ABSTRACT
Chronic kidney disease (CKD) is a syndrome caused by the progressive reduction of renal function. This study aimed to systematically examine the effects of supplementation with probiotics in the treatment of CKD. Searches were carried out on databases MEDLINE (PubMed), SciELO, Cochrane, and Clinical Trials. Two independent reviewers selected the studies from which data was extracted. The search included papers written in English and Portuguese published in the 2012-2016 period describing randomized clinical trials. Eight of the 82 eligible articles met the inclusion criteria. Sample size ranged from 18 to 101 individuals with CKD. The duration of the included studies varied from four to 24 weeks. Most of the included articles reported positive effects in renal function and decreased levels of urea, blood urea nitrogen, ammonia, plasma p-cresol, p-cresyl sulfate, and indoxyl sulfate.
Keywords: Renal Insufficiency, Chonic; Probiotics; Lactobacillus
RESUMO
A insuficiência renal crônica (IRC) é definida como uma síndrome causada pela redução progressiva da função renal. O objetivo deste trabalho foi revisar sistematicamente o efeito da suplementação probiótica no tratamento da IRC. Foi realizada uma busca nas bases de dados MEDLINE (PubMed), SciELO, Cochrane e Clinical Trials. Dois revisores independentes realizaram a seleção dos estudos e a extração de dados. A pesquisa incluiu estudos entre 2012-2016, do tipo estudo clínico randomizado, em inglês e em português. Dos 82 artigos elegíveis, 8 artigos preencheram os critérios de inclusão. O número amostral variou de 18 a 101 pacientes com IRC, com duração de 4 a 24 semanas de estudo. A maioria dos estudos relatados mostraram efeitos benéficos na redução das concentrações de ureia, nitrogênio ureico, amônia, p-cresol plasmático, sulfato de p-cresil e sulfato de indoxil, ou seja, os probióticos parecem estar relacionados à melhora da função de renal.
Palavras-chave: Insuficiência Renal Crônica, Probióticos, Lactobacillus
INTRODUCTION
Chronic kidney disease (CKD) may be defined as a syndrome caused by the progressive reduction of renal function.1 It is characterized by progressive deterioration of organic biochemical and physiological function secondary to the accumulation of catabolites, disturbed fluid-electrolyte and acid-base balance, metabolic acidosis, hypovolemia, hyperkalemia, hyperphosphatemia, anemia, hormonal disorders, hyperparathyroidism, infertility, and growth failure, to name a few.2 , 3 , 4
More than 20 million people have kidney disorders in the United States, and more than 600,000 suffer from kidney failure.5 In Brazil an estimated 10 million people have some degree of renal disorder, while the global incidence of kidney disease grows at a mean rate of 10% a year.6 The number of individuals affected by kidney disease has increased significantly, partly on account of population aging and partly due to the growth in the number of people with hypertension and diabetes mellitus, two morbidities strongly correlated with the development of renal involvement.7 , 8
Patients with CKD and end-stage renal disease (ESRD) present quantitative and qualitative alterations in the gut microbiota such as increased concentration of urea and ammonia in the bowel, compromised integrity of the intestinal barrier, and increased levels of inflammation.9 Considering the bacteria living in the lower gastrointestinal (GI) tract, an estimated 100 trillion microorganisms live in the human bowel,10 a number ten times greater than the number of cells in a living organism. The members of this microbiome play a key role in immune response development and function.11
Probiotics include a vast array of products with living microorganisms12 whose purpose is to improve intestinal microbial balance and produce beneficial effects on one's health.13 To do so, they must be administered in proper dosages.14 In order for probiotics to produce actual benefits to one's organism, they must be viable when used, i.e., the microorganisms in them have to survive contact with gastric juice and bile, to then fixate in the intestinal lining to compete against pathogenic microorganisms and satisfactorily modulate inflammation and immunity.15
Patients with CKD with increased plasma levels of uremic solutes in need of kidney transplantation or chronic dialysis have in probiotics an alternative therapy to treat ESRD and attenuate uremia.11
The effects arising from the use of probiotics by patients with CKD are unclear, and few studies have been carried out in this area. This systematic review was based on the following guiding question: "Are probiotics beneficial when used as an adjuvant element in the treatment of CKD?" This systematic review aims to examine the effects of probiotics used in the treatment of patients with CKD.
