Abstract
OBJECTIVE:
Asparagus adscendens Roxb. (Liliaceae), a traditional herbal medicine, has been used as an aphrodisiac and brain tonic in Asian countries. The aim of the present study is to investigate the antidepressant-like effect of standardized hydroethanolic extract of A. adscendens root and its possible mechanisms.
MATERIALS AND METHODS:
Mice administered with vehicle, imipramine (15 mg/kg/day; i.p.), and A. adscendens extract (AAE) (25, 50, and 100 mg/kg/day; i.p.) for 14 days were subjected to behavioral tests including forced swimming test (FST), tail suspension test (TST), and open-field test (OFT) on the 14th day. In order to explore the underlying mechanism behind an antidepressant effect of AAE, the brain monoamine levels, oxidative stress parameters, and serum corticosterone levels were monitored.
RESULTS:
Our results indicated that pretreatment of AAE (25, 50, and 100 mg/kg) for 14 days statistically significantly (P < 0.01) demonstrated antidepressant-like effect as evidenced by reduced immobility time in both FST (105, 78.6, and 53.6 s) and TST (97.6, 73.5, and 54.67 s), with no significant change in spontaneous locomotor activities as observed in OFT. Further, the behavioral improvement was supported by the statistically significantly (P < 0.05) enhanced levels of monoamines and reduced corticosterone level along with amelioration of oxidative stress in AAE-treated animals as compared to vehicle control group.
Conclusion:
Our findings clearly demonstrated the antidepressant-like effect of AAE, which might have been mediated through the modulation of monoaminergic system and by regulating hypothalamic–pituitary–adrenal axis with amelioration of oxidative stress.
Keywords: Corticosterone, hypothalamic–pituitary–adrenal axis, monoamines, oxidative stress
Introduction
Depression is a chronic, recurrent psychiatric disorder affecting nearly 21% of the world's population.[1,2] According to the World Health Organization reports, it will become the fourth leading cause of disability worldwide by the year 2020.[3,4] The monoaminergic theory states that depletion of brain neurotransmitters such as 5-hydroxytyrptamine (5-HT), norepinephrine (NE), and dopamine (DA) assumes to play a key role in the pathophysiology of depression.[5] Clinically, at the early stages of depression, noradrenaline reuptake inhibitors (e.g., desipramine and reboxetine) and selective serotonin reuptake inhibitors (SSRIs) (e.g., fluoxetine and citalopram) have been mainly exploited for the monotherapy treatment.[6] However, as the monotherapy ended up with the emergence of resistance to treatment in many patients, inevitably, the combined use of antidepressant drugs with different mechanism becomes an advanced option for the patient's resistance to monotherapy treatment.[7,8] However, the combination of SSRIs with tricyclic antidepressants (TCAs) has been reported to show the drug–drug interactions and undesirable side effects, which may limit the use of the most preferred TCA–SSRI combination.[9] Hence, there is a necessity to develop the newer antidepressant drugs with more safety and efficacy. Medicinal plants could be considered an excellent source for the antidepressant novel drug discovery by virtue of its proven safety and presence of multiple phytoconstituents which may exert synergistic effects. Recently, medicinal plants have been explored as complementary and alternative medicine for the treatment of depression.[10,11]
Asparagus adscendens Roxb. (Liliaceae), commonly known as safed musali or dholi musali, is a climbing herb found mainly in Asian countries.[12] Its medicinal uses have been well documented in various traditional records as an aphrodisiac and brain tonic.[13,14] Literature reveal that roots of A. adscendens have been investigated for antifilarial,[15] antidiabetic,[16] antistress,[17] chemomodulatory,[18] aphrodisiac,[19] antifungal,[20] and antioxidant activities.[18,21,22] Pertaining to the phytochemical analysis, several studies showed the presence of 3-heptadecanone, 3-hexadecenoic acid, methyl pentacosanoate, tetratriacontane, tritriacontane, methyl palmitate, tetracosyl tetracosanoate, palmitic acid, stearic acid, asparanin C, asparanin D, asparoside C, asparoside D, 3-β-O-(β-D-2-tetracosylxylopyranosyl) -stigmasterol, 3-β-O-(β-D-glucopyranosyl (1-2)-α-L-arabinopyranosyl)-stigmasterol, β-sitosterol-β-D-glucoside, Vitamin C, xanthophylls, Vitamin E, β-carotene, and Shatavarin IV.[23,24,25,26,27,28] Moreover, clinically, A. adscendens has been used for the treatment of depression and other neurological disorders, as an ingredient in one of the famous pharmaceutical formulations (Geriforte).[29,30] Despite being a part of clinically used formulation, the A. adscendens per se has never been evaluated for the treatment of depression. Furthermore, earlier studies suggested that many medicinal plants possessing aphrodisiac and antioxidant potential have been found to possess antidepressant activity also.[18,31,32] Hence, the current study was envisaged to explore the antidepressant effect of root extract of A. adscendens and its possible mechanisms.
Materials and Methods
Drugs and chemicals
All standard chemicals used in this study were of analytical grade. Shatavarin IV was procured from Natural Remedies Pvt. Ltd., Bengaluru, India. Serotonin, DA, NE, and Griess reagent were procured from Sigma-Aldrich, Co., St. Louis, MO, USA. Imipramine was obtained from Troikaa Pharmaceuticals, Dehradun, Uttarakhand, India.
Animals
Swiss Albino mice of either sex weighing 20–30 g (3–4 months of age) were purchased from Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, Haryana. The animals were housed on a 12-h light/dark cycle under controlled temperature (22°C ± 2°C) and humidity (50 ± 10%). They were allowed to acclimate for 1 week with access to food and water ad libitum. The procedures in this study were conducted in accordance to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India, and approved by the Institutional Animal Ethical Committee (107/99/CPCSEA-2016-13).
Plant collection and preparation of extract
Roots of A. adscendens were procured from “Council of Scientific and Industrial Research -Institute of Himalayan Bioresource Technology” (CSIR-IHBT), Palampur (Himachal Pradesh), India, and authenticated by Dr. Bikram Singh (CSIR-IHBT), Palampur (Himachal Pradesh) in May–June, 2013 (Voucher no. PLP16565). The authenticated roots were washed with water, shade-dried, and ground to a moderately coarse powder. The powdered roots were subjected to extraction by percolation method with ethanol: water (50:50 v/v) at room temperature. The resultant extract was evaporated to dryness using a rotary evaporator (Buchi Type Rotary Vacuum Evaporator, Axiva, Shanghai, China) followed by lyophilization (Delvac, Chennai, Tamilnadu, India) and stored at 4°C for further use. The percentage yield of the extract was found to be 44.7% w/w.
