Abstract
Background
Traditional surgical treatment for patients with atrial fibrillation (AF) is performed via sternotomy and on cardiopulmonary bypass. It is very effective in regard to rhythm control, but remains unpopular due to its invasiveness. Truly endoscopic AF treatments have decreased the threshold for electrophysiologists (and cardiologists) to refer, and the reluctance of patients to accept a standalone surgical approach. Practice guidelines from around the world have recognized this as an acceptable therapeutic approach. Current guidelines recommend the HeartTeam approach in treating these complex AF cases. In this study we report our experience with AF HeartTeam approach for surgical stand-alone AF ablation.
Methods
The AF HeartTeam Program began in 2013, patients qualified for inclusion if either of the following was present: failed catheter ablation and/or medication, not suitable for catheter ablation, contraindication to anticoagulation, or patients preferring such an approach. All patients with a complex AF history were assessed by the AF HeartTeam, from which 42 patients were deemed suitable for a totally endoscopic AF procedure (epicardial ablation and LAA closure). Endpoints were intraoperative bidirectional block of the pulmonary veins and closure of left atrial appendage confirmed by transesophageal echocardiography (TEE). Post discharge rhythm follow-up was performed after 3 and 12, 24 and 36 months. Anticoagulation was discontinued 6 weeks after the procedure in patients after documented LAA closure.
Results
In total 42 patients underwent the endoscopic procedure (Median CHA2DS2-VASC=3 (1-6), HAS-BLED=2 (1-6)) for paroxysmal (15/42) and non-paroxysmal AF (27/42) respectively. Bidirectional block was obtained in all patients and complete LAA closure was obtained in all but one Patient on TEE (41/42). In one patient the LAA was not addressed due to extensive adhesions. Two patients underwent median sternotomy because of bleeding during the endoscopic surgery early in the series. There were no deaths. Procedure duration was a median of 124min (Range 83-211) and duration of hospitalization was median of 5 days (Range 3-12). During 36 months follow-up survival free of mortality, thromboembolic events or strokes was 100%. Twelve month freedom from atrial arrhythmia off anti-arrhythmic medication was 93% and 89% for paroxysmal and non-paroxysmal patients respectively. 6/42 patients who had an AF recurrence during the follow-up underwent touch-up catheter ablation.
Conclusions
Atrial fibrillation heart team approach provides excellent outcomes for patients with AF. This approach is beneficial for patients after failed catheter ablation or not candidates for such and offers a very effective mid-term outcome data. In addition to effective rhythm control the protective effect of epicardial LAA closure may play an important role in effectively reducing stroke. The creation of an AF HeartTeam as recommended by the guidelines insures unbiased therapies and provides access to this minimally invasive but effective therapeutic option for AF patients.
Keywords: Atrial Fibrillation, ablation , left Atrial Appendage, Catheter Ablation
Background
Atrial fibrillation (AF) is a very common arrhythmia with increasing incidence and disease burden[1,2]. Rhythm control strategies are manifold varying from electrical cardioversion, anti-arrhythmic pharmacologic therapy, catheter (endocardial) ablation (CA), surgical AF (epicardial) ablation, and hybrid approaches[2]. Self eased diagnosis and patient empowerment will lead to a significant increase in AF diagnosis and awareness[3]. Since the development of the Maze procedure by James Cox in 1982 the role of surgical ablation has become more and more important in the treatment of atrial fibrillation (AF)[4]. As such concomitant surgical ablation of AF at the time of mitral valve surgery has a class Ia indication in the most recent guidelines[2]. Recently catheter based pulmonary vein isolation has established itself as the first line of invasive therapy to treat paroxysmal AF[2].However, it is apparent that some patients with paroxysmal AF fare less well than others. Certain risk factors for worse outcome are well known, such as duration of atrial fibrillation, extent of left atrial dilatation and left atrial fibrosis. Results of catheter ablation for the persistent forms of AF remain suboptimal[5]. It is established that in the setting of non-paroxysmal AF pulmonary vein isolation is not sufficient[6] and this is why more extensive lesions obtained with an epicardial approach may provide more durable results as the LAA is also targeted [7] during this procedure[2,8,9]. And finally the latest guidelines recommend the creation of AF HeartTeams to pose the indication for complex ablations[8]. In our clinic we applied the HeartTeam approach from our TAVI practice to the field of complex AF ablations and report our results herein.
Material and Methods
This is a prospective registry approved by the local institutional review board. All patients included in this study provided an informed consent prior to surgery.