MATERIALS AND METHODS
SEARCH FOR LITERATURE AND SELECTION OF STUDIES
The search for literature was carried out from December of 2016 to May of 2017 on the following databases: Scientific Electronic Library Online (SciELO), MEDLINE (accessed via PubMed), Clinical Trials, and Cochrane Library. A search for more recent papers published since May of 2017 was made on June of 2017. An additional search was made on Google Scholar to make sure no relevant paper was missed. The searches were carried out using the following terms: (Chronic renal disease) OR (Chronic kidney disease) OR (Chronic renal failure) OR (Chronic kidney failure) AND (Lactobacillus) OR (Lactobacilli) OR (Probiotic) OR (Probiotics). All terms were used in the searches without using quotation marks (").
INCLUSION AND EXCLUSION CRITERIA
This systematic review included studies published between 2012 and 2016 in the English and Portuguese languages, designed as randomized clinical trials (RCT) enrolling humans to examine the effect of probiotic supplements, independent clinical trials phases I, II, III, IV enrolling patients with CKD, aged 18 years or older of both sexes, on hemodialysis or not. Review papers, systematic, narrative and integrative reviews, editorials, book chapters and abstracts, experimental trials with animals, and abstracts published in meeting proceedings were excluded.
REFERENCE PROTOCOL
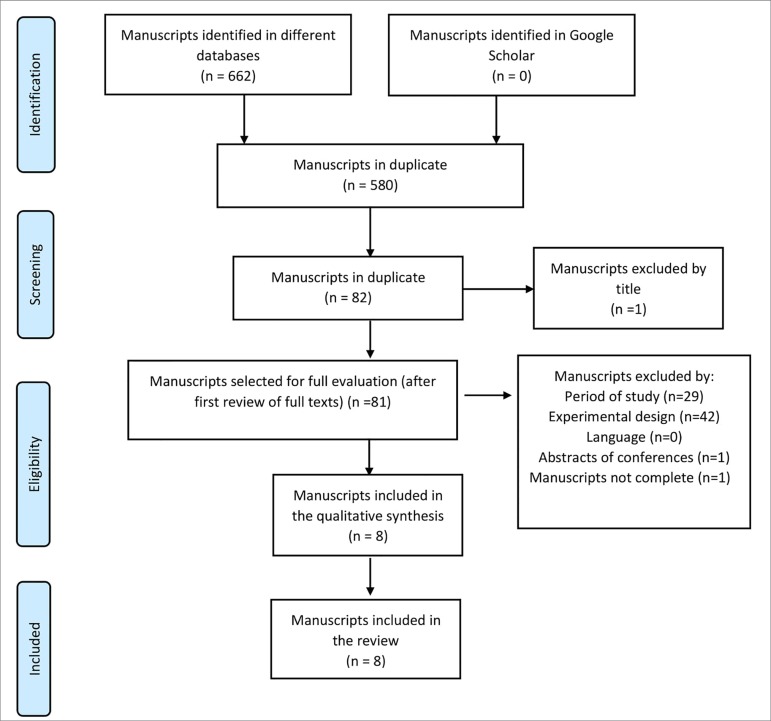
This study was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA - Figure 1).16 A protocol for this review as registered with PROSPERO (CRD42017064068). As described in Carvalho et al.,17 "risk of bias" was assessed for each RCT included in the systematic review through a protocol built in accordance with the recommendations of the Cochrane Collaboration.18
Figure 1. Flowchart describing the identification and selection of articles (PRISMA).
DATA EXTRACTION
Two independent reviewers (R.A.B.F. and T.F.S.) searched the databases for papers and selection was made based solely on the titles of the articles. The abstracts of the selected studies were read and assessed based on the eligibility criteria. The disagreements between the two reviewers were discussed until consensus was reached.
RESULTS
SEARCH FOR LITERATURE AND SELECTION OF STUDIES
Database searches yielded 662 articles, 580 of which were duplicates. After the analysis of titles and abstracts, 75 records were excluded for not meeting the inclusion criteria and eight were selected and read in their entirety (Figure 1). The eight papers selected were on PubMed. All the studies included in this review were RCTs written in English. The selected studies were performed in Mexico19 , 20, Italy21, the United States22, Malaysia23, China24, Iran25 and Australia26.
