Abstract
Background: Penta-brominated diphenyl ethers (PentaBDEs) are endocrine-disrupting chemicals that structurally resemble thyroid hormones and were widely used as flame retardants in household consumer products from 1975 to 2004. Polybrominated diphenyl ethers (PBDEs) cross the placenta, and evidence suggests that for many children, body burdens may peak during the toddler years. This study aimed to understand the impact of exposure timing by examining both pre- and postnatal exposure to BDE-47, the predominant penta-brominated diphenyl ether congener detected in humans, in relation to thyroid hormone parameters measured during early childhood.
Methods: The Columbia Center for Children's Environmental Health Mothers and Newborns Study is a prospective birth cohort of African American and Dominican maternal–child pairs. Pregnant women were recruited from two prenatal clinics in Northern Manhattan and the South Bronx between 1998 and 2006. Participants included 158 children with (i) plasma PBDE concentrations measured at birth and in the toddler years (age 2–3 years), and (ii) serum thyroid parameters measured at three and/or five years of age. Outcomes included concentrations of serum thyrotropin, free thyroxine, and total thyroxine.
Results: Children with high exposure to BDE-47 during the prenatal period (−17% [confidence interval −29 to −2]) or toddler age (−19% [confidence interval −31 to −5]) had significantly lower geometric mean thyrotropin levels compared to children with low BDE-47 exposure throughout early life. Associations with thyroxine were also inverse; however, they did not reach statistical significance at the p = 0.05 level. Sex-stratified models suggest associations with postnatal exposure may be stronger among boys compared to girls.
Conclusions: The thyroid regulatory system may be sensitive to BDE-47 during pre- and postnatal periods.
Keywords: flame retardants, organohalogen, endocrine disruption, prenatal, childhood, thyroid hormone
Introduction
Endocrine-disrupting chemicals (EDCs) contribute substantially to human morbidity and are estimated to result in hundreds of billions in costs per year (1). EDCs are defined by their ability to cause changes in endocrine function and consist of several classes of chemicals with varying structures, actions, and endocrine targets (2). Many EDCs were introduced into U.S. commerce beginning in the 1970s to augment food packaging, personal-care products, and other household items (3). For example, following reports indicating improperly extinguished cigarettes were the leading cause of household fires (4), polybrominated diphenyl ethers (PBDEs) began to be used as flame retardants in electronics and household furnishings in 1975 (5). PBDEs were used as three technical mixtures known as penta-brominated diphenyl ether (PentaBDE), octa-brominated diphenyl ether, and deca-brominated diphenyl ether, each comprised of several congeners. PentaBDEs, which are estimated to make up 90% of the human body burden, were primarily applied to products containing polyurethane foam, including couches, car seats, carpet padding, and other upholstered items (6). PentaBDEs were often used in large volumes. For example, reports indicate they comprised up to 3% by weight of the polyurethane foam contained within a couch (7). During manufacturing, PBDEs are not chemically bonded to base polymers and thus have a propensity to migrate into the indoor environment and settle in house dust (8). Human exposure occurs primarily through incidental ingestion of dust, placing young children at risk for elevated exposure due to their frequent hand to mouth behavior and often close proximity to the floor (5,9–11). Owing to their lipophilic properties, PBDEs have long half-lives (PentaBDE congeners: 1.6–6.5 years) (12) and are known to penetrate the fat-soluble placenta, as well as partition into breast milk (13,14). It is estimated that >46,000 tons of PentaBDEs were used in North America until their phase-out in 2004, leading to nearly ubiquitous exposure and body burdens that are the highest in the world (15,16). Despite the phase-out, exposure to PBDEs continues because of their resistance to environmental degradation and ongoing release from consumer products that are infrequently replaced (15).
PBDE congeners consist of a diphenyl ether backbone around which varying numbers of bromine atoms are attached (5). This molecular structure closely resembles that of the halogenated (iodine) thyroid hormones triiodothyronine (T3) and thyroxine (T4) (17), supporting the putative interaction of PBDEs with thyroid hormone transport proteins, receptors, and/or degradation enzymes (18). Thyroid hormones bind to receptors in nearly every organ in the human body and play critical roles in the regulation of growth, metabolism, and brain development (19). In vitro research indicates hydroxylated metabolites of BDE-47, the predominant congener detected in humans, markedly inhibit the capacity of T3 to bind with receptors (20), and evidence from animal research suggests PBDE exposure alters thyroid hormone homeostasis (18) as well as other thyroid-dependent processes. For example, research conducted in Xenopus laevis has demonstrated BDE-47 exposure arrests thyroid-dependent metamorphosis of tadpoles into froglets and disrupts thyroid hormone-related gene expression in the brain (21,22). Likewise, research conducted in avian and murine models has consistently demonstrated associations between prenatal exposure to PentaBDE congeners, including BDE-47, with reductions in circulating levels of T4 (reviewed by Costa et al. and Birnbaum and Staskal) (18,23).
Widespread research supports classification of PBDEs as developmental neurotoxicants (11,24–27), with disruption of thyroid hormones as a leading putative mechanism underlying observed relationships (18,28). However, despite convincing evidence from in vitro and animal research, results from studies investigating PBDEs in relation to thyroid hormone function in humans include a mix of negative, positive, and null associations (29–34). Notably, previous studies have measured thyroid hormone parameters in maternal blood (29,30,34) collected during pregnancy or parturition, cord blood (29,32,34), or infant blood (31,32) collected within hours to weeks of birth—periods when transient yet substantial endocrine system changes occur, including profound alterations to the thyroid regulatory system (35). It is plausible that inconsistencies across previous human studies are partially attributable to misclassification introduced by the timing of thyroid hormone measurement. The present study addresses this limitation by examining plasma PBDE concentrations in samples collected at birth and during the toddler years (age 2–3 years) in relation to thyrotropin (TSH), free T4, and total T4 levels measured in serum samples collected during early childhood (age 3–5 years). Based on results from animal research, it was hypothesized that prenatal exposure to BDE-47 would be associated with lower T4 levels at birth (reviewed by Costa et al. and Birnbaum and Staskal) (18,23). It was further hypothesized that these associations would act through a programming pathway, leading to effects that persist throughout childhood. Because of the typically higher exposure of young children compared to fetuses (via direct interaction with the external environment), it was also hypothesized that children with low prenatal exposure but high postnatal exposure would show evidence of a dysregulated thyroid regulatory system during childhood. This is the largest prospective study to examine both pre- and postnatal exposure to PBDEs in relation to thyroid endpoints.
