Abstract
Alcohol self-administration produces brain and behavior adaptations that facilitate a progressive loss of control over drinking and contribute to relapse. One possible adaptation is the ability of antecedent environmental stimuli that are consistently paired with alcohol to trigger alcohol seeking behaviors. We previously modeled this adaptation in rats using a Pavlovian conditioning procedure in which illumination of a houselight preceded the presentation of a sipper tube that produced unsweetened alcohol when licked. However, in our previous work we did not demonstrate whether this adaptation represented a consequence of repeated exposure to alcohol or the houselight, or whether it was the consequence of associative learning and memory. Thus, in the present study, we tested the associative basis of alcohol seeking in response to houselight illumination in our task using adult male rats that were not food or water deprived and were not dependent on alcohol. Separate groups of rats received houselight illumination that was explicitly paired or unpaired with presentation of the retractable sipper that provided access to unsweetened alcohol. Our primary dependent variable was appetitive alcohol-directed behavior: the frequency of movement toward and interaction with the hole in the wall of the chamber through which the sipper was presented during the period of houselight illumination before each sipper presentation. However, we also analyzed consummatory sipper licking behavior and blood ethanol concentration in the same rats. Finally, we explored the brain basis of cue-elicited alcohol seeking using c-Fos immunohistochemistry. Our findings confirmed the associative basis of cue-elicited alcohol seeking in our paradigm and mapped these onto the insular cortex, suggesting a role for this brain region in early stages of brain and behavior adaptation to regular alcohol use.
Keywords: cues, alcohol seeking, Pavlovian conditioning, low dose alcohol, Fos
INTRODUCTION
Classical conditioning allows antecedent sensory stimuli to become associated with alcohol availability, ingestion, and/or pharmacological effects. Conditioned behavioral and physiological reactivity to these alcohol-predictive cues contributes to the risk for relapse to problem drinking. We recently developed a model of alcohol cue reactivity in rats (Cofresí et al., 2017; Cofresí et al., 2018). Here, we verify whether reactivity to the alcohol cue in our model stems from associative learning and whether memory for that cue-alcohol association maps onto brain regions that are implicated in alcohol addiction.
In our paradigm, conditioning trials involve illumination of a houselight (the cue) and brief availability of a retractable sipper from which rats can lick out unsweetened alcohol. Houselight illumination appears to gain the ability to elicit anticipatory approach to the sipper. If this response reflects a learned association with alcohol availability, then it should only be acquired when illumination is explicitly paired with alcohol availability, in a classic “Paired” conditional-unconditional stimulus (CS-US) arrangement. However, if the same alcohol-seeking response is acquired when alcohol availability is explicitly “unpaired” with illumination by presenting the sipper only during the interval between illuminations (“Unpaired” CS/US arrangement), then it may stem from a non-associative learning process (e.g., sensitization). The associative basis of cue-alcohol associations has been shown previously in studies that used Paired and Unpaired groups (Srey et al., 2015); however, in that task alcohol delivery occurred immediately after presentation of the CS and the CS was a retractable lever.
Another reason for comparing a Paired and Unpaired group in our paradigm is that since alcohol delivery occurs via a sipper tube, the presentation of this sipper tube may itself be learned as a cue, and acquire the ability to initiate drinking bouts. However, sipper presentation is always experienced during houselight illumination. As such, what looks like a response to another distinct cue (sipper presentation) may actually represent facilitation of consummatory sipper licking behavior by the houselight cue-alcohol association. If so, then once the houselight cue-alcohol association is formed, rats in the “Paired” cue group should initiate drinking bouts faster than rats in the “Unpaired” cue group.
Cue-related behavioral reactivity in other rodent models of alcohol cue reactivity has been shown to reflect associative learning. However, we cannot assume that cue-related reactivity in our procedure also reflects associative learning, as our procedure differs substantially from others. In the conditioned place avoidance/preference procedure, which has taught us that alcohol-associated cues can acquire both appetitive and aversive properties in rodents, the cue predicts experimenter-administered alcohol (e.g., Bormann & Cunningham, 1988; Bozarth, 1990; Ciccociopo, Panocka, Froldi, et al., 1999; Cunningham, 1979, 1981; Fidler, Bakner, & Cunningham, 2004; Nentwig, Myers, & Grisel, 2017; Torres, Walker, Beas, & O’Dell, 2014). In procedures where the rodent self-administers alcohol, cues typically acquire appetitive properties such as the ability to invigorate instrumental responding (Corbit & Janak, 2007; Glasner, Overmier, & Balleine, 2005; Krank, O’Neill, Squarey, et al., 2008; Milton, Schramm, Wawrzynski, et al., 2012), the ability to elicit sign-tracking conditioned responses (e.g., Krank, 2003; Krank, O’Neill, Squarey, et al., 2008; Srey et al., 2015; Villaruel et al., 2016), and the ability to act as conditioned positive reinforcers for the acquisition of new instrumental responses (e.g., Srey et al., 2015; Schramm, Everitt, & Milton, 2016). However, in some cases, even cues paired with self-administered alcohol can acquire aversive properties (e.g., Stewart & Grupp, 1986). In most of the self-administration models, the cue predicts delivery of a fixed amount of alcohol, which is available for ingestion even when the cue is not present (e.g., Krank et al., 2008; Remedios et al., 2014). In many, access to food and/or water in the homecage is restricted or alcohol is sweetened to motivate rodents to drink alcohol in the conditioning chamber (e.g., Krank, 2003; Tomie et al., 2002; Millan et al., 2013). In our procedure, rats have free-access to food and water in the homecage and alcohol is never sweetened. Moreover, rats are free to drink as much alcohol they want during conditioning trials. Importantly, our procedure also allows us to measure drinking behavior (consummatory sipper licking) directly for each conditioning trial.
Many therapies for alcohol use disorder (e.g., naltrexone, cue exposure, counterconditioning) are predicated on the idea that maladaptive alcohol-associative memories (implicit or not) drive relapse. Knowing whether alcohol-related behavior stems from associative or non-associative memory is important because different approaches (behavioral and pharmacological) are needed to target associative and non-associative aspects of behavior. Ideally, we would know how (associative v. non-associative), as well as where alcohol-related cue memories are encoded in the brain. Consequently, in the present study, using the same rats in which we characterized behavior and blood ethanol, we evaluated expression of the immediate-early gene product c-Fos as an index of cellular activity in brain regions that may be involved in maintaining and/or expressing alcohol-related cue memories, with a focus on those regions implicated in cue-induced relapse to alcohol-seeking (Koob & Volkow, 2010).
METHODS & MATERIALS
Subjects
Adult male Long-Evans rats were used (Envigo; Indianapolis, IN, USA). Upon arrival, rats weighed 250-275 g. All were singly housed in a temperature- and humidity-controlled room (22±2 °C; 12hr light cycle). The homecage contained Sani-Chips® bedding and a Bio-Serv Gummy Bone (polyurethane; 5 cm L × 2.5 cm W). Metal wire cage-tops were used. Standard chow pellets were loaded into a large cup inside the cage. Tap water was provided via gravity-fed sipper inserted at approximately 45° from the cage top. Chow and water were replenished daily. Bedding was replaced weekly. All procedures took place during the light phase of the light/dark cycle. The colony room was adjacent to the procedure rooms. Ethanol (v/v) solutions were prepared every 3 days from 95% ethyl alcohol (ACS/USP grade, Pharmco-AAPER, Brookfield, CT, USA) and tap water. Solutions were kept and served at room temperature (20 °C). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin, and conducted in accordance with NIH guidelines.
Behavioral Methods
Paired and Unpaired Cue-Ethanol Conditioning
All conditioning took place in chambers housed in cubicles equipped with a digital video camera (for detailed description of the apparatus, see Cofresí et al., 2017, 2018). Rats were acclimated to the conditioning chambers and stimuli 48 hr after familiarization with unsweetened ethanol in the homecage (see Supplemental Information). Rats were first familiarized with a retractable sipper in the conditioning chamber by giving a single session in which the sipper was presented for 35 min, during which time rats were free to lick out unsweetened ethanol. The next day, rats were habituated to houselight illumination in the chamber by giving a single session in which the houselight was illuminated 8 times (described below). During this session, the bottle assembly was never activated and ethanol was absent from the room.
Next, 2 groups matched on ingested doses across the homecage familiarization phase were created. One group of rats was assigned to our usual conditioning procedure: houselight illumination was explicitly paired with presentations of the alcohol sipper (creating group “Paired”). For the other group, the houselight stimulus was explicitly unpaired with presentations of the alcohol sipper (creating group “Unpaired”).