SAMPLE SIZE, STUDY DURATION, INTERVENTION CHARACTERISTICS
Sample size ranged between 18 and 101 patients with CKD, and study duration varied from four to 24 weeks. Probiotic agent dosages ranged from 2.0 x 1012 to 16 x 109 CFU and 15g; dosages did not follow a specific standard. The form of presentation of the probiotic agents varied significantly; some were administered in bags/envelopes to be dissolved in water, while others had to be added to yogurt and a few were given in capsules. In all studies, groups of patients were prescribed probiotics in different presentations - capsules, bags, etc. - and times in relation to meals -right after or while eating.
ASSESSMENT OF STUDY RISK OF BIAS
Table 1 describes the methodological quality of the included studies. The only criterion established by the Cochrane Collaboration to assess methodological quality referred to other sources of bias, an area in which all studies presented low levels of risk. However, all studies were designed to answer clear focal questions. Five studies offered detailed descriptions of the method used to generate random sequences, while three described in detail the method used to hide patient allocation. The methods used to define the blinding of patients or researchers were reported in seven studies. Seven studies were described as blinded experiments, but failed to describe all the measures taken to enforce blinding. Results were completely assessed in seven of the eight studies.
Table 1. Assessment of risk of bias in randomized clinical trials (Cochrane Collaboration).
| Study | Randomization | Allocation | Blinding of participants or researchers | Blinding for outcomes | Incomplete outcomes (losses) | Selective outcome report | Other sources of bias |
|---|---|---|---|---|---|---|---|
| Alatriste et al., 2014 | ↓ | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ |
| Mora et al., 2014 | ? | ? | ↓ | ↓ | ↓ | ↓ | ↓ |
| Guida et al., 2014 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Natarajan et al., 2014 | ? | ? | ↓ | ↓ | ↓ | ↑ | ↓ |
| Firouzi et al., 2015 | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ |
| Wang et al., 2015 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Dehghani et al., 2015 | ? | ? | ↓ | ↓ | ↑ | ↓ | ↓ |
| Rossi et al., 2016 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
↑: High risk; ↓: Low risk; ?: Unknown risk.
TYPES AND DURATION OF THE INTERVENTIONS
Only one study tested the effects of yogurt with added probiotics;19 another study tested a protocol with one daily symbiotic gel capsule.20 Probiotics were also presented in envelopes dissolved in water and ingested three times a day, not on meal times.21 Natarajan et al.22 prescribed two capsules three times a day ingested during meals. Bags with probiotics dissolved in water and ingested promptly were also prescribed twice a day (in the morning and in the evening) accompanied or not by a meal.23 Wang et al.24 prescribed one capsule a day before going to bed. Dehghani et al.25 prescribed two capsules a day after meals, while Rossi et al.26 offered 15g of probiotics daily in a symbiotic formulation.
MOST PREDOMINANT MICROORGANISMS USED IN PROBIOTIC AGENTS
Most of the microorganisms used in the studies belonged to the Lactobacillus (100% = 8 studies) and Bifidobacterium genera (87.5% = 7 studies). Table 2 shows the data extracted from the selected studies.
Table 2. Characteristics of the studies included in the review.
| Study | N | Treatment duration | Probiotic strain | Dosages | Beneficial effects | Side effects | Nutritional counseling | Outcome |
|---|---|---|---|---|---|---|---|---|
| Alatriste et al. (2014) | 30 | 8 weeks | Lactobacillus casei Shirota (LcS) | Group A was given a fermented dairy drink in an 80-mL bottle with 8 x 109 CFU of LcS; Group B was given two 80-mL bottles de 80 mL of a fermented dairy drink with 16 x 109 CFU of LcS. | Yes. The patients presented decreased levels of ammonia (a precursor of urea, with which bacteria are involved).' | None | Yes | Decrease greater than 10% in serum urea levels after dietary intervention with LcS for patients with CKD stages 3 and 4. |