Methods
Study sample
The sample includes a subset of participants enrolled in the Columbia Center for Children's Environmental Health (CCCEH) Mothers and Newborns birth cohort, which recruited African American and Dominican women from New York City between 1998 and 2006. Women were ineligible for study participation if they were outside the ages of 18–35 years, initiated prenatal care after the 20th week of pregnancy, had a multiple pregnancy, used tobacco products or illicit drugs, had diabetes, had hypertension, or were HIV positive. Women were considered fully enrolled if a maternal or umbilical cord blood sample was collected at the child's delivery. During pregnancy and at each postnatal study visit, a bilingual research worker conducted a structured interview to collect information about socio-demographic and life-style factors. At delivery, study staff collected umbilical cord blood; at follow-up visits at the ages of two, three, and five years, a pediatric phlebotomist collected child venous blood. All samples were transported to the CCCEH laboratory immediately following collection, where the buffy coat, packed red blood cells, and plasma were separated and frozen at −70°C. Additional details describing the cohort design, recruitment, and follow-up have been previously published (36–38).
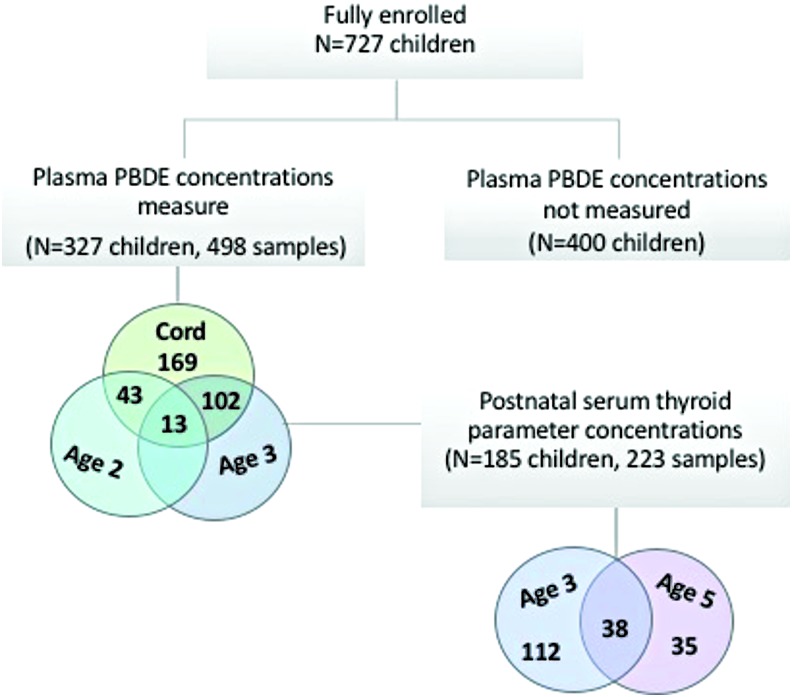
At delivery, 727 mothers remained eligible and were fully enrolled in the cohort. At the two-, three-, and five-year follow-up visits, 566 (78%), 562 (77%), and 551 (76%) maternal–child pairs remained in the study, respectively. At these postnatal follow-up visits, blood was collected from 92–98% of children. PBDE concentrations were measured in all available stored cord plasma samples and in early childhood samples among children with follow-up data (cord plasma n = 327, 2-year follow-up n = 43, 3-year follow-up n = 102, or both 2- and 3-year follow-up n = 13). Thyroid hormone parameters were measured in 185 of the 327 children with a measure of PBDE concentrations at three years of age (n = 112), five years of age (n = 35), or both three and five years of age (n = 38; Fig. 1). The study protocol was approved by the Institutional Review Board of Columbia University Medical Center. It was determined at the Centers for Disease Control and Prevention (CDC) that the agency was not engaged in human subject research. Before each study visit, mothers gave written informed consent for herself and for her child.
FIG. 1.
Flow diagram of participant selection from the Columbia Center for Children's Environmental Health Mothers and Newborns birth cohort study. Color images are available online.
PBDE analysis
The CDC's Persistent Organic Pollutants Biomonitoring Laboratory measured PBDE concentrations in umbilical cord and venous plasma samples. Detailed analytic methods are available elsewhere (39,40) and information pertaining to analysis of PBDEs in this cohort has been previously described (9,41). Briefly, samples were fortified with internal standards followed by automated liquid–liquid extraction using a Gilson 215 liquid handler (Gilson, Inc., Middleton, WI). Final analytical determinations were made by gas chromatography isotope dilution high-resolution mass spectrometry using a DFS instrument (Thermo Fisher Scientific, Bremen, Germany). Each analytical batch was comprised of method blanks (n = 3), quality-control samples (n = 3), and study samples (n = 26). All reported data were subtracted from the median concentration detected in method blank samples. Co-extracted lipids were removed using a silica gel/silica sulfuric acid column with automation on a Rapid Trace SPE workstation (Biotage, Uppsala, Sweden), and total cholesterol and triglycerides were determined on a Roche Hitachi 912 Chemistry Analyzer (GMI, Inc., Ramsey, MN). Total cord blood lipid levels, including unmeasured free cholesterol and phospholipids, were estimated by summation of individual lipid components using an umbilical cord blood–specific formula (total cord blood lipids = 2.657 × total cord blood cholesterol + cord blood triglycerides +0.268, in g lipids/L plasma; A. Sjodin, pers. commun., November 2016). Child total blood lipids were estimated using the short formula developed by Phillips et al. (42). PBDEs were examined as a lipid standardized variable in all models (ng/g lipid).
Thyroid hormone analysis
TSH, free T4, and total T4 were measured in child serum samples by the Clinical and Epidemiologic Research Laboratory at Boston Children's Hospital. All analyses were performed by automated immunoassay using a competitive electrochemiluminescence detection system (Roche Diagnostics, Indianapolis, IN). The lowest detection limits were 0.005 μIU/mL, 0.26 pmol/L, and 5.4 nmol/L for TSH, free T4, and total T4, respectively. Day-to-day imprecision values ranged from 1.8% to 5.4% for 0.09–3.96 μIU/mL of TSH, from 3.5% to 6.6% for 8.75–50.70 pmol/L of free T4, and from 3.0% to 6.9% for 33.4–237 nmol/L of total T4. Maternal iodide concentrations, an essential substrate for thyroid hormone biosynthesis (43), were measured in maternal spot urine samples collected during the third trimester. Before performing statistical analyses, iodide was adjusted for specific gravity to control for variation in urinary dilution.
Statistical analysis
The study focused on BDE-47 (percent detected: 80%), which was the only congener for which cord plasma concentrations were detectable in >50% of samples; concentrations in child plasma (age 2–3 years) were detectable in 99% of samples. Consistent with other studies, in the samples, cord plasma BDE-47 concentrations were moderately to highly correlated with the other primary congeners that comprise the PentaBDE formulation (Spearman's ρ: BDE-99, 0.83; BDE-100, 0.76; BDE-153, 0.47; p < 0.01). As previously described (41), a distribution-based approach was used to multiply impute values for plasma BDE-47 concentrations below the sample-specific limit of detection (LOD), which is determined by the sample's volume and lipid content.
