Short abstract
Introduction
We aimed to compare the characteristics and vascular outcomes between Asian and non-Asian patients with non-cardioembolic stroke/transient ischaemic attack receiving antiplatelet monotherapy and to identify population-specific predictors for recurrent events.
Patients and methods
We conducted a post-hoc analysis of data from the PERFORM study, in which 19,100 patients (mean age, 67.2 years; male, 63%; 2178 Asian and 16,922 non-Asian patients) with non-cardioembolic ischaemic stroke/transient ischaemic attack were randomised to aspirin or terutroban and followed for two years. The primary outcome was a composite of major adverse cardiovascular events (non-fatal myocardial infarction, non-fatal stroke and cardiovascular death).
Results
There was no difference in major adverse cardiovascular events risk between Asian and non-Asian populations (11.1% vs. 10.5%; p = 0.39). However, Asian patients were at significantly higher risk of intracranial haemorrhage (2.4% vs. 1.3%; hazard ratio (HR) 1.87; 95% confidence interval (CI) 1.34–2.60; p < 0.001) and major bleeding (5.4% vs. 4.1%; HR 1.30; 95% CI 1.04–1.61; p = 0.02). Stroke risk was significantly higher in Asian than in non-Asian populations among patients with lacunar stroke (7.4% vs. 4.5%; p = 0.02). In multivariable analysis, diastolic blood pressure (HR per 5 mm Hg 1.08; 95% CI 1.01–1.16; p = 0.03) and diabetes (HR 1.36; 95% CI 1.22–1.52; p < 0.001) were independent predictors of major adverse cardiovascular events for Asian and non-Asian patients, respectively.
Conclusion: Compared with non-Asian patients, Asian patients had significantly higher risk of haemorrhagic events when given antiplatelet monotherapy for secondary prevention after non-cardioembolic stroke/transient ischaemic attack. Lacunar stroke and elevated diastolic blood pressure were more associated with recurrence risk in Asian patients.
Keywords: Antiplatelet, bleeding, cerebrovascular disease, intracranial haemorrhage, race and ethnicity
Introduction
Asian people are at disproportionately higher risk of stroke and related mortality than non-Asian people.1 Collectively, Asian countries account for almost two-thirds of the world’s total mortality due to stroke.2 Because of recent population growth and aging, the burden of stroke in Asia is still increasing, giving rise to an urgent need for better prevention and management of stroke in this region.
Non-cardioembolic stroke, mostly caused by large arterial atherosclerosis or small vessel disease, accounts for >70% of ischaemic strokes. Compared with non-Asian people, Asian people are more likely to develop lacunar stroke due to small vessel disease.3 Furthermore, recent westernisation and urbanisation have increased the incidence of atherothrombotic stroke in many Asian societies.4,5 These facts underscore the growing burden of non-cardioembolic stroke in Asia. However, racial-ethnic or regional disparities in the nature and prognosis of non-cardioembolic stroke, possibly related to genetic, behavioural and socioeconomic contributions, have been poorly understood. Further, Asian people are generally at higher risk of intracranial haemorrhage than non-Asian people,6 although no studies have directly compared haemorrhagic risk with antiplatelet medication in secondary prevention populations.
The Prevention of cerebrovascular and cardiovascular Events of ischaemic origin with teRutroban in patients with a history oF ischaemic strOke or tRansient ischaeMic attack (PERFORM) study is the second largest randomised trial so far to test antiplatelet drugs for secondary prevention after non-cardioembolic stroke/transient ischaemic attack (TIA).7 We aimed to elucidate the population-level disparities in characteristics, prognosis and risk factors for recurrent events between Asian and non-Asian patients with non-cardioembolic stroke/TIA using data from the PERFORM study.
Patients and methods
Study population
The PERFORM study was a multicentre randomised controlled trial, designed to compare terutroban versus aspirin in the prevention of cerebro-cardiovascular events in patients with either a non-cardioembolic ischaemic stroke (≤3 months) or a TIA (≤8 days). The design, baseline characteristics and main findings have been reported elsewhere.7 Briefly, 19,100 participants from 802 sites in 46 countries (Supplementary Table I) were enrolled between February 2006 and April 2008. The minimum follow-up duration was two years. The major efficacy and safety outcomes were not found to be statistically different between the drugs studied. In the present analysis, patients were classified into two groups according to their countries of origin (2178 Asian; 16,922 non-Asian).
The study protocol was approved by independent ethics committees in all countries. All participants provided written informed consent before enrolment. PERFORM was registered, number ISRCTN66157730.
Outcomes
The primary outcome was the occurrence of major adverse cardiovascular events (MACE) – a composite of non-fatal myocardial infarction, non-fatal stroke or cardiovascular death. Secondary outcomes included all-cause death, myocardial infarction, stroke, intracranial haemorrhage and bleeding. Tertiary outcome was new occurrence of atrial fibrillation.
