Abstract
Objective: The goal was to test whether stone composition and kidney phantom configuration affected comminution in extracorporeal shockwave lithotripsy (SWL) laboratory tests. Confinement may enhance the accumulation of dust and associated cavitation bubbles in the fluid surrounding the stone. It is known that high shockwave delivery rates in SWL are less effective because bubbles generated by one shockwave do not have sufficient time to dissolve, thereby shielding the next shockwave.
Materials and Methods: Experiments were conducted with a lithotripter coupled to a water bath. The rate of comminution was measured by weighing fragments over 2 mm at 5-minute time points. First, plaster and crystal stones were broken in four phantoms: a nylon wire mesh, an open polyvinyl chloride (PVC) cup, a closed PVC cup, and an anatomical kidney model—the phantoms have decreasing fluid volumes around the stone. Second, the fluid volume in the kidney model was flushed with water at different rates (0, 7, and 86 mL/min) to remove dust.
Results: The efficiency of breakage of stones decreases for the dust emitting plaster stones (percentage of breakage in 5 minutes decreased from 92% ± 2% [n = 3] in wire mesh to 19% ± 3% [n = 3] in model calix) with increasing confinement, but not for the calcite crystal stones that produced little dust (percentage of breakage changed from 87% ± 3% [n = 3] in wire mesh to 81% ± 3% [n = 3] in kidney model). Flushing the kidney phantom at the fastest rate improved comminution of smaller plaster stones by 27%.
Conclusions: Phantoms restricting dispersion of dust were found to affect stone breakage in SWL and in vitro experiments should replicate kidney environments. The dust around the stone and potential cavitation may shield the stone from shockwaves and reduce efficacy of SWL. Understanding of stone composition and degree of hydronephrosis could be used to adapt patient-specific protocols.
Keywords: shockwave lithotripsy, renal calculi, cavitation, kidney phantom
Introduction
Extracorporeal shockwave lithotripsy (SWL) research often involves in vitro assessment of stone fragmentation. The experimental setup involves several factors that can affect stone breakage and may not mimic a biologic environment. For instance, shockwaves may generate a cavitation bubble cluster proximal to the stone, which scatters energy and reduces effective comminution.1–4 Cavitation shielding is affected by conditions of the shockwave, such as power setting or amplitude, wave shape, and wave frequency and fluid conditions such as volume, gas content, and level of filtration.5–8 As a result, water is often filtered and degassed for in vitro experiments to remove bubbles and suspended submillimeter particles. Others have used castor oil to suppress cavitation and/or held stones in confined spaces to mimic the enclosed space of a calix.9 There is limited ability to affect fluid conditions in treatment, but if they are not mimicked accurately in an in vitro stone breakage experiment, shielding that occurs in vivo may not be accurately simulated.
Although small dispersed dust particles themselves have little effect on shockwaves because their small size causes minimal reflection of acoustic energy, such particles can form niduses for bubbles.10 Because bubbles contain gas and oscillate in the pressure field, they are more reflective than similarly sized dust particles.11 Consequently, if stone comminution by SWL produces dust and bubbles, then bubble proliferation with successive shocks can ultimately shield the stone from effective comminution.8
In an effort to develop accurate models and also understand the SWL comminution process within the kidney collecting system, we researched the impact of dust (suspended submillimeter particles) and fluid confinement on comminution through multiple experiments. We compare two types of artificial stones, one that creates a significant amount of dust and the other that creates minimal dust. In addition, we fragmented these stones in different types of tissue phantoms, which varied in the degree of confinement of fluid and dust around the stone. The least confined phantom allowed unhindered dispersion of dust into water in the tank, whereas the most confined phantom—an anatomic kidney model—did not allow the dust to leave a calix holding the stone. Furthermore, we irrigated the anatomic kidney phantom to displace the dust actively during fragmentation and observed changes in rate of comminution. In general, broken stone fragments can also exist in the acoustic path and shield distal fragments from SWL energy; however, in this study, incident shockwaves only passed through dust dissipating away from the stone.