Phytochemical analysis and standardization of the plant extract using high-performance-thin-layer chromatography
A. adscendens extract (AAE) was subjected to preliminary phytochemical screening tests to determine the presence of alkaloids, carbohydrates, glycosides, saponins, steroids, triterpenoids, flavonoids, tannins, proteins, and amino acids.[33]
Shatavarin IV being one of the constituents present in A. adscendens was used as a biomarker for the standardization of the hydroethanolic extract. The presence of Shatavarin IV in the hydroethanolic extract was confirmed by using high-performance-thin-layer chromatography (HPTLC) method as reported by Gohel et al.[34] Initially, the presence of Shatavarin IV was confirmed by TLC using ethyl acetate: methanol: water (7.5:1.5:0.5, v/v/v) as a mobile phase. The plate was derivatized by spraying with freshly prepared anisaldehyde in sulfuric acid and then dried in an oven at 100°C for 10 min. Further, A. adscendens was standardized with Shatavarin IV using HPTLC. A stock solution of hydroethanolic extract (30 mg/mL) and Shatavarin IV (1 mg/mL) was prepared in methanol. The mobile phase and spraying reagent for developing the chromatogram were same as that used in TLC. Detection was done by the measurement of absorbance at 426 nm. The study was carried out using precoated silica gel aluminum plate 60F254(20 cm × 20 cm, E. Merck, Germany); Camag-HPTLC instrumentation (Camag, Muttenz, Switzerland) equipped with Camag Linomat V sample applicator, Camag TLC scanner IV and CAMAG Win CATS (1.4.8.2031, Camag, Muttenz, Switzerland) for data interpretation. The Rf values were recorded, and the developed plate was screened at a wavelength (λmax) of 426 nm. Standard curve was prepared with Shatavarin IV (y = 2.239x + 712.7) with a regression coefficient (R2) of 0.997.
Acute toxicity test of the extract
The AAE was administered to six different groups of mice (n = 6) at different doses, up to ten times of effective dose, i.e., 25, 50, and 100 mg/kg; i.p. One group served as vehicle control and received equal volume of vehicle. Percentage mortality and gross behavioral changes were observed during 24 h after treatment.[35,36]
Experimental protocol
Six groups of animals (n = 6) were investigated. Group I: naive (incorporated for the biochemical studies only); Group II: saline (vehicle control) (10 mL/kg/day, i.p.); Group III: imipramine (standard drug) (15 mg/kg/day, i.p.);[10] Groups IV, V, and VI: AAE(25, 50, and 100 mg/kg/day, i.p., respectively) (based on the results of pilot studies carried out on limited number of animals, three doses were selected for the current study). AAE and imipramine were dissolved in normal saline. The animals were treated for 14 days with respective treatments. The behavioral assessment was made after half an hour of AAE (25, 50, and 100 mg/kg) and imipramine (15 mg/kg) administration on 14th day. The animals were sacrificed by decapitation, and their brains were removed after behavioral assessments. The regions of brain (cortex and hippocampus) were isolated immediately and then stored at − 80°C for later analysis. The blood was immediately collected, separated, and centrifuged at 4°C. Serum was stored at − 20°C until assay was performed.
Behavioral assessments
Forced swimming test
The modified forced swimming test (FST) was conducted on the mice as reported previously,[37,38] with slight modification. Briefly, mice were individually placed in a glass cylinder (25 cm in height, 14 cm in diameter) filled with 20-cm high water (25°C ± 2°C), and the duration of immobility was observed for 6 min (out of which 2 min is for habituation). The test session was recorded by a video camera and scored by a blind observer. During the test session, some behavioral parameters were recorded in seconds such as immobility time (i.e., cessation of limb movement necessary to keep the mouse afloat), climbing time (i.e., upward directed movements of the forepaws along the side of the swim chamber), and swimming time (i.e., movement usually horizontal throughout the swim chamber).
Tail suspension test
The tail suspension test (TST) was conducted as previously described,[37,39] with some modifications. Briefly, mice were individually suspended by tail with a clamp (1 cm from the tip of the tail). Testing was carried out in a noise-free room. A mouse was suspended for a total of 6 min, and the duration of immobility was recorded during the final 4-min interval of the test. Mice were considered immobile only when they hung passively and completely motionless. The test session was recorded by a video camera and scored by a blind observer.
Open-field test
To assess the possible effects of the extract on locomotor and exploratory behaviors, mice were individually evaluated in the open field paradigm.[9,40] The apparatus consisted of a wooden box measuring 40 cm × 40 cm × 30 cm, with the floor divided into 25 equal squares (8 cm × 8 cm). The number of squares crossed with all paws (crossings) and of standing on the hind legs (rearing) was observed for 5 min after 1 min acclimatization in each session. The apparatus was cleaned with 10% alcohol to prevent auditory cue. The test session was recorded by a video camera and scored by a blind observer.
Estimation of monoamines using high-performance liquid chromatography with electrochemical detection
The levels of brain monoamine neurotransmitters were measured by high-performance liquid chromatography with electrochemical detection (Zapata et al., 2009, with slight modification).[41] Briefly, the brain samples were homogenized in an ice-cold solution of 0.1 M perchloric acid and then centrifuged at 14,500 g for 30 min at 4°C. The clear supernatants were filtered through a 0.45-μm pore membrane. Standard solution or samples were injected into the column (5 μm, 250 mm × 4.6 mm). The separation was done in an isocratic elution mode at a column temperature of 35°C using mobile phase consisting of 0.15 M sodium dihydrogen phosphate, 2.28 mM heptane sulphonic acid, and 1 mM ethylenediaminetetraacetic acid (pH 5.4), containing 10% methanol at a flow rate of 0.9 mL/min. The contents of monoamine neurotransmitters were expressed in ng/g wet weight tissue.[42] The order of elution of monoamines in the chromatogram was norepineprine (6.481 min), DA (15.437 min), and 5-HT (38.144 min). The amount of monoamines in standard and samples was quantified using area under the curve of the corresponding sample using their straight line equation: NE (y = 13363x − 12625; R2= 0.998); DA (y = 45909x − 26031; R2= 0.997); and 5-HT (y = 55131x + 38031; R2= 0.998).
Biochemical estimation
The brain samples were homogenized in an ice-cold 10% w/v (0.05 M, pH 7.4) phosphate buffer and then centrifuged at 6000 g for 20 min at 4°C. The clear supernatant obtained was used for the estimation of thiobarbituric acid-reactive substances (TBARS), reduced glutathione (GSH), catalase (CAT), and total nitrite levels.
