c & d). Additionally, we observed a slight increase in p27 levels in the EIF4EBP1 knockdown cells compared to control cells (Fig. 5c & d). The alterations of Cyclin D1 and p27 expression that we found are consistent with the cell cycle arrest phenotype that we observed in 4EBP1 knockdown cells.
4EBP1 knockdown inhibits proliferation of ER- 8p11-p12 amplified breast cancer cells
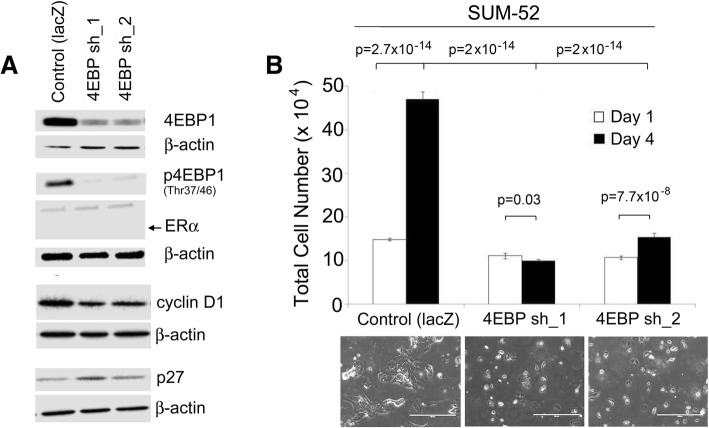
Because we saw only a small effect of everolimus on the proliferation of the ER- 8p11-p12 SUM-52 breast cancer cell line, we also wanted to test the effect of EIF4EBP1 knockdown on these cells. Using the same two shRNAs targeted to EIF4EBP1 as we used on the previous cell lines, we knocked down 4EBP1 mRNA in the SUM-52 cells and likewise, saw a reduction in 4EBP1 protein levels (Fig. 6a). EIF4EBP1 knockdown in SUM-52 cells resulted in a dramatic reduction in proliferation of SUM-52 cells, similar to what we observed with the two ER+ cell lines. In LacZ control cells, there was a highly significant increase in cell number between days 1 and 4, whereas in the sh4EBP1 cells, there was a slight reduction in cell number in the sh1 group and a slight increase in cell number is the sh2 group. These differences most likely reflect different levels of knockdown achieved with the two vectors. Of greatest importance is the three to four-fold difference in the number of cells per dish at the 4 day time point between the shLacZ and sh4EBP1 groups again with p-values on the order of 10− 14. (Fig. 6b). We also probed these control and knockdown cells for Cyclin D1 and p27 expression. We saw a similar effect on these two proteins as in the SUM-44 and Cama-1 cells where Cyclin D1 levels were decreased and p27 levels were increased (Fig. 6a). We also evaluated the effect of 4EBP1 knockdown on the non-amplicon bearing models, MCF7 (ER+) (Additional file 1: Figure S1 a), T47D (ER+) (Additional file 1: Figure S1, b), SUM-229 (ER-) (Additional file 2: Figure S2 a), and SUM-149 (ER-) (Additional file 2: Figure S2 b). These experiments showed that knockdown of 4EBP1 in MCF7 and T47D also significantly inhibited proliferation (Additional file 1: Figure S1 c & d). By contrast, 4EBP1 knock-down in the triple negative SUM-149 and SUM-229 cells was less effective at reducing proliferation of these cells (Additional file 2: Figure S2 c & d).
Fig. 6.
4EBP1 knockdown inhibits proliferation of ER- 8p11-p12 breast cancer cells. (a) Western blot of 4EBP1, phospho-4EBP1 on residues Thr 37/46, ERα, cyclin D1, and p27 in SUM-52 cells engineered with either control shRNA to lacZ or two individual shRNAs to EIF4EBP1 (4EBP sh_1 or sh_2). (b) Cell proliferation was assessed in SUM-52 control and EIF4EBP1 knockdown cells on day 1 and day 4 in culture after selection in puromycin-containing medium. Error bars represent standard deviation among replicates and significance is the comparison between each corresponding group
EIF4EBP1 expression levels correlate with reduced relapse free survival in human breast cancer
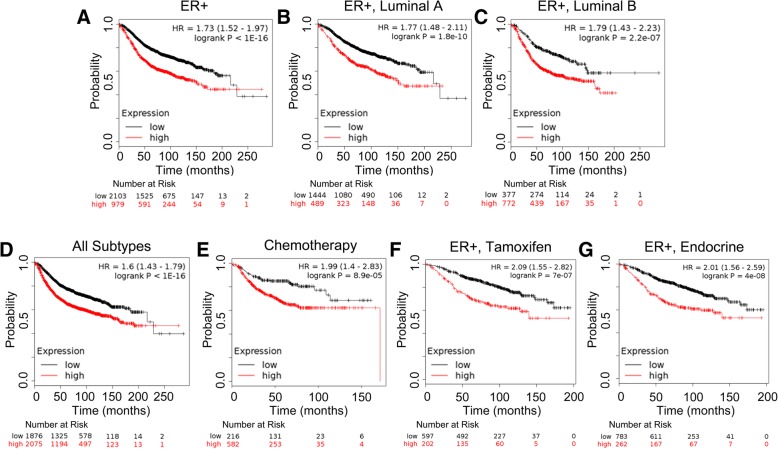
To determine the overall impact of EIF4EBP1 on survival and to assess whether treatment affects the outcomes, we used the online Kaplan-Meier plotter database tool (kmplot.com) to assess the relationship between EIF4EBP1 gene expression and relapse free survival. This tool uses gene expression data from Gene Expression Omnibus (GEO), the European Genome-phenome Archive (EGA), and The Cancer Genome Atlas (TCGA) [88].The JetSet probe set for EIF4EBP1 (probe ID: 221539_at) was used for all analyses. We found that high EIF4EBP1 gene expression significantly correlated with reduced relapse free survival not only in ER+ populations (Fig. 7a), including when separated by luminal A (Fig. 7b) and luminal B (Fig. 7c) subtypes, but also across all subtypes (Fig. 7d). Furthermore, this was also true post treatment with chemotherapy (Fig. 7e) and following either tamoxifen (Fig. 7f) or other endocrine therapy (Fig. 7g). Altogether, these analyses point to a role of 4EBP1 overexpression in breast cancer development and response to therapy.