Materials and Methods
Setup
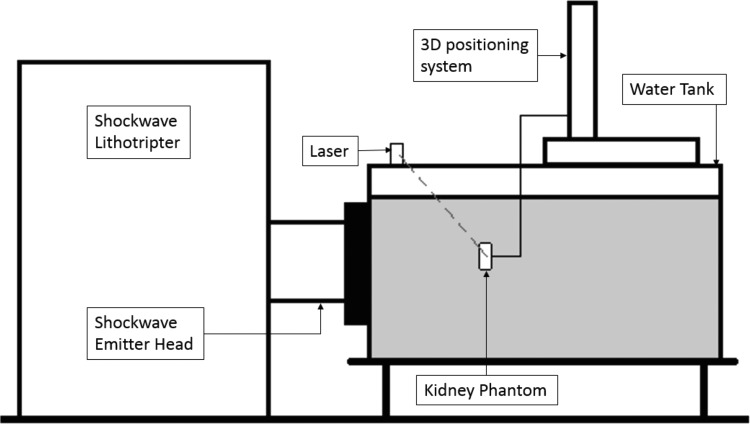
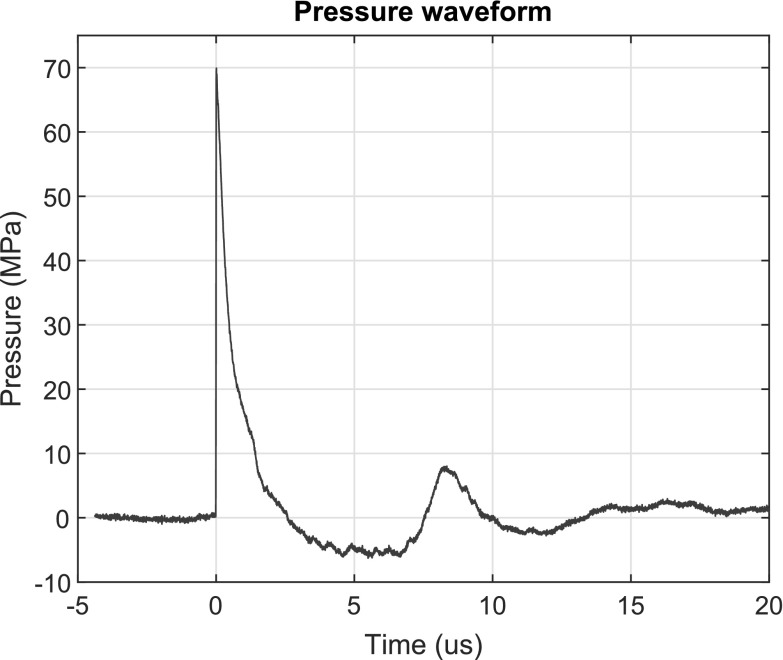
The experimental configuration is shown in Figure 1. A 190-L water tank was filled with deionized water filtered through a 0.5-μm sediment filter. The water was held at 18°C, degassed to maintain a gas concentration corresponding to 50% of the saturation level of dissolved oxygen4,12 (Oxi 330i meter with a CellOx 325 probe; WTW, Weilheim, Germany). A shockwave emitter (Compact S; Dornier MedTech, Wessling, Germany) was coupled to the water tank to deliver shocks along a horizontal beam axis. The shockwave lithotripter was operated at a power level of 5 (range 1–6) and a repetition rate of 70 shocks per minute. The free-field focal pressure waveform of the lithotripter at this power level was measured using a fiber optic hydrophone (Model FOPH 2000; RP Acoustics, Leutenbach, Germany) and is shown in Figure 2.
FIG. 1.
The figure shows setup of the experiment. A shockwave lithotripter was coupled with a water tank such that the shockwaves traveled horizontally. A kidney phantom holding the stone to be broken was positioned at the focus of the lithotripter with the help of 3D positioning system. The focus coincided with the intersection of two laser beams. The two lasers were placed on the side walls of the tank.
FIG. 2.
Pressure waveform of Dornier Compact S lithotripter in free field. The lithotripter was operated at power level 5 and 70 shocks per minute.