Statistical analysis
Quantitative variables are expressed as mean±standard deviation in case of normal distribution or median (interquartile range) for other distributions. Qualitative variables are expressed as counts (percentages). The normality of distributions was assessed graphically using the Shapiro-Wilk test.
Cumulative event curves were constructed using the Kaplan-Meier method. We compared the two-year event rates between the groups using Cox proportional hazard models adjusted for age and sex. Adjusted event rates were calculated using the corrected group prognosis method.8 Patients from non-Asian countries were used as the reference group in the Cox proportional hazard models to determine hazard ratios (HRs) as effect size measures, with their 95% confidence intervals (CIs). We assessed between-group heterogeneity according to the antiplatelet treatment assignment (aspirin or terutroban) by including the interaction term between the regional group and treatment allocation. Since no interaction across treatment groups was found, Cox proportional hazard models were further adjusted by the antiplatelet treatment allocation and three predefined confounding factors (modified Rankin Scale at admission, hypertension and statin use at randomisation), given their known importance on vascular outcomes. The proportional hazard assumptions were checked using the log–log survival plots and by introducing a time-dependent variable into models. Sensitivity analyses were conducted firstly by comparing Asian and non-Asian patients based on self-identified ethnic origins, secondly by restricting the study sample to patients allocated to aspirin and thirdly by restricting the study sample to patients with new-onset atrial fibrillation occurring during the study. In further analysis, we calculated the age-sex-adjusted stroke risk according to the PERFORM stroke classification in patients from Asian and non-Asian countries; heterogeneity according to the stroke subtype was tested by including interaction term into a Cox proportional hazard models.
We identified predictors of MACE for Asian and non-Asian populations separately, using a Cox proportional hazard regression model adjusted for age and sex. We used all the baseline variables expect for glucose and lipid values due to high rates of missing data. Heterogeneities in the associations of risk factors with MACE risk between Asian and non-Asian patients were tested by formal interaction tests. Since some significant heterogeneities were found, further multivariable analyses were performed in Asian and non-Asian patients separately. According to region, risk factors that were associated with MACE with a p-value <0.10 were included in a backward-selection Cox regression analysis using removal criteria of 0.10. Age and sex were forced into the model.
Statistical testing was done at the two-tailed α level of 0.05. Data were analysed using SAS software package, release 9.3 (SAS Institute, Cary, NC).
Results
Baseline characteristics
Of the 19,100 patients (mean age, 67.2 years; male, 63%), 2178 (11.4%) were from Asian countries. As shown in Table 1, 99.9% and 0.4% of patients from Asian and non-Asian countries identified themselves as Asian ethnicity, respectively. Patients from Asian countries were younger and more likely to have diabetes, but less likely to have hypertension, coronary artery disease, peripheral artery disease, atrial fibrillation or flutter and congestive heart failure. They were less well educated and had lower body mass index, higher blood pressure (BP), lower total, low- and high-density lipoprotein cholesterol, a higher prevalence of atherogenic dyslipidaemia and higher modified Rankin scale scores. Lacunar strokes were more prevalent in Asian populations. Supplementary Table II shows medication use before and at randomisation.
Table 1.
Baseline characteristics of patients from non-Asian and Asian countries.
