ABSTRACT
MYH9-related disease is an autosomal dominant disorder caused by mutations of the MYH9 gene, which encodes the non-muscle myosin heavy chain IIA on chromosome 22q12. It is characterized by congenital macrothrombocytopenia, bleeding tendency, hearing loss, and cataracts. Nephropathy occurs in approximately 30% of MYH9-related disease in a male patient carrier of a de novo missense mutation in exon 1 of the MYH9 gene [c.287C > T; p.Ser(TCG)96(TTG)Leu]. He presented all phenotypic manifestations of the disease, but cataracts. Renal alterations were microhematuria, nephrotic-range proteinuria (up to 7.5 g/24h), and rapid loss of renal function. The decline per year of the glomerular filtration rate was 20 mL/min/1.73m2 for five years. Blockade of the renin-angiotensin system, the only recommended therapy for slowing the progression of this nephropathy, was prescribed. Although MYH9-related disease is a rare cause of glomerulopathy and end-stage renal disease, awareness of rare genetic kidney disorders is essential to ensure accurate diagnosis and proper management of orphan disease patients.
Keywords: Renal Insufficiency, Chronic; Thrombocytopenia; Nephrotic Syndrome; Genetic Diseases, Inborn; Rare Diseases
RESUMO
A doença relacionada ao MYH9 é um distúrbio autossômico dominante causado por mutações no gene MYH9 que codifica a cadeia pesada da miosina não muscular IIA no cromossomo 22q12. Ela é caracterizada por macrotrombocitopenia congênita, tendência a sangramento, perda auditiva e catarata. A nefropatia ocorre em aproximadamente 30% dos pacientes. O presente artigo relata o caso de um paciente com doença relacionada ao MYH9 portador de mutação missense de novo no exon 1 do gene MYH9 [c.287C > T; p.Ser(TCG)96(TTG)Leu]. Com a exceção de catarata, o paciente apresentou todas as manifestações fenotípicas da doença. As alterações renais incluíram micro-hematúria, proteinúria nefrótica (até 7,5 g/24h) e perda rápida da função renal. O declínio anual da taxa de filtração glomerular foi de 20 mL/min/1,73 m2 durante cinco anos. Foi receitado bloqueio do sistema renina-angiotensina, a única terapia recomendada para retardar a progressão dessa nefropatia. Embora a doença relacionada ao MYH9 seja uma causa rara de glomerulopatia e doença renal terminal, a conscientização sobre distúrbios genéticos renais raros é essencial para garantir o diagnóstico preciso e o manejo adequado dos pacientes com tal doença órfã.
Palavras-chave: Insuficiência Renal Crônica, Trombocitopenia, Síndrome Nefrótica, Doenças Genéticas Inatas, Doenças Raras
INTRODUCTION
MYH9-related disease (MYH9-RD) is a genetic disorder of autosomal dominant inheritance caused by mutations of the MYH9 gene, which encodes the non-muscle myosin heavy chain IIA (NMMHC-IIA) on chromosome 22q12. Around 200 affected families have been described in the literature, which suggest a very low prevalence of this disease.
MYH9-RD is characterized by congenital macrothrombocytopenia, leading to bleeding tendency, along with cytoplasmic inclusion bodies within leukocytes (Döhle-like inclusions), sensorineural deafness, cataracts, and nephropathy. The latter usually presents at a juvenile age with proteinuria, sometimes causing nephrotic syndrome, with or without microhematuria. It often progresses to end-stage renal disease (ESRD).1
Herein, we sought to describe the case of a young male patient affected by MYH9-RD that developed nephrotic-range proteinuria, microhematuria, and rapid loss of kidney function.