Fig. 7.
Kaplan-Meier analysis of breast cancer outcomes in patients with and without overexpression of 4EBP1. KM plotter analysis of EIF4EBP1 (probe ID: 221539_at) gene expression and overall survival in (a) ER+ populations (b) separated by luminal A (c) luminal B subtypes (d) all subtypes (no parameters selected) (e) post treatment with chemotherapy (f) tamoxifen or (g) endocrine therapy
Discussion
We and others have determined that a number of oncogenes reside within the 8p11-p12 region and are amplified in human breast cancer. Genes found within this region such as WHSC1L1 [11], DDHD2 [11], LSM1 [10, 11, 18], BAG4 [10, 11], and KAT6A [16, 28] have all been shown to have transforming properties in vitro. Of significance, the 8p11-p12 amplicon is implicated in endocrine resistance [1]. Consistent with this implication, NSD3 (aka WHSC1L1) was shown to drive high levels of ER expression, and to enhance proliferation in an estrogen independent manner [29]. Reminiscent of this finding, hyperactive mTOR is often observed in endocrine resistant cells and can activate ERα [95–99]. Interestingly, the EIF4EBP1 gene which encodes the mTOR effector protein 4EBP1 is located on the short arm of chromosome 8 within the 8p11 region of the amplicon. It is highly overexpressed but rarely mutated in breast cancer, regardless of amplification, and has been suggested to be an essential driving gene in many cancer cell lines in vitro which we [93]and others have witnessed [94]using genome-wide gene essentiality screens. Consequently, our study initially aimed to determine whether 4EBP1 overexpression influences proliferation in ER+ 8p11-p12 amplicon positive breast cancer cells. Our findings show that 4EBP1 is a critical protein for luminal breast cancer cell proliferation regardless of amplicon and/or ER status. However, shRNA mediated knockdown of 4EBP1 in non-transformed mammary epithelial cells did not affect proliferation. It is possible that complete knock-out of 4EBP1 in non-tumorigenic breast epithelial cells could affect their proliferative capacity, but our results indicate that the changes in 4EBP1 expression in luminal breast cancer cells achieved by shRNA knockdown is sufficient to profoundly affect their proliferative capacity. Consistent with the idea that 4EBP1 has a potential role in regulating ERα expression, as well as a potential role outside of ERα regulation, we found that downregulation of 4EBP1 reduces not only ERα expression but also affects Cyclin D1 expression and p27 expression. These observations are consistent with the reduced proliferation and cell cycle arrest phenotypes that we report in our present study. There is no indication that Cyclin D1 or p27 levels would change in non-transformed cells because cell proliferation was not compromised with 4EBP1 knockdown in these models. Future studies should further explore the relationship between 4EBP1 and Cyclin D1 in cancer cells and non-transformed cells. There is a consistently demonstrated occurrence between co-amplification of genomic loci harboring 4EBP1 (EIF4EBP1) and Cyclin D1 (CCND1) in breast cancer patients such as the recent report by Giltnane and colleagues [27], so further studies should assess how these two oncogenes together can influence cell cycle states, meiotic progression, and the regulation of aneuploidy. Because 4EBP1 is required for coupling mTORC1 signaling to Cyclin D1 expression [101] and translational inhibition can result in the loss of cell cycle regulators like the D-cyclins [105], we plan to determine the predictive value of 4EBP1 levels to CDK inhibition in breast tumors, especially in the context of dual inhibition with PI3K/AKT/mTOR inhibitors.
Amplification of EIF4EBP1 leads to increased 4EBP1 expression and phosphorylation suggesting that mechanisms are in place to promote 4EBP1 mediated translation and post-translational regulation during breast cancer initiation and progression. Consequently, targeting of 4EBP1 either directly or via inhibition of mTOR could relieve repressive effects of phosphorylated 4EBP1 on translation as well as any capacity of 4EBP1 to stabilize mTORC1 [106] or other proteins like p21 [107]. Several Phase II clinical trials have evaluated use of mTOR inhibitors for ER+ breast cancer [108–110]. While promising, results from trials in patients with ER+ breast cancer who experience aromatase inhibitor failure were only somewhat successful [108]. However, a current clinical trial is underway to determine if the phosphorylation status of 4EBP1 can be used to predict everolimus response in breast tumors (NCT00855114).