Rats received 1 conditioning session per day. Each conditioning session was preceded by a 5 min wait period. Session start was signaled to the rat by turning on the cubicle exhaust fan. At session start, the pre-trial interval (preTI) was selected randomly without replacement from a list of possible intervals (160 s, 240 s, 250 s, 320 s, 350 s, 360 s). Upon conclusion of the 1st trial, an inter-trial interval (ITI) was selected randomly without replacement from the same list above. Each session consisted of 8 trials and each trial was 20 s long. After the 8th trial, the post-trial interval (postTI) was selected randomly without replacement from the same list above. The session ended once the postTI had elapsed. This was signaled by turning off the cubicle exhaust fan. On average, total session duration was 45 min (8 trials + 7 ITI + 1 preTI + 1 postTI). Sessions were given across consecutive days and at approximately the same time every day by the same experimenter.
During each trial, the houselight was illuminated for 20 s. In group Unpaired, there was no consequent event. However, in group Paired, the retractable bottle assembly was activated 10 s into the illumination to present the metal sipper such that alcohol availability and illumination co-terminated. The sipper insertion hole was 8.5 cm rightward and 16 cm downward from the houselight fixture (installed on the same wall). For group Unpaired, sipper presentations instead occurred mid-ITI, beginning in ITI 2 and ending in the postTI. Houselight illumination onset, offset, and intervals were yoked between groups. Retractable bottle assembly activations were yoked within groups. On average, time between the 1st and 8th sipper presentation was 35 min.
Licking the sipper produced the ethanol solution each rat was drinking at the end of homecage pre-exposure. The ethanol solution was either 10 or 15% ethanol (vol/vol tap water; 10E or 15E). Of the 17 rats for which data are presented in the main text, 6 drank 10E. These 6 were evenly split between groups Paired and Unpaired. The sipper contained a ball bearing to prevent fluid spill upon insertion and retraction. When inserted, the tip was typically in the plane of the wall. In other words, the sipper did not protrude far into the chamber. At most, 500 ms elapsed between activation of the bottle assembly and availability of the sipper.
Brain Collection Day
On brain collection day, rats in 2 groups (Paired Run, Unpaired Run) were weighed and transferred into the conditioning chambers for a conditioning session. Rats in 2 other groups (Paired Not Run, Unpaired Not Run) were not handled and stayed in the homecage. Groups were matched for ingested doses across all conditioning sessions. Rats in the Not Run condition had 15 conditioning sessions total prior to brain collection. Due to technical limitations, rats in the Run condition had either 16 (n=2/group) or 18 (n=2/group) conditioning sessions total prior to brain collection. Houselight-elicited behavioral reactivity on brain collection day was not different between rats in the Run condition with 16 v. 18 sessions, so their data were pooled.
All brains were collected 90 min from the beginning of the conditioning session given to the Run groups. All rats were anesthetized with isoflurane gas and administered euthanasia III solution (Med-Pharmex, Inc., Ponoma, CA, USA). Following euthanasia, tissues were fixed by perfusing phosphate buffered saline (pH 7.2) (PBS) then 4% para-formaldehyde w/v in PBS (fixative solution). Brains were kept in 20% sucrose m/v in fixative solution overnight. Brains were flash-frozen on dry ice the next day and stored at −80°C until processing for Fos immunohistochemistry.
Behavioral Measurements
Trials from conditioning sessions 6, 9, and 12 as well as the sessions given on brain collection day and the day before brain collection were sampled for appetitive behavioral state from digital video recordings by making instantaneous observations every 1.25 s starting 5 s before houselight onset as in (Cofresí et al., 2017, 2018; Lee et al., 2005) such that there were 4 observations per trial phase. Trial phase or bin “−1” refers the 5 s period before houselight onset and trial phase or bins 1-4 refer to 5 s periods across the houselight illumination. At each observation, the mutually exclusive rating options were “sipper site approach” (approaching, attending to, or exploring the sipper insertion hole, including sniffing, gnawing, and clawing at the hole; Figure 1 A), “orienting” (rearing: both forepaws off the floor, supported by hindlimbs, facing any direction; Figure 1 B) or “other” (e.g., grooming, resting). Consummatory drinking behavior (sipper licking; Figure 1 C) was recorded automatically using a contact lickometer circuit. Behavioral observations were made by highly trained judges (intra- and inter-rater agreement ≥95%). However, since within-trial events differed dramatically, it was impossible to blind raters to “Paired” v. “Unpaired” status, thus bias was possible. In order to corroborate “sipper site approach” observations, after the 12th conditioning session, we customized the metal panel on which the retractable bottle assembly was mounted. The portion of the panel bearing the sipper hole was cut, trimmed, and seated in a polylacticacid plastic frame (7.11 cm L, 0.32 cm W, 3.71 cm H) in order to electrically isolate it from the rest of the conditioning chamber. The exposed metal surface (6.91 cm L, 0.32 cm W, 3.66 cm H) was then wired into a second contact lickometer circuit, allowing automated recording of forepaw contact with the area around the sipper hole. In the session where both were collected, device-based sipper site contact (data not shown) exhibited the same within-session patterns as scored sipper site approach.
Figure 1. Behavioral measurements.
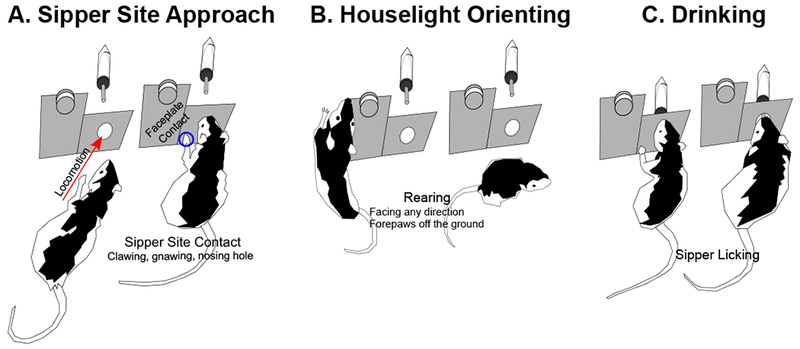
A-B: Digital video recordings of conditioning trials were scored for (A) “sipper site approach” (defined as approach and/or contact: locomotion toward the hole in the wall at which the sipper was presented or exploring the hole, including sniffing, gnawing, and clawing at the hole) or (B) “houselight orienting” (defined as rearing: forepaws off the floor, supported by hindlimbs, facing any direction). C: A contact lickometer circuit was used to automatically record the latency and intensity of alcohol drinking bouts (sipper licking) during conditioning trials.
The total dose of ethanol ingested in each conditioning session was also monitored for each rat. Drinking solution intake was measured as the mass difference in bottle weight pre- and post-session after correcting for spillage. The grams of solution ingested were then converted to g ethanol and ingested dose was expressed as g/kg body weight for each rat. For every conditioning session, a weighboat underneath the retractable sipper tube/bottle assembly collected spillage such that solution intake values were corrected at the level of each rat.
Blood ethanol analysis
To determine the relationship between ingested doses and blood ethanol levels, we took blood samples after the 8th sipper presentation in a session. Blood was sampled on different days for different rats (session 13 or 14) in order to measure blood ethanol concentration at approximately the same point along the blood ethanol concentration timecourse given logistical constraints (i.e., only 1 experimenter for 3 groups of 5-6 rats undergoing a yoked conditioning session at a specific time of day). As on any day, rats had unlimited chow and water in the homecage until they were transferred into conditioning chambers for a conditioning session. Rats were removed from the chamber immediately after the 8th sipper presentation and anesthetized with isoflurane gas. Blood was then collected from the lateral saphenous vein (3 replicates per rat). Ethanol concentration (mg/dL) in 10 μL whole blood mixed with 90 μL saturated saline was determined using gas chromatography with flame ionization detection (GC-FID) as in (Carrillo et al., 2008).
Fos Immunohistochemistry
Brain sectioning, tissue processing, and c-Fos immunostaining was performed as in (Lee et al., 2005). For details, see Supplemental Information.
Statistical Analysis & Data Visualization
Behavior data were analyzed using mixed factorial analysis of variance (ANOVA). The threshold for statistical significance was p<0.05. Significant effects in the omnibus ANOVAs were followed up as appropriate (e.g., ANOVA F-tests to decompose interactions of 2 or more factors, T-tests to decompose the main effect of a factor). Bonferroni correction was applied at every follow-up stage to prevent false discovery (i.e., the threshold p-value used to evaluate significance in a batch of tests was divided by the number of tests in the batch; e.g., a corrected threshold p-value of 0.017 would be used to evaluate the 3 F-tests for the simple 2-way interaction effect of two factors at each level of a 3-level third factor and a corrected threshold p-value of 0.006 would be used to evaluate a batch of 8 pairwise t-tests).
Ingested dose, ethanol bottle preference ratio, and blood ethanol concentration data were analyzed using ANOVA and simple linear regression. The threshold for statistical significance was p<0.05. Bonferroni corrected follow-ups were conducted as described above.
