Abstract
Background
Catheter ablation technology has evolved rapidly in recent years. There is a need to understand the impact of these advances on efficiency, safety, and effectiveness in real-world populations. The objective of this study was to evaluate a standardized workflow that integrates a contact force (CF) catheter and stability module in an attempt to optimize efficiency and clinical outcomes of paroxysmal atrial fibrillation (PAF) ablation, and to compare the outcomes of this workflow with existing ablation technologies at a high-volume center.
Methods
Consecutive ablations for PAF from July 2013 - June 2016 were included. Radiofrequency (RF) ablations were performed with the ThermocoolSF Catheter (SF) through April 2014, after which a change was made to the ThermocoolSmarttouchCatheter (ST)with a standardized workflow. Cryoballoon ablations (CA) were performed with theArctic FrontAdvancebetween July 2013 and March 2016. Systematic collection of 12-month effectiveness data began in July 2014. Prior to that time, only acute outcomes and reablations were captured.
Results
Procedural data for 32 SF, 232 ST, and 59 CA procedures for PAF were available. Mean procedure times were similar across SF and CA, and moderately shorter with ST (p=0.0201). Fluoroscopy times were substantially reduced with ST (p<0.0001). Complication rates were low and similar across all cohorts (p=0.4744), whereas reablation rates were lowest in the ST cohort (p=0.0194).
Conclusions
PAF ablation using integrated CF and catheter stability technology with a systematic ablation workflow maylead to improvements in both procedural efficiency and reablation rates, without compromising patient safety.
Keywords: Atrial Fibrillation Ablation, Catheter Ablation, Workflow, Contact Force, Fluoroscopy
Introduction
Catheter ablation is a well-established treatment option for patients with atrial fibrillation (AF). AF ablation techniques and technologies have improved greatly over the past 15 years;nevertheless,AF ablation remains a technically challenging procedure with room for improvement in both efficiency and efficacy[1].Reported procedure times arestill lengthy,[2-4] and fluoroscopy exposure remainsa concern for patients, physicians, and electrophysiology (EP) lab staff. Innovative strategies and technologies that increase efficiency while maintaining or optimizing efficacy and safety are thus welcomed by the EP community.
Recent years have seen significant improvements in catheter technology and electroanatomic mapping (EAM) systems. Contact force (CF)-sensing catheters have improved procedural efficiency and efficacy[5].Meanwhile, improvements to EAM systems now allow objective measurement of catheter stability and CF during radiofrequency (RF) energy delivery. As a consequence of these latest technological advances,operators are challengedwitha paucity of data on how to better incorporate them into an effective, efficient, and safe workflow.
The objective of this study is to describe a single-center experience witha workflow developed to integrate and optimize the use of new CFcatheter technologyand compare it with prior technologies.Our goal is toprovide a strategy to help minimize the learning curve required for full incorporation of the new technologies, such that procedural efficiency and effectiveness are optimized, and safety is uncompromised.
Methods
This study represents the experience of a real-world paroxysmal atrial fibrillation (PAF) population presenting for an index catheter ablation at a single high-volume EP practice between July 2013 and June 2016. All patients were treated according to standard clinical practice, and were ablated by an operator experienced in both RF and cryoballoon ablation (>100 cases with each technology). Baseline patient characteristics, procedural details, and acute outcomes were collected for all ablations during the study period. Effectiveness outcomes at 12 months were collected systematically beginning inJuly 2014.
Procedural efficiency and safety outcomes were analyzed for ablations performed under the new workflow utilizing the ThermocoolSmarttouchCatheter(Biosense Webster, Inc.) (ST) and CartoVisitag Module (Biosense Webster, Inc.) (Visitag), and were compared with prior and concurrent ablations at the same site utilizing the ThermocoolSF Catheter (Biosense Webster, Inc.)(SF) or the second-generation cryoballoon, the Arctic Front Advance Cardiac Cryoablation Catheter (Medtronicplc).Local institutional review board approval was obtained.
Patient population
We analyzed the prospectively collected data of all adult PAF patients with index catheter ablations performed by two operators at our center during the 3-year study period. Patients with prior catheter or surgical ablations were excluded from the analysis. Cohorts were defined based on the catheter technology utilized in these procedures. RF ablations were performed with the SF catheter through April 2014, at which time the ST catheter was adopted. Cryoablation (CA)procedures were performed with no technology changebetween July 2013 and March 2016. Data were extracted for the SF and CA cohorts at the end of 2016, and for the ST cohort at the end of 2017. The selection of RF energy versus CA for the index ablation was at the discretion of the operator and shared with the patient.
RF ablation procedure
After obtaining patient informed consent, general anesthesia with endotracheal intubation was administered. The procedure was performed with uninterrupted oral anticoagulation. Most patients were on direct oral anticoagulants (DOACs), which were continued the morning of the procedure without a single dose interruption. For patients on warfarin, INR >2.0 was targeted. A pre-procedure transesophageal echocardiogram was obtained to rule out left atrial appendage thrombus.
A decapolar deflectable catheter was placed in the coronary sinus (CS).Intravenous heparin was administered before and after transseptal catheterization to target an activated clotting time of > 350 seconds. Transseptal catheterization was performed with a Fast-Cath SL2preformed sheath (St. Jude Medical, Inc.) and a Brockenbrough needle, and guided by intracardiac echocardiography (ICE)with minimal or nofluoroscopy, as previously described[6].Whentransseptal access was achieved, a J-tipped wire was advanced to the left superior pulmonary vein (PV), guided by ICE,and the trajectory of the wire across the fossa ovalis was marked with Cartosound Module(Biosense Webster, Inc.) technology. The ablation catheter was then advanced to the left atrium (LA), guided by ICE and Cartosound System, viathe same access site as the initial transseptal access.
