Short abstract
Background
The relationship between different patterns of atrial fibrillation and early recurrence after an acute ischaemic stroke is unclear.
Purpose
In a prospective cohort study, we evaluated the rates of early ischaemic recurrence after an acute ischaemic stroke in patients with paroxysmal atrial fibrillation or sustained atrial fibrillation which included persistent and permanent atrial fibrillation.
Methods
In patients with acute ischaemic stroke, atrial fibrillation was categorised as paroxysmal atrial fibrillation or sustained atrial fibrillation. Ischaemic recurrences were the composite of ischaemic stroke, transient ischaemic attack and symptomatic systemic embolism occurring within 90 days from acute index stroke.
Results
A total of 2150 patients (1155 females, 53.7%) were enrolled: 930 (43.3%) had paroxysmal atrial fibrillation and 1220 (56.7%) sustained atrial fibrillation. During the 90-day follow-up, 111 ischaemic recurrences were observed in 107 patients: 31 in patients with paroxysmal atrial fibrillation (3.3%) and 76 with sustained atrial fibrillation (6.2%) (hazard ratio (HR) 1.86 (95% CI 1.24–2.81)). Patients with sustained atrial fibrillation were on average older, more likely to have diabetes mellitus, hypertension, history of stroke/ transient ischaemic attack, congestive heart failure, atrial enlargement, high baseline NIHSS-score and implanted pacemaker. After adjustment by Cox proportional hazard model, sustained atrial fibrillation was not associated with early ischaemic recurrences (adjusted HR 1.23 (95% CI 0.74–2.04)).
Conclusions
After acute ischaemic stroke, patients with sustained atrial fibrillation had a higher rate of early ischaemic recurrence than patients with paroxysmal atrial fibrillation. After adjustment for relevant risk factors, sustained atrial fibrillation was not associated with a significantly higher risk of recurrence, thus suggesting that the risk profile associated with atrial fibrillation, rather than its pattern, is determinant for recurrence.
Keywords: Stroke, atrial fibrillation, paroxysmal atrial fibrillation, sustained atrial fibrillation, stroke recurrence, anticoagulation
Introduction
Atrial fibrillation (AF) is the most common chronic cardiac arrhythmia and the number of patients with AF is predicted to rise steeply in the coming years.1,2 Despite remarkable progresses in the management of patients with AF, this arrhythmia remains one of the major causes of stroke and thromboembolic events.3
The relationship between the different patterns of AF and the risk of stroke has been analysed in several studies with conflicting results. Various studies reported similar risks of thromboembolism for patients with paroxysmal AF (PAF) and those with sustained AF (SAF).4–10 However, several other studies indicated a higher risk for ischaemic events in patients with non-paroxysmal AF compared to those with PAF.11–21 Current guidelines on AF management recommend that the pattern of AF should not influence the decision on whether to treat patients with anticoagulants.3,22
The association of AF pattern with the risk of early recurrence after an acute ischaemic stroke is unclear. By using Early Recurrence and Cerebral Bleeding in Patients with Acute Ischemic Stroke and Atrial Fibrillation (RAF) and Early Recurrence and Cerebral Bleeding in Patients with Acute Ischemic Stroke and Atrial Fibrillation treated with Non Vitamin K Oral Anticoagulants (RAF-NOAC) study databases,23,24 we evaluated the rates of ischaemic recurrences in patients with PAF and those with SAF within 90 days after an acute ischaemic stroke. The ultimate aim of the study was to consider if after an acute ischaemic stroke different treatment approaches are needed in the presence of different patterns of AF.
Methods
For the purpose of this analysis, we combined the databases of the RAF study and the RAF-NOAC study. RAF and RAF-NOAC were prospective observational studies carried out between January 2012 and March 2014 in 29 Stroke Units and between April 2014 and June 2016 in 35 Stroke Units respectively, across Europe, United States and Asia. Both studies enrolled consecutive patients with acute ischaemic stroke and known or newly diagnosed AF without contraindications to anticoagulation. The studies were approved by the local Institutional Review Boards, if required.