Estimation of thiobarbituric acid-reactive substance
The quantitative measurement of TBARS, an index of lipid peroxidation, was performed using the method described by Oakes and Van der Kraak with slight modifications. The absorbance was measured at 532 nm using a microplate reader (BIORAD-Microplate Reader, Logotech, ISE Group, Germany). A standard calibration curve was prepared using 1–10 nM of 1,1,3,3-tetra methoxy propane (y = 0.0105x + 0.0562; R2= 0.992). TBARS value was expressed as nanomoles/mg of protein.[43]
Estimation of reduced glutathione
The reduced GSH content in the brain was estimated by using the method described by Beutler et al. with slight modifications. Absorbance was measured using a microplate reader (BIORAD-Microplate Reader, Logotech, ISE Group, Germany) at 412 nm. Reduced GSH standard curve was plotted using a 10–100 μM of reduced GSH. The results were calculated using the following equation: y = 0.001x + 0.085; R2= 0.994. All the values were expressed as micromoles per gram of wet tissue.[44]
Estimation of catalase
CAT activity was determined by using the modified method of Chance and Maehly CAT activity was calculated by using the molar extinction coefficient of H2O2(0.071 mM/cm) and was expressed as micromoles of H2O2 oxidized per minute per milligram protein.[45]
Estimation of total nitrite level
The total nitrite level was estimated employing the method described by Choudhary et al. Absorbance was noted using a microplate reader (BIORAD-Microplate Reader, Logotech, ISE Group, Germany) at 540 nm. The standard curve was plotted using 10–100 mM of sodium nitrite. The results were calculated using the following equation: y = 0.0472x + 0.1034; R2= 0.9982). All the values were expressed as ng/g of wet tissue.[46]
Serum corticosterone measurement
The corticosterone level was assessed by employing the modified method described by Kumar et al. and Lim et al. using spectrofluorometer (Perkin Elmer, US) at 475 nm excitation and 525 nm emissions. The standard curve was plotted using 10–100 ng of corticosterone. The results were calculated using the following equation: y = 0.785x + 8.777; R2= 0.992). All the values were expressed as ng/mL.[47,48]
Statistical analysis
Statistical analysis of significance was carried out by using one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls post hoc multiple comparison test using GraphPad prism® version 5 (Graph-Pad Software Inc., San Diego, CA, USA).
Results
Phytochemical analysis and standardization of plant extract
Preliminary phytochemical screening confirmed the presence of carbohydrates, glycosides, flavanoid, saponins, steroids, triterpenoids, and amino acids in the hydroethanolic extract. The HPTLC analysis depicted well-resolved peaks of A. adscendens showing the presence of Shatavarin IV. The spots of the entire chromatogram were visualized at 426 nm, and the quantity of Shatavarin IV was found to be 9.4 mg/kg of the hydroethanolic extract [Supplementary Figure 1A and B (219.4KB, tif) ].
Acute toxicity studies
In acute toxicity test, no mortality was observed in animals treated with the extract at all doses. There was no change in the behavior of animals during 24 h of administration of the extract. The extract was found to have no neurotoxic effects with respect to disturbances in motor coordination up to the highest dose (i.e., 100 mg/kg).
Effect of Asparagus adscendens extract on forced swimming test
The results illustrated in Figure 1A show the effect of AAE on the duration of immobility time in the FST model. One-way ANOVA revealed that there were significant differences between the treatment groups (F4,25]= 20.03, P < 0.0001). Student–Newman–Keuls test showed that AAE (25, 50, and 100 mg/kg) significantly decreased the duration of immobility time as compared to vehicle control group.
Figure 1.
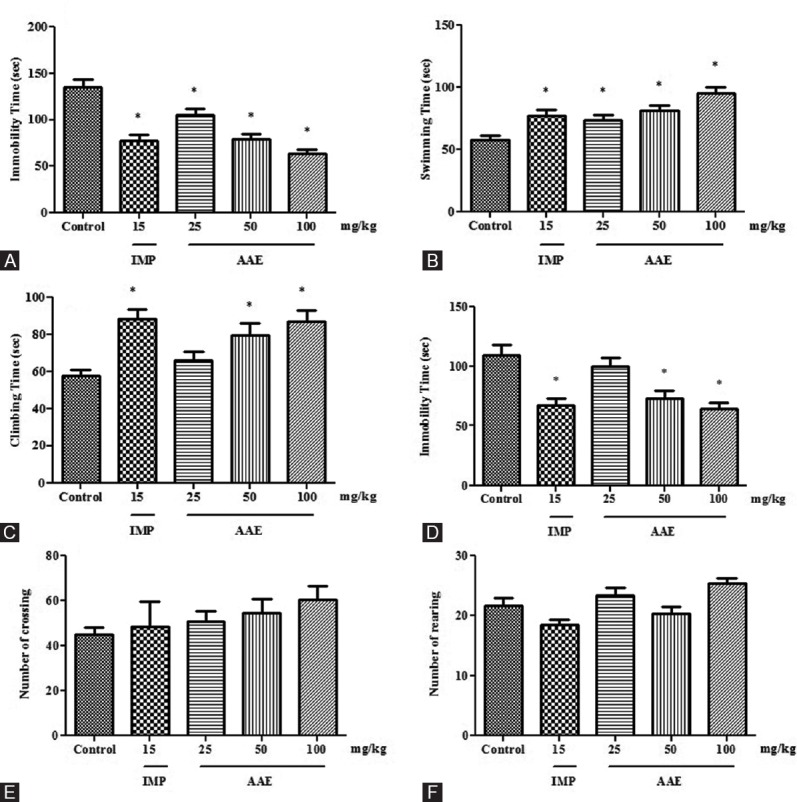
Effect of Asparagus adscendens extract treatment on the behaviors in mice. Immobility (panel A), swimming (panel B), and climbing (panel C) time in the modified forced swimming test. Immobility time (panel D) in the tail suspension test. Crossing (panel E) and rearing (panel F) numbers in the open-field test. Values were expressed as mean ± standard error of the mean. The significance level was considered as P< 0.05 (Student–Newman–Keuls test) * = As compared with naïve animals. IMP 15 = Imipramine 15 mg/kg; i.p. AAE 25, 50, and 100 =Asparagus adscendens extract 25, 50, and 100 mg/kg; i.p., respectively
Treatment with AAE revealed the significant effect on the swimming time in the modified FST (F4,25]= 8.956, P < 0.0001). The extract significantly increased the swimming time at 50 and 100 mg/kg as compared to vehicle control group [Figure 1B]. Moreover, imipramine also elicited the swimming time after administration of 15 mg/kg dose.
A significant effect of the treatment on the climbing time was seen in modified FST [Figure 1C]. AAE significantly increased the climbing time at 50 and 100 mg/kg when compared to vehicle control group (F4,25]= 6.637, P = 0.0009). Similarly, imipramine also increased the climbing time in the test.
