Abstract
In the past 25 years, the tissue engineering field has made incredible strides in developing tangible therapies for patients in need. Applications of the tissue engineering paradigm, involving varying configurations of cells, materials, and biochemical factors, have been explored for their regenerative capacity of virtually all tissue types. The impact and learning opportunities of current tissue engineering that inspired clinical successes are summarized. In addition, challenges associated with the translation and scale-up of therapies and replacements for complex organs, such as the heart and liver, are addressed. Platforms of research thrusts, specifically cell source, materials, fabrication, scalability, and Food and Drug Administration regulatory changes, and their respective innovations are identified for their potential to address these problems. Ideally, through their progress, tissue engineering strategies can be used to create a diverse range of easily accessible patient-specific treatments that more effectively improve quality of life.
Impact Statement
In this Perspective, we discuss the impact of the past 25 years of tissue engineering on the development of clinical therapies. Based on their success and other significant research accomplishments, platforms of innovation were identified. Their discoveries will enable tissue engineering inspired therapies to meet the requirements necessary for large-scale manufacturing and Food and Drug Administration (FDA) approval for a diverse range of indications.
Keywords: tissue engineering, regenerative medicine, 3D bioprinting, clinical, personalized medicine
Foundation
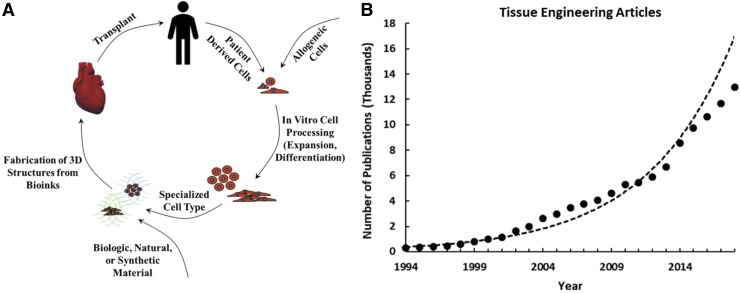
A prolific quarter of a century has been dedicated to the development and clinical translation of tissue engineering-inspired therapies, leading to an increase in methods and technology with capacity for patient impact. In 1994, 18,000 organ transplants were performed with almost double the number of patients on the waiting list for a lifesaving procedure.1 Easily identified as unsustainable statistics, this initiated the synergy of engineering and life sciences fields to develop biologic substitutes that facilitate tissue functional recovery.2 The foundation of tissue engineering involves the use of engineered materials in the presence or absence of cells and biochemical factors to restore, maintain, or improve biological tissues2 (Fig. 1A). Today, over 115,000 patients remain on the organ waiting list, and with each year the feasibility of patient-specific organ fabrication has improved.1 The increased understanding and technological toolbox have expanded the tissue engineering paradigm to new fields such as organ models, drug discovery, and cancer models.3 This has resulted in an exponential yearly increase in related discoveries and publications; 50% of the total number of tissue engineering related articles were published in the last 5 years (Fig. 1B). In parallel to the growth of technology, improvement to medical practices, and spread of information and funding, tissue engineering has made significant strides in developing tangible treatments and inspiring the next generation of medicine.4–6
FIG. 1.
(A) The tissue engineering paradigm based on discoveries in cell source, cell differentiation, material choice, and fabrication. (B) The number of published tissue engineering associated articles from 1994 to 2018, demonstrating an exponential increase [Data from pubmed.gov, PubMed database keyword search: tissue engineering].
The successes and failures of tissue engineering approaches have been learning opportunities for future research and product development. Great achievements have been made in terms of engineering materials with biochemical factors to stimulate regenerative processes and facilitate proper healing in patients. However, as of 2018, a limited number of cellular strategies have been approved by the Food and Drug Administration (FDA), with only a handful that include a combination of cells with materials.4,7,8 This Perspective will focus on the strategies that promote the translation of tissue engineered therapies with direct patient impact. Discussion points will include why the few have succeeded and what is needed to reach more indications and support full organ therapies at commercial scales.