On admission, stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS). A non-contrast cerebral computed tomography (CT) or cerebral magnetic resonance (MR) scan was performed on admission for all patients to exclude intracranial haemorrhage. Revascularisation treatments were given as per standard local protocol, when appropriate. Standard Stroke Unit care, monitoring and treatment were provided according to current international recommendations for acute ischaemic stroke. Attending physicians made decisions regarding the type of anticoagulant to be used for secondary prevention, as well as the day of initiation of anticoagulant treatment. The RAF study included patients treated with either vitamin K antagonists or NOACs, while the RAF-NOAC study included only patients who received NOACs.
A full history and clinical examination, admission ECG and prolonged ECG monitoring for 48 h after the index stroke were performed to detect non-valvular AF. AF was categorised as:
Paroxysmal: associated with episodes terminating spontaneously within seven days;
Persistent: associated with episodes lasting more than seven days or requiring pharmacological and/or electrical cardioversion;
Permanent: persisting for more than one year, either because cardioversion failed or was not pursued.25
For the purpose of the present study, AF was categorised into two types: paroxysmal AF or sustained (persistent or permanent) AF.
A second brain CT scan or MR was scheduled to be performed 24–72 h from stroke onset in all patients. The sites and sizes of the qualifying infarcts were determined based on standard templates as:
Small: when a lesion was ≤1.5 cm in the anterior or posterior circulation;
Medium: when a lesion was in a cortical superficial branch of middle cerebral artery, in the middle cerebral artery deep branch, in the internal border zone territories, in a cortical superficial branch of posterior cerebral artery, in a cortical superficial branch of the anterior cerebral artery;
Large anterior: when a lesion involved the complete territory of middle, posterior, or anterior cerebral artery; in two cortical superficial branches of middle cerebral artery; in a cortical superficial branch of middle cerebral artery associated to the middle cerebral artery deep branch, or in more than one artery territory;
Large posterior: when a lesion was ≥1.5 cm in the brain stem or cerebellum.26
Left atrial enlargement and its severity was defined following the American Society of Echocardiography guidelines measuring the left atrial diameter or volume taking into account the difference between sexes.27
Risk factors
Data on known stroke risk factors were collected as follows: age, gender, history of hypertension (blood pressure of ≥140/90 mmHg at least twice before stroke or already under treatment with antihypertensive drugs), history of diabetes mellitus (fasting glucose level ≥126 mg/dL pre-prandial on two examinations, glucose level ≥200 mg/dL postprandial, or HbA1c ≥6.5%, or under antidiabetic treatment), current cigarette smoking, past smoking (cessation less than five years ago), hyperlipidaemia (total cholesterol ≥200 mg/dL or triglyceride ≥140 mg/dL or already under lipid-lowering therapy), history of symptomatic ischaemic heart disease (myocardial infarction, history of angina or existence of multiple lesions on thallium heart isotope scan or evidence of coronary disease on coronary angiography), history of symptomatic peripheral arterial disease (intermittent claudication of presumed atherosclerotic origin; or ankle/arm systolic blood pressure ratio <0.85 in either leg at rest; or history of intermittent claudication with previous leg amputation, reconstructive surgery, or angioplasty), alcohol abuse (≥300 g per week), obesity (body mass index ≥30 kg/m2), or previous stroke/TIA. White matter changes (leukoaraiosis defined on the first CT (or MR) examination as ill-defined and moderately hypodense (or hyperintensity on T2-weighted on MR) areas of ≥5 mm according to published criteria) were investigated. Leukoaraiosis in the deep white matter was dichotomised into absent versus present (independently if mild, moderate or severe).28 Other baseline variables obtained at admission for all patients included: fasting serum glucose, fasting serum cholesterol (total, HDL and LDL), platelet count, international normalised ratios, activated partial thromboplastin time, systolic blood pressure and diastolic blood pressure.
Data on the use of any antiplatelet, anticoagulants or thrombolytic agent, prior to admission, at baseline and during the follow-up period, were recorded.
The CHA2DS2-VASc score (two points for history of stroke or age ≥75 years and one point each for congestive heart failure, hypertension, diabetes, vascular disease, age 65 to 74 years and female sex) was calculated before and after the index event.
Evaluation of outcome
Patients were followed up prospectively by face-to-face or telephone interviews. Whether an outcome event occurred, patients were requested to bring full documentation of it to a face-to-face appointment. Study outcome at 90 days was the composite of: recurrent ischaemic cerebrovascular events (stroke or TIA) and symptomatic systemic embolism.