Fos expression data were analyzed by running ANOVA within structures considering the between-subject factor of group (Paired v. Unpaired), the within-subject factor of brain region (e.g., in the Accumbens ANOVA, shell v. core), and their interaction. Known Fos expression differences between structures (e.g., dorsal striatum v. basolateral amygdalar complex) and missing data precluded ANOVA considering all sampled structures. Separate ANOVA were done for the “Run” and “Not Run” conditions because our primary interest was differential induction of Fos in groups Paired and Unpaired. The threshold for statistical significance was p<0.05. Bonferroni corrected follow-ups were conducted as described above.
All analysis was done in R version 3.41 (R Core Team, 2017) using the car package (Fox & Weisberg, 2011). Data were plotted in R using the ggplot2 package (Wickham, 2009) and finalized in Inkscape version 0.92.2 (Inkscape Team, 2017).
RESULTS
Acquisition of alcohol drinking and behavioral reactivity
A total 35 rats were obtained for this study. Of those screened, 29 ingested doses ≥ 1 g/kg/24hr on average over the last week of homecage drinking, and were retained for conditioning. Of those conditioned, 17 ingested doses ≥ 0.30 g/kg/session on average across the last 3 sessions (“Learners”), which is the dose threshold for conditioning effects of ingested ethanol that we observed in our previous study (Cofresí et al., 2018). The other 12 rats ingested doses that were consistently below that threshold (“NonLearners”). In Supplemental Information, we provide the following: ingested doses and ethanol bottle preference ratio across homecage sessions for both Learners and NonLearners, ingested doses across conditioning sessions for NonLearners, scored sipper site approach and orienting to the light across conditioning sessions for both Learners and NonLearners, sipper licking (latency and intensity) across conditioning sessions for Learners and NonLearners, and trial-by-trial scored orienting for Learners. In the main text, we present trial-by-trial scored sipper site approach and sipper licking data for Learners in conditioning sessions 6, 9, and 12 as well as the conditioning sessions given on brain collection day and the day before. We also present blood ethanol concentrations obtained after a conditioning session alongside ingested dose and estimated blood ethanol concentrations across conditioning sessions.
Blood ethanol concentrations and drinking across conditioning sessions
Both groups of rats (Paired and Unpaired) ingested similar doses across conditioning. Doses increased across sessions 1-9 and were relatively stable thereafter (F14, 210=32.17, p<0.001; Figure 2 A). ANOVA detected a significant interaction of session × group (group × session interaction: F14, 210=2.47, p<0.01), but follow-up revealed that while groups differed at session 1 and 8, these differences were not statistically significant after Bonferroni correction. Additionally, groups ingested similar doses on brain collection day (n=4/group, group main effect: F1, 6<0.5, NS). Collapsing group, rats (n=17) drank (mean ± sem) 0.48 ± 0.03 g/kg per session across session 10-15. On brain collection day, collapsing group, rats (n=8) drank (mean ± sem) 0.58 ± 0.04 g/kg.
Figure 2. Ethanol exposure across conditioning.
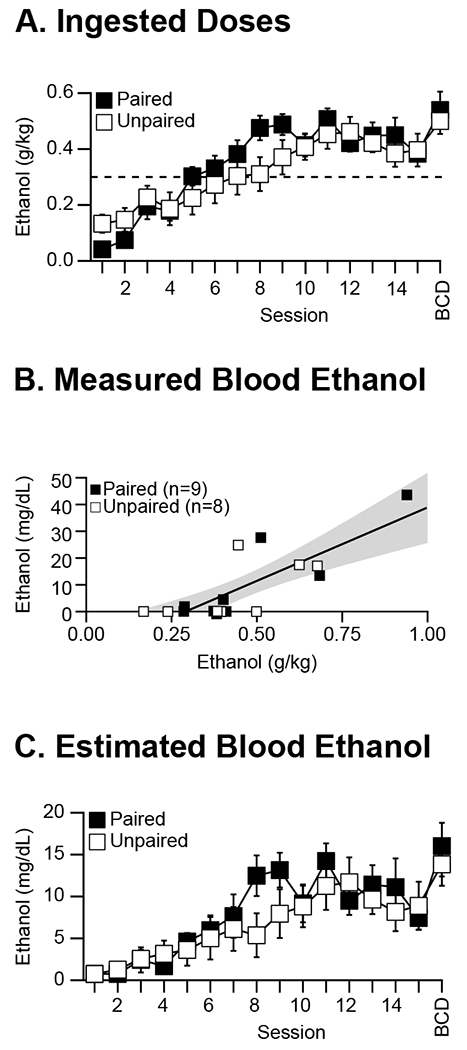
A: Mean ± sem ingested ethanol doses across conditioning sessions for adult, male Long-Evans rats. Dashed horizontal line shows a priori study inclusion criterion (≥0.30 g/kg across sessions 10-12). Black and white squares represent group Paired (n=9) and Unpaired (n=8), respectively. Data on brain collection day (session “BCD”) are from n=4/group. B: Relationship between ethanol concentrations in whole blood samples taken 8-12 min after the 8th drinking opportunity in a conditioning session and ingested ethanol doses for the same 17 rats. Regression line and 95% confidence limits shown as solid and dashed lines, respectively. C: Mean ± sem estimated blood ethanol concentrations across conditioning sessions (8-12 min after the 8th drinking opportunity in the session) using ingested doses from A and regression equation from B for the same 17 rats.
Blood samples were taken after the 8th sipper presentation in session 13 or 14 for ethanol analysis (only one session per rat). Body weights ranged from 409 to 505 g. Time between the 1st and 8th sipper presentation in the session was 32-37 min (median = 34 min). Sampling time ranged from 8 to 12 min after the 8th sipper presentation. Blood ethanol concentration (BEC) ranged from 0 to 43 mg/dL with mean ± sem of 9 ± 3 mg/dL (n=17). Total ingested dose across the 8 sipper presentations ranged from 0.17 to 0.94 g/kg with mean ± sem. of 0.45 ± 0.05 g/kg (n=17). BEC at 8-12 min after the 8th sipper presentation was significantly correlated with the total ingested dose across the 8 sipper presentations (Pearson’s r=+0.80, t15=5.14, p<0.001) (Figure 2 B). The dose-BEC relationship was independent of group (F<1, NS).
We noticed that many rats had non-detectable or zero BEC 8-12 min after the 8th sipper presentation on the sampled day. Specifically, 5 rats in each group had non-detectable or zero BEC (in group Paired, we counted 1 rat with BEC 1.85 mg/dL as “zero” to be conservative). Whether BECs was zero or non-zero was independent of group (X2<1, NS). The mean ± sem across the 4 rats in group Paired with detectable BEC was 22 ± 8 mg/dL, and the mean ± sem across the 3 rats in group Unpaired with detectable BEC was 20 ± 2 mg/dL. These group-mean BEC did not differ statistically (t5<1, NS).
To get a better sense for possible levels of ethanol exposure across conditioning and differences between groups in exposure levels, we applied the equation for the regression line in Figure 2 B to estimate BEC after the 8th sipper presentation per session for each rat based on ingested dose for each session. In both groups Paired and Unpaired, estimated BEC increased across sessions 1-4 to non-zero levels, continued to increase across sessions 5-9, and then remained relatively stable (F14, 210=13.43, p<0.001; group main effect and group × session interaction: F<1.5, NS; Figure 2 C). Additionally, there was no group difference in estimated BEC on brain collection day (n=4/group; group main effect: F<1, NS). Across session 10-15, collapsing group, rats (n=17) could be expected to have BEC (mean ± sem) of 10 ± 1 mg/dL after the 8th sipper presentation. On brain collection day, collapsing group, rats (n=8) could be expected to have BEC (mean ± sem) of 16 ± 1 mg/dL after the 8th sipper presentation. Thus, the potential level of ethanol exposure was modest, but likely non-zero, and similar between groups.
Reactivity to the houselight depends on the relationship between the houselight and ethanol
In our paradigm, houselight illumination elicits anticipatory approach and contact with hole in the wall of the conditioning chamber where the alcohol sipper is presented. To characterize this form of reactivity in our paradigm, we analyzed ethanol-directed approach and contact (i.e., scored sipper site approach) before and during the first half of houselight illumination (specifically, trial phases −1, 1, and 2) on a per trial basis for 9 well-trained rats in group Paired and 8 well-trained rats in group Unpaired across conditioning sessions 6, 9, and 12, as well as the session before brain collection day (Figure 3 A–D). The data from brain collection day (Figure 3 E) were also analyzed, but these were treated separately because only a subset of rats (n=4 per group) received a conditioning session on brain collection day.
Figure 3. Houselight cue-triggered ethanol sipper-seeking per trial.