Past Successes
While a majority of therapies are based on the fabrication of tissues outside the body, various attempts have aimed to manipulate the body's own regenerative capacity. This involves the use of natural or synthetic materials, with or without additional biological cues, to stimulate specific responses and promote healing in vivo.9 FDA approval has been achieved for innovations in both the material choice and the inclusion of biochemical factors to support tissue-specific regeneration. The Integra Dermal Regeneration Template, an acellular device made of collagen, glycosaminoglycans, and polysiloxane, was approved in 2002 for burn treatment and has since expanded to other wound healing indications.10–12 Also, the INFUSE bone graft, which uses bone morphogenic protein-2 on a collagen sponge to support bone formation, was approved in 2002 for use in lumbar fusion.13 These strategies are mostly focused on the tissues with high intrinsic regenerative capacity, such as bone, while tissues with limited regenerative capacity like cartilage may still require cellular components.9,14 The use of naturally derived extracellular matrix (ECM) or growth factor/material combinations can jump-start the body's reparatory processes to facilitate healing of critical size defects, where healing would normally not occur independently.15,16 These material-based therapeutics have demonstrated the benefit of appropriate ECM and other biological cues for regenerating different tissue types.17–19
Biologic materials have also demonstrated an incredible therapeutic potential for both allogenic and xenogeneic tissues. For example, strategies to generate decellularized tissues have led to the development of FDA-approved ECM materials for various applications, such as wound healing and nerve repair.20,21 In addition, placental tissue allograft products, which retain native tissue ECM, growth factors, and contain low immunogenicity, have demonstrated positive outcomes in treating chronic wounds.22,23 These examples demonstrate the potential of engineered methods to preserve allogeneic tissues that can provide regenerative signals upon implantation to stimulate healing.
While more challenging to create, regulate, and distribute, fabricated cell-material therapies have also made significant strides in the past 25 years. Initial successes were seen in developing skin substitutes used to facilitate wound healing. One of the first processes to use allogeneic cells for treating burns was TransCyte, which was prepared by culturing foreskin fibroblasts on nylon mesh. Their technique, which was FDA approved in 1997, solely relied on the benefits of cell-derived ECM and did not preserve cell viability following frozen storage.24,25 However, the incorporation of cellular components in implants is important for improving the extent of regeneration, increasing integration, and reducing the need for immunosuppressive drugs.26 To this aim, Apligraf, which combined foreskin-derived fibroblasts and keratinocytes on a collagen matrix, was FDA approved in 1998 for use on venous ulcers.27,28 This was followed by the FDA approval of Dermagraft, made of cryopreserved foreskin derived fibroblasts on a polyglycolic acid mesh, in 2001 for use on diabetic foot ulcers.4,29 These products were the first FDA-approved products with scaled-up manufacturing processes in which cells were cultured on a matrix in vitro before application and maintained viability upon implantation.27 To meet FDA guidelines of safety, these allogeneic cell banks are extensively tested for diseases, pathogens, and immunologic reactivity.28 Their manufacturing procedures to expand cell banks to necessary cell numbers and mature tissues show great promise for the future of cell-based applications. In addition, the products remain viable upon shipment at both 37°C and −80°C.27,30 The efficacy of these early applications was promoted by the use of allogeneic immune-privileged cells that produce cytokines and growth factors to stimulate healing in chronic wounds.27
One application of patient-derived cells/scaffold therapies is the matrix-associated autologous chondrocyte implantation (MACI), which was FDA approved for full-thickness knee cartilage defects.4 Marketed by Vericel, MACI was approved under Section 351 of the Public Service Health Act and required extensive Phase III clinical trials for premarket approval and commercial availability. MACI is prepared by seeding in vitro expanded biopsy-derived chondrocytes on a porcine type I/type III collagen matrix. Upon implantation, MACI has demonstrated efficacy and safety for up to 15 years.4,31,32 By adding the material component, this product avoids the problems of the cellular therapy (ACI), such as cell leakage, chondrocyte dedifferentation, and postoperative hypertrophy.31,33,34 This is another facet of the tissue-engineering approach where cell phenotype and functional recovery can be improved through the use of three-dimensional (3D) materials compared to two-dimensional culture alone.35,36 In this approach, the FDA approved processes include autologous cells and an in vitro cultured device—opening the door for future adaptions of these methods.
