Abstract
Introduction
Atrial fibrillation (AF) and heart failure (HF) often coexist with an increase in morbidity and mortality. AF catheter ablation (CA) has proved to be a safe and efficient option for HF patients, but long-term evolution and prognosis remain uncertain. The aim is to assess the efficacy and safety of CA in HF patients with AF, and analyze HF long-term evolution.
Methods
We prospectively analyzed consecutive patients with AF and congestive HF or left ventricular ejection fraction (EF) less than 45%, who underwent CA of AF between 2011 and 2016. We excluded patients who did not complete one year of follow-up.
Results
Seventy-nine patients were included. Mean age was 62.1 years, 72.4% were men, 67.2% had hypertension and 8.6% were diabetics. Mean EF was 49%, left atrial area was 26.5 cm2 and mean CHA2DS2-VASc score was 2. 70.6% were on NYHA FC II-III.
The recurrence rate of AF was 60%, and after a second CA the rate decreased to 27.8%. Only persistent AF prior to the procedure was identified as independent predictor of recurrence. There was a significant NYHA FC improvement in the sinus rhythm (SR) group vs those with recurrence (63.6% vs 36.4%; p=0.047). None of the patients in SR were hospitalized, whereas six with recurrence were hospitalized due to HF (0% vs. 18.2%; p = 0.07). The rate of complications was 9.1%.
Conclusions
Catheter ablation of atrial fibrillation in heart failure presents an adequate success rate, improving symptoms and reducing rehospitalizations due to heart failure.
Keywords: Atrial Fibrillation, Heart Failure, Preserved Ejection Fraction, Catheter Ablation, Hospitalization, Prognostic
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in patients with and without heart failure (HF), affecting 1-2% of the population and up to 50% of the patients with heart failure.[1,2] AF is associated with increased mortality, with a 5-fold increased risk of stroke and a 3-fold increase in the risk of HF and hospitalizations.[3,4]
The association between both conditions is well-known. There is a direct correlation between left ventricular ejection fraction (LVEF), New York Heart Association functional class (NYHA) and the prevalence as well as the duration of AF,[1,3] generating a loop that perpetuates both conditions, increasing morbidity and mortality.[5]
Several studies comparing rhythm control versus rate control failed to demonstrate significant differences favoring either strategy in terms of NYHA or mortality.[6-8]This could be due to the fact that sinus rhythm (SR) was difficult to maintain in the rhythm control group.Atrial fibrillation catheter ablation (CA) has proved to be a safe and efficient option for patients with HF.[9-11]Although the efficacy of this procedure is lower in HF patients compared with those without structural heart disease, recent studies have indicated that the rate of SR at the long-term is higher than that achieved with medical therapy.[12,13] Several studies have analyzed CA in HF patients with reduced LVEF (with different cut-off points), but few have included those with preserved LVEF (HFpEF). We believe that improving the SR rate with CA will improve NYHA, quality of life, morbidity and mortality by reducing hospitalizations in HF patients.
The aim of the study is to evaluate the success rate, freedom from AF and complications associated with AF CA in HF patients with preserved or reduced LVEF. The predictors of AF recurrence, the rate of HF hospitalizations and the NYHA functional class after one year of follow-up according to the presence or absence of SR will also be analyzed.
Methods
Study design and population
We conducted a prospective, observational and single-center study. Consecutive patients with a history of AF and signs and symptoms of HF or LVEF less than 45%, who underwent CA between July 2011 and March 2016, were included. The patients were refractory to or did not tolerate therapy, presenting inadequate ventricular response, recurrent AF or adverse events associated with the treatment.
The exclusion criteria were previous CA of AF, cardiogenic shock, contraindication to anticoagulation, left atrial appendage thrombus, pregnancy or severe comorbidities. Patients who did not complete one year follow-up were also excluded.
HFpEF was defined as LVEF equal to or greater than 50% and at least one of the following: - Signs and symptoms of HF (dyspnea, fatigue or fluid retention).- Echocardiographic evidence of diastolic dysfunction (left atrial enlargement, engorged inferior vena cava, pulmonary hypertension or elevated E/e´ filling velocity). - and elevated brain natriuretic peptide (BNP).
Strategy of anticoagulation
All the patients presented a CHA2DS2-VASc score ≥ 1 and received oral anticoagulation with new oral anticoagulants or vitamin K antagonists (VKAs) for at least 3 weeks. In patients receiving VKAs, therapeutic international normalized ratio (INR) was monitored and the anticoagulant was replaced by subcutaneous enoxaparin (1 mg/kg bid) before the procedure. In those cases receiving new oral anticoagulants, only the last dose was stopped.
During the procedure and before transseptal puncture, an intravenous bolus of unfractionated heparin was administered (200 UI/kg) and activated clotting time (ACT) was monitored every 30 minutes until it reached 350 seconds or greater. Intravenous protamine was administered after the procedure to revert anticoagulation before removing the intravascular introducers and sheaths. Two hours after the procedure ended, unfractionated intravenous heparin was administered by infusion pump. The following morning, subcutaneous low-molecular-weight-heparin (LMWH) and a VKA were administered. LMWH was not administered in patients taking new oral anticoagulants.
