Short abstract
Background
Subfebrile temperatures and fever in the first days after stroke are associated with a greater risk of a poor outcome. If this relation is causal, prevention of hyperthermia may improve outcome. Causality can be tested in animal models. We therefore assessed the effects of hyperthermia on outcomes in animal models of ischaemic stroke and explored under which conditions prevention of hyperthermia could be most effective.
Methods
We performed a systematic review and meta-analysis of data from animal experiments testing the effect of spontaneous or induced hyperthermia on outcome after focal cerebral ischaemia. Our primary outcome measure was infarct size. Normalised mean differences were combined using the random effects model and stratified meta-analysis was used to explore the impact of study characteristics.
Results
We included 19 publications, reporting on 49 comparisons involving 603 animals. Overall, hyperthermia increased infarct size by 43.4% (95% confidence interval, 29.8–56.9%) and worsened neurobehavioral outcomes by 48.5% (17.2–79.8%). The increase in infarct size was larger with higher temperatures. Hyperthermia was most harmful if present for more than 2 h and when started at the time of artery occlusion rather than later.
Conclusion
Hyperthermia substantially increased infarct size in animal models of ischaemic stroke, suggesting that the relation between fever and poor outcome observed in patients is at least in part causal. These data provide support to trials testing the effect of the prevention of fever with antipyretic drugs in patients with acute stroke.
Keywords: Stroke, animal studies, fever, hyperthermia
Introduction
In the first days after stroke, one fourth to half of the patients develop subfebrile temperatures or fever.1–4 Clinical studies have shown that an elevated body temperature early after stroke is associated with an increased infarct volume and a greater risk of a poor clinical outcome.1–9 In a meta-analysis of these studies, hyperthermia in the first 24 h after stroke was associated with a twofold increase in short-term mortality.10 Despite the suggested negative effects of hyperthermia, a phase III clinical trial on the prevention of hyperthermia with high-dose paracetamol in patients with acute stroke failed to demonstrate improvements in functional outcome or survival.11 However, this trial was underpowered to detect a small but clinically relevant benefit, and it has remained uncertain whether the relation between an elevated body temperature early after stroke and poor outcome is causal.
Animal models allow us to carefully study the effects of changes in physiological variables in a controlled setting and they are therefore suitable to assess the causality between hyperthermia and poor outcome after stroke. In addition, it is possible to identify the conditions under which hyperthermia is most harmful, such as different levels, durations, and temporal patterns of hyperthermia. Knowledge of these factors might provide further insights in which patients prevention or treatment of hyperthermia could be most beneficial, which could be used to guide the design of clinical trials in patients with ischaemic stroke. Therefore, we performed a systematic review and meta-analysis to assess the effects of hyperthermia in animal models of ischaemic stroke.
Methods
Search strategy and study inclusion
We performed a systematic literature search in Medline, EMBASE, and Web of Science up to July 2017 for synonyms of ischaemic stroke, hyperthermia, and animal experiments (see Appendix 1 for the exact search strategy). After removing duplicates, two authors independently screened titles and abstracts for relevance and, where appropriate, evaluated full-text versions of the selected articles for eligibility. Any discrepancy was resolved by consensus discussion. We included articles if (1) ischaemic stroke was modelled in an animal by means of a cerebral artery occlusion; (2) hyperthermia was compared to controlled normothermia; and (3) at least one of the following outcomes was assessed: infarct size, cerebral oedema, neurobehavioral outcome, or mortality. Both studies which assessed induced hyperthermia (by means of internal or external heating) or spontaneous hyperthermia (due to the surgical procedure or the occurrence of cerebral ischaemia) were included. Only studies assessing intra- or post-ischaemic hyperthermia were considered; studies which assessed the effect of hyperthermia before the onset of ischemia alone were excluded. Studies were also excluded if animal models of forebrain or global ischemia were used and if the data were presented in a way not suitable for meta-analysis.
Data-extraction and outcome assessment
The following data were, if available, extracted from the included studies: author, year of publication, journal, country, language, title; species, strain, sex, weight, age and number of animals used; excluded animals and reasons of exclusion; type of anaesthetic; method of ischaemia induction, duration of ischaemia; method and duration of temperature monitoring; induced or spontaneous hyperthermia; time, duration, and method of hyperthermia induction; level of hyperthermia; definition of normothermia, method of maintaining normothermia; time of animal sacrifice; infarct size, functional outcome, mortality, and cerebral oedema, method of assessment of the outcomes; administration of additional medication or therapies; the ten points of the CAMARADES quality checklist – see below.
Study quality was assessed according to the CAMARADES quality checklist with 10 points in total: (1) peer-reviewed publication; (2) statement of control of temperature; (3) use of animals with co-morbidities; (4) use of anaesthetic without prominent intrinsic neuroprotective abilities; (5) reporting of sample size calculation; (6) randomisation of treatment allocation; (7) concealment of treatment allocation; (8) blinded assessment of outcome; (9) statement of compliance with animal welfare regulations; and (10) statement of possible conflicts of interest.12
The number of animals and the mean outcome and its standard deviation (SD) or standard error (SE) were extracted for each intervention and control group. In the case if data were only presented graphically, explicit values were requested from the authors. If unavailable, values were estimated by measurement from the graphs. Also, in cases where data required for meta-analysis were not available from abstracts or publications, they were requested from the authors.