Current State of Clinical Advances
New technologies for the clinical translation of larger vascularized tissues have been developed due to the success of tissue engineering. Current clinical trials are investigating acellular and cellular solutions for a variety of treatments—growing the tissue engineering market.6 Unique engineering of material components targeting intrinsic regeneration have expanded the reach of tissue engineered materials for clinical applications in cardiac, musculotendinous, and nerve repair.37–39 Devices made of biodegradable polymers, such as the Neuro-Spinal Scaffold prepared by the solvent casting and porogen leaching of poly-lactic-co-glycolic acid and poly-L-lysine, are currently under clinical investigation for spinal cord injury repair.40,41 By tailoring the degradation rate and including positively charged functional groups, this device recently demonstrated improved spinal cord recovery in large animal models by supporting neuronal cell attachment, cell migration, and nutrient diffusion for functional tissue remodeling.41
In addition, FDA-approved processes, such as in vitro expansion and tissue maturation, have been applied to new applications. This includes products from cellular-derived ECM, such as the Humacyte acellular vascular graft, where allogeneic smooth muscle cells are seeded on polyglycolic acid scaffolds, cultured in bioreactors to deposit ECM, and decellularized to remove cellular components.42 This device has demonstrated safety and efficacy greater than that of synthetic grafts in a recent phase II trial, and, due to the decellularization procedure, maintains the off the shelf availability.42,43 Other tissues, such as bladder and vascular graft replacements, that combine both cells and materials have already received significant recognition for successfully treating patients.44,45
Despite these successes, following FDA approval concerns have surfaced regarding the use of exogenous growth factors. For example, off-label use of INFUSE has been responsible for side effects, such as ectopic bone formation and increased reoperation rate.46 Questions of effective dose, extended release, side effects, and cytotoxicity have limited the translation of other growth factor therapies.19 The identification of optimal materials that can localize growth factors to limit side effects and support tissue specific regeneration is still under investigation.19
The development of therapies for solid organs, such as the heart and liver, requires more complex organization of materials and growth factors to support multiple cell types, tissue structure, and vascular networks.47 Earlier cell-material liver implants, which relied on in vivo angiogenesis to provide vascular structures for newly formed tissue, had success in animal models but did not effectively translate to larger structures for human applications.48 The last decade of tissue engineering has seen incredible discoveries in engineering cells, materials, and tissue architecture to promote vasculature and organ-specific cellular phenotypes in implantable constructs.49 For patient availability, these need to meet FDA guidelines for host compatibility, sterility, and functionality. Even with such an urgent need, tissue-engineering therapies will require extended approval timelines, requiring identification of complex mechanisms and overcoming funding challenges.4 The challenge of the next decennium will focus on the scalability of these discoveries to commercially available therapies. Ideally, these therapies will encompass the use of cells and materials to form implantable matured tissues that can incorporate into native tissue for better healing and long-term outcomes.
Future Directions
Specific platforms stand out for solving issues required for translating more complex organ therapies. Tissue engineering thrusts are aimed at combining these different technologies for specific tissue restoration. The following have been identified as critical components that will ideally allow for clinical availability of many tissue engineered products.
Cell source
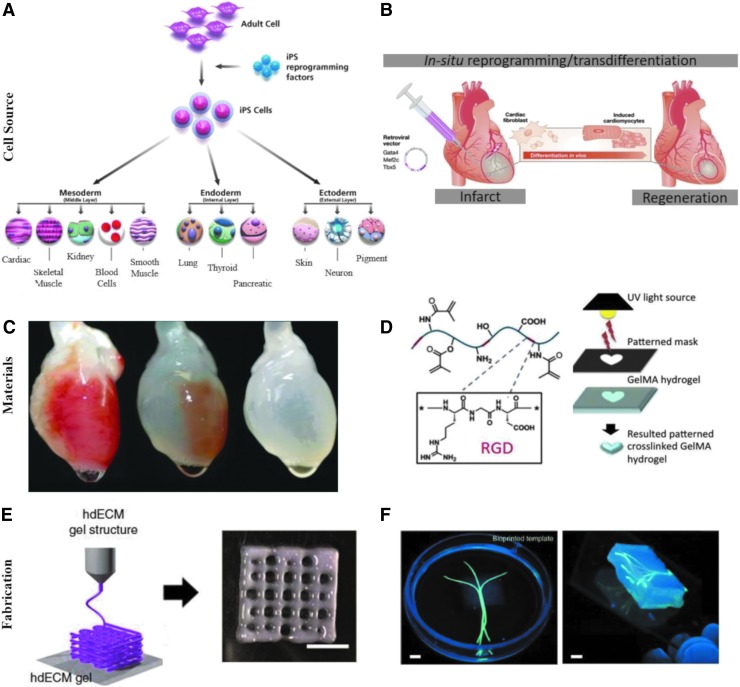
Currently, Section 351-approved devices use either autologous cells that require invasive biopsies and extended culture times or allogeneic differentiated cells with safety concerns. The identification of an optimal source of cells that do not lead to immune rejection is critical for scalable translation. Many of these cell origins are currently under clinical investigation as cell therapies. Autologous bone marrow or adipose derived mesenchymal stem cells (MSCs) with multilineage potential have demonstrated safety and efficacy for the treatment of many diseases and organs.50 Most importantly, the potential of induced pluripotency introduced by Yamanaka in 2007 opened the doors for a new generation of patient-specific cells51,52 (Fig. 2A). The ability to generate functional differentiated cells of any tissue type from patient-derived fibroblasts holds incredible potential for tissue engineering applications.53 Although these cells require complicated methods that lead to side effects, his work inspired modifications for clinical accessibility and safety, such as use of nonviral vectors and direct reprogramming.54 Recently, large efforts have been focused on in situ direct reprogramming with interesting applications for cell/material therapies55,56 (Fig. 2B). In addition, there have been numerous studies to identify an allogeneic stem cell source. For example, placental derived stem cells have been shown to behave similarly to MSCs without inducing an immune response.50 Other strategies include the use of viral vectors to remove the human leukocyte antigen expression of stem cells, creating an “off the shelf” donor cell that can be applied to any patient without an immune response.57
FIG. 2.