Latent class growth analysis (LCGA) was performed using the SAS Proc Traj procedure (44) to estimate trajectories of BDE-47 concentration between birth and three years. LCGA is a group-based modeling technique that empirically clusters individuals with a shared temporal pattern of change for a given characteristic (i.e., change in PBDE concentration over early life) (45). Before estimating trajectories, continuous BDE-47 concentrations (ng/g lipid) were log10-transformed to improve normality of the distribution, and non-detected concentrations were replaced with the sample-specific mean value across the 10 imputed data sets. Models were iteratively tested with varying numbers of groups (n = 2–5) and shapes (linear–cubic), and the optimal number of trajectories was determined based on: (i) visual confirmation of distinct trajectories, each of which comprised >10% of the data; (ii) evaluation of the Bayesian Information Criterion; and (iii) evaluation of the average posterior probability of group membership. Additional details describing LCGA model fitting are provided in Supplementary Table S1.
Multivariable linear regression was used to examine associations between trajectories of BDE-47, treated as a categorical variable, and thyroid hormone parameters collected between three and five years. The generalized estimating equations (GEE) approach was used with an exchangeable working correlation to account for repeated thyroid measures within a child over time. An exchangeable working correlation was selected based on evaluation of the empirical correlation matrix, which did not indicate an autoregressive relationship, as well as evaluation of the quasi-likelihood information criterion. In all models, TSH, free T4, and total T4 were expressed as continuous variables, and TSH was log10-transformed to improve normality of the distribution. Models including an interaction term between age and BDE-47 trajectory did not indicate that the association between PBDEs and thyroid parameters significantly varies by age at blood collection. Separate models were further examined for thyroid hormone parameters measured at three and five years of age in sensitivity analyses.
Intra-individual thyroid hormone concentrations decrease with age (46). Therefore, the study a priori included exact age at blood draw as a time-varying covariate. Directed acyclic graphs (DAGs) were constructed based on substantive knowledge and previously published research to identify the minimal set of covariates sufficient to estimate the unconfounded effect of PBDEs on thyroid hormone parameters, which included only race/ethnicity (Supplementary Fig. S1). The set of potential confounders considered included: sex, race/ethnicity (African American/Dominican), date of birth, gestational age (in weeks), birth weight (in grams), prenatal environmental tobacco smoke exposure (yes/no, as previously described) (47), breastfeeding history (<12 weeks/≥12 weeks), parity (nulliparous/multiparous), relationship status (unmarried/married or with the same partner for seven years or more), maternal age (in years), material hardship (none/unable to afford food, clothing, or housing), maternal education (less than high school/high school or equivalent), and maternal employment (employed/not employed). All variables relating to the mother or household were collected during the prenatal period, and variables relating to the delivery were extracted from hospital medical records. Information on breast-feeding history was collected at 3-, 6-, 12-, 24-, and 36-month follow-up visits. In sensitivity analyses, the influence of covariate selection was further evaluated by examining a priori (age at blood draw only) and fully adjusted models.
Given sex differences in the incidence of many thyroid-related diseases (48,49), potential effect modification by child sex was explored using cross-product terms and sex-stratified models. The influence of maternal iodide status during pregnancy was examined by stratifying participants by the pregnancy-specific threshold for population iodine sufficiency (≥150 μg/L) and examining models within each stratum (50). Finally, to compare the results with findings from other cohort studies, the GEE approach was used to examine associations between plasma BDE-47 concentrations treated as a continuous, log10-transformed variable measured in cord blood or three-year-old blood in relation to repeated thyroid hormone parameters. Statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC) or RStudio v0.99.891 and constructed DAGs using DAGitty v2.3 (51).
Results
Table 1 presents characteristics of the study population. All maternal–child pairs were African American (45%) or Dominican (55%), and at delivery 37% of mothers had less than a high school education, 76% were not in a stable relationship, and 36% reported experiencing material hardship. Socio-demographic and life-style characteristics were similarly distributed between children included in the analysis and those excluded due to missing PBDE or thyroid hormone data, with the following exceptions: the excluded sample had a higher proportion of Dominican participants (68% vs. 55%; p < 0.01) and fewer nulliparous mothers (42% vs. 51%; p = 0.04), and on average newborns had lower birth weights (mean difference: 119 g; p < 0.01). The difference in birth weight may reflect racial/ethnic variation between the included and excluded samples, as African American newborns weighed on average 148 g less than Dominican infants (p < 0.01). Among included children, BDE-47 concentrations (at birth, 2 years of age, and 3 years of age) and thyroid hormone parameters (at 3 and 5 years of age) were not significantly associated with parity or birthweight. At two and three years of age, BDE-47 concentrations were significantly higher among children included in the analysis compared to excluded children (p = 0.05), which likely reflects that included children were more likely to be born earlier during the enrollment period (i.e., prior to the 2004 phase-out of BDE-47) to allow time to age into later blood draws (9).
Table 1.
Participant Characteristics, BDE-47 Concentrations, and Thyroid Parameter Levels Among Maternal–Child Pairs Included in the Analysis (n = 185)
| n (%) or M ± SD | ||||
|---|---|---|---|---|
| Included | N sample | Excluded | N sample | |
| African American* | 83 (45) | 185 | 171 (32) | 542 |
| Dominican* | 102 (55) | 185 | 371 (68) | 542 |
| Nulliparous* | 95 (51) | 185 | 229 (42) | 538 |
| Maternal age (years) | 24.9 ± 4.8 | 185 | 25.3 ± 5.0 | 542 |
| Less than high school education | 68 (37) | 185 | 190 (36) | 532 |
| Stable relationship | 44 (24) | 185 | 150 (28) | 538 |
| Employed | 117 (63) | 185 | 282 (52) | 539 |
| Material hardship | 66 (36) | 185 | 220 (42) | 530 |
| Male | 85 (46) | 185 | 266 (49) | 542 |
| Prenatal ETS exposure | 70 (38) | 185 | 184 (34) | 540 |
| Gestational age (weeks) | 39.3 ± 1.2 | 185 | 38.5 ± 6.3 | 542 |
| Birthweight (kg)* | 3.5 ± 0.5 | 185 | 3.3 ± 0.6 | 535 |
| Breast-fed ≥12 weeks | 64 (35) | 185 | 173 (34) | 502 |
| BDE-47 (ng/g lipid)a | ||||
| Prenatal | 14.2 ± 1.2 | 185 | 14.1 ± 1.2 | 142 |
| 2 years* | 43.9 ± 7.4 | 45 | 20.4 ± 6.2 | 11 |
| 3 years* | 38.0 ± 5.3 | 60 | 26.6 ± 3.6 | 55 |
| TSH (μIU/mL)a | ||||
| 3 years | 2.4 ± 0.1 | 150 | 2.3 ± 0.10 | 125 |
| 5 years | 2.1 ± 0.1 | 73 | 2.3 ± 0.2 | 36 |
| Free T4 (pmol/L) | ||||
| 3 years | 18.3 ± 2.2 | 150 | 18.3 ± 2.5 | 125 |
| 5 years | 18.1 ± 2.2 | 73 | 18.2 ± 1.8 | 36 |
| Total T4 (nmol/L) | ||||
| 3 years | 140.6 ± 25.3 | 150 | 140.8 ± 26.8 | 125 |
| 5 years | 146.4 ± 31.8 | 73 | 148.9 ± 31.3 | 36 |
Geometric mean.