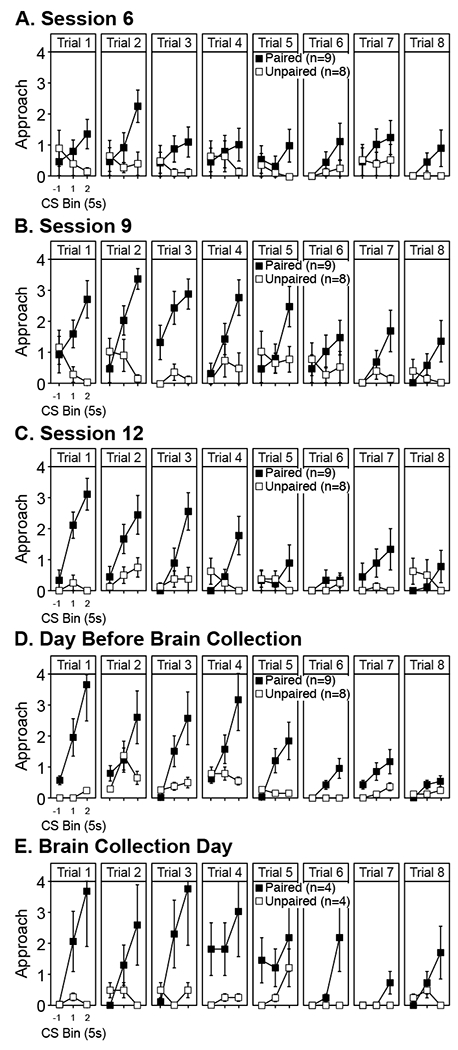
Mean ± sem level of anticipatory sipper site approach state in the 5 s before light onset (CS bin −1) and 10 s post-light onset but pre-sipper onset (CS bin 1 and 2) paneled by trial (1-8) within select conditioning sessions for adult, male Long-Evans rats. Approach data (maximum response level was 4) were derived from offline manual videoscoring.
In brief, we found that houselight illumination was able to elicit ethanol (sipper)-seeking behavior only in group Paired. The vigor of this response exhibited within-session decay as early as session 9, and this within-session pattern appeared to be present in subsequent sessions. Statistical results are presented below.
In the analysis considering data from session 6, 9, 12, and the day before collection, we saw that the overall level of sipper site approach was greater in group Paired than Unpaired (main effect: F1, 15=26.85, p<0.002). Additionally, the pattern of approach across trials varied across sessions (trial × session interaction: F21, 315=1.92, p<0.01), and this variation was similar within groups (group × trial × session interaction: F<1, NS). Specifically, trial-by-trial variation did not emerge until session 9 (simple trial main effect within all session except 6: F7,112≥5.51 , p<0.001). In all sessions except 6, the level of sipper site approach was greater across trials 1-4 than 5-8 (t16≥2.70, p<0.01). On average across sessions, however, within-session trial-by-trial variation depended on group (group × trial interaction: F7, 105=20.71, p<0.001). Specifically, only group Paired exhibited trial-by-trial changes in approach level (simple trial main effect: F7, 56=32.33, p<0.001). Sipper site approach varied across trial phases within trials differently depending on trial (trial phase × trial interaction: F14, 210=6.13, p<0.001), and this pattern of variation depended on group (group × trial phase × trial interaction: F14, 210=4.64, p<0.001), but not session (trial phase × trial × session and group × trial phase × trial × session interaction: F<1.5, NS). Sipper site approach varied within trials differently across trials only in group Paired (simple trial phase × trial interaction: F14, 112=7.69, p<0.001). Moreover, only sipper site approach during houselight illumination exhibited variation across trials in group Paired (simple trial main effect within bin 1 and 2: F7, 56≥5.58, p<0.001). Specifically, sipper site approach during houselight illumination (bin 1 and 2) was greater across trials 1-4 than 5-8 (collapsing session, t8≥4.26, p<0.01).
In the analysis of data from brain collection day, we also saw that the overall level of sipper site approach was greater in group Paired than Unpaired (main effect: F1, 5=12.38, p<0.05). We also saw that the pattern of sipper site approach across trial phases within trials varied across trials differently between groups (group × trial phase × trial interaction: F14, 70=3.68, p<0.001). Specifically, trial and trial phase-dependent variation in sipper site approach level was only detected in group Paired (simple trial phase × trial interaction: F14, 28=3.18, p<0.01). However, after Bonferroni correction, the simple main effect trial was not significant within any of the trial phases, and the simple main effect of trial phase was significant only within trial 3 (F2, 6=30.18, p<0.01).
Ethanol-directed reactivity to ethanol sipper presentation does not depend on relationship between ethanol sipper presentation and houselight illumination
Ethanol sipper presentation involves auditory and visual stimuli, so it is conceivable that sipper presentation could condition initiation of consummatory sipper licking (drinking). To characterize reactivity to sipper presentation, we analyzed the latency to start licking the sipper (Paired group n=9; Unpaired group n=7 out of 8 due to data loss) in well-trained rats across conditioning sessions 6, 9, and 12, as well as the session before brain collection day (Figure 4 A–D). The data from brain collection day (Figure 4 E) were also analyzed, but these were treated separately because only a subset of rats received a conditioning session on brain collection day.
Figure 4. Latency to initiate drinking per trial.
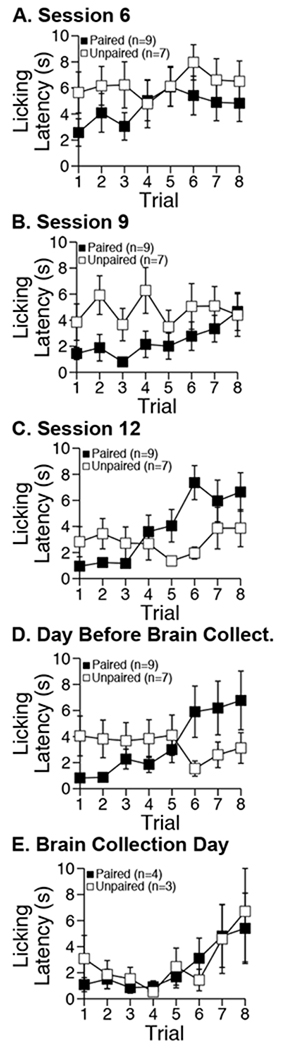
Mean ± sem latency (s) to start licking (viz., initiate drinking bout) by trial within select conditioning sessions for adult, male Long-Evans rats. Latencies (maximum latency was 10 s; omissions were recorded as maximum latency) were derived from lickometer data.
In brief, we found that sipper presentation was able to elicit rapid initiation of consummatory licking (drinking) in both groups. The within-session pattern of this response also appeared to converge across sessions such that by brain collection day response vigor was subject to within-session decay in both groups. Statistical results are presented below.
In the analysis considering data from session 6, 9, 12, and the day before collection, we saw that the overall latency to start licking the sipper was similar between groups (group main effect: F1, 14=1.03, NS). Additionally, latency varied by session (F3, 42=4.95 p<0.01) and by trial (F7, 98=4.84, p<0.01). Although these two patterns of variation were independent of each other (trial × session interaction: F21, 294<1, NS), each pattern of variation was separately dependent on group (group × trial × session interaction: F21, 294<1.5, NS; group × trial interaction: F7, 98=4.59, p<0.01; group × session interaction: F3, 42=3.26, p<0.05). Latency varied across sessions only within group Unpaired (simple session main effect: F3, 24=6.80, p<0.01). Specifically, latency decreased between session 9 and 12 (t6=4.18, p<0.01). On average across sessions, latency varied by trial only within group Paired (simple trial main effect: F7, 56=11.26, p<0.001). Specifically, latency was lower across trials 1-4 than 5-8 (t8=5.59, p<0.001).
In the analysis of data from brain collection day, we found that rats in group Paired (n=4) and Unpaired (n=3 out of 4 due to data loss) now exhibited the same trial-by-trial variation (trial main effect: F7, 35=3.20, p<0.05; group main effect and group × trial interaction: F<1, NS).
Drinking pattern does not depend on relationship between ethanol availability and houselight illumination
Although total ingested dose per session was equivalent between groups across conditioning sessions, we were curious whether the within-session pattern of consummatory sipper licking (drinking) changed across sessions and whether it differed between groups. Consequently, we characterized total licks per trial across trials for 9 well-trained rats in group Paired and 7 out of 8 well-trained rats in group Unpaired (due to equipment-related data loss) across conditioning sessions 6, 9, and 12, as well as the conditioning session given the day before brain collection (Figure 5 A–D). The data from brain collection day (Figure 5 E) were also analyzed, but these were treated separately because only a subset of rats received a conditioning session on brain collection day
Figure 5. Drinking intensity per trial.