Phantoms
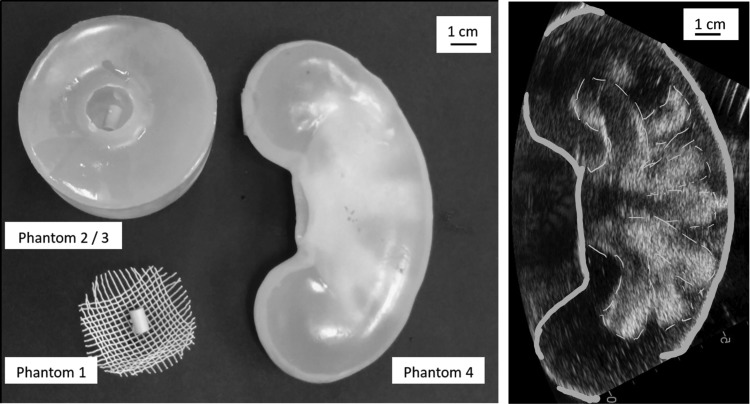
Different types of phantoms (Fig. 3) were used for holding stones. Each phantom confined the fluid around the stone to a different degree. Phantom 1 was a hemispherical-shaped mesh basket made from nylon strands with 2 mm pores and a 35 mm diameter. Phantom 2 was a 60-mm-diameter × 70-mm-tall cylindrical “cup” made of polyvinyl chloride (PVC). The “cup” descriptor refers to the presence of a depression (15 mm diameter × 40 mm height) in the top flat surface. The base of the depression was hemispherical to maintain all fragments near the center of the focus, as Qin et al. have shown reduced comminution when fragments have sufficient space to move out of the focus.13 Shockwaves entered from the side of the cup and traversed 22.5 mm of phantom before reaching the stone within the depression. We anticipate little change in the waveforms due to attenuation between phantoms (5% loss over 15 mm at 1 MHz in standard tissue14). To address potential waveform differences between phantoms, the experiment was also performed with the top surface of phantom 2 covered with Paraffin® tape to further confine fluid around the stone and restrict the exchange of water between the depression and the bulk water in the tank (phantom 3). The sound speed in the PVC is 1400 m/s and has attenuation comparable with human tissue.15 Phantom 4 was an anatomical kidney model made from gelatin powder and water (Blue Phantom, Redmond, WA). The sound speed in this phantom is 1450 m/s and has little attenuation compared with human tissue. The kidney phantom had a small slit at the ureteropelvic junction through which stones could be placed into the same interpolar minor calix for all experiments. The shockwaves entered at a 90° angle to the anterior surface of kidney, and the thickness of phantom traversed was 15 mm. Although the mesh basket from phantom 1 is not associated with a volume per se, phantoms 2–4 provide differing amounts of confinement for respective volumes of 7, 7, and 3 mL, but with acoustic properties similar to tissue. A combination of ultrasound imaging, a pointer, and lasers was used to assure alignment of the stone at the lithotripter focus.
FIG. 3.
Tissue phantoms—phantom 1: 2 mm mesh basket, phantom 2: PVC cup, phantom 3: PVC cup covered with Paraffin® tape, and phantom 4: anatomic kidney phantom. The ultrasound image shows internal structures of the anatomic kidney phantom. PVC = polyvinyl chloride.
We used plaster stones that emit dust during fragmentation and calcite crystal stones that emit dust in insignificant quantity. Artificial stones made from BegoStone Plus (Bego USA, Lincoln, RI), a gypsum-based plaster, were formed into cylinders with two dimensions: 10 mm length × 6 mm diameter (avg. weight = 0.462 ± 0.011 g) and 4 mm length × 6 mm diameter (avg. weight = 0.120 ± 0.002 g).16 Before experiments, stones were hydrated for more than 48 hours as recommended in the literature. Liu and Zhong have demonstrated that the artificial stones made from BegoStone Plus plaster have mechanical properties similar to natural stones and can be used as stone phantoms.17 Calcite stones of Iceland spar (Educational Innovations, Inc., Bethel, CT), a crystalline form of calcium carbonate, were hammered and broken into pieces with avg. weight = 0.450 ± 0.019 g and had sizes comparable with that of large plaster stones. Blitz and colleagues have demonstrated reproducible fragmentation of these stones and use as a stone model for lithotripsy.18
Experiment
Experiment 1 compared the comminution rate for the four phantoms using the larger plaster stones and calcite stones. For each phantom, stones were exposed for 15 minutes (1050 shocks) in steps of 5 minutes. Three stones of each type were broken in each phantom.
Experiment 2 measured the comminution rate of plaster stones within phantom 4 with different rates of water flow into and out of the phantom to remove dust and debris. The smaller plaster stones were exposed for 5 minutes (350 shocks) and 5 stones were broken in each of the following 3 flow rates: 0, 7, and 86 cc/min. The 7 cc/min rate represents a rate near the upper bound for the maximum rate of induced urine production (9.5 cc/min) in the human kidney,19 while 86 cc/min rate is superphysiologic and is the maximum capable with the available infusion pump (Compact Infusion Pump—Model No. 975; Harvard Apparatus Co., Inc., MA). Water was injected through an 18-gauge needle into the interpolar calix of phantom 4. At the highest rate of flow of water, the volume of calix was replaced every 2 seconds, and thus, suspended dust particles were removed from the calix. The irrigation system and the needle were present even when irrigant was not introduced.