Statistical analysis
Normalised mean difference was used as the effect size. The effect size, SE, and 95% confidence interval (95%) were calculated for each comparison. In case of serial infarct sizes or neurobehavioral outcomes in the same group of animals, the effect size of the last observation was used for meta-analysis. If one normothermia group was compared to multiple hyperthermia groups, the amount of animals in the normothermia group was divided by the amount of hyperthermic groups. By means of the inverse variance method, weighted effect sizes were calculated for each comparison, with greater weight for more precise studies with smaller standard errors. All effect sizes were pooled by means of the random effects model instead of the fixed effects model, because of anticipated heterogeneity between studies. The amount of heterogeneity between studies was estimated by means of I2 and Cochran’s Q.13,14 A stratified analysis was performed for the following pre-specified study characteristics to assess their impact on the overall effect size: level of hyperthermia, duration of hyperthermia, start of induction of hyperthermia in relation to the onset of ischaemia, method of ischaemia induction, duration of ischaemia, strain, and sex; and study quality items, focusing on randomisation and blinding. Significance was assessed by Chi-square statistic of the residual heterogeneity with n−1 degrees of freedom (df). To allow for multiple comparisons, we adjusted our significance level using Bonferroni correction to a critical value of p < 0.004 for each comparison of infarct volume. Publication bias was assessed by funnel plotting and with the Egger regression method.15
Results
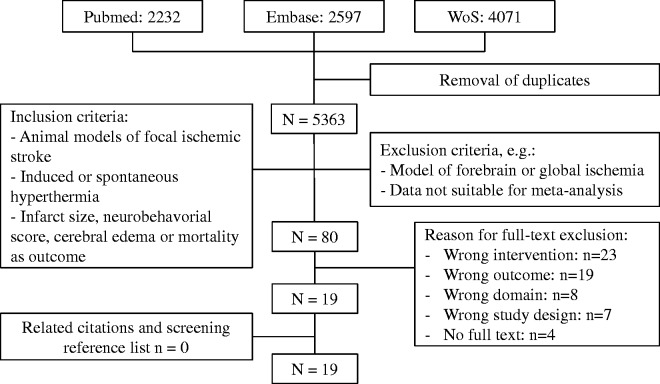
Of 5363 screened articles, 19 were included in the meta-analysis (Figure 1), reporting on 49 comparisons involving 603 animals.16–34 Data for infarct size were reported in 36 comparisons (572 animals), for neurobehavioral score in 20 comparisons (150 animals), and for cerebral oedema in 5 comparisons (73 animals).
Figure 1.
Flowchart of literature search.
Study characteristics
All experiments involved male rats. Four studies assessed the effect of spontaneous hyperthermia after focal ischaemia 16,21,28,29 and 15 studies assessed the effect of controlled induced hyperthermia. Of these 15 studies, hyperthermia was induced before the onset of ischaemia in three,17–19 during ischaemia in nine23,25–27,30–34 and after the onset of ischemia in six.16,18,20,24,28,29 Body temperatures during the anaesthesia and the surgical procedure were measured and controlled in 17 out of the 19 studies. Monitoring of temperature during the experiment occurred by measuring the temperature in the rectum (n = 11),16,18,19,21–23,25,28,29,33,34 brain (n = 5),17,18,20,24,26,27 or peri-cranial muscles (n = 2).31,32 The median temperature during hyperthermia was 39.0°C (range, 38–40.0°C). The median time of start of hyperthermia was 0 min, i.e. at the onset of ischaemia (range: −60 to 1500 min). The median duration of induced hyperthermia was 120 min (range: 30–270 min) and the median time to infarct size measurement 48 h (range: 1.5–169.5 h), with no studies performing temperature measurements between these two moments. By contrast, three of the four studies which assessed the effects of a spontaneous rise in body temperature after focal ischaemia provided information on body temperature until animal sacrifice (median 48 h). In addition, none of these four studies reported on the occurrence of infections as a potential cause of the ‘spontaneous’ increase in body temperature.
Effect of hyperthermia on outcomes
Hyperthermia increased infarct size by 43.4% (95% CI, 29.8% to 56.9%; Figure 2(a)). There was considerable heterogeneity in the estimates of efficacy between the studies (I2 = 88.7%; Cochran’s Q = 291.7; df = 33; P < 10−43). Neurobehavioral scores worsened by 48.5% (95% CI, 17.2 to 79.8%) in the presence of hyperthermia (Figure 3). In the seven studies which reported neurobehavioral outcomes, there was considerable heterogeneity (I2 = 84.0%; Cochran’s Q = 56.1; df = 9; P = 0.007). Four studies reported on the effect of hyperthermia on cerebral oedema after ischaemia, expressed as the increase of ipsilateral hemisphere volume in relation to the unaffected contralateral hemisphere. Pooled results showed an increase of 64.2% (95% CI, 15.8 to 112.6%), with significant heterogeneity between studies (I2 = 85.3%; Cochran’s Q = 27.2; df = 4; P <0.0001). Just seven studies reported on mortality in each of the experimental groups. The risk of premature death was higher with hyperthermia than with normothermia (7/110 vs. 44/152; P < 0.001).