The LA geometry and voltage were acquired with theCarto(Biosense Webster, Inc.) system using parameters designed for rapid acquisition of geometric and voltage data, as follows:
1. For patients in sinus rhythm, atrial pacing was performed from the CS at 500 ms during geometry and voltage acquisition. For patients in AF,cardioversion was performed beforehand. 2. A Lasso2515 or PentarayCatheter (Biosense Webster, Inc.)was used to create the LA geometry and acquire voltage data with fast anatomic mappingin the Confidense Module ofCarto3 System Version 4 (Biosense Webster, Inc.). 3. For rapid data acquisition, ventilator parameterswere set at 16 breaths per minute ata 1:4 ratio of inspiration to expiration.
The CartounivuModule(Biosense Webster, Inc.) (Univu) was used to integrate still fluoroscopy images as the background for the EAM system. A quadripolar catheter was tied to a standard esophageal temperature probe with silk 2:0 and inserted in the esophagus to monitor location and temperature without fluoroscopy. Wide area circumferential ablations (WACA) around ipsilateral PVs were performed for patients undergoing RF ablation with the SF or ST catheters.
blation workflow for ST ablations
In the ST cohort,the followingworkflowwas followed to optimize the use of the CF and mapping technologies for:
T1. prospective tagging of lesions, 2. real-time monitoring of impedance and CF, and 3. validation of lesion sets.
Initial Visitagparameters included only the stability of 2.5 mm maximum range and 4 seconds minimum timeto quickly obtain tags. Ablation was performed at 40 watts throughout the lesion set. When ablating the posterior wall, RF time was decreased at the operator’s discretion. During ablation, the CF goal was 10 to 20 grams, and Visitagautomatically generated ablation tags for each application meeting the stability parameters. The catheter was moved every 15-20 seconds in the anterior aspects of the ipsilateral veins and every 10-15 secondsin the posterior wall, regardless of whether a tag was obtained. Parameters were chosen to avoid inappropriately prolonging RF delivery while waiting on the appearance of a lesion tag.
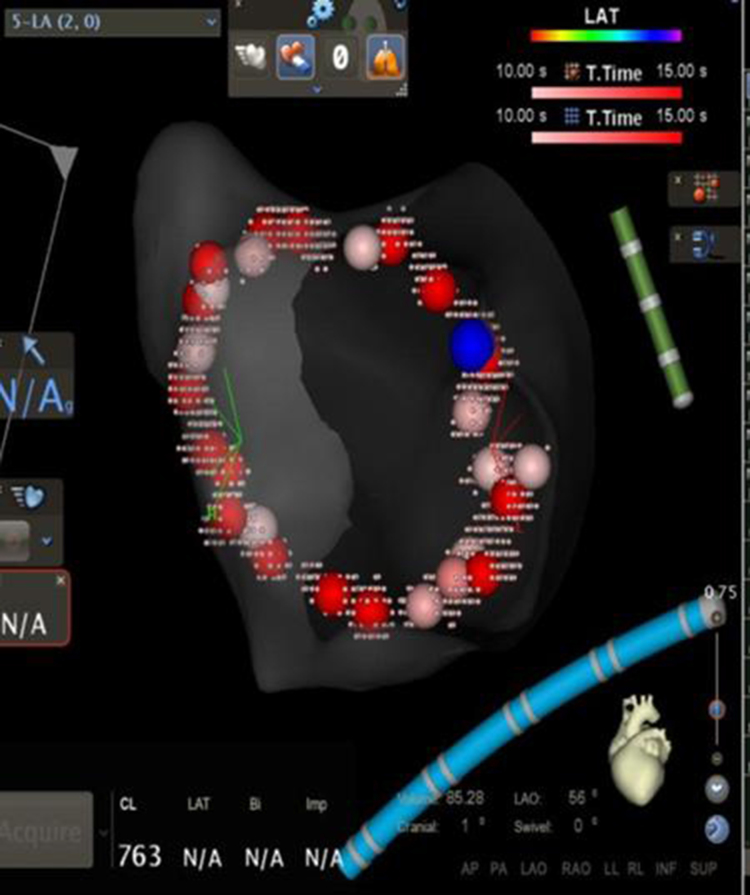
To validate the lesion set after PVisolation [Figure 1a], force over time (FOT) assessment was used to filter and display only tags for locations where a minimum force of 10 grams was maintained for at least 50% of the time[Figure 1b]. All visual gaps where the CF target was not met were targeted for touch-up. At the end of the procedure, the complete pulmonary vein isolation (PVI) lesion set met stability and CF goals [Figure 1c].
Figure 1a. Procedural Workflow for Pulmonary Vein Isolation with Contact Force Sensing in AF Ablation (a) After Isolation.
WACA lesion set after PV isolation without force filters; the blue tag denotes isolation
Ablation with SF catheter
The SF ablation procedure was similar, including stability and time criteria for Visitag.The ablation was continued until the local electrogram was abolished or significantly reduced. Univu was not available for the entire span of this cohort.
Cryoablation
CA procedures were performed by the standard technique using a second generation cryoballoonwith uninterrupted oral anticoagulation. Single transseptal access was obtained using an SL2 preformed sheath and a Brockenbrough needle guided by fluoroscopy. Neither ICEnor EAM were used. A quadripolar catheter was used to pace the phrenic nerve during ablation of the right-sided veins. The Achieve(Medtronic, plc.) multipolar catheter was used to cannulate the veins and confirm PVI.
PV occlusion was confirmed with pressure monitoring and, if needed, angiography. All veins were isolated using a minimum oftwo freeze-thaw cycles, with freeze durations ofat least three minutes. After isolation of a vein, a bonus freeze was always performed for three minutes.