Direct targeting of 4EBP1 or targeting of multiple upstream kinases that target 4EBP1 may provide additional benefit. Recently, several kinases were identified to phosphorylate 4EBP1 in both mTOR dependent as well as independent manners [41, 42]. Of note, GSK3β phosphorylation of 4EBP1 plays a similar role as mTOR, whereby phosphorylation decreases 4EBP1 association with eIF4E [50]. Contrary to this observation, CDK1 is a mitotic kinase that also phosphorylates 4EBP1 [67, 70]. However, phosphorylation by CDK1 does not alter the cap-dependent translation functions of 4EBP1. Interestingly, a phospho-deficient mutant of 4EBP1 that is resistant to phosphorylation by CDK1 partially reverses rodent cell transformation. It is suggested that 4EBP1 phosphorylation by CDK1 could result in a gain of function, which opposes the canonical form of regulation set forth by studies evaluating mTOR-mediated inhibition of 4EBP1 through phosphorylation. Regulation of phosphorylated 4EBP1 especially the intertwined dynamics between CDK1 and mTOR should be further explored, as CDK1 can phosphorylate mTOR and co-localize with phosphorylated 4EBP1 [59]. Whether the distinct effects of the different phosphorylation states of 4EBP1, determined by distinct phosphorylation events driven by individual kinases, affects 4EBP1’s ability to drive breast cancer progression or endocrine resistance would be of significant interest for future studies particular in the context of therapeutic interventions.
Conclusions
EIF4EBP1 is a candidate oncogene in breast cancer because it is commonly amplified and overexpressed, and is part of a genomic region that, when amplified, confers poor prognosis for patients. Overexpression of 4EBP1 drives proliferation of luminal breast cancer cells by mechanisms involving cell cycle regulators such as cyclin D1 and the cdk inhibitor p27. In some cells, 4EBP1 phosphorylation occurs with high level activity of the mTORC pathway, which also is common in estrogen-receptor positive breast cancer, and indeed, knockdown of EIF4EBP1 results in reduced expression of ERα. Based on these results, we conclude that 4EBP1, and particularly phosphorylated 4EBP1 plays a dominant role in breast cancer by mechanisms distinct from its role in regulating cap-dependent translation.
Additional files
Figure S1 4EBP1 knockdown inhibits proliferation of MCF7 and T47D breast cancer cells. (a) Western blot of 4EBP1 in MCF7 cells and (b) T47D cells engineered with either control shRNA to lacZ or two individual shRNAs to EIF4EBP1 (4EBP sh_1 or sh_2). (c) Cell proliferation was assessed in MCF7 and (d) T47D control and EIF4EBP1 knockdown cells on day 1 and day 4 in culture. Error bars represent standard deviation among replicates and p-values represent the comparison between each corresponding group. (TIF 2538 kb)
Figure S2 4EBP1 knockdown slows proliferation of SUM-229 and SUM-149 breast cancer cells. (a) Western blot of 4EBP1 in SUM-229 cells and (b) SUM-149 cells engineered with either control shRNA to lacZ or two individual shRNAs to EIF4EBP1 (4EBP sh_1 or sh_2). (c) Cell proliferation was assessed in SUM-229 and (d) SUM-149 control and EIF4EBP1 knockdown cells on day 1 and day 4 in culture. Error bars represent standard deviation among replicates and signficance is shown between each corresponding group. (TIF 2688 kb)
GrowthResults_2–15-19.xlsx Results and statistical analysis of experiments in which EIF4EBP1 was knocked down in three breast cancer cell lines. (XLSX 16 kb)
Acknowledgements
The authors’ gratefully acknowledge Mr. Zack Kratche who worked on the shRNA screening, to the Genomics Sequencing laboratory and Dr. Bob Wilson and Ms. Jennifer Schulte for sequence analysis of the shRNA screens, and the Flow Cytometry Core of the Hollings Cancer Center for performing cell cycle analysis.
Funding
This work was supported by grant number CA100724 from the National Cancer Institute which specifically funded this project as well as the first author, and by funds from the Allicia Spaulding-Paolozzi endowment for Breast Cancer Research to SPE which supported the final phases of the work and the writing of the manuscript. This research was also supported in part by the shRNA Technology Shared Resource, the Biostatistics Shared Resource, and the Cell Evaluation & Therapy Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313).
Availability of data and materials
Data relevant to the SUM lines, including shRNA screening data as well as gene expression data, and other information relevant to these cell lines are freely available at our web site, The SUM Breast Cancer Cell Line Knowledge Base (SLKBase) www.sumlineknowledgebase.com
Abbreviations
- 4EBP1
Eukaryotic Initiation Factor 4E-Binding Protein
- EIF4EBP1
Eukaryotic Initiation Factor 4E-Binding Protein
- ER
Estrogen receptor alpha
- RFS
Relapse Free Survival
Authors’ contributions
Experiments reported in this paper were carried out by ACR, with the exception of the shRNA screening experiments which were carried out by STG. ESY, RCM-H, and VJF played critical roles in experimental design and strategy. KA performed all of the statistical analyses. SPE oversaw the entire project and is the Ph.D. mentor of ACR. All authors have read and approve of this manuscript.
Ethics approval and consent to participate
NA
Consent for publication
NA
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexandria C. Rutkovsky, Email: rutkovsk@musc.edu
Elizabeth S. Yeh, Email: yeh@musc.edu
Stephen T. Guest, Email: stguest@umich.edu
Victoria J. Findlay, Email: findlay@musc.edu
Robin C. Muise-Helmericks, Email: musehelm@musc.edu
Kent Armeson, Email: armeson@musc.edu.