| Non-Asian (n = 16,922) | Asian (n = 2178) | pa | |
|---|---|---|---|
| Age, y, mean±SD | 67 ± 8 | 66 ± 7 | <0.001 |
| Male sex, n (%) | 10,574 (62.5) | 1376 (63.2) | 0.46 |
| Ethnic origin, n (%) | NA | ||
| White | 16,025 (94.7) | 1 (0.05) | |
| Asian | 68 (0.4) | 2176 (99.9) | |
| Black | 319 (1.9) | 0 (0.0) | |
| Other | 510 (2.0) | 1 (0.05) | |
| Medical history, n (%) | |||
| Hypertension | 14,221 (84.0) | 1743 (80.0) | <0.001 |
| Diabetes | 4502 (26.6) | 797 (36.6) | <0.001 |
| Former smoking | 4361 (25.8) | 350 (16.1) | <0.001 |
| Current smoking | 4431 (26.2) | 643 (29.5) | 0.31 |
| Stroke, TIA | 3603 (21.3) | 444 (20.4) | 0.51 |
| Coronary artery disease | 3871 (22.9) | 248 (11.4) | <0.001 |
| Peripheral artery disease | 733 (4.3) | 6 (0.3) | <0.001 |
| Atrial fibrillation or flutter | 557 (3.3) | 37 (1.7) | 0.001 |
| Congestive heart failure | 857 (5.1) | 15 (0.7) | <0.001 |
| Educational level, n (%) | <0.001 | ||
| 0–4 Years | 2161 (12.8) | 680 (31.2) | |
| 5–8 Years | 5139 (30.4) | 590 (27.1) | |
| 9–13 or High school diploma | 6450 (38.1) | 587 (27.0) | |
| College experience or higher degree | 2890 (17.1) | 294 (13.5) | |
| Examinations | |||
| Body mass index, kg/m2, mean ± SD | 27.4 ± 4.3 | 24.6 ± 3.5 | <0.001 |
| Systolic BP, mm Hg, mean ± SD | 138 ± 17 | 141 ± 18 | <0.001 |
| Diastolic BP, mm Hg, mean ± SD | 80 ± 9 | 81 ± 9 | <0.001 |
| Glucose, mg/dL,b median (IQR) | 103 (94–121) | 103 (93–126) | 0.33 |
| Total cholesterol, mg/dL, mean ± SD | 181 ± 45 | 169 ± 42 | <0.001 |
| LDL cholesterol, mg/dL, mean ± SD | 109 ± 38 | 102 ± 35 | <0.001 |
| HDL cholesterol, mg/dL, mean ± SD | 49 ± 14 | 45 ± 12 | <0.001 |
| Triglycerides, mg/dL,b median (IQR) | 128 (96–174) | 127 (86–173) | 0.11 |
| Atherogenic dyslipidaemia, n (%) | 2233 (13.2) | 350 (16.1) | 0.004 |
| Metabolic syndrome, n (%) | 5086 (33.7) | 700 (34.3) | 0.78 |
| Modified Rankin Scale, n (%) | <0.001 | ||
| 0 (no symptoms) | 3992 (23.6) | 245 (11.2) | |
| 1 (no significant disability) | 6406 (37.9) | 910 (41.8) | |
| 2 (slight disability) | 3838 (22.7) | 457 (21.0) | |
| 3 (moderate disability) | 1735 (10.3) | 311 (14.3) | |
| 4 (moderately severe disability) | 944 (5.6) | 254 (11.7) | |
| Ischaemic stroke subtype, n (%) | <0.001 | ||
| Atherothrombotic | 1485 (9.9) | 328 (15.7) | |
| Likely atherothrombotic | 6408 (42.7) | 693 (33.2) | |
| Lacunar | 1417 (9.4) | 316 (15.2) | |
| Cardioembolic | 151 (1.0) | 18 (0.9) | |
| Coexisting | 2227 (14.8) | 331 (15.9) | |
| Unknown | 3322 (22.1) | 398 (19.1) |
BP: blood pressure; HDL: high-density lipoprotein; IQR: interquartile range; LDL: low-density lipoprotein; NA: not applicable; SD: standard deviation; TIA: transient ischaemic attack.
aAdjusted for age and sex.
bAnalysed using logarithmic values.
Follow-up characteristics
At 24 months, patients from Asian countries still had lower total, low-, and high-density lipoprotein cholesterol, whereas BP levels were similar (mean, 136 mm Hg) (Supplementary Table III).
Two-year outcomes
During the two-year follow-up, there were 230 non-fatal myocardial infarctions, 1462 non-fatal strokes and 344 cardiovascular deaths. Overall, 1964 patients experienced at least one MACE, giving an event rate of 10.6% (95% CI, 10.1–11.0%). The risk of MACE was not different between patients from Asian and non-Asian countries (Figure 1(a) and Table 2; 11.1% vs. 10.5%; p = 0.39), whereas the between-group difference in the risk of non-fatal myocardial infarction was nearly statistically significant in the direction of a higher risk for non-Asian patients (0.8% vs. 1.3%; p = 0.07). Asian populations had a significantly higher risk of intracranial haemorrhage (Figure 1(b); 2.4% vs. 1.3%; HR, 1.87; 95% CI, 1.34–2.60; p < 0.001) and major bleeding (5.4% vs. 4.1%; HR, 1.30; 95% CI, 1.04–1.61; p = 0.02). No significant heterogeneity was found between patients from Asian and non-Asian countries according to the antiplatelet treatment assignment (p = 0.26 and 0.37 for intracranial haemorrhage and major bleeding, respectively). In addition, when limiting the analysis to patients receiving aspirin, the same trend was found in terms of the risk of MACE and haemorrhagic events (Supplementary Table IV). The results were also consistent when comparing the event risk between patients of Asian and non-Asian (predominantly white) ethnic origins (Supplementary Table V).
Figure 1.