CASE DESCRIPTION
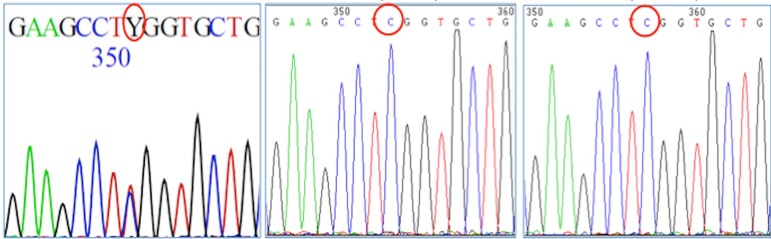
A twenty-year-old male has been followed up at the Clinic Hospital of Federal University of Paraná due to medical history of epistaxis, ecchymosis, and petechiae since infancy. At first, Bernard-Soulier syndrome was suspected due to macrothrombocytopenia and tendency of bleeding. When he was 17 years old, hearing loss and hypertension were detected along with mild renal failure, microhematuria and nephrotic-range proteinuria. Renal biopsy could not be performed due to risk of bleeding (platelets count: 7000/µL). Cataracts were excluded by ophthalmological evaluation. Due to the clinical suspicion of MYH9-RD, genotyping of the patient and of his parents was performed. A de novo missense mutation in exon 1 of the MYH9 gene [c.287C > T; p.Ser(TCG)96(TTG)Leu] was detected (Figure 1). Actually, neither his parents nor his brother and sister had clinical manifestation of MYH9-RD. Enalapril (20 mg/day) was initiated for renal protection. The patient did not adhere to treatment and was lost to follow-up. Two years later, he returned to the outpatient clinic complaining of foamy urine, peripheral edema, and hypertension (160/120 mmHg). Laboratory tests detected worsening of renal function and persistent proteinuria. Table 1 shows the evolution of laboratory parameters during the follow-up.
Figure 1. Molecular test of the index case and of his parents; Footnote: a de novo heterozygous c.287C>T in exon 1 of MHY9 [p.Ser(TCG)96(TTG) Leu] was detected (i). His father (ii) and his mother (iii) did not have the mutation.
Table 1. Laboratory evolution.
| Laboratory | June 2012 | March 2013 | June 2015 | June 2017 |
|---|---|---|---|---|
| creatinine (mg/dL) | 0.9 | 1.2 | 1.7 | 3.4 |
| eGFR (mL/min/1.73m2) | 126 | 88.4 | 57.2 | 24.4 |
| urea (mg/dL) | 34 | 27 | 32 | 80 |
| proteinuria (g/24h) | NA | 7.5 | 5.5 | 5.7 |
| albumin (g/dL) | 3.4 | 3.1 | 3.5 | 3.1 |
| cholesterol (mg/dL) | 185 | 205 | 249 | 241 |
| platelets count (n/µL) | 4000 | 7000 | 6500 | 3000 |
Abbreviations: eGFR: CKD-EPI estimated glomerular filtration rate; NA: not available.
DISCUSSION
MYH9-RD is characterized by congenital macrothrombocytopenia associated with variable degrees of sensorineural hearing loss, pre-senile cataract, and renal disease. Nephropathy occurs in approximately 30% of the patients with MYH9-RD and has a progressive and severe evolution. It usually presents at a juvenile age with proteinuria, sometimes causing nephrotic syndrome, with or without microhematuria. Our patient presented all clinical manifestation of MYH9-RD, but cataract. In most patients, nephropathy progresses to ESRD before the fourth decade of life.1
A genotype-phenotype correlation has been recognized in MYH9-RD. A higher incidence and a worse prognosis of kidney impairment have been associated with mutations affecting the head domain of NMMHC-IIA, compared with mutations in tail domain.2 Most patients with MYH9-RD present an autosomal dominant inheritance, and around 30% of them have a de novo mutation.1 Our patient presented a de novo missense mutation in exon 1 of MYH9 [c.287C > T; p.Ser(TCG)96(TTG)Leu] in the head domain. To date, more than 30 mutations within the 40 exons of the MYH9 gene have been detected, among them the one of our patient.3 In agreement with the genotype-phenotype correlation, our patient developed a rapid deterioration of renal function. The decline per year of the glomerular filtration rate was 20 mL/min/1.73m2 during the last five years.
