Abstract
Objective: Parental attention-deficit/hyperactivity disorder (ADHD) is associated with suboptimal parenting and reduces the effectiveness of child ADHD treatments. We conducted a Pilot Sequential, Multiple Assignment, Randomized Trial (SMART Pilot) to evaluate the feasibility and acceptability of sequencing medication and behavioral treatments for mothers with ADHD to target outcomes, including maternal ADHD, parenting, and child ADHD symptoms/impairment in multiplex ADHD families.
Methods: Thirty-five mothers with ADHD and their 5- to 8-year-old child with ADHD symptoms were enrolled. Mothers were randomized to 8 weeks of individually titrated stimulant medication (MSM) or behavioral parent training (BPT), followed by rerandomization to 8 weeks of continued first-line treatment (with as-needed modifications) or combined treatment, leading to four treatment sequences (MSM-MSM, MSM-BPT, BPT-MSM, and BPT-BPT).
Results: Recruitment of multiplex ADHD families came primarily from child providers. Mothers were adherent to medication and had high therapy session attendance. Mothers and clinicians found both treatments to be acceptable and preferred combination treatment, especially receiving medication before BPT. Monotherapy treatment visits were viewed as more burdensome (MSM-MSM, BPT-BPT).
Conclusions: Maternal stimulant medication and BPT are acceptable and feasible interventions for families in which both the mother and child have ADHD symptoms. Mothers with concerns about their children's ADHD symptoms are receptive to receiving treatment themselves as an initial strategy for improving their children's health and functioning. Fully powered SMART designs show promise in evaluating the sequencing of interventions and helping clinicians develop algorithms for treating multiplex families in real-world practice settings.
Keywords: ADHD, stimulant treatment, behavioral treatment, sequential randomized trial
Introduction
Up to one-half of parents with attention-deficit/hyperactivity disorder (ADHD) have at least one child with ADHD (Johnston et al. 2012). Viewed from the perspective of the child, 25%–50% of children with ADHD have a mother or father with ADHD (Johnston et al. 2012). Maternal ADHD is found to limit response to behavioral treatments for child ADHD, namely behavioral parent training (BPT), and to be associated with aspects of parenting that predict a more pernicious course of child ADHD (Wang et al. 2014; Chronis-Tuscano et al. 2017). Yet, despite the prevalence of ADHD in mothers and its relevance to parenting, ADHD in mothers is often unrecognized and seldom treated.
Several studies over the last decade have examined the acute impact of treating parental ADHD with stimulant medication (Chronis-Tuscano et al. 2017). Two studies found that both maternal ADHD and some measures of parenting behaviors improved with maternal stimulant medication (MSM) (Chronis-Tuscano et al. 2008; Waxmonsky et al. 2014). The majority of studies, however, have found no effect of MSM on parenting or child behavior, despite clinical improvement of maternal ADHD (Wang et al. 2014; Chronis-Tuscano et al. 2017). In the largest study to date, mothers with ADHD received either multimodal treatment for ADHD (group psychotherapy plus methylphenidate medication) or supportive therapy, after which all mothers received BPT (Jans et al. 2015). Although maternal ADHD improved more for mothers who received the multimodal adult ADHD treatment first, BPT was associated with equivalent improvements in child externalizing behaviors regardless of mothers' prior treatment condition.
It is noteworthy that in the aforementioned studies the average age of the children was 9, and ∼75% of the children were treated first or concurrently with stimulant medication, perhaps limiting the ability to detect an effect in children with ADHD of treating mothers initially. Thus, there is a need to examine the approach of treating mothers with younger children and children naive to medication for ADHD to determine whether early intervention may delay or reduce the need for child medication.
Treating mothers with ADHD of younger children at risk for ADHD (vs. older children who are already medicated) might improve child and family outcomes and disrupt at an earlier age the negative parent–child transactional processes that occur as a result of ADHD symptoms, thereby improving family outcomes. Such a strategy (i.e., treating mothers first) is aligned with the American Academy of Pediatrics (AAP) recommendation of employing behavioral interventions before initiating ADHD medication, particularly for young children with ADHD under the age of 6 (Subcommittee on Attention-Deficit/Hyperactivity Disorder et al. 2011).
Consistent with the AAP recommendation, a recent school-based study comparing sequences of child stimulant treatment and BPT for children ages 5–12 (Pelham et al. 2016) found that parents who received an 8-week BPT intervention before child stimulant treatment had higher session attendance and more improved child classroom behavior than families in which the child received stimulant medication first. In other words, child stimulant medication may have reduced motivation and engagement in behavioral treatment targeting parenting skills. The age range in this study was similar to the aforementioned maternal ADHD treatment studies, so it is unclear whether behavioral treatment targeting a younger age group may delay the need for medication. Nonetheless, these results support the strategy of starting with behavioral treatment for children with ADHD more generally before initiating child medication. However, what still remains unknown is whether it is better to treat maternal ADHD with medication before (or concurrently) with BPT, or to deliver BPT to mothers before maternal ADHD medication to try to reduce the need for maternal ADHD medication.
Questions about sequencing and combining treatments in multiplex ADHD families (families in which when a parent and a child have ADHD) can be answered with a Sequential, Multiple Assignment, Randomized Trial (SMART) design (Almirall and Chronis-Tuscano 2016). Participants in a SMART are initially randomized to receive a treatment and then are rerandomized to either intensified or augmented treatment or a different intervention modality during a second phase of treatment (i.e., the second randomization). SMART trials allow for an investigation of whether treatments should be sequenced in a particular order, as well as how to tailor treatment sequences for an individual child or family through the examination of moderators such as baseline characteristics, or initial response during the first phase of treatment. The ultimate goal of a SMART is to guide real-world clinical decision making.
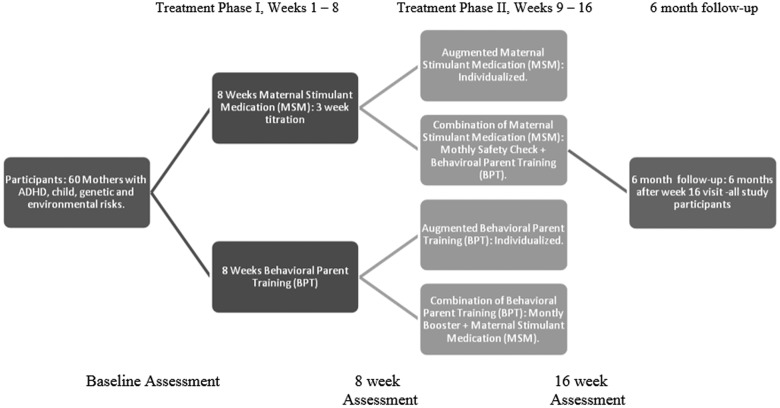
We undertook a pilot SMART to examine feasibility and acceptability of our study procedures and treatment as an initial step toward conducting a fully powered SMART to evaluate sequences of treatment for mothers with ADHD who have young children with ADHD symptoms (Almirall et al. 2012). The goal of a fully powered future trial would be to improve both parenting and child ADHD and functioning through the pathway of improving maternal ADHD. A SMART trial is necessary to evaluate the impact of treating mothers first, and would inform treatment algorithms for multiplex families. The four adaptive treatment strategies in the current study (Table 1) consisted of eight initial weeks (Phase I) of individually titrated MSM or BPT, followed by 8 weeks (Phase II) of the same intervention continued with as-needed modifications, or both treatments combined (MSM-BPT or BPT-MSM). Modifications added to monotherapy in Phase II, included adding a short-acting booster to increase duration of effect in MSM, or incorporating communication training or parent organizational skills in BPT. In this article, we evaluate four feasibility and acceptability aims.