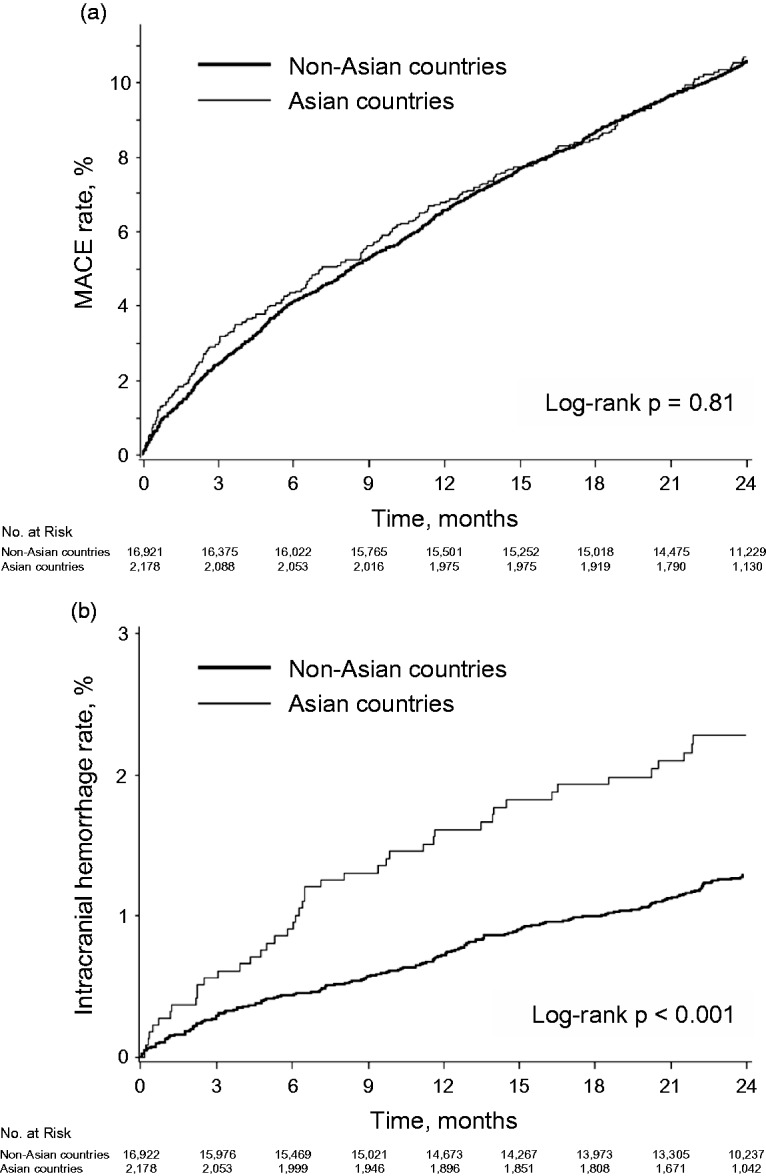
Unadjusted Kaplan-Meier curves for (a) MACE and (b) intracranial haemorrhage for patients from non-Asian and Asian countries.
MACE: major adverse cardiovascular events.
Table 2.
Two-year primary, secondary and tertiary outcomes in patients from non-Asian and Asian countries.
|
Events, n (%)a |
Adjusteda HR(95% CI) | pa | Fully adjustedb HR(95% CI) | pb | ||
|---|---|---|---|---|---|---|
| Non-Asian(n = 16,922) | Asian(n = 2178) | |||||
| Primary outcome | ||||||
| MACE | 1740 (10.5) | 224 (11.1) | 1.06 (0.92–1.22) | 0.39 | 1.02 (0.89–1.18) | 0.74 |
| Non-fatal myocardial infarction | 214 (1.3) | 16 (0.8) | 0.63 (0.38–1.05) | 0.07 | 0.63 (0.37–1.05) | 0.07 |
| Non-fatal stroke | 1289 (7.8) | 173 (8.5) | 1.09 (0.93–1.28) | 0.27 | 1.07 (0.91–1.26) | 0.38 |
| Cardiovascular death | 301 (1.8) | 43 (2.3) | 1.27 (0.92–1.75) | 0.14 | 1.11 (0.80–1.54) | 0.52 |
| Secondary outcomes | ||||||
| All-cause death | 820 (4.9) | 102 (5.3) | 1.08 (0.88–1.33) | 0.45 | 0.98 (0.79–1.21) | 0.87 |
| Myocardial infarction | 221 (1.4) | 17 (0.9) | 0.65 (0.39–1.06) | 0.08 | 0.64 (0.38–1.05) | 0.07 |
| Stroke | 1298 (7.9) | 176 (8.7) | 1.11 (0.94–1.29) | 0.21 | 1.09 (0.92–1.28) | 0.31 |
| Intracranial haemorrhage | 192 (1.3) | 45 (2.4) | 1.88 (1.36–2.61) | <0.001 | 1.87 (1.34–2.60) | <0.001 |
| Bleeding | 2238 (14.7) | 304 (16.0) | 1.10 (0.98–1.24) | 0.12 | 1.08 (0.95–1.23) | 0.20 |
| Minor bleeding | 1753 (11.6) | 224 (11.9) | 1.03 (0.90–1.19) | 0.66 | 1.02 (0.88–1.17) | 0.84 |
| Major bleeding | 606 (4.1) | 98 (5.4) | 1.33 (1.08–1.65) | 0.008 | 1.30 (1.04–1.61) | 0.02 |
| Tertiary outcomes | ||||||
| New-onset atrial fibrillation | 390 (2.4) | 33 (1.8) | 0.75 (0.53–1.07) | 0.12 | 0.84 (0.58–1.19) | 0.32 |
| MACEc | 69 (18.4) | 6 (18.3) | 0.92 (0.39–2.14) | 0.99 | 0.98 (0.42–2.30) | .96 |
CI: confidence interval; HR: hazard ratio; MACE: major adverse cardiovascular events.
