Abstract
Objective: To guide clinicians in selecting the “next line” selective serotonin reuptake inhibitor (SSRI) for adolescents with treatment-resistant major depressive disorder, we sought to compare response rates among SSRIs in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) study and to jointly model tolerability and efficacy for the specific SSRI comparisons.
Methods: Efficacy and tolerability data for paroxetine, citalopram, and fluoxetine were extracted from the TORDIA study. Using a joint bivariate normal likelihood for response and tolerability (based on the maximum implied variance from the 95% credible intervals previously reported for the three SSRIs), a Monte Carlo pseudorandom sample (100,000 draws) was obtained, from which credible intervals, means, posterior tail probabilities, etc. were determined. Joint null hypotheses of no difference in efficacy and tolerability were then evaluated with regard to superiority of each SSRI over the others.
Results: No significant differences in response were observed for citalopram compared with fluoxetine (p = 0.247) or for fluoxetine compared with paroxetine (p = 0.110), although citalopram trended toward being superior to paroxetine (mean difference: 0.2, p = 0.055). For efficacy–tolerability models, citalopram and fluoxetine were superior to paroxetine (p = 0.029 and p = 0.022, respectively) but did not differ between each other (p = 0.146).
Conclusions: Joint efficacy–tolerability models suggest that citalopram and fluoxetine were statistically significantly superior to paroxetine while citalopram trended toward superiority over paroxetine in the efficacy model. These findings provide a more granular and practical evidence base for clinicians faced with treatment sequencing decisions in adolescents with SSRI-resistant depression.
Keywords: depression, major depressive disorder, paroxetine, sertraline, fluoxetine
Introduction
Two in five adolescents with major depressive disorder (MDD) fail to respond to initial treatment with a selective serotonin reuptake inhibitor (SSRI) (Emslie et al. 2002, 2009). In fact, current treatments produce remission in only 30% of adolescents (March et al. 2004, 2007; Curry et al. 2011). Moreover, adolescents with persistent depressive symptoms are more likely to be psychiatrically hospitalized and to attempt suicide; they are more likely to have impaired peer and family relationships, lower academic achievement, and are more likely to develop additional psychiatric morbidity. However, we have few studies to inform “next step” interventions for youth with SSRI-resistant MDD. The largest trial comparing antidepressant switching, the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) Study, randomized adolescents aged 12–17 years (n = 334) to four evidence-based treatments for 12 weeks (March et al. 2004). Patients were randomized to switch to (1) a different SSRI (paroxetine, citalopram, or fluoxetine); (2) a different SSRI + cognitive behavioral therapy (CBT); (3) venlafaxine; or (4) venlafaxine + CBT. Improvement was assessed with the Clinical Global Impressions-Improvement (CGI-I) scale (Guy 1976). CBT + medication switch (either to another SSRI or venlafaxine) was associated with a marked increase in response compared with simply changing medication, and no differences were detected between switching to venlafaxine relative to another SSRI. Longer-term follow-up of the TORDIA patients suggests that by 72 weeks >60% of youth reached remission and that the initial TORDIA treatment assignment did not predict remission rate or time to remission. Interestingly, patients who were switched to a second SSRI (as opposed to venlafaxine) experienced a more rapid improvement in depressive symptoms and suicidal ideation than those assigned to venlafaxine (Vitiello et al. 2011).
In TORDIA, post hoc analyses of response rates for the three SSRIs suggested no differences among the SSRIs (paroxetine, 19/50 [38.0%; 95% confidence interval (CI), 25%–52%]; fluoxetine, 41/84 [48.8%; 95% CI, 38%–60%]; citalopram, 19/34 [55.9%; 95% CI, 39%–73%]; χ2 = 2.81, p = 0.25). However, these frequentist estimates may fail to detect group differences and suffer limitations with regard to small samples and distributional assumptions of symmetry. Over the past decade, advances in Bayesian modeling have facilitated analyses of efficacy and tolerability of psychopharmacologic interventions in youth (Cohen et al. 2012) and the impact of clinical characteristics on antidepressant treatment response (Fountoulakis et al. 2013).