Figure 2.
Effect of hyperthermia on infarct size. (a) Forrest plot of the effect of hyperthermia on infarct size. The Y-axis shows the different comparisons (n = 34) in order of effect size (non-chronological). The X-axis depicts the increase in infarct size (in percentage) compared to normothermic animals. The grey band is the 95% CI of the pooled effect size. (b) The effect of different levels of hyperthermia on infarct size. The Y-axis illustrates the worsening of infarct size compared to normothermic animals. The X-axis shows the different ranges of hyperthermia. The size of the circles is a representation of the number of animals in that study.
Figure 3.
Neurobehavioral scores. Forrest plot of the effect of hyperthermia on neurobehavioral scores. The Y-axis shows the different comparisons (n = 10) in order of effect size (non-chronological). The X-axis depicts the worsening of neurobehavioural outcome compared to normothermic animals. The grey band is the 95% CI of the pooled effect size.
Study characteristics and effect on infarct size
Higher temperatures resulted in larger infarct sizes, with a clear ‘dose–response’ relationship (R2 = 0.298, P = 0.010; Figure 2(b)). Compared to rats with normothermia, hyperthermia in the ranges of 38.0–38.9°C, 39–39.9°C, and >40.0°C resulted in an increase in infarct size of 34.3% (5.5–63.1%), 62.8% (45.4–80.2%) and 159.1% (14.8–303.4%), respectively. Stratifying outcomes by duration or timing of hyperthermia, or by the timing of the outcome assessment explained a significant proportion of heterogeneity (Figure 4(a) and (b)). Infarct sizes were largest in rats in which hyperthermia was maintained for more than 120 min, if hyperthermia was started before MCA occlusion, or if outcome was assessed later than 48 h after the onset of ischaemia. Hyperthermia also had a slightly larger effect on infarct size in models of temporary MCA occlusion as compared with permanent occlusion.
Figure 4.
Factors modifying the effect of hyperthermia on infarct size. (a) Estimated effected sizes, stratified by different methodological factors. The size of the marker reflects the amount of comparisons. The grey band is the 95% CI of the pooled effect size. The column on the right shows the mean effect size and 95% CI. The Y-axis illustrates different characteristics of the studies. The X-axis shows the increase in infarct size. (b) Estimated effected sizes, stratified by different methodological factors. The size of the marker reflects the amount of comparisons. The grey band is the 95% CI of the pooled effect size. The column on the right shows the mean effect size and 95% CI. The Y-axis illustrates different characteristics of the studies. The X-axis shows the increase in infarct size.
Study quality and outcomes
The median score on the CAMARADES checklist was 4 out of a possible 10 (interquartile range [IQR] = 3–5). Randomisation and blinding were reported in just six and seven of the 19 studies, respectively. Stratifying the data according to blinding or randomisation revealed no significant differences in the effects on infarct size. None of the included studies reported on a sample size calculation. The CAMARADES score explained a significant proportion of the observed heterogeneity (p < 0.001). However, there was no clear linear relationship between lower scores on the CAMARADES quality checklist and infarct size (R2 = 0.0037, p = 0.92). Visual inspection of the funnel plot (Figure 5(a)) showed asymmetry with greater effect sizes in more imprecise studies, suggesting publication bias. This was supported by Egger regression (Figure 5(b)).
Figure 5.
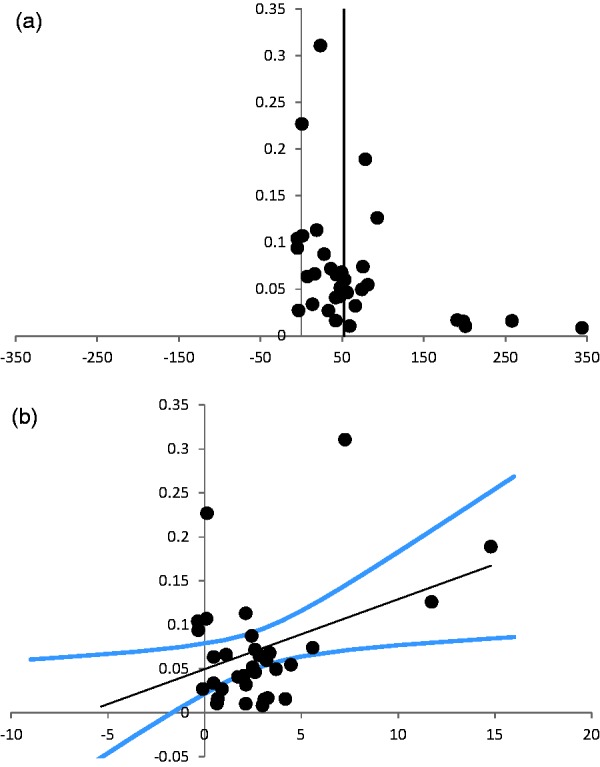
Assessment of publication bias. (a) Funnel plot of the studies on infarct size. The Y-axis shows the precision of each study defined as the inverse of the standard error (1/standard error). The X-axis shows the effect size per study. There is a clear asymmetry in the less precise studies, suggesting publication bias. (b) Egger regression of the studies on infarct size. The Y-axis shows the precision of each study defined as the inverse of the standard error (1/standard error). The X-axis shows the ratio between effect size and standard error (effect size/standard error). The black line is the linear regression line, with a 95% confidence interval (blue lines). The 95% confidence interval of the linear regression line does not include 0, which suggests publication bias.