Patient selection
The Atrial Fibrillation Heart Team Program began in 2013 and since then a total of 69 patients have been evaluated for an ablation procedure. Referrals for AF ablation were accepted from primary care doctors, cardiologists and from within our clinic. Self-referrals from patients directly were also accepted after screening of their medical history. Patients with paroxysmal AF and no additional risk factors or not requiring discontinuation of anticoagulation were triaged to catheter ablation as a first line of therapy. All other patients were considered for the endoscopic procedure. Most common inclusion criteria were: failed catheter ablation, or not suitable for such and patients with contraindication to anticoagulation. We did also take into consideration patient’s choice for such a procedure after informed consent about both therapies. Contraindications for the endoscopic procedure were previous cardiac or thoracic surgery, severely impaired pulmonary capacity and/or contraindication for endotracheal intubation and poor mobility.
AF HeartTeam
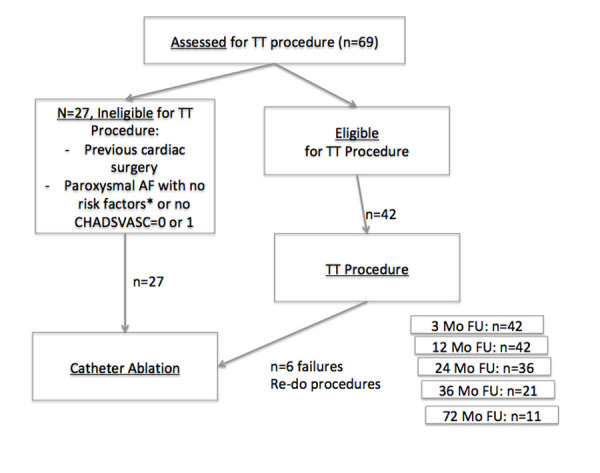
Our institutional AF HeartTeam is an interdisciplinary team composed of several specialists who have no competing financial interest in the treatment of any given patient, identical to the TAVI board. Specialists composing this AF HeartTeam are: non invasive Cardiologist, interventional cardiologists, electrophysiology/device specialist, cardiac surgeon with AF experience and a nurse involved in patient care. All patients are discussed after a first consultation and prior to them receiving an appointment for any procedure. [Figure 1] depicts patient flow.
Figure 1. Patient flow directed by the AF Heart team.
Preoperative Workup
Preoperative workup is composed of pulmonary function testing, 9-day/Holter/ECG, cardiac CT to assess anatomy of the left atrium and the LAA. All patients had echocardiographic evaluation. The presence of significant risk factors for coronary artery disease, or a high calcium score on cardiac CT, mandated diagnostic coronary angiography.
Surgical Procedure
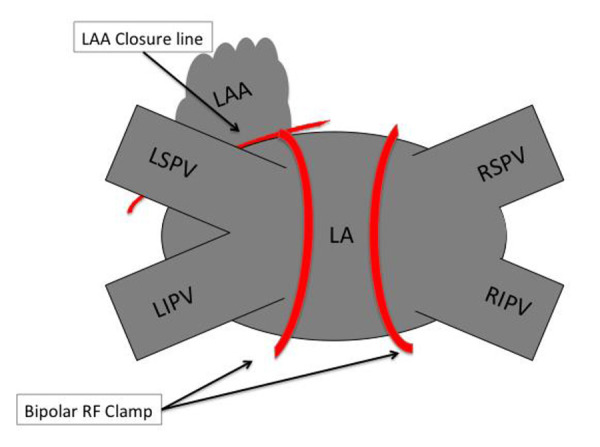
Surgery was performed by two dedicated AF surgeons (SPS and WJvB). The patient was prepared in suppine position with the patient draped for a median sternotomy. Cardio pulmonary bypass was always on standby. Double lumen endotracheal tube, central venous line and arterial line were used in all. All patients underwent intraoperative transesophageal echocardiography (TEE) to assess the left atrial appendage in particular by a cardiologist. The procedure, has previously been described [10], it is begun on the right side with the insertion of 3 ports, one 5mm and two 12mm Ports. CO2 insufflation is used to displace the mediastinum and lung. The pericardium is opened 1cm dorsally to the phrenic and 2 stay sutures are applied. In cases of prior ablation the bipolar pen is used in the sensing mode (Isolator Transpolar Pen™, Atricure, Westchester (OH), USA) to assess entrance and exit block over the pulmonary veins. After which the oblique and transverse sinus are opened. The transverse sinus is then dissected all the way to the left atrial appendage (LAA). With the GlidePath™ Dissector (Atricure, Westchester (OH), USA) the right pulmonary veins are encircled and then ablated with the bipolar radiofrequency clamp (Isolator Synergy Clamp™, Atricure, Westchester (OH), USA). Energy application was done (7-16 Applications) several times until the tissue impedance as measured on the ablation console dropped in under 5 seconds. Once the ablation procedure complete entrance and exit block were confirmed with the bipolar pen (Isolator Transpolar Pen™, Atricure, Westchester (OH), USA) in pacing/sensing mode, the pericardium was closed, chest tube were inserted and the procedure repeated on the left side in the same manner. In all patients a connection from the left superior pulmonary vein to the base of the LAA was also done with the bipolar pen prior to addressing the left atrial appendage. Finally the left atrial appendage was addressed in all. The first 20 patients underwent left atrial appendage resection with a stapler (Endo GIA™ Reinforced Reload with Tri-Staple™, Medtronic, Mineapolis (MN), USA). After this we used the Atriclip Pro2 Device (Atricure, Westchester (OH), USA) which can be inserted through a port. The Stapler and Atriclip were only deployed when adequate closure was documented on TEE with a stump less than 1.0cm in all. A chest tube was inserted on the left side. The patients were extubated in the Operating Room and transferred thereafter. [Figure 2] depicts a schematic of the used lesion set in the patients. We assessed all LAA prior to incision to screen for LAA Thrombi. LAA closure was confirmed by Transesophageal echocardiography (TEE) in all patients.