Previously, we employed Bayesian models to evaluate placebo response in pediatric anxiety disorders (Strawn et al. 2017) and to analyze abandoned clinical trials (Strawn et al. 2018). This approach provides clinically significant insight into the efficacy and tolerability of treatments and, compared with traditional statistical approaches, requires fewer restrictive assumptions regarding the relative variance of treatment groups. Furthermore, other restrictions inherent to the data can be easily incorporated (e.g., bounds above 0).
In the decade since the TORDIA trial was completed, there has been increased attention given to the heterogeneity of SSRI treatment response in internalizing disorders and to SSRI-specific differences in tolerability (Dobson et al. 2019). In this regard, antidepressant response rates in adolescents with MDD vary substantially (March et al. 2004; Emslie et al. 2006, 2009), although contemporary work suggests that fluoxetine produces the greatest magnitude of antidepressant effect compared with placebo (Cipriani et al. 2016). In addition, pediatric patients who are treated with tricyclic antidepressants, duloxetine, and venlafaxine have higher rates of adverse effects than placebo (Cipriani et al. 2016). Taken together, these data underscore the need for evidence-based approaches for treatment-resistant depression in adolescents, particularly given that at least 50% of adolescents receiving antidepressant in acute or continuation treatment are nonadherent (Fontanella et al. 2011).
With these considerations in mind, we sought to comparatively evaluate SSRI response rates in the TORDIA study and then to jointly model tolerability and efficacy for the specific SSRI comparisons to guide clinicians in selecting the “next line” SSRI for adolescents with MDD who have failed to respond to an initial course of SSRI treatment of adequate dose and duration. Given previous randomized controlled trials of paroxetine in children and adolescents with MDD (Emslie et al. 2006), we hypothesized that response rates would be lower in paroxetine-treated patients (compared with those youth receiving fluoxetine or citalopram). In addition, prior reports highlight tolerability concerns in pediatric patients receiving paroxetine with 30% of patients withdrawing from one prior trial (Emslie et al. 2006). In the context of these concerns, we hypothesized that tolerability–response profiles for paroxetine would be inferior to citalopram or fluoxetine. Lastly, given the expansive evidence supporting the salutary effects of psychotherapy in adolescents with depressive disorders, we focused on the medication-related aspects of the TORDIA study.
Methods
Data extraction
We extracted summary response data (CGI-I score ≤2 and a CDRS-R decline ≥50%) from the TORDIA study (Brent et al. 2008) as well as pooled adverse event-related discontinuation data (discontinuation secondary to an adverse event) for paroxetine (κ = 5), citalopram (κ = 2), and fluoxetine (κ = 10) from network meta-analyses of these SSRIs in pediatric MDD to generate the joint distributions described herein. In addition, efficacy and tolerability data for the three SSRIs were extracted from the same network meta-analysis to validate the statistical approach (described in Response modeling below). However, it should be noted that the SSRI tolerability data (discontinuation due to an adverse event) that were extracted from the NMA represent tolerability data in adolescents with MDD, rather than SSRI-resistant MDD.
Response modeling
As response (i.e., efficacy) is inherently linked to tolerability and vice versa, a joint model of both variables is necessary to account for their interaction. Nonetheless, although these factors are related, the relative strength of the relationship is unknown. Therefore, the model specified allows us to examine a range of correlations between efficacy and tolerability, ranging from no dependence to a relatively strong relationship between the two. Moreover, a Bayesian inferential approach was selected given its advantages over the frequentist approach. Specifically, the Bayesian approach allows direct probability statements and comparisons for the unknown parameters, which enables simulation across potential dependencies even in the absence of data that would allow direct estimation of dependencies (i.e., evaluating covariance). In addition, the Bayesian approach obviates the need to adjust for multiple comparisons given the sequential relationship between inference and hypothesis testing (Kruschke 2015).