Stroke was defined as the sudden onset of a new focal neurological deficit of vascular origin in a site consistent with the territory of a major cerebral artery and categorised as ischaemic or haemorrhagic. TIA was defined as a transient episode of neurological dysfunction caused by focal brain ischaemia without acute infarction. Systemic embolism was defined as an acute vascular occlusion of an extremity or organ confirmed by imaging, surgery or autopsy.
Statistical analyses
Population characteristics were summarised as mean and SD for continuous variables and as absolute numbers and percentages for categorical variables.
Differences in the baseline characteristics of patients with PAF or SAF were tested using χ2 test for nominal variables, or ANOVA for continuous variables. Specifically, univariate tests were utilised to compare both clinical characteristics on admission and pre-existing risk factors for PAF.
A multivariate analysis was performed using logistic regression to determine independent predictors of PAF.
Differences in the characteristics of patients with or without ischaemic recurrence at 90 days were tested using χ2 test for nominal variables or ANOVA for continuous variables. Specifically, univariate tests were used to compare both clinical characteristics on admission and pre-existing risk factors for ischaemic events.
The relationship between the survival function and the set of explanatory variables were explored with Cox proportional hazard model. Cox model provided estimates of AF pattern influence on survival after adjusting for other explanatory variables.
In order to measure associations, we used odds ratios (OR) for multivariate logistic models and hazard ratios (HR) for survival curve analyses with a 95% confidence interval; a two-sided p-value < 0.05 was considered significant.
All statistical analyses were performed using the IBM SPSS Statistics version 22.0 (IBM Corporation, Somers, NY).
Results
PAF versus SAF patients
A total of 2150 patients (1155 women, 53.7%) were enrolled. Among these, 930 (43.3%) had paroxysmal (360 in RAF, 570 in RAF-NOAC) and 1220 (56.7%) sustained AF (660 in RAF, 560 in RAF-NOAC).
A total of 812 patients with PAF (87.3%) and 976 patients with SAF (80.0%) received OAC, of which 592 and 630 were non-vitamin K antagonist oral anticoagulants (NOACs), respectively. Vitamin K antagonists (VKAs) included warfarin (518) and acenocumarol (49), whereas NOACs included rivaroxaban (414), dabigatran (410) and apixaban (396).
The baseline characteristics of the patients with PAF and SAF are summarised in Table 1. Patients with SAF were on average older and more likely to have the following: higher baseline NIHSS-score, diabetes mellitus, hypertension, a history of stroke/TIA, congestive heart failure, pacemaker implanted, CHA2DS2-VASc > 4 and atrial enlargement (all p < 0.05). Patients with PAF were more likely to be on OAC treatment after the index stroke.
Table 1.
Baseline characteristics of patients with PAF and SAF.
| PAF (n = 930) | SAF (n = 1220) | pa | |
|---|---|---|---|
| Age (mean, years) | 74.5 ± 9.8 | 77.7 ± 9.5 | <0.0001 |
| Sex, M | 422 (45.4%) | 573 (47.0%) | |
| NIHSS (mean) | 8.0 ± 6.7 | 8.7 ± 6.8 | 0.026 |
| Diabetes mellitus | 181 (19.5%) | 301 (24.7%) | 0.004 |
| Hypertension | 709 (76.2%) | 978 (80.2%) | 0.026 |
| Hyperlipidaemia | 318 (34.2%) | 405 (33.2%) | |
| History stroke/TIA | 211 (22.7%) | 357 (29.3%) | 0.001 |
| Current smoker | 110 (11.8%) | 95 (7.8%) | |
| Alcoholism | 50 (5.4%) | 92 (7.5%) | |
| History congestive heart failure | 89 (9.6%) | 281 (23.0%) | <0.0001 |
| History myocardial infarction | 116 (12.5%) | 180 (14.8%) | |
| History peripheral artery disease | 71 (7.6%) | 111 (9.1%) | |
| Pacemaker | 39 (4.2%) | 111 (9.1%) | <0.0001 |
| Lesion size | |||
| Small | 350 (37.6%) | 471 (38.6%) | |
| Medium | 310 (33.3%) | 428 (35.1%) | |
| Large anterior | 173 (18.6%) | 225 (18.4%) | |
| Large posterior | 62 (6.7%) | 74 (6.1%) | |
| Leukoaraiosis | 465 (50.0%) | 632 (51.8%) | |
| OAC | 812 (87.3%) | 976 (80.0%) | <0.0001 |
| VKA | 220 (23.7%) | 346 (28.4%) | |
| NOAC | 592 (63.7%) | 630 (51.6%) | <0.0001 |
| CHA2DS2-VASc > 4 | 682 (73.3%) | 988 (81.0%) | <0.0001 |
| Atrial enlargementb | 510 (62.5%) | 739 (74.6%) | <0.0001 |
NIHSS: National Institutes of Health Stroke Scale; PAF: paroxysmal atrial fibrillation; SAF: sustained atrial fibrillation; TIA: transient ischaemic attack.