Table 1.
Sequential, Multiple Assignment, Randomized Trial Pilot Adaptive Treatment Strategies
| Condition | Phase 1 MSM | Phase 1 BPT | Phase 2 MSM | Phase 2 BPT |
|---|---|---|---|---|
| MSM/MSM | MSM protocol—minimum of 7 visits | — | Weekly medication visits with prescriber, continued titration as needed | — |
| Addition of late-afternoon immediate release stimulant as needed | ||||
| MSM/BPT | MSM protocol—minimum of 7 visits | — | Monthly med safety check-ins with prescriber by phone, continued titration as needed | BPT protocol—8 visits |
| BPT/MSM | — | BPT protocol—8 visits | MSM protocol—minimum of 5 visits | Booster 1-hour sessions with therapist at weeks 12 and 16 focused on skill maintenance and trouble-shooting |
| BPT/BPT | — | BPT protocol—8 visits | — | Eight additional weekly therapy sessions focused on personalized content beyond manual depending on maternal needs (e.g., child emotion regulation skills; maternal organization and planning) |
BPT, behavioral parent training; MSM, maternal stimulant medication.
-
1.
Evaluate strategies for recruiting and retaining participants for a 16-week trial and 6-month follow-up period.
-
2.
Determine acceptability to families and clinicians of the overall study protocol, randomization, and measurement procedures.
-
3.
Pilot the feasibility, acceptability, and clinical utility of the four adaptive treatment strategies, specifically MSM and BPT protocols, as stand-alone treatments and in combination.
-
4.
Assess the feasibility of the monitoring and rescue strategy in cases of worsening child ADHD and need for child medication during the study.
Methods
Recruitment and screening
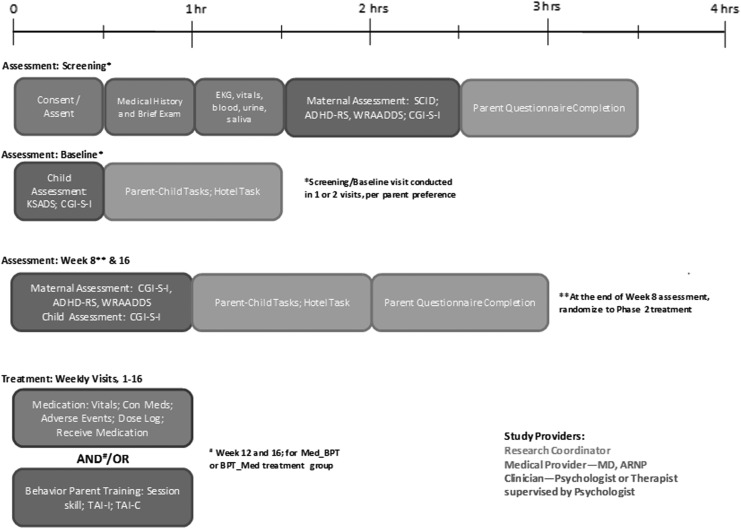
Mother–child dyads consisting of a mother with ADHD and her 3- to 8-year-old child with ADHD symptoms were recruited from internal clinic referrals, clinic newsletters, flyers, media advertisements (e.g., radio; website; magazine), and community support groups. Screening and assessment procedures are outlined in Figure 1. Research staff administered a telephone screening to assess maternal ADHD symptoms using six items from the Adult ADHD Self-Report Symptom Scale, age of mother and child, and past or current ADHD treatment. Eligible mothers (those with current symptoms who were not currently receiving medication for ADHD) were invited to an in-person diagnostic and baseline assessment visit requiring ∼5 hours spread across one to two visits depending on individual preference. The baseline diagnostic assessment included a clinical interview of the mother and child, rating scales and questionnaires, maternal physical exam, medical history, and laboratory studies (electrocardiogram [ECG], pregnancy, and toxicology screen). See Table 2 for baseline and follow-up assessment measures.
FIG. 1.
Treating mothers first study assessment and treatment guide.
Table 2.
Study Measures
| Measures and assessment | Screening/baseline | 8 Weeks | 16 Weeks | 6 Months |
|---|---|---|---|---|
| Maternal functioning | ||||
| CAARS | P | P | P | P |
| CAARS-O | O | O | O | — |
| WRAADDS | C | C | C | — |
| ACDS | C | C | C | — |
| BDI-II | P | P | P | — |
| WURS | P | P | P | — |
| Structured clinical interview for DSM-IV | C | — | — | — |
| CGI-S; CGI-I | C (CGI-S) | C | C | C |
| BFIS | P, O | P, O | P, O | P |
| Parenting and family functioning | ||||
| APQ-PR | P | P | P | P |
| FRI | P | P | P | — |
| DPICS-IV | O | O | O | — |
| DAS | P | P | P | — |
| Child functioning | ||||
| K-SADS-PL | C | — | — | — |
| Conners early childhood or 3 | P, T | P, T | P, T | P, T |
| Children's Impairment Rating Scale | P, T | P, T | P, T | — |
| CGI-S; CGI-I | C | C | — | C |
| Treatment engagement | ||||
| MSM pill count | — | P | P | — |
| Treatment attendance | — | C | C | — |
| Treatment adherence inventory (BPT groups) | — | P, C | P, C | P |
| Treatment acceptability and feasibility questionnaire | — | — | — | P |
| Other | ||||
| Informed consent | P, C/M | — | — | — |
| Demographic form | P | — | — | — |
| Maternal medical history | M | — | — | — |
| Maternal physical exam and vitals | M | — | — | — |
| Maternal vitals | M | M* | M* | |
| Maternal concomitant medication | M | M* | M* | — |
| Maternal adverse events | M | M* | M* | — |
| Maternal EKG, laboratories (pregnancy, CBC), urine toxicity screen | N | — | — | — |
M*, MSM groups only.
C, Clinician; M, Medical provider; N, Nurse; O, Observer; P, parent; T, Teacher.