After each shockwave exposure, stone fragments remaining in the phantom were collected and passed through a 2 mm sieve. The ratio of weight of fragments greater than 2 mm to the weight of the stone before exposure was calculated as a fragmentation metric. The ratio (Wi − Wf)/Wi reflects efficiency of the fragmentation as a fraction of the stone considered sufficiently fragmented for natural passage through the urinary tract, where Wi is the initial weight and Wf is the weight of remaining fragments greater than 2 mm after the exposure. Except for phantom 1, all the pieces were returned to the phantom, where they were realigned for subsequent exposure. All the stones broke completely within 10 minutes in phantom 1, and hence, we collected data in 5-minute steps for comparing different phantoms. The results are reported in mean values and standard deviations of percentage of fragmentation of each type of stone after every 5-minute step. The means and standard deviations of different conditions were analyzed statistically with a Student's t-test and p-values are reported.
Results
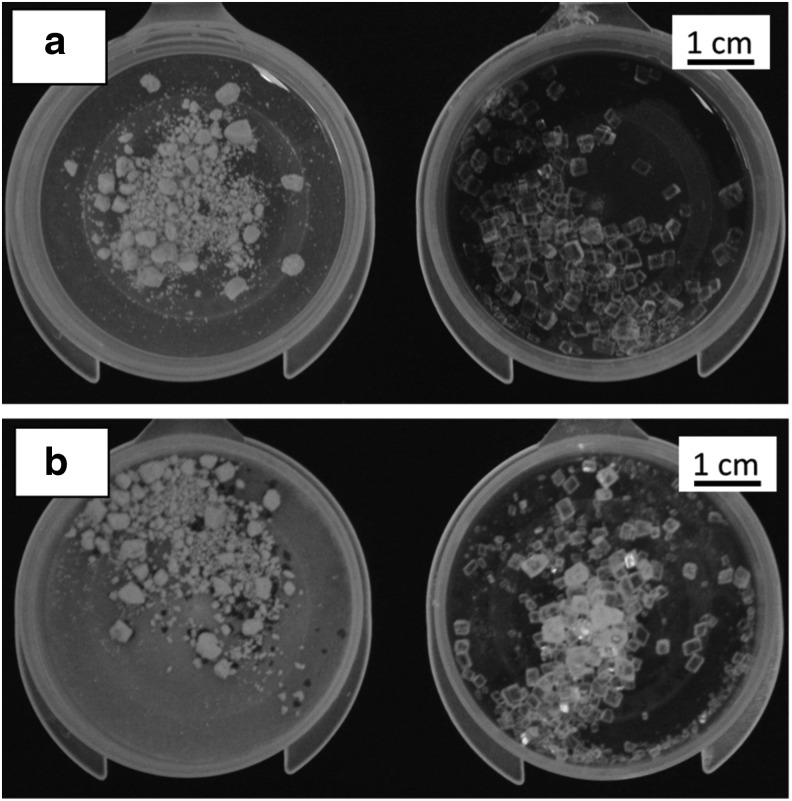
In the first experiment, it was observed that dust clouds from plaster stones drifted away from stones in phantoms 1 and 2 and remained trapped within phantoms 3 and 4. Figure 4 shows wet and dry residual plaster and crystal material from phantom 3 after an experiment. When the plaster dries, the dust covers the bottom of the container. By contrast, the crystal stones produce negligible apparent dust. When a fragment was smaller than the mesh size of phantom 1, it fell through the mesh and was collected in a plate at the bottom of the tank. Fragments did not leave the confined space in other phantoms. However, the incidence of each SW was observed to cause stones to jump around within each phantom, which necessitated occasional phantom repositioning to ensure that stones remained at the lithotripter focus. All stones required about two to three pauses of ∼5 seconds during a treatment.
FIG. 4.
(a) Wet and (b) dry fragments of plaster and crystal stones were collected after treatments of stones in phantom 3. When the plaster dried, the dust covering the bottom of the container was apparent. By contrast, the crystal stones produced negligible dust.