ap-Values are given only if < 0.05.
b1806 Patients with trans-thoracic echocardiogram performed.
The results from the multivariate analysis for factors associated with SAF are listed in Table 2. Older age, diabetes mellitus, history of stroke/TIA, alcoholism, history of congestive heart failure and pacemaker implant were all significant factors associated with SAF.
Table 2.
Multivariate logistic regression analysis of factors potentially associated with SAF.
| OR | 95% CI | pa | |
|---|---|---|---|
| Age (for each year increase) | 1.03 | 1.02–1.04 | <0.0001 |
| Sex | 1.15 | 0.94–1.39 | |
| NIHSS | 1.01 | 1.00–1.03 | |
| Diabetes mellitus | 1.28 | 1.03–1.61 | 0.026 |
| Hypertension | 1.05 | 0.79–1.25 | |
| Hyperlipidaemia | 3.85 | 0.73–1.09 | |
| History stroke/TIA | 1.25 | 1.01–1.54 | 0.042 |
| Smoking | 3.85 | 0.79–1.06 | |
| Alcoholism | 1.69 | 1.14–2.50 | 0.009 |
| History congestive heart failure | 2.70 | 2.04–3.57 | <0.0001 |
| History myocardial infarction | 3.85 | 0.64–1.12 | |
| Pacemaker | 1.85 | 1.23–2.78 | 0.003 |
CI: confidence interval; OR: odds ratios; TIA: transient ischaemic attack; NIHSS: National Institutes of Health Stroke Scale.
ap-Values are given only if <0.05.
Ischaemic recurrences
Over 90 days of follow-up, 111 ischaemic recurrences were recorded in 107 patients (82 ischaemic strokes, 18 TIAs and 11 systemic embolisms). The baseline characteristics of patients with and without ischaemic events within 90 days from acute stroke are listed in Table 3. Patients with ischaemic recurrence were on average older and were more likely to have: higher baseline NIHSS-score, diabetes mellitus, hypertension, congestive heart failure, pacemaker implanted and CHA2DS2-VASc score > 4. Patients without ischaemic recurrence were more likely to have small size lesions, to be on OAC treatment after the index stroke and to have PAF.
Table 3.
Baseline characteristics of patients with and without ischaemic recurrences.