| Mora et al. (2014) | 18 | 8 weeks | Lactobacillus acidophilus and Bifidobacterium bifidum, prebiotic inulin fiber, omega-3 fatty acids, and vitamins (B vitamins, folic acid, ascorbic acid, and vitamin E). | Nutrihealth: 1 capsule/day – 2.0 x 1012 CFU | Yes. Increased counts of bifidobacteria. | None | Yes | Symbiotic gel used for two months may be potentially in the therapy of patients with kidney disease and may increase the population of bifidobacteria. |
| Guida et al. (2014) | 30 | 4 weeks | Lactobacillus plantarum, Lactobacillus casei subsp. Rhamnosus, Lactobacillus gasseri, Bifidobacterium infantis, Bifidobacterium longum, Lactobacillus acidophilus, Lactobacillus salivarius, Lactobacillus sporogenes, Streptococcus thermophilus, previotic inulin (VB Beneo Synergy 1), and resistant tapioca starch. | Probinul neutro® (5g powder bags dissolved in water, three times a day, away from meal times). | Yes. The symbiotic agent significantly decreased plasma p-cresol levels of non-dialysis patients with CKD. | None | None | Symbiotic agent may significantly decrease total plasma p-cresol levels in patients with CKD stages 3 and 4. |
| Natarajan et al. (2014) | 22 | 24 weeks | S. thermophilus KB 19, L. acidophilus KB 27, B. longum KB 31. | Two capsules three times a day with meals. Each capsule contained a probiotic formulation with 30 billion CFU. | None. | Yes | None | Efficacy could not be confirmed primarily due to small sample size and low statistical power - additional studies are needed. |
| Firouzi et al. (2015) | 10 1 | 12 weeks | Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus lactis, Bifidobacterium bifidum, Bifidobacterium longum and Bifidobacterium infantis. | Daily dosage of 6 × 1010 CFU (Hexbio B-Crobes). Probiotic agent in bags were poured into a glass with approximately 250 mL of water; twice a day (morning and evening) accompanied or not by a meal. | Yes Urea levels improved significantly after supplementation with probiotics. | Yes | Yes | This study showed that probiotics may improve urea levels, particularly of OW/OB individuals and subjects with high urea levels. However, other renal parameters and liver function were not altered by the administration of probiotics. |
| Wang et al. (2015) | 39 | 24 weeks | B. bifidum A218, B. catenulatum A302, B. longum A101, and L. plantarum A87. | Daily dosage: 1 capsule 4 × 109 CFU/day before going to bed. | Yes. Significant decreases were seen in the levels of TNF-α, IL-5, IL-6, and endotoxins, along with increased levels of IL-10; residual renal function of patients on PD was preserved after six months of treatment with oral probiotics. | None | None | Probiotics may significantly reduce serum levels of endotoxins, proinflammatory cytokines (TNF-α and IL-6), IL-5, and increase serum levels of proinflammatory cytokines (IL-10) and preserve residual renal function of individuals on PD. |
| Dehghani et al. (2016) | 66 | 6 weeks | Lactobacillus casei, Lactobacillus cidophilus, Lactobacillus bulgarigus, Lactobacillus rhamnosus, Bifido,bacterium breve, Bifidobacterium longum, Streptococcus thermophilus and fructooligosaccharide prebiotic agent. | Familact 500mg - two capsules a day after meals. | Yes. Blood urea nitrogen levels of patients with CKD were decreased. | None | None | Treatment with symbiotic probiotics for six weeks led to significant decreases in mean blood urea levels of patients with CKD stages 3 and 4 compared to controls; effects were not seen in other renal function indicators. |
| Rossi et al. (2016) | 31 | 18 weeks | Nine different strains from the Lactobacillus, Bifidobacterium and Streptococcus genera. High molecular weight inulin, fructooligosaccharides, and galacto-oligosaccharides, and probiotic component. | In the first three weeks, participants ingested 7.5g of prebiotic powder and one capsule with probiotics containing 45 billion CFU in the morning with a meal. In the last three weeks, participants took an additional dose (7.5 g of powder and one capsule) with a meal in the evening, yielding a daily dosage of 15g. | Yes. Symbiotic therapy significantly decreased serum levels of PCS and, to a lesser extent, IS levels in patients with moderate to severe CKD. | None | Yes | Symbiotic therapy led to statistically significant and potentially clinically relevant decreases in serum levels of IS and PCS. |
CFU: colony forming unit; LcS: Lactobacillus casei Shirota; PCS: p-cresyl sulfate; IS: indoxyl sulfate; OW: overweight; OB: obese; PD: peritoneal dialysis. CKD: chronic kidney disease.