Assessing acute procedural success
After PVI was confirmed in all cohorts,and after all visual gaps in the VisitagFOT tags were ablated in theST cohort, adenosine 18 mg was injected intravenously to assess dormant conduction. If dormant conduction was detected, further ablation was performed until the PVs were re-isolated. Thereafter,continuous isoproterenol infusion was administeredat up to 20 mcg/min to identify any PV reconnectionor non-PV triggersfor a minimum of 20 minutes after the last ablation lesion. If non-PV triggers were identified, they were targeted foradditional RF ablation.
Follow-up
Follow-up visits were performed at 3 and 12 months,with a96-hour Holtermonitoring at 6 and 12 months after the ablation. Event monitors were used as needed for patients with symptoms. Any hospitalizations or unscheduled office visitsthat documented an atrial arrhythmia recurrence were also captured. Though a comprehensive clinical follow-up schedule was in place throughout the study period, standardized data collection forms were only used to capture the effectiveness detail systematically for the ST cohort. Earlier ablation follow-up, including all of the SF and most of the CA ablations, did not capture recurrence information comprehensively or cumulatively.
Study endpoints
The primary study endpoints were the procedural efficiency measures of total procedure time and fluoroscopy duration and dose. Procedure time was defined as time from vascular access toremoval of catheters. The safety endpoint was occurrence of anyserious procedure-related complication, including pericardial effusion requiring intervention, periprocedural stroke, death, need for emergent thoracic surgery, oran acute vascular complication. The key effectiveness endpoint wassurvival time without repeat ablation. A secondary effectiveness endpoint, captured only for the later procedures, was treatmentsuccess at the 12-month visit. The 12-month treatment success criteriawas defined as freedom fromany atrial arrhythmia lasting >30 seconds within 3-12 months after the index ablation without antiarrhythmic drugs (AADs),or with an AAD that was previously ineffective or became effective at a lower dose than required previously.
Statistical analysis
Survival analysis modeling methodology, including Kaplan-Meier and Cox regression, was used to model time to repeat ablation by cohort. Product limit estimates of the proportion of patients who did not requirea repeat procedure were calculated through one year post-ablation for each cohort with Kaplan-Meier survival models. Cox regression models were used to test for the significance of additional patient and procedural characteristics in explaining reablation rates, as well as for calculating hazard ratios to compare reablation risk by cohort. The characteristics tested for significance included age, sex, CHA2DS2-VASc score, congestive heart failure, LA size and volume, left ventricular ejection fraction (LVEF), operator, procedure time, and the ability to pharmacologically induce latent arrhythmia.
All statistical analyses in this study were performed using SAS software, Version 9.2 (SAS Institute, Inc., Cary, NC). All data were de-identified and accessed in compliance with the Health Insurance Portability and Accountability Act.
Results
A total of 323 patients had an index ablation for PAF during the study period and were eligible for inclusion in the analysis. An additional 38 procedures performed within the study period were excluded from the analysis population due to missing catheter information. The RF cohorts consisted of 32 patients with SF ablation from July 2013 to March 2014, followed by 232 patients with ST ablation from April 2014 through June 2016. The CA cohort consisted of 59 patients with ablation dates from July 2013 to March 2016.
Patient characteristics
The patient population comprised 50% males, with significantly more females (73%) in the CA cohort (chi-square p=0.0006) compared with the SF or ST cohorts (53% and 55%). The mean age was 63 years with similar age distributions across cohorts. CHADS2 scores were also similar across cohorts, averaging 1.4 for the SF and CA cohorts and 1.3 for the ST cohort. Mean CHA2DS2-VASc risk scores were slightly higher in the CA cohort due to the higher percentage of females in this cohort,while prevalence rates of individual comorbidities, LVEF, and LA size(parasternal long axis view) were similar across the three cohorts [Table 1].
Table 1. Baseline Patient Characteristics.
Statistical tests: ANOVA for numeric variables and chi-squared for categorical variables; significance denoted by * for p<0.05, ** for p<0.01, *** for p<0.001; Note: sample sizes for a particular measurement may be different from the overall sample size due to missing data
| SF (N=32) | ST (N=232) | CA (N=59) | ||||
|---|---|---|---|---|---|---|
| n | Percent | n | Percent | n | Percent | |
| Age (mean, SD) | 63.8 | 11.5 | 62.9 | 10.9 | 63.7 | 12.6 |
| Male Sex*** | 17 | 53.1 | 128 | 55.2 | 16 | 27.1 |
| Left Ventricular Ejection Fraction (%) | ||||||
| <35% | 2 | 6.3 | 9 | 3.9 | 0 | 0.0 |
| 35-44% | 3 | 9.4 | 14 | 6.0 | 1 | 1.7 |
| 45-54% | 4 | 12.5 | 25 | 10.8 | 9 | 15.3 |
| 55% and higher | 15 | 46.9 | 116 | 50.0 | 35 | 59.3 |
| Unknown | 8 | 25.0 | 68 | 29.3 | 14 | 23.7 |
| Left Atrial Size, cm (mean, SD) | 3.8 | 0.7 | 3.9 | 0.8 | 3.9 | 0.7 |
| Medical History | ||||||
| Congestive Heart Failure | 5 | 15.6 | 26 | 11.2 | 9 | 15.3 |
| Hypertension | 22 | 68.8 | 154 | 66.4 | 44 | 74.6 |
| Diabetes | 5 | 15.6 | 50 | 21.6 | 9 | 15.3 |
| Stroke/Transient Ischemic Attack | 4 | 12.5 | 19 | 8.2 | 6 | 10.2 |
| Vascular Disease* | 13 | 40.6 | 57 | 24.6 | 22 | 37.3 |
| Renal Disease* | 3 | 9.4 | 7 | 3.0 | 6 | 10.2 |
| Liver Disease | 0 | 0.0 | 2 | 0.9 | 0 | 0.0 |
| Pre-ablation Medications | ||||||
| Antiarrhythmic drugs | 23 | 71.9 | 145 | 62.5 | 50 | 84.8 |
| Beta-blockers | 18 | 56.3 | 131 | 56.5 | 24 | 40.7 |
| CHA2DS2-VASc Score | ||||||
| 0 | 0 | 0.0 | 17 | 7.3 | 2 | 3.4 |
| 1 | 6 | 18.8 | 52 | 22.4 | 11 | 18.6 |
| 2 | 9 | 28.1 | 58 | 25.0 | 11 | 18.6 |
| 3 | 6 | 18.8 | 49 | 21.1 | 10 | 17.0 |
| 4 | 8 | 25.0 | 30 | 12.9 | 13 | 22.0 |
| 5 | 1 | 3.1 | 18/ | 7.8 | 6 | 10.2 |
| 6 | 2 | 6.3 | 5 | 2.2 | 5 | 8.5 |
| 7 | 0 | 0.0 | 3 | 1.3 | 1 | 1.7 |
| Follow-up Time After Ablation | ||||||
| Patients with ≥ 1 Year | 32 | 100.0 | 232 | 100.0 | 58 | 98.3 |
| Patients with ≥ 2 Years*** | 32 | 100.0 | 145 | 62.5 | 52 | 88.1 |
Since the SF cohorthad ablations completed during the earliest portion of the study period, all 32 (100%) of this cohort had at least 2 years of follow-up time between their index procedure and the data extraction in December 2016. The ST cohort had the most recent ablations on average, but had a later data extraction date in December 2017, such that 100% of the 232 cases had1 year or more of follow-up time and 62.5% had2 or more years. The CA cohort included 58 (98.3%) patients with at least 1 year and 52 (88.1%) with at least 2 years of follow-up.