Included and excluded significantly different at p = 0.05.
BDE, brominated diphenyl ether; ETS, environmental tobacco smoke; T4, thyroxine; TSH, thyrotropin.
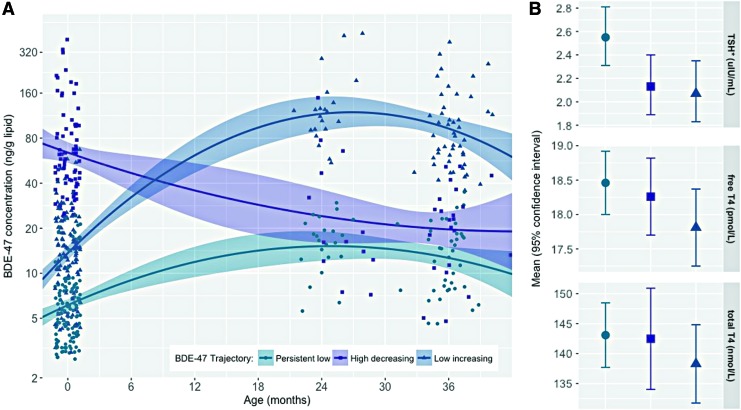
BDE-47 was detected in 80% of cord plasma samples and 99% of toddler plasma samples. The lower detection frequency in cord blood is consistent with results from an independent cohort based in New York City (BDE-47: 81%) and a cohort based in Baltimore (BDE-47: 90%), which both measured PBDE concentrations in cord blood (52,53). As expected, plasma BDE-47 concentrations were significantly lower at birth (14.2 ± 0.9; n = 327) compared to the toddler years (2 years of age: GM±GSE = 37.8 ± 5.8, n = 56, paired t-test using log10-transformed BDE-47 in ng/g lipids, t = −4.07, p = 0.0002; 3 years of age: GM ± GSE = 32.0 ± 3.1, n = 115, paired t-test using log10-transformed BDE-47 in ng/g lipid, t = −6.90, p < 0.0001). BDE-47 in cord plasma correlated poorly with BDE-47 in child plasma measured at two years of age (ρ = −0.03, p = 0.82) or three years of age (ρ = 0.09, p = 0.36). However, BDE-47 measured at two years of age was strongly correlated with BDE-47 measured at three years of age (ρ = −0.79, p < 0.01). Exposure percentiles for each group are provided in Supplementary Table S2. As illustrated by Figure 2A, the best-fitting LCGA model revealed three trajectories of BDE-47 exposure characterized by (i) persistent low (34%), (ii) high-decreasing (28%), and (iii) low-increasing (38%) plasma concentrations across early childhood.
FIG. 2.
(A) Trajectories of BDE-47 concentration from birth to three years of age. (B) Age- and ethnicity-adjusted mean thyroid parameter levels by BDE-47 trajectory. *Thyrotropin (TSH) is the geometric mean. Color images are available online.
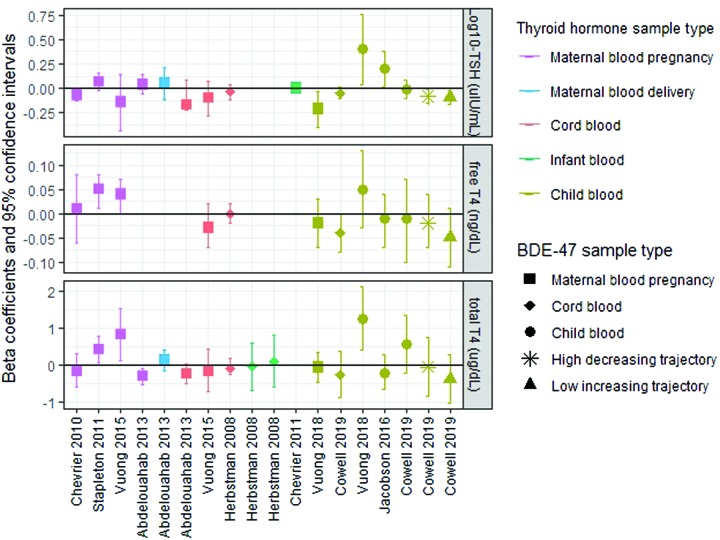
In models examining BDE-47 trajectories in relation to serum thyroid parameters measured between three and five years, children assigned to the high-decreasing or low-increasing trajectory had 17% [confidence interval (CI) −29 to −2] and 19% [CI −31 to −5] lower geometric mean TSH levels compared to children assigned to the persistent low trajectory, respectively. Associations between each of these trajectories and T4 levels (free and total) were also inverse. However, they did not reach statistical significance at the p = 0.05 level (Table 2, which presents estimates from GEE models, and Figure 2B, which plots adjusted mean thyroid parameter concentrations stratified by BDE-47 trajectory). Results from fully adjusted models, as well as models examining thyroid hormones at three and five years of age separately, did not substantially deviate from these results (see Supplementary Tables S3 and S4). Models examining cord plasma BDE-47 as a continuous variable are presented in Figure 3 and Supplementary Table S5.
Table 2.
Age- and Ethnicity-Adjusted Associations Between BDE-47 Trajectories (ng/g Lipid) and Serum Thyroid Parameters
| BDE-47 trajectory | Log10TSH (μIU/mL) | Free T4 (pmol/L) | Total T4 (nmol/L) |
|---|---|---|---|
| Persistent low (34%) | Reference | Reference | Reference |
| High decreasing (28%) | –0.08 [−0.15 to −0.01] | –0.20 [−0.92 to 0.52] | –0.61 [−10.66 to 9.44] |
| Low increasing (38%) | –0.09 [−0.16 to −0.02] | –0.65 [−1.37 to 0.07] | –4.80 [−13.11 to 3.51] |
Data shown as β [CI]. 185 children and 223 observations.
CI, confidence interval.
FIG. 3.
Results from multiple linear regression models examining associations between log10BDE-47 (ng/g lipid) and thyroid hormone parameters (log10TSH: μIU/mL; free thyroxine [T4]: ng/dL; total T4: μg/dL) measured as continuous variables reported by nine North American birth cohort studies. Herbstman et al. (32) and Stapleton et al. (33) applied a natural-log transformation to BDE-47 and TSH rather than a log10-transformation. Stapleton et al. (33) additionally natural-log transformed free T4. To facilitate comparison of the present results to others, final models expressing free T4 and total T4 were reanalyzed in units of ng/dL and μg/dL, respectively. Abdelouahab et al. (29) modeled free T4 measured in cord blood on a pmol/L basis. therefore, these results were excluded from the figure to accommodate the y-axis scale. Supplementary Table S8 presents summary data for BDE-47 and thyroid hormone parameters measured by each study. Color images are available online.