ACDS, Adult ADHD Clinical Diagnostic Scale; ADHD, attention-deficit/hyperactivity disorder; APQ-PR, Alabama Parenting Questionnaire Preschool Revision; BDI-II, Beck Depression Inventory-II; BFIS, Barkley Functional Impairment Scale; BPT, behavioral parent training; CAARS, Conners Adult ADHD Rating Scale; CAARS-O, CAARS-Observer; CBC, complete blood count; CGI-S, CGI-I, Clinical Global Impressions Scale-Severity/Improvement; DAS, Dyadic Adjustment Scale; DPICS-IV, Dyadic Parent–Child Interaction Coding System; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-IV; EKG, electrocardiogram; FRI, Family Routines Inventory; KSADS-PL, Kiddie-SADS-Present and Lifetime Version; MSM, maternal stimulant medication; WRAADDS, Wender Reimherr Adult Attention Deficit Disorder Scale; WURS, Wender Utah ADHD Rating Scale.
Inclusion criteria for mothers included: (1) signed consent; (2) between the ages of 21–55 years (inclusive) at the screening visit; (3) English-speaking; (4) Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) diagnosis of ADHD, any subtype, at screening (or after medication washout, if required); and (5) current Clinical Global Impressions scale-ADHD severity (CGI-S) rating ≥4 and <7. Exclusion criteria included mothers who were (1) currently taking medication for ADHD; (2) pregnant or breastfeeding; (3) if taking an antidepressant, had had a dose change within the past 30 days; and (4) physical exam, laboratory studies, vital signs, or ECG results judged to be abnormal for age or contraindicative for stimulant treatment. ADHD diagnosis and psychiatric comorbidity were assessed with the Structured Interview for DSM-IV (SCID-IV) (First et al. 1996) and Beck Depression Inventory (BDI-II) (Beck et al. 1996).
In our previous study (Chronis-Tuscano et al. 2008), mothers with a history of, or current, mood disorder were excluded. This contributed to marked difficulty recruiting participants and limited generalization of findings, since the comorbidity of adult ADHD and depression is quite high in adult women (Kessler et al. 2006). Consequently, to enhance generalizability, we decided to include mothers with a comorbid depressive disorder as long as they were not actively suicidal, and to include those on a stable antidepressant dose. Although the majority of mothers were stimulant naive, we did not exclude mothers who had had a prior trial of stimulant medication. Mothers with and without a prior trial of stimulant medication went through the same randomization protocol, as described below.
Children ages 3–8 were eligible if they (1) displayed elevated ADHD symptoms on the Conners-3 Parent Rating Scale or Early Childhood Parent Rating Scale (Conners 2008) as indicated by a Hyperactivity or Inattention Index T-score >60; and (2) were not previously treated with an ADHD medication. As with the mothers, we did not exclude children with psychiatric comorbidities to enhance generalizability of the sample to community populations with ADHD.
As shown in the study timeline (Fig. 1), evaluation of mother–child dyads occurred at baseline and weeks 8 and 16. Additionally, a 6-month follow-up included parent questionnaires and a 20-minute phone assessment with a study clinician unaware of treatment sequence. Families received up to $20 transportation reimbursement for each completed treatment visit and 6-month follow-up phone call, and $50 each for the week 8 and 16 assessment visits.
Randomization and treatments
Before consent, mothers were informed of the possible treatment sequences. Following baseline assessment, mother–child dyads were randomized to receive either BPT or MSM in Phase I, and after 8 weeks were rerandomized to Phase II of treatment (Chronis-Tuscano et al. 2016). Mothers were informed in person of the results of randomization immediately following their completion of the baseline and 8-week assessments. Due to lack of prior information regarding maternal treatment sequencing or obvious tailoring variables based on initial treatment response, we decided to conduct a nonrestricted pilot SMART, meaning that all families were rerandomized in the second phase of the trial regardless of initial response. The four sequences of MSM and BPT treatment are found in Table 1 and treatments are briefly described below and in a previous article (Chronis-Tuscano et al. 2016).
Maternal stimulant medication
The initial 8-week phase of MSM consisted of lisdexamphetamine (LDX) titrated during weekly visits beginning at 20 mg, with dose increases until Clinical Global Impressions Scale-Improvement (CGI-I) was ≤2 (Improved or Very Much Improved), with minimal associated adverse events. Titration visits lasted ∼1 hour (30 minutes with the provider and 30 minutes for the hospital pharmacy to process the prescription). In the event of poor tolerability or nonresponse after week 4, an alternative methylphenidate stimulant could be tried. For mothers in the MSM-MSM condition, the 8-week Phase II consisted of titration visits in person or by phone weekly or on an as-needed basis. If there was inadequate duration of response reported by a mother, an immediate release formulation was added in the late afternoon. Mothers receiving BPT in Phase II (MSM-BPT) continued stimulant medication during BPT and received safety checks at weeks 12 and 16 with continued dose adjustments if needed.
Behavioral parent training
The initial 8-week phase of BPT included weekly one-hour individual BPT sessions provided using Barkley's Defiant Children Third Edition (Barkley 2013) manual. Skills presented included differential attention (e.g., paying attention to positive behaviors; ignoring minor misbehaviors), child-directed play, providing effective commands, use of a token system to reward compliance, time-out discipline, managing misbehavior in public, and creating a daily report card for use in school to complement the home token reward system. Children were invited to attend up to three sessions (Session 2 to practice Special Time; Session 4 to introduce Time Out; and Session 8 if more direct practice was deemed helpful) unless scheduling or practical concerns prevented this. In Phase II, mothers in the BPT-BPT condition were offered eight additional therapy sessions with a flexible curriculum depending on remaining needs. Potential topics included further enhancement of the reward system, active listening skills, emotion coaching, parent–child problem solving, or behavioral organizational skills to address mother's difficulty with task completion. In Phase II, mothers in the BPT-MSM condition received two additional follow-up BPT sessions (week 12 and 16) focused on reinforcing parenting skill use from Phase I.
Outcome measures
Feasibility of recruitment and retention
A screening form included documentation of how potential participants first heard about the study (e.g., study flyer; primary care clinician; website; magazine article; etc.). Study staff tracked contacts with potential participants as well as percentage who attended a screening visit and number screened into the study. Dropout, percentage completion of study assessments and time to complete the study were recorded.
Acceptability and feasibility of SMART design and protocol
The acceptability and feasibility questionnaire (AFQ) was developed specifically for this study to assess each treatment and study component (e.g., sequencing of treatments) and was collected during the week 16 assessment. Mothers completed quantitative (Likert-type scales) and qualitative (open-ended written) questions regarding assessments, treatment assignment/randomization satisfaction, benefits, interactions with study staff, clinicians, and medical providers. Additionally, a study staff member not affiliated with treatment or assessment completed a Clinician and Medical Provider Exit Interview over the phone with providers and study coordinators regarding their perceptions of assessment administration and treatment delivery to study participants. Questions addressed clinician training and supervision and ease of implementation and perceived benefits of the treatment protocols. Providers were asked to (1) provide numerical ratings; (2) answer open-ended questions regarding the most and least beneficial aspects of each study component; (3) and submit suggestions which could improve the study design for future, large-scale trials.