Figure 2. Schematic description of the lesion set used during the TT procedure.
Postoperative Management
Postoperative management was conducted on an individual level, with increased experience by the entire team the threshold to no longer transfer patients from the operating room to the intensive care unit (ICU) decreased. Either in the ICU or the wake-up ward, the chest drains were removed rapidly (2h postoperatively) followed by a chest x-ray. Transfer to the regular ward with telemetry was then done. Patients were ambulating on the day of surgery. Anticoagulation management was tailored individually. Patients with Coumadin were kept on an INR 2-3 and Coumadin was not discontinued. Patient not on anticoagulation were given only sub-cutaneous low molecular weight heparin 6h postoperatively and then anticoagulated for 6 weeks. Patients on NOAC where discontinued according to our institutional policy and guidelines. Postoperatively on Day one LMWH was given and on day 2 NOAC was reinstated for 6 weeks
Validation of PV isolation was performed using adenosine boluses, isoproterenol infusion at high doses for up to 20 mcg/min and pacing maneuvers, where appropriate. When necessary for additional validation, post RFA voltage mapping of the left atrial was performed.
Antiarrhythmic medications were only given when severe arrhythmias were present postoperatively. In cases where hemodynamic compromise was severe; readmission to the ICU with IV Amiodarone and/or DC electroconversion was instituted.
Follow-up
Long-term rhythm follow-up was obtained in all patients. As per clinical standard all patients were seen by the referring cardiologists (or at our clinic) after 3, 12, 24 and 36 months. Clinical evaluation, echocardiography and Holter and/or 9 Day EKG were performed either at our clinic (9day Holter) or by the referring cardiologists (24h Holter). During follow-up if the patient had an AF recurrence antiarrhythmics were started and a DC electroconversion was organized. If this failed a re-ablation by catheter was discussed with the patient and the referring cardiologist.
Results are reported according to the Guidelines for reporting data and outcomes for the surgical treatment of atrial fibrillation[11].
Results
Patient selection
Out of 69 patients evaluated, a total of 42 patients (Median CHA2DS2-VASC=3 (1-6), HAS-BLED=2 (1-6)) were treated (15 paroxysmal AF, 27 non-paroxysmal AF). [Table 1] depicts general patient’s demographics ablated in this series. The 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation[9] and the 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS[8] where applied for patient selection.
Table 1. Patient Demographics.
LVEF=Left Ventricular Ejection Fraction, LA=Left Atrium, LAVI=Left Atrium Volume Index
| n= | 42 |
|---|---|
| Age (y) | 65 +/- 7 |
| Gender (M) | 14 (54%) |
| HTN | 32 (76%) |
| DM | 13 (31%) |
| CAD | 12 (29%) |
| Stroke/TIA | 4 (10%) |
| CHF | 6 (19%) |
| CHA2DS2-VASc | median 2 (R: 0-4) |
| LVEF (%) | 60 (40-77) |
| LA Diameter (mm) | 48+/-10 |
| LAVI (ml/m2) | 34+/-6 |
| Medications: | |
| Beta-Blocker | 26 (62%) |
| Ca-Antagonists | 7 (21%) |
| Cordarone | 9 (23%) |
| Marcoumar | 14 (33%) |
| NOAC | 24 (57%) |
| no Anticoag | 4 (10%) |
| Aspirin | 10 (38%) |
| Paroxysmal AF | 15/42 |
| Non Paroxysmal AF | 27/42 |
| mean +/- stdv |
A subset of 17 patients despite paroxysmal AF were not deemed suitable for catheter ablation by the AF HeartTeam and addressed for catheter ablation at our institution. Reason for this was mainly patient choice as these patients presented already with the desire not to undergo catheter ablation. The second reason was the ability to discontinue oral anticoagulation after the thoracoscopic ablation procedure because the LAA is managed. All patients were highly symptomatic and/or had intolerance to antiarrhythmic medications. In addition patient’s choice played an important part in decision-making.[Table 2] shows Atrial Fibrillation characteristics.