No significant sex differences were observed in the proportion of children assigned to each trajectory. Likewise, thyroid hormone parameters did not significantly differ between girls and boys at three or five years of age (Supplementary Table S6). In sex-stratified models (see Supplementary Fig. S2 and Supplementary Table S7), the inverse association observed between the low-increasing BDE-47 trajectory (vs. persistent low trajectory) and childhood thyroid parameters (TSH and free T4) was augmented among boys (percent change TSH: −30 [CI −45 to −11]; p-interaction: 0.12; unit change free T4: −1.18 [CI −2.18 to −0.20]; p-interaction: 0.21) and attenuated among girls (percent change TSH: −8 [CI −26 to 13.3]; unit (nmol/L) change free T4: −0.19 [CI −1.19 to 0.82]). While the interaction terms did not reach statistical significance at the p = 0.05 level, these findings suggest sex may modify the association between postnatal BDE-47 exposure and thyroid hormone parameters. Given the relatively small sample size for investigating interactions, it will be important that these findings are replicated by other research groups.
Specific-gravity adjusted urinary iodide concentrations among the 115 mothers with an available urine sample ranged from 45.4 to 425.9 μg/L; 27% of mothers had a concentration below the pregnancy-specific threshold for population iodine sufficiency (150 μg/L). In age, ethnicity, and specific gravity adjusted models, no significant interaction was detected between BDE-47 and maternal urinary iodine status, treated as a continuous or categorical variable (<150 vs. ≥150 μg/L) for any thyroid hormone parameter.
Discussion
Compared to children with low cord plasma BDE-47 concentrations (GM ± GSD = 5.8 ± 0.4 ng/g lipid) that remained low throughout early childhood (GM ± GSD = 13.8 ± 1.2 ng/g lipid), children with high prenatal exposure (GM ± GSD = 66.6 ± 6.1 ng/g lipid) that decreased after birth had significantly lower circulating TSH levels measured between three and five years of age. TSH is a key effector and stimulus of the hypothalamic–pituitary–thyroid (HPT) axis, which maintains circulating thyroid hormone levels around an intra-individual set point. Briefly, low levels of circulating T3 and T4 stimulate the pituitary gland to release TSH, which in turn stimulates the thyroid gland to produce and secrete T3 and T4 (43,54). Evidence from animal models and human clinical studies suggests the set point around which this negative feedback mechanism responds may be partially determined during gestation (55–58). The present findings suggest prenatal exposure to PBDEs may program a “reactive HPT axis” phenotype such that less TSH is required to stimulate production and release of adequate T4.
Children with low cord plasma BDE-47 concentrations (GM ± GSD = 13.8 ± 1.2 ng/g lipid) that increased during the toddler years (GM ± GSD = 106.9 ± 9.2 ng/g lipid) also had significantly lower TSH concentrations between three and five years of age compared to children with low cord plasma BDE-47 concentrations (GM ± GSD = 5.8 ± 0.4 ng/g lipid) that remained low during the toddler years (GM ± GSD = 13.8 ± 1.3 ng/g lipid). Interestingly, the magnitude of this association was stronger among boys, despite the finding of no significant difference in BDE-47 concentrations or TSH levels between girls and boys. Boys with high exposure during the toddler years also showed significantly lower free and total T4 levels compared to boys with persistent low BDE-47 exposure. The finding of depressed T4 levels is consistent with research conducted in murine models, which have consistently found PBDE exposure to be associated with reduced serum T4 levels (59). Putative mechanisms underlying this finding include PBDE interference with thyroid hormone transport and metabolism. For example, research conducted in mice suggests PBDEs induce upregulation of thyroid hormone metabolizing enzymes, resulting in enhanced clearance of T4 (60), and both animal and in vitro studies suggest PBDEs or their hydroxylated metabolites may bind and displace T4 from protein transporters, thereby disrupting circulating levels (18).
Several birth cohort studies (n > 100) have investigated cross-sectional associations between prenatal exposure to PBDEs and thyroid hormone parameters measured in maternal or cord blood collected during pregnancy, delivery, or early postnatal life with a combination of positive, negative, and null findings (Fig. 3 and Supplementary Table S8) (29–34). Results from these studies are inconsistent and difficult to compare to the present findings, given variation in the measurement of thyroid hormones during developmental periods when normal fluctuations in HPT axis homeostasis occur. For example, an estrogen-induced elevation of thyroid-binding globulin and placental production of chorionic gonadotrophin triggers maternal T4 levels to increase sharply and TSH levels to fall during the first trimester of pregnancy, and during delivery, a stress and cold-evoked surge in TSH occurs in the newborn, followed by a reflexive increase in T4 over the next 24–48 hours (35,43,61).
Similar to studies focused on pregnancy and infancy, results from research investigating cross-sectional associations between PBDEs and thyroid dysregulation during childhood are inconsistent and difficult to compare due to differences in study design and variation in both the distribution of PBDE concentrations and congeners detected. Specifically, of the eight studies identified that examined postnatal exposure to PBDEs and thyroid parameters, three focused on special populations with unusually high exposure levels (i.e., children living and working near electronics recycling facilities in China) (62–64), two detected unusually low PBDE concentrations for unexplained reasons (65,66), and one was conducted among a small sample (n < 30) of older children (aged 14–18 years) (67). Among 80 children admitted to a hospital for a non-endocrine-related disease between one and five years of age, Jacobson et al. detected a significant positive association between BDE-47 (ng/g lipid) and TSH but no significant associations with total or free T4 or T3, reverse T3, or T3 uptake (68). A number of factors could underlie the differences in the findings, including study design (cross-sectional vs. prospective), variation in age at time of thyroid parameter measurement (1–5 years vs. 3–5 years), or differences in the source populations (i.e., general population recruitment vs. hospital-based recruitment).
Only one other study has prospectively examined associations between both pre- and postnatal BDE-47 concentrations and thyroid hormone levels measured during early childhood. Using multiple informant models, Vuong et al. detected significant inverse associations between maternal log10-BDE-47 concentrations measured during pregnancy and ln-TSH measured at three years of age (β = −0.20 [CI −0.38 to −0.03]) among 158 maternal–child pairs living in Cincinnati, Ohio (69). Also consistent with our finding of a trend toward a stronger association between the early prenatal high BDE-47 trajectory and TSH among girls, Vuong et al. found that inverse associations between prenatal BDE-47 and ln-TSH were only statistically significant among girls. Vuong et al. found that inverse associations between prenatal BDE-47 and ln-TSH were only statistically significant among girls. While the present study did not detect evidence of a sex-specific effect between prenatal BDE-47 and free T4, Vuong et al. found a significant inverse association only among boys (69). Additionally, in contrast to the present observation of inverse associations between postnatal BDE-47 and ln-TSH, the researchers detected significant positive associations between serum BDE-47 measured at two years of age (n = 71) but not one (n = 77) or three years of age (n = 71), and both ln-TSH and total T4 measured at three years of age (69). Results of sex interactions with postnatal exposure were not reported.