aAdjusted for age and sex.
bAdjusted for age, sex, modified Rankin Scale, hypertension, statin use and antiplatelet treatment allocation (aspirin or terutroban).
cCalculated for patients with new onset atrial fibrillation.
The risk of new-onset atrial fibrillation was not different between patients from Asian and non-Asian countries. In the sensitivity analysis restricted to patients with new atrial fibrillation, there was no difference in the MACE risk between the populations.
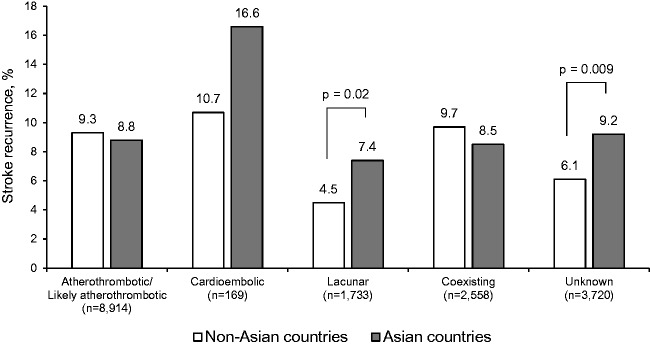
The associations between PERFORM stroke classification and stroke risk did not differ between patients from Asian and non-Asian countries (p for interaction = 0.20). However, stroke risk was significantly higher in those from Asian countries among patients with lacunar stroke and those with stroke of unknown cause subtypes (Figure 2).
Figure 2.
Two-year risk of stroke according to stroke subtype among patients from non-Asian and Asian countries. Event rates were adjusted for age and sex.
There were potential interactions between the two populations in terms of atherogenic dyslipidaemia, diabetes and diastolic BP (Supplementary Table VI; p for interaction = 0.05, 0.06, and 0.06, respectively). Diastolic BP was more associated with an increased MACE risk in Asian populations, whereas the opposite was true for atherogenic dyslipidaemia and diabetes.
In multivariable analysis (Table 3), a modified Rankin scale score of 4, previous stroke or TIA, age, male sex and diastolic BP (HR per 5 mm Hg, 1.08; 95% CI, 1.01–1.16) were independent predictors of MACE for patients from Asian countries. In patients from non-Asian countries, 11 parameters, including diabetes (HR, 1.36; 95% CI, 1.22–1.52), were independently associated with MACE (Table 4).
Table 3.
Multivariable analysis for two-year risk of major adverse cardiovascular events in patients from Asian countries.
| HR (95% CI) | p | |
|---|---|---|
| Modified Rankin Scale | ||
| 0 (no symptoms) | 1.00 (ref) | – |
| 1 (no significant disability) | 1.38 (0.81–2.37) | 0.24 |
| 2 (slight disability) | 1.74 (0.99–3.07) | 0.05 |
| 3 (moderate disability) | 1.82 (1.01–3.26) | 0.05 |
| 4 (moderately severe disability) | 2.70 (1.51–4.84) | <0.001 |
| Previous stroke, TIA | 1.58 (1.18–2.10) | 0.002 |
| Age, per 10-year increase | 1.29 (1.08–1.54) | 0.005 |
| Male sex | 1.42 (1.06–1.89) | 0.02 |
| Diastolic BP, per 5 mm Hg increase | 1.08 (1.01–1.16) | 0.03 |
| Previous PAD | 4.01 (0.98–16.38) | 0.05 |
BP: blood pressure; CI: confidence interval; HR: hazard ratio; PAD: peripheral artery disease; TIA: transient ischaemic attack.
Table 4.