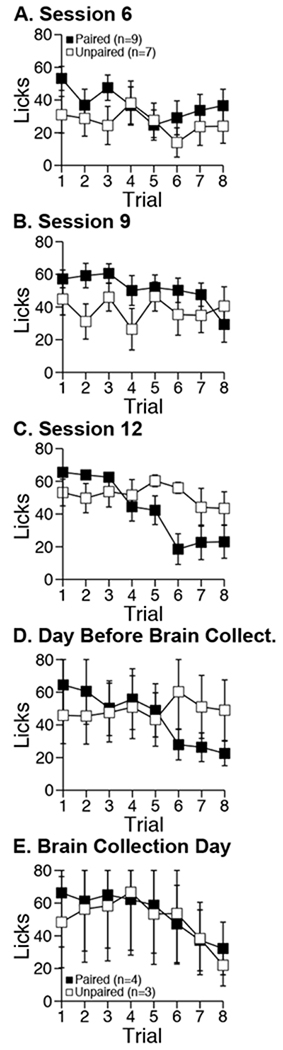
Mean ± sem total licks (viz., drinking bout size or intensity) by trial within select conditioning sessions for adult, male Long-Evans rats. Licking was measured directly using a lickometer.
In brief, the within-session pattern of consummatory sipper licking (drinking) appeared to converge across sessions such that by brain collection day both groups exhibited trial-by-trial decreases in the vigor of sipper licking.
In the analysis considering data from session 6, 9, 12, and the day before collection, we saw that overall licking levels were similar between groups (group main effect: F1, 14=0.21, NS). However, we also saw that licking varied by session (F3, 42=5.61, p<0.01) and by trial (F7, 98=5.74, p<0.001). Although these two patterns of variation were independent of each other (trial × session interaction: F21, 294<1, NS), each pattern of variation was separately dependent on group (group × trial × session interaction: F21, 294<1.5, NS; group × trial interaction: F7, 98=4.17, p<0.001; group × session interaction: F3, 98=3.05, p<0.05). Licking varied across sessions only in group Unpaired (simple session main effect: F3,18=6.50, p<0.01). Specifically, licking increased from session 9 and 12 (t6=3.79, p<0.01). Licking varied by trial only in group Paired (simple trial main effect: F7, 56=11.72, p<0.001). Specifically, licking was greater across trials 1-4 than 5-8 (t8=5.29, p<0.001).
In the analysis of data from brain collection day, we found that rats in group Paired (n=4) and Unpaired (n=3 out of 4 due to data loss) now exhibited the same trial-by-trial variation (trial main effect: F7, 35=3.77, p<0.01; group main effect and group × trial interaction: F<1, NS).
Different brain regions are responsive to Paired v. Unpaired cue-ethanol conditioning
To begin mapping the substrates of ethanol-associated cue memories in our models, we obtained brain tissue for all 17 rats that consistently drank ≥ 0.30 g/kg and evaluated expression of the immediate-early gene product c-Fos. Following immunostaining for c-Fos protein, sections were imaged and Fos+ cells counted. For each brain region, cell counts were averaged across sampling regions, atlas levels, and hemispheres to index regional activation.
We found no significant difference between group Paired and Unpaired (group main effect and group × sub-region interaction: NS) in either the Run or Not Run condition for mean Fos+ cell counts in the following brain regions: the medial and lateral divisions of the orbitofrontal cortex (Figure 6 A), the prelimbic and infralimbic divisions of the medial prefrontal cortex (Figure 6 B), the core and shell compartments of the nucleus accumbens (Figure 6 C), the medial and lateral aspects of the dorsal striatum (Figure 6 D), the medial and lateral divisions of the amygdalar central nucleus (Figure 6 E), and the substantia nigra pars compacta/ventral tegmental area complex (Figure 6 F).
Figure 6. Brain c-Fos expression after a conditioning session or its omission in well-trained rats.
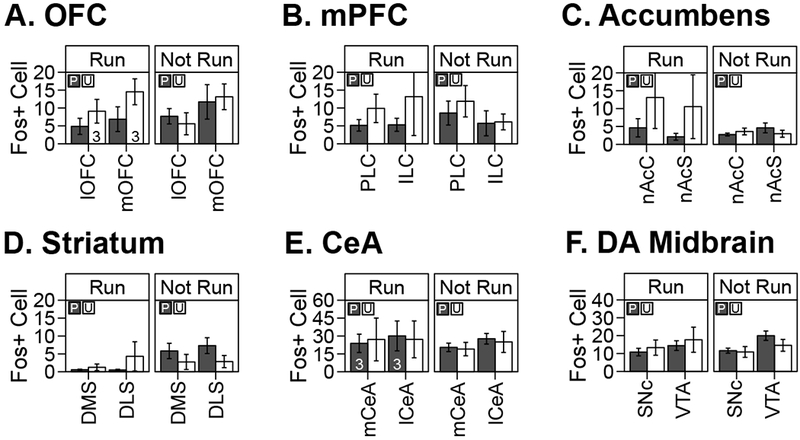
Group-level mean ± sem of Fos-positive cell counts (averaged across sampling region, level, and hemisphere) across specific structures in the adult, male Long-Evans rat brain. See Supplemental Information for immunostaining details. Data from rats that underwent conditioning on brain collection day (“Run” condition) and rats that remained in homecage on brain collection day (“Not Run”) are shown in separate panels for each brain structure. Dark gray bars represent data from group Paired. White bars represent data from group Unpaired. Unless otherwise indicated, sample sizes were: Run Paired n=4, Run Unpaired n=4, Not Run Paired n=5, and Not Run Unpaired n=4.
However, we did find that in the Run condition, there was a significantly greater mean Fos+ cell count in group Paired across the anterior and posterior divisions of the insular cortex (group main effect: F1, 6=13.17, p<0.02; group × sub-region interaction: F1, 6<1, NS; Figure 7 A). In the Not Run condition, mean Fos+ cell counts did not differ significantly between groups Paired and Unpaired (group main effect and group × sub-region interaction: F1, 7<1, NS).
Figure 7. Brain c-Fos expression after a conditioning session or its omission in well-trained rats.
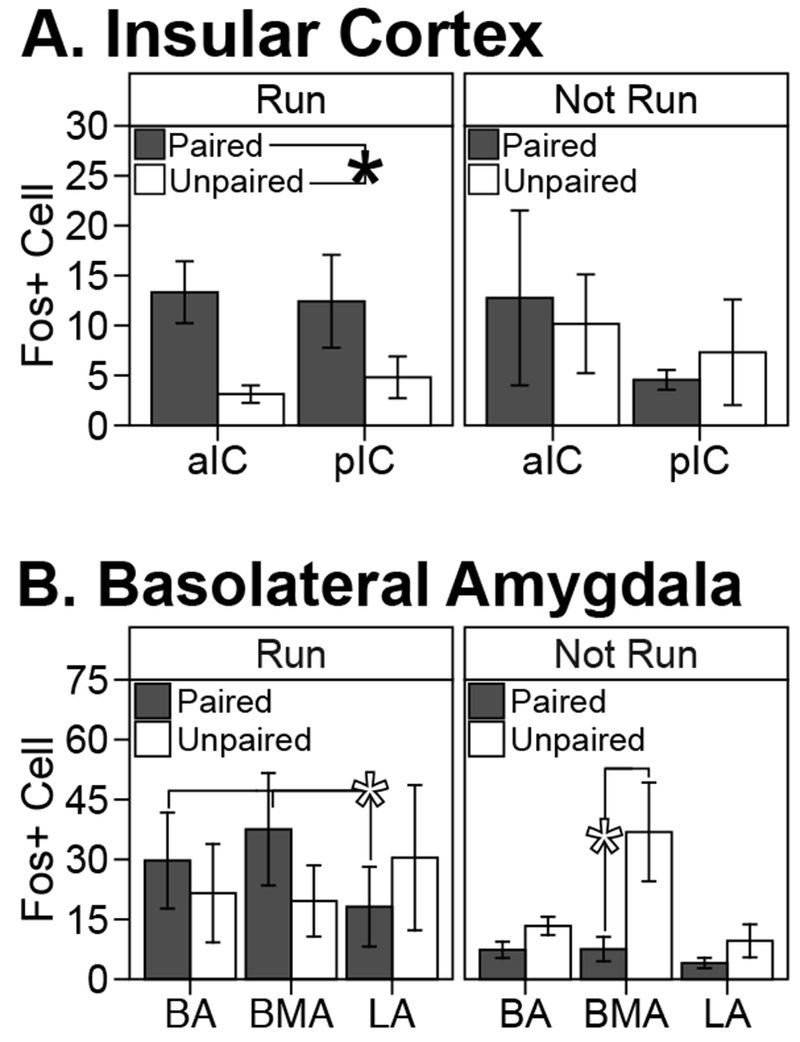
Group-level mean ± sem of Fos-positive cell counts (averaged across sampling region, level, and hemisphere) in the insular cortex (A) and the basolateral amygdala (B). Data from rats that underwent conditioning on brain collection day (“Run” condition) and rats that remained in homecage on brain collection day (“Not Run”) are shown in separate panels for each brain structure. Dark gray bars represent data from group Paired. White bars represent data from group Unpaired. Sample sizes were: Run Paired n=4, Run Unpaired n=4, Not Run Paired n=5, and Not Run Unpaired n=4. Black-filled asterisk indicates Bonferroni-corrected p<0.05, white-filled asterisk indicates p<0.05 before correction.