Effect of Asparagus adscendens extract on tail suspension test
The results illustrated in Figure 1D show the effect of AAE on the duration of immobility time in the tail suspension model. One-way ANOVA revealed a significant effect of the treatment on the immobility time in the TST (F4,25]= 8.817, P = 0.0001). Student–Newman–Keuls test indicated a significant decrease in the immobility time attenuated by the administration of AAE at 50 and 100 mg/kg. Moreover, imipramine also decreased the immobility time after administration of 15 mg/kg dose.
Effect of Asparagus adscendens extract on open-field test
No significant effect of the AAE treatment (25, 50, and 100 mg/kg) was observed on the number of crossing [Figure 1E] and rearing [Figure 1F] as compared to that of the vehicle control group in this test.
Effect of Asparagus adscendens extract on monoamine neurotransmitters levels in cortical and hippocampal areas of mouse brain
Changes in norepinephrine levels
A significant difference observed in the concentration of NE in both cortical (F5,30]= 17.48, P < 0.0001) and hippocampal (F5,30)= 9.479, P < 0.0001) areas in between the groups [Figure 2A and B]. Student–Newman–Keuls test indicated a significant decrease in the cortical and hippocampal NE levels of vehicle control as compared to naive group. However, a significant increase in cortical and hippocampal NE levels was observed in AAE-treated animals, whereas no significant change was observed in imipramine-treated group as compared to vehicle control group. Moreover, the cortical and hippocampal NE levels increased significantly only at AAE (100 mg/kg) dose as compared to vehicle control group.
Figure 2.
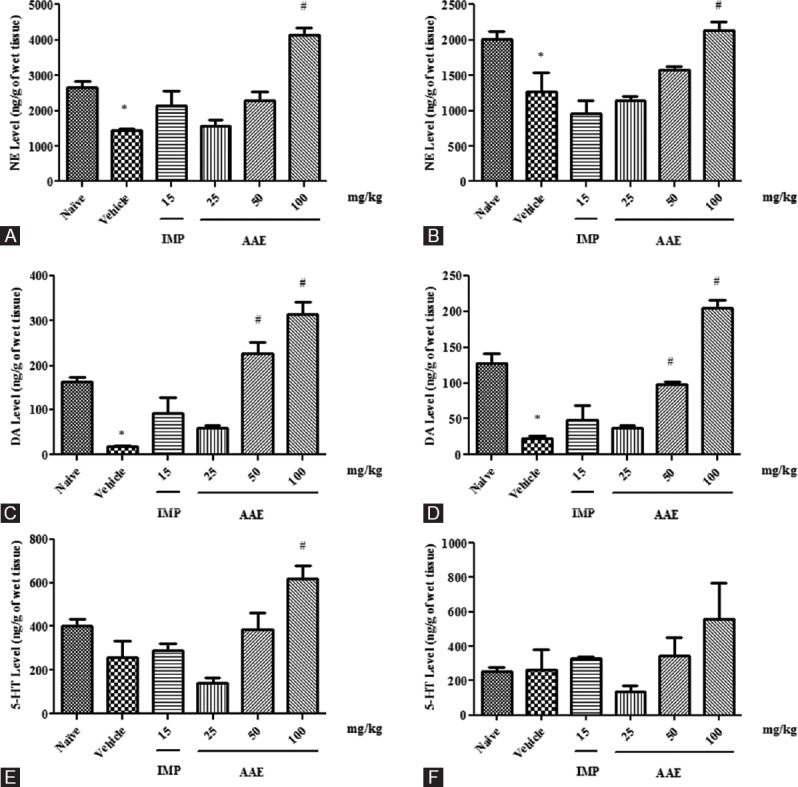
Effect of Asparagus adscendens extract treatment on monoamine neurotransmitters. Norepinephrine level cortex (panel A) and hippocampus (panel B); Dopamine level cortex (panel C) and hippocampus (panel D); 5-hydroxytyrptamine level cortex (panel E); and hippocampus (panel F) in (ng/g of wet tissue). Values were expressed as mean ± standard error of the mean. The significance level was considered as P< 0.05 (Student–Newman–Keuls test) * = As compared with naïve; # = As compared with vehicle control; IMP 15 = Imipramine 15 mg/kg; i.p. AAE 25, 50, and 100 =Asparagus adscendens extract 25, 50, and 100 mg/kg; i.p., respectively
Changes in dopamine levels
There was a significant difference between the groups in the concentration of DA in cortical (F5,30]= 25.78, P < 0.0001) and hippocampal (F5,30]= 38. 47, P < 0.0001) areas in between the groups. Student–Newman–Keuls test suggested a significant decrease in cortical and hippocampal DA levels of vehicle control as compared to naive group. However, a significant increase was observed in the cortical and hippocampal DA levels of AAE-treated groups as compared to vehicle control group. Moreover, no significant change in DA level was recorded in imipramine-treated group as compared to vehicle control group [Figure 2C and D].
Changes in serotonin levels
There was a statistically significant difference in the concentration of serotonin in the cortex (F5,30]= 9.044, P < 0.0001), whereas no significant change was observed in hippocampal serotonin level in between the groups. Student–Newman–Keuls test revealed a significant decrease in cortical serotonin level of vehicle control as compared to naive group. However, a significant increase was observed in cortical serotonin level of AAE-treated groups as compared to vehicle control group. Moreover, no significant change in serotonin level was recorded compared in imipramine-treated group to vehicle control group [Figure 2E and F].
Effect of Asparagus adscendens extract on lipid peroxidation, reduced glutathione, catalase, and total nitrite levels in cortical and hippocampal areas of mouse brain
There was a significant difference in lipid peroxidation level in the cortical (F5,30]= 22.65, P < 0.0001) and hippocampal (F5,30]= 17.86, P < 0.0001) areas in between the groups. Student–Newman–Keuls test suggested a significant increase in the cortical and hippocampal lipid peroxidation levels of vehicle control as compared to naive group. However, a significant decrease was observed in cortical and hippocampal lipid peroxidation levels of AAE-treated group as compared to vehicle control group. Similarly, a significant decrease in cortical and hippocampal lipid peroxidation levels was observed in imipramine-treated group as compared to vehicle control group [Table 1].
Table 1.