Stephen P. Ethier, Email: ethier@musc.edu
References
- 1.Turner N, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70:2085–2094. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van 't Veer LJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 3.Courjal F, et al. Mapping of DNA amplifications at 15 chromosomal localizations in 1875 breast tumors: definition of phenotypic groups. Cancer Res. 1997;57:4360–4367. [PubMed] [Google Scholar]
- 4.Adelaide J, et al. Chromosome region 8p11-p21: refined mapping and molecular alterations in breast cancer. Genes Chromosomes Cancer. 1998;22:186–199. doi: 10.1002/(SICI)1098-2264(199807)22:3<186::AID-GCC4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Gelsi-Boyer V, et al. Comprehensive profiling of 8p11-12 amplification in breast cancer. Mole Cancer Res. 2005;3:655–667. doi: 10.1158/1541-7786.mcr-05-0128. [DOI] [PubMed] [Google Scholar]
- 6.Yang ZQ, Albertson D, Ethier SP. Genomic organization of the 8p11-p12 amplicon in three breast cancer cell lines. Cancer Genet Cytogenet. 2004;155:57–62. doi: 10.1016/j.cancergencyto.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Kwek SS, et al. Co-amplified genes at 8p12 and 11q13 in breast tumors cooperate with two major pathways in oncogenesis. Oncogene. 2009;28:1892–1903. doi: 10.1038/onc.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard-Pierrot I, et al. Characterization of the recurrent 8p11-12 amplicon identifies PPAPDC1B, a phosphatase protein, as a new therapeutic target in breast cancer. Cancer Res. 2008;68:7165–7175. doi: 10.1158/0008-5472.CAN-08-1360. [DOI] [PubMed] [Google Scholar]
- 9.Holland DG, et al. ZNF703 is a common luminal B breast cancer oncogene that differentially regulates luminal and basal progenitors in human mammary epithelium. EMBO Mol Med. 2011;3:167–180. doi: 10.1002/emmm.201100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang ZQ, Streicher KL, Ray ME, Abrams J, Ethier SP. Multiple interacting oncogenes on the 8p11-p12 amplicon in human breast cancer. Cancer Res. 2006;66:11632–11643. doi: 10.1158/0008-5472.can-06-2946. [DOI] [PubMed] [Google Scholar]
- 11.Yang ZQ, Liu G, Bollig-Fischer A, Giroux CN, Ethier SP. Transforming properties of 8p11-12 amplified genes in human breast cancer. Cancer Res. 2010;70:8487–8497. doi: 10.1158/0008-5472.can-10-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazarov AV, Yaswen P. Who is in the driver's seat in 8p12 amplifications? ZNF703 in luminal B breast tumors. Breast Cancer Res. 2011;13:308. doi: 10.1186/bcr2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sircoulomb F, et al. ZNF703 gene amplification at 8p12 specifies luminal B breast cancer. EMBO Mol Med. 2011;3:153–166. doi: 10.1002/emmm.201100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, et al. RCP is a human breast cancer-promoting gene with Ras-activating function. J Clin Invest. 2009;119:2171–2183. doi: 10.1172/JCI37622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray ME, et al. Genomic and expression analysis of the 8p11-12 amplicon in human breast cancer cell lines. Cancer Res. 2004;64:40–47. doi: 10.1158/0008-5472.CAN-03-1022. [DOI] [PubMed] [Google Scholar]
- 16.Turner-Ivey B, et al. KAT6A, a chromatin modifier from the 8p11-p12 amplicon is a candidate oncogene in luminal breast cancer. Neoplasia (New York, NY) 2014;16:644–655. doi: 10.1016/j.neo.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reis-Filho JS, et al. FGFR1 emerges as a potential therapeutic target for lobular breast carcinomas. Clin Cancer Res. 2006;12:6652–6662. doi: 10.1158/1078-0432.ccr-06-1164. [DOI] [PubMed] [Google Scholar]
- 18.Streicher KL, Yang ZQ, Draghici S, Ethier SP. Transforming function of the LSM1 oncogene in human breast cancers with the 8p11-12 amplicon. Oncogene. 2007;26:2104–2114. doi: 10.1038/sj.onc.1210002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia MJ, et al. A 1 Mb minimal amplicon at 8p11-12 in breast cancer identifies new candidate oncogenes. Oncogene. 2005;24:5235–5245. doi: 10.1038/sj.onc.1208741. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, et al. ERLIN2 promotes breast cancer cell survival by modulating endoplasmic reticulum stress pathways. BMC Cancer. 2012;12:225. doi: 10.1186/1471-2407-12-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmood SF, et al. PPAPDC1B and WHSC1L1 are common drivers of the 8p11-12 amplicon, not only in breast tumors but also in pancreatic adenocarcinomas and lung tumors. Am J Pathol. 2013;183:1634–1644. doi: 10.1016/j.ajpath.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Bilal E, et al. Amplified loci on chromosomes 8 and 17 predict early relapse in ER-positive breast cancers. PLoS One. 2012;7:e38575. doi: 10.1371/journal.pone.0038575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Kuraya K, et al. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res. 2004;64:8534–8540. doi: 10.1158/0008-5472.can-04-1945. [DOI] [PubMed] [Google Scholar]