Tolerability outcomes were assumed to be log-normally distributed while response outcomes were assumed to be normally distributed given the results of large pediatric MDD trials (Emslie et al. 2009). We specified a joint bivariate normal likelihood for response and log tolerability. Sample variances were based on the maximum implied variance from the 95% credible intervals previously reported in youth with MDD for citalopram, paroxetine, and fluoxetine. A Monte Carlo (MC) pseudorandom sample (M = 100,000 draws) from the joint conditional posterior distribution was obtained, and credible intervals, means, posterior tail probabilities, etc. were computed from the MC sample of M values from the posterior of differences in means (Lancaster 2004; Greenberg 2008). We assumed that the observed mean response, 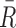 , and mean log tolerability,
, and mean log tolerability, 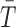 , were jointly Gaussian with mean vector
, were jointly Gaussian with mean vector  and covariance matrix,
and covariance matrix, 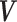 ,
,
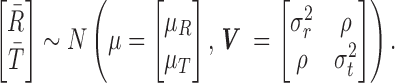 |
Combining this likelihood with an uninformative prior leads to a joint normal conditional posterior distribution for the means, 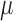 ,
,
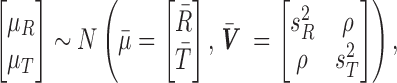 |
with mean vector 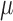 and covariance matrix,
and covariance matrix, 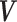 . Since only sample means and 95% CIs were available, values for the variances were computed using the normality assumption, that is,
. Since only sample means and 95% CIs were available, values for the variances were computed using the normality assumption, that is, 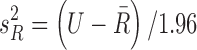 , where U is the 95% CI upper bound. Since tolerance is log-normally distributed, the bounds from the reported CI were not symmetric, so we chose the larger computed variance from transforming to log values and computed the implied variance using the upper and lower bounds, that is,
, where U is the 95% CI upper bound. Since tolerance is log-normally distributed, the bounds from the reported CI were not symmetric, so we chose the larger computed variance from transforming to log values and computed the implied variance using the upper and lower bounds, that is, 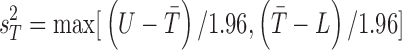 , where L is the 95% CI lower bound. This provides a conservative estimate of the precision of posterior inference for the tolerance efficacy. Since the covariance,
, where L is the 95% CI lower bound. This provides a conservative estimate of the precision of posterior inference for the tolerance efficacy. Since the covariance, 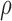 , is unknown and cannot be estimated from the data available, we computed joint posterior regions conditional on correlation coefficient values
, is unknown and cannot be estimated from the data available, we computed joint posterior regions conditional on correlation coefficient values 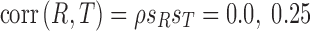 and
and 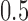 to allow for a range of possible dependence. This specifies a range of correlation from no dependence to a relatively strong relationship between tolerability and efficacy. In other words, at ρ = 0, tolerability and efficacy are statistically unrelated, whereas at ρ = 0.5, there is a moderate relationship between efficacy and tolerability. To evaluate the joint null hypothesis that average efficacy and tolerability of one SSRI is no better than that of one of the other two SSRIs,
to allow for a range of possible dependence. This specifies a range of correlation from no dependence to a relatively strong relationship between tolerability and efficacy. In other words, at ρ = 0, tolerability and efficacy are statistically unrelated, whereas at ρ = 0.5, there is a moderate relationship between efficacy and tolerability. To evaluate the joint null hypothesis that average efficacy and tolerability of one SSRI is no better than that of one of the other two SSRIs, 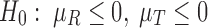 , the joint posterior probability
, the joint posterior probability 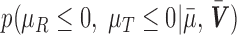 was computed, which is the Bayesian posterior equivalent of a frequentist p-value for evidence against the null hypothesis. For all analyses, Bayesian posterior p-values ≤0.05 were considered statistically significant. Statistical analyses were performed using Julia (version 1.0.1).
was computed, which is the Bayesian posterior equivalent of a frequentist p-value for evidence against the null hypothesis. For all analyses, Bayesian posterior p-values ≤0.05 were considered statistically significant. Statistical analyses were performed using Julia (version 1.0.1).
The use of the Bayesian approach allows multiple comparisons without Bonferonni correction. In this regard, the posterior distribution of the modeled parameters (e.g., average response rate) represents the complete implication of the data and is unaffected by subsequent testing (Kruschke 2015). This contrasts with the frequentist approach in which there is no separation between inference and testing. Thus, with a frequentist approach, each test necessitates a new estimate from the same data, whereas with the Bayesian inferential approach, the data are used to generate the posterior distribution and then, this distribution is used for hypothesis testing. This eliminates the need for multiple comparison adjustments (Kruschke 2015).