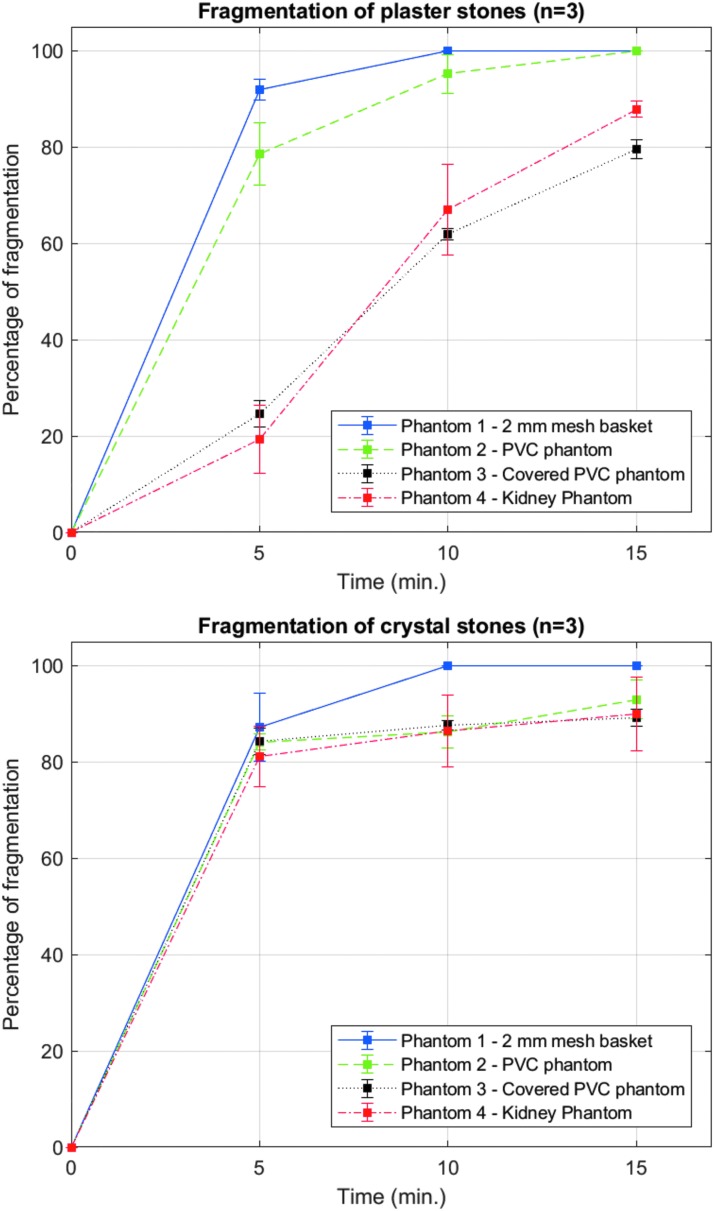
Figure 5 shows the results of experiment 1 as comminution vs time for the 4 phantoms. For plaster stones, comminution effectiveness varied between phantoms: fragmentation after 5 minutes was 92% ± 2% in phantom 1, 79% ± 6% in phantom 2, 25% ± 3% in phantom 3, and 19% ± 3% in phantom 4. Thus, the phantoms where the dispersion of dust and convection of water were restricted showed lower comminution. Calcite stone fragmentation did not show the same trend, and after 5 minutes was 87% ± 3% in phantom 1, 84% ± 1% in phantom 2, 84% ± 1% in phantom 3, and 81% ± 3% in phantom 4. There was a significant difference between percentage of fragmentation of phantom 1 and either phantom 3 or phantom 4 for plaster stones (p < 0.001 in both cases), but not a significant difference for the analogous cases with crystal stones (p = 0.27 for phantom 1 and phantom 3 and p = 0.16 for phantom 1 and phantom 4).
FIG. 5.
Effect of confinement on fragmentation of the stone—as the volume of confinement and dispersion of dust decreased, efficiency of the fragmentation of the plaster stones, which were observed to emit significant amount of dust during fragmentation, decreased. Whereas fragmentation of calcite crystal stones, which emitted minimal dust, was not affected by the phantom. Note, there was no significant difference in weight of fragments larger than 2 mm for any of the conditions above 5 minutes. The calcite stones appeared to be broken as finely as they are going to be broken within 5 minutes. Zhong and colleagues as well as Sapozhnikov and colleagues12–14 have previously described a process where stones can be broken into fragments on the size of the SWL pulse length by elastic wave effects and then ground to smaller pieces by a cavitation mechanism. SWL = extracorporeal shockwave lithotripsy.