Platforms of tissue engineering innovation that will facilitate clinical translation. Examples include cell sources of induced pluripotent stem cells (A) [Photo courtesy of Millipore Sigma. Used with permission] and direct/in situ reprogramming strategies (B) [Adapted from Dewitt, N.D., and Trounson A., with permission56] materials of decellularized organs (C) [Adapted from Ott et al., with permission63] or natural hydrogels (D) [Adapted from Yue et al., with permission67], and 3D printing fabrication techniques using decellularized extracellular matrix inks (E) [Adapted from Pati et al.70] or hydrogels (F) [Adapted from Bertassoni et al., with permission from the Royal Society of Chemistry79]. 3D, three-dimensional.
Materials
A critical requirement is adaptation of these cell therapies onto effective scaffolds to promote tissue repair through controlling cell behavior and in vivo responses. The ideal scaffold should include biological and mechanical factors to support specific tissue formation.58 Various 3D platforms are standouts in their ability to support cell viability, support tissue-specific differentiation, and integrate upon implantation. Decellularized tissues, both intact or processed from allogeneic or xenogeneic origin, allow for the application of native ECM that positively influences cellular behavior.59,60 By removing cellular components, the matrix can be transplanted into any patient without rejection, which enhances its ability to be broadly used.61,62 Various efforts have been made for both ideal decellularization protocols and storage parameters. Decellularized matrices of many tissue types have been clinically tested and are commercially available for many indications.61,62 In addition, decellularized whole organs keep vascular networks intact and have the potential to be reseeded with autologous cells for tissue maturation before implantation63–65 (Fig. 2C).
Hydrogels can also be used for complex tissue formation. Both natural and synthetic materials, or their combination, have been used to synthesize hydrogels with tailored properties. For example, gelatin methacryloyl has tunable mechanical properties, functionalization, cell encapsulation, drug elution, degradation, and smart responsive behavior, which allow for adaptions to many different organ systems66,67 (Fig. 2D). Tuning the compositions of material properties and growth factors in acellular and cellular approaches to recapitulate the native environment have been effective in facilitating healing.68 Recent efforts investigating the generation of personalized hydrogels from patient biopsies seeded with autologous cells have potential applications for many organ types with reduced risk of immune rejection.69
Fabrication and maturation
Another important aspect is the fabrication of these previously mentioned materials into complex structures. Vascular network development is essential for fabrication of complex organ replacements. 3D printing provides exciting potential to construct either decellularized ECM (Fig. 2E) or hydrogels into tissue-specific structures for in vitro modeling or facilitating proper in vivo tissue formation.70,71 Through the development of bioinks, precise control over deposition location, different printing techniques, materials with specific functionalization and mechanical properties, and distinct cell populations, 3D printing can create structures resembling complex organs.72–75 For example, recent efforts have used 3D printing techniques to model the central nervous system and spinal cord or create implantable devices that improve functional recovery following spinal cord injury.76,77 In addition, recent advances in vascularization strategies, including use of sacrificial inks or endothelial cell layers, will allow for fabrication and cell survival in larger constructs78,79 (Fig. 2F). Current innovations of thick vascularized tissues with extended viability of multiple cell types demonstrate the potential development of complex, functional ex vivo organs that mimic native architecture.80 Combined with personalized cell sources and applicable materials, engineered tissues could be developed that are tailored to the patient.
The use of large-scale culturing techniques in bioreactors to generate sufficient quantities of relevant cell populations (e.g., induced pluripotent stem cells) have been previously investigated.81–84 In addition, bioreactor culture conditions and stimuli are being optimized for cell differentiation and ex vivo tissue maturation.83
Manufacturing and scalability
Many tissue strategies have been successful on the benchtop, but producing these tissues at commercially relevant scales continues to be a challenge. The generation of cells85 and 3D printing of larger tissues with viable processes75 remain as ongoing efforts. The latter is the current focus of many companies such as Organovo, CELLINK, and Allevi.86 In addition, recent initiatives such as Advanced Regenerative Manufacturing Institute are supporting the necessary studies to identify critical components needed to scale-up effective technologies to reach patients.87 The transition of discovered technologies and therapies to a commercial production scale is crucial for the widespread application of tissue engineering.
FDA regulatory changes
As technology has advanced in the past 25 years, it has been difficult for the FDA to adjust regulations for combination products of materials and cells with demonstrated efficacy in animal models. Requirement of extensive phase III clinical trials for therapies involving autologous cells in previously demonstrated safe-to-use materials has been a topic of debate.4,18 Proposed regulation updates, if adopted by the FDA, suggest a new classification that will allow these materials to be available for patients earlier and reach full approval after 7 years of demonstrated safety and efficacy.88 This will aid in funding concerns, which have precluded many developed technologies from reaching the market due to the high cost of Phase III clinical trials.4,88 This will increase the feasibility of synergizing independently approved cell and material therapies for more effective treatments.