| With event (n = 107) | Without event (n = 2040) | pa | |
|---|---|---|---|
| Age (mean, years) | 78.3 ± 9.4 | 76.2 ± 9.8 | 0.034 |
| Sex, M | 47 (43.9%) | 945 (46.3%) | |
| NIHSS (mean) | 10.4 ± 8.0 | 8.3 ± 6.7 | 0.001 |
| Diabetes mellitus | 38 (35.5%) | 443 (21.7%) | 0.020 |
| Hypertension | 93 (86.0%) | 1597 (78.3%) | 0.026 |
| Hyperlipidaemia | 37 (34.6%) | 697 (34.2%) | |
| History stroke/TIA | 35 (32.7%) | 528 (25.9%) | |
| Current smoker | 8 (7.5%) | 196 (9.6%) | |
| Alcoholism | 9 (8.4%) | 131 (6.4%) | |
| History congestive heart failure | 29 (27.1%) | 340 (16.7%) | 0.008 |
| History myocardial infarction | 17 (15.9%) | 275 (13.5%) | |
| History peripheral artery disease | 14 (13.1%) | 163 (8.0%) | |
| Pacemaker | 13 (12.1%) | 133 (6.5%) | 0.029 |
| Lesion size | |||
| Small | 31 (29.0%) | 796 (39.0%) | 0.050 |
| Medium | 42 (39.3%) | 705 (34.6%) | |
| Large anterior | 27 (25.2%) | 368 (18.0%) | |
| Large posterior | 3 (2.8%) | 132 (6.5%) | |
| Leukoaraiosis | 61 (57.0%) | 1013 (49.7%) | |
| PAF | 31 (29.0%) | 886 (43.4%) | 0.003 |
| OAC | 73 (68.2%) | 1710 (83.9%) | 0.001 |
| VKA | 38 (35.5%) | 525 (25.7%) | 0.041 |
| NOAC | 35 (32.7%) | 1185 (58.1%) | 0.001 |
| CHA2DS2-VASc > 4 | 94 (87.9%) | 1573 (77.0%) | 0.008 |
CHA2DS2-VASc: congestive heart failure, hypertension, 75 years of age and older, diabetes mellitus, previous stroke or transient ischaemic attack, vascular disease, 65 to 74 years of age, female; OAC: oral anticoagulation; NIHSS: National Institutes of Health Stroke Scale; PAF: paroxysmal atrial fibrillation; SAF: sustained atrial fibrillation; TIA: transient ischaemic attack.
ap-Values are given only if <0.05.
Among patients who suffered an ischaemic recurrence, 9.1% (34/372) had not started anticoagulation therapy, 6.7% (38/563) were treated with a VKA and 2.9% (35/1220) with an NOAC. The median time for starting OAC was eight days in patients with PAF and six days in patients with SAF.
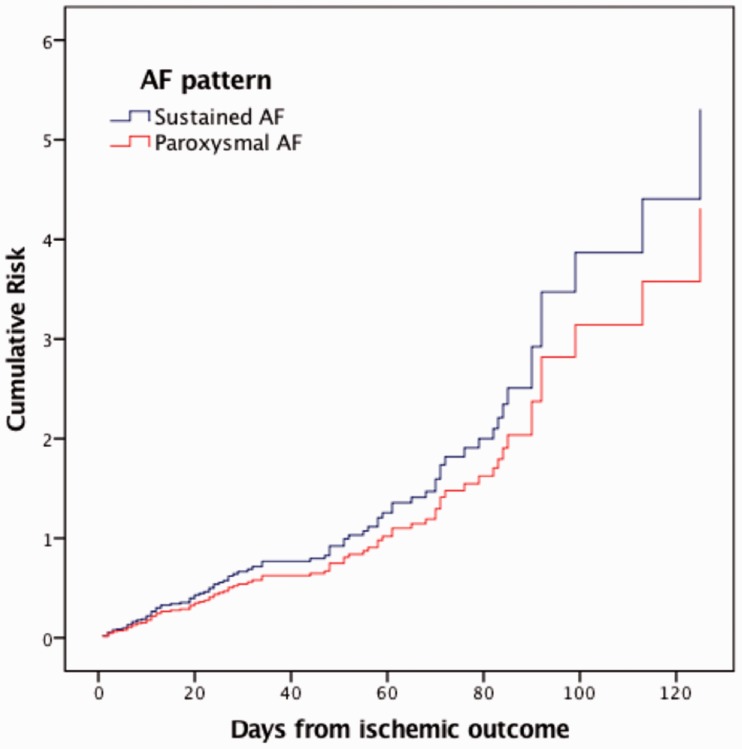
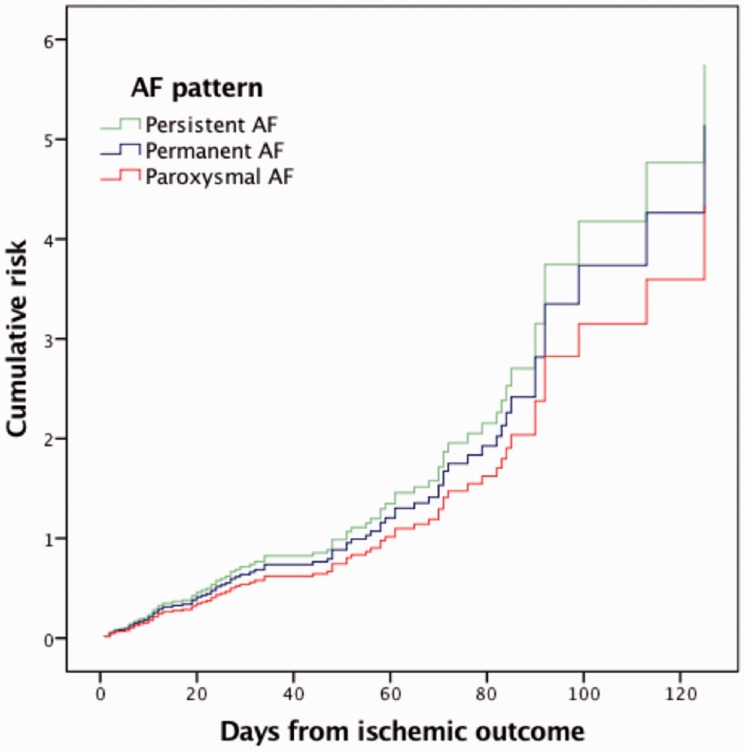
