Abstract
Inherited retinal diseases (IRDs) are a group of rare, heterogenous eye disorders caused by gene mutations that result in degeneration of the retina. There are currently limited treatment options for IRDs; however, retinal gene therapy holds great promise for the treatment of different forms of inherited blindness. One such IRD for which gene therapy has shown positive initial results is choroideremia, a rare, X-linked degenerative disorder of the retina and choroid. Mutation of the CHM gene leads to an absence of functional Rab escort protein 1 (REP1), which causes retinal pigment epithelium cell death and photoreceptor degeneration. The condition presents in childhood as night blindness, followed by progressive constriction of visual fields, generally leading to vision loss in early adulthood and total blindness thereafter. A recently developed adeno-associated virus-2 (AAV2) vector construct encoding REP1 (AAV2-REP1) has been shown to deliver a functional version of the CHM gene into the retinal pigment epithelium and photoreceptor cells. Phase 1 and 2 studies of AAV2-REP1 in patients with choroideremia have produced encouraging results, suggesting that it is possible not only to slow or stop the decline in vision following treatment with AAV2-REP1, but also to improve visual acuity in some patients.
Keywords: AAV2-REP1, choroideremia, gene therapy, retina
RETINAL GENE THERAPY
Gene therapy aims to treat the underlying cause of inherited retinal diseases (IRDs), a group of rare, heterogenous eye disorders in which a genetic mutation has caused a dysfunctional or nonmissing protein (1), through therapeutic delivery of the specific deoxyribonucleic acid (DNA) into patients’ cells to produce the functional protein (2). A new gene is introduced to the target cells via a viral vector and transcribed using the host’s transcription machinery, resulting in expression of the functional protein (2,3). Exploiting the natural capability of viruses, adeno-associated virus (AAV) vectors are increasingly being used to deliver the gene of interest into the target cell (2). To date, mutations in more than 250 genes have been implicated in IRDs, the majority of which are associated with dysfunction or death of photoreceptor cells (4).
Retinal gene therapy holds great promise in treating different forms of inherited blindness (3). The eye is a particularly attractive target for gene therapy for several reasons. First, owing to