Treatment acceptability, feasibility, and adherence
The AFQ evaluated participant acceptability and qualitative feedback for MSM and BPT approaches, including benefits, drawbacks, burdensomeness of treatments, and suggested changes. The provider exit interview assessed provider perceptions of both treatments and requested qualitative feedback.
Adherence for the medication treatment groups was collected using the study coordinator's medication dosing log and pill counts collected at each medication visit, as well as a log of medication visits attended. Medication providers inquired and logged participants' reports of side effects at each in-person titration visit or phone call.
Adherence to BPT was assessed with session attendance, overall BPT skill use rated on the AFQ, the treatment adherence inventory—Caregiver [TAI; adapted from Kazdin et al. (1997)], and the TAI—Therapist, which were administered to mothers and clinicians after each weekly session during the BPT protocol. The TAI—Caregiver includes 23 skill-specific questions (e.g., “How often did you use the chip/point system to reward positive behaviors?”) with 5 Likert-scale choices ranging from “Almost Never” to “Almost Always.” It included seven overarching questions (e.g., “Overall, how would you rate your skill in using the techniques?”) with five answer choices ranging from “Not Very” to “Extremely,” as well as one open-ended comment question for treatment improvement suggestions. The TAI—Therapist is similarly structured with 17 skill-specific questions (e.g., “Does the caregiver practice special time with her child?”) and 7 general treatment questions (e.g., “Overall, how receptive is the caregiver to treatment?”). Caregivers and therapists were instructed not to rate items that did not apply and/or for skills that had not yet been taught.
Regarding acceptability of sequencing of treatments, mothers completed a Likert-style question on the AFQ [“Were you pleased to receive the treatment in the order you received it (e.g., medication first or medication after parent training)?”] followed by two open-ended questions asking mothers for their perceived benefits and drawbacks of the treatment sequence they received. Clinicians also reported qualitative information about participant responses to the different treatment sequences.
Monitoring of child severity and urgent need for medication
Clinical severity of child ADHD was tracked using the CGI-S at baseline, 8 weeks, and 16 weeks, and through verbal check-ins at weekly treatment visits. If CGI-S worsened, or parents at any time voiced concern regarding deterioration or need for medication, they were offered an “ASAP evaluation” (Abikoff et al. 2002) with a clinician other than their study treatment provider to assess the concerns, obtain a CGI, and to provide clinical recommendations.
Results
Key results related to each dimension of feasibility and acceptability are summarized in Table 3, along with implications for the design of a future trial of treatment sequences for mothers with ADHD.
Table 3.
Study Demographics
| Characteristic | Mother (n = 35) | Child (n = 35) |
|---|---|---|
| Mean age | 39.63 | 6.00 |
| Married or living with partner, n (%) | 28 (80) | n/a |
| Female gender, n (%) | 35 (100) | 16 (46) |
| Caucasian, n (%) | 30 (88)a | 31 (89) |
| Not Hispanic/Latino, n (%) | 33 (94) | 32 (91) |
| ADHD inattentive presentation, n (%) | 21 (60) | 11 (31) |
| ADHD combined presentation, n (%) | 10 (29) | 18 (51) |
| ADHD hyperactive–impulsive presentation, n (%) | 4 (11) | 0 (0) |
| ADHD not otherwise specified, n (%) | 0 (0) | 1 (3) |
| Did not meet ADHD criteria, n (%) | 0 (0) | 5 (14) |
| Current comorbid depressive disorder, n (%) | 8 (23) | 0 (0) |
| Current comorbid anxiety disorder, n (%) | 5 (14) | 1 (3) |
| Current oppositional defiance disorder, n (%) | 0 (0) | 8 (23) |
| History of ADHD medication treatment, n (%) | 5 (14) | 0 (0) |
n = 34.
ADHD, attention-deficit/hyperactivity disorder.
Study recruitment and retention
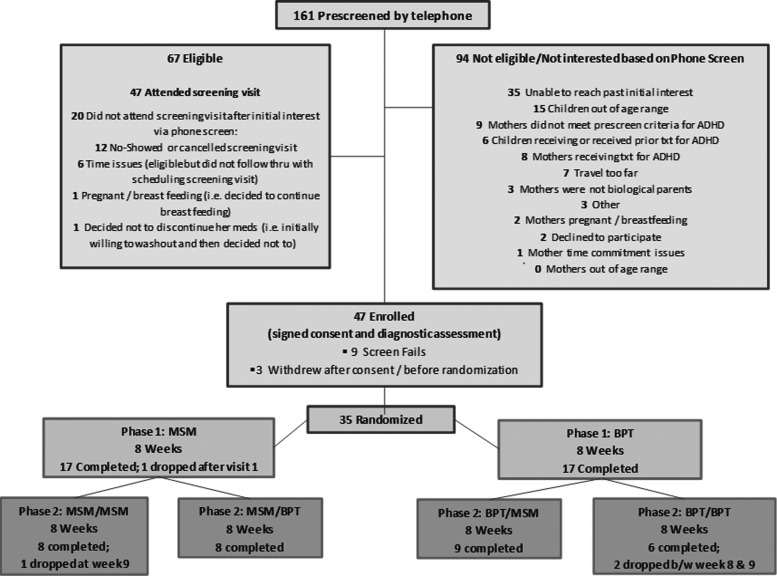
Over 31 months, 161 mothers were prescreened by telephone (see Fig. 2 for Consort Diagram). Despite attempts to recruit mothers with ADHD through adult psychiatrist colleagues and adult primary care, these efforts were largely unsuccessful as those recruited through adult psychiatric settings were often receiving ADHD medication already. Rather, most participants were recruited through media advertisements or referrals in an outpatient child psychiatric clinic. Of 67 mothers determined to be eligible in the phone screen, 47 agreed to attend a screening visit and 35 were randomized to treatment; demographic information for mothers and their children is found in Table 3. The most common reason that mothers declined to participate was a desire for direct treatment for their child (medication or psychotherapy). Fourteen percent of enrolled mothers had had a prior unsuccessful stimulant trial with frequencies in each intervention arm as follows: MSM-MSM 1 of 9; MSM-BPT 0 of 9; BPT-MSM 1 of 9; BPT-BPT 3 of 8. Retention was strong among those who attended the baseline visit with one drop-out in Phase I, but 3/35 participants dropped out after being rerandomized to Phase II treatment following the 8-week assessment (two in MSM-MSM, one in BPT-BPT). The mother who dropped out after one MSM visit in Phase I attributed her withdrawal to medication side effects. Of those who dropped out after 8 weeks, two mothers cited time commitment or length of drive as their reason and the third (randomized to MSM-MSM) stated that she did not want to continue medication despite not reporting side effects to her prescriber during Phase I (although she did note insomnia in her retrospective AFQ).
FIG. 2.
Treating mothers first study consort diagram.