Discussion
In this systematic review and meta-analysis, the presence of hyperthermia in animals with focal cerebral ischaemia increased infarct size and worsened neurobehavioral scores by 43.4% and 48.5%, respectively. Infarct size increased with each degree Celsius increase in body temperature. Hyperthermia was most harmful with longer durations of hyperthermia, in models of temporary ischemia, and if rats were hyperthermic during vessel occlusion rather than if hyperthermia was initiated after vessel occlusion. These findings strongly suggest that the relationship between hyperthermia and worse outcome in stroke patients is at least partially causal, and warrants further trials on the prevention of fever in patients with acute stroke.
In several prospective clinical studies, summarised in three meta-analyses, the presence of hyperthermia in the first days after stroke has been associated with a worse functional outcome and an increased risk of death.10,35,36 Prevention of hyperthermia with high-dose paracetamol is safe and simple,37,38 but has so far not convincingly improved outcomes in randomised trials in patients with acute stroke,11,38 which may be explained by several factors. In the phase III Paracetamol (Acetaminophen) In Stroke (PAIS) trial, the majority of patients had a mild stroke (median NIHSS, 6). Since both the risk of hyperthermia and the risk of a poor outcome are larger in patients with a more severe deficit,8 the trials missed the opportunity to assess the effects of fever prevention in patients with the largest chance of benefit.11,38 Moreover, all trials were underpowered to detect a small but clinically relevant benefit of fever prevention. In a post-hoc analysis of PAIS, preventive treatment with acetaminophen in patients with a baseline temperature of 37 to 39°C did result in an improvement in functional outcome (OR 1.43; 95% CI, 1.02–1.97). This finding is consistent with ours, and both findings provide support to the hypothesis that prevention or treatment of hyperthermia in these patients improves functional outcome. This hypothesis is currently tested in the phase III trial PRECIOUS: PREvention of Complications to Improve OUtcome in elderly patients with acute Stroke. This trial assesses whether prevention of aspiration, infections, or fever with metoclopramide, ceftriaxone, paracetamol, or any combination of these in elderly patients with a moderately severe to severe acute stroke is more effective at improving functional outcome than current clinical practice of waiting until these complications are manifest before initiating treatment.39
The results of this meta-analysis do not provide extensive information on the relationship between the timing of hyperthermia and outcome in animals. Almost all studies induced hyperthermia within the first hours after MCA occlusion, and maintained higher temperatures for a median of just 2 h. The single study that delayed the induction of hyperthermia to 24 h after the onset of ischaemia demonstrated a detrimental effect of an increase in body temperature to 40°C, but not of an increase to 39°C.20
Three of the four studies that assessed the effects of the spontaneous increase in body temperature after focal ischaemia performed temperature measurements until animal sacrifice. Temperatures generally remained elevated during the first 24 h, with apparent normalisation after 48 h.21,28,29 In clinical studies, elevated body temperatures have been observed in almost 10% of the patients after one week, and the relation between hyperthermia and poor outcome or larger infarct size persisted for up to one week.8,9
There are several proposed mechanisms by which hyperthermia enlarges infarct size, including enhanced neurotoxic neurotransmitter release,40,41 blood-brain barrier breakdown,42 increased radical production,43 increased metabolism44 and increased inflammation.45 In our meta-analysis, we found a modest difference in the increase in infarct size between temporary ischemia and permanent ischemia. In rats with temporary ischemia, hyperthermia could increase the infarct core before revascularisation or reduce the potential for recovery of penumbral tissue despite adequate reperfusion. This has also recently been suggested in patients with ischaemic stroke, in whom fever after successful mechanical thrombectomy was associated with larger infarct sizes.46
The current meta-analysis has limitations. There was considerable heterogeneity between the included studies and funnel plotting and Egger regression suggested publication bias. For these reasons, the overall pooled effect size should be interpreted with caution. Furthermore, no study reported body temperatures in the period between cessation of induced hyperthermia and animal sacrifice. Since the median duration of hyperthermia was 2 h, and the median duration until sacrifice was 24 h, there is a considerable time window in which spontaneous hyperthermia could have occurred in any experimental group. It is not known whether there were differences in the severity or duration of spontaneous hyperthermia between these groups. In addition, in three studies,22,26,28 the reported mortality rate was not specified for each experimental arm, or it was not reported if deceased animals were excluded from infarct size assessment. Therefore, in these studies, the exact number of remaining animals in the outcome assessment could not be inferred, and we had to use the predefined number of animals, which might be an overestimation. Finally, we limited our search to publications written in English, which may have led to selection bias.
Conclusion
In rats with focal cerebral ischaemia, hyperthermia increases infarct size and worsens neurobehavioral outcomes. This provides additional evidence to the assumption that the relation between hyperthermia and poor outcome in patients with acute stroke is at least in part causal, and supports the conduct of clinical trials testing the effects of fever prevention in these patients.