Catheter ablation procedure
All the procedures were performed under general anesthesia. All the patients underwent a 64-detector row computed tomography scan or cardiac magnetic resonance imaging if the patient had contraindications. Transesophageal echocardiography was performed during the procedure only if the levels of anticoagulation were inconsistent to exclude left atrial thrombi or to guide difficult transseptal punctures. 12-lead electrocardiogram (ECG) and intracardiac bipolar electrograms were recorded using electronic calibrators (EP-WorkMate 4.2 System, St. Jude Medical, Inc.) at a screen speed of 50 to 200 mm/s and were filtered at band-pass settings of 50 to 500 Hz.
A non-fluoroscopic mapping navigation system was used in all the cases (Ensite® Velocity® cardiac mapping system, St.Jude Medical Inc.). After both femoral veins were punctured, a decapolar catheter was placed in the coronary sinus. Under radioscopic guidance in the 40° left anterior oblique projection, two transseptal punctures were performed with Brockenbrough needles; then, two long preshaped introducers SL1 and SL2 (St. Jude Medical Inc.) were positioned. A circular duodecapolar Optima Plus® catheter and an irrigated-tip ablation catheter Therapy-Cool® (St. Jude Medical Inc.) were advanced through the introducers. The anatomical reconstruction was performed using the circular Optima Plus® mapping catheter which is capable of simultaneous recording from multiple points. The ablation catheter was used to identify the ostia and the antrum of the pulmonary vein (PV).
The electric activity of each PV was obtained using the circular catheter. Isolation started in the left superior PV and continued in the left inferior PV.The same sequence was used for the right PVs. Radiofrequency energy was delivered at the anterior and posterior aspect of each pulmonary vein with a power output of 40 W and of 35 W, respectively. The lesions were applied to the antrum but not the ostia of the veins. The electrograms recorded by the ablation catheter before and after applying the ablation lesion were analyzed in each patient. The target was a reduction of the potential amplitude by 75% and the elimination or dissociation between atrial and PV activity. Once the isolation was completed, the presence of persistent block in each PV was evaluated.If necessary, ablation was repeated to consolidate the line of bidirectional block.
We used all the methods available to discriminate local or remote electrical activity: After pulmonary veins isolation, all the patients with persistent AF underwent electrical cardioversion. In sinus rhythm , other AF ablation techniques were used at the discretion of the treating physician: ablation lines at the cavotricuspid isthmus in the case of atrial flutter history; superior venae cavae or coronary sinus were mapped using the circular duodecapolar catheter and ablated in case of electric activity; complex fractionated atrial electrograms (CFAEs) were mapped with the circular duodecapolar catheter; finally a voltage map was made and those areas with intermediate voltage values (between 0.1 and 0.5 mV) in the left atrium were homogenized.
Patients underwent neurological examination after recovery from anesthesia at the electrophysiology laboratory and before discharge. Upon discharge, the patient continued with the same antiarrhythmic and anticoagulant agent prior to the procedure.
Follow-up
Follow-up visits were scheduled with the electrophysiology service at 1, 3, 6, 9 and 12 months with ECG and 24-hour Holter.In all the cases, the patients underwent physical examination and were interrogated about symptoms suggestive of arrhythmia and HF. If necessary, medical treatment was adjusted and laboratory tests or cardiac imaging tests were ordered. All the visits to the emergency department and hospitalizations due to HF or arrhythmia were also recorded.
Blanking Period
Due to the inflammatory process after catheter ablation, episodes of AF, atrial flutter (AFL) or atrial tachycardia (AT) within the first three months are not considered arrhythmia recurrence.
Success
Success at follow up was defined as the absence of episodes of AF, AFL or AT lasting for more than 30 seconds and documented by Holter monitoring, ECG or centralized monitoring station after the 3-month blanking period. In case of AF recurrence, a second ablation procedure was suggested to the patient.
Clinical improvement
Clinical improvement was defined as improvement by at least one NYHA and a reduction of signs of venous congestion without need of drug adjustment.
Complications
The following events related to the procedure were included: 1. Vascular complications: groin hematoma with a 5-point fall in hematocrit, pseudoaneurysm or femoral arteriovenous fistula. 2. Cardiac tamponade. 3. Stroke or transient ischemic attack (TIA). 4. Worsening of heart failure during the procedure-related hospitalization. 5. Prolonged hospitalization not due to social issues.
Statistical analysis
Discrete variables are expressed as percentages and continuous variables as mean with its corresponding standard deviation. The chi square test was used to compare discrete variables and continuous variables were analyzed using the Student's t test or the Mann-Withney test depending on the distribution of the sample. A multivariate analysis was performed using the Cox proportional hazard regression model. The Kaplan-Meier method was used to compare the groups with and without success. A p value < 0.05 was considered statistically significant. All the statistical procedures were performed using the software package SPSS 21.0.