Feasibility of study protocol
On the AFQ, participants' main qualitative concern regarding the assessment was a desire for more flexibility in scheduling/availability (n = 3). All other comments were noted once: desire for more comprehensive, personalized assessment; more personalized feedback about the assessment; more preparation regarding the sensitivity of assessment questions; more information/resources on ADHD diagnosis and strategies to manage it; too time consuming; wanting questionnaires to be sent out sooner; request to have the Principal Investigator conduct all assessments; request for a visual chart to denote study timeline.
Obtaining collateral report on maternal and child functioning proved to be quite difficult, especially teacher ratings, although teachers were provided with a $20 payment for questionnaire completion. At baseline, 37% (n = 13) of teacher data were missing; 62% (n = 21) were not collected at week 8, and 55% (n = 17) of data were missing at week 16. Teacher measures were not solicited for three children who were on summer break during initial assessment.
In their exit interview, assessing clinicians (three clinical psychologists) reported high satisfaction with the validity of the assessment visits (M = 4.67 out of 5, SD = 0.58). However, they noted that lengthy assessments appeared to be a burden for study participants (M = 4.67, SD = 0.58) and somewhat of a burden for clinicians (M = 3.33, SD = 1.53). Open-ended comments included observed redundancy in ADHD symptom measures and suggestions to send rating scales ahead of time. In contrast to initial (baseline) evaluations, all three assessors agreed that the follow-up assessments were streamlined and useful.
Regarding randomization, three mothers who were phone screened declined to participate in the study after learning about randomization. Of those who enrolled, none reported concern about randomization procedures, although anecdotally several mothers expressed initial disappointment at being randomized to MSM in either Phase I or II and as previously mentioned, three mothers withdrew after randomization to the same treatment in Phase II. On the AFQ, there was a quantitative trend that mothers reported feeling more “pleased” at being assigned to BPT either in a monotherapy (M = 5.42 out of 6) or a combined condition (M = 5.9), compared to being assigned to MSM monotherapy (M = 3.42) or combined treatment (M = 4.25). Slightly longer duration to complete all study and treatment visits, which may be a sign of participant burden, was found for participants in BPT conditions, with median time to complete the study of 15.5 weeks (MSM-MSM), 17.0 weeks (MSM-BPT), 18.1 weeks (BPT-MSM), and 19.9 weeks (BPT-BPT).
Feasibility of study treatments
Medication treatment
Regarding adherence, 90% (146) of the 163 medication “minimally required visits” (i.e., not including visits to continue titration or to begin a different medication) were completed in the clinic; the others were completed by phone or rescheduled. Medication adherence as measured by pill counts was high (range 89.2%–98.9%). A logistic regression with condition predicting percent of doses taken, clustered by participant, was used to compare medication adherence across treatment condition. Medication adherence did not differ significantly across the three MSM conditions.
Regarding the adequacy of the MSM strategy, 22 of 27 mothers assigned to a MSM sequence were optimized on LDX. The mean LDX dose to optimize was 45.45 mg and the mean number of weeks to optimize was 6.57 weeks for MSM-MSM, 9.67 for MSM-BPT, and 4 weeks for BPT-MSM (although three mothers in BPT-MSM switched to another formulation). Of note, only 7 of the 22 mothers made a medication change after 8 weeks. Of the five mothers who did not remain on LDX, one dropped from the study before the second titration visit in Phase I due to side effects (MSM-BPT condition), one discontinued all medication due to side effects (after trying LDX, osmotic release oral system [OROS] methylphenidate, and bupropion), two were optimized on a different stimulant (mixed amphetamine salts, OROS methylphenidate), and one was optimized on atomoxetine after experiencing an exacerbation of tics on LDX and OROS methylphenidate.
Mothers who enrolled in the study on either an antidepressant (n = 6) or antianxiety (n = 4) medication had no difficulties tolerating LDX, and all completed both phases of treatment. There were no serious adverse events, and adverse events reported were consistent with the study medication insert, and as outlined in the consent form. There was a single report of suicidal ideation (without plan or intent) at week 10. The mother reported that the ideation co-occurred with a stressful life event and persisted for less than 1 day. She continued in the study without incident.
Of the mothers in the MSM-MSM group who completed both phases of treatment (n = 7 of 9), changes during Phase II included the following: short-acting stimulant added to increase duration of effect (n = 2), 10 mg increase in LDX to improve efficacy (n = 2, at week 9 and 12, respectively), and LDX discontinued after week 8 due to orthostatic hypotension and tachycardia (medication was switched to methylphenidate; n = 1).
Regarding acceptability of MSM, most mothers reported on the AFQ that they experienced improved focus and decreased restlessness when they were medicated on an optimal dose. The most common negative comment was that the duration of each of the 1-hour titration visits was too long (n = 7). Other qualitative suggestions mentioned once included: provide medication after the study for gap coverage; provide natural medication options; give more information about the medication and more personal consultation; reduce repetitive medical questions at each titration visit (relating to concomitant medications and adverse events); and request for phone visits.
In exit interviews with the four medication providers (MD = 3; ARNP = 1) the MSM condition was viewed as beneficial for mothers and none suggested changes to the medication protocol. Medication providers responded they were “extremely satisfied” with the titration procedures (M = 5.75 out of 6, SD = 0.5), “extremely satisfied” with augmenting medication as needed for increased duration (M = 6.0, SD = 0), found the treatment algorithm “very acceptable” to administer (M = 5.5, SD = 0.58), and “quite acceptable” for their clients (M = 4.5, SD = 1.0). Providers rated their training (M = 1.0, SD = 1.0) and consultation (M = 1.75, SD = 0.96) as carrying low burden and that titration carried a fairly low burden for participants (M = 2.0, SD = 0.71). Two providers noted some concern about stimulant side effects. One provider requested increased clarity about making changes during Phase II for MSM-BPT group, who had fewer medication follow-up visits.
Behavioral parent training
BPT clinicians were two PhD psychologists, one predoctoral intern, and three predoctoral clinical psychology student therapists (all with Master's degrees). Of the 266 scheduled BPT sessions, 234 (88%) of the sessions occurred as planned and all but 4 were rescheduled (3 were for a participant who ultimately dropped out of the study). All mothers who began an 8-week BPT condition attended all 8 sessions, and mothers in BPT-BPT averaged 15.83 out of 16 sessions.
Regarding BPT acceptability, the most common qualitative comment on the AFQ about benefits of BPT was that it helped the mother understand their child and improved child behavior. The most common drawback mentioned was the desire for more personalization of skills taught. After one participant requested that her partner join the BPT sessions, an IRB amendment was approved to include spouses/partners in BPT sessions. However, no other mothers elected to include their spouses/partners. Other comments reported by one person included: check in process to the hospital clinic was too lengthy; desire for materials to take notes; more focus on helping the parent manage her own organizational and coping skills; provide social or group support; challenges sitting still and focusing on material; more role play; and need for more sessions.