Acknowledgements
We thank Emily S Sena and Malcom R Macleod (Department of Clinical Neurosciences, University of Edinburgh, UK) for their careful review of the manuscript and useful suggestions.
Appendix 1. Search strategy
The applied search string for Pubmed, Embase and Web of Science:
(((Stroke [tiab] OR ((cerebral [tiab] OR brain [tiab] OR focal [tiab]) AND … (ischemia [tiab]OR ischemic [tiab] OR ischaemia [tiab] OR ischaemic [tiab])) OR cerebrovascular [tiab] OR middle cerebral artery [tiab] OR MCA [tiab] OR middle cerebral artery occlusion [tiab] OR MCAO [tiab] OR anterior cerebral artery [tiab] OR ACA [tiab] OR anterior cerebral artery occlusion [tiab] OR ACAO [tiab] OR experimental stroke [tiab])) AND (Hyperthermia [tiab] OR hyperthermic [tiab] OR fever [tiab] OR temperature* [tiab])) AND ((Animal* [tiab] OR experimental [tiab] OR preclinical [tiab] OR invertebrates [tiab] OR chordata [tiab] OR vertebrates [tiab] OR amphibians [tiab] OR birds [tiab] OR fishes [tiab] OR reptiles [tiab] OR mammals [tiab] OR primates [tiab] OR artiodactyla [tiab] OR carnivore [tiab] OR cetacean [tiab] OR chiroptera [tiab] OR elephants [tiab] OR hyraxes [tiab] OR insectivora [tiab] OR lagomorpha [tiab] OR marsupialia [tiab] OR monotremata [tiab] OR perissodactyla [tiab] OR rodentia [tiab] OR scandentia [tiab] OR sirenia [tiab] OR xenarthra [tiab] OR haplorhini [tiab] OR strepsirhini [tiab] OR platyrrhini [tiab] OR tarsii [tiab] OR catarrhini [tiab] OR cercopithecidae [tiab] OR hylobatidae [tiab] OR hominidae [tiab] OR gorilla [tiab] OR “pan paniscus” [tiab] OR “pan troglodytes” [tiab] OR “pongo pygmaeus” [tiab] OR mice [tiab] OR mus [tiab] OR mouse [tiab] OR murine [tiab] OR woodmouse [tiab] OR rats [tiab] OR rat [tiab] OR murinae [tiab] OR muridae [tiab] OR cottonrat [tiab] OR cottonrats [tiab] OR hamster [tiab] OR hamsters [tiab] OR cricetinae [tiab] OR rodentia [tiab] OR rodent [tiab] OR rodents [tiab] OR pigs [tiab] OR pig [tiab] OR swine [tiab] OR swines [tiab] OR piglets [tiab] OR piglet [tiab] OR boar [tiab] OR boars[tiab] OR “sus scrofa” [tiab] OR ferrets [tiab] OR ferret [tiab] OR polecat [tiab] OR polecats [tiab] OR “mustela putorius” [tiab] OR “guinea pigs” [tiab] OR “guinea pig” [tiab] OR cavia [tiab] OR callithrix [tiab] OR marmoset [tiab] OR marmosets [tiab] OR cebuella [tiab] OR hapale [tiab] OR octodon [tiab] OR chinchilla [tiab] OR chinchillas [tiab] OR gerbillinae [tiab] OR gerbil [tiab] OR gerbils [tiab] OR jird [tiab] OR jirds [tiab] OR merione [tiab] OR meriones [tiab] OR rabbits [tiab] OR rabbit [tiab] OR hares [tiab] OR hare [tiab] OR diptera [tiab] OR flies [tiab] OR fly [tiab] OR dipteral [tiab] OR drosophila [tiab] OR drosophilidae [tiab] OR cats [tiab] OR cat [tiab] OR carus [tiab] OR felis [tiab] OR nematoda [tiab] OR nematode [tiab] OR nematodes [tiab] OR sipunculida [tiab] OR dogs [tiab] OR dog [tiab] OR canine [tiab] OR canines [tiab] OR canis [tiab] OR sheep [tiab] OR sheeps [tiab] OR mouflon [tiab] OR mouflons [tiab] OR ovis [tiab] OR goats [tiab] OR goat [tiab] OR capra [tiab] OR capras [tiab] OR rupicapra [tiab] OR rupicapras [tiab] OR chamois [tiab] OR haplorhini [tiab] OR monkey [tiab] OR monkeys [tiab] OR anthropoidea [tiab] OR anthropoids [tiab] OR saguinus [tiab] OR tamarin [tiab] OR tamarins [tiab] OR leontopithecus [tiab] OR hominidae [tiab] OR ape [tiab] OR apes [tiab] OR “pan paniscus” [tiab] OR bonobo [tiab] OR bonobos [tiab] OR “pan troglodytes” [tiab] OR gibbon [tiab] OR gibbons [tiab] OR siamang [tiab] OR siamangs [tiab] OR nomascus [tiab] OR symphalangus [tiab] OR chimpanzee [tiab] OR chimpanzees [tiab] OR prosimian [tiab] OR prosimians [tiab] OR “bush baby” [tiab] OR bush babies [tiab] OR galagos [tiab] OR galago [tiab] OR pongidae [tiab] OR gorilla [tiab] OR gorillas [tiab] OR “pongo pygmaeus” [tiab] OR orangutan [tiab] OR orangutans [tiab] OR lemur [tiab] OR lemurs [tiab] OR lemuridae [tiab] OR horse [tiab] OR horses [tiab] OR equus [tiab] OR cow [tiab] OR calf [tiab] OR bull [tiab] OR chicken [tiab] OR chickens [tiab] OR gallus [tiab] OR quail [tiab] OR bird [tiab] OR birds [tiab] OR quails [tiab] OR poultry [tiab] OR poultries [tiab] OR fowl [tiab] OR fowls [tiab] OR reptile [tiab] OR reptilia [tiab] OR reptiles [tiab] OR snakes [tiab] OR snake [tiab] OR lizard [tiab] OR lizards [tiab] OR alligator [tiab] OR alligators [tiab] OR crocodile [tiab] OR crocodiles [tiab] OR turtle [tiab] OR turtles [tiab] OR amphibian [tiab] OR amphibians [tiab] OR amphibia [tiab] OR frog [tiab] OR frogs [tiab] OR bombina [tiab] OR salientia [tiab] OR toad [tiab] OR toads [tiab] OR “epidalea calamita” [tiab] OR salamander [tiab] OR salamanders [tiab] OR eel [tiab] OR eels [tiab] OR fish [tiab] OR fishes [tiab] OR pisces [tiab] OR catfish [tiab] OR catfishes [tiab] OR siluriformes [tiab] OR arius [tiab] OR heteropneustes [tiab] OR sheatfish [tiab] OR perch [tiab] OR perches [tiab] OR percidae [tiab] OR perca [tiab] OR trout [tiab] OR trouts [tiab] OR char [tiab] OR chars [tiab] OR salvelinus [tiab] OR minnow [tiab] OR cyprinidae [tiab] OR carps [tiab] OR carp [tiab] OR zebrafish [tiab] OR zebrafishes [tiab] OR goldfish [tiab] OR goldfishes [tiab] OR guppy [tiab] OR guppies [tiab] OR chub [tiab] OR chubs [tiab] OR tinca [tiab] OR barbels [tiab] OR barbus [tiab] OR pimephales [tiab] OR promelas [tiab] OR “poecilia reticulata” [tiab] OR mullet [tiab] OR mullets [tiab] OR eel [tiab] OR eels [tiab] OR seahorse [tiab] OR seahorses [tiab] OR mugil curema [tiab] OR atlantic cod [tiab] OR shark [tiab] OR sharks [tiab] OR catshark [tiab] OR anguilla [tiab] OR salmonid [tiab] OR salmonids [tiab] OR whitefish [tiab] OR whitefishes [tiab] OR salmon [tiab] OR salmons [tiab] OR sole [tiab] OR solea [tiab] OR lamprey [tiab] OR lampreys [tiab] OR pumpkinseed [tiab] OR sunfish [tiab] OR sunfishes [tiab] OR tilapia [tiab] OR tilapias [tiab] OR turbot [tiab] OR turbots [tiab] OR flatfish [tiab] OR flatfishes [tiab] OR sciuridae [tiab] OR squirrel [tiab] OR squirrels [tiab] OR chipmunk [tiab] OR chipmunks [tiab] OR suslik [tiab] OR susliks [tiab] OR vole [tiab] OR voles [tiab] OR lemming [tiab] OR lemmings [tiab] OR muskrat [tiab] OR muskrats [tiab] OR lemmus [tiab] OR otter [tiab] OR otters [tiab] OR marten [tiab] OR martens [tiab] OR martes [tiab] OR weasel [tiab] OR badger [tiab] OR badgers [tiab] OR ermine [tiab] OR mink [tiab] OR minks [tiab] OR sable [tiab] OR sables [tiab] OR gulo [tiab] OR gulos [tiab] OR wolverine [tiab] OR wolverines [tiab] OR mustela [tiab] OR llama [tiab] OR llamas [tiab] OR alpaca [tiab] OR alpacas [tiab] OR camelid [tiab] OR camelids [tiab] OR guanaco [tiab] OR guanacos [tiab] OR chiroptera [tiab] OR chiropteras [tiab] OR bat [tiab] OR bats [tiab] OR fox [tiab] OR foxes [tiab] OR iguana [tiab] OR iguanas [tiab] OR xenopus laevis [tiab] OR parakeet [tiab] OR parakeets [tiab] OR parrot [tiab] OR parrots [tiab] OR donkey [tiab] OR donkeys [tiab] OR mule [tiab] OR mules [tiab] OR zebra [tiab] OR zebras [tiab] OR shrew [tiab] OR shrews [tiab] OR bison [tiab] OR bisons [tiab] OR buffalo [tiab] OR buffaloes [tiab] OR deer [tiab] OR deers [tiab] OR bear [tiab] OR bears [tiab] OR panda [tiab] OR pandas [tiab] OR “wild hog” [tiab] OR “wild boar” [tiab] OR fitchew [tiab] OR fitch [tiab] OR beaver [tiab] OR beavers [tiab] OR jerboa [tiab] OR jerboas [tiab] OR capybara [tiab] OR capybaras [tiab]))
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors de JCdJ and HBvdW are trial coordinator and chief investigator, respectively, of the trial PRECIOUS: PREvention of Complications to Improve Outcome in elderly patients with acute stroke. PRECIOUS has received funding from the European Union’s Horizon, 2020 research and innovation programme under grant no. 634809.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JCdeJ is funded by the European Union’s Horizon, 2020 research and innovation programme (grant no. 634809).