Ethical considerations
The study protocol was evaluated and approved by the Committee on Ethics of the Instituto Cardiovascular de Buenos Aires. The Argentine personal data protection law 25,326 ensures the confidentiality of all the information. The study was conducted following the recommendations of the Declaration of Helsinki. The principles of the Declaration of Helsinki are fulfilled as the study was approved by the Committee on Ethics, underwent risk benefit assessment and each individual involved in this study was qualified by training to perform the task.
Results
A total of 796 consecutive drug-refractory patients who underwent an AF CA were enrolled. Of these, 99 patients presented signs and symptoms of HF or LVEF less than 45%. We excluded 4 patients with cardiogenic shock, 7 with contraindication to anticoagulation, and 6 with left atrial appendage thrombus, leaving 82 patients in the final analysis.
Baseline characteristics of the population
The cohort was made up of 82 patients undergoing AF CA with signs and symptoms of HF or a LVEF < 45%. Three patients did not complete the 1-year follow-up period and were excluded from the recurrence analysis. Mean age was 62.1 ± 10.5 years and 72.4% were men; 67.2% had hypertension and 8.6% were diabetics. Left ventricular ejection fraction was 49 ± 13.1% and 48.3% had a LVEF < 45%. 25 patients had HFpEF (45%). Left atrial area was 26.5 ± 7 cm2, mean CHA2DS2-VASc score was 2, 44.8% presented paroxysmal AF, 38% persistent and 17.2% long-standing persistent AF (total persistent 55.2%). NYHA II-III was present in 70.6%. The antiarrhythmic treatment included amiodarone (63.8%), propafenone (15.5%) and sotalol (6.9%), and 13.8% were not receiving any antiarrhythmic drug due to intolerance. [Table 1]
Table 1. Baseline characteristics according to atrial fibrillation recurrence or freedom from atrial fibrillation. Data are presented as mean ± standard deviation and percentages.
AF: Atrial fibrillation. HF: Heart failure. NYHA: New York Heart Association. LVEF: Left ventricular ejection fraction. LA: Left atrium.
| Total(82) | Recurrence(47) | Freedom from AF (32) | p | |
|---|---|---|---|---|
| Age | 62.1 +/- 10.2 | 61.0 +/- 11.5 | 63.8 +/- 9.1 | 0.41 |
| Male sex | 72.4% | 72.7% | 81.8% | 0.43 |
| Hypertension | 67.2% | 63.6% | 77.3% | 0.28 |
| Diabetes | 8.6% | 6.1% | 13.6% | 0.33 |
| Smoking habit | 58.6% | 63.6% | 54.5% | 0.50 |
| Coronary artery disease | 20.7% | 24.2% | 13.6% | 0.33 |
| Idiopathic cardiomyopathy | 43.3% | 41.2% | 36.4% | 0.79 |
| Myocarditis. | 13.3% | 11.8% | 18.2% | 0.77 |
| Tachycardiomyopathy | 13.3% | 17.6% | 9.1% | 0.52 |
| Stroke | 5.2% | 6.1% | 4.5% | 0.80 |
| CHA₂DS₂-VASc score | 2 | 2 | 2 | 0.48 |
| Preserved ejection fraction | 43% | 40% | 54% | 0.59 |
| Previous HF hospitalization | 41.4% | 33.3% | 54.5% | 0.11 |
| Type of atrial fibrillation | ||||
| Paroxysmal | 44.8% | 33.3% | 59.1% | 0.59 |
| Persistent | 55.2% | 66.7% | 40.9% | 0.6 |
| NYHA | ||||
| I | 25.9% | 24.2% | 31.8% | 0.53 |
| II | 67.2% | 69.7% | 68.2% | 0.63 |
| III | 3.4% | 6.1% | 0.0% | 0.23 |
| Medication | ||||
| Amiodarone | 63.8% | 78.8% | 40.9% | 0.004 |
| Beta blockers | 55.2% | 57.6% | 54.5% | 0.82 |
| Propafenone | 15.5% | 9.1% | 22.7% | 0.15 |
| Oral anticoagulant | 91.4% | 93.9% | 90.9% | 0.67 |
| Echocardiography | ||||
| LVEF (%) | 49 +/- 13 | 48 +/- 14 | 50 +/- 11 | 0.61 |
| LA (cm2) | 26.5 +/- 6 | 29 +/- 6 | 26 +/- 6 | 0.11 |
| LVEF <= 45% | 48.3% | 48.5% | 45.5% | 0.82 |
Procedure and complications
The acute procedural success rate was 90%. In one year of follow-up, 47 presented AF after the blanking period (success rate of 40% after one procedure).