RENAL FUNCTION MARKERS
Renal function biomarkers such as serum creatinine, urea, ammonia, and uric acid were analyzed in six studies.19 , 22 - 26 Positive effects were reported for some renal function markers based on decreases in blood urea nitrogen levels.19 , 25 Other markers did not decrease significantly (creatinine, creatinine clearance, uric acid, glomerular filtration rate, sodium, potassium) before or after the intervention.22 , 23 , 24 , 25 , 26
INFLAMMATORY MARKERS
Inflammatory biomarkers such as C-reactive protein (CRP) and interleukins were assessed in three studies.22 , 24 , 26 When compared to controls, decreases were reported in endotoxins and proinflammatory cytokines TNF-α, IL-5, IL-6, and increases in serum IL-10 levels.24 Narajan22 did not report changes in inflammatory marker concentrations as a result of probiotic administration. Rossi et al.27 reported that probiotic therapy produced positive effects on the serum levels of free and total p-cresol sulfate and indoxyl sulfate.
DISCUSSION
This systematic review revealed the main microorganisms targeted in the treatment of CKD. The list includes bacteria belonging to the Lactobacillus and Bifidobacterium genera, with reported beneficial effects such as decreasing urea, blood urea nitrogen, and ammonia levels and plasma concentrations of p-cresol and indoxyl sulfate. Another benefit described for these probiotic strains is that they reportedly help increase bifidobacteria populations, a genus known to play a key role in the function of the intestinal mucosal barrier26 and to help decrease cytokine and endotoxin concentrations and increase serum levels of IL-10. Other strains, such as the ones belonging to genera S. thermophilus KB 19, L. acidophilus KB 27, B. longum KB 31, did not produce beneficial effects to human health.
Alatriste et al.19 observed that higher dosages of probiotic agents with LcS yielded better outcomes. Probiotic dosages in the range of 16 x 109 CFU administered for eight weeks in combination with diet and protein intake led to decreases in blood urea levels. The benefits of offering probiotics to patients with CKD included a protective effect stemmed from decreased levels of inflammatory markers. Natarajan et al.22 reported that the administration of Renadyl to patients with CKD at a dosage of 180 billion CFU/day was safe and well tolerated. Non-significant trends were observed in white blood cell count, CRP, total indoxyl glucuronide, uremic toxins, markers of oxidative stress, and quality of life measures.
Investigations on the effects of a multi-strain microbial cell preparation on the renal profiles and liver function of individuals with diabetes type 2 for 12 weeks revealed that probiotics might potentially improve urea levels, particularly in overweight and obese individuals with elevated urea levels. Blood sodium and potassium levels and liver function tests remained unaltered after supplementation with probiotics, while creatinine levels increased in case and control groups, a finding possibly related to the administration of angiotensin-converting-enzyme inhibitors. Contrastingly, urea levels decreased in the group offered probiotics. A few adverse effects were reported, including expected minor gastric disorders, sexual impotence, and a carbuncle in one individual.23
Supplementation with probiotics appears to be associated with significant decreases in serum levels of proinflammatory cytokines TNF-α, IL-5, and IL-6 and endotoxins in patients on PD, and increased levels of anti-inflammatory cytokine IL-10, in addition to preservation of residual renal function after six months of oral supplementation; urea, creatinine, and uric acid levels remained unaltered after probiotic administration.24
The administration of probiotics (Familact 500, twice a day after meals for six weeks) reportedly decreased blood urea levels in patients with CKD stages 3 and 4, although no effects on other indicators of renal function were observed.25
Symbiotic supplementation also yielded positive effects to the gut microbiota of patients with CKD. A prescription of one symbiotic capsule for eight weeks increased Bifidobacterium and preserved Lactobacillus populations;20 supplementation for four weeks with probiotics given three times a day away from meal times led to considerable decreases in the plasma p-cresol levels of patients with CKD stages 3 and 4,21 while supplementation with 15g a day of symbiotic probiotics resulted in effective decreases in serum p-cresol levels and lesser decreases in indoxyl sulfate levels of patients with moderate to severe CKD treated for 18 weeks.27
Choosing the right strain might help maintain the balance between the different populations of microorganisms and thus decrease the potential for overgrowth and pathogenicity.28 When the populations of pathogenic bacteria increase, patients with renal impairment develop dysbiosis, which may contribute to increase the levels of uremic toxins often associated with progression of CKD.29 Gut colonization is highly affected by eating habits, host phenotype, mental health, and host living habits.30
With a growing number of lifestyle-related diseases, healthy eating habits and physical exercise have become more important than drug therapy.8 Diet is one of the better known lifestyle factors and an important regulator of the intestinal microbiota.31 , 32 Dietary intervention is an adjuvant strategy used to restore microbial balance and suppress circulating levels of p-cresol and indoxyl sulfate,33 as described in the study published by Rossi et al.27
However, studies indicated that diet has a strong impact on colonic bacterial metabolism and on the progression of CKD. When associated with dietary fiber in the treatment of CKD, benefits are expected in relation to the integrity of the gastrointestinal wall, along with decreases in the systemic concentrations of hazardous uremic toxins.34 Isocaloric (30 kcal/kg of ideal body weight) e isoprotein (0.8 g/kg of ideal body weight) diets benefit patients with renal impairment on account of the impacts they may produce primarily on serum urea levels, a parameter significantly affected by protein intake.19
Interventions with clearly presented dietary factors shed light on the possible causal relationships between diet and disease mediated by the intestinal microbiota. The administration of probiotics and prebiotics may be seen as a preventive or therapeutic measure, since it promotes the establishment of a healthier, better functioning intestinal microbiota.30
The limitations of this review include the short follow-up period featured in some studies and the usually small size of the enrolled populations. Another limitation was the methodological heterogeneity of the studies, which precluded the production of a meta-analysis. Therefore, this review was limited to a descriptive presentation of the data.