Effect of Asparagus adscendens extract treatment on oxidative parameters in the cortex and hippocampus
| Treatment | TBARS (nmol/mg protein) | Reduced GSH (µmol/g of wet tissue) | Catalase (µ moles of H2O2 oxidized/mg of protein) | Nitrite level (ng/g of wet tissue) |
|---|---|---|---|---|
| Cortex | ||||
| Naïve | 10.26±0.82 | 48.33±4.04 | 2.87±0.26 | 277±21.28 |
| Vehicle control | 25.24±1.11* | 22.91±1.36* | 1.69±0.15* | 825±5.14* |
| IMP 15 mg/kg | 13.05±1.10# | 30.84±1.15 | 2.14±0.14 | 521±9.62# |
| AAE 25 mg/kg | 19.88±0.81# | 21.92±2.47 | 1.59±0.14 | 712.3±12.4# |
| AAE 50 mg/kg | 15.35±2.07# | 25.24±1.71 | 2.12±0.12 | 626.83±12.94# |
| AAE 100 mg/kg | 10.87±0.89# | 35.12±3.06$ | 2.99±0.07# | 472±20.57# |
| Hippocampus | ||||
| Naïve | 10.12±1.03 | 42.66±5.06 | 2.58±0.23 | 242.66±17.17 |
| Vehicle control | 22.33±1.56* | 21.25±1.59* | 1.61±0.13* | 814.16±8.41* |
| IMP 15 mg/kg | 11.73±1.11# | 28.45±2.15 | 2±0.04 | 529±15.68# |
| AAE 25 mg/kg | 18.62±0.97# | 22.48±1.96 | 1.32±0.08 | 718.2±15.12# |
| AAE 50 mg/kg | 14.67±0.79# | 24.11±1.45 | 1.86±0.05 | 605.33±12.31# |
| AAE 100 mg/kg | 12.50±0.94# | 33.18±2.94ψ | 2.76±0.09# | 513±10.88# |
Values were expressed as mean±SEM. The significance level was considered as P<0.05 (Student-Newman-Keuls test). *As compared with Naïve, #As compared with vehicle control. Vehicle control=Saline; intraperitoneal, IMP 15=Imipramine 15 mg/kg; intraperitoneal, AAE 25, 50, and 100=Asparagus adscendens extract 25, 50, and 100 mg/kg; i.p., SEM=Standard error of mean, TBARS=Thiobarbituric acid-reactive substance, GSH=Glutathione, $As compared to AAE 25 mg/kg; ψAs compared to AAE 50 mg/kg
There was a statistically significant difference in reduced GSH level in the cortical (F5,30]= 15.71, P < 0.0001) and hippocampal (F5,30]= 8.251, P < 0.0001) areas in between the groups. Student–Newman–Keuls test suggested a significant decrease in cortical and hippocampal reduced GSH levels of vehicle control as compared to naive group. However, a significant increase was observed in cortical and hippocampal reduced GSH levels of AAE-treated groups as compared to vehicle control group. There was no significant change observed in cortical and hippocampal reduced GSH levels in imipramine-treated group as compared to vehicle control group [Table 1].
One-way ANOVA showed that CAT level was significantly different in both cortical (F5,30]= 19.33, P < 0.0001) and hippocampal (F5,30]= 12.79, P < 0.0001) areas in between the groups. Student–Newman–Keuls test revealed a significant decrease in cortical and hippocampal CAT levels of vehicle control as compared to naive group. However, a significant increase in the CAT levels was elicited by the administration of AAE in both cortical and hippocampal areas as compared to vehicle control group. There was no significant change observed in cortical and hippocampal CAT levels in imipramine-treated group as compared to vehicle control group [Table 1].
One-way ANOVA showed that total nitrite levels were significantly different in both cortical (F5,30]= 169.7, P < 0.0001) and hippocampal (F5,30]= 210.6, P < 0.0001) areas in between the groups. Student–Newman–Keuls test revealed a significant increase in cortical and hippocampal total nitrite levels of vehicle control as compared to naive group. However, a significant decrease in the total nitrite levels was attenuated by the administration of AAE in both cortex and hippocampus as compared to vehicle control group. Similarly, imipramine also significantly decreased the total nitrite levels in both cortex and hippocampus as compared to vehicle control group [Table 1].
Effect of Asparagus adscendens extract on serum corticosterone level
There was statistically significant difference in the serum corticosterone level (F5,30]= 14.39, P < 0.0001) in between the groups. Student–Newman–Keuls test showed an increase in serum corticosterone level of vehicle control as compared to naive group. However, AAE (25, 50, and 100 mg/kg) significantly decreased the serum corticosterone level in a dose-dependent manner as compared to vehicle control group [Figure 3]. Similarly, imipramine also decreased the serum corticosterone level as compared to vehicle control group.
Figure 3.
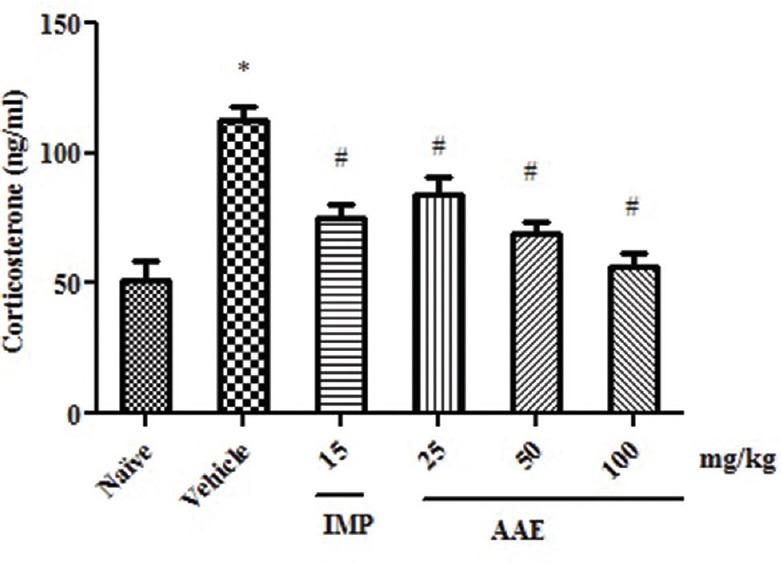
Effect of Asparagus adscendens extract treatment on serum corticosterone levels. Values were expressed as mean ± standard error of the mean. The significance level was considered as P< 0.05 (Student–Newman–Keuls test) * = As compared with naïve; # = As compared with vehicle control
Discussion
This is the first attempt, to our knowledge, to scientifically screen the antidepressant effect of hydroethanolic root extract of A. adscendens. The results of the current study demonstrate the significant antidepressant-like effect of AAE as observed in the behavioral tests on mice showing its involvement on monoaminergic neurotransmitters, oxidative stress markers, and serum corticosterone levels.