The AF freedom rate in HFpEF was lower than in the general population and there was no significant difference compared with reduced LVEF (48% vs 32% respectively; p= 0.28). Median time to recurrence was 4 ± 3.2 months in patients with reduced ejection fraction and 6 ± 2.8 months in HFpEF (p= 0.67). Twenty eight patients underwent a second ablation procedure. The recurrence rate after the second procedure was 44.4% at one year. Therefore, the success rate of SR maintenance after two procedures increased to 72.2%. In addition to pulmonary veins isolation, ablation of extrapulmonary foci was performed in 25 patients (43%). There were no significant differences between patients with recurrence or AF freedom (48% vs. 40%; p= 0.12).
Of the 110 ablations (82 in the first and 28 in a second procedure), 10 complications occurred (five in reduced and five in HFpEF; total 9.1%): 8 after the first procedure and 2 after the second ablation, and included: 4 HF worsening, 3 vascular complications, 2 cardiac tamponade requiring pericardiocentesis and 1 extreme bradycardia. None of the patients presented stroke, atrioesophageal fistula, symptomatic pulmonary vein stenosis or procedure-related mortality. [Table 2]
Table 2. Major complications.A higher rate of complications is observed due to the inclusion of worsening of heart failure. The same is not a reported complication in the general population.
| Complications: 10 in 110 procedures (9.1%) | |
|---|---|
| Worsening of Heart Failure | 4 (3.63%) |
| Cardiac tamponade | 2 (1.81%) |
| Groin hematoma | 3 (2.72%) |
| Extreme bradicardia | 1 (0.91%) |
The rate of complications was 9.1%. There were no significant differences between cardiac tamponade (1.81% vs. 1.9%; p= 0.36) or vascular complications (2.72% vs. 2.3%; p = 0.62%) compared with the trials published for the general population in our country. [14]
Predictors of recurrence and follow-up
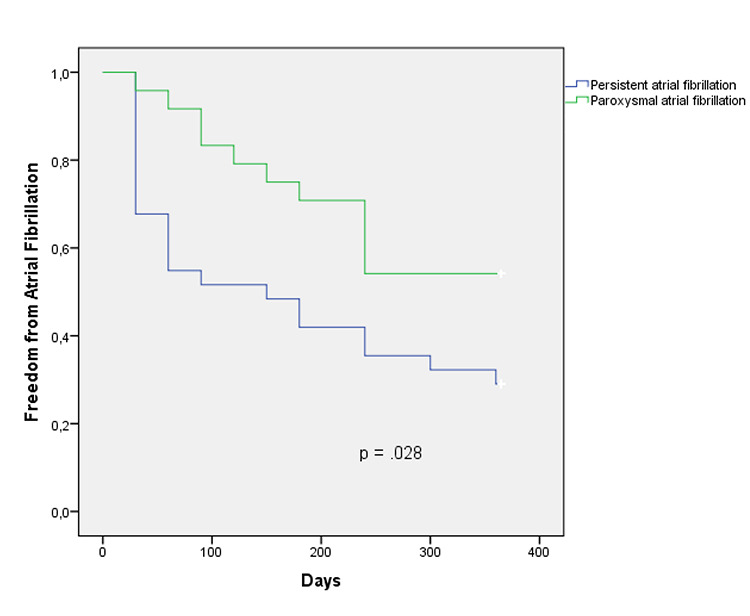
Patients with recurrence of AF were compared with those who remained free from AF to identify predictors of recurrence and to evaluate the clinical outcome during follow-up. Baseline characteristics such as NYHA functional class, persistent AF, left atrial area or LVEF were analyzed by multivariate analysis using Cox regression. Only persistent AF prior to the procedure was identified as an independent predictor of AF recurrence at 1-year follow-up. [Table 3] A Kapplan-Meier survival curve was performed with the Log-Rank test to compare the free survival from AF according to paroxysmal or persistent AF. [Figure 1] Finally, 79 patients who completed 12 months of follow-up after the first procedure were analyzed. Fourteen patients who remained free from AF presented significant improvement of their functional class, while only twelve patients with recurrent AF had improvement (63.6% vs. 36.4%; p = 0.047).
Table 3. Multivariate Cox regression analysis of predictive factors in recurrence for overall survival. Persistent atrial fibrillation was the only independent predictor (p = 0.042).
AF: Atrial fibrillation. NYHA: New York Heart Association. LVEF: Left ventricular ejection fraction. LA: Left atrium
| log HR | (95% IC) | p | |
|---|---|---|---|
| Age | 0.98 | (0.95 – 1.01) | .33 |
| Persistent AF | 2.41 | (1.38 – 5.47) | .042 |
| LVEF | 0.99 | (0.96 – 1.02) | .5 |
| LA size | 0.99 | (0.94 – 1.06) | .97 |
| NYHA | 1.24 | (0.66 – 2.55) | .54 |
Figure 1. Freedom from atrial fibrillation compared by the Kaplan-Meier curves with the log-rank test for overall survival. Persistent atrial fibrillation showed a higher recurrence rate compared to paroxysmal atrial fibrillation.