In the basolateral complex of the amygdala (Figure 7 B), we found significant group × subregion interaction effects for both the Run (F2, 10=5.87, p<0.025) and Not Run condition (F2, 4=3.96, p<0.05). In the Run condition, the mean Fos+ cell count differed by region for group Paired (simple effect of region: F2, 4=8.02; specifically, basal and basomedial ≥ lateral nucleus), but not Unpaired (F2, 5=1.45). However, this within-group by-region difference did not survive Bonferroni correction and none of the simple effects of group within regions were significant (even before Bonferroni correction). In the Not Run condition, group Unpaired had much greater mean Fos+ cell count in the BMA (simple effect of group: F1, 7=6.61); however, this too did not survive Bonferroni correction. In addition, neither simple effect of region within group was significant (even before Bonferroni correction) in the Not Run condition.
DISCUSSION
In the present study, we characterized the behavioral reactions of rats to specific alcohol-related stimuli (houselight and sipper) in an oral alcohol conditioning task to determine whether reactivity was driven by associative or non-associative memory. We also sampled blood after a conditioning session to characterize alcohol exposure level in the same rats. Finally, we used cFos expression as an index of cellular activation to map what brain areas might contribute to alcohol-related stimulus reactivity in the oral alcohol conditioning task.
Acquisition of houselight-elicited behavioral reactivity depends on the relationship between alcohol availability and houselight illumination
After a period of intermittent access to unsweetened alcohol in the homecage that was sufficient to promote alcohol drinking and apparent habituation of initial taste aversion (Supplemental Figure 7 A–B), rats were presented with an alcohol sipper intermittently in a different context (a conditioning chamber). Sipper presentations were accompanied by the sound of the bottle assembly motor activation. Some rats were also provided with an antecedent houselight stimulus (group Paired), while others were exposed to the same houselight stimulus explicitly unpaired with alcohol sipper presentation (group Unpaired). These conditioning stimulus arrangements were designed to test whether any resulting behavioral reactivity to houselight illumination reflected its learned association with alcohol availability.
In keeping with the correspondence between the two stimulus arrangements, rats in both groups learned to approach the sipper quickly upon its presentation to initiate and sustain consummatory licking across the period of alcohol availability (Supplemental Figure 3 A–B). However, in keeping with the key difference between the two stimulus arrangements, anticipatory approach to the site of alcohol availability was only conditioned as a reaction to houselight illumination in group Paired (Supplemental Figure 6 A). This is behavioral evidence that alcohol seeking behavior in response to houselight illumination in our paradigm reflects cue-alcohol associative memory, and not adaptation to repeated exposure to either the alcohol or the houselight. In previous work from one of our labs (Srey et al., 2015; Villaruel et al., 2016), we compared the learned behavioral responses of rats that received cue presentations explicitly paired and unpaired with alcohol delivery using a completely different paradigm. In that paradigm, a retractable lever was inserted into the conditioning chamber and immediately upon its retraction, unsweetened alcohol was delivered into an adjacent fluid port for consumption at any point during conditioning session. Using that paradigm, we found that alcohol seeking behavioral responses were only conditioned to the cue when the cue was explicitly paired with alcohol delivery, demonstrating the associative basis of alcohol seeking behaviors in the paradigm. Additionally, the cue only gained the ability to act as a secondary or conditional reinforcer if it had previously been explicitly paired with alcohol delivery. Together, our findings add to a growing body of preclinical work in rodent models that suggests that the potentially problematic ability of alcohol-predictive cues to elicit alcohol seeking behaviors in people may be a property that arises as a result of naturally-occurring associative learning across the alcohol use history.
It is worth mentioning that although the stimulus arrangement for group Unpaired was meant to eliminate the capacity of houselight onset to serve as a predictor for alcohol availability, it was possible for these rats to have learned that houselight offset predicted alcohol availability. One way this latter learning could have manifested is as conditioning of the overt attentional response (orienting) (Holland, 1980; Delamater & Holland, 2008). There was some behavioral evidence for this adventitious conditioning in group Unpaired in the form of persistent, albeit low frequency, orienting toward the time of houselight offset (Supplemental Figure 4 A); however, we are not convinced that this behavior is related to the consequences of alcohol drinking (Supplemental Figure 4 B). Thus, given equivalent alcohol ingestion, the stimulus arrangement in group Paired conditioned an appetitive response whereas the stimulus arrangement in group Unpaired may have conditioned an attentional response.
Trial-by-trial vigor of behavioral reactivity depends on the relationship between alcohol availability and houselight illumination
After conditioning session 9, rats in group Paired exhibited a decrease in houselight illumination-elicited sipper site approach across trials (Figure 3), an increase in latency to initiate drinking across trials (Figure 4), and a decrease in overall drinking intensity or rate across trials (Figure 5). Our present findings tell us that within-session patterns in group Paired are present as early as conditioning session 9 and are stable once they emerge, replicating and extending our previous finding (Cofresí et al., 2018). This within-session decrease in behavior might reflect the slow-onset of alcohol’s sedative-like effects (Chuck et al., 2006; Frye & Breese, 1981). If so, then we would have predicted trial-by-trial decreases in the vigor of consummatory licking (drinking behavior) not only in group Paired, but also in group Unpaired given similar total ingested doses. This was only true on brain collection day. Alternatively, within-session decrease in behavior might reflect the progressive devaluation of alcohol reinforcer (e.g., trial-by-trial decreases in the hedonic or incentive value of oral alcohol receipt or ingestion) (Samson et al., 1998; Samson et al., 2000). Cue-elicited reinforcer-directed responses are sensitive to between-session reinforcer devaluation procedures (e.g., satiety, pairing with illness) in food and sugar cue conditioning paradigms (Holland & Rescorla, 1975; Morrison et al., 2015). If within-session reinforcer devaluation were taking place in our paradigm, then we might expect the level of houselight-elicited sipper site approach in group Paired to decrease across trials. This was always the case. Tentatively, our present findings allow us to rule out alcohol-induced sedation and accept progressive within-session reinforcer devaluation as the explanation for within-session decrease in the vigor of cue-elicited alcohol seeking in group Paired. In turn, this suggests that the reason why we consistently observed within-session decreases in overall consummatory vigor in group Paired, but not group Unpaired, is that drinking behavior in group Paired is in part determined by the houselight-alcohol association. However, it is important to keep in mind that we observed neither consistently faster initiation of drinking bouts per trial nor consistently more intense drinking per trial in group Paired compared to group Unpaired.
Alcohol drinking, blood alcohol concentrations, and the motivation for learning about alcohol-predictive cues
In the present study, as in others (Chaudhri et al., 2010; Remedios et al., 2014), we were able to observe conditioning effects of self-administered alcohol on alcohol-related behavior in rats without ever depriving them of food or fluid or sweetening the alcoholic beverage. We can therefore rule out any confounding motivation for sweet taste or hydration as the source of reinforcement. We cannot, however, rule out the possibility that the additional calories provided by metabolism of ingested alcohol played some role in alcohol-related learning. The reality is that extra calories and intoxication both contribute to the reinforcing effects of ingested alcohol. However, the within-session behavior patterns exhibited by group Paired here and in our previous study (Cofresí et al., 2018) are not observed in studies with food-deprived rats presented with cues that predict food pellet delivery where the homeostatic drive for calories likely provides the primary motivation for learning (e.g., Nasser et al., 2018).
Another possibility is that the post-ingestive pharmacological effects of alcohol may have motivated alcohol-related learning in our procedure. The blood alcohol results in our previous studies support this suggestion (Cofresí et al., 2018; LeCocq et al., 2018), and the blood alcohol results in the present study also provide some support (Figure 2 B), although the evidence is less strong. Specifically, in the present study, over half rats had zero or non-detectable blood alcohol. One explanation for this observation is that we sampled blood too late after the last trial or because we only sampled blood once, which is why we then used the regression equations derived from the blood alcohol measurement data to estimate possible blood alcohol concentrations across sessions (Figure 2 C). However, we acknowledge that we cannot rule out the alternative interpretation, which is that the majority of rats in our present study had zero contact with alcohol’s post-ingestive pharmacological effects, at least during the cue conditioning phase.