The behavioral stress paradigms are most widely used to assess antidepressant effect because of their reliability, ease of use, sensitivity, and specificity.[49,50] Both TST and FST are most widely accepted behavioral tools with good predictive value for assessing pharmacological antidepressant activity, besides the limitation of assessing only monoaminergic mechanisms.[51,52] In both the models, animals were placed in an inescapable situation and became immobile, resembling that behavioral despair represents a condition that mimics human depression.[53,54] Moreover, the well-known antidepressants were found to be effective in both the models as evidenced by decreased immobility time.[50,55] In line with this, our data showed that treatment with AAE reduced the duration of immobility in the FST and TST, with peak effect observed at 100 mg/kg in both the models. Furthermore, the swimming and climbing time were also increased after the treatment with AAE in mice. The psychostimulant agents such as caffeine, decreases the immobility time similar to antidepressant drugs but causes the motor stimulation. Therefore, to rule out this false positive result, we had evaluated the locomotor activity in Open Field test (OFT).[56,57,58] However, none of the test doses affect the locomotor activity. Thus, the results indicated that anti-immobility effect of AAE might not be attributed to any psychostimulant effect as evidenced in OFT.
The classic depression hypothesis of monoamines postulates that the major neurochemical process in depression is the impairment of monoamine neurotransmitter levels.[59] It has been recognized as a multifactorial disease with changes in many different biochemical parameters involving not only monoamines levels, but also causing the hyperactivity of hypothalamic–pituitary–adrenal (HPA) axis along with oxidative damage by inducing an imbalance between the in vivo pro-oxidant and antioxidant status.[60] In line to this, several human studies have shown that depression causes HPA dysfunction followed by elevated corticosterone levels and reduced monoamines levels along with some behavioral and biochemical changes that are characteristic of depression.[61,62] Thus, this prompted us to investigate the above factors in the current study.
Enhancement of 5-HT and NE levels in AAE-treated animals complements the observed antidepressant effect. In addition, these neurotransmitters have also involved in the expression of antidepressant effect in behavioral despair models of depression[63,64] as described by Detke et al. The antidepressant drugs enhanced swimming and climbing acts through serotonergic neurotransmission, such as fluoxetine, and catecholaminergic neurotransmission, such as desipramine,[37] respectively. In the current study, our results showed enhanced swimming and climbing time in AAE-treated animals, indicating that the antidepressant-like effect might be mediated through both serotonergic and catecholaminergic neurotransmissions. The above findings were further confirmed by enhanced monoamines levels, namely, NE, DA, and 5-HT in the cortex and hippocampus of AAE-treated animals as compared to vehicle control group. Moreover, imipramine also enhanced the levels of monoamines as compared to vehicle control group. Similarly, our results are corroborated with earlier evidence which demonstrated that conventional antidepressant drugs produced their pharmacological effect through enhancement of noradrenergic, serotonergic, and/or dopaminergic neurotransmission.[65] Hence, the antidepressant activity produced by AAE treatment might be due to enhanced monoaminergic levels.
In addition to monoaminergic system, a role of oxidative stress has also been reported in depression. A numerous studies have demonstrated that free radical generation increased during depression, which play an important role in the pathogenesis of depression.[32] Interestingly, several medicinal plants showing antidepressant activity such as Bacopa monniera Wettst. (Scrophulariaceae)[66] and Withania somnifera Dunal (Solanaceae)[67] have been reported to possess antidepressant effect due to its ameliorative antioxidant activity. Moreover, A. adscendens has already known for its antioxidant potential.[18] Thus, we evaluated the oxidative parameters in this study which might be having an ameliorative effect on depression. Based on our results, AAE significantly increased the activity of CAT and reduced GSH levels in both cortex and hippocampus. Additionally, it also showed significant reduction in the level of lipid peroxidation marker (MDA) and total nitrite levels in both cortex and hippocampus. Thus, the observed antidepressant effect of AAE might be supported by its antioxidant effect.
Hyperactivation of HPA axis is another additional pathogenic factor for depression.[68,69] Furthermore, several studies have been well documented for the increase in corticosterone levels in response to overactivation of HPA axis, which correlated with behavioral alterations observed in depressed patients including anxiety, fear, and other psychological alterations.[70] In this study, our data suggest that AAE exerted antidepressant effect, at least in part, by downregulating the corticosterone levels, thus normalizing the HPA hyperactivity. Similarly, imipramine also decreased the corticosterone level by regulating the HPA axis hyperactivity.
Conclusion
The current study has demonstrated an antidepressant-like effect of AAE by facilitating monoaminergic neurotransmission and also by regulating HPA axis along with amelioration of oxidative stress.
Financial support and sponsorship
Financial assistance for this study was provided by the CSIR, Pusa, New Delhi, India (Vide F. No. 38 (1339)/12/EMR-II).
Conflicts of interest
There are no conflicts of interest.
Representative high-performance-thin-layer chromatography chromatograms of a standard, Shatavarin IV (a) and hydroethanolic extract of Asparagus adscendens (b)
Acknowledgment
The authors are deeply grateful to the CSIR, Pusa, New Delhi, India, for providing financial assistance (Vide F. No. 38 (1339)/12/EMR-II) for the current study.