Also, none of the patients in SR were hospitalized, whereas 6 patients with recurrent AF were hospitalized due to HF (0% vs. 18.2%; p = 0.07) [Figure 2]. Thus, 95.5% of the patients in SR and 54.5% of those with recurrence of AF were free from HF or in functional class I (p = 0.001). [Figure 3]
Figure 2. Differences at one year follow-up between patients with recurrence or freedom from atrial fibrillation. Heart failure functional class improvement was statistically significant using Chi-Square Test.
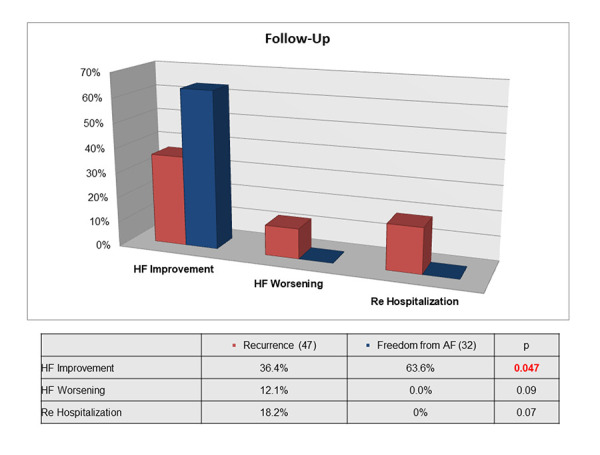
Figure 3. NYHA Functional Class at one year follow-up. Patients free from atrial fibrillation have better functional class than those with recurrence using Chi-Square Test.

Discussion
Main findings
The results reported in this study indicate that AF ablation in HF patients with reduced or preserved LVEF is a safe and acceptable therapeutic option. The acute success rate was high, 90% after the procedure, and although 47 patients had arrhythmia recurrence, a second procedure was successful in maintaining SR at one year in 72.2% of the cases, without increasing the incidence of complications. The improvement of symptoms and the hospitalization rate showed that SR maintenance had significant benefits in these patients. Of the variables analyzed, persistent AF before the procedure had a significant association with AF recurrence at one year. Probably, early intervention of AF in HF patients will improve SR rate during follow-up.
Our study also analyzed the population with preserved LVEF. The recurrence rate of AF at one year of follow-up was higher than in the general population, however, they also benefited clinically with catheter ablation.[14]
The rate of complications was higher in our study due to HF worsening, a complication that was not included in publications evaluating the general population (9.1% vs. 4.6%; p = 0.03). [14] We did not register major events such as death or stroke. Also, no patient required inotropic agents, vasopressors or mechanical ventilation.
Pathophysiology and previous studies
Atrial fibrillation and HF are two conditions that impair quality of life and reduce longevity. The prevalence of AF in patients with HF enrolled in clinical trials ranges between 15 and 45%.[15-19] Atrial fibrillation increases the risk of embolic stroke and tachycardiomyopathy, and is associated with reduced survival. The association between HF and AF worsens the prognosis, with a survival rate between 25 and 40% at 5 years according to the previous NYHA. Each condition increases the severity and worsens the outcome of the other.
There are several pathophysiological mechanisms that perpetuate both pathologies. Heart failure produces diastolic insufficiency, electromechanical remodeling of the left atrium, increased sympathetic tone and hydrosaline retention. This increases the rate of episodes of AF. Atrial fibrillation begins as an isolated ectopic activity mainly from the pulmonary veins. Subsequently, with the chronicity of both pathologies, the electrical and mechanical remodeling of the left atrium worsens, causing the perpetuity of the arrhythmia from multiple wave fronts. The electrical and anatomical changes associated with AF worsen heart failure, a situation that also worsens the evolution of atrial fibrillation, generating a vicious loop.[20]
Several studies have compared the efficacy of rate control versus rhythm control in HF patients, but did not show better outcomes with one strategy over the other. However, these studies only used medical treatment for rhythm control with suboptimal efficacy to maintain patients in SR.[6] In addition, 21% of the patients in the rhythm control group crossed over to the rate control group due to impossibility to maintain SR, and 10% of those in the rate control group crossed over to the rhythm control group, mostly due to HF worsening.[21] The adverse events of the medications and the presence of contraindications in patients with structural heart disease were other factors that failed to maintain SR.
The superiority of CA over antiarrhythmic treatment in patients with symptomatic AF has been already demonstrated.[22-25] several studies analyzed the role of CA in HF patient using functional endpoints with different results.
The study by McDonald et al., included patients with persistent AF and advanced HF and failed to demonstrate significant improvement in LVEF and in other secondary endpoints as six-minute walk distance, quality of life and NTproBNP, compared with a rate control strategy. After 14 months, only 50% of the patients in the CA group remained in SR The inclusion of patients with persistent AF and advanced HF could explain the high rate of recurrence without achieving the final endpoints.