Results
Effectiveness of SSRIs
In terms of efficacy (response), the mean difference for citalopram compared with fluoxetine was 0.067 (p = 0.247), whereas the mean difference between fluoxetine and paroxetine was 0.104 (p = 0.11). Citalopram trended toward being superior to paroxetine in terms of mean difference (0.171, p = 0.055) with a posterior probability ratio of 17.3 to 1 against the null hypothesis. In other words, a patient would be 17 times more likely to respond to citalopram than to paroxetine. Regarding tolerability, discontinuation due to adverse events was extracted from a previously reported network meta-analysis of double-blind placebo-controlled trials of the three antidepressants (fluoxetine vs. citalopram: odds ratio [OR] = 0.69 [CrI: 0.24–3.50]; citalopram vs. paroxetine: OR = 0.93 [0.20–2.77]; and fluoxetine vs. paroxetine: OR = 0.78 [0.21–2.18]) (Cipriani et al. 2016).
Joint efficacy–tolerability comparisons
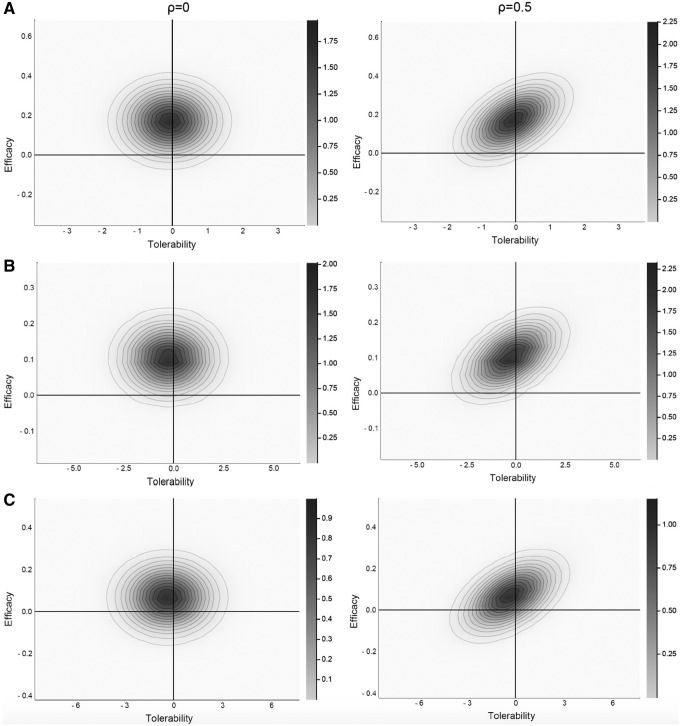
For efficacy–tolerability models, citalopram was superior to paroxetine regardless of ρ (ρ = 0, p = 0.029; ρ = 0.25, p = 0.041; ρ = 0.5, p = 0.047). Fluoxetine was superior to paroxetine at all values of ρ (ρ = 0, p = 0.022; ρ = 0.25, p = 0.030; ρ = 0.5, p = 0.035). Last, there were no statistically significant differences between fluoxetine and citalopram at any ρ (ρ = 0, p = 0.146; ρ = 0.25, p = 0.175; ρ = 0.5, p = 0.204) (Fig. 1).
FIG. 1.
Joint probability density for antidepressant-related improvement and tolerability (pooled discontinuation due to adverse events) in adolescents with major depressive disorder. Citalopram (A) and fluoxetine (B) are statistically superior to paroxetine (C) at ρ = 0 and ρ = 0.5. Citalopram and fluoxetine do not significantly differ at either ρ = 0 or ρ = 0.5. The lower left quadrant reflects the posterior probability that the two treatments do not differ on the efficacy–tolerability diathesis. The posterior p-values reflect: 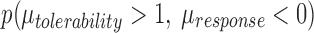 .
.