Table 2. Preoperative Atrial Fibrillation.
CA=Catheter Ablations
| Paroxysmal(n=15) | non Paroxysmal(n=27) | |
|---|---|---|
| Duration(years) | 3.7 ± 3.5 | 4.4 ± 4.6 |
| no Ablation | 15 (33%) | 7 (26%) |
| CA 1 | 5 (33%) | 10 (37%) |
| CA 2 | 6 (40%) | 8 (30%) |
| CA 3+ | 4 (27%) | 2 (7%) |
Operative details
All procedures were performed successfully. No mortality occurred in this series. Median duration of the procedure was 124 minutes (Range 83-211). Pulmonary vein isolation was successful in 41 patients (98%). In one patient a bleeding occurred during lumitip encirclement of the left pulmonary veins. The procedure was aborted and the patient came back after 3 months for a redo thoracoscopic pulmonary vein isolation on the left side – this time successful. All 42 patients (100%) received a connecting line to the left atrial appendage. LAA closure was successful and complete 40 patients, while one 1 patient had an insufficient LAA closure due to technical problems during application and in one patient with severe adherences LAA amputation with the stapler was not attempted.
All but one patient were extubated in the Operating theatre. One patient showed prolonged effect of sedatives and was extubated 5h postoperatively and discharged to the regular ward the next day.
The first 20 patients spent the first night in the intensive care unit (ICU) thereafter all patients were individually assessed for the intensive care unit or just to transit through the wake-up ward. Median duration of stay in the ICU and Recovery-Ward was 18h (Range 2-72) and 4h (Range 3-48). Chest-tubes were removed shortly after arrival in the intensive care unit /wakeup ward, after chest X-ray Patients were transferred to the regular ward on the same day in some, or on postoperative day one. A summary of the postoperative Outcomes is depicted in [Table 3].
Table 3. Postoperative Outcomes.
MACCE=Major Adverse Cardiac and Cerebrovascular Event
| Outcomes | Perioperative | Follow-up |
|---|---|---|
| Sternotomy | 2 | 0 |
| Bleeding* | 2 | 0 |
| Intubation > 6h | 1 | 0 |
| Stroke/TIA | 0 | 0 |
| Pacemaker | 0 | 1 |
| MACCE | 1 | 0 |
| Pleural Effusion | 2 | 7 (16%) |
| Pleurodhesis | 0 | 1 |
| Death | 0 | 0 |
Complications
Conversion to a sternotomy was necessary in 2 patients. In one case it was due to bleeding from a tear in the right upper pulmonary vein during encircling with the dissector. In the second case this occurred in a patient with severe scoliosis while inserting the bipolar clamp. Both cases were managed without cardiopulmonary bypass and had an uneventful postoperative course leaving the hospital after 5 and 8 days in sinus Rhythm. In one patient a bleeding occurred during encircling of the left pulmonary veins. In a second patient on postoperative day 1 a hematothorax occurred which required placement of a drain. One patient suffered ECG changes on the regular ward on postoperative day 1. Emergency PCI was performed on a LAD occlusion. Pleural effusions was a “common” postoperative problem. Effusion was managed conservatively with diuretics. When the chest X-rax prior to discharge demonstrated effusion a pleural ultrasound was performed for quantitative evaluation. In patients with >500ml a thoracentesis was performed. In one patient who developed chronic pleural effusions, which did not disappear despite pharmacologic management and several thoracentesis a pleurodhesis was necessary 18months out.
Figure 3. Rhythm outcomes.