- 24.Dib A, et al. Characterization of the region of the short arm of chromosome 8 amplified in breast carcinoma. Oncogene. 1995;10:995–1001. [PubMed] [Google Scholar]
- 25.Theillet C, et al. FGFRI and PLAT genes and DNA amplification at 8p12 in breast and ovarian cancers. Genes Chromosomes Cancer. 1993;7:219–226. doi: 10.1002/gcc.2870070407. [DOI] [PubMed] [Google Scholar]
- 26.Ugolini F, et al. Differential expression assay of chromosome arm 8p genes identifies frizzled-related (FRP1/FRZB) and fibroblast growth factor receptor 1 (FGFR1) as candidate breast cancer genes. Oncogene. 1999;18:1903–1910. doi: 10.1038/sj.onc.1202739. [DOI] [PubMed] [Google Scholar]
- 27.Giltnane Jennifer M., Hutchinson Katherine E., Stricker Thomas P., Formisano Luigi, Young Christian D., Estrada Monica V., Nixon Mellissa J., Du Liping, Sanchez Violeta, Ericsson Paula Gonzalez, Kuba Maria G., Sanders Melinda E., Mu Xinmeng J., Van Allen Eliezer M., Wagle Nikhil, Mayer Ingrid A., Abramson Vandana, Gόmez Henry, Rizzo Monica, Toy Weiyi, Chandarlapaty Sarat, Mayer Erica L., Christiansen Jason, Murphy Danielle, Fitzgerald Kerry, Wang Kai, Ross Jeffrey S., Miller Vincent A., Stephens Phillip J., Yelensky Roman, Garraway Levi, Shyr Yu, Meszoely Ingrid, Balko Justin M., Arteaga Carlos L. Genomic profiling of ER+ breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Science Translational Medicine. 2017;9(402):eaai7993. doi: 10.1126/scitranslmed.aai7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L, et al. Identification of MYST3 as a novel epigenetic activator of ERalpha frequently amplified in breast cancer. Oncogene. 2017;36:2910–2918. doi: 10.1038/onc.2016.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irish JC, et al. Amplification of WHSC1L1 regulates expression and estrogen-independent activation of ERalpha in SUM-44 breast cancer cells and is associated with ERalpha over-expression in breast cancer. Mol Oncol. 2016;10:850–865. doi: 10.1016/j.molonc.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner-Ivey B, et al. Development of mammary hyperplasia, dysplasia, and invasive ductal carcinoma in transgenic mice expressing the 8p11 amplicon oncogene NSD3. Breast Cancer Res Treat. 2017;164:349–358. doi: 10.1007/s10549-017-4258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Formisano L, et al. Association of FGFR1 with ERalpha maintains ligand-independent ER transcription and mediates resistance to estrogen deprivation in ER(+) breast Cancer. Clin Cancer Res. 2017;23:6138–6150. doi: 10.1158/1078-0432.CCR-17-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin K, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Parris TZ, et al. Genome-wide multi-omics profiling of the 8p11-p12 amplicon in breast carcinoma. Oncotarget. 2018;9:24140–24154. doi: 10.18632/oncotarget.25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modrak-Wojcik A, et al. Eukaryotic translation initiation is controlled by cooperativity effects within ternary complexes of 4E-BP1, eIF4E, and the mRNA 5′ cap. FEBS Lett. 2013;587:3928–3934. doi: 10.1016/j.febslet.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 35.Feigenblum D, Schneider RJ. Cap-binding protein (eukaryotic initiation factor 4E) and 4E-inactivating protein BP-1 independently regulate cap-dependent translation. Mol Cell Biol. 1996;16:5450–5457. doi: 10.1128/MCB.16.10.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol. 1995;15:4990–4997. doi: 10.1128/MCB.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elia A, Constantinou C, Clemens MJ. Effects of protein phosphorylation on ubiquitination and stability of the translational inhibitor protein 4E-BP1. Oncogene. 2008;27:811–822. doi: 10.1038/sj.onc.1210678. [DOI] [PubMed] [Google Scholar]
- 38.Beugnet A, Wang X, Proud CG. Target of rapamycin (TOR)-signaling and RAIP motifs play distinct roles in the mammalian TOR-dependent phosphorylation of initiation factor 4E-binding protein 1. J Biol Chem. 2003;278:40717–40722. doi: 10.1074/jbc.M308573200. [DOI] [PubMed] [Google Scholar]
- 39.Gingras AC, et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gingras AC, et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musa J, et al. Eukaryotic initiation factor 4E-binding protein 1 (4E-BP1): a master regulator of mRNA translation involved in tumorigenesis. Oncogene. 2016;35:4675–4688. doi: 10.1038/onc.2015.515. [DOI] [PubMed] [Google Scholar]
- 42.Qin X, Jiang B, Zhang Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle. 2016;15:781–786. doi: 10.1080/15384101.2016.1151581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karlsson E, et al. The mTOR effectors 4EBP1 and S6K2 are frequently coexpressed, and associated with a poor prognosis and endocrine resistance in breast cancer: a retrospective study including patients from the randomised Stockholm tamoxifen trials. Breast Cancer Res. 2013;15:R96. doi: 10.1186/bcr3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braunstein S, et al. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell. 2007;28:501–512. doi: 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Rojo F, et al. 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res. 2007;13:81–89. doi: 10.1158/1078-0432.CCR-06-1560. [DOI] [PubMed] [Google Scholar]