Expanding applications
The combined use of cells and material technology expands beyond tissue fabrication and restoration. Tissue-engineered approaches for the treatment of genetic conditions and systemic diseases are the focus of many academic and commercial clinical efforts. Examples include the application of polymer-encapsulated engineered insulin-producing cells, which recently demonstrated long-term glycemic control in a diabetic rat model.89 Commercial efforts are working toward applying the encapsulated engineered cell platform in clinical trials for the treatment of diabetes, hemophilia, or lysosomal storage disorders.90
Tissue engineering has affected the medical community beyond regenerative medicine. Combinations of previously listed technologies can be developed into clinically available patient-specific diagnostic tools, such as organ on a chip or organoid systems. These models can mimic the structure and function of specific organs on smaller scales and be used to determine drug responses on patient-derived cells.49,91,92 This technology is currently being evaluated by the FDA and is expected to have dramatic impacts on the medical field, leading to more efficacious use of drugs, lowering costs of approval studies, and predicting negative side effects.92,93
In addition, the reach expands beyond direct patient impact. The applications of tissue engineering techniques to the in vitro production of cultured meat for consumption and leather have significant societal benefits by decreasing environmental impact, risk of disease, animal use, and ethical concerns.94 Interestingly, the cultured meat industry is also investigating optimal cell sources, material choices, and fabrication processes to best recreate muscle tissue.94
Enabling future success
Other platforms of regenerative medicine offer exciting potential for tissue engineering-inspired applications. SiRNA as a mechanism of influencing cellular behavior for regeneration has been demonstrated using in vitro and in vivo models.95 Recently, the first application of a siRNA-based treatment was FDA approved as ONPATTRO for the treatment of peripheral nerve disease.96 In addition, gene therapy can be adapted to achieve desired cell behavior and in vivo tissue repair.97 The strides in FDA approval and positive clinical results of gene therapies indicate an exciting future for adaptations to tissue engineering applications.7 Furthermore, CRISPR technology opens the door for high accuracy gene editing for cell differentiation, angiogenesis, immunoengineering, or even increasing the transplantable organ supply.98,99
Conclusions
Although the many successes demonstrated in publications are not yet available to treat patients, the past 25 years of both scientific and clinical discoveries pave the way for more effective clinically translated therapies. Tissue engineering continues to inspire the collaboration of many fields to create biologically relevant applications. As discoveries are made in individual fields, such as a better understanding of developmental biology, the combined efforts of multiple fields can recapitulate these findings into therapies or medical treatments at a much greater rate. In the upcoming decade, the previously mentioned pillars can be further developed and enable the clinical translation of therapies for many organ systems. With this development, the goal of easily distributed and patient-specific treatments can be achieved—alleviating a wide range of problems, from injuries with limited healing capacity to treatment of genetic disorders, and improving quality of life for millions of patients.
Acknowledgments
The authors acknowledge funding from the National Institutes of Health (EB021857, AR066193, AR057837, HL137193, EB023052, EB021857, and EB021857) and Air Force Office of Sponsored Research under award #FA9550-15-1-0273.
Disclosure Statement
No competing financial interests exist for T.H. and A.K.
R.L. has commercial associations with Tara Biosystems, Humacyte, In Vivo Therapeutics, Frequency Therapeutics, Aleph Farms, Allevi, Vivitex, Sigilon Therapeutics, Alnylam, and StemBioSys.
References
- 1. Department of Health and Human Services. Organ Donation and Transplantation Statistics: Graph Data. 2018. Available at: www.organdonor.gov/statistics-stories/statistics/data.html (last accessed December19, 2018).
- 2. Langer R., and Vacanti J.P. Tissue engineering. Science 260, 920, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Zorlutuna P., Vrana N.E., and Khademhosseini A. The expanding world of tissue engineering: the building blocks and new applications of tissue engineered constructs. IEEE Rev Biomed Eng 6, 47, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yano K., Speidel A.T., and Yamato M. Four Food and Drug Administration draft guidance documents and the regrow act: a litmus test for future changes in human cell- and tissue-based products regulatory policy in the United States? J Tissue Eng Regen Med 12, 1579, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lysaght M.J., and Reyes J. The growth of tissue engineering. Tissue Eng 7, 485, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Kim Y.S., Smoak M.M., Melchiorri A.J., and Mikos A.G. An overview of the tissue engineering market in the United States from 2011 to 2018. Tissue Eng Part A 25, 1, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Food and Drug Administration. Approved Cellular and Gene Therapy Products. 2018. Available at: www.fda.gov/biologicsbloodvaccines/cellulargenetherapyproducts/approvedproducts/default.htm (last accessed January4, 2019).