In patients with AF refractory to antiarrhythmic treatment, left ventricular dysfunction and HF in NYHA class II-III, pulmonary veins isolation showed significantly better quality of life, longer six-minute walk distance and higher LVEF compared with ablation of the AV node and biventricular pacing after 6 months.[11]
The CAMTAF trial analyzed CA in patients with persistent AF, HF and LVEF < 50% and showed significant improvement of LVEF, oxygen consumption and quality of life at 6 months compared with rate control. Freedom from AF was achieved in 81% of patients at 6 months of follow-up without antiarrhythmic drugs.[12] Despite the short follow-up period, the high rate of freedom from AF is associated with clinical improvement.
More recently, a study compared CA versus rate control in patients with persistent AF, HF and LVEF < 35%.The primary endpoint -improvement in oxygen consumption at 12 months- was significantly higher in patients undergoing CA. The Minnesota score and BNP also showed significant improvement.[26] None of the studies mentioned above analyzed rehospitalization due to HF, a significant prognostic indicator.
Finally, the CASTLE-AF study,[27] a randomized trial recently presented in the 2017 ESC congress, enrolled 363 patients with symptomatic paroxysmal or persistent AF, intolerance to take at least one antiarrhythmic drug, LVEF less than 35% and NYHA FC ≥2 with implantable cardioverter-defibrillator or cardiac resynchronization therapy-defibrillator with home monitoring capabilities. This study showed that catheter ablation was superior at preventing death or heart failure admissions (28.5% vs. 44.6%; p = 0.007). However, unlike our work, HF patients with preserved LVEF were not included. It is known that atrial fibrillation is a frequent cause of diastolic failure and that diastolic failure predisposes to AF recurrence after medical treatment or radiofrequency ablation. [28,29] Therefore, HFpEF patients would also benefit from catheter ablation.
Clinical Implications
The clinical impact found in our work is due to keeping patients with HF in SR. The success achieved with catheter ablation improves the functional class and reduces re-admissions for heart failure in HF patients with reduced or preserved LVEF.
This is achieved without increasing the rate of complications, as has occurred in pharmacological treatment trials to maintain SR. As we have previously seen, most of the studies evaluating CA in HF patients showed benefits in terms of quality of life, six-minute walk distance and LVEF. Our initial experience shows that the success rate at one year in patients with HF and AF treated with CA was acceptable, and that the patients who remained in SR had better NYHA functional class and fewer re-hospitalizations. Symptoms relief and reduction of hospitalizations are endpoints with a positive impact on the evolution of the disease and on the healthcare system. Likewise, patients with preserved LVEF, a poorly studied population, benefited as much as those with reduced ejection fraction.
Study Limitations
This study has several potential limitations. Firstly, it is a single-center and observational study. The rate of success and of complications could be different in each center. In addition, the ablation technique varies according to each case and to the discretion of the attending physician. Secondly, although we recorded the complications in our database, those occurring after discharge could have been lost. Thirdly, the information about patients living in other cities or neighboring countries followed-up by telephone calls could be underestimated.
Conflicts of interest
None declared.
Conclusions
Catheter ablation of atrial fibrillation in heart failure patients presents an adequate success rate in patients refractory or intolerant to antiarrhythmic treatment, improving symptoms and reducing re-hospitalizations due to heart failure. The benefit was observed both in preserved and reduced ejection fraction. Persistent atrial fibrillation is an independent predictor of recurrence.
References
- 1.Fonarow Gregg C. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4 Suppl 7 ():S21–30. [PubMed] [Google Scholar]
- 2.Nieuwlaat Robby, Capucci Alessandro, Camm A John, Olsson S Bertil, Andresen Dietrich, Davies D Wyn, Cobbe Stuart, Breithardt Günter, Le Heuzey Jean-Yves, Prins Martin H, Lévy Samuel, Crijns Harry J G M. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur. Heart J. 2005 Nov;26 (22):2422–34. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 3.Wang Thomas J, Larson Martin G, Levy Daniel, Vasan Ramachandran S, Leip Eric P, Wolf Philip A, D'Agostino Ralph B, Murabito Joanne M, Kannel William B, Benjamin Emelia J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003 Jun 17;107 (23):2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 4.Naccarelli Gerald V, Hynes B John, Wolbrette Deborah L, Bhatta Luna, Khan Mazhar, Samii Soraya, Luck Jerry C. Atrial fibrillation in heart failure: prognostic significance and management. J. Cardiovasc. Electrophysiol. 2003 Dec;14 (12 Suppl):S281–6. doi: 10.1046/j.1540-8167.2003.90404.x. [DOI] [PubMed] [Google Scholar]