In clinician exit interviews, all six BPT clinicians reported overall satisfaction with the curriculum, length of treatment, and benefits of the intervention for families. BPT providers were satisfied with their training (M = 4.42 out of 6, SD = 1.11). Therapist burden in completing training was overall low (M = 2.17, SD = 1.51); however, one student clinician rated training as “very burdensome.” Therapists rated quality of feedback and supervision as high (M = 4.90, SD = 1.24) and burden of supervision as low (M = 2.0, SD = 1.09). Therapists reported feeling comfortable (M = 4.25, SD = 0.5) implementing an individualized treatment plan in weeks 9–16 for the BPT-BPT treatment arm.
Clinicians perceived that BPT was somewhat burdensome for participants (M = 3.83, SD = 0.68). On average, therapists felt the number of BPT sessions was appropriate (M = 4.0, SD = 1.67); however, the clinician responses to this question ranged widely from a score of 2 to 6. This range may have reflected whether their clients received 8 or 16 weeks of BPT, or whether complicating factors were present (e.g., parental depression; child with severe symptoms).
In open-ended exit interview questions, all clinicians reported that training and supervision was adequate, although the student therapists specifically noted logistical difficulties with long-distance supervision and a desire for on-site supervision as well. Two student clinicians felt that BPT sessions were too rushed and suggested that longer, or extra, sessions were needed in the 8-week protocol. Nearly all clinicians mentioned a desire to tailor the skills for families or skip content that was not relevant to a particular family. A majority of clinicians mentioned concern about the lack of a “planned ignoring” skill, which is a staple of many parent training programs, in the Barkley manual (although a similar concept is included in the section on differential attention). Several clinicians also suggested providing parents with more information on BPT and expectations for participating in BPT before beginning the program.
Supervisors documented clinicians' questions and treatment challenges during supervision. The most common challenge was that mothers reported being overwhelmed with BPT homework, particularly with implementing the token system and using time out procedures. Some mothers needed time management prompts to remember to use skills, for example, using their mobile phone calendar for reminders about special playtime or to provide labeled praise.
Combination treatment
On the AFQ, most participants who received combination treatment rated both BPT and stimulant medication treatments as helpful, and there were no dropouts in combined conditions. Results using a Kruskal–Wallis test with AFQ data indicated no significant differences between groups regarding acceptability. The Kruskal–Wallis test using maternal AFQ report indicated a trend (p = 0.09) that, among parents who received BPT, mothers in BPT-BPT practiced behavioral parenting skills less (M = 4.1, SD = 1.1) than those who were in either the MSM-BPT (M = 5.3, SD = 1.0) or BPT-MSM groups (M = 5.1, SD = 1.6). Another trend (p = 0.08) indicated mothers were more pleased with assignment to medication if they had received BPT first, compared with those assigned to MSM in Phase I. A Fischer's exact test compared BPT skill adherence as measured by the TAI-Therapist questionnaire across conditions and found no difference in skill adherence across conditions.
Of mothers who responded to the AFQ regarding whether they would change the order of combination treatment (n = 13), six preferred medication first (three who received medication first noted no change to treatment order) and three who received BPT first suggested that medication should be received first so as to improve their implementation of the behavioral skills taught in BPT. Four mothers who received BPT first preferred that order. Two mothers who received medication first, suggested treatments be started concurrently. One mother suggested offering mothers a choice of which to begin first.
Child monitoring strategy
During the course of the study, only one ASAP assessment was conducted to address severe or worsening child behavior during the course of the study that might require child medication. In this case, the mother told her BPT therapist during week 14 of BPT-BPT that the child's teacher reported behavioral concerns during his first week of kindergarten and suggested a special education evaluation. The BPT therapist helped this parent communicate with the teacher and school counselor about classroom accommodations. The ASAP evaluation concluded that no immediate child medication treatment was required.
Discussion
The main objective of the study was to examine feasibility and participant/provider acceptability of conducting a pilot SMART to evaluate treating maternal ADHD using medication, BPT, or a combination of treatments offered in one of four sequences. This pilot feasibility study is the first step toward the broader objective of determining the best treatment combination and sequencing for parents with ADHD and their young children with the goal of improving maternal ADHD, parenting, and child outcomes. Results indicated that the SMART study procedures were feasible to implement as designed but that feasibility of the study protocol may be improved with alternative recruitment resources, shorter duration and number of assessment and treatment visits, alternative modalities (telehealth) for administering assessment measures and obtaining collateral reports, and inclusion of more detailed evaluation feedback and psychoeducation about ADHD (Table 4). Feasibility of and adherence to the two treatment strategies alone and in combination were high, and both strategies were acceptable to participants and providers with some differences in perceived burden. These findings have implications for the design of a subsequent treatment sequencing trial for mothers with ADHD.
Table 4.
Feasibility Issues and Changes for Full-Scale Sequential, Multiple Assignment, Randomized Trial
| Feasibility dimension | Lessons learned in pilot SMART | Changes for full-scale SMART |
|---|---|---|
| Recruitment | Most mothers contacting study were seeking treatment for their child (recruited through psychiatric clinics) Most children diagnosed with ADHD before study |
Recruit/screen in primary care (e.g., pediatrics, family medicine) Recruit younger children in “at risk” range for ADHD |
| Study assessments and protocol | Lengthy clinic visits High participant burden Low return of collateral report (i.e., teacher) measures Request further information and evaluation feedback on ADHD |
Administer questionnaires digitally Streamline assessment measures; briefer battery Administer collateral report digitally Provide evaluation feedback session with psychoeducation on ADHD |
| Medication treatment | Weekly sessions not consistently needed for titration Minimal titration after 8 weeks Only a minority needed a booster for increased duration LDX was effective and well tolerated for the majority |
Offer med titration visits through telehealth Switch to every-other week once optimized Abbreviate length of titration visits to be more feasible in real-world |
| Behavioral treatment | Clinician and family desire for more personalization and tailoring Diminishing acceptability and lower skill use for 16 weeks over 8 weeks BPT Some mother struggled to complete weekly home skills practice Clinician preference for on-sight supervision |
Allow selection of supplemental modules based on family needs Tailor number of sessions to treatment response Increase clinician support for home skill practice Combined supervision through local supervisor plus expert/remote supervisor |
| Combination treatment | Drop out occurred only with monotherapy Generally, mother preference for meds before BPT |
Consider tailoring of treatment sequence based on initial response (SMART design) Assess treatment preference and whether that moderates adherence and treatment response |
| Child monitoring | Procedure was adequate No children required medication during 16-week study period |
It is feasible to treat mothers first |
ADHD, attention-deficit/hyperactivity disorder; BPT, behavioral parent training; LDX, lisdexamphetamine; SMART, Sequential, Multiple Assignment, Randomized Trial.
There were no cases where the child's behavior worsened to the point of requiring stimulant treatment during the study period, and parents were willing to engage in 16 weeks of study treatment despite lack of direct intervention with the child. The threshold for considering stimulant treatment in the current study was quite high, but was appropriate for all participants, even the many children who already had diagnoses of ADHD.
