Abstract
Left atrial appendage occlusion (LAAO) is a promising alternative for stroke risk reduction in patients with non-valvular atrial fibrillation who are not suitable for long-term oral anticoagulation (OAC). Current practice mandates use of post-procedural OAC for 45 days after WatchmanTM placement during which complete device endothelialization is expected to occur. However, most of the evidence supporting this strategy stem from animal studies. Incomplete device endothelialization are often encountered after 6-weeks of procedure and its therapeutic implications are less clear. Here, we present two cases of incomplete endothelialization after 1.5- and 2-year of Watchman implantation. In one of the cases, we believe that an eccentric mitral regurgitation jet caused shearing force on the Watchman device and impeded with normal endothelialization. In the other case, we found a device related thrombus possibly favored by prothrombotic environment created by lack of endothelialization. Further studies are warranted to find predictors and better diagnostic tool of LAAO endothelialization.
Keywords: Atrial fibrillation, Oral anticoagulation, left atrial appendage occlusion
Introduction
Atrial fibrillation (AF) is the most common cause of stroke. Oral anticoagulation (OAC) is used to mitigate stroke risk in patient with non-valvular AF [1]. However, a significant number of patients cannot be started on OAC due to bleeding complications. Percutaneous left atrial appendage occlusion (LAAO) has emerged as a potential option for such group of patients. Post-LAAO anticoagulation is maintained until device endothelialization. However, there is no systematic human study on device endothelialization and currently recommended OAC strategy mainly stem from animal evidence. There can be variability in endothelialization of the devices and delayed or lack of endothelialization of devices are often encountered. There are no standard techniques to ensure device endothelization and little is known about the predictors of delayed endothelization after LAAO.
Here we present two cases of delayed endothelialization after watchman device and discuss potential mechanisms.
Cases
Case 1
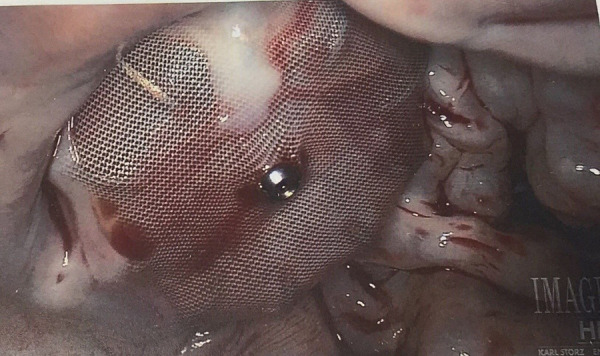
A 70-year-old woman with diabetes, hypertension, moderate to severe mitral regurgitation (MR) and persistent atrial fibrillation had a history of recurrent gastrointestinal bleeding. As she was not a good candidate for long-term oral anticoagulation, she underwent Watchman device implantation. Adequate deployment of the device was confirmed both by transesophageal echocardiography (TEE )and fluoroscopy using the PASS criteria (Position, Anchoring Size, and Seal). No residual flow into the appendage was observed by TEE at the conclusion of the case. She received warfarin and aspirin for 6 weeks after the procedure. TEE at 45 days after the procedure showed good device apposition and no evidence of thrombus or device leak. She was then treated with aspirin and clopidogrel for the next 6 months followed by aspirin therapy alone thereafter. TEE after a year post-procedure did not reveal any thrombus or device leak. Patient developed severe symptoms from MR and needed open mitral valve replacement surgery 1.5 years after watchman procedure. Intraoperatively, on visual inspection of the Watchman device the cardiac surgeon noted that there was complete lack of endothelialization ([Figure 1]). A jet of blood stream from posterior mitral leaflet was seen to hit the left atrial appendage on the echocardiogram.
Figure 1. Intraoperative picture showing complete non-endothelialization of Watchman device after 1.5 years of implantation. Patient had severe posterior mitral regurgitant jet hitting on the Watchman device.
Case 2
A 57-year-old man with a history of permanent AF and lower GI bleed while on warfarin. underwent implantation of a 30 mm Watchman LAAO device. Adequate deployment of the device was confirmed both by TEE and fluoroscopy using the PASS criteria. There was no leak and an inferior shoulder noted was deemed to be acceptable with respect to the device’s final position. The patient was maintained on warfarin and aspirin therapy following the procedure for 45 days. Follow up TEE after approximately 45 days following implant revealed no thrombus on the device or residual leak. Warfarin was stopped, and the patient was placed on clopidogrel 75 mg daily in addition aspirin 81 mg daily. Follow up TEE at 1-year revealed stable device position, no leak or thrombus on the device. clopidogrel was discontinued, and the patient was maintained on ASA therapy. Two years after the procedure the patient underwent a transthoracic echocardiogram that revealed a large thrombus along the superior and lateral region of left atrium as well as the surface of the Watchman device. A TEE was performed that confirmed a large mass, consistent with thrombus, that appeared to originate at the center of the Watchman device and extended along the wall of the Left Atrium. The patient was initially restarted on warfarin therapy with (target INR 2.5-3.5). When this failed to resolve the thrombus, aspirin 325 mg daily, and clopidogrel 75 daily were added. Several months later, the patient developed acute lower gastrointestinal bleeding and warfarin, aspirin and clopidogrel were discontinued. The patient underwent surgical removal of the LAA thrombus, the Watchman device, and excision of the LAA. Gross inspection of the Watchman device revealed patchy and incomplete endothelization of the device ([Figure 2]). The patient recovered from the surgery without incident and is doing well.