Safety
Acute procedure-related serious complications were infrequent, and occurred at similar rates between the three cohorts (SF: 2/32 [6%]; ST: 9/232 [4%]; CA: 1/59 [2%]; p=0.5311). The SF events were a case of pericardial effusion requiring treatment and a stroke with complete resolution after 3-4 days. The ST events included six cases of effusion or tamponade requiring treatment or extending the patient’s hospital stay; one vascular access event requiring a blood transfusion; and two cases of transient symptoms potentially indicating a cerebrovascular event, neither of which showed MRI findings nor required treatment. The CA event was a case of pericardial effusion requiring treatment.
Procedural efficiency
Fluoroscopy times and radiation doses were significantly lower in the ST cohort (ANOVA p-values<0.0001) [Table 2]. Mean procedure times were similar for the SF cohort (92 minutes) and CA cohort (94 minutes), whileRF ablations using the newer ST technology resulted in a mean time savings of 10 minutes over the prior SF technology (82 minutes, ANOVA p-value=0.0201).
Table 2. – Procedural Detail.
Statistical test: ANOVA; significance denoted by* for p<0.05, ** for p<0.01, *** for p<0.001
| SF (N=32) | ST (N=232) | CA (N=59) | ||||
|---|---|---|---|---|---|---|
| Efficiency Measure | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD |
| Total Procedure Time*(minutes) | 31 | 92.2 ± 25.0 | 222 | 81.9 ± 31.7 | 50 | 93.6 ± 29.5 |
| Total Fluoroscopy Time***(minutes) | 28 | 1.5 ± 1.7 | 229 | 0.2 ± 0.4 | 41 | 12.4 ± 6.9 |
| Radiation Dose*** (mGy) | 30 | 247.7 ± 423.6 | 225 | 9.5 ± 26.0 | 53 | 676.4 ± 949.1 |
| Ablation Time (minutes) | 30 | 35.8 ± 11.1 | 227 | 31.5 ± 11.8 | 24 | 25.0 ± 11.9 |
| Ablation Procedure | n | Percent | n | Percent | n | Percent |
| Additional Non-PVI Triggers*** | 7 | 21.9 | 55 | 23.7 | 0 | 0.0 |
| DC Cardioversion Required | 2 | 6.3 | 10 | 4.3 | 7 | 11.9 |
Recurrence
Recurrence of any atrial arrhythmia was captured at 12-month follow-up visits starting approximately at the time of the change from SF to ST technology for RF ablations, and thus was not available for the SF cohort and was only available for CA procedures that occurred after systematic data collection was instituted. Since sample size with 12-month follow-up data was further reduced due to some patients not returning for a 12-month visit, only the ST population had sufficient sample size for reporting this outcome.
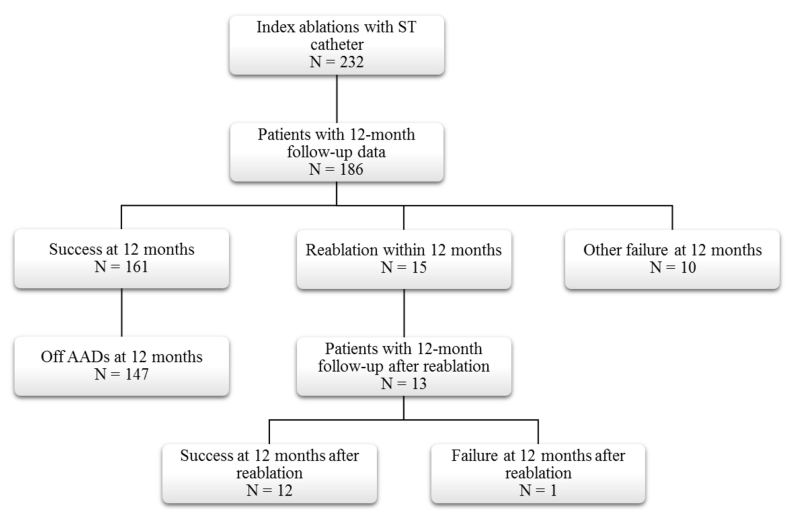
As shown in [Figure 2], 186 (80.2%) of the 232 ST patientswith an index ablation during the study period had 12-month follow-up visit data. Of the patients with follow-up, 161 (86.6%) met the 12-month success endpoint and 147 (91.3%) of these were also off AADs. Fifteen (8.1%) of the ST patients with 12 months of follow-up hadreablations within the first year, and another 10 (5.4%) were considered to be failures per the 12-month effectiveness endpoint. Thirteen of the 15 patients with reablations had 12-month follow-up visits after the second ablation, at which time 12 met the success criteria for the effectiveness endpoint.