Due to the overlap of clinical manifestations, MYH9-RD associated with renal impairment was considered a variant of Alport syndrome, designated as Fechtner syndrome. Recently, these syndromes were recognized as distinct disorders. They can be distinguished by the presence of thrombocytopenia, the hallmark of MYH9-RD and not a feature of Alport syndrome. Moreover, the latter is caused by mutations in the COL4A3, COL4A4, and COL4A5 genes, leading mainly to alterations in the glomerular basement membrane.4 The clinical features together with the presence of a pathogenic mutation in the MYH9 gene allowed a prompt and reliable diagnosis of MYH9-RD.
Renal biopsy is not usually performed in MYH9-nephropathy because of the risk of bleeding, reserved for cases in which the differential diagnosis is necessary. Renal histopathological findings are variable and unspecific, encompassing mesangial expansion or proliferation and segmental glomerulosclerosis. Electron microscopy commonly reveals glomerular basement membrane thickening and podocyte foot process effacement1. The pathogenesis of MYH9-nephropathy is not completely understood. NMMHC-IIA is an important component of podocyte foot process. Thus, MYH9-nephropathy may result from an alteration in the podocyte cytoskeleton.5
Blockade of the renin-angiotensin system might be effective in reducing proteinuria and slowing the progression of MYH9 nephropathy.6 As our patient did not adhere to the treatment, we could not evaluate the efficacy of this strategy, though.
To the best of our knowledge, this is the first case of MYH9-nephropathy described in Brazil. The learning points of this case need to be highlighted. In case of macrothrombocytopenia of uncertain diagnosis, urinalysis must be performed and proteinuria should be monitored to start renin-angiotensin system blockage as early as possible. Genotyping is a valuable tool for guiding diagnosis and prognosis. Finally, awareness of rare genetic kidney disorders is essential to ensure accurate diagnosis and proper management of orphan disease patients.
ACKNOWLEDGEMENTS
This study was carried out at the Clinic Hospital of the Federal University of Paraná and supported by a grant (HI12C0014) from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea.
REFERENCES
- 1.Oh T, Jung Seo H, Taek Lee K, Jo Kim H, Jun Kim H, Lee JH, et al. MYH9 nephropathy. Kidney Res Clin Pract. 2015;34:53–56. doi: 10.1016/j.krcp.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pecci A, Klersy C, Gresele P, Lee KJ, De Rocco D, Bozzi V, et al. MYH9-related disease: a novel prognostic model to predict the clinical evolution of the disease based on genotype-phenotype correlations. Hum Mutat. 2014;35:236–247. doi: 10.1002/humu.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrondel C, Vodovar N, Knebelmann B, Grünfeld JP, Gubler MC, Antignac C, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65–74. doi: 10.1681/ASN.V13165. [DOI] [PubMed] [Google Scholar]
- 4.Savige J, Storey H, Il Cheong H, Gyung Kang H, Park E, Hilbert P, et al. X-Linked and Autosomal Recessive Alport Syndrome: Pathogenic Variant Features and Further Genotype-Phenotype Correlations. PLoS One. 2016;11:e0161802. doi: 10.1371/journal.pone.0161802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostrom MA, Freedman BI. The spectrum of MYH9-associated Nephropathy. Clin J Am Soc Nephrol. 2010;5:1107–1113. doi: 10.2215/CJN.08721209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pecci A, Granata A, Fiore CE, Balduini CL. Renin-angiotensin system blockade is effective in reducing proteinuria of patients with progressive nephropathy caused by MYH9 mutations (Fechtner-Epstein syndrome) Nephrol Dial Transplant. 2008;23:2690–2692. doi: 10.1093/ndt/gfn277. [DOI] [PubMed] [Google Scholar]