- 5.Mamas Mamas A, Caldwell Jane C, Chacko Sanoj, Garratt Clifford J, Fath-Ordoubadi Farzin, Neyses Ludwig. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur. J. Heart Fail. 2009 Jul;11 (7):676–83. doi: 10.1093/eurjhf/hfp085. [DOI] [PubMed] [Google Scholar]
- 6.Roy Denis, Talajic Mario, Nattel Stanley, Wyse D George, Dorian Paul, Lee Kerry L, Bourassa Martial G, Arnold J Malcolm O, Buxton Alfred E, Camm A John, Connolly Stuart J, Dubuc Marc, Ducharme Anique, Guerra Peter G, Hohnloser Stefan H, Lambert Jean, Le Heuzey Jean-Yves, O'Hara Gilles, Pedersen Ole Dyg, Rouleau Jean-Lucien, Singh Bramah N, Stevenson Lynne Warner, Stevenson William G, Thibault Bernard, Waldo Albert L. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 2008 Jun 19;358 (25):2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 7.Wyse D G, Waldo A L, DiMarco J P, Domanski M J, Rosenberg Y, Schron E B, Kellen J C, Greene H L, Mickel M C, Dalquist J E, Corley S D. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 Dec 05;347 (23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 8.Van Gelder Isabelle C, Hagens Vincent E, Bosker Hans A, Kingma J Herre, Kamp Otto, Kingma Tsjerk, Said Salah A, Darmanata Julius I, Timmermans Alphons J M, Tijssen Jan G P, Crijns Harry J G M. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002 Dec 05;347 (23):1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 9.Hsu Li-Fern, Jaïs Pierre, Sanders Prashanthan, Garrigue Stéphane, Hocini Mélèze, Sacher Fréderic, Takahashi Yoshihide, Rotter Martin, Pasquié Jean-Luc, Scavée Christophe, Bordachar Pierre, Clémenty Jacques, Haïssaguerre Michel. Catheter ablation for atrial fibrillation in congestive heart failure. N. Engl. J. Med. 2004 Dec 02;351 (23):2373–83. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald Michael R, Connelly Derek T, Hawkins Nathaniel M, Steedman Tracey, Payne John, Shaw Morag, Denvir Martin, Bhagra Sai, Small Sandy, Martin William, McMurray John J V, Petrie Mark C. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011 May;97 (9):740–7. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- 11.Khan Mohammed N, Jaïs Pierre, Cummings Jennifer, Di Biase Luigi, Sanders Prashanthan, Martin David O, Kautzner Josef, Hao Steven, Themistoclakis Sakis, Fanelli Raffaele, Potenza Domenico, Massaro Raimondo, Wazni Oussama, Schweikert Robert, Saliba Walid, Wang Paul, Al-Ahmad Amin, Beheiry Salwa, Santarelli Pietro, Starling Randall C, Dello Russo Antonio, Pelargonio Gemma, Brachmann Johannes, Schibgilla Volker, Bonso Aldo, Casella Michela, Raviele Antonio, Haïssaguerre Michel, Natale Andrea. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N. Engl. J. Med. 2008 Oct 23;359 (17):1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 12.Hunter Ross J, Berriman Thomas J, Diab Ihab, Kamdar Ravindu, Richmond Laura, Baker Victoria, Goromonzi Farai, Sawhney Vinit, Duncan Edward, Page Stephen P, Ullah Waqas, Unsworth Beth, Mayet Jamil, Dhinoja Mehul, Earley Mark J, Sporton Simon, Schilling Richard J. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014 Feb;7 (1):31–8. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- 13.Di Biase Luigi, Mohanty Prasant, Mohanty Sanghamitra, Santangeli Pasquale, Trivedi Chintan, Lakkireddy Dhanunjaya, Reddy Madhu, Jais Pierre, Themistoclakis Sakis, Dello Russo Antonio, Casella Michela, Pelargonio Gemma, Narducci Maria Lucia, Schweikert Robert, Neuzil Petr, Sanchez Javier, Horton Rodney, Beheiry Salwa, Hongo Richard, Hao Steven, Rossillo Antonio, Forleo Giovanni, Tondo Claudio, Burkhardt J David, Haissaguerre Michel, Natale Andrea. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation. 2016 Apr 26;133 (17):1637–44. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 14.L Tomas , A Orosco, JM Vergara , S Rivera , N Vecchio , I Mondragón , M Caro , A Giniger , G Albina , F Scazzuso . Predictores de recurrencia y resultados en la ablación de la fibrilación auricular paroxística. Rev Arg Cardiol. 2017;0:240–246. [Google Scholar]