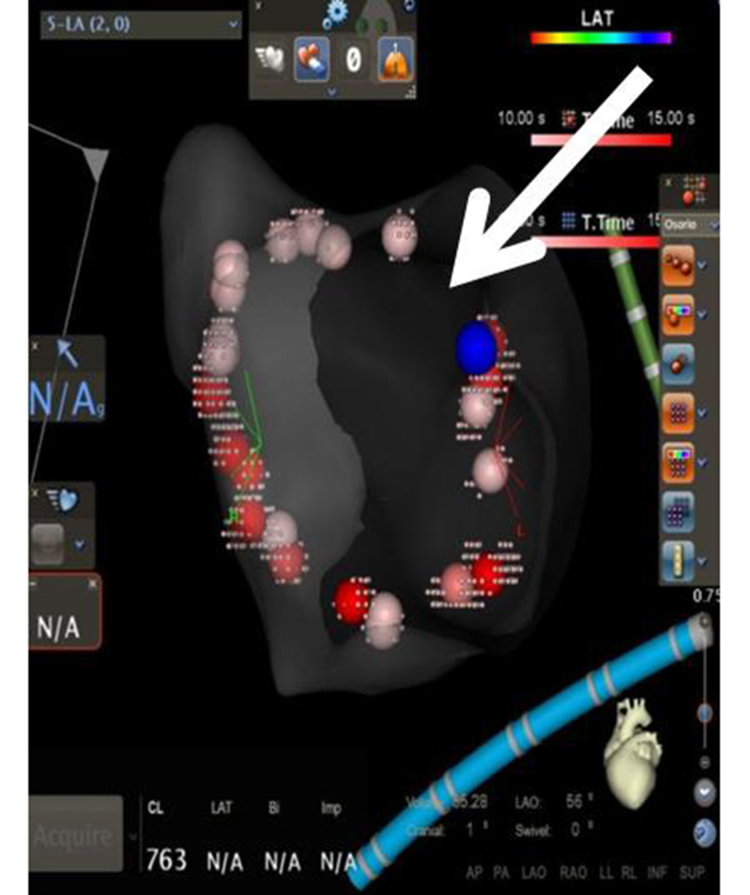
Figure 1b. Procedural Workflow for Pulmonary Vein Isolation with Contact Force Sensing in AF Ablation(b) FOT 50% at 10g .
The same lesion set after FOT was used to remove lesion tags where a force >10 grams was not maintained for at least 50% of the time; the arrow shows a gap denoting return of conduction
Figure 2. ST Cohort Disposition.
AAD: Antiarrhythmic drug ST: ThermocoolSmarttouchCatheter
Repeat ablation procedures
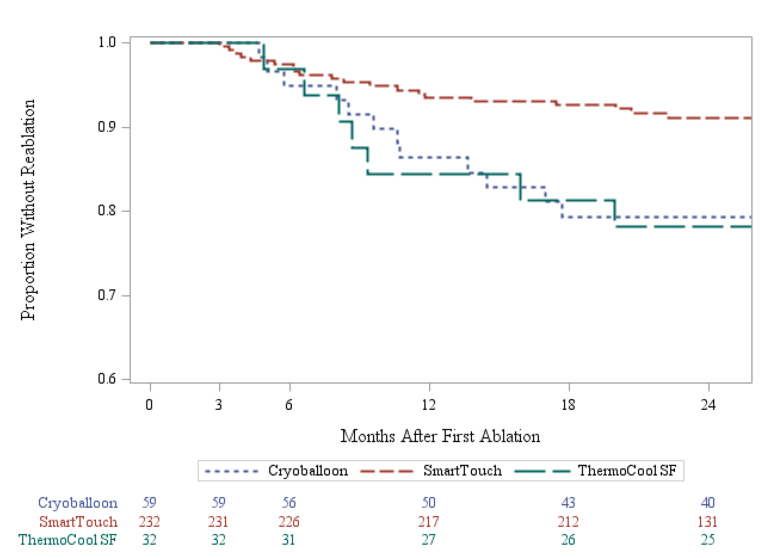
Kaplan-Meier survival modeling showed that ST ablation technology was associated with a significant reduction in repeat procedure rates (log-rank p-value=0.0194). Product limit survival estimates of repeat procedure rates at one year were 15.6% for SF,13.7% for CA, and 6.5% for ST. This reduction persisted at 2 years with cumulative reablation rate estimates of 21.9% for SF, 20.7% for CA, and 9.0% for ST [Figure 3].