- 46.Armengol G, et al. 4E-binding protein 1: a key molecular "funnel factor" in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 47.Loibl S, et al. Integrated analysis of PTEN and p4EBP1 protein expression as predictors for pCR in HER2-positive breast Cancer. Clin Cancer Res. 2016;22:2675–2683. doi: 10.1158/1078-0432.CCR-15-0965. [DOI] [PubMed] [Google Scholar]
- 48.Shin S, Wolgamott L, Roux PP, Yoon SO. Casein kinase 1epsilon promotes cell proliferation by regulating mRNA translation. Cancer Res. 2014;74:201–211. doi: 10.1158/0008-5472.can-13-1175. [DOI] [PubMed] [Google Scholar]
- 49.Deng C, et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kdelta and CK1epsilon in hematological malignancies. Blood. 2017;129:88–99. doi: 10.1182/blood-2016-08-731240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin S, et al. Glycogen synthase kinase-3beta positively regulates protein synthesis and cell proliferation through the regulation of translation initiation factor 4E-binding protein 1. Oncogene. 2014;33:1690–1699. doi: 10.1038/onc.2013.113. [DOI] [PubMed] [Google Scholar]
- 51.Li M, et al. eRF3b, a biomarker for hepatocellular carcinoma, influences cell cycle and phosphoralation status of 4E-BP1. PloS One. 2014;9:e86371. doi: 10.1371/journal.pone.0086371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chauvin C, Salhi S, Jean-Jean O. Human eukaryotic release factor 3a depletion causes cell cycle arrest at G1 phase through inhibition of the mTOR pathway. Mol Cell Biol. 2007;27:5619–5629. doi: 10.1128/MCB.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morita M, et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013;18:698–711. doi: 10.1016/j.cmet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Coffman K, et al. Characterization of the raptor/4E-BP1 interaction by chemical cross-linking coupled with mass spectrometry analysis. J Biol Chem. 2014;289:4723–4734. doi: 10.1074/jbc.M113.482067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG. Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol. 2005;25:2558–2572. doi: 10.1128/MCB.25.7.2558-2572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nojima H, et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278:15461–15464. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- 57.Heesom KJ, Denton RM. Dissociation of the eukaryotic initiation factor-4E/4E-BP1 complex involves phosphorylation of 4E-BP1 by an mTOR-associated kinase. FEBS Lett. 1999;457:489–493. doi: 10.1016/S0014-5793(99)01094-7. [DOI] [PubMed] [Google Scholar]
- 58.Brunn GJ, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 59.Jansova D, et al. Regulation of 4E-BP1 activity in the mammalian oocyte. Cell Cycle. 2017;16:927–939. doi: 10.1080/15384101.2017.1295178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dennis MD, Kimball SR, Jefferson LS. Mechanistic target of rapamycin complex 1 (mTORC1)-mediated phosphorylation is governed by competition between substrates for interaction with raptor. J Biol Chem. 2013;288:10–19. doi: 10.1074/jbc.M112.402461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shang ZF, et al. 4E-BP1 participates in maintaining spindle integrity and genomic stability via interacting with PLK1. Cell Cycle. 2012;11:3463–3471. doi: 10.4161/cc.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Renner AG, et al. A functional link between polo-like kinase 1 and the mammalian target-of-rapamycin pathway? Cell Cycle. 2010;9:1690–1696. doi: 10.4161/cc.9.9.11295. [DOI] [PubMed] [Google Scholar]
- 63.Severance AL, Latham KE. Meeting the meiotic challenge: specializations in mammalian oocyte spindle formation. Mol Reprod Dev. 2018;85:178–187. doi: 10.1002/mrd.22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun GD, et al. The endoplasmic reticulum stress-inducible protein Niban regulates eIF2alpha and S6K1/4E-BP1 phosphorylation. Biochem Biophys Res Commun. 2007;360:181–187. doi: 10.1016/j.bbrc.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 65.Foukas LC, Shepherd PR. eIF4E binding protein 1 and H-Ras are novel substrates for the protein kinase activity of class-I phosphoinositide 3-kinase. Biochem Biophys Res Commun. 2004;319:541–549. doi: 10.1016/j.bbrc.2004.04.191. [DOI] [PubMed] [Google Scholar]
- 66.O'Brien C, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res. 2010;16:3670–3683. doi: 10.1158/1078-0432.CCR-09-2828. [DOI] [PubMed] [Google Scholar]
- 67.Shuda M, et al. CDK1 substitutes for mTOR kinase to activate mitotic cap-dependent protein translation. Proc Natl Acad Sci U S A. 2015;112:5875–5882. doi: 10.1073/pnas.1505787112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heesom KJ, Gampel A, Mellor H, Denton RM. Cell cycle-dependent phosphorylation of the translational repressor eIF-4E binding protein-1 (4E-BP1) Curr Biol. 2001;11:1374–1379. doi: 10.1016/S0960-9822(01)00422-5. [DOI] [PubMed] [Google Scholar]