Additional support for the idea that cue-alcohol learning in our procedure was motivated by alcohol’s post-ingestive pharmacological effects can come from the fact that in our previous study (Cofresí et al., 2018) as well as in our present study, no alcohol seeking behavior was conditioned in rats that consistently drank below 0.30 g/kg across conditioning sessions (Supplemental Figure 2+6). These rats may have been drinking enough to experience alcohol’s peripheral but not central pharmacological effects. However, some may argue that these rats may not have experienced even alcohol’s peripheral pharmacological effects. At the very least, these rats licked the sipper enough to experience alcohol as a taste stimulus (Supplemental Figure 3D). It is thus plausible to suggest that these rats were simply more sensitive to the aversive taste of unsweetened alcohol. These rats did not differ reliably from those that consistently drank above 0.30 g/kg across conditioning sessions in terms of initial or final alcohol preference in homecage two bottle-choice phase preceding conditioning. They did, however, exhibit lower alcohol preference and ingest lower doses on average across the homecage two bottle-choice phase (Supplemental Figure 7 C–D), which is consistent with the idea that this group of rats may have had greater aversion to the taste of alcohol.
Another possibility is that the houselight cue became associated with the flavor of alcohol. This explanation hinges on the assumption that a flavor preference was first conditioned and that this then allowed the flavor of alcohol to serve as a secondary reinforcer for conditioning of an alcohol approach response to the houselight illumination (an idea first proposed by Cunningham & Niehus, 1997). This assumption and the explanation based on it are supported by the fact that rats in our paradigm drank increasingly larger doses and appeared to lose their initial aversion to the taste of unsweetened alcohol across the homecage two-bottle choice phase (Supplemental Figure 7 A–B). We have no blood alcohol data from the homecage phase, so we do not know the extent to which that acquired reinforcing value is a function of exposure to alcohol’s post-ingestive pharmacological effects. However, the literature suggests that rats drink enough in this paradigm to experience a range of blood alcohol concentrations (see Carnicella, Ron, & Barak, 2014).
The cue-alcohol associative memory formation or expression may involve the insular cortex
Overall, despite differences in study design, mean Fos+ cell counts in the present study were in line with those reported by others using rat models of oral alcohol conditioning (Dayas et al., 2007; Radwanska et al., 2008; Jupp et al., 2011). Our Fos expression findings corroborate the idea that some, but not all, brain regions involved in tests for alcohol cue-induced relapse-like behavior after extinction training may be those involved in maintaining or expressing alcohol-related cue memories before extinction training (Figure 6 + Figure 7) (Chaudhri et al., 2010, 2013; Gass et al., 2011; Millan et al., 2015). The only brain region in which we observed noteworthy differential Fos expression between groups Paired and Unpaired was the insula (Figure 7 A). Activity in the insula appears to be important for maintaining cue-reward associative memory (Nasser et al., 2018). Additionally, deactivation of the insula appears to be important for the interoceptive effects of alcohol (Jaramillo et al., 2016). Since “Run” rats ingested equivalent alcohol doses and exhibited similar drinking behavior within the session on brain collection day, our findings suggest that either memory for the learned association of alcohol availability with houselight illumination in group Paired involves cells in the insula or that ingested alcohol has different effects in the insula of rats in group Paired and Unpaired.
Our Fos findings come with critical caveats. First, we do not know the identity of the cells activated (i.e., expressing Fos) in each brain region. Projection neurons and interneurons, and their neurochemically-defined subtypes, play important roles in communication within and between brain regions. Furthermore, Fos induction also has been observed in astrocytes (Arenander et al., 1989; Edling et al., 2007; Hermann & Rogers, 2009). Second, our study considered only 4-5 rat brains per group. Third, we lack some control groups such as alcohol and/or conditioning-naïve control groups, and groups exposed to CS without alcohol ingestion. Fourth, it is important to remember that over half the rats in the present study had zero blood alcohol when sampled. However, among rats that received a conditioning session prior to brain collection (viz., those in the “Run” condition), non-zero blood alcohol had been detected in 3 out of 4 rats in group Paired (we counted 1 rat with BEC 1.85 mg/dL as “zero” to be conservative) and 2 out of 4 rats in group Unpaired. Given these caveats, more work will be needed to illuminate the brain bases of reactivity to alcohol-related cues present in voluntary oral self-administration paradigms, including manipulation of putative substrates in order to confirm their involvement in alcohol-associative versus non-associative memory.
Conclusion
In the present study, we showed that behavioral reactivity to an antecedent visual stimulus signaling the opportunity to self-administer alcohol resulted from associative learning, rather than from non-specific effects of repeated exposure to the oral alcohol or visual stimulus. We also showed that memory for this conditioned alcohol cue reactivity may involve cells in the insular cortex. Our findings support continued investigation of the progression of brain and behavioral adaptations to chronic voluntary oral alcohol self-administration.
Supplementary Material
Highlights.
In rats that received light cue-alcohol sipper pairings, but not rats exposed to the light cue explicitly unpaired with the alcohol sipper, the light cue gained the ability to elicit anticipatory alcohol seeking
In both groups of rats, the alcohol sipper gained the ability to elicit the initiation of alcohol drinking
In rats that received light cue-alcohol sipper pairings, the vigor of alcohol seeking and drinking was subject to progressive devaluation of the alcohol reinforcer within the conditioning session
In rats that received light cue-alcohol sipper pairings, behavioral expression or maintenance of memory for association of the light cue with alcohol access induced c-Fos expression in the insular cortex
ACKNOWLEDGEMENTS
Funding was provided by NIH NIAAA R37AA11852 (RAG), NIH NIAAA T32AA007471 (RUC), NIHM R01MH091147 (MHM), CIHR MOP-137030 (NC), and a University of Texas undergraduate research fellowship (DJG). We thank Dr. Greg Loney for suggesting the idea to automate sipper site/faceplate contact measurement. We thank Jonathan Rosenzweig for his help with designing, prototyping, and fabricating the frame used to automate sipper site/faceplate contact measurement in conjunction with the Longhorn Maker Studio, the Aerospace Research Machine Shop, the Mechanical Engineering Machine Shop, and Mechanical Engineering Wood Shop. We thank Jarod Chaney and Lauren Schneider for their help with microscopy and cell counts. We thank Dr. Rheall Roquet is thanked for her help with brain collection.