Multivariable analysis for two-year risk of major adverse cardiovascular events in patients from non-Asian countries.
| HR (95% CI) | p | |
|---|---|---|
| Male sex | 1.27 (1.42–1.42) | <0.001 |
| Age, per 10-year increase | 1.36 (1.27–1.45) | <0.001 |
| Diabetes | 1.36 (1.22–1.52) | <0.001 |
| Current smoking | 1.17 (1.04–1.32) | <0.001 |
| Previous stroke or TIA | 1.35 (1.21–1.51) | <0.001 |
| Previous CAD | 1.54 (1.37–1.73) | <0.001 |
| Previous PAD | 1.41 (1.16–1.72) | <0.001 |
| Previous atrial fibrillation | 1.79 (1.46–2.19) | <0.001 |
| Modified Rankin Scale | ||
| 0 (no symptoms) | 1.00 (ref) | – |
| 1 (no significant disability) | 1.16 (0.99–1.34) | 0.05 |
| 2 (slight disability) | 1.57 (1.35–1.83) | <0.001 |
| 3 (moderate disability) | 1.34 (1.11–1.62) | 0.002 |
| 4 (moderately severe disability) | 1.58 (1.26–1.98) | <0.001 |
| Educational level | ||
| 0–4 Years | 1.00 (ref) | |
| 5–8 Years | 0.89 (0.76–1.05) | 0.16 |
| 9–13 Years or high school diploma | 0.91 (0.78–1.07) | 0.27 |
| College experience or higher degree | 0.74 (0.61–0.90) | 0.003 |
| Congestive heart failure | 1.31 (1.08–1.58) | 0.005 |
CAD: coronary arteries disease; CI: confidence interval; HR: hazard ratio; PAD: peripheral artery disease; TIA: transient ischaemic attack.
Discussion
The PERFORM study provided a unique opportunity to investigate Asian versus non-Asian disparities in a large number of patients receiving antiplatelet monotherapy after non-cardioembolic stroke/TIA. Despite no difference in the overall MACE risk between the populations, Asian patients were at significantly higher risk of intracranial haemorrhage and major bleeding. The risk of recurrent stroke was also significantly higher in Asian patients among those with lacunar stroke. Furthermore, multivariable analysis identified different predictors of MACE; of note, diastolic BP and diabetes were independently predictive in those from Asian and non-Asian countries, respectively. Our findings could help to guide population-specific approaches to the secondary prevention of non-cardioembolic stroke/TIA.
Although higher incidence and mortality of stroke in Asian versus non-Asian people have been well documented in population-based settings,1,3 limited data are available on the differences in vascular outcomes among secondary prevention populations. The patients in the PERFORM study appear to represent the current risk characteristics after stroke under standard antiplatelet therapy and risk factor managements. In fact, the event rate was remarkably similar to that in the Prevention Regimen for Effectively Avoiding Second Strokes study,9 which is the largest study to test antiplatelet therapy in patients with non-cardioembolic stroke. The PERFORM reported no differences between aspirin and terutroban in the risk of major endpoints, including MACE, stroke, intracranial haemorrhage and major bleeding, whereas only minor bleeding occurred more with terutroban (12% vs. 11%).7 We therefore tested the heterogeneity in outcome differences between the treatment assignments and found no significant interactions. In addition, the increased risk in haemorrhagic events for Asian patients was consistent after adjustments on the antiplatelet allocation, supporting that the bleeding tendency in terutroban did not influence our results.
Our study demonstrated that patients from Asian countries had a 1.9-fold higher risk of intracranial haemorrhage than those from non-Asian countries taking antiplatelet monotherapy after non-cardioembolic stroke/TIA. A similar increased risk has been reported in the general population – according to a previous meta-analysis, the incidence of intracerebral haemorrhage per 100,000 person-years is 51.8 in Asian, 24.2 in white, 22.9 in black and 19.6 in Hispanic people.6 Moreover, Asian patients have been reported to be at higher risk of intracranial haemorrhage during warfarin therapy for atrial fibrillation10 and after thrombolytic therapy for acute ischaemic stroke.11 All of these data are comparable with our findings.
We observed a higher haemorrhagic risk in Asian versus non-Asian patients despite the presence of similar BP values. Furthermore, a nearly significant regional interaction was observed for the effect of diastolic BP on MACE risk, and elevated diastolic BP was an independent predictor of MACE for Asian populations, but not for non-Asian populations. These results may be indicative of stronger relationships between BP and vascular risk in Asian people. Indeed, the linear relationship between BP level and stroke risk was reported to be steeper in Asians than in Caucasians.12 Moreover, in the Asia Pacific Cohort Studies Collaboration population-based study, the association between systolic BP and stroke risk was significantly stronger for the Asian versus Australia/New Zealand cohorts.13 We found it interesting that not systolic but diastolic BP was an independent determinant for recurrences in Asian patients, although this might be due to chance in the context of multiple comparisons. There is a generally stronger relation of systolic BP to vascular risk than is that of diastolic BP,14,15 whereas some studies reported that systolic and diastolic BPs have similar predictive values for certain endpoints.16,17 The prognostic impact of diastolic BP in Asians should be further assessed by future studies.