The SMART study procedures, including recruitment, randomization, and study evaluations, were successful but require some changes for a fully powered SMART. It took longer to recruit families than anticipated, and the majority of interested mothers did not enroll in the study. Mothers who declined participation often reported concern about the burden of study visits or a preference for direct child treatment. We believe this may be attributed in part to most referrals coming through an outpatient child psychiatry clinic, where parents were seeking child treatment (rather than treatment for themselves). To study whether medication and/or parent training of mothers with ADHD delays the need for child medication treatment by reducing the environmental risk component of ADHD in this population, we will need to enroll more children who are at-risk for ADHD, rather than those who are already diagnosed. One way to achieve this goal is to recruit a younger sample, perhaps through pediatric primary care, where parents often first raise concerns about their young children's attention and behavior (Bernal 2003) and parents of children with disruptive behavior could feasibly be screened for parental ADHD. Recruitment and implementation in primary care should also be more efficient and less burdensome to families. Furthermore, our sample consisted of more Caucasian and middle-class families than is typical of the area, in which the study was conducted (66.3% Caucasian; “Seattle Race & Ethnicity,” 2018), although on par with ethnic demographics (6.6% Hispanic/Latino ethnicity reported). This limitation may also be addressed in a subsequent trial by recruiting through primary care and in other regions of the United States.
Time constraints and commitment were raised as factors that inhibited mothers from completing a screening visit. The 5 hours required for this visit was too burdensome for study participants and would not be not feasible in the real world. A future trial may employ strategies that would be realistic to use in community settings, such as reducing questionnaires, sending questionnaires digitally to be completed at home, streamlining parent and child diagnostic assessment, and conducting interviews using telehealth technology. To increase participants' understanding of the study process, it will be helpful to provide them with a study assessment and treatment guide visually explaining the visit time points and duration (Fig. 3).
FIG. 3.
Treating mothers first study schematic.
The low rate of collateral report data from teachers and mothers' significant others is a limitation of the current study. To address low rates of gathering collateral report, we will rely on direct contact to collect information in future work. Directly emailing questionnaires to teachers and significant others through a secure web-based program (e.g., REDCAP) instead of relying on mothers to organize distribution and collection of these paper materials will likely increase response rates.
Overall, there were no significant differences in acceptability and adherence across intervention groups, and mothers rated treatments as good to extremely satisfying, which speaks well to the future delivery of these treatments. Study participants were not compensated for session attendance but did receive compensation for transportation costs (up to $20 per session), which must be taken into account when evaluating satisfaction compared with families in the community who incur financial burden to access treatment. Based on qualitative data, there was higher acceptability for families that received both MSM and BPT treatment arms as opposed to one modality, and the only mothers to withdraw mid study did so after being rerandomized to monotherapy in Phase II. Thus, there is indication that multimodal treatment is beneficial and perhaps preferable for many families and that 8 weeks is likely an appropriate time frame for delivery of both pharmacological and behavioral ADHD treatments. The high acceptability of combined treatment is noteworthy compared with prior multimodal treatment sequencing studies, such as the Sequence Treatment Alternatives to Relieve Depression (STAR*D) Study, in which only 1/3 of participants who had received a medication for depression consented to being randomized to a second treatment phase that included medication or cognitive therapy (Thase et al. 2007). Some patients may be hesitant to receive behavioral rather than pharmacological treatment, but our trial and other recent ADHD treatment trials (Pelham et al. 2016) indicate that satisfaction may indeed be improved with combined versus unimodal treatment. Indeed, mothers in our study reported feeling more pleased about randomization to BPT than MSM. In a future study, an adaptive design similar to the aforementioned sequencing studies could be used, wherein only treatment nonresponders are randomized to a second phase of treatment with a focus on implementing and evaluating combined treatments. Despite being initially more pleased about randomization to BPT than MSM, numerous mothers suggested during exit interviews that it would be most beneficial to receive medication before (or concurrently with) BPT, to improve their ability to implement BPT skills consistently. Concurrent medication and BPT may be considered in a future trial.
Regarding perceived burdensomeness and adequacy of medication treatment, several participants in the medication treatment group noted the titration visits were too frequent or lengthy. Indeed, the majority of mothers were optimized on their medication by 8 weeks, indicating that weekly medication titration sessions were likely not needed after optimization. Titration visits could also be shortened to more closely resemble real-world medication follow-up visits. Mothers who received medication over a 16-week period took longer to optimize than those who received only 8 weeks of titration, especially those who received MSM-BPT. It may be that more nuanced changes could be made over longer time periods, or possibly that mothers realized they needed dose adjustments as they began to attempt using BPT skills. Also, more mothers in the BPT-MSM condition did not optimize on LDX compared with the other two MSM conditions. Perhaps after receiving BPT, mothers were more comfortable expressing dissatisfaction about tolerability, better able to focus on and notice side effects in light of improved family functioning, or had higher expectations for medication during the study.
For mothers in BPT conditions, those who received 16 weeks of BPT monotherapy took longer to complete the study, rated visits as somewhat more burdensome, and reported less skill use than those who received 8 weeks of BPT. Many families may not need such lengthy BPT treatment, and the number of sessions should be tailored to treatment response and individual needs in a future trial. Furthermore, mothers in the medication groups had opportunities to skip weekly visits if their medication was titrated correctly, so including this option for the BPT condition may be helpful. Despite allowing fathers to participate in BPT sessions, only one opted to do so. Prior studies have found challenges of engaging fathers in BPT (Fabiano 2007), and this is an important area for further study.
Regarding parent and clinician desire for personalization of BPT, we can address this by teaching core BPT concepts to all parents (e.g., increased attention to positive behaviors; special time play; differential attending; token system) and then tailoring additional treatment modules to needs. For example, clinicians may select an emotion regulation or parent emotion coaching module (Chronis-Tuscano et al. 2016), or a time management module, depending on what the parent would find more beneficial. Allowing parent participation in selecting supplemental treatment modules is likely to increase skill practice and engagement in session. Some mothers also struggled with homework completion. This trend may be related to the decision to alter delivery from 10 to 8 sessions by combining treatment modules. Suggested modifications to reduce the burdensomeness of session homework include adjusting the token system to focus on a particular time of day (e.g., nighttime routine) to make the home point system more manageable and less intimidating initially and allocating extra session time to review and troubleshoot skill use. Text messages or phone calls (perhaps using telehealth technology) between sessions may boost skills practice and may also prevent missed appointments.
Conducting both MSM and BPT visits through telehealth in a future trial would substantially reduce participant treatment burden and increase access for families. Numerous studies have demonstrated that mental health treatment can be feasibly delivered through telehealth, including BPT for parents of children with ADHD specifically (Xie et al. 2013; Myers et al. 2015).