Discussion
In models considering tolerability and efficacy jointly, citalopram and fluoxetine were both superior to paroxetine, whereas in the efficacy only model, citalopram trended toward superiority over paroxetine (p = 0.055). This finding is consistent with several lines of accumulating clinical experience and data. First, in the Food and Drug Administration meta-analysis, paroxetine was associated with greater suicidality than the other antidepressants (Hammad et al. 2006), whereas in another meta-analysis, fluoxetine and citalopram had superior pooled effect sizes than paroxetine (Bridge et al. 2007). Second, clinical trials of paroxetine in pediatric patients suggest that this antidepressant may be poorly tolerated (Keller et al. 2001; Wagner et al. 2004; Emslie et al. 2006). In one of these randomized controlled trials, younger patients had higher dropout rates and it was suggested that, in this trial, paroxetine doses were too high in younger patients (Emslie et al. 2006). Third, paroxetine has greater anticholinergic liability than other SSRIs and this affinity is comparable with desipramine (Nemeroff and Owens 2003). Fourth, paroxetine exhibits nonlinear pharmacokinetics in children and adolescents. Thus, the increase (or decrease) in plasma paroxetine concentration resulting from a dose increase (or decrease) is difficult to predict. Fifth, paroxetine clearance (maximum plasma concentration [Cmax] and exposure (i.e., area under the curve during a 24-hour dosing period [AUC24]) are remarkably variable, a phenomenon that is likely subtended by polymorphic cytochrome CYP2D6 activity (Findling et al. 2006a, 2006b). Sixth, compared with other SSRIs, paroxetine has a greater likelihood of discontinuation symptoms (Rosenbaum et al. 1998).
Although this is the first probabilistic efficacy or efficacy–tolerability comparison of SSRIs in treatment-resistant MDD and yields findings that are consistent with general recommendations, at least with regard to paroxetine use in pediatric patients, there are several important limitations. First, summary data—rather than patient-level data—were used. Because we lack patient-level data, we are unable to examine the impact of age or other patient characteristics on efficacy–tolerability differences. Second, we assumed log normally distributed outcomes within the data set. This assumption is informed by previously reported distributions of tolerability and efficacy in other antidepressant trials in both MDD and anxiety disorders, and from a central limit theorem standpoint, the sample means of moderately large samples are generally approximated by a normal distribution (even when data are not normally distributed). Third, the covariance, 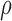 , of efficacy and tolerability is unknown; however, in our models, we computed joint posterior regions conditional on correlation coefficient values
, of efficacy and tolerability is unknown; however, in our models, we computed joint posterior regions conditional on correlation coefficient values 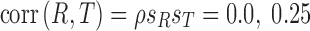 and
and 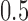 to allow for a range of possible dependence and, at even the most conservative
to allow for a range of possible dependence and, at even the most conservative 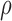 (0.5), our findings remained statistically significant.
(0.5), our findings remained statistically significant.
Conclusions
The results from these analyses raise the possibility that for adolescents, fluoxetine and citalopram may have a superior joint efficacy–tolerability profile compared with paroxetine. Given the challenges in the design and execution of clinical trials in children and adolescents with treatment-resistant MDD, this study highlights the importance of Bayesian and alternative approaches to analyzing clinical psychopharmacologic trials. Finally, prospective studies and patient-level examinations of individual factors that may influence differences in SSRI tolerability–efficacy are needed to inform real-world treatment sequencing in clinical practice.
Clinical Significance
In these analyses, fluoxetine and citalopram are superior with regard to the efficacy–tolerability diathesis to paroxetine for adolescents with SSRI-resistant MDD. When considering an SSRI switch in an adolescent with treatment-resistant MDD, clinicians might consider citalopram or fluoxetine in lieu of paroxetine as the “next step.” Finally, although this report focuses on pharmacotherapy in adolescents with SSRI-resistant MDD, clinicians treating youth with depressive disorders should consider the critical role of psychotherapy in increasing the likelihood of remission (March et al. 2004), overall response (Brent et al. 2008; The Treatment for Adolescents With Depression Study (TADS) Team et al. 2009), and maintenance of response (Kennard et al. 2008; Curry et al. 2011).