Genetic differences in predisposition to hypertension may in part explain the higher proportion of lacunar strokes in Asian patients, given that small vessel disease is more related to hypertension than other subtypes.18 In addition, we found that the risk of stroke recurrence was higher in patients from Asian countries among lacunar stroke patients. According to the Secondary Prevention of Small Subcortical Strokes trial,19 lacunar stroke patients benefit from intensive BP control. The study randomised patients with recent lacunar stroke/TIA to two systolic BP targets, higher (130–150 mm Hg) or lower (<130 mm Hg), and showed that the lower target was more beneficial, as all recurrent strokes were non-significantly reduced, and intracerebral haemorrhage was significantly reduced by about two-thirds. Hence, the mean BP of 136–138 mm Hg in our cohort was potentially inadequate for patients with lacunar stroke. In particular, Asian patients with lacunar stroke might need a lower BP target, although further studies are warranted to verify this hypothesis.
Limitations and strengths
The present study has several limitations, mainly related to the inherent nature of post-hoc subgroup analysis. Because the analysis was conducted in the population from a single randomised trial, our results should not be generalisable to the community-based stroke population. Nevertheless, this can also be a strength – in that a large, well-characterised and multinational population was analysed together based on a standardised methodology. In addition, we achieved a high follow-up rate (less than 1% of patients were lost to follow-up), with outcome events systematically assessed by experts.
Inadequate definition of racial-ethnic groups would be a key limitation of this study. We primarily assumed the residence in Asian countries as a surrogate for ‘Asian’ populations, despite a wide heterogeneity in genotypes, behaviours, culture, socioeconomic factors or health policies between the countries in which patients were recruited. While an analysis according to self-identified ethnic origin demonstrated consistent results, we used relatively broad categories (i.e. white, Asian, black and other) that might also mask within-group differences. Further, self-report of ethnicity is often inaccurate20 and could be complicated by having a substantial proportion of people of various multiracial-ethnic backgrounds (e.g. mixed races). Practical categorisations might allow us to easily recognise and differentiate disease patterns between population subgroups, but potentially limit the validity and generalisability.
We had no data on the types of atherosclerotic lesions in patients with large artery disease. Asian people are more susceptible to intracranial arterial atherosclerosis than other races,21 whereas Caucasians are more likely to have extracranial carotid artery disease.22 Such racial disparities potentially have some effect on MACE risk, but were not taken in the current analysis.
Finally, because of a number of comparisons within a study, we cannot exclude the possibilities of some false associations.
Conclusion
Asian patients were at significantly higher haemorrhagic risk than non-Asian patients while receiving antiplatelet monotherapy for secondary prevention after non-cardioembolic stroke/TIA. Lacunar type of stroke and high diastolic BP at baseline were important determinants of recurrence risk for Asians.
Supplemental Material
Supplemental material for Non-cardioembolic stroke/transient ischaemic attack in Asians and non-Asians: A post-hoc analysis of the PERFORM study by Takao Hoshino, Leila Sissani, Julien Labreuche, Marie-Germaine Bousser, Angel Chamorro, Marc Fisher, Ian Ford, Kim M Fox, Michael G Hennerici, Heinrich P Mattle, Peter M Rothwell, Philippe Gabriel Steg, Eric Vicaut and Pierre Amarenco in European Stroke Journal
Acknowledgements
The manuscript was edited for non-intellectual content by Jenny Lloyd (MedLink Healthcare Communications Limited).
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KMF collaborates with Servier as consultant to EMEA and advisory boards. EV reports an advisory relationship with Abbot, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Fresenius, LFB, Lilly, Medtronic, Pfizer and Sorin group (consultancy), Novartis (lectures), European Cardiovascular Research Center (DSMB), and Boehringer (grants for Hospital). The other authors report no conflicts to declare.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: TH reports research grants from AstraZeneca and SOS-ATTAQUE CEREBRALE Association. KMF reports honoraria from Servier (lectures). PGS reports research grants from Sanofi and Servier (steering committee chair) and honoraria from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, CSL-Behring, Daiichi-Sankyo, GSK, Janssen, Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi, Servier, The Medicines Company, Amarin (steering committee), and Pfizer (event adjudication committee). PA reports research grants from Pfizer (TST trial), Sanofi, and AstraZeneca (TIAregistry.org), research support from AstraZeneca (SOCRATES), GSK (SUMMIT adjudication committee), Fibrogen (Roxadustat DSMB), and Pfizer (SPIRE executive committee), and honoraria form Pfizer, Sanofi, and Bayer (speaking activities). The PERFORM study was funded by Servier, Suresnes, France. Jenny Lloyd (MedLink Healthcare Communications Limited), who edited the manuscript for non-intellectual content, was funded by SOS-ATTAQUE CEREBRALE Association.