- 8. Mao A.S., and Mooney D.J. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A 112, 14452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dzobo K., Thomford N.E., Senthebane D.A., et al. Advances in regenerative medicine and tissue engineering: innovation and transformation of medicine. Stem Cells Int 2018, 2495848, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Food and Drug Administration. Premarket Approval (Pma) Integra Dermal Regeneration Template. 2002. Available at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P900033 (last accessed January7, 2019).
- 11. Heimbach D.M., Warden G.D., Luterman A., et al. Multicenter postapproval clinical trial of integra dermal regeneration template for burn treatment. J Burn Care Rehabil 24, 42, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Food and Drug Administration. FDA Approves Integra Omnigraft Dermal Regeneration Matrix to Treat Diabetic Foot Ulcers. 2016. Available at: www.fda.gov/newsevents/newsroom/pressannouncements/ucm480564.htm (last accessed January7, 2019).
- 13. Summary of Safety and Effectiveness Data for Infuse Bone Graft/Lt-Cage™ Lumbar Tapered Fusion Device (Pma Number P000058). 2002. Available at: www.accessdata.fda.gov/cdrh_docs/pdf/p000058b.pdf (last accessed January4, 2019).
- 14. Karuppal R. Current concepts in the articular cartilage repair and regeneration. J Orthop 14, A1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee K., Silva E.A., and Mooney D.J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8, 153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vo T.N., Kasper F.K., and Mikos A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev 64, 1292, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tatara A.M., and Mikos A.G. Tissue engineering in orthopaedics. J Bone Joint Surg Am 98, 1132, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Webber M.J., Khan O.F., Sydlik S.A., Tang B.C., and Langer R. A perspective on the clinical translation of scaffolds for tissue engineering. Ann Biomed Eng 43, 641, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z.M., Wang Z.F., Lu W.W., Zhen W.X., Yang D.Z., and Peng S.L. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Materials 9, e435, 2017 [Google Scholar]
- 20. Means K.R., Jr., Rinker B.D., Higgins J.P., Payne S.H., Jr, Merrell G.A., and Wilgis E.F. A multicenter, prospective, randomized, pilot study of outcomes for digital nerve repair in the hand using hollow conduit compared with processed allograft nerve. Hand (N Y) 11, 144, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kimmel H., and Gittleman H. Retrospective observational analysis of the use of an architecturally unique dermal regeneration template (Derma Pure®) for the treatment of hard-to-heal wounds. Int Wound J 14, 666, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavery L.A., Fulmer J., Shebetka K.A., et al. The efficacy and safety of Grafix((R)) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J 11, 554, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duan-Arnold Y., Gyurdieva A., Johnson A., Uveges T.E., Jacobstein D.A., and Danilkovitch A. Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv Wound Care (New Rochelle) 4, 523, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noordenbos J., Dore C., and Hansbrough J.F. Safety and efficacy of transcyte for the treatment of partial-thickness burns. J Burn Care Rehabil 20, 275, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Transcyte Human Fibroblast-Derived Temporary Skin Substitute. 1997. Available at: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P960007 (last accessed January18, 2019).
- 26. Heath C.A. Cells for tissue engineering. Trends Biotechnol 18, 17, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Zaulyanov L., and Kirsner R.S. A review of a bi-layered living cell treatment (Apligraf®) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin Interv Aging 2, 93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilkins L.M., Watson S.R., Prosky S.J., Meunier S.F., and Parenteau N.L. Development of a bilayered living skin construct for clinical applications. Biotechnol Bioeng 43, 747, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Marston W.A. Dermagraft®, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev Med Devices 1, 21, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Marston W.A., Hanft J., Norwood P., Pollak R., and Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26, 1701, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Behrens P., Bitter T., Kurz B., and Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 13, 194, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Gille J., Behrens P., Schulz A.P., Oheim R., and Kienast B. Matrix-associated autologous chondrocyte implantation: a clinical follow-up at 15 years. Cartilage 7, 309, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marlovits S., Zeller P., Singer P., Resinger C., and Vecsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol 57, 24, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Schuette H.B., Kraeutler M.J., and McCarty E.C. Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid- to long-term clinical outcomes. Orthop J Sports Med 5, 2325967117709250, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng M.H., Willers C., Kirilak L., et al. Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment. Tissue Eng 13, 737, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Flaningan D.C., Everhart J.S., and Early N.A. Autologous chondrocyte implantation: scaffold-based solutions. In: Zorzi A.R., and Miranda J.B., eds. Cartilage Repair and Regeneration. London, UK: IntechOpen Limited, 2018. DOI: 10.5772/intechopen.70276 [DOI] [Google Scholar]
- 37. ClinicalTrials.gov A Study of Ventrigel in Post-Mi Patients. NCT02305602, 2014. Available at: https://clinicaltrials.gov/ct2/show/NCT02305602 (last accessed January7, 2019).