Figure 2. Gross inspection of Watchman device that was removed after 2 years of implantation. It shows a large area of non-endothelialization.

Discussion
Discussion LAAO is being increasingly used for stroke risk reduction in patients not suitable for long term oral anticoagulation. It is important to understand the endothelialization process in these devices as it has clinical implications in the management of these patients. Here, we report two cases of incomplete endothelialization after more than 1.5 year of watchman LAAO. While the cause of delayed endothelialization in one is less clear we hypothesized that chronic force from posterior mitral regurgitant jet into the watchman could be the significant contributor in the other case.
Most of the information about endothelialization of LAAO devices stem from animal studies. In animal studies, LAAO devices have been found to undergo complete endothelialization in 3 months [2]. Except for a few case reports, there are no systematic studies about endothelialization in human. Massarenti et al reported a case of incomplete endothelialization that was observed after 10 months of Watchman implantation in a patient with hereditary hemorrhagic telengectesesia. Patient had stroke with device and when the device was analyzed, incomplete endothelialization was noted [3]. Another report from Canada reports a post mortem finding of incomplete endothelialization of Watchman device in a patient with multiple thromboembolic events [4]. Another report describes a direct visualization of an area of incomplete endothelialization of Amplatzer Cardiac Plug by a cardiac surgeon during open heart surgery for CABG after 1.5 years [5]. More publications exist in the sphere of Amplatzer Septal Occluder (ASO). Animal studies on ASO showed that it gets endothelialized in 3 months [6]. However, many human studies have found incomplete endothelialization of septal occluder device on autopsy or surgery after 5 months to 7 years post implantation [7-12]. Such discrepancy in endothelialization of endovascular devices between animals and humans clearly point to the presence of different regeneration mechanisms and calls for systemic studies in humans.
Clinically, it is important to clearly understand the process of endothelialization for better management of LAAO patients. Postprocedural antithrombotic therapy largely prevents thrombus formation until endothelialization is complete. Incomplete endothelialization could provide a persistent prothrombotic surface for thrombus formation. Delayed device surface healing could potentially be related to thrombus formation that accompanies any device implantation [13]. Device related thrombus (DRT) formation may be related to degree of LAAO device endothelialization [14].
There is no standard test to determine the degree of endothelialization of LAAO devices in current practice. In a study by Granier et al, incomplete endothelialization was defined as residual permeability on cardiac computed tomography without peridevice leak on TEE at follow-up [15]. Using such definition, they found 61% of the implants to have incomplete endothelialization after the mean follow up of 10 ± 6 months. Behenes et al recently presented a standardized cardiac computed tomography angiography (cCTA) protocol for systematic follow-up and comparison of patients after LAAO device implantation. They have proposed a protocol to correlate contrast enhancement with degree of endothelialization after LAAO device placement. Complete neo-endothelialization is characterized by the absence of contrast enhancement within the LAA without any peri-device leak. Contrast enhancement in the LAA of less than 50 Hounsfield units compared to the left atrium suggests incomplete neo-endothelialization while equal contrast enhancement in both LAA and Left atrium signifies no or very early endothelialization [16]. However, it is unknown at this time, if cardiac CT would provide additional benefits over standard TEE after LAAO. Nevertheless, growing interest in the use of alternative imaging modalities after LAAO may be able to provide with better test to assess neoendothelialization after endocardial LAA closure devices.
In addition to the need of better diagnostic tool for detecting device endothelialization, understanding factors associated with device endothelialization is equally important. The optimal neoendothelialization of any implanted cardiac device might largely depend on the internal milieu such as hemodynamics of the heart and endothelial function. Dysfunctional endothelial cells due to abnormalities in transforming growth factor-B in a patient with hereditary telengectesia has been a putative cause of incomplete endothelialization that was observed 10 months after a watchman implant [3]. The high shear force near vascular stenotic area is known to affect cellular adherence and cause deleterious effects on endothelial cells [17]. Similarly, turbulent blood flow caused by high velocity regurgitant jet has been known to invoke spatial and temporal disorganization that can thwart normal compensatory endothelial realignment [17]. It is our speculation that turbulent flow caused by posterior MR jet might have impeded normal endothelialization in response to LAAO device in our first case. Granier et al had found patients with incomplete endothelialization tended to be more likely to have diabetes (36% vs 11%) and permanent AF (57% vs 22%), and to have been implanted with larger devices (86% vs 44%) [15].