- 69.Greenberg VL, Zimmer SG. Paclitaxel induces the phosphorylation of the eukaryotic translation initiation factor 4E-binding protein 1 through a Cdk1-dependent mechanism. Oncogene. 2005;24:4851–4860. doi: 10.1038/sj.onc.1208624. [DOI] [PubMed] [Google Scholar]
- 70.Velasquez C, et al. Mitotic protein kinase CDK1 phosphorylation of mRNA translation regulator 4E-BP1 Ser83 may contribute to cell transformation. Proc Natl Acad Sci U S A. 2016;113:8466–8471. doi: 10.1073/pnas.1607768113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–898. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 72.Kim ST, Lim DS, Canman CE, Kastan MB. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem. 1999;274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- 73.Haystead TA, Haystead CM, Hu C, Lin TA, Lawrence JC., Jr Phosphorylation of PHAS-I by mitogen-activated protein (MAP) kinase. Identification of a site phosphorylated by MAP kinase in vitro and in response to insulin in rat adipocytes. J Biol Chem. 1994;269:23185–23191. [PubMed] [Google Scholar]
- 74.Liu G, Zhang Y, Bode AM, Ma WY, Dong Z. Phosphorylation of 4E-BP1 is mediated by the p38/MSK1 pathway in response to UVB irradiation. J Biol Chem. 2002;277:8810–8816. doi: 10.1074/jbc.M110477200. [DOI] [PubMed] [Google Scholar]
- 75.Dufner A, Andjelkovic M, Burgering BM, Hemmings BA, Thomas G. Protein kinase B localization and activation differentially affect S6 kinase 1 activity and eukaryotic translation initiation factor 4E-binding protein 1 phosphorylation. Mol Cell Biol. 1999;19:4525–4534. doi: 10.1128/MCB.19.6.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pons B, et al. Association between LRRK2 and 4E-BP1 protein levels in normal and malignant cells. Oncol Rep. 2012;27:225–231. doi: 10.3892/or.2011.1462. [DOI] [PubMed] [Google Scholar]
- 77.Hu Y, Zhu Q, Tang L. MiR-99a antitumor activity in human breast cancer cells through targeting of mTOR expression. PLoS One. 2014;9:e92099. doi: 10.1371/journal.pone.0092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pola C, Formenti SC, Schneider RJ. Vitronectin-alphavbeta3 integrin engagement directs hypoxia-resistant mTOR activity and sustained protein synthesis linked to invasion by breast cancer cells. Cancer Res. 2013;73:4571–4578. doi: 10.1158/0008-5472.can-13-0218. [DOI] [PubMed] [Google Scholar]
- 79.Muranen T, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21:227–239. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coleman LJ, et al. Combined analysis of eIF4E and 4E-binding protein expression predicts breast cancer survival and estimates eIF4E activity. Br J Cancer. 2009;100:1393–1399. doi: 10.1038/sj.bjc.6605044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi Ae-Ran, Kim Ju-Hwa, Yoon Sungpil. Sensitization of Cancer Cells through Reduction of Total Akt and Downregulation of Salinomycin-Induced pAkt, pGSk3β, pTSC2, and p4EBP1 by Cotreatment with MK-2206. BioMed Research International. 2014;2014:1–8. doi: 10.1155/2014/295760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ethier SP, Mahacek ML, Gullick WJ, Frank TS, Weber BL. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. Cancer Res. 1993;53:627–635. [PubMed] [Google Scholar]
- 83.Ethier SP. Human breast cancer cell lines as models of growth regulation and disease progression. J Mammary Gland Biol Neoplasia. 1996;1:111–121. doi: 10.1007/BF02096306. [DOI] [PubMed] [Google Scholar]
- 84.Forozan F, et al. Molecular cytogenetic analysis of 11 new breast cancer cell lines. Br J Cancer. 1999;81:1328–1334. doi: 10.1038/sj.bjc.6695007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tait L, Soule HD, Russo J. Ultrastructural and immunocytochemical characterization of an immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6087–6094. [PubMed] [Google Scholar]
- 86.Band V, et al. Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res. 1990;50:7351–7357. [PubMed] [Google Scholar]
- 87.Moffa AB, Tannheimer SL, Ethier SP. Transforming potential of alternatively spliced variants of fibroblast growth factor receptor 2 in human mammary epithelial cells. Mol Cancer Res. 2004;2:643–652. [PubMed] [Google Scholar]
- 88.Gyorffy B, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 89.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Curtis C, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guest ST, et al. Functional oncogene signatures guide rationally designed combination therapies to synergistically induce breast cancer cell death. Oncotarget. 2016;7:36138–36153. doi: 10.18632/oncotarget.9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ethier, S. The SUM Breast Cancer Cell Line Knowledge Base (SLKBase), <https://sumlineknowledgebase.com/>(. [DOI] [PMC free article] [PubMed]
- 94.Broad Institute & Wellcome Sanger Institute. The Cancer Dependency Map Consortium (DepMap). CRISPR (Avana) Public 19Q2 data accessed spring 2018 for EIF4EBP1. <https://depmap.org/portal/>.