Source of Support: NIH NIAAA R37AA11852 (RAG), NIH NIAAA T32AA007471 (RUC), NIHM R01MH091147 (MHM), CIHR MOP-137030 (NC), University of Texas Undergraduate Research Fellowship (DJG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arenander AT, de Vellis J, & Herschman HR (1989). Induction of c-fos and TIS genes in cultured rat astrocytes by neurotransmitters. Journal of Neuroscience Research, 24(1), 107–114. 10.1002/jnr.490240115 [DOI] [PubMed] [Google Scholar]
- Bormann NM, & Cunningham CL (1998). Ethanol-induced conditioned place aversion in rats: Effect of interstimulus interval. Pharmacology Biochemistry and Behavior, 59(2), 427–432. 10.1016/S0091-3057(97)00455-3 [DOI] [PubMed] [Google Scholar]
- Bozarth M (1990). Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacology Biochemistry and Behavior, 35, 485–487. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S (2014). Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48, 243–252. doi: 10.1016/j.alcohol.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, Howard EC, Moten M, Houck BD, Czachowski CL, & Gonzales RA (2008). A 3-day exposure to 10% ethanol with 10% sucrose successfully initiates ethanol self-administration. Alcohol, 42(3), 171–8. 10.1016/j.alcohol.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, & Janak PH (2010). Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35(3), 783–91. 10.1038/npp.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Woods CA, Sahuque LL, Gill TM, & Janak PH (2013). Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. The European Journal of Neuroscience, 38(5), 2751–61. 10.1111/ejn.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, & Correa M (2006). Comparison between multiple behavioral effects of peripheral ethanol administration in rats: Sedation, ataxia, and bradykinesia. Life Sciences, 79(2), 154–161. 10.1016/j.lfs.2005.12.045 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Quitadamo E, & Massi M (1999). Ethanol induces conditioned place preference in genetically selected alcohol-preferring rats. Psychopharmacology, 141(3), 235–241. 10.1007/s002130050830 [DOI] [PubMed] [Google Scholar]
- Cofresí RU, Lee HJ, Monfils M-H, Chaudhri N, & Gonzales RA (2018). Characterizing conditioned reactivity to sequential alcohol-predictive cues in well-trained rats. Alcohol, 69, 41–49. 10.1016/j.alcohol.2017.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofresí RU, Lewis SM, Chaudhri N, Lee HJ, Monfils M-H, & Gonzales RA (2017). Postretrieval Extinction Attenuates Alcohol Cue Reactivity in Rats. Alcoholism: Clinical & Experimental Research, 41(3), 608–617. 10.1111/acer.13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, & Janak PH (2007). Ethanol-associated cues produce general pavlovian-instrumental transfer. Alcoholism: Clinical & Experimental Research, 31(5), 766–74. 10.1111/j.1530-0277.2007.00359.x [DOI] [PubMed] [Google Scholar]
- Cunningham CL (1979). Flavor and location aversions produced by ethanol. Behavioral and Neural Biology, 27(3), 362–367. 10.1016/S0163-1047(79)92440-3 [DOI] [PubMed] [Google Scholar]
- Cunningham CL (1981). Spatial aversion conditioning with ethanol. Pharmacology, Biochemistry and Behavior, 14(2), 263–264. 10.1016/0091-3057(81)90255-0 [DOI] [PubMed] [Google Scholar]
- Cunningham CL, & Niehus JS (1997). Flavor preference conditioning by oral self-administration of ethanol. Psychopharmacology, 134(3), 293–302. 10.1007/s002130050452 [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, & Weiss F (2007). Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biological Psychiatry, 61(8), 979–89. 10.1016/j.biopsych.2006.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, Holland PC, 2008. The influence of CS-US interval on several different indices of learning in appetitive conditioning. J. Exp. Psychol. Behav. Process. 34, 202–222. doi:Doi 10.1037/0097-7403.34.2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edling Y, Ingelman-Sundberg M, & Simi A (2007). Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM. Glia, 55(3), 328–340. 10.1002/glia.20464 [DOI] [PubMed] [Google Scholar]
- Fidler TL, Bakner L, & Cunningham CL (2004). Conditioned place aversion induced by intragastric administration of ethanol in rats. Pharmacology Biochemistry and Behavior, 77(4), 731–743. 10.1016/j.pbb.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Fox J, & Weisberg S (2011). An {R} Companion to Applied Regression, Second Edition Thousand Oaks CA: Sage; URL: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion [Google Scholar]
- Frye GD, & Breese GR (1981). An evaluation of the locomotor stimulating action of ethanol in rats and mice. Psychopharmacology, 75(4), 372–379. 10.1007/BF00435856 [DOI] [PubMed] [Google Scholar]
- Gass JT, Sinclair CM, Cleva RM, Widholm JJ, & Olive MF (2011). Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addiction Biology, 16(2), 215–228. 10.1111/j.1369-1600.2010.00262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner S, Overmier J, & Balleine B (2005). The role of Pavlovian cues in alcohol seeking in dependent and nondependent rats. Journal of Studies on Alcohol and Drugs, 66(1), 53–61. [DOI] [PubMed] [Google Scholar]
- Hermann GE, & Rogers RC (2009). TNF activates astrocytes and catecholaminergic neurons in the solitary nucleus: Implications for autonomic control. Brain Research, 1273(Dmv), 72–82. 10.1016/j.brainres.2009.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC (1980). CS-US interval as a determinant of the form of Pavlovian appetitive conditioned responses. J. Exp. Psychol. Anim. Behav. Process. 6, 155–174. doi : 10.1037//0097-7403.6.2.155 [DOI] [PubMed] [Google Scholar]
- Holland PC, & Rescorla RA (1975). The effect of two ways of devaluing the unconditioned stimulus after first- and second-order appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes, 1(4), 355–363. 10.1037//0097-7403.1.4.355 [DOI] [PubMed] [Google Scholar]
- Inkscape Team (2017). Inkscape: free, open-source SVG editor. URL: https://www.inkscape.org/.
- Jupp B, Krstew E, Dezsi G, & Lawrence AJ (2011). Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin receptors. British Journal of Pharmacology, 162(4), 880–9. 10.1111/j.1476-5381.2010.01088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–38. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krank MD (2003). Pavlovian conditioning with ethanol: sign-tracking (autoshaping), conditioned incentive, and ethanol self-administration. Alcoholism: Clinical & Experimental Research, 27(10), 1592–8. 10.1097/01.ALC.0000092060.09228.DE [DOI] [PubMed] [Google Scholar]
- Krank MD, O’Neill S, Squarey K, & Jacob J (2008). Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology, 196(3), 397–405. 10.1007/s00213-007-0971-0 [DOI] [PubMed] [Google Scholar]
- LeCocq MR, Lahlou S, Chahine M, Padillo NL, & Chaudhri N (2018). Modeling relapse to Pavlovian alcohol-seeking in rats using reinstatement and spontaneous recovery paradigms. Alcoholism: Clinical and Experimental Research, 0–3 10.1111/acer.13825 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, & Holland PC (2005). Role of amygdalo-nigral circuitry in conditioning of a visual stimulus paired with food. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(15), 3881–8. 10.1523/JNEUR0SCI.0416-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Reese RM, Grossman CD, Chaudhri N, & Janak PH (2015). Nucleus Accumbens and Posterior Amygdala Mediate Cue-Triggered Alcohol Seeking and Suppress Behavior During the Omission of Alcohol-Predictive Cues. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(11), 2555–65. 10.1038/npp.2015.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Schramm MJW, Wawrzynski JR, Gore F, Oikonomou-Mpegeti F, Wang NQ, Everitt BJ (2012). Antagonism at NMDA receptors, but not β-adrenergic receptors, disrupts the reconsolidation of pavlovian conditioned approach and instrumental transfer for ethanol-associated conditioned stimuli. Psychopharmacology, 219(3), 751–61. 10.1007/s00213-011-2399-9 [DOI] [PubMed] [Google Scholar]
- Morrison SE, Bamkole MA, & Nicola SM (2015). Sign tracking, but not goal tracking, is resistant to outcome devaluation. Frontiers in Neuroscience, 9(December), 1–12. 10.3389/fnins.2015.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser HM, Lafferty DS, Lesser EN, Bacharach SZ, & Calu DJ (2018). Disconnection of basolateral amygdala and insular cortex disrupts conditioned approach in Pavlovian lever autoshaping. Neurobiology of Learning and Memory, 147, 35–45. 10.1016/j.nlm.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nentwig TB, Myers KP, & Grisel JE (2017). Initial subjective reward to alcohol in Sprague-Dawley rats. Alcohol, 58, 19–22. 10.1016/j.alcohol.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2017). R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: URL: https://www.R-project.org/. [Google Scholar]
- Radwanska K, Wrobel E, Korkosz A, Rogowski A, Kostowski W, Bienkowski P, & Kaczmarek L (2008). Alcohol relapse induced by discrete cues activates components of AP-1 transcription factor and ERK pathway in the rat basolateral and central amygdala. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 33(8), 1835–46. 10.1038/sj.npp.1301567 [DOI] [PubMed] [Google Scholar]
- Remedios J, Woods C, Tardif C, Janak PH, & Chaudhri N (2014). Pavlovian-conditioned alcohol-seeking behavior in rats is invigorated by the interaction between discrete and contextual alcohol cues: implications for relapse. Brain and Behavior, 4(2), 278–289. 10.1002/brb3.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, & Chappell A (1998). Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcoholism: Clinical & Experimental Research, 22(8), 1783–7. [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, & Slawecki CJ (2000). A New Assessment of the Ability of Oral Ethanol to Function as a Reinforcing Stimulus. Alcoholism: Clinical & Experimental Research, 24(6), 766–773. 10.1111/j.1530-0277.2000.tb02054.x [DOI] [PubMed] [Google Scholar]
- Schramm MJW, Everitt BJ, & Milton AL (2016). Bidirectional Modulation of Alcohol-Associated Memory Reconsolidation through Manipulation of Adrenergic Signaling. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(4), 1103–11. 10.1038/npp.2015.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srey CS, Maddux J-MN, & Chaudhri N (2015). The attribution of incentive salience to Pavlovian alcohol cues: a shift from goal-tracking to sign-tracking. Frontiers in Behavioral Neuroscience, 9(March), 54 10.3389/fnbeh.2015.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, & Grupp LA (1986). Conditioned Place Aversion Mediated by Orally Self-Administered Ethanol in the Rat. Pharmacology Biochemistry & Behavior, 24(5), 1369–1375. [DOI] [PubMed] [Google Scholar]
- Tomie A, di Poce J, Derenzo CC, & Pohorecky LA (2002). Autoshaping of ethanol drinking: an animal model of binge drinking. Alcohol & Alcoholism, 37(2), 138–146. 10.1093/alcalc/37.2.138 [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, & O’Dell LE (2014). Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcoholism: Clinical & Experimental Research, 38(1), 108–15. 10.1111/acer.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaruel FR, & Chaudhri N (2016). Individual Differences in the Attribution of Incentive Salience to a Pavlovian Alcohol Cue. Frontiers in Behavioral Neuroscience, 10(December), 1–13. 10.3389/fnbeh.2016.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2009). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