Conclusions
In summary, mothers of multiplex families with ADHD were well engaged in a pilot SMART providing pharmacology and BPT. MSM, BPT, and the combination are feasible and acceptable to this population. There appears to be a preference for combined treatment compared with the increased burden of offering a single strategy for longer than 8 weeks. Our findings indicate that mothers with concerns about their children's ADHD symptoms are receptive to receiving treatment themselves as an initial strategy for improving their children's health and functioning. Subsequent analysis will investigate effects of these multimodal treatments on maternal ADHD symptoms and functioning, parenting, and child functioning, and the need for direct child treatment strategies (e.g., medication).
Clinical Significance
Treating parental ADHD may improve the course and treatment response of child ADHD. The current study evaluated the feasibility and acceptability of treating mothers with ADHD with stimulant medication alone or in combination with BPT through a sequential randomized design. The study contributes knowledge about how to evaluate optimal sequencing of medications and behavioral treatment in this population with the goal of ultimately reducing the need for child ADHD treatment.
Acknowledgments
The authors are grateful to Kelsey E. Woods for data collection and synthesis, to the medication prescribers William French, MD, Soo Kim, MD, Lindsey Miller, ARNP, and Sam Zinner, MD as well as our behavioral therapy providers Joy Kawamura, PhD, Melanie Klein, BA, Michelle Kuhn, MS, Erika Ruberry, MS, Michael Vitulano, PhD, and Erin Underbrink, PhD.
Disclosures
E.S., A.C.-T., J.S., and D.A., have no disclosures to report. M.A.S., has received research support from Shire and Supernus, and is a consultant for Akili Interactive Labs, Arbor, Ironshore, KenPharm, Medicie, Neos, NLS, Shire, Supernus, and Sunovian.
References
- Abikoff H, Arnold LE, Newcorn JH, Elliott GR, Hechtman L, Severe JB, Wigal T, Shapiro C, Cantwell DP, Conners CK, Greenhill LL, Hinshaw SP, Hoza B, Jensen PS, Kraemer HC, March JS, Pelham WE, Swanson JM, Vitiello B, Wells KC: Emergency/adjunct services and attrition prevention for randomized clinical trials in children: The MTA manual-based solution. J Am Acad Child Adolesc Psychiatry 41:498–504, 2002 [DOI] [PubMed] [Google Scholar]
- Almirall D, Chronis-Tuscano A: Adaptive interventions in child and adolescent mental health. J Clin Child Adolesc Psychol 45:383–395, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almirall D, Compton SN, Gunlicks-Stoessel M, Duan N, Murphy SA: Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Stat Med 31:1887–1902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA: Defiant Children: A Clinician's Manual for Assessment and Parent Training. New York, Guilford Press, 2013 [Google Scholar]
- Beck AT, Steer RA, Brown GK: Beck depression inventory-II. San Antonio 78:490–498, 1996 [Google Scholar]
- Bernal P: Hidden morbidity in pediatric primary care. Pediatr Ann 32:415–420, 2003 [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Lewis-Morrarty E, Woods KE, O'Brien KA, Mazursky-Horowitz H, Thomas SR: Parent-child interaction therapy with emotion coaching for preschoolers with attention-deficit/hyperactivity disorder. Cogn Behav Pract 23:62–78, 2016 [Google Scholar]
- Chronis-Tuscano A, Raggi VL, Clarke TL, Rooney ME, Diaz Y, Pian J: Associations between maternal attention-deficit/hyperactivity disorder symptoms and parenting. J Abnorm Child Psychol 36:1237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Wang CH, Woods KE, Strickland J, Stein MA: Parent ADHD and evidence-based treatment for their children: Review and directions for future research. J Abnorm Child Psychol 45:501–517, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK: Conners 3rd Edition: Manual: Multi-Health Systems. North Tonawanda, NY, MHS Assessments, 2008 [Google Scholar]
- Fabiano GA: Father participation in behavioral parent training for ADHD: Review and recommendations for increasing inclusion and engagement. J Fam Psychol 21:683, 2007 [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB: User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders—Research Version. New York: Biometrics Research Department, New York State Psychiatric Institute, 1996 [Google Scholar]
- Jans T, Jacob C, Warnke A, Zwanzger U, Groß-Lesch S, Matthies S, Borel P, Hennighausen K, Haack-Dees B, Rösler M, Retz W, von Gontard A, Hänig S, Sobanski E, Alm B, Poustka L, Hohmann S, Colla M, Gentschow L, Jaite C, Kappel V, Becker K, Holtmann M, Freitag C, Graf E, Ihorst G, Philipsen A: Does intensive multimodal treatment for maternal ADHD improve the efficacy of parent training for children with ADHD? A randomized controlled multicenter trial. J Child Psychol Psychiatry 56:1298–1313, 2015 [DOI] [PubMed] [Google Scholar]
- Johnston C, Mash EJ, Miller N, Ninowski JE: Parenting in adults with attention-deficit/hyperactivity disorder (ADHD). Clin Psychol Rev 32:215–228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE, Holland L, Crowley M, Breton S: Barriers to treatment participation scale: Evaluation and validation in the context of child outpatient treatment. J Child Psychol Psychiatry 38:1051–1062, 1997 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM: The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry 163:716–723, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers K, Vander Stoep A, Zhou C, McCarty CA, Katon W: Effectiveness of a telehealth service delivery model for treating attention-deficit/hyperactivity disorder: A community-based randomized controlled trial. J Am Acad Child Adolesc Psychiatry 54:263–274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA, Waxmonsky JG, Greiner AR, Gnagy EM, Pelham WE, III, Coxe S, Verley J, Bhatia I, Hart K, Karch K, Konijnendijk E, Tresco K, Nahum-Shani I, Murphy SA: Treatment sequencing for childhood ADHD: A multiple-randomization study of adaptive medication and behavioral interventions. J Clin Child Adolesc Psychol 45:396–415, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management, Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S: ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, Fava M, Nierenberg AA, McGrath PJ, Warden D, Niederehe G, Hollon SD, Rush AJ: Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: A STAR* D report. Am J Psychiatry 164:739–752, 2007 [DOI] [PubMed] [Google Scholar]
- Wang CH, Mazursky-Horowitz H, Chronis-Tuscano A: Delivering evidence-based treatments for child attention-deficit/hyperactivity disorder (ADHD) in the context of parental ADHD. Curr Psychiatry Rep 16:474, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxmonsky JG, Waschbusch D, Babinski D, Humphrey H, Alfonso A, Crum KI, Bernstein M, Slavec J, Augustus JN, Pelham WE: Does pharmacological treatment of ADHD in adults enhance parenting performance? Results of a double-blind randomized trial. CNS Drugs 28:665–677, 2014 [DOI] [PubMed] [Google Scholar]
- Xie Y, Dixon JF, Yee OM, Zhang J, Chen YA, DeAngelo S, Yellowlees P, Hendren R, Schweitzer JB: A study on the effectiveness of videoconferencing on teaching parent training skills to parents of children with ADHD. TelemedJ E Health 19:192–199, 2013 [DOI] [PubMed] [Google Scholar]