Cox proportional hazard model was adjusted for age, sex, diabetes, hypertension, hyperlipidaemia, history of stroke, current smoking, alcoholism, history of congestive heart failure, myocardial infarction, pacemaker implant, small lesion size and type of anticoagulant. SAF did not result as a risk for ischaemic recurrence when compared with PAF (HR 1.23; 95% CI 0.74–2.04; p = 0.418) (Figure 1). The same result was obtained excluding TIAs from outcomes (HR 1.11; CI 0.60–2.12; p = 0.7). A sensitivity analysis performed to evaluate if our results were consistent when SAF was categorised as either persistent (HR 1.33; 95% CI 0.70–2.51; p = 0.387) or permanent AF (HR 1.12; 95% CI 0.61–2.04; p = 0.715) confirmed that AF pattern is not associated with ischaemic recurrence (Figure 2).
Figure 1.
Adjusted cumulative risk for ischaemic recurrence (stroke, transient ischaemic attack, systemic embolism) in patients with SAF compared with patients with PAF (HR 1.23; 95% CI: 0.74–2.04; p = 0.418). AF: atrial fibrillation; HR: hazard ratio; PAF: paroxysmal atrial fibrillation; SAF: sustained atrial fibrillation.
Figure 2.
Adjusted cumulative risk for ischaemic recurrence in patients with paroxysmal, permanent and persistent AF. AF: atrial fibrillation.
Discussion
Among patients with acute ischaemic stroke, those with SAF had a higher rate of early ischaemic recurrence than patients with PAF. After adjustment for risk factors for early ischaemic recurrence, SAF was not associated with a significantly higher risk of recurrence.
Recent studies have reported a higher risk for ischaemic events in patients with non-paroxysmal AF compared to those with PAF.11–14,17,19–21 The large majority of the patients included in these studies did not have a previous stroke and were evaluated for a long-term follow-up. In contrast, our study was focused on early recurrence in patients with recent ischaemic stroke followed up for 90 days.
The vast majority of patients enrolled in the RAF and RAF-NOAC studies were prescribed with anticoagulation therapy (81.6%) after the index stroke. Anticoagulation reduces the rates of ischaemic outcome and therefore, although our study mirrors clinical practice, it might not reflect the natural history of the disease. Evaluating a group of AF patients in absence of anticoagulation might better reflect the recurrence risk in different types of AF,21 but such a study design would not be feasible in populations at high risk of ischaemic events, like cohorts on secondary prevention.
On average, in this cohort, patients with SAF started OAC therapy two days before those with PAF; this could have influenced the event rate. However, this effect was, in our opinion, marginal.