Trial registration
The PERFORM study: ISRCTN66157730.
Informed consent
Written informed consent was obtained from all subjects before the study.
Ethical approval
Ethical approval for this study was obtained from independent ethics committees in all countries.
Guarantor
PA.
Contributorship
MGB, AC, MF, IF, KMF, MGH, HPM, PMR, PGS, EV, and PA were involved in protocol development, gaining ethical approval, patient recruitment of the PERFORM study. TH and PA designed this substudy. LS and JL analysed the data. TH wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation 2008; 118: 2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehndiratta MM, Khan M, Mehndiratta P, et al. Stroke in Asia: geographical variations and temporal trends. J Neurol Neurosurg Psychiatry 2014; 85: 1308–1312. [DOI] [PubMed] [Google Scholar]
- 3.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs. white populations: a systematic review. Neurology 2013; 81: 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo KS, Chook P, Raitakari OT, et al. Westernization of Chinese adults and increased subclinical atherosclerosis. Arterioscler Thromb Vasc Biol 1999; 19: 2487–2493. [DOI] [PubMed] [Google Scholar]
- 5.Kim YD, Choi HY, Cho HJ, et al. Increasing frequency and burden of cerebral artery atherosclerosis in Korean stroke patients. Yonsei Med J 2010; 51: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010; 9: 167–176. [DOI] [PubMed] [Google Scholar]
- 7.Bousser MG, Amarenco P, Chamorro A, et al. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet 2011; 377: 2013–2022. [DOI] [PubMed] [Google Scholar]
- 8.Ghali WA, Quan H, Brant R, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA 2001; 286: 1494–1497. [DOI] [PubMed] [Google Scholar]
- 9.Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008; 359: 1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen AY, Yao JF, Brar SS, et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007; 50: 309–315. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RH, Cox M, Smith EE, et al. Race/ethnic differences in the risk of hemorrhagic complications among patients with ischemic stroke receiving thrombolytic therapy. Stroke 2014; 45: 2263–2269. [DOI] [PubMed] [Google Scholar]
- 12.Perkovic V, Huxley R, Wu Y, et al. The burden of blood pressure-related disease: a neglected priority for global health. Hypertension 2007; 50: 991–997. [DOI] [PubMed] [Google Scholar]
- 13.Hyun KK, Huxley RR, Arima H, et al. A comparative analysis of risk factors and stroke risk for Asian and non-Asian men: the Asia Pacific Cohort Studies Collaboration. Int J Stroke 2013; 8: 606–611. [DOI] [PubMed] [Google Scholar]
- 14.Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation 1999; 100: 354–360. [DOI] [PubMed] [Google Scholar]
- 15.Miura K, Dyer AR, Greenland P, et al. Pulse pressure compared with other blood pressure indexes in the prediction of 25-year cardiovascular and all-cause mortality rates: the Chicago Heart Association Detection Project in Industry Study. Hypertension 2001; 38: 232–237. [DOI] [PubMed] [Google Scholar]
- 16.Lawes CM, Rodgers A, Bennett DA, et al. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens 2003; 21: 707–716. [DOI] [PubMed] [Google Scholar]
- 17.Tziomalos K, Giampatzis V, Bouziana SD, et al. Elevated diastolic but not systolic blood pressure increases mortality risk in hypertensive but not normotensive patients with acute ischemic stroke. Am J Hypertens 2015; 28: 765–771. [DOI] [PubMed] [Google Scholar]
- 18.Ohira T, Shahar E, Chambless LE, et al. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke 2006; 37: 2493–2498. [DOI] [PubMed] [Google Scholar]
- 19.Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAuley J, De Souza L, Sharma V, et al. Describing race, ethnicity, and culture in medical research. Self defined ethnicity is unhelpful. BMJ 1996; 313: 425–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshino T, Uchiyama S, Wong LKS, et al. Differences in characteristics and outcomes between Asian and Non-Asian patients in the TIAregistry.org. Stroke 2017; 48: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 22.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke 1986; 17: 648–655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Non-cardioembolic stroke/transient ischaemic attack in Asians and non-Asians: A post-hoc analysis of the PERFORM study by Takao Hoshino, Leila Sissani, Julien Labreuche, Marie-Germaine Bousser, Angel Chamorro, Marc Fisher, Ian Ford, Kim M Fox, Michael G Hennerici, Heinrich P Mattle, Peter M Rothwell, Philippe Gabriel Steg, Eric Vicaut and Pierre Amarenco in European Stroke Journal