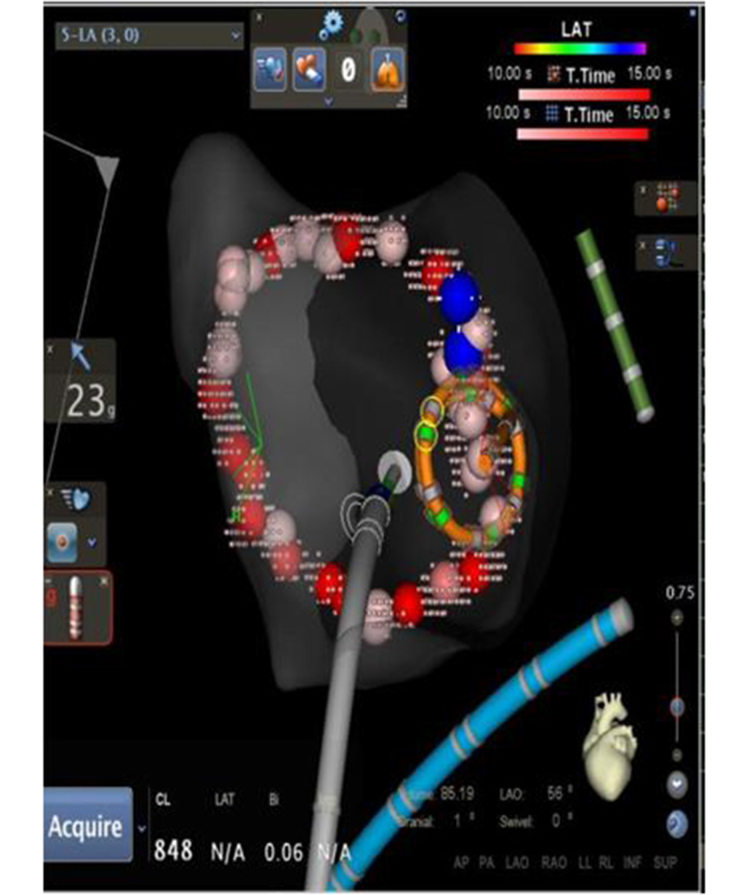
Figure 1c. Procedural Workflow for Pulmonary Vein Isolation with Contact Force Sensing in AF Ablation (c) After Touch-up, FOT 80% at 10g.
WACA lesion set after touch-up using the FOT filter shows that stable and adequate force was achieved in the entire lesion set
Figure 3. Freedom from Reablation across Technology Based Cohorts.
CA: Cryoablation SF: Thermocool SF Catheter ST: ThermocoolSmarttouchCatheter
Cox regression models were used to test for significance of additional baseline patient characteristics on risk of a repeat ablation procedure. None of the available demographics or co-morbidity indicators were significant after adjusting for the catheter cohort. In addition, separate models for each cohort showed no significant effect on need for reablation within any of the technologies.
Discussion
Main finding
Our main finding is that CF-sensing catheters, combined with Visitagtechnology andtheproposed standardized ablation protocol could decrease both total procedure and fluoroscopy times in PAF ablation procedures, compared to anon-CF-sensing catheter or cryoballoon. Our study also showed that integrating a CF-sensing catheter (ST) and an automated ablation annotation algorithm with a rigorous workflow led to good clinical outcomes, including a significant reduction in the need for reablation compared to procedures using the SF catheter or second-generation CA.
New technologies for radiofrequency AF ablation
Over the past decade, several technologies have advanced the field of AF ablation. Improvements in EAM systems, such as continuous multi-electrode mapping, have allowed us to obtain more information in a shorter time[7].CF-sensing catheters have also shown great promise in clinical trials, and now allow us to better estimate the quality of lesions delivered, ultimately achieving more durable lesions that can substantially improve procedural outcomes[8].The amount of information available to physicians through these technological advances in AF ablation has increased significantly; nevertheless, there exists no clear consensus on how to use, apply, and combine the new technologies for optimizing procedural outcomes.
A recent study by Anter et al. reported on effectiveness of automated annotation of RF ablation lesions usingVisitag software with different predefined filters and irrigated, non CF-sensing catheter technology[9].These researchers reported a higher PVI success rate after completion of the initial anatomical linewith Visitagand pre-defined catheter stability versus operator-guided ablation (90.5% vs. 66.7%, p=0.0001). This translated to a lower recurrence rate in the Visitagcohort (9.5%) compared to the cohort with empirical lesion tag annotation (23.8%).
Our workflow with CF-sensing technologies (ST cohort) resulted in lower RF time to achieve PVI compared to the Anter study (32 vs. 50 minutes). This reduction is likely due to: a) the addition of CF-sensing minimizing energy delivery when contact is sub-optimal; b) our minimalVisitag filters preventing unnecessarily long ablation times; and c) higher RF power used in our study. The ablation settings of 40 watts for 15 seconds that were used during our study correspond to a common trend toward higher power with shorter duration of energy application that has been proven safe and effective in invivo models.[10,11]
Several studies have also evaluated the synergistic role of Visitag with CF technology. The first study used different stability filters and when CF was not added to Visitag,only 74% of PVs were isolated with the initial PV encirclement, despite open access to real-time CF information[12].When a FOT criterion of 10 g was introduced, and further ablation was performed at the sites of automated annotation visual gaps, the PVI rate increased to 92%. This report did not includelong-term clinical results, but anotherstudy using similar strategiesresulted in clinical success rates of 81% at 12.9 months[13].
A more recent study found that use ofaVisitag software algorithm synergistically with CF ablation catheters resulted ina higher PVI rate during the first encircling lesion set compared to no Visitag use (66 vs. 37%, p=0.0006), with a corresponding improvement in freedom from AF at one year (91.8% vs. 76.2%)[14].Our study is the first to compare three widely used technologies, reportingimprovedprocedural efficiency with ST technology combined with a proposed lesion tagging workflow.
Although these technological advances are all intended to increase safety and durability of results, a standardized ablation method has not been validated and adopted widely within the electrophysiology community. The development of Ablation Index (AI) is a recentattempt to provide the missing piece of the equation, a single value thatobjectivelysummarizesthe total energy used. Preliminary studies using AI are promising, revealing improvements in clinical outcomes such as PV reconnection rates and tachyarrhythmia recurrence rates[15]. Our study was conducted prior to publication of the AI literature, but our goal of standardizingRF ablation strategies in order to reduce RF ablation time and the need for repeat ablation procedures was the same.