Informed consent
Not applicable.
Ethical approval
Not applicable.
Guarantor
JCdJ.
Contributorship
All co-authors have contributed to the trial design, protocol development, and the writing of this manuscript.
References
- 1.Castillo J, Dávalos A, Marrugat J, et al. Timing for fever-related brain damage in acute ischemic stroke. Stroke 1998; 29: 2455–2460. [DOI] [PubMed] [Google Scholar]
- 2.Azzimondi G, Bassein L, Nonino F, et al. Fever in acute stroke worsens prognosis. A prospective study. Stroke 1995; 26: 2040–2043. [DOI] [PubMed] [Google Scholar]
- 3.Reith J, Jørgensen HS, Pedersen PM, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet (London, England) 1996; 347: 422–425. [DOI] [PubMed] [Google Scholar]
- 4.Kammersgaard LP, Jørgensen HS, Rungby JA, et al. Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke 2002; 33: 1759–1762. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen HS, Reith J, Pedersen PM, et al. Body temperature and outcome in stroke patients. Lancet (London, England) 1996; 348: 193–131. [DOI] [PubMed] [Google Scholar]
- 6.Boysen G, Christensen H. Stroke severity determines body temperature in acute stroke. Stroke 2001; 32: 413–417. [DOI] [PubMed] [Google Scholar]
- 7.Leira R, Rodríguez-Yáñez M, Castellanos M, et al. Hyperthermia is a surrogate marker of inflammation-mediated cause of brain damage in acute ischaemic stroke. J Intern Med 2006; 260: 343–349. [DOI] [PubMed] [Google Scholar]
- 8.Saini M, Saqqur M, Kamruzzaman A, et al. VISTA investigators. Effect of hyperthermia on prognosis after acute ischemic stroke. Stroke 2009; 40: 3051–3059. [DOI] [PubMed] [Google Scholar]
- 9.Geurts M, Scheijmans FEV, van Seeters T, et al. Temporal profile of body temperature in acute ischemic stroke: relation to infarct size and outcome. BMC Neurol 2016; 16: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad K, Krishnan PR. Fever is associated with doubling of odds of short-term mortality in ischemic stroke: an updated meta-analysis. Acta Neurol Scand 2010; 122: 404–408. [DOI] [PubMed] [Google Scholar]
- 11.den Hertog HM, van der Worp HB, van Gemert HMA, et al. The Paracetamol (Acetaminophen) In Stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol 2009; 8: 434–440. [DOI] [PubMed] [Google Scholar]
- 12.Macleod MR, O’collins T, Howells DW, et al. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004; 35: 1203–1208. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochran W. The combination of estimates from different experiments. Biometrics 1954; 10: 101–129. [Google Scholar]
- 15.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001; 323: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham H, Somogyvári-Vigh A, Maderdrut J, et al. Filament size influences temperature changes and brain damage following middle cerebral artery occlusion in rats. Exp Brain Res 2002; 142: 131–138. [DOI] [PubMed] [Google Scholar]
- 17.Campos F, Pérez-Mato M, Agulla J, et al. Glutamate excitoxicity is the key molecular mechanism which is influenced by body temperature during the acute phase of brain stroke. PLoS ONE 2012; 7: e44191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Chopp M, Welch KM. Effect of mild hyperthermia on the ischemic infarct volume after middle cerebral artery occlusion in the rat. Neurology 1991; 41: 1133–1135. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Chopp M, Bodzin G, et al. Temperature modulation of cerebral depolarization during focal cerebral ischemia in rats: correlation with ischemic injury. J Cereb Blood Flow Metab 1993; 13: 389–394. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Busto R, Dietrich WD, et al. Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke 1996; 27: 2274- 80–2281. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Omae T, Fisher M. Spontaneous hyperthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats. Stroke 1999; 30: 2464–2470. [DOI] [PubMed] [Google Scholar]
- 22.Meden P, Overgaard K, Pedersen H, et al. The influence of body temperature on infarct volume and thrombolytic therapy in a rat embolic stroke model. Brain Res 1994; 647: 131–138. [DOI] [PubMed] [Google Scholar]
- 23.Meng Q, He C, Shuaib A, et al. Hyperthermia worsens ischaemic brain injury through destruction of microvessels in an embolic model in rats. Int J Hyperthermia 2012; 28: 24–32. [DOI] [PubMed] [Google Scholar]
- 24.Morikawa E, Ginsberg MD, Dietrich WD, et al. The significance of brain temperature in focal cerebral ischemia: histopathological consequences of middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 1992; 12: 380–389. [DOI] [PubMed] [Google Scholar]