- 15.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N. Engl. J. Med. 1987 Jun 04;316 (23):1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 16.Bardy Gust H, Lee Kerry L, Mark Daniel B, Poole Jeanne E, Packer Douglas L, Boineau Robin, Domanski Michael, Troutman Charles, Anderson Jill, Johnson George, McNulty Steven E, Clapp-Channing Nancy, Davidson-Ray Linda D, Fraulo Elizabeth S, Fishbein Daniel P, Luceri Richard M, Ip John H. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005 Jan 20;352 (3):225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 17.Kadish Alan, Dyer Alan, Daubert James P, Quigg Rebecca, Estes N A Mark, Anderson Kelley P, Calkins Hugh, Hoch David, Goldberger Jeffrey, Shalaby Alaa, Sanders William E, Schaechter Andi, Levine Joseph H. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N. Engl. J. Med. 2004 May 20;350 (21):2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 18.McMurray John J V, Ostergren Jan, Swedberg Karl, Granger Christopher B, Held Peter, Michelson Eric L, Olofsson Bertil, Yusuf Salim, Pfeffer Marc A. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003 Sep 06;362 (9386):767–71. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 19.Zannad Faiez, McMurray John J V, Krum Henry, van Veldhuisen Dirk J, Swedberg Karl, Shi Harry, Vincent John, Pocock Stuart J, Pitt Bertram. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011 Jan 06;364 (1):11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 20.Janse Michiel J. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc. Res. 2004 Feb 01;61 (2):208–17. doi: 10.1016/j.cardiores.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Talajic Mario, Khairy Paul, Levesque Sylvie, Connolly Stuart J, Dorian Paul, Dubuc Marc, Guerra Peter G, Hohnloser Stefan H, Lee Kerry L, Macle Laurent, Nattel Stanley, Pedersen Ole D, Stevenson Lynne Warner, Thibault Bernard, Waldo Albert L, Wyse D George, Roy Denis. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J. Am. Coll. Cardiol. 2010 Apr 27;55 (17):1796–802. doi: 10.1016/j.jacc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Wazni Oussama M, Marrouche Nassir F, Martin David O, Verma Atul, Bhargava Mandeep, Saliba Walid, Bash Dianna, Schweikert Robert, Brachmann Johannes, Gunther Jens, Gutleben Klaus, Pisano Ennio, Potenza Dominico, Fanelli Raffaele, Raviele Antonio, Themistoclakis Sakis, Rossillo Antonio, Bonso Aldo, Natale Andrea. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005 Jun 01;293 (21):2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 23.Wilber David J, Pappone Carlo, Neuzil Petr, De Paola Angelo, Marchlinski Frank, Natale Andrea, Macle Laurent, Daoud Emile G, Calkins Hugh, Hall Burr, Reddy Vivek, Augello Giuseppe, Reynolds Matthew R, Vinekar Chandan, Liu Christine Y, Berry Scott M, Berry Donald A. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010 Jan 27;303 (4):333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 24.Cosedis Nielsen Jens, Johannessen Arne, Raatikainen Pekka, Hindricks Gerhard, Walfridsson Håkan, Kongstad Ole, Pehrson Steen, Englund Anders, Hartikainen Juha, Mortensen Leif Spange, Hansen Peter Steen. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N. Engl. J. Med. 2012 Oct 25;367 (17):1587–95. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 25.Morillo Carlos A, Verma Atul, Connolly Stuart J, Kuck Karl H, Nair Girish M, Champagne Jean, Sterns Laurence D, Beresh Heather, Healey Jeffrey S, Natale Andrea. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014 Feb 19;311 (7):692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 26.Jones David G, Haldar Shouvik K, Hussain Wajid, Sharma Rakesh, Francis Darrel P, Rahman-Haley Shelley L, McDonagh Theresa A, Underwood S Richard, Markides Vias, Wong Tom. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J. Am. Coll. Cardiol. 2013 May 07;61 (18):1894–903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 27.Marrouche Nassir F, Brachmann Johannes, Andresen Dietrich, Siebels Jürgen, Boersma Lucas, Jordaens Luc, Merkely Béla, Pokushalov Evgeny, Sanders Prashanthan, Proff Jochen, Schunkert Heribert, Christ Hildegard, Vogt Jürgen, Bänsch Dietmar. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018 Feb 01;378 (5):417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 28.Kosiuk Jedrzej, Van Belle Yves, Bode Kerstin, Kornej Jelena, Arya Arash, Rolf Sascha, Husser Daniela, Hindricks Gerhard, Bollmann Andreas. Left ventricular diastolic dysfunction in atrial fibrillation: predictors and relation with symptom severity. J. Cardiovasc. Electrophysiol. 2012 Oct;23 (10):1073–7. doi: 10.1111/j.1540-8167.2012.02368.x. [DOI] [PubMed] [Google Scholar]
- 29.Kosiuk Jedrzej, Breithardt Ole-A, Bode Kerstin, Kornej Jelena, Arya Arash, Piorkowski Christopher, Gaspar Thomas, Sommer Philipp, Husser Daniela, Hindricks Gerhard, Bollmann Andreas. The predictive value of echocardiographic parameters associated with left ventricular diastolic dysfunction on short- and long-term outcomes of catheter ablation of atrial fibrillation. Europace. 2014 Aug;16 (8):1168–74. doi: 10.1093/europace/eut415. [DOI] [PubMed] [Google Scholar]
- 30.Anselmino Matteo, Grossi Stefano, Scaglione Marco, Castagno Davide, Bianchi Francesca, Senatore Gaetano, Matta Mario, Casolati Dario, Ferraris Federico, Cristoforetti Yvonne, Negro Alessandro, Gaita Fiorenzo. Long-term results of transcatheter atrial fibrillation ablation in patients with impaired left ventricular systolic function. J. Cardiovasc. Electrophysiol. 2013 Jan;24 (1):24–32. doi: 10.1111/j.1540-8167.2012.02419.x. [DOI] [PubMed] [Google Scholar]