Despite evidence indicating (i) sex-specific associations between PBDEs and thyroid gland function in birds (70), (ii) interactions with sex hormone receptors in fish and rodents (71–73), and (iii) altered sex hormone levels in children (74) and pregnant women (75), few studies have investigated sexually dimorphic effects of PBDEs on thyroid hormone disruption. Specific mechanisms underlying the present observation of stronger associations with postnatal exposure among boys compared to girls are unknown. However, the high degree of overlap between the HPT axis and the hypothalamic–pituitary–gonadal axis, which regulates circulating sex hormone levels, suggests disruption in one system may have downstream consequences for the other (76). For example, evidence suggests hypogonadism in hypothyroid men reflects a hypothyroidism-induced blunted pituitary response to gonadotropin-releasing hormone secreted by the hypothalamus (77). Further, male reproductive organs, including the prepubertal testes, are thyroid-responsive tissues (78), and animal studies have demonstrated that experimentally induced hypothyroidism results in testicular damage, decreased testosterone concentrations, and arrest of sexual maturity (79,80). Research designed to investigate the effect of PBDEs on overlapping pathways central to both thyroid and reproductive hormone homeostasis is needed to understand more fully the mechanisms underlying the current sex-specific findings.
In addition to the longitudinal design, the present study has several strengths. First, PBDE concentrations were comparable to other geographically and temporally similar birth cohorts and reflect general population exposure (52,53). Additionally, the relatively large sample size allowed many potential confounders to be examined, as well as effect modification by child sex and maternal iodide status during pregnancy to be explored. Unfortunately, it was not possible to evaluate selenium, which is known to be an important determinant of thyroid status (81). Additional limitations include the lack of T3 levels, thyroid-binding protein levels, and PBDE metabolite data, which structurally resemble endogenous thyroid hormones more closely than parent congeners (18). Finally, thyroid parameters were analyzed by immunoassay, which may be affected by variation in serum thyroid-binding protein levels (43).
Overall, the findings suggest the thyroid regulatory system may be sensitive to disruption by PBDEs during both the pre- and postnatal periods. Pregnant women and young children should minimize exposure to these EDCs. While research on PBDE exposure intervention studies is limited, results from observational research suggest several behavioral modifications for reducing contact with dust may be effective in limiting PBDE exposure, including wet or damp mopping the home (9,41), wiping down plastic toys (82), vacuuming with a HEPA filter and/or wearing a dust mask while vacuuming, washing hands frequently (83), avoiding hand-to-mouth behaviors (82) (i.e., thumb sucking, nail biting), and purchasing flame-retardant-free furniture and furnishings, which can be readily identified in the United States by examining product tags.
Supplementary Material
Acknowledgments
We thank Dr. Lori Hoepner for assistance with data management and Pat Vuguin for review of manuscript drafts. This work was supported by National Institutes of Health (grant numbers R01ES021806, R01ES013543, P50ES009600, T32ES023772 and T32ES007322) and Environmental Protection Agency FP-91779001.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services. This publication was developed under STAR Fellowship Assistance Agreement no. FP-91779001 awarded by the U.S. Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this publication are solely those of the study authors.
Author Disclosure Statement
The authors report no conflicts of interest in this work.
Supplementary Material
References
- 1. Trasande L, Zoeller RT, Hass U, Kortenkamp A, Grandjean P, Myers JP, DiGangi J, Bellanger M, Hauser R, Legler J, Skakkebaek NE, Heindel JJ. 2015. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European Union. J Clinical Endocrinol Metab 100:1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. 2012. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 153:4097–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergman A, Heindel JJ, Jobling S, Kidd KA, Zoeller RT. 2013. State of the Science of Endocrine Disrupting Chemicals 2012. United Nations Environment Programme and World Health Organization, Geneva, Switzerland [Google Scholar]
- 4. Callahan P, Roe S, Hawthorne M. 2012. Playing with Fire. Chicago Tribune, Chicago, IL [Google Scholar]
- 5. EPA 2010. An Exposure Assessment of Polybrominated Diphenyl Ethers. National Center for Environmental Assessment. Environmental Protection Agency, Washington, DC [Google Scholar]
- 6. Talsness CE. 2008. Overview of toxicological aspects of polybrominated diphenyl ethers: a flame-retardant additive in several consumer products. Environ Res 108:158–167 [DOI] [PubMed] [Google Scholar]
- 7. Cobb D. 2005. Analysis of flame retardant chemicals added to foams, fabric, batting, loose fill and barriers. Memorandum to Dale R Ray, Project Manager, Upholstered Furniture, Consumer Products Safety Commission. Available at https://www.cpsc.gov/s3fs-public/uff6.pdf (accessed April30, 2019)
- 8. Zhang X, Diamond ML, Robson M, Harrad S. 2011. Sources, emissions, and fate of polybrominated diphenyl ethers and polychlorinated biphenyls indoors in Toronto, Canada. Environ Sci Technol 45:3268–3274 [DOI] [PubMed] [Google Scholar]
- 9. Cowell WJ, Sjodin A, Jones R, Wang Y, Wang S, Herbstman JB. 2018. Temporal trends and developmental patterns of plasma polybrominated diphenyl ether concentrations over a 15-year period between 1998 and 2013. J Expo Sci Environ Epidemiol 29:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toms LM, Sjodin A, Harden F, Hobson P, Jones R, Edenfield E, Mueller JF. 2009. Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2–5 years of age) than in infants and adults. Environ Health Perspect 117:1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vuong AM, Braun JM, Yolton K, Xie C, Webster GM, Sjodin A, Dietrich KN, Lanphear BP, Chen A. 2017. Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children. Environ Res 153:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geyer H, Schramm K, Darnerud P, Aune M, Feicht E, Fried K. 2004. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Comp 66:5 [Google Scholar]
- 13. Adgent MA, Hoffman K, Goldman BD, Sjodin A, Daniels JL. 2014. Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months. Paediatr Perinat Epidemiol 28:48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leonetti C, Butt CM, Hoffman K, Hammel SC, Miranda ML, Stapleton HM. 2016. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environ Health 15:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbasi G, Buser AM, Soehl A, Murray MW, Diamond ML. 2015. Stocks and flows of PBDEs in products from use to waste in the U.S. and Canada from 1970 to 2020. Environ Sci Technol 49:1521–1528 [DOI] [PubMed] [Google Scholar]