- 25.Nategh M, Shaveisi K, Shabanzadeh AP, et al. Systemic hyperthermia masks the neuroprotective effects of MK-801, but not rosiglitazone in brain ischaemia. Basic Clin Pharmacol Toxicol 2010; 107: 724–729. [DOI] [PubMed] [Google Scholar]
- 26.Noor R, Wang CX, Shuaib A. Effects of hyperthermia on infarct volume in focal embolic model of cerebral ischemia in rats. Neurosci Lett 2003; 349: 130–132. [DOI] [PubMed] [Google Scholar]
- 27.Noor R, Wang CX, Shuaib A. Hyperthermia masks the neuroprotective effects of tissue plaminogen activator. Stroke 2005; 36: 665–669. [DOI] [PubMed] [Google Scholar]
- 28.Reglodi D, Somogyvari-Vigh A, Vigh S, et al. Neuroprotective effects of PACAP38 in a rat model of transient focal ischemia under various experimental conditions. Ann N Y Acad Sci 2000; 921: 119–128. [DOI] [PubMed] [Google Scholar]
- 29.Reglodi D, Somogyvari VA, Maderdrut JL, et al. Postischemic spontaneous hyperthermia and its effects in middle cerebral artery occlusion in the rat. Exp Neurol 2000; 163: 399–407. [DOI] [PubMed] [Google Scholar]
- 30.Shabanzadeh AP, Shuaib A, Wang CX. Reduction of ischemic brain injury in rats with normothermic and hyperthermic conditions. J Neurosurg 2008; 109: 522–529. [DOI] [PubMed] [Google Scholar]
- 31.Sick TJ, Tang R, Pérez-Pinzón MA. Cerebral blood flow does not mediate the effect of brain temperature on recovery of extracellular potassium ion activity after transient focal ischemia in the rat. Brain Res 1999; 821: 400–406. [DOI] [PubMed] [Google Scholar]
- 32.Warner DS, McFarlane C, Todd MM, et al. Sevoflurane and halothane reduce focal ischemic brain damage in the rat. Possible influence on thermoregulation. Anesthesiology 1993; 79: 985–992. [DOI] [PubMed] [Google Scholar]
- 33.Xue D, Huang ZG, Smith KE, et al. Immediate or delayed mild hypothermia prevents focal cerebral infarction. Brain Res 1992; 587: 66–72. [DOI] [PubMed] [Google Scholar]
- 34.Yip PK, Koh AT, Lin CT, et al. Effects of mild whole body hyperthermia on graded focal ischaemia-reperfusion in a rat stroke model. J Clin Neurosci 1998; 5: 428–431. [DOI] [PubMed] [Google Scholar]
- 35.Hajat C, Hajat S, Sharma P. Effects of poststroke pyrexia on stroke outcome : a meta-analysis of studies in patients. Stroke 2000; 31: 410–414. [DOI] [PubMed] [Google Scholar]
- 36.Greer DM, Funk SE, Reaven NL, et al. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke 2008; 39: 3029–3035. [DOI] [PubMed] [Google Scholar]
- 37.Dippel DWJ, van Breda EJ, van der Worp HB, et al. Timing of the effect of acetaminophen on body temperature in patients with acute ischemic stroke. Neurology 2003; 61: 677–679. [DOI] [PubMed] [Google Scholar]
- 38.de Ridder IR, den Hertog HM, van Gemert HMA, et al. PAIS 2 (Paracetamol [Acetaminophen] in Stroke 2). Stroke 2017; 48: 977–982. [DOI] [PubMed] [Google Scholar]
- 39.Reinink H, de Jonge JC, Bath PM, et al. PRECIOUS: PREvention of Complications to Improve OUtcome in elderly patients with acute Stroke. Rationale and design of a randomised, open, phase III, clinical trial with blinded outcome assessment. European Stroke Journal 2018; 3: 291–298. [DOI] [PMC free article] [PubMed]
- 40.Campos F, Blanco M, Barral D, et al. Influence of temperature on ischemic brain: basic and clinical principles. Neurochem Int 2012; 60: 495–505. [DOI] [PubMed] [Google Scholar]
- 41.Castillo J, Dávalos A, Noya M. Aggravation of acute ischemic stroke by hyperthermia is related to an excitotoxic mechanism. Cerebrovasc Dis. 2017; 9: 22–27. [DOI] [PubMed] [Google Scholar]
- 42.Wang CX, Shuaib A. Critical role of microvasculature basal lamina in ischemic brain injury. Prog Neurobiol 2007; 83: 140–148. [DOI] [PubMed] [Google Scholar]
- 43.Wang CX, Stroink A, Casto JM, et al. Hyperthermia exacerbates ischaemic brain injury. Int J Stroke 2009; 4: 274–284. [DOI] [PubMed] [Google Scholar]
- 44.Karaszewski B, Wardlaw JM, Marshall I, et al. Early brain temperature elevation and anaerobic metabolism in human acute ischaemic stroke. Brain 2008; 132: 955–964. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Yáñez M, Castillo J. Role of inflammatory markers in brain ischemia. Curr Opin Neurol 2008; 21: 353–357. [DOI] [PubMed] [Google Scholar]
- 46.Dehkharghani S, Bowen M, Haussen DC, et al. Body temperature modulates infarction growth following endovascular reperfusion. Ajnr Am J Neuroradiol 2017; 38: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]