- 95.Alayev A, et al. mTORC1 directly phosphorylates and activates ERalpha upon estrogen stimulation. Oncogene. 2016;35:3535–3543. doi: 10.1038/onc.2015.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584:124–128. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miller TW, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamnik Rachel L., Digilova Alla, Davis Daphne C., Brodt Z. Nilly, Murphy Christopher J., Holz Marina K. S6 Kinase 1 Regulates Estrogen Receptor α in Control of Breast Cancer Cell Proliferation. Journal of Biological Chemistry. 2008;284(10):6361–6369. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 99.Maruani DM, et al. Estrogenic regulation of S6K1 expression creates a positive regulatory loop in control of breast cancer cell proliferation. Oncogene. 2012;31:5073–5080. doi: 10.1038/onc.2011.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qi J, Huo L, Zhu YT, Zhu YJ. Absent, small or homeotic 2-like protein (ASH2L) enhances the transcription of the estrogen receptor alpha gene through GATA-binding protein 3 (GATA3) J Biol Chem. 2014;289:31373–31381. doi: 10.1074/jbc.M114.579839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Averous J, Fonseca BD, Proud CG. Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene. 2008;27:1106–1113. doi: 10.1038/sj.onc.1210715. [DOI] [PubMed] [Google Scholar]
- 102.Yu ZJ, et al. Stabilization of 4E-BP1 by PI3K kinase and its involvement in CHK2 phosphorylation in the cellular response to radiation. Int J Med Sci. 2017;14:452–461. doi: 10.7150/ijms.18329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Azar R, Susini C, Bousquet C, Pyronnet S. Control of contact-inhibition by 4E-BP1 upregulation. Cell Cycle. 2010;9:1241–1245. doi: 10.4161/cc.9.7.11047. [DOI] [PubMed] [Google Scholar]
- 104.Romasko EJ, Amarnath D, Midic U, Latham KE. Association of maternal mRNA and phosphorylated EIF4EBP1 variants with the spindle in mouse oocytes: localized translational control supporting female meiosis in mammals. Genetics. 2013;195:349–358. doi: 10.1534/genetics.113.154005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.She QB, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L, Rhodes CJ, Lawrence JC., Jr Activation of mammalian target of rapamycin (mTOR) by insulin is associated with stimulation of 4EBP1 binding to dimeric mTOR complex 1. J Biol Chem. 2006;281:24293–24303. doi: 10.1074/jbc.M603566200. [DOI] [PubMed] [Google Scholar]
- 107.Llanos S, Garcia-Pedrero JM, Morgado-Palacin L, Rodrigo JP, Serrano M. Stabilization of p21 by mTORC1/4E-BP1 predicts clinical outcome of head and neck cancers. Nat Commun. 2016;7:10438. doi: 10.1038/ncomms10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Massarweh S, et al. A phase II study of combined fulvestrant and everolimus in patients with metastatic estrogen receptor (ER)-positive breast cancer after aromatase inhibitor (AI) failure. Breast Cancer Res Treat. 2014;143:325–332. doi: 10.1007/s10549-013-2810-9. [DOI] [PubMed] [Google Scholar]
- 109.Baselga J, et al. A phase II study of combined ridaforolimus and dalotuzumab compared with exemestane in patients with estrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2017;163:535–544. doi: 10.1007/s10549-017-4199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piccart M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann Oncol. 2014;25:2357–2362. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aviad Tsherniak, Francisca Vazquez, Phil G. Montgomery, Barbara A. Weir, Gregory Kryukov, Glenn S. Cowley, Stanley Gill, William F. Harrington, Sasha Pantel, John M. Krill-Burger, Robin M. Meyers, Levi Ali, Amy Goodale, Yenarae Lee, Guozhi Jiang, Jessica Hsiao, William F.J. Gerath, Sara Howell, Erin Merkel, Mahmoud Ghandi, Levi A. Garraway, David E. Root, Todd R. Golub, Jesse S. Boehm, William C. Hahn, (2017) Defining a Cancer Dependency Map. Cell 170 (3):564-576.e16. [DOI] [PMC free article] [PubMed]
- 112.Robin M Meyers, Jordan G Bryan, James M McFarland, Barbara A Weir, Ann E Sizemore, Han Xu, Neekesh V Dharia, Phillip G Montgomery, Glenn S Cowley, Sasha Pantel, Amy Goodale, Yenarae Lee, Levi D Ali, Guozhi Jiang, Rakela Lubonja, William F Harrington, Matthew Strickland, Ting Wu, Derek C Hawes, Victor A Zhivich, Meghan R Wyatt, Zohra Kalani, Jaime J Chang, Michael Okamoto, Kimberly Stegmaier, Todd R Golub, Jesse S Boehm, Francisca Vazquez, David E Root, William C Hahn, Aviad Tsherniak, (2017) Computational correction of copy number effect improves specificity of CRISPR–Cas9 essentiality screens in cancer cells. Nature Genetics 49 (12):1779-1784. [DOI] [PMC free article] [PubMed]
- 113.James M. McFarland, Zandra V. Ho, Guillaume Kugener, Joshua M. Dempster, Phillip G. Montgomery, Jordan G. Bryan, John M. Krill-Burger, Thomas M. Green, Francisca Vazquez, Jesse S. Boehm, Todd R. Golub, William C. Hahn, David E. Root, Aviad Tsherniak, (2018) Improved estimation of cancer dependencies from large-scale RNAi screens using model-based normalization and data integration. Nature Communications 9 (1). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 4EBP1 knockdown inhibits proliferation of MCF7 and T47D breast cancer cells. (a) Western blot of 4EBP1 in MCF7 cells and (b) T47D cells engineered with either control shRNA to lacZ or two individual shRNAs to EIF4EBP1 (4EBP sh_1 or sh_2). (c) Cell proliferation was assessed in MCF7 and (d) T47D control and EIF4EBP1 knockdown cells on day 1 and day 4 in culture. Error bars represent standard deviation among replicates and p-values represent the comparison between each corresponding group. (TIF 2538 kb)
Figure S2 4EBP1 knockdown slows proliferation of SUM-229 and SUM-149 breast cancer cells. (a) Western blot of 4EBP1 in SUM-229 cells and (b) SUM-149 cells engineered with either control shRNA to lacZ or two individual shRNAs to EIF4EBP1 (4EBP sh_1 or sh_2). (c) Cell proliferation was assessed in SUM-229 and (d) SUM-149 control and EIF4EBP1 knockdown cells on day 1 and day 4 in culture. Error bars represent standard deviation among replicates and signficance is shown between each corresponding group. (TIF 2688 kb)
GrowthResults_2–15-19.xlsx Results and statistical analysis of experiments in which EIF4EBP1 was knocked down in three breast cancer cell lines. (XLSX 16 kb)
Data Availability Statement
Data relevant to the SUM lines, including shRNA screening data as well as gene expression data, and other information relevant to these cell lines are freely available at our web site, The SUM Breast Cancer Cell Line Knowledge Base (SLKBase) www.sumlineknowledgebase.com