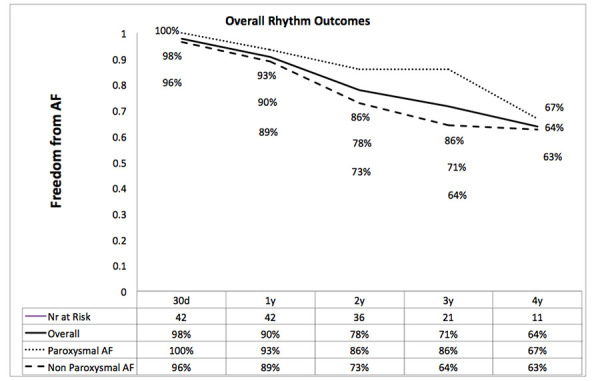
Cardiac Rhythm Outcomes
During the early postoperative period Direct-Current (DC) cardioversion was aggressively performed in all patients, when AF recurrence was symptomatic or a hemodynamically relevant. This occurred in 13 patients once, in 1 Patient three times and in 1 patient four times. After DC Conversion pharmacologic treatment with Amiodarone and/or Betablockers was started. Overall 9 Patients (21%) required antiarrhythmic medication after hospital discharge for atrial arrhythmias. A total of 6 patients (14%) required a redo procedure for AF recurrence. [Table 4] shows Rhythm status at Follow-up.
Table 4. Rhythm Outcomes.
AAD=Antiarrhythmic Drugs
| Paroxysmal (n=15) | Non Paroxysmal(n=27) | |
|---|---|---|
| Freedom from AF off AAD | 93% | 89% |
| Follow-up (months) Median(range) | 15 (11-43) | 16 (11-43) |
| DC Electroconversion (during Blanking P.) | 5 | 10 (37%) |
| re-Ablation - Right | 0 | 1 |
| re-Ablation – Left + Right | 1 | 4 |
| NOAC during FU | 1 | 4 (15%) |
Follow-up
Median follow-up was 20 Months (11-43). During follow-up no mortality, thromboembolic events, TIA or stroke occurred. Overall (12 month) freedom from AF and antiarrhythmic medication was 93% and 89% for paroxysmal and non-paroxysmal patients respectively.
In the paroxysmal AF group, one patient had a symptomatic atrial arrhythmia recurrence after 18 month, which required a catheter ablation. This was due to a peri-mitral flutter and a reconnection of the right inferior pulmonary vein. In addition left sided substrate modification and a right sided cavo-tricuspid-isthmus line were performed. Only one patient with paroxysmal AF remained under oral anticoagulants due to an incomplete LAA closure with a CHADSVASC score of 3.
In the non-paroxysmal group overall 5 patients (12%) required a redo procedure. This was due to pulmonary vein reconnection and mitral isthmus flutter. Despite optimal LAA closure 15% of patients remained under oral anticoagulation with NOACS.
Discussion
This is the first study that highlights the role of an AF HeartTeam Program (AFHTP) in appropriately utilizing both catheter and minimally invasive surgical ablations in the management of AF. Our study shows that AFHTP can yield excellent patient outcomes for patients with improved communication between cardiologists and cardiac surgeons.
Too often, surgeons and electrophysiologists work in silos taking care of the same problem. With increased communication between specialists and increased awareness about quality and outcomes the surgical community has slowly begun to embraces the HeartTeam as it has become evident from the TAVI HeartTeams and team assessment of coronary artery disease.
The results for the surgical approach when randomized to either catheter or totally thoracoscopic ablation demonstrated a high rate of freedom of AF at 12 months, 77 vs. 42% in favor of the thoracoscopic procedure[12] . Other studies have shown a 2-year success rate of 70–80% for preventing AF recurrence in these complex patients[13]. Furthermore the relatively high complication rate initially described in the FAST trial[12] was demonstrated to become significantly lower in a very recent report by Vos et al.[14]. Over a period of 11 years in a dedicated AF center n=558 patients underwent thorascopic ablations, the freedom from complications was 89% and 97% for complications with life-long affecting consequences[15].
We believe that the surgical procedure comes with some advantages which are: bipolar RF as energy source, large antral epicardial bites isolation, inclusion of the entire epicardial fat pads and the ganglia within the wide antral ablation, and most importantly electrical isolation the LAA by amputation. The importance of this is confirmed by interesting EP data on targeting the LAA during first time catheter ablation[16]. As we have demonstrated, the Atriclip exclusion of the LAA leads to it’s electrical silencing [17].
The most recent guidelines ESC suggest that a dedicated AF Heartteam setting and integrated AF care chain may be crucial for good outcomes[8]. Our’s is the first report of such an integrated care chain.
Anticoagulation management after ablation procedures is directed by the guidelines[8]. However LAA closure plays a crucial role in this setting, as this is done as an add-on to the ablation procedure. Though the interventional cardiology literature suggests that we can discontinue anticoagulation when the LAA is closed[18], after ablation procedures the recommendation is different[8]. Anticoagulation in this setting is to prevent thromboembolism from thrombi accumulating on the endocardial ablation lines due to the transmural nature of the epicardial ablation. However we believe that in the presence of LAA closure the risk of such thromboemboli is lower. There is currently some evidence indicating that the LAA closure line may present a thrombotic surface and that LAA closure in itself might warrant oral anticolagulation during a limited period. Further insight in this matter is necessary. In accordance to the guidelines referring doctors are reluctant to discontinue oral anticoagulation despite documented epicardial LAA closure. In a series of 291 Atriclip patients, Caliskan et al demonstrated that in a sub-group of 166 patients with no oral anticoagulation during benefited from a relative risk reduction of 87.5% with an observed ischemic stroke-rate of 0.5/100 patient-years compared with what would have been expected in a group of patients with similar CHA2DS2-VASc scores (expected rate of 4.0/100 patient-years). This is where endoscopic ablation techniques offer safe[15], effective[12] and durable[13] results, hence providing an additional option in the therapeutic armamentarium of invasive AF therapies. In addition to effective rhythm control, left atrial appendage (LAA) closure diminishes stroke risk and affords the possibility to discontinue anticoagulation. Together, these latter two factors potentially provide improved long-term survival not attainable with catheter ablation alone. This is an important factor in favor of the endoscopic treatment option as the epicardial techniques provide safe, effective and durable left atrial appendage closure[19].
The Star AF II demonstrated an absence of reduction in the rate of recurrent atrial fibrillation when either linear ablation or ablation of complex fractionated electrograms was performed in addition to pulmonary-vein isolation in patients with persistent AF[6]. Based on this and our experience, we believe that obtaining bidirectional block over all pulmonary veins is the cornerstone of any ablation procedure. Isolation from the epicardium, through the fat to the endothelium is done with very effective energy delivery. In fact the only energy source to have proven transmurality is the bipolar clamp [20].
Thoracoscopic ablation could be the first line of therapy due to the nature of transmural lesions on the left atrium leading to a high success rate of pulmonary vein isolation.
From recent studies[21] it appears that up to 20% of pulmonary veins seem isolated when measured with the pen, but when more sophisticated EP tools are used there appears to be a significant amount of incomplete lesions. This again leads to another argument in favor of a combined EP/surgical approach.
Limitations of this study are that we were not able to compare catheter ablation to thoracoscopic ablation. This was not the scope of the work as we believe that these therapeutic pathways are complimentary.
Conclusions
In patients who have either failed catheter ablation or not candidates for such, evaluation by a multidisciplinary HeartTeam can lead to effective combined therapy. Thoracoscopic bipolar ablation is safe and effective. In addition to effective rhythm control the protective effect of LAA closure decreases the risk of stroke significantly.
References
- 1.Lane Deirdre A, Skjøth Flemming, Lip Gregory Y H, Larsen Torben B, Kotecha Dipak. Temporal Trends in Incidence, Prevalence, and Mortality of Atrial Fibrillation in Primary Care. J Am Heart Assoc. 2017 Apr 28;6 (5) doi: 10.1161/JAHA.116.005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirchhof Paulus, Benussi Stefano, Kotecha Dipak, Ahlsson Anders, Atar Dan, Casadei Barbara, Castella Manuel, Diener Hans-Christoph, Heidbuchel Hein, Hendriks Jeroen, Hindricks Gerhard, Manolis Antonis S, Oldgren Jonas, Popescu Bogdan Alexandru, Schotten Ulrich, Van Putte Bart, Vardas Panagiotis. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016 Oct 07;37 (38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 3.Schnabel Renate B, Yin Xiaoyan, Gona Philimon, Larson Martin G, Beiser Alexa S, McManus David D, Newton-Cheh Christopher, Lubitz Steven A, Magnani Jared W, Ellinor Patrick T, Seshadri Sudha, Wolf Philip A, Vasan Ramachandran S, Benjamin Emelia J, Levy Daniel. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015 Jul 11;386 (9989):154–62. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox J L, Boineau J P, Schuessler R B, Ferguson T B, Cain M E, Lindsay B D, Corr P B, Kater K M, Lappas D G. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991 Oct 09;266 (14):1976–80. [PubMed] [Google Scholar]
- 5.Ganesan Anand N, Shipp Nicholas J, Brooks Anthony G, Kuklik Pawel, Lau Dennis H, Lim Han S, Sullivan Thomas, Roberts-Thomson Kurt C, Sanders Prashanthan. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013 Mar 18;2 (2) doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma Atul, Jiang Chen-yang, Betts Timothy R, Chen Jian, Deisenhofer Isabel, Mantovan Roberto, Macle Laurent, Morillo Carlos A, Haverkamp Wilhelm, Weerasooriya Rukshen, Albenque Jean-Paul, Nardi Stefano, Menardi Endrj, Novak Paul, Sanders Prashanthan. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015 May 07;372 (19):1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 7.Di Biase Luigi, Burkhardt J David, Mohanty Prasant, Mohanty Sanghamitra, Sanchez Javier E, Trivedi Chintan, Güneş Mahmut, Gökoğlan Yalçın, Gianni Carola, Horton Rodney P, Themistoclakis Sakis, Gallinghouse G Joseph, Bailey Shane, Zagrodzky Jason D, Hongo Richard H, Beheiry Salwa, Santangeli Pasquale, Casella Michela, Dello Russo Antonio, Al-Ahmad Amin, Hranitzky Patrick, Lakkireddy Dhanunjaya, Tondo Claudio, Natale Andrea. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J. Am. Coll. Cardiol. 2016 Nov 01;68 (18):1929–1940. doi: 10.1016/j.jacc.2016.07.770. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhof Paulus, Benussi Stefano, Kotecha Dipak, Ahlsson Anders, Atar Dan, Casadei Barbara, Castella Manuel, Diener Hans-Christoph, Heidbuchel Hein, Hendriks Jeroen, Hindricks Gerhard, Manolis Antonis S, Oldgren Jonas, Popescu Bogdan Alexandru, Schotten Ulrich, Van Putte Bart, Vardas Panagiotis, Agewall Stefan, Camm John, Baron Esquivias Gonzalo, Budts Werner, Carerj Scipione, Casselman Filip, Coca Antonio, De Caterina Raffaele, Deftereos Spiridon, Dobrev Dobromir, Ferro José M, Filippatos Gerasimos, Fitzsimons Donna, Gorenek Bulent, Guenoun Maxine, Hohnloser Stefan H, Kolh Philippe, Lip Gregory Y H, Manolis Athanasios, McMurray John, Ponikowski Piotr, Rosenhek Raphael, Ruschitzka Frank, Savelieva Irina, Sharma Sanjay, Suwalski Piotr, Tamargo Juan Luis, Taylor Clare J, Van Gelder Isabelle C, Voors Adriaan A, Windecker Stephan, Zamorano Jose Luis, Zeppenfeld Katja. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016 Nov;18 (11):1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 9.Calkins Hugh, Hindricks Gerhard, Cappato Riccardo, Kim Young-Hoon, Saad Eduardo B, Aguinaga Luis, Akar Joseph G, Badhwar Vinay, Brugada Josep, Camm John, Chen Peng-Sheng, Chen Shih-Ann, Chung Mina K, Nielsen Jens Cosedis, Curtis Anne B, Davies D Wyn, Day John D, d'Avila André, de Groot N M S Natasja, Di Biase Luigi, Duytschaever Mattias, Edgerton James R, Ellenbogen Kenneth A, Ellinor Patrick T, Ernst Sabine, Fenelon Guilherme, Gerstenfeld Edward P, Haines David E, Haissaguerre Michel, Helm Robert H, Hylek Elaine, Jackman Warren M, Jalife Jose, Kalman Jonathan M, Kautzner Josef, Kottkamp Hans, Kuck Karl Heinz, Kumagai Koichiro, Lee Richard, Lewalter Thorsten, Lindsay Bruce D, Macle Laurent, Mansour Moussa, Marchlinski Francis E, Michaud Gregory F, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Okumura Ken, Packer Douglas, Pokushalov Evgeny, Reynolds Matthew R, Sanders Prashanthan, Scanavacca Mauricio, Schilling Richard, Tondo Claudio, Tsao Hsuan-Ming, Verma Atul, Wilber David J, Yamane Teiichi. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace. 2018 Jan 01;20 (1):157–208. doi: 10.1093/europace/eux275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgerton James R, Jackman Warren M, Mack Michael J. A new epicardial lesion set for minimal access left atrial maze: the Dallas lesion set. Ann. Thorac. Surg. 2009 Nov;88 (5):1655–7. doi: 10.1016/j.athoracsur.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Shemin Richard J, Cox James L, Gillinov A Marc, Blackstone Eugene H, Bridges Charles R. Guidelines for reporting data and outcomes for the surgical treatment of atrial fibrillation. Ann. Thorac. Surg. 2007 Mar;83 (3):1225–30. doi: 10.1016/j.athoracsur.2006.11.094. [DOI] [PubMed] [Google Scholar]
- 12.Boersma Lucas V A, Castella Manuel, van Boven Wimjan, Berruezo Antonio, Yilmaz Alaaddin, Nadal Mercedes, Sandoval Elena, Calvo Naiara, Brugada Josep, Kelder Johannes, Wijffels Maurits, Mont Lluís. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012 Jan 03;125 (1):23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 13.Saini Aditya, Hu Yuhning L, Kasirajan Vigneshwar, Han Frederick T, Khan Muhammad Z, Wolfe Luke, Gunda Sampath, Koneru Jayanthi N, Ellenbogen Kenneth A. Long-term outcomes of minimally invasive surgical ablation for atrial fibrillation: A single-center experience. Heart Rhythm. 2017 Sep;14 (9):1281–1288. doi: 10.1016/j.hrthm.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Vos Lara M, Kotecha Dipak, Geuzebroek Guillaume S C, Hofman Frederik N, van Boven Wim Jan P, Kelder Johannes, de Mol Bas A J M, van Putte Bart P. Totally thoracoscopic ablation for atrial fibrillation: a systematic safety analysis. Europace. 2018 Nov 01;20 (11):1790–1797. doi: 10.1093/europace/eux385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vos Lara M, Kotecha Dipak, Geuzebroek Guillaume S C, Hofman Frederik N, van Boven Wim Jan P, Kelder Johannes, de Mol Bas A J M, van Putte Bart P. Totally thoracoscopic ablation for atrial fibrillation: a systematic safety analysis. Europace. 2018 Nov 01;20 (11):1790–1797. doi: 10.1093/europace/eux385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero Jorge, Michaud Gregory F, Avendano Ricardo, Briceño David F, Kumar Saurabh, Carlos Diaz Juan, Mohanty Sanghamitra, Trivedi Chintan, Gianni Carola, Della Rocca Domenico, Proietti Riccardo, Perrotta Laura, Bordignon Stefano, Chun Julian K R, Schmidt Boris, Garcia Mario, Natale Andrea, Di Biase Luigi. Benefit of left atrial appendage electrical isolation for persistent and long-standing persistent atrial fibrillation: a systematic review and meta-analysis. Europace. 2018 Aug 01;20 (8):1268–1278. doi: 10.1093/europace/eux372. [DOI] [PubMed] [Google Scholar]
- 17.Starck Christoph T, Steffel Jan, Emmert Maximilian Y, Plass Andre, Mahapatra Srijoy, Falk Volkmar, Salzberg Sacha P. Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interact Cardiovasc Thorac Surg. 2012 Sep;15 (3):416–8. doi: 10.1093/icvts/ivs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy Vivek Y, Gibson Douglas N, Kar Saibal, O'Neill William, Doshi Shephal K, Horton Rodney P, Buchbinder Maurice, Gordon Nicole T, Holmes David R. Post-Approval U.S. Experience With Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation. J. Am. Coll. Cardiol. 2017 Jan 24;69 (3):253–261. doi: 10.1016/j.jacc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Caliskan Etem, Cox James L, Holmes David R, Meier Bernhard, Lakkireddy Dhanunjaya R, Falk Volkmar, Salzberg Sacha P, Emmert Maximilian Y. Interventional and surgical occlusion of the left atrial appendage. Nat Rev Cardiol. 2017 Dec;14 (12):727–743. doi: 10.1038/nrcardio.2017.107. [DOI] [PubMed] [Google Scholar]
- 20.Saint Lindsey L, Lawrance Christopher P, Okada Shoichi, Kazui Toshinobu, Robertson Jason O, Schuessler Richard B, Damiano Ralph J. Performance of a novel bipolar/monopolar radiofrequency ablation device on the beating heart in an acute porcine model. Innovations (Phila) 2013 Oct 23;8 (4):276–83. doi: 10.1097/IMI.0b013e3182a77f2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haldar Shouvik K, Jones David G, Bahrami Toufan, De Souza Anthony, Panikker Sandeep, Butcher Charlie, Khan Habib, Yahdav Rashmi, Jarman Julian, Mantziari Lilian, Nyktari Eva, Mohiaddin Raad, Hussain Wajid, Markides Vias, Wong Tom. Catheter ablation vs electrophysiologically guided thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: The CASA-AF Study. Heart Rhythm. 2017 Nov;14 (11):1596–1603. doi: 10.1016/j.hrthm.2017.08.024. [DOI] [PubMed] [Google Scholar]