The EORP-AF General Pilot Registry reported a worse outcome in patients with SAF in comparison to patients with PAF. However, this difference did not seem to be associated to the pattern of the arrhythmia, but to the worse clinical risk profile in terms of age, underlying cardiac disease and other clinical risk factors.29 Likewise, a sub-analysis of the J-RHYTHM Registry reported that thromboembolic events occurred more frequently in the permanent AF group, particularly in patients with higher CHA2DS2-VASc score. In that study, after adjusting for CHA2DS2-VASc score components and anticoagulation treatment, the risk of ischaemic events did not differ between PAF and permanent AF.9 Our present findings in patients on secondary prevention are in line with the results of these two primary prevention studies: in particular, patients with SAF were on average older and were more likely to have diabetes mellitus, hypertension, history of stroke/TIA and congestive heart failure. After adjusting for established risk factors, the early thromboembolic risk in patients with acute ischaemic stroke was not associated with a particular AF pattern. These findings suggest that AF pattern should not influence the decision regarding the timing of initiating anticoagulation treatment after an acute ischaemic stroke.
Study limitations
Our study had several limitations. First, the associations shown in this non-randomised study were most likely influenced by several confounders, although adjusted statistical models were used to partially control them. Second, a central adjudication of the outcome events was not performed but rather these events were assessed by the local investigators. This approach is quite common in investigator-initiated cohort prospective studies and may be justified provided that validated and internationally recognised definitions of events are actually used. This was the case of our study. Third, the inclusion of TIA as ischaemic recurrence, which is considered as a soft endpoint, may have also influenced the reported associations, but sensitivity analysis performed excluding TIAs as outcome gave similar results.
Strengths of our study included the prospective design, the multicentre nature and its relatively large sample size.
Conclusions
Among patients with acute ischaemic stroke, patients with PAF had a lower rate of early ischaemic recurrence than patients with SAF. After adjustment for risk factors for early ischaemic recurrence, SAF was not associated with a significantly higher risk of recurrence. Our results suggest that, when making decision about the timing to initiate anticoagulation treatment after an acute ischaemic stroke, the patient’s risk profile should be given greater relevance than the pattern of AF. These results support the need for additional comparative studies, including randomised trials.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MP received honoraria as a member of the speaker bureau of Sanofi-Aventis, Boehringer Ingelheim, Bayer, Bristol Meyer Squibb and Pfizer. GA received honoraria as a member of the speaker bureau of Boehringer Ingelheim and Bayer. CB received honoraria as a member of the speaker bureau of Bristol Meyer Squibb and Bayer. VC received honoraria as a member of the speaker bureau and as consultant or advisory board of Boehringer Ingelheim. JP received honoraria for lectures related to atrial fibrillation and anticoagulants for Orion Pharma, Bristol Meyer Squibb, Pfizer, Bayer and Boehringer Ingelheim. Turgut Tatlisumak received honoraria as consultant or advisory relationship by Lundbeck and Boehringer Ingelheim. KL reports fees and expenses for data monitoring committee work and lectures from Boehringer Ingelheim. WA has received speaker’s honoraria from, and participated in scientific advisory boards for, Boehringer Ingelheim, Bayer, Bristol-Myers Squibb/Pfizer, and Daiichi Sankyo, and has received research support from Bayer and Boehringer Ingelheim. DT received honoraria as a member of speaker bureau and as advisory board of Boehringer Ingelheim, Pfizer, Bristol Meyer Squibb and Bayer.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
The studies were approved by the local Institutional Review Boards.
Informed consent
Written or verbal informed consent was obtained from the patients for their anonymised information to be published in this article.
Guarantor
MP.