- 38. ClinicalTrials.gov Musculotendinous Tissue Repair Unit and Reinforcement (Mturr). NCT01292876, 2011. Available at: https://clinicaltrials.gov/ct2/show/NCT01292876 (last accessed January6, 2019).
- 39. ClinicalTrials.gov Study of Nerve Reconstruction Using Avance in Subjects Who Undergo Robotic Assisted Prostatectomy for Treatment of Prostate Cancer. NCT00953277, 2009. Available at: https://clinicaltrials.gov/ct2/show/NCT00953277 (last accessed January7, 2019).
- 40. ClinicalTrials.gov The Inspire Study: Probable Benefit of the Neuro-Spinal Scaffold for Treatment of Ais a Thoracic Acute Spinal Cord Injury. NCT02138110, 2014. Available at: https://clinicaltrials.gov/ct2/show/NCT02138110 (last accessed January6, 2019).
- 41. Slotkin J.R., Pritchard C.D., Luque B., et al. Biodegradable scaffolds promote tissue remodeling and functional improvement in non-human primates with acute spinal cord injury. Biomaterials 123, 63, 2017 [DOI] [PubMed] [Google Scholar]
- 42. Dahl S.L., Kypson A.P., Lawson J.H., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 3, 68ra9, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Lawson J.H., Glickman M.H., Ilzecki M., et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet 387, 2026, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Atala A., Bauer S.B., Soker S., Yoo J.J., and Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367, 1241, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Shin'oka T., Imai Y., and Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med 344, 532, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Epstein N.E. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg Neurol Int 4, S343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Atala A., Kasper F.K., and Mikos A.G. Engineering complex tissues. Sci Transl Med 4, 160rv12, 2012 [DOI] [PubMed] [Google Scholar]
- 48. Langer R., and Vacanti J. Advances in tissue engineering. J Pediatr Surg 51, 8, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khademhosseini A., and Langer R. A decade of progress in tissue engineering. Nat Protoc 11, 1775, 2016 [DOI] [PubMed] [Google Scholar]
- 50. Trounson A., and McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17, 11, 2015 [DOI] [PubMed] [Google Scholar]
- 51. Takahashi K., Tanabe K., Ohnuki M., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Takahashi K., and Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Pagliuca F.W., Millman J.R., Gurtler M., et al. Generation of functional human pancreatic beta cells in vitro. Cell 159, 428, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu J., Du Y., and Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell 16, 119, 2015 [DOI] [PubMed] [Google Scholar]
- 55. Srivastava D., and DeWitt N. In vivo cellular reprogramming: the next generation. Cell 166, 1386, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. DeWitt N.D., and Trounson A. Direct conversion in the heart: a simple twist of fate. EMBO J 31, 2244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gornalusse G.G., Hirata R.K., Funk S.E., et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol 35, 765, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kretlow J.D., and Mikos A.G. 2007 Aiche Alpha Chi Sigma Award: From Material to Tissue: Biomaterial Development, Scaffold Fabrication, and Tissue Engineering. AIChE J 54, 3048, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Badylak S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol 12, 367, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Dahms S.E., Piechota H.J., Dahiya R., Lue T.F., and Tanagho E.A. Composition and biomechanical properties of the bladder acellular matrix graft: comparative analysis in rat, pig and human. Br J Urol 82, 411, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Parmaksiz M., Dogan A., Odabas S., Elcin A.E., and Elcin Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed Mater 11, 022003, 2016 [DOI] [PubMed] [Google Scholar]
- 62. Gilpin A., and Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed Res Int 2017, 9831534, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ott H.C., Matthiesen T.S., Goh S.K., et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14, 213, 2008 [DOI] [PubMed] [Google Scholar]
- 64. Chen K.L., Eberli D., Yoo J.J., and Atala A. Bioengineered corporal tissue for structural and functional restoration of the penis. Proc Natl Acad Sci U S A 107, 3346, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Petersen T.H., Calle E.A., Zhao L., et al. Tissue-engineered lungs for in vivo implantation. Science 329, 538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang Y.S., and Khademhosseini A. Advances in engineering hydrogels. Science 356, eaaf3627, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., and Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (gelma) hydrogels. Biomaterials 73, 254, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Klotz B.J., Lim K.S., Chang Y.X., et al. Engineering of a complex bone tissue model with endothelialised channels and capillary-like networks. Eur Cell Mater 35, 335, 2018 [DOI] [PubMed] [Google Scholar]
- 69. Edri R., Gal I., Noor N., et al. Personalized hydrogels for engineering diverse fully autologous tissue implants. Adv Mater 31, e1803895, 2019 [DOI] [PubMed] [Google Scholar]