Acknowledgment
The authors thank the Yung Family Foundation for their generous support of this work.
Disclosures
Dr. Strawn has received research support from Edgemont, Eli Lilly, Shire, Forest Research Institute, Lundbeck, and the National Instituted of Health (NIMH and NIEHS). He receives royalties from Springer Publishing for two texts, and has received material support from Assurex. Dr. Croarkin receives research support from the National Institute of Mental Health, NeuroStar Advanced Therapy, NeoSync, and Genesight/Assurex. Dr. Mills has no potential financial conflicts of interest.
References
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller M, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, DeBar L, McCracken J, Strober M, Suddath R, Spirito A, Leonard H, Melhem N, Porta G, Onorato M, Zelazny J: Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. JAMA 299:901–913, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA: Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. JAMA 297:1683–1696, 2007 [DOI] [PubMed] [Google Scholar]
- Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, Coghill D, Zhang Y, Hazell P, Leucht S, Cuijpers P, Pu J, Cohen D, Ravindran AV, Liu Y, Michael KD, Yang L, Liu L, Xie P: Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet 388:881–890, 2016 [DOI] [PubMed] [Google Scholar]
- Cohen D, Bonnot O, Bodeau N, Consoli A, Laurent C: Adverse effects of second-generation antipsychotics in children and adolescents: A Bayesian meta-analysis. J Clin Psychopharmacol 32:309–316, 2012 [DOI] [PubMed] [Google Scholar]
- Curry J, Silva S, Rohde P, Ginsburg G, Kratochvil C, Simons A, Kirchner J, May D, Kennard B, Mayes T, Feeny N, Albano AM, Lavanier S, Reinecke M, Jacobs R, Becker-Weidman E, Weller E, Emslie G, Walkup J, Kastelic E, Burns B, Wells K, March J: Recovery and recurrence following treatment for adolescent major depression. Arch Gen Psychiatry 68:263–269, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson ET, Bloch M, Strawn JR: Network meta-analysis: efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders. Journal Clin Psychiatry 80: 17r12064, 2019 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, Nilsson M, Jacobson JG: Fluoxetine for acute treatment of depression in children and adolescents: A placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry 41:1205–1215, 2002 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S: Escitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry 48:721–729, 2009 [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Wagner KD, Kutcher S, Krulewicz S, Fong R, Carpenter DJ, Lipschitz A, Machin A, Wilkinson C: Paroxetine treatment in children and adolescents with major depressive disorder: A randomized, multicenter, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 45:709–719, 2006 [DOI] [PubMed] [Google Scholar]
- Findling RL, McNamara NK, Stansbrey RJ, Feeny NC, Young CM, Peric FV, Youngstrom EA: The relevance of pharmacokinetic studies in designing efficacy trials in juvenile major depression. J Child Adolesc Psychopharmacol 16:131–145, 2006a [DOI] [PubMed] [Google Scholar]
- Findling RL, Nucci G, Piergies AA, Gomeni R, Bartolic EI, Fong R, Carpenter DJ, Leeder JS, Gaedigk A, Danoff TM: Multiple dose pharmacokinetics of paroxetine in children and adolescents with major depressive disorder or obsessive-compulsive disorder. Neuropsychopharmacology 31:1274–1285, 2006b [DOI] [PubMed] [Google Scholar]
- Fontanella CA, Bridge JA, Marcus SC, Campo JV: Factors associated with antidepressant adherence for Medicaid-enrolled children and adolescents. Ann Pharmacother 45:898–909, 2011 [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Veroniki AA, Siamouli M, Möller H-J: No role for initial severity on the efficacy of antidepressants: Results of a multi-meta-analysis. Ann Gen Psychiatry 12:26, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg E: Introduction to Bayesian Econometrics. New York (New York), Cambridge University Press, 2008 [Google Scholar]