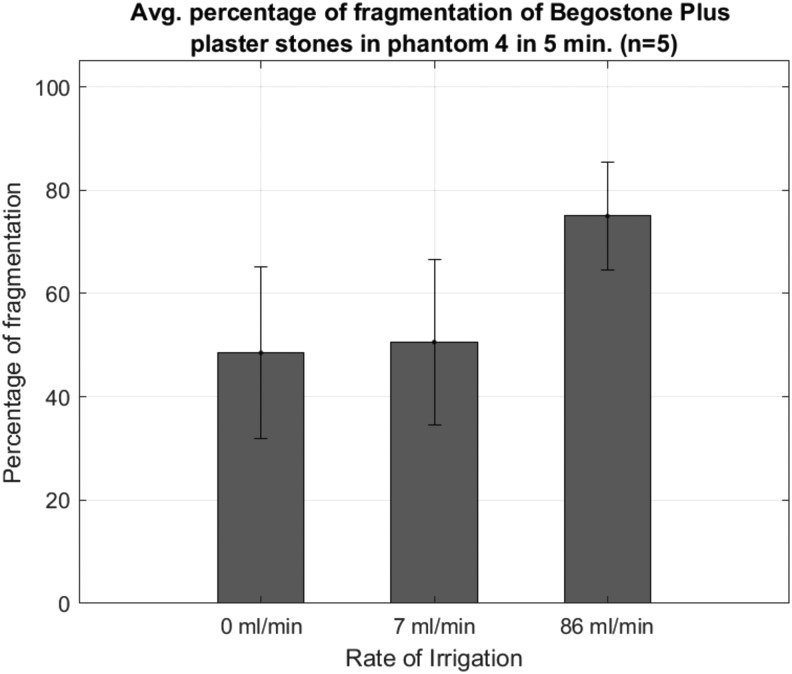
Figure 6 shows the results of experiment 2 as comminution vs irrigation rate. Fragmentation of plaster stones was 48% ± 17% without irrigation, 51% ± 16% with 7 mL/min, and 75% ± 10% with 86 mL/min. The stones with the highest irrigation rate broke 27% better than stones without irrigation and the difference was statistically significant (p < 0.01). While irrigating at 86 cc/min, it was found that a few small fragments of the broken stone left the calix with the flow of water and deposited in a calix below it. However, these fragments were found to be smaller than 2 mm.
FIG. 6.
Effect of irrigation within phantom 4. When water was infused in the kidney phantom continuously through a channel (and allowed to drain from ureteropelvic junction), the fluid containing stone dust and cavitation bubbles was washed away resulting in increase in efficient stone breakage.
Discussion
This study demonstrates that when dispersion of suspended dust particles and convection of fluid are restricted, the efficiency of breakage of stones decreases for the plaster stones but not for the calcite crystal stones. The decrease in efficiency is likely due to stone dust that forms clouds around the stones and may facilitate cavitation. Although other factors vary among phantoms, such as small differences in acoustic impedance, these differences had no apparent impact on the fragmentation of calcite stones. In contrast, the fragmentation of plaster stones changes when flow confinement is provided by either the PVC or kidney phantom. These phantoms impede dispersion of dust out of the area immediately surrounding the plaster stone, and thus, clouds surround the stone compactly. If there is sufficient removal of fluid around the plaster stone, the density of dust decreases and efficiency of treatment increases. This is demonstrated by removal of the stone dust with active irrigation, under which efficiency of breakage of the stone increases. It should be noted that smaller stones with less mass were used in experiment 2 relative to experiment 1 to facilitate the study of confinement in experiment 2. This difference in stone size and mass explains the associated discrepancy in the fractions of stone breakage for these experiments.
Because the efficiency of breakage increases with less fluid confinement around the stone, it can be useful to move kidney stones from confined areas such as small calices to larger areas such as the renal pelvis to treat them more effectively. With medication, humans can produce urine up to 9.5 mL/min in a kidney.19 However, this rate of irrigation was not sufficient for significant improvement in efficiency in experiment 2. Nonetheless, the result of experiment 2 demonstrates that removal of the fluid containing dust at a sufficient rate can improve lithotripsy. Certain acoustic exposures, such as those used in ultrasonic propulsion, could induce acoustic fluid streaming in the urine20,21 and aid in the removal of such dust. Such exposures have been shown to enhance stone breakage during lithotripsy in vitro,22 as both SWL and burst wave lithotripsy (BWL) have to work around the same issues of dust.23 Hence, potential future work may study how fluid streaming can be induced in the kidney such that there is considerable improvement in the efficiency of the treatment of stone. Other approaches may also be developed—for example, a past approach added irrigation through a nephrostomy tube or catheter to wash out fragments during SWL.24,25
One of the limitations of the study is that it was conducted in phantoms and shockwaves reached the phantom directly. In reality, the shocks pass through overlying tissues before reaching the kidney. Although all the phantoms had similar acoustic properties to most tissues, they were made from different materials, which may produce a slightly different field on the stone. In addition, the study was conducted with plaster stones, which produce a large amount of dust or suspended particles. Natural kidney stones vary in composition, and the relative quantity of dust produced during treatment also varies; in our experience, the amount of dust from natural stone is between what is observed with plaster and with crystal artificial stones.