FINAL CONSIDERATIONS
This systematic review stressed the relevance of probiotics in decreasing urea, blood urea nitrogen, ammonia, plasma p-cresol, IS, PCS levels of individuals with CKD. The main strains used in the treatment of patients with CKD belonged to the Lactobacillus and Bifidobacterium genera, with dosages ranging from 2.0 x 1012 to 16 x 109 CFU and 15g; dosages did not follow a specific standard. Some of the probiotic agents were administered in bags/envelopes dissolved in water, while other agents were given in capsules and one was added to yogurt. Each of the formulations had specific ingestion instructions devised to preserve the properties of the probiotic agents. The limited number of articles on the topic precluded the generalization of the obtained results. Therefore, additional long-term studies are needed to further elucidate the possible role of probiotics in the treatment of individuals with CKD.
REFERENCES
- 1.Barreto FC, Stinghen AE, de Oliveira RB, Franco AT, Moreno AN, Barreto DV, et al. The quest for a better understanding of chronic kidney disease complications: an update on uremic toxins. J Bras Nefrol. 2014;36:221–235. doi: 10.5935/0101-2800.20140033. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro RCHM, Oliveira GASA, Ribeiro DF, Bertolin DC, Cesarino CB, Lima LCEQ, et al. Characterization and etiology of the chronic renal failure in a countryside nephrology unit of São Paulo State. Acta Paul Enferm. 2008;21:207–211. [Google Scholar]
- 3.Mascarenhas CHM, Reis LA, Lyra JE, Peixoto AV, Teles MS. Insuficiência Renal Crônica: Caracterização Sociodemográfica e de Saúde de Pacientes em Tratamento Hemodialítico no Município de Jequié/BA. Espaç Saúde. 2010;12:30–37. [Google Scholar]
- 4.Peres BLA, Biela R, Herrmann M, Matsuo T, Ann HK, Camargo MT, et al. Epidemiological study of end-stage renal disease in western Paraná. An experience of 878 cases in 25 years. J Bras Nefrol. 2010;32:49–54. [PubMed] [Google Scholar]
- 5.Kidney Health Initiative . Annual Review. Washington: KHI; 2015. [2017 Jul 18]. Available from: https://www.asn-online.org/khi/KHI_Annual_Report_2015.pdf. [Google Scholar]
- 6.Sociedade Brasileira de Nefrologia . SBN Informa - Publicação Oficial da Sociedade Brasileira de Nefrologia. São Paulo: SBN; 2016. [2017 Jul 3]. Disponível em: https://sbn.org.br/app/uploads/sbninforma105_2016_bx-1.pdf. [Google Scholar]
- 7.Siviero PCL, Machado CJ, Cherchiglia ML. Insuficiência renal crônica no Brasil segundo enfoque de causas múltiplas de morte. Cad Saúde Colet. 2014;22:75–85. [Google Scholar]
- 8.Watanabe S. Low-protein diet for the prevention of renal failure. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:1–9. doi: 10.2183/pjab.93.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ericsson AC, Franklin CL. Manipulating the Gut Microbiota: Methods and Challenges. ILAR J. 2015;56:205–217. doi: 10.1093/ilar/ilv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Cerbo A, Palmieri B, Aponte M, Morales-Medina JC, Iannitti T. Mechanisms and therapeutic effectiveness of lactobacilli. J Clin Pathol. 2016;69:187–203. doi: 10.1136/jclinpath-2015-202976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Gastroenterology Organisation Diretrizes Mundiais da Organização Mundial de Gastroenterologia - Probióticos e prebióticos. Oct, 2011. [2017 Jul 18]. Available from: http://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-portuguese-2011.pdf.