Contributorship
MP and FA contributed equally to this article. MP and FA researched literature, conceived the study, were responsible for data analysis and wrote the first draft of the manuscript. All the authors were involved in protocol development, gaining ethical approval and patient recruitment. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013; 112: 1142–1147. [DOI] [PubMed] [Google Scholar]
- 2.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013; 34: 2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee A, Taillandier S, Olesen JB, et al. Pattern of atrial fibrillation and risk of outcomes: The Loire Valley Atrial Fibrillation Project. Int J Cardiol 2013; 167: 2682–2687. [DOI] [PubMed] [Google Scholar]
- 5.Disertori M, Franzosi MG, Barlera S, et al. Thromboembolic event rate in paroxysmal and persistent atrial fibrillation: data from the GISSI-AF trial. BMC Cardiovasc Disord 2013; 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of atrial fibrillation. Eur Heart J 2010; 31: 967–975. [DOI] [PubMed] [Google Scholar]
- 7.Hart RG, Pearce LA, Rothbart RM, et al. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. J Am Coll Cardiol 2000; 35: 183–187. [DOI] [PubMed] [Google Scholar]
- 8.Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy. An ACTIVE W substudy. J Am Coll Cardiol 2007; 50: 2156–2161. [DOI] [PubMed] [Google Scholar]
- 9.Inoue H, Atarashi H, Okumura K, et al. Thromboembolic events in paroxysmal vs. permanent non-valvular atrial fibrillation. Circ J 2014; 78: 2388–2393. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwlaat R, Dinh T, Olsson SB, et al. Should we abandon the common practice of withholding oral anticoagulation in paroxysmal atrial fibrillation? Eur Heart J 2008; 29: 915–922. [DOI] [PubMed] [Google Scholar]
- 11.Al-Khatib SM, Thomas L, Wallentin L, et al. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J 2013; 34: 2464–2471. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Zhao Y, Dang G, et al. Stroke event rates and the optimal antithrombotic choice of patients with paroxysmal atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015; 94: e2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang CE, Naditch-Brûlé L, Murin J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circulation 2012; 5: 632–639. [DOI] [PubMed] [Google Scholar]
- 14.Deguchi I, Hayashi T, Fukuoka T, et al. Features of cardioembolic stroke with persistent and paroxysmal atrial fibrillation – a study with the Japan Stroke Registry. Eur J Neurol 2015; 22: 1215–1219. [DOI] [PubMed] [Google Scholar]
- 15.Koga M, Yoshimura S, Hasegawa Y, et al. Higher risk of ischemic events in secondary prevention for patients with persistent than those with paroxysmal atrial fibrillation. Stroke 2016; 47: 2582–2588. [DOI] [PubMed] [Google Scholar]
- 16.Lip GYH, Frison L, Grind M. Stroke event rates in anticoagulated patients with paroxysmal atrial fibrillation. J Intern Med 2008; 264: 50–61. [DOI] [PubMed] [Google Scholar]
- 17.Ntaios G, Vemmou A, Koroboki E, et al. The type of atrial fibrillation is associated with long-term outcome in patients with acute ischemic stroke. Int J Cardiol 2013; 167: 1519–1523. [DOI] [PubMed] [Google Scholar]
- 18.Senoo K, Lip GYH, Lane DA, et al. Residual risk of stroke and death in anticoagulated patients according to the type of atrial fibrillation: AMADEUS trial. Stroke 2015; 46: 2523–2528. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J 2015; 36: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takabayashi K, Hamatani Y, Yamashita Y, et al. Incidence of stroke or systemic embolism in paroxysmal versus sustained atrial fibrillation: the Fushimi atrial fibrillation registry. Stroke 2015; 46: 3354–3361. [DOI] [PubMed] [Google Scholar]
- 21.Vanassche T, Lauw MN, Eikelboom JW, et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in active-a and averroes. Eur Heart J 2015; 36: 281–287. [DOI] [PubMed] [Google Scholar]
- 22.January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of Cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol 2014; 64: e1–e76. [DOI] [PubMed] [Google Scholar]
- 23.Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke 2015; 46: 2175–2182. [DOI] [PubMed] [Google Scholar]
- 24.Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-K oral anticoagulants (RAF-NOACs) study. J Am Heart Assoc 2017; 6: e007034. [DOI] [PMC free article] [PubMed]
- 25.Lévy S, Camm AJ, Saksena S, et al. International consensus on nomenclature and classification of atrial fibrillation: a collaborative project of the Working Group on Arrhythmias and the Working Group of Cardiac Pacing of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol 2003; 14: 443–445. [DOI] [PubMed] [Google Scholar]
- 26.Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008; 39: 2249–2256. [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 28.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001; 32: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 29.Boriani G, Laroche C, Diemberger I, et al. Real-world” management and outcomes of patients with paroxysmal vs. non-paroxysmal atrial fibrillation in Europe: The EURObservational Research Programme-Atrial Fibrillation (EORP-AF) General Pilot Registry. Europace 2016; 18: 648–657. [DOI] [PubMed] [Google Scholar]