References
- 1.Gu X, Zhou Y, Wu X, Wang F, Zhang CY, Du C, et al. Antidepressant-like effects of auraptenol in mice. Sci Rep. 2014;4:4433. doi: 10.1038/srep04433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. 2014;99:181–97. doi: 10.3945/ajcn.113.069880. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. The World Health Report 2001: Mental Health: New Understanding, New Hope. World Health Organization. 2001. [Last accessed on 2016 Jun 16]. Available from: http://www.who.int/whr/2001/en/
- 4.Wang QS, Ding SL, Mao HP, Cui YL, Qi XJ. Antidepressant-like effect of ethanol extract from Zuojin Pill, containing two herbal drugs of Rhizoma Coptidis and Fructus Evodiae, is explained by modulating the monoaminergic neurotransmitter system in mice. J Ethnopharmacol. 2013;148:603–9. doi: 10.1016/j.jep.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Patel JS, Galani VJ. Investigation of noradrenaline and serotonin mediated antidepressant action of Mucuna pruriens (L) D.C seeds using various experimental models. Orient Pharm Exp Med. 2013;13:143–8. [Google Scholar]
- 6.Dhingra D, Joshi P, Gupta A, Chhillar R. Possible involvement of monoaminergic neurotransmission in antidepressant-like activity of Emblica officinalis fruits in mice. CNS Neurosci Ther. 2012;18:419–25. doi: 10.1111/j.1755-5949.2011.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68(Suppl 8):17–25. [PubMed] [Google Scholar]
- 8.Richelson E. Multi-modality: A new approach for the treatment of major depressive disorder. Int J Neuropsychopharmacol. 2013;16:1433–42. doi: 10.1017/S1461145712001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishola IO, Ochieng CO, Olayemi SO, Jimoh MO, Lawal SM. Potential of novel phytoecdysteroids isolated from Vitex doniana in the treatment depression: Involvement of monoaminergic systems. Pharmacol Biochem Behav. 2014;127:90–100. doi: 10.1016/j.pbb.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Singh GK, Garabadu D, Muruganandam AV, Joshi VK, Krishnamurthy S. Antidepressant activity of Asparagus racemosus in rodent models. Pharmacol Biochem Behav. 2009;91:283–90. doi: 10.1016/j.pbb.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Pan SY, Zhou SF, Gao SH, Yu ZL, Zhang SF, Tang MK, et al. New perspectives on how to discover drugs from herbal medicines: CAM's outstanding contribution to modern therapeutics. Evid Based Complement Alternat Med. 2013;2013:627375. doi: 10.1155/2013/627375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan I, Nisar M, Khan N, Saeed M, Nadeem S, Fazal-ur-Rehman, et al. Structural insights to investigate conypododiol as a dual cholinesterase inhibitor from Asparagus adscendens. Fitoterapia. 2010;81:1020–5. doi: 10.1016/j.fitote.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Devi U, Sharma P, Rana JC. Assessment of ethnomedicinal plants in Shivalik hills of Northwest Himalaya, India. Am J Ethnomed. 2014;1:186–205. [Google Scholar]
- 14.Yaseen G, Ahmad M, Sultana S, Suleiman Alharrasi A, Hussain J, Zafar M, et al. Ethnobotany of medicinal plants in the Thar Desert (Sindh) of Pakistan. J Ethnopharmacol. 2015;163:43–59. doi: 10.1016/j.jep.2014.12.053. [DOI] [PubMed] [Google Scholar]
- 15.Singh R, Khan NU, Singhal KC. Potential antifilarial activity of roots of Asparagus adscendens roxb, against Setaria cervi in vitro. Indian J Exp Biol. 1997;35:168–72. [PubMed] [Google Scholar]
- 16.Mathews JN, Flatt PR, Abdel-Wahab YH. Asparagus adscendens (Shweta Musali) stimulates insulin secretion, insulin action and inhibits starch digestion. Br J Nutr. 2006;95:576–81. doi: 10.1079/bjn20051650. [DOI] [PubMed] [Google Scholar]
- 17.Kanwar AS, Bhutani KK. Effects of Chlorophytum arundinaceum, Asparagus adscendens and Asparagus racemosus on pro-inflammatory cytokine and corticosterone levels produced by stress. Phytother Res. 2010;24:1562–6. doi: 10.1002/ptr.3218. [DOI] [PubMed] [Google Scholar]
- 18.Singh M, Singh S, Kale RK. Chemomodulatory potential of Asparagus adscendens against murine skin and forestomach papillomagenesis. Eur J Cancer Prev. 2011;20:240–7. doi: 10.1097/CEJ.0b013e3283447410. [DOI] [PubMed] [Google Scholar]
- 19.Bansode FW, Arya KR, Singh RK, Narender T. Dose-dependent effects of Asparagus adscendens root (AARR) extract on the anabolic, reproductive, and sexual behavioral activity in rats. Pharm Biol. 2015;53:192–200. doi: 10.3109/13880209.2014.913295. [DOI] [PubMed] [Google Scholar]
- 20.Khattak AA, Ahmad A, Naeem R, Sohaib M, Bilal M, Iqbal A, et al. Extracts from Asparagus adscendens exhibit potential antifungal activity. J Appl Environ Biol Sci. 2014;4:47–54. [Google Scholar]
- 21.Guleria S, Tiku AK, Singh G, Koul A, Gupta S, Rana S. In vitro antioxidant activity and phenolic contents in methanol extracts from medicinal plants. J Plant Biochem Biotechnol. 2013;22:9–15. [Google Scholar]
- 22.Surveswaran S, Cai YZ, Corke H, Sun M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007;102:938–53. [Google Scholar]
- 23.Sharma OP, Kumar N, Singh B, Bhat TK. An improved method for thin layer chromatographic analysis of saponins. Food Chem. 2012;132:671–4. doi: 10.1016/j.foodchem.2011.10.069. [DOI] [PubMed] [Google Scholar]
- 24.Sharma SC, Chand R, Bhatti BS, Sati OP. New oligospirostanosides and oligofurostanosides from Asparagus adscendens roots. Planta Med. 1982;46:48–51. doi: 10.1055/s-2007-970018. [DOI] [PubMed] [Google Scholar]
- 25.Tandon M, Shukla YN, Thakur RS. Steroid glycosides from Asparagus adscendens. Phytochemistry. 1990;29:2957–9. [Google Scholar]
- 26.Tandon M, Shukla YN, Thakur RS. Constituents of Asparagus adscendens. Fitoterapia. 1990;61:473. [Google Scholar]
- 27.Jadhav AN, Bhutani KK. Steroidal saponins from the roots of Asparagus adscendens roxb and Asparagus racemosus willd. Indian J Chem. 2006;45B:1515–24. [Google Scholar]
- 28.Prashad D, Moulekhi K, Bisht G. Preliminary investigation on antioxidant phytochemical in some medicinal plants of Kumaon region. Res J Phytochem. 2014;8:199–204. [Google Scholar]
- 29.Malhotra SK. A study of the efficacy of Geriforte in depressive disorders. Med Surg. 1986;6:21–5. [Google Scholar]
- 30.Velmurugendran CU, Virudhagirinatha BS. Geriforte in neurological disorders. Probe. 1980;1:13–7. [Google Scholar]
- 31.Oketch-Rabah HA. Mondia whitei, a medicinal plant from Africa with aphrodisiac and antidepressant properties: A review. J Diet Suppl. 2012;9:272–84. doi: 10.3109/19390211.2012.726704. [DOI] [PubMed] [Google Scholar]
- 32.Bilici M, Efe H, Köroǧlu MA, Uydu HA, Bekaroǧlu M, Deǧer O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 33.Trease G, Evans SM. Pharmacognosy. 15th ed. London, UK: Bailer Tindal; 2002. [Google Scholar]
- 34.Gohel R, Solanki B, Gurav N, Patel G, Patel B. Isolation and characterization of Shatavarin IV from root of Asparagus racemosus willd. Int J Pharm Pharm Sci. 2015;7:362–5. [Google Scholar]
- 35.Pahwa P, Goel RK. Absence of anticonvulsant activity in Asparagus adscendens Roxb. hydroethanolic root extract against acute pentylenetetrazol and maximal electroshock-induced convulsions mice models. J Pharm Negat Results. 2016;7:25–8. [Google Scholar]