- 13.Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária Informações Técnicas Probióticos. Jun 21, 2016. [2017 Jul 18]. Available from: http://portal.anvisa.gov.br.
- 14.Flesch AGT, Poziomyck AK, Damin DC. O Uso Terapêutico dos Simbióticos. ABCD Arq Bras Cir Dig. 2014;27:206–209. doi: 10.1590/S0102-67202014000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueira JCR, Gonçalves MCR. Probióticos - Revisão da Literatura. Rev Bras Ciênc Saúde. 2011;15:487–492. [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho APV, Silva V, Grande AJ. Avaliação do risco de viés de ensaios clínicos randomizados pela ferramenta da colaboração Cochrane. Diagn Tratamento. 2013;18:38–44. [Google Scholar]
- 18.Higgins J, Green S, Cochrane Collaboration Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. [2017 Jul 18]. [Internet] Available from: http://handbook.cochrane.org/
- 19.Miranda Alatriste PV, Urbina Arronte R, Gómez Espinosa CO, Espinosa Cuevas Mde L. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp. 2014;29:582–590. doi: 10.3305/nh.2014.29.3.7179. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Mora J, Martínez-Hernández NE, Campo-López MF, Viramontes-Hörner D, Vizmanos-Lamotte B, Muñoz-Valle JF, et al. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr. 2014;24:330–335. doi: 10.1053/j.jrn.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Guida B, Germanò R, Trio R, Russo D, Memoli B, Grumetto L, et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis. 2014;24:1043–1049. doi: 10.1016/j.numecd.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A, Liang P, et al. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int. 2014;2014:568571. doi: 10.1155/2014/568571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firouzi S, Mohd-Yusof BN, Majid HA, Ismail A, Kamaruddin NA. Effect of microbial cell preparation on renal profile and liver function among type 2 diabetics: a randomized controlled trial. BMC Complement Altern Med. 2015;15:433–433. doi: 10.1186/s12906-015-0952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang IK, Wu YY, Yang YF, Ting IW, Lin CC, Yen TH, et al. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Benef Microbes. 2015;6:423–430. doi: 10.3920/BM2014.0088. [DOI] [PubMed] [Google Scholar]
- 25.Dehghani H, Heidari F, Mozaffari-Khosravi H, Nouri-Majelan N, Dehghani A. Synbiotic Supplementations for Azotemia in Patients With Chronic Kidney Disease: a Randomized Controlled Trial. Iran J Kidney Dis. 2016;10:351–357. [PubMed] [Google Scholar]
- 26.Stürmer ES, Casasola S, Gall MC, Gall MC. A importância dos probióticos na microbiota intestinal humana. Rev Bras Nutr Clin. 2012;27:264–272. [Google Scholar]
- 27.Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, et al. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin J Am Soc Nephrol. 2016;11:223–231. doi: 10.2215/CJN.05240515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salonen A, Lahti L, Salojärvi J, Holtrop G, Korpela K, Duncan SH, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Khodor S, Shatat IF. Gut microbiome and kidney disease: a bidirectional relationship. Pediatr Nephrol. 2017;32:921–931. doi: 10.1007/s00467-016-3392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moraes ACF, Silva IT, Almeida-Pititto B, Ferreira SR. Microbiota intestinal e risco cardiometabólico: mecanismos e modulação dietética. Arq Bras Endocrinol Metab. 2014;58:317–327. doi: 10.1590/0004-2730000002940. [DOI] [PubMed] [Google Scholar]
- 31.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graf D, Cagno RD, Fak F, Flint HJ, Nyman M, Saarela M, et al. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis. 2015;26:26164. doi: 10.3402/mehd.v26.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evenepoel P, Poesen R, Meijers B. The gut-kidney axis. Pediatr Nephrol. 2016;32:2005–2014. doi: 10.1007/s00467-016-3527-x. [DOI] [PubMed] [Google Scholar]
- 34.Rossi M, Johnson DW, Campbell KL. The Kidney-Gut Axis: Implications for Nutrition Care. J Ren Nutr. 2015;25:399–403. doi: 10.1053/j.jrn.2015.01.017. [DOI] [PubMed] [Google Scholar]