- 70. Pati F., Jang J., Ha D.H., et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5, 3935, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bajaj P., Schweller R.M., Khademhosseini A., West J.L., and Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng 16, 247, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jang J., Park H.J., Kim S.W., et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 112, 264, 2017 [DOI] [PubMed] [Google Scholar]
- 73. Derakhshanfar S., Mbeleck R., Xu K., Zhang X., Zhong W., and Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater 3, 144, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yan Q., Dong H.H., Su J., et al. A review of 3D printing technology for medical applications. Engineering 4, 729, 2018 [Google Scholar]
- 75. Murphy S.V., and Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 32, 773, 2014 [DOI] [PubMed] [Google Scholar]
- 76. Joung D., Truong V., Neitzke C.C., et al. 3D printed stem-cell derived neural progenitors generate spinal cord scaffolds. Adv Funct Mater 28, 1801850, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Koffler J., Zhu W., Qu X., et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med 25, 263, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li X., Liu L., Zhang X., and Xu T. Research and development of 3D printed vasculature constructs. Biofabrication 10, 032002, 2018 [DOI] [PubMed] [Google Scholar]
- 79. Bertassoni L.E., Cecconi M., Manoharan V., et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 14, 2202, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kolesky D.B., Homan K.A., Skylar-Scott M.A., and Lewis J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 113, 3179, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Krawetz R., Taiani J.T., Liu S., et al. Large-scale expansion of pluripotent human embryonic stem cells in stirred-suspension bioreactors. Tissue Eng Part C Methods 16, 573, 2010 [DOI] [PubMed] [Google Scholar]
- 82. Kropp C., Massai D., and Zweigerdt R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process Biochem 59, 244, 2017 [Google Scholar]
- 83. Martin I., Wendt D., and Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol 22, 80, 2004 [DOI] [PubMed] [Google Scholar]
- 84. Kempf H., Olmer R., Kropp C., et al. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Reports 3, 1132, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pigeau G.M., Csaszar E., and Dulgar-Tulloch A. Commercial scale manufacturing of allogeneic cell therapy. Front Med (Lausanne) 5, 233, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. The Medical Futurist. The Top Bioprinting Companies. 2018. Available at: https://medicalfuturist.com/top-biopriting-companies (last accessed December29, 2018).
- 87. Advanced Regenerative Manufacturing Institute. Manufacturing the Future of Biofabrication. 2018. Available at: www.armiusa.org (last accessed December29, 2018).
- 88. Bipartisan Policy Center. Advancing Regenerative Cellular Therapy: Medical Innovation for Healthier Americans. 2015. Available at: https://bipartisanpolicy.org/wp-content/uploads/2015/12/BPC-Advancing-Regenerative-Cellular-Therapies.pdf (last accessed December19, 2018).
- 89. Vegas A.J., Veiseh O., Gurtler M., et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 22, 306, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sigilon Therapeutics, Inc. Pipeline. 2019. Available at: http://sigilon.com/pipeline (last accessed January13, 2019).
- 91. Lancaster M.A., Renner M., Martin C.A., et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Esch E.W., Bahinski A., and Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 14, 248, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Food and Drug Administration. FDA Researchers to Evaluate “Organs-on-Chips” Technology. 2017. Available at: www.fda.gov/food/newsevents/constituentupdates/ucm551503.htm (last accessed January13, 2019).
- 94. Stephens N., Di Silvio L., Dunsford I., Ellis M., Glencross A., and Sexton A. Bringing cultured meat to market: technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci Technol 78, 155, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cheema S.K., Chen E., Shea L.D., and Mathur A.B. Regulation and guidance of cell behavior for tissue regeneration via the SiRNA mechanism. Wound Repair Regen 15, 286, 2007 [DOI] [PubMed] [Google Scholar]
- 96. Food and Drug Administration. FDA Approves First-of-Its Kind Targeted Rna-Based Therapy to Treat a Rare Disease. 2018. Available at: www.fda.gov/newsevents/newsroom/pressannouncements/ucm616518.htm (last accessed January4, 2019).
- 97. Heyde M., Partridge K.A., Oreffo R.O., Howdle S.M., Shakesheff K.M., and Garnett M.C. Gene therapy used for tissue engineering applications. J Pharm Pharmacol 59, 329, 2007 [DOI] [PubMed] [Google Scholar]
- 98. Pulgarin D.A.V., Nyberg W.A., Bowlin G.L., and Espinosa A. CRISPR/CAS systems in tissue engineering: a succinct overview of current use and future opportunities. Curr Trends Biomedical Eng Biosci 5, 555670, 2017 [Google Scholar]
- 99. Yang L., Guell M., Niu D., et al. Genome-wide inactivation of porcine endogenous retroviruses (Pervs). Science 350, 1101, 2015 [DOI] [PubMed] [Google Scholar]