- 16. Fromme H, Becher G, Hilger B, Volkel W. 2016. Brominated flame retardants—exposure and risk assessment for the general population. Int J Hyg Environ Health 219:1–23 [DOI] [PubMed] [Google Scholar]
- 17. Mary EG, Zoeller RT. 2010. Thyroid hormones' impact on the developing brain: possible mechanisms of neurotoxicity. In: Harry GJ, Tilson HA. (eds) Neurotoxicology. Third edition. Informa Healthcare, New York, NY, pp 79–111 [Google Scholar]
- 18. Costa LG, de Laat R, Tagliaferri S, Pellacani C. 2014. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett 230:282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams GR. 2008. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 20:784–794 [DOI] [PubMed] [Google Scholar]
- 20. Kitamura S, Shinohara S, Iwase E, Sugihara K, Uramaru N, Shigematsu H, Fujimoto N, Ohta S. 2008. Affinity for thyroid hormone and estrogen receptors of hydroxylated polybrominated diphenyl ethers. J Health Sci 54:607–614 [Google Scholar]
- 21. Balch GC, Velez-Espino LA, Sweet C, Alaee M, Metcalfe CD. 2006. Inhibition of metamorphosis in tadpoles of Xenopus laevis exposed to polybrominated diphenyl ethers (PBDEs). Chemosphere 64:328–338 [DOI] [PubMed] [Google Scholar]
- 22. Yost AT, Thornton LM, Venables BJ, Sellin Jeffries MK. 2016. Dietary exposure to polybrominated diphenyl ether 47 (BDE-47) inhibits development and alters thyroid hormone-related gene expression in the brain of Xenopus laevis tadpoles. Environ Toxicol Pharmacol 48:237–244 [DOI] [PubMed] [Google Scholar]
- 23. Birnbaum LS, Staskal DF. 2004. Brominated flame retardants: cause for concern? Environ Health Perspect 112:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lam J, Lanphear BP, Bellinger DC, Axelrad DA, McPartland J, Sutton P, Davidson L, Daniels N, Sen S, Woodruff TJ. 2017. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ Health Perspect 125:086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linares V, Belles M, Domingo JL. 2015. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol 89:335–356 [DOI] [PubMed] [Google Scholar]
- 26. Roth N, Wilks MF. 2014. Neurodevelopmental and neurobehavioural effects of polybrominated and perfluorinated chemicals: a systematic review of the epidemiological literature using a quality assessment scheme. Toxicol Lett 230:271–281 [DOI] [PubMed] [Google Scholar]
- 27. Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A. 2017. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: current findings and future directions. Horm Behav 101:94–104 [DOI] [PubMed] [Google Scholar]
- 28. Mughal B, Fini J, Demeneix B. 2018. Thyroid-disrupting chemicals and brain development: an update. Endocr Connect 7:R160–R186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abdelouahab N, Langlois MF, Lavoie L, Corbin F, Pasquier JC, Takser L. 2013. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol 178:701–713 [DOI] [PubMed] [Google Scholar]
- 30. Chevrier J, Harley KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. 2010. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect 118:1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chevrier J, Harley KG, Bradman A, Sjodin A, Eskenazi B. 2011. Prenatal exposure to polybrominated diphenyl ether flame retardants and neonatal thyroid-stimulating hormone levels in the CHAMACOS study. Am J Epidemiol 174:1166–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Halden RU, Patterson DG, Panny SR, Needham LL, Goldman LR. 2008. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ Health Perspect 116:1376–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. 2011. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect 119:1454–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vuong AM, Webster GM, Romano ME, Braun JM, Zoeller RT, Hoofnagle AN, Sjodin A, Yolton K, Lanphear BP, Chen A. 2015. Maternal polybrominated diphenyl ether (PBDE) exposure and thyroid hormones in maternal and cord sera: the HOME Study, Cincinnati, USA. Environ Health Perspect 123:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glinoer D. 1997. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 18:404–433 [DOI] [PubMed] [Google Scholar]
- 36. Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P. 2006. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect 114:1287–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. 2006. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118:e1845–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whyatt RM, Camann DE, Kinney PL, Reyes A, Ramirez J, Dietrich J, Diaz D, Holmes D, Perera FP. 2002. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect 110:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones R, Edenfield E, Anderson S, Zhang Y, Sjodin A. 2012. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Comp 74:97–98 [Google Scholar]
- 40. Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, Patterson DG, 2004. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem 76:1921–1927 [DOI] [PubMed] [Google Scholar]
- 41. Cowell WJ, Sjodin A, Jones R, Wang Y, Wang S, Herbstman JB. 2018. Determinants of prenatal exposure to polybrominated diphenyl ethers (PBDEs) among urban, minority infants born between 1998–2006. Environ Pollut 223:774–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol 18:495–500 [DOI] [PubMed] [Google Scholar]
- 43. Braverman LE, Cooper D. (eds) 2013. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. Tenth edition. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 44. Jones B, Nagin D, Roeder K. 2001. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methodol Res 29:374–393 [Google Scholar]
- 45. Nagin D. 2005. Group-Based Modeling of Development. Harvard University Press, Cambridge, MA [Google Scholar]
- 46. Elmlinger MW, Kuhnel W, Lambrecht HG, Ranke MB. 2001. Reference intervals from birth to adulthood for serum thyroxine (T4), triiodothyronine (T3), free T3, free T4, thyroxine binding globulin (TBG) and thyrotropin (TSH). Clin Chem Lab Med 39:973–979 [DOI] [PubMed] [Google Scholar]
- 47. Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, Diaz D, Camann D, Perera FP. 2004. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol 26:373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrin Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 49. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, et al. 1995. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol 43:55–68 [DOI] [PubMed] [Google Scholar]
- 50. World Health Organization 2007. Assessment of the Iodine Deficiency Disorders and Monitoring their Elimination. World Health Organization, Geneva, Switzerland [Google Scholar]
- 51. Textor J, Hardt J, Knuppel S. 2011. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 22:745. [DOI] [PubMed] [Google Scholar]
- 52. Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, Jones RS, Park A, Zhang Y, Heidler J, Needham LL, Goldman LR. 2007. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ Health Perspect 115:1794–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect 118:712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Andersen S, Pedersen KM, Bruun NH, Laurberg P. 2002. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrin Metab 87:1068–1072 [DOI] [PubMed] [Google Scholar]