Furthermore, shockwave delivery rate is a method to affect proliferation of bubbles,8 and for simplicity we chose only one clinically relevant rate. Likewise, the gas concentration in a confined water volume was chosen to match cavitation threshold behaviors observed in pig kidneys in vivo for BWL.12 Different threshold limits can be expected for SWL for cavitation. We note that the gas concentration of 50% saturation used in this study, which is less than the 70% concentration present in urine,26 promotes bubble dissolution to reduce cavitation effects.2 Combined, this may result in variations in required treatment durations for phantoms and the human body.
Finally, we did not record images of the dust or cavitation during these experiments because filming in the phantoms was not possible and probing for cavitation with ultrasound imaging affects the bubbles being measured. Nevertheless, we have presented a large body of data to demonstrate that selection of the stone composition and confining volume of the phantom for in vitro/ex vivo SWL experiments has an effect on stone comminution. Based on our observations of dust particles associated with the tested stone types (Fig. 4) and previous SWL research that has correlated shielding with cavitation2–4,7 and cavitation with dust,5,6 we speculate that the presence of dust and associated cavitation is the underlying mechanism. We have described dust as suspended submillimeter particles because this corresponds to direct visual observations as in Figure 4. However, consideration of simple viscous drag force calculations suggests that the most impactful particles may be on the order of 100 μm or smaller, to stay suspended in fluid near the stone between successive shockwaves.27 Future work is required to determine the size of these fragments and also validate the hypothesis with natural kidney stones in a phantom and eliminate factors related to artificial stones.
Conclusions
Phantoms confining fluid motion around the stone were found to affect stone breakage in SWL. These observations imply that the suspended submillimeter particles around the stone reduce comminution effectiveness. Thus, it is important in water tank experiments to replicate the dust conditions seen in vivo by proper selection of stone model and phantom volume among perhaps other things. Likewise, there may be some opportunity to tune clinical SWL effectiveness through fluid flow around the stone to wash out dust. However, the flow due to normal urine production in human kidney appears unlikely to be sufficient to enhance breakage.
Acknowledgments
The work was supported by NIDDK P01-DK043881 and K01-DK104854. Furthermore, this material is also the result of work supported by resources from the Veterans Affairs Puget Sound Health Care System, Seattle, Washington.
Abbreviations Used
- BWL
burst wave lithotripsy
- PVC
polyvinyl chloride
- SW
shock wave
- SWL
extracorporeal shockwave lithotripsy
Author Disclosure Statement
M.R.B., M.D.S., and A.D.M. have equity in and consulting agreements with SonoMotion, Inc., which has licensed technology related to this work from the University of Washington.
References
- 1. Rassweiler JJ, Knoll T, Köhrmann KU, McAteer JA, Lingeman JE, Cleveland RO, Bailey MR, Chaussy C. Shock wave technology and application: An update. Eur Urol 2011;59:784–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sapozhnikov OA, Khokhlova VA, Bailey MR, Williams JC, McAteer JA, Cleveland RO, Crum LA. Effect of overpressure and pulse repetition frequency on cavitation in shock wave lithotripsy. J Acoust Soc Am 2002;112:1183–1195 [DOI] [PubMed] [Google Scholar]
- 3. Sapozhnikov OA, Maxwell AD, MacConaghy B, Bailey MR. A mechanistic analysis of stone fracture in lithotripsy. J Acoust Soc Am 2007;121:1190–1202 [DOI] [PubMed] [Google Scholar]
- 4. Pishchalnikov YA, Zancanaro AJ, Williams JC, McAteer JA. Gas content of the medium surrounding a stone has a significant effect on the efficiency of stone breakage in shock wave lithotripsy. J Acoust Soc Am 2010;127:1761–1761 [Google Scholar]
- 5. Pishchalnikov YA, McAteer JA, Bailey MR, Williams JC, Sapozhnikov OA. Bubble proliferation in shock wave lithotripsy. J Acoust Soc Am 2007;121:3081–3081 [Google Scholar]