Cryoablationvs.force-sensing RF technology
Three studies have addressed procedural and clinical benefitsof CF-guided RF ablation versusCA.[16-18]All three studies failed to show a difference in atrial arrhythmia recurrences between the strategies, and they reported conflicting procedural outcomes. Two of the studies reported longer procedural time in the RF group compared to the CA,[16-17]with one suggesting that the reason was time spent generating a reliable 3D reconstruction of the LA geometry[16].Fluoroscopy time in this study, though longer than in our study, was not significantly different between the RF and CA cohorts[16]. Only the study by Kardos used Visitag software in a standardized way, in a non-specified number of patientsspanning approximately 80% of the study duration[17].Although total RF time was not reported, the mean procedural time of 120 minutes was substantially longer than reported in our study[17].Jourda et al. reported shorter procedure time and radiation exposure with CF-guided RF ablation, though values were considerably higher than we observed[18].This could be explained by the lack of standardized lesion tagging, thus supporting our hypothesis that AF ablation procedures could be more efficient with a standardized workflow and our proposed Visitag tagging strategy.
Our study is important as the first to comparethreewidely used AF ablation strategies while standardizing the use of the new CF technologiesin order to achieve reproducible results with minimal variability across physicians and mapping technologist. One clinically relevant benefit to both operators and patients is the minimization of fluoroscopy, particularly with CF-sensing technology where both Visitag and Univu are consistently used, averaging only a few seconds of exposure and mostly lead-free procedures for the operators.
Overall, our automated annotation strategy achieved the desired results of safety, efficiency and effectiveness. Procedural efficiency was achieved throughreducing RF ablation time due to a more lenient initial tag filter criteria. Effectiveness was improved through increasing our ability to identify areas with insufficient CF after the first lesion set, in which further ablation was required, thusresulting in a higher success rate and a lower repeat procedure rate compared to the other two strategies.
Limitations
The primary limitations of this study are consequences of the non-randomized nature of the retrospective unmatched cohorts. In particular, the timing of SF procedures is earlier than the ST procedures. With rapid advancements in the field, the later procedures incorporate some learnings beyond those attributable specifically to CF technology. Alternatively, the ST procedures include a learning curve for this site on new technology since they represent the first ablations using the new workflow. CA procedures span both SF and ST procedures in time with no significant workflow changes, so theyare unlikely to contribute significantly to any time effects. Arrhythmia recurrence, which was collected only after the switch from SF to ST, could not be compared across technologies.
Another limitation is the potential for selection bias between RF ablation and CA. However, it is reasonable to assume that any selection bias would persist over the entire study timeframe, thus would not explain differences seen between the SF and ST cohorts. Additional limitations include potential confounding by unmeasured variables and the possibility of insufficient statistical power to detect significant differences for factors with low prevalence.
Conclusion
Adoption of the proposed workflow, using CF catheter technology and Visitagautomated annotation software,may result in improved procedural outcomes, reductions in procedure time, fluoroscopy exposure, and reablation ratescompared to non-CFRF ablation and second-generation CA, without compromising procedural safety.
References
- 1.Latchamsetty Rakesh, Morady Fred. Catheter ablation of atrial fibrillation. Cardiol Clin. 2014 Nov;32 (4):551–61. doi: 10.1016/j.ccl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Beukema Rypko P, Beukema Willem P, Smit Jaap Jan J, Ramdat Misier Anand R, Delnoij Peter Paul H M, Wellens Hein, Elvan Arif. Efficacy of multi-electrode duty-cycled radiofrequency ablation for pulmonary vein disconnection in patients with paroxysmal and persistent atrial fibrillation. Europace. 2010 Apr;12 (4):502–7. doi: 10.1093/europace/euq023. [DOI] [PubMed] [Google Scholar]
- 3.Packer Douglas L, Kowal Robert C, Wheelan Kevin R, Irwin James M, Champagne Jean, Guerra Peter G, Dubuc Marc, Reddy Vivek, Nelson Linda, Holcomb Richard G, Lehmann John W, Ruskin Jeremy N. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013 Apr 23;61 (16):1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 4.Wilber David J, Pappone Carlo, Neuzil Petr, De Paola Angelo, Marchlinski Frank, Natale Andrea, Macle Laurent, Daoud Emile G, Calkins Hugh, Hall Burr, Reddy Vivek, Augello Giuseppe, Reynolds Matthew R, Vinekar Chandan, Liu Christine Y, Berry Scott M, Berry Donald A. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010 Jan 27;303 (4):333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 5.Natale Andrea, Reddy Vivek Y, Monir George, Wilber David J, Lindsay Bruce D, McElderry H Thomas, Kantipudi Charan, Mansour Moussa C, Melby Daniel P, Packer Douglas L, Nakagawa Hiroshi, Zhang Baohui, Stagg Robert B, Boo Lee Ming, Marchlinski Francis E. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J. Am. Coll. Cardiol. 2014 Aug 19;64 (7):647–56. doi: 10.1016/j.jacc.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 6.Reddy Vivek Y, Morales Gustavo, Ahmed Humera, Neuzil Petr, Dukkipati Srinivas, Kim Steve, Clemens Janet, D'Avila Andre. Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm. 2010 Nov;7 (11):1644–53. doi: 10.1016/j.hrthm.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Liang Jackson J, Elafros Melissa A, Muser Daniele, Pathak Rajeev K, Santangeli Pasquale, Supple Gregory E, Schaller Robert D, Frankel David S, Dixit Sanjay. Comparison of Left Atrial Bipolar Voltage and Scar Using Multielectrode Fast Automated Mapping versus Point-by-Point Contact Electroanatomic Mapping in Patients With Atrial Fibrillation Undergoing Repeat Ablation. J. Cardiovasc. Electrophysiol. 2017 Mar;28 (3):280–288. doi: 10.1111/jce.13151. [DOI] [PubMed] [Google Scholar]