- 55. Azizi F, Vagenakis AG, Bollinger J, Reichlin S, Braverman LE, Ingbar SH. 1974. Persistent abnormalities in pituitary function following neonatal thyrotoxicosis in the rat. Endocrinology 94:1681–1688 [DOI] [PubMed] [Google Scholar]
- 56. Bagattini B, Cosmo CD, Montanelli L, Piaggi P, Ciampi M, Agretti P, Marco GD, Vitti P, Tonacchera M. 2014. The different requirement of L-T4 therapy in congenital athyreosis compared with adult-acquired hypothyroidism suggests a persisting thyroid hormone resistance at the hypothalamic–pituitary level. Eur J Endocrinol 171:615–621 [DOI] [PubMed] [Google Scholar]
- 57. Cavaliere H, Medeiros-Neto GA, Rosner W, Kourides IA. 1985. Persistent pituitary resistance to thyroid hormone in congenital versus later-onset hypothyroidism. J Endocrinol Invest 8:527–532 [DOI] [PubMed] [Google Scholar]
- 58. Walker P, Courtin F. 1985. Transient neonatal hyperthyroidism results in hypothyroidism in the adult rat. Endocrinology 116:2246–2250 [DOI] [PubMed] [Google Scholar]
- 59. Zhou T, Ross DG, DeVito MJ, Crofton KM. 2001. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci 61:76–82 [DOI] [PubMed] [Google Scholar]
- 60. Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PR, Birnbaum LS. 2009. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci 107:27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fisher DA, Klein AH. 1981. Thyroid development and disorders of thyroid function in the newborn. New Engl J Med 304:702–712 [DOI] [PubMed] [Google Scholar]
- 62. Han G, Ding G, Lou X, Wang X, Han J, Shen H, Zhou Y, Du L. 2011. Correlations of PCBs, DIOXIN, and PBDE with TSH in children's blood in areas of computer E-waste recycling. Biomed Environ Sci 24:112–116 [DOI] [PubMed] [Google Scholar]
- 63. Xu P, Lou X, Ding G, Shen H, Wu L, Chen Z, Han J, Han G, Wang X. 2014. Association of PCB, PBDE and PCDD/F body burdens with hormone levels for children in an e-waste dismantling area of Zhejiang Province, China. Sci Total Environ 499:55–61 [DOI] [PubMed] [Google Scholar]
- 64. Xu X, Liu J, Zeng X, Lu F, Chen A, Huo X. 2014. Elevated serum polybrominated diphenyl ethers and alteration of thyroid hormones in children from Guiyu, China. PLoS One 9:e113699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gascon M, Vrijheid M, Martinez D, Forns J, Grimalt JO, Torrent M, Sunyer J. 2011. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int 37:605–611 [DOI] [PubMed] [Google Scholar]
- 66. Kicinski M, Viaene MK, Den Hond E, Schoeters G, Covaci A, Dirtu AC, Nelen V, Bruckers L, Croes K, Sioen I, Baeyens W, Van Larebeke N, Nawrot TS. 2012. Neurobehavioral function and low-level exposure to brominated flame retardants in adolescents: a cross-sectional study. Environ Health 11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leijs MM, ten Tusscher GW, Olie K, van Teunenbroek T, van Aalderen WM, de Voogt P, Vulsma T, Bartonova A, Krayer von Krauss M, Mosoiu C, Riojas-Rodriguez H, Calamandrei G, Koppe JG. 2012. Thyroid hormone metabolism and environmental chemical exposure. Environ Health 11:S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jacobson MH, Barr DB, Marcus M, Muir AB, Lyles RH, Howards PP, Pardo L, Darrow LA. 2016. Serum polybrominated diphenyl ether concentrations and thyroid function in young children. Environ Res 149:222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vuong AM, Braun JM, Webster GM, Thomas Zoeller R, Hoofnagle AN, Sjodin A, Yolton K, Lanphear BP, Chen A. 2018. Polybrominated diphenyl ether (PBDE) exposures and thyroid hormones in children at age 3 years. Environ Int 117:339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fernie KJ, Marteinson SC. 2016. Sex-specific changes in thyroid gland function and circulating thyroid hormones in nestling American kestrels (Falco sparverius) following embryonic exposure to polybrominated diphenyl ethers by maternal transfer. Environ Toxicol Chem 35:2084–2091 [DOI] [PubMed] [Google Scholar]
- 71. Lefevre PL, Wade M, Goodyer C, Hales BF, Robaire B. 2016. A mixture reflecting polybrominated diphenyl ether (PBDE) profiles detected in human follicular fluid significantly affects steroidogenesis and induces oxidative stress in a female human granulosa cell line. Endocrinology 157:2698–2711 [DOI] [PubMed] [Google Scholar]
- 72. Noyes P, Stapleton H. 2014. PBDE flame retardants: toxicokinetics and thyroid hormone endocrine disruption in fish. Endocr Disruptors 2:e29430 [Google Scholar]
- 73. Sarkar D, Chowdhury JP, Singh SK. 2016. Effect of polybrominated diphenyl ether (BDE-209) on testicular steroidogenesis and spermatogenesis through altered thyroid status in adult mice. Gen Comp Endocrinol 239:50–61 [DOI] [PubMed] [Google Scholar]
- 74. Eskenazi B, Rauch SA, Tenerelli R, Huen K, Holland NT, Lustig RH, Kogut K, Bradman A, Sjodin A, Harley KG. 2017. In utero and childhood DDT, DDE, PBDE and PCBs exposure and sex hormones in adolescent boys: the CHAMACOS study. Int J Hyg Environ Health 220:364–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gao Y, Chen L, Wang C, Zhou Y, Wang Y, Zhang Y, Hu Y, Ji L, Shi R, Cui C, Ding G, Jin J, Tian Y. 2016. Exposure to polybrominated diphenyl ethers and female reproductive function: a study in the production area of Shandong, China. Sci Total Environ 572:9–15 [DOI] [PubMed] [Google Scholar]
- 76. Krassas GE, Pontikides N. 2004. Male reproductive function in relation with thyroid alterations. Best Pract Res Clin Endocrinol Metab 18:183–195 [DOI] [PubMed] [Google Scholar]
- 77. Velazquez EM, Bellabarba Arata G. 1997. Effects of thyroid status on pituitary gonadotropin and testicular reserve in men. Arch Androl 38:85–92 [DOI] [PubMed] [Google Scholar]
- 78. Jannini EA, Ulisse S, D'Armiento M. 1995. Thyroid hormone and male gonadal function. Endocr Rev 16:443–459 [DOI] [PubMed] [Google Scholar]
- 79. Asker ME, Hassan WA, El-Kashlan AM. 2015. Experimentally induced hyperthyroidism influences oxidant and antioxidant status and impairs male gonadal functions in adult rats. Andrologia 47:644–654 [DOI] [PubMed] [Google Scholar]
- 80. Chandrasekhar Y, Holland MK, D'Occhio MJ, Setchell BP. 1985. Spermatogenesis, seminal characteristics and reproductive hormone levels in mature rams with induced hypothyroidism and hyperthyroidism. J Endocrinol 105:39–46 [DOI] [PubMed] [Google Scholar]
- 81. Arthur JR, Beckett GJ, Mitchell JH. 1999. The interactions between selenium and iodine deficiencies in man and animals. Nutr Res Rev 12:55–73 [DOI] [PubMed] [Google Scholar]
- 82. Hoffman K, Webster TF, Sjodin A, Stapleton HM. 2017. Toddler's behavior and its impacts on exposure to polybrominated diphenyl ethers. J Expo Sci Environ Epidemiol 27:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjodin A, Webster TF. 2011. Exposure to PBDEs in the office environment: evaluating the relationships between dust, handwipes, and serum. Environ Health Perspect 119:1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.