- 6. Pishchalnikov YA, McAteer JA, Williams JC, Pishchalnikova IV, Vonderhaar RJ. Why stones break better at slow shockwave rates than at fast rates: In vitro study with a research electrohydraulic lithotripter. J Endourol 2006;20:537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frank S, Lautz J, Sankin GN, Szeri AJ, Zhong P. Bubble proliferation or dissolution of cavitation nuclei in the beam path of a shock-wave lithotripter. Phys Rev Appl 2015;3:034002 [Google Scholar]
- 8. Pishchalnikov YA, McAteer JA. Cavitation-induced streaming in shock wave lithotripsy. J Acoust Soc Am 2013;133:3315–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu S, Cocks FH, Preminger GM, Zhong P. The role of stress waves and cavitation in stone comminution in shock wave lithotripsy. Ultrasound Med Biol 2002;28:661–671 [DOI] [PubMed] [Google Scholar]
- 10. Crum LA, Fowlkes JB. Acoustic cavitation generated by microsecond pulses of ultrasound. Nature 1986;319:52–54 [Google Scholar]
- 11. Sboros V, MacDonald CA, Pye SD, Moran CM, Gomatam J, McDicken WN. The dependence of ultrasound contrast agents backscatter on acoustic pressure: Theory versus experiment. Ultrasonics 2002;40:579–583 [DOI] [PubMed] [Google Scholar]
- 12. Hunter C, Ahn JS, Kreider W, et al. Evaluation of in vitro burst wave lithotripsy exposure conditions. Scientific Program of 35th World Congress of Endourology Program Book and Abstracts. J Endourol 2017;31(Suppl 2):paper BRPRS4-20 [Google Scholar]
- 13. Qin J, Simmons WN, Sankin G, Zhong P. Effect of lithotripter focal width on stone comminution in shock wave lithotripsy. J Acoust Soc Am 2010;127:2635–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. AIUM/NEMA UD2 FDA Recognition Number 12–105: Acoustic Output Measurement Standard for Diagnostic Ultrasound Equipment, Revision 3. NEMA Standards Publication UD 2-2004; American Institute of Ultrasound in Medicine. Laurel, MD: National Electrical Manufacturers Association; Rosslyn, VA, 2004 [Google Scholar]
- 15. Spirou GM, Oraevsky AA, Vitkin IA, Whelan WM. Optical and acoustic properties at 1064 nm of polyvinyl chloride-plastisol for use as a tissue phantom in biomedical optoacoustics. Phys Med Biol 2005;50:N141–N153 [DOI] [PubMed] [Google Scholar]
- 16. Simmons WN, Cocks FH, Zhong P, Preminger G. A composite kidney stone phantom with mechanical properties controllable over the range of human kidney stones. J Mech Behav Biomed Mater 2010;3:130–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y, Zhong P. BegoStone—A new stone phantom for shock wave lithotripsy research (L). J Acoust Soc Am 2002;112:1265–1268 [DOI] [PubMed] [Google Scholar]
- 18. Blitz BF, Lyon ES, Gerber GS. Applicability of iceland spar as a stone model standard for lithotripsy devices. J Endourol 1995;9:449–452 [DOI] [PubMed] [Google Scholar]
- 19. Upsdell SM, Leeson SM, Brooman PJ, O'Reilly PH. Diuretic-induced urinary flow rates at varying clearances and their relevance to the performance and interpretation of diuresis renography. Br J Urol 1988;61:14–18 [DOI] [PubMed] [Google Scholar]
- 20. Sorensen MD, Bailey MR, Hsi RS, et al. Focused ultrasonic propulsion of kidney stones: Review and update of preclinical technology. J Endourol 2013;27:1183–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Starritt HC, Duck FA, Humphrey VF. An experimental investigation of streaming in pulsed diagnostic ultrasound beams. Ultrasound Med Biol 1989;15:363–373 [DOI] [PubMed] [Google Scholar]
- 22. Zwaschka TA, Cunitz BW, Bailey MR, Dunmire B, Maxwell AD. Combining burst wave lithotripsy and ultrasonic propulsion for enhanced kidney stone comminution. J Acoust Soc Am 2016;140:3307–3307 [Google Scholar]
- 23. Maxwell AD, Cunitz BW, Kreider W, Sapozhnikov OA, Hsi RS, Harper JD, Bailey MR, Sorensen MD. Fragmentation of urinary calculi in vitro by burst wave lithotripsy. J Urol 2015;193:338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham JB, Nelson JB. Percutaneous caliceal irrigation during extracorporeal shock wave lithotripsy for lower pole renal calculi. J Urol 1994;152:2227. [DOI] [PubMed] [Google Scholar]
- 25. Nicely ER, Maggio MI, Kuhn EJ. The use of a cystoscopically placed cobra catheter for directed irrigation of lower pole caliceal stones during extracorporeal shock wave lithotripsy. J Urol 1992;148:1036–1039 [DOI] [PubMed] [Google Scholar]
- 26. Hwang EY, Fowlkes JB, Carson PL. Variables controlling contrast generation in a urinary bladder model. J Acoust Soc Am 1998;103:3706–3716 [DOI] [PubMed] [Google Scholar]
- 27. Motion with Linear Drag. Available at: http://hyperphysics.phy-astr.gsu.edu/hbase/lindrg.html#c1 (Accessed on November 12, 2018)