- 8.Kimura Masaomi, Sasaki Shingo, Owada Shingen, Horiuchi Daisuke, Sasaki Kenichi, Itoh Taihei, Ishida Yuji, Kinjo Takahiko, Tomita Hirofumi, Okumura Ken. Comparison of lesion formation between contact force-guided and non-guided circumferential pulmonary vein isolation: a prospective, randomized study. Heart Rhythm. 2014 Jun;11 (6):984–91. doi: 10.1016/j.hrthm.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Anter Elad, Tschabrunn Cory M, Contreras-Valdes Fernando M, Buxton Alfred E, Josephson Mark E. Radiofrequency ablation annotation algorithm reduces the incidence of linear gaps and reconnection after pulmonary vein isolation. Heart Rhythm. 2014 May;11 (5):783–90. doi: 10.1016/j.hrthm.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Bhaskaran Abhishek, Chik William, Pouliopoulos Jim, Nalliah Chrishan, Qian Pierre, Barry Tony, Nadri Fazlur, Samanta Rahul, Tran Ying, Thomas Stuart, Kovoor Pramesh, Thiagalingam Aravinda. Five seconds of 50-60 W radio frequency atrial ablations were transmural and safe: an in vitro mechanistic assessment and force-controlled in vivo validation. Europace. 2017 May 01;19 (5):874–880. doi: 10.1093/europace/euw077. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon Gurpreet, Ahsan Syed, Honarbakhsh Shohreh, Lim Wei, Baca Marco, Graham Adam, Srinivasan Neil, Sawhney Vinit, Sporton Simon, Schilling Richard J, Chow Anthony, Ginks Matthew, Sohal Manav, Gallagher Mark M, Hunter Ross J. A multicentered evaluation of ablation at higher power guided by ablation index: Establishing ablation targets for pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2018 Dec 16; () doi: 10.1111/jce.13813. [DOI] [PubMed] [Google Scholar]
- 12.Asbach Stefan, Lang Corinna, Trolese Luca, Bode Christoph, Schluermann Fabienne. Automated lesion annotation during pulmonary vein isolation: influence on acute isolation rates and lesion characteristics. J Interv Card Electrophysiol. 2016 Dec;47 (3):349–356. doi: 10.1007/s10840-016-0173-y. [DOI] [PubMed] [Google Scholar]
- 13.Okumura Yasuo, Watanabe Ichiro, Iso Kazuki, Nagashima Koichi, Sonoda Kazumasa, Sasaki Naoko, Kogawa Rikitake, Takahashi Keiko, Ohkubo Kimie, Nakai Toshiko, Nakahara Shiro, Hori Yuuichi, Hirayama Atsushi. Clinical utility of automated ablation lesion tagging based on catheter stability information (VisiTag Module of the CARTO 3 System) with contact force-time integral during pulmonary vein isolation for atrial fibrillation. J Interv Card Electrophysiol. 2016 Nov;47 (2):245–252. doi: 10.1007/s10840-016-0156-z. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Nobuaki, Inoue Koichi, Tanaka Koji, Toyoshima Yuko, Oka Takafumi, Okada Masato, Inoue Hiroyuki, Nakamaru Ryo, Koyama Yasushi, Okamura Atsunori, Iwakura Katsuomi, Sakata Yasushi, Fujii Kenshi. Automated Ablation Annotation Algorithm Reduces Re-conduction of Isolated Pulmonary Vein and Improves Outcome After Catheter Ablation for Atrial Fibrillation. Circ. J. 2017 Oct 25;81 (11):1596–1602. doi: 10.1253/circj.CJ-17-0195. [DOI] [PubMed] [Google Scholar]
- 15.Hussein Ahmed, Das Moloy, Chaturvedi Vivek, Asfour Issa Khalil, Daryanani Niji, Morgan Maureen, Ronayne Christina, Shaw Matthew, Snowdon Richard, Gupta Dhiraj. Prospective use of Ablation Index targets improves clinical outcomes following ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2017 Sep;28 (9):1037–1047. doi: 10.1111/jce.13281. [DOI] [PubMed] [Google Scholar]
- 16.Squara Fabien, Zhao Alexandre, Marijon Eloi, Latcu Decebal Gabriel, Providencia Rui, Di Giovanni Giacomo, Jauvert Gaël, Jourda Francois, Chierchia Gian-Battista, De Asmundis Carlo, Ciconte Giuseppe, Alonso Christine, Grimard Caroline, Boveda Serge, Cauchemez Bruno, Saoudi Nadir, Brugada Pedro, Albenque Jean-Paul, Thomas Olivier. Comparison between radiofrequency with contact force-sensing and second-generation cryoballoon for paroxysmal atrial fibrillation catheter ablation: a multicentre European evaluation. Europace. 2015 May;17 (5):718–24. doi: 10.1093/europace/euv060. [DOI] [PubMed] [Google Scholar]
- 17.Kardos Attila, Kis Zsuzsanna, Som Zoltan, Nagy Zsofia, Foldesi Csaba. Two-Year Follow-Up after Contact Force Sensing Radiofrequency Catheter and Second-Generation Cryoballoon Ablation for Paroxysmal Atrial Fibrillation: A Comparative Single Centre Study. Biomed Res Int. 2016;2016 () doi: 10.1155/2016/6495753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jourda François, Providencia Rui, Marijon Eloi, Bouzeman Abdeslam, Hireche Hassiba, Khoueiry Ziad, Cardin Christelle, Combes Nicolas, Combes Stéphane, Boveda Serge, Albenque Jean-Paul. Contact-force guided radiofrequency vs. second-generation balloon cryotherapy for pulmonary vein isolation in patients with paroxysmal atrial fibrillation-a prospective evaluation. Europace. 2015 Feb;17 (2):225–31. doi: 10.1093/europace/euu215. [DOI] [PubMed] [Google Scholar]