- Guy W. (ed): CGI Clinical Global Impressions. In: ECDEU Assessment Manual. Rockville, MD, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976, pp 217–222 [Google Scholar]
- Hammad TA, Laughren T, Racoosin J: Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 63:332–339, 2006 [DOI] [PubMed] [Google Scholar]
- Keller MB, Ryan ND, Strober M, Klein RG, Kutcher SP, Birmaher B, Hagino OR, Koplewicz H, Carlson GA, Clarke GN, Emslie GJ, Feinberg D, Geller B, Kusumakar V, Papatheodorou G, Sack WH, Sweeney M, Wagner KD, Weller EB, Winters NC, Oakes R, McCafferty JP: Efficacy of paroxetine in the treatment of adolescent major depression: A randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 40:762–772, 2001 [DOI] [PubMed] [Google Scholar]
- Kennard BD, Stewart SM, Hughes JL, Jarrett RB, Emslie GJ: Developing cognitive behavioral therapy to prevent depressive relapse in youth. Cogn Behav Pract 15:387–399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke JK: Doing Bayesian Data Analysis, 2nd Edition: A Tutorial with R, JAGS and Stan. Waltham, MA, Academic Press/Elsevier, 2015 [Google Scholar]
- Lancaster T: Introduction to Modern Bayesian Econometrics. Oxford, Blackwell, 2004 [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J, Treatment for Adolescents with Depression Study (TADS) Team: Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA J Am Med Assoc 292:807–820, 2004 [DOI] [PubMed] [Google Scholar]
- March JS, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J: The Treatment for Adolescents with Depression Study (TADS): Long-term effectiveness and safety outcomes. Arch Gen Psychiatry 64:1132–1143, 2007 [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ: Neuropharmacology of paroxetine. Psychopharmacol Bull 37(Suppl. 1):8–18, 2003 [PubMed] [Google Scholar]
- Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB: Selective serotonin reuptake inhibitor discontinuation syndrome: A randomized clinical trial. Biol Psychiatry 44:77–87, 1998 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Dobson ET, Mills JA, Cornwall GJ, Sakolsky D, Birmaher B, Compton SN, Piacentini J, McCracken JT, Ginsburg GS, Kendall PC, Walkup JT, Albano AM, Rynn MA: Placebo response in pediatric anxiety disorders: Results from the child/adolescent anxiety multimodal study. J Child Adolesc Psychopharmacol 27:501–508, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Mills JA, Cornwall GJ, Mossman SA, Varney ST, Keeshin BR, Croarkin PE: Buspirone in children and adolescents with anxiety: A review and bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol 28:2–9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Treatment for Adolescents With Depression Study (TADS) Team, March J, Silva S, Curry J, Wells K, Fairbank J, Burns B, Domino M, Vitiello B, Severe J, Riedal K, Goldman M, Feeny N, Findling R, Stull S, Baab S, Weller EB, Robbins M, Weller RA, Jessani N, Waslick B, Sweeney M, Dublin R, Walkup J, Ginsburg G, Kastelic E, Koo H, Kratochvil C, May D, LaGrone R, Vaughan B, Albano AM, Hirsch GS, Podniesinki E, Chu A, Reincecke M, Leventhal B, Rogers G, Jacobs R, Pathak S, Wells J, Lavanier SA, Danielyan A, Rohde P, Simons A, Grimm J, Frank S, Emslie G, Kennard B, Hughes C, Mayes TL, Rosenberg D, Benazon N, Butkus M, Bartoi M: The Treatment for Adolescents With Depression Study (TADS): Outcomes over 1 year of naturalistic follow-up. Am J Psychiatry 166:1141–1149, 2009 [DOI] [PubMed] [Google Scholar]
- Vitiello B, Emslie G, Clarke G, Wagner KD, Asarnow JR, Keller MB, Birmaher B, Ryan ND, Kennard B, Mayes TL, DeBar L, Lynch F, Dickerson J, Strober M, Suddath R, McCracken JT, Spirito A, Onorato M, Zelazny J, Porta G, Iyengar S, Brent DA: Long-term outcome of adolescent depression initially resistant to selective serotonin reuptake inhibitor treatment: A follow-up study of the TORDIA sample. J Clin Psychiatry 72:388–396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KD, Berard R, Stein MB, Wetherhold E, Carpenter DJ, Perera P, Gee M, Davy K, Machin A: A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry 61:1153–1162, 2004 [DOI] [PubMed] [Google Scholar]