- 36.Sonavane GS, Palekar RC, Kasture VS, Kasture SB. Anticonvulsant and behavioral actions of Myristica fragrans seeds. Indian J Pharmacol. 2002;34:332–8. [Google Scholar]
- 37.Li J, Geng D, Xu J, Weng LJ, Liu Q, Yi LT. Antidepressant-like effect of macranthol isolated from Illicium dunnianum Tutch in mice. Eur J Pharmacol. 2013;707:112–9. doi: 10.1016/j.ejphar.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 39.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 40.Brown RE, Corey SC, Moore AK. Differences in measures of exploration and fear in MHC-congenic C57BL/6J and B6− H-2K mice. Behav Genet. 1999;29:263–71. [Google Scholar]
- 41.Zapata A, Chefer VI, Shippenberg TS, Denoroy L. Detection and quantification of neurotransmitters in dialysates. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns0704s48. doi: 10.1002/0471142301.ns0704s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pahwa P, Goel RK. Ameliorative effect of Asparagus racemosus root extract against pentylenetetrazol-induced kindling and associated depression and memory deficit. Epilepsy Behav. 2016;57:196–201. doi: 10.1016/j.yebeh.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 43.Oakes KD, Van Der Kraak GJ. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat Toxicol. 2003;63:447–63. doi: 10.1016/s0166-445x(02)00204-7. [DOI] [PubMed] [Google Scholar]
- 44.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 45.Chance B, Maehly AC. Assay of catalase and peroxidases. Met Enzymol. 1955;11:764–75. [Google Scholar]
- 46.Choudhary KM, Mishra A, Poroikov VV, Goel RK. Ameliorative effect of curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur J Pharmacol. 2013;704:33–40. doi: 10.1016/j.ejphar.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Kumar N, Singh N, Jaggi AS. Anti-stress effects of cilnidipine and nimodipine in immobilization subjected mice. Physiol Behav. 2012;105:1148–55. doi: 10.1016/j.physbeh.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Lim TS, Putt N, Safranski D, Chung C, Watson RR. Effect of Vitamin E on cell-mediated immune responses and serum corticosterone in young and maturing mice. Immunology. 1981;44:289–95. [PMC free article] [PubMed] [Google Scholar]
- 49.Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol. 2002;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- 50.Jin ZL, Gao N, Li XR, Tang Y, Xiong J, Chen HX, et al. The antidepressant-like pharmacological profile of Yuanzhi-1, a novel serotonin, norepinephrine and dopamine reuptake inhibitor. Eur Neuropsychopharmacol. 2015;25:544–56. doi: 10.1016/j.euroneuro.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 52.Duman CH. Models of depression. Vitam Horm. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- 53.Bourin M, Chenu F, Ripoll N, David DJ. A proposal of decision tree to screen putative antidepressants using forced swim and tail suspension tests. Behav Brain Res. 2005;164:266–9. doi: 10.1016/j.bbr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 54.Renard CE, Dailly E, David DJ, Hascoet M, Bourin M. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fundam Clin Pharmacol. 2003;17:449–55. doi: 10.1046/j.1472-8206.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 55.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 56.Bourin M, Fiocco AJ, Clenet F. How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol. 2001;16:9–21. doi: 10.1002/hup.178. [DOI] [PubMed] [Google Scholar]
- 57.Borsini F, Lecci A, Mancinelli A, D'Aranno V, Meli A. Stimulation of dopamine D-2 but not D-1 receptors reduces immobility time of rats in the forced swimming test: Implication for antidepressant activity. Eur J Pharmacol. 1988;148:301–7. doi: 10.1016/0014-2999(88)90107-0. [DOI] [PubMed] [Google Scholar]
- 58.de Oliveira KN, Costa P, Santin JR, Mazzambani L, Bürger C, Mora C, et al. Synthesis and antidepressant-like activity evaluation of sulphonamides and sulphonyl-hydrazones. Bioorg Med Chem. 2011;19:4295–306. doi: 10.1016/j.bmc.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 59.Schildkraut JJ. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am J Psychiatry. 1965;122:509–22. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 60.Pålhagen S, Qi H, Mårtensson B, Wålinder J, Granérus AK, Svenningsson P. Monoamines, BDNF, IL-6 and corticosterone in CSF in patients with Parkinson's disease and major depression. J Neurol. 2010;257:524–32. doi: 10.1007/s00415-009-5353-6. [DOI] [PubMed] [Google Scholar]
- 61.Detka J, Kurek A, Basta-Kaim A, Kubera M, Lasoń W, Budziszewska B, et al. Neuroendocrine link between stress, depression and diabetes. Pharmacol Rep. 2013;65:1591–600. doi: 10.1016/s1734-1140(13)71520-2. [DOI] [PubMed] [Google Scholar]
- 62.Zafir A, Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009;12:167–77. doi: 10.1080/10253890802234168. [DOI] [PubMed] [Google Scholar]
- 63.Freitas AE, Budni J, Lobato KR, Binfaré RW, Machado DG, Jacinto J, et al. Antidepressant-like action of the ethanolic extract from Tabebuia avellanedae in mice: Evidence for the involvement of the monoaminergic system. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:335–43. doi: 10.1016/j.pnpbp.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:435–51. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Li LF, Lu J, Li XM, Xu CL, Yang J, Qu R, et al. Antidepressant-like effects of the saponins extracted from Chaihu-Jia-Longgu-Muli-Tang in a rat unpredictable chronic mild stress model. Fitoterapia. 2012;83:93–103. doi: 10.1016/j.fitote.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Sairam K, Dorababu M, Goel RK, Bhattacharya SK. Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine. 2002;9:207–11. doi: 10.1078/0944-7113-00116. [DOI] [PubMed] [Google Scholar]
- 67.Bhattacharya SK, Bhattacharya A, Sairam K, Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: An experimental study. Phytomedicine. 2000;7:463–9. doi: 10.1016/S0944-7113(00)80030-6. [DOI] [PubMed] [Google Scholar]
- 68.Manthey L, Leeds C, Giltay EJ, van Veen T, Vreeburg SA, Penninx BW, et al. Antidepressant use and salivary cortisol in depressive and anxiety disorders. Eur Neuropsychopharmacol. 2011;21:691–9. doi: 10.1016/j.euroneuro.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Ali SH, Madhana RM, Athira K V, Kasala ER, Bodduluru LN, Pitta S, et al. Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids. 2015;101:37–42. doi: 10.1016/j.steroids.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Doron R, Lotan D, Versano Z, Benatav L, Franko M, Armoza S, et al. Escitalopram or novel herbal mixture treatments during or following exposure to stress reduce anxiety-like behavior through corticosterone and BDNF modifications. PLoS One. 2014;9:e91455. doi: 10.1371/journal.pone.0091455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative high-performance-thin-layer chromatography chromatograms of a standard, Shatavarin IV (a) and hydroethanolic extract of Asparagus adscendens (b)