Conclusions
Incomplete endothelialization of LAAO devices are often encountered clinically. There is paucity of understanding regarding the causes of incomplete endothelialization. Incomplete endothelialization may provide a persistent prothrombotic surface for thrombus formation and may warrant prolonged anticoagulation use post-implant. Based on our case, we hypothesize that MR could be one of the predictors of delayed endothelialization. The predictive factors associated with delayed endothelialization should be determined in a large prospective cohort.
References
- 1.RG Hart , LA Pearce , MI Aguilar . Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007;0:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz Robert S, Holmes David R, Van Tassel Robert A, Hauser Robert, Henry Timothy D, Mooney Michael, Matthews Ray, Doshi Shephal, Jones Russell M, Virmani Renu. Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc Interv. 2010 Aug;3 (8):870–7. doi: 10.1016/j.jcin.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 3.L Massarenti , A Yilmaz . Incomplete endothelialization of left atrial appendage occlusion device 10 months after implantation. . J. Cardiovasc. Electrophysiol. 2012;0:1384–1385. doi: 10.1111/j.1540-8167.2012.02360.x. [DOI] [PubMed] [Google Scholar]
- 4.G Prosperi-Porta, G Schnell , J Colbert , A Franko , SB Wilton , VP Kuriachan . Multiple Thromboembolic Events from a Left Atrial Appendage Occlusion Device. Can J Cardiol . 2017;0:0–0. doi: 10.1016/j.cjca.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 5.S Schiettekatte , J Czapla , J Nijs , M La Meir. Unmasking a naked left atrial appendage closure device: a case of a silent embolic threat. Heart Rhythm. 2014;11:2314–2315. doi: 10.1016/j.hrthm.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 6.JE Lock , JJ Rome , R Davis . Transcatheter closure of atrial septal defects. Experimental studies. Circulation. 1989;79:1091–1099. doi: 10.1161/01.cir.79.5.1091. [DOI] [PubMed] [Google Scholar]
- 7.F Chen , X Zhao , X Zheng , S Chen, R Xu , Y Qin. Incomplete endothelialization and late dislocation after implantation of an Amplatzer septal occluder device. Circulation. 2011;124:0–189. doi: 10.1161/CIRCULATIONAHA.110.991836. [DOI] [PubMed] [Google Scholar]
- 8.M Chessa , G Butera , A Frigiola , M Carminati . Endothelialization of ASD devices for transcatheter closure: possibility or reality? Int. J. Cardiol. . 2004;97:563–564. doi: 10.1016/j.ijcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 9.A Kawamura , H Kigasawa , H Kamma . Autopsy findings of Amplatzer septal occluder at 5 months after closure of atrial septal defect: how long does it take to be endothelialized? J Invasive Cardiol . 2013;25:0–168. [PubMed] [Google Scholar]
- 10.Z Astroulakis, A El-Gamel, JM Hill . Failed endothelialisation of a percutaneous atrial septal defect closure device. Heart . 2008;94:580–0. doi: 10.1136/hrt.2007.135251. [DOI] [PubMed] [Google Scholar]
- 11.TC Slesnick , AW Nugent , CD Fraser , BC Cannon . Images in cardiovascular medicine. Incomplete endothelialization and late development of acute bacterial endocarditis after implantation of an Amplatzer septal occluder device. Circulation . 2008;117:0–327. doi: 10.1161/CIRCULATIONAHA.107.754069. [DOI] [PubMed] [Google Scholar]
- 12.F Zahr , WE Katz , Y Toyoda , WD Anderson . Late bacterial endocarditis of an amplatzer atrial septal defect occluder device. Am. J. Cardiol. 2010;105:279–280. doi: 10.1016/j.amjcard.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 13. J Lammers , T Elenbaas , A Meijer . Thrombus formation on an Amplatzer closure device after left atrial appendage closure. Eur. Heart J. 2013;34:741–0. doi: 10.1093/eurheartj/ehs437. [DOI] [PubMed] [Google Scholar]
- 14. S Shamim, A Magalski, AK Chhatriwalla, KB Allen, KC Huber , ML Main. Transesophageal echocardiographic diagnosis of a WATCHMAN left atrial appendage closure device thrombus 10 years following implantation. Echocardiography . 2017;34:128–130. doi: 10.1111/echo.13413. [DOI] [PubMed] [Google Scholar]
- 15.M Granier , G Laugaudin , F Massin . Occurrence of Incomplete Endothelialization Causing Residual Permeability After Left Atrial Appendage Closure. J Invasive Cardiol . 2018;30:245–250. [PubMed] [Google Scholar]
- 16.M Behnes , I Akin , B Sartorius . LAA Occluder View for post-implantation Evaluation (LOVE)--standardized imaging proposal evaluating implanted left atrial appendage occlusion devices by cardiac computed tomography. BMC Med Imaging. 2016;16:25–0. doi: 10.1186/s12880-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LM Baddour , MA Bettmann , AF Bolger. Nonvalvular cardiovascular device-related infections. Circulation . 2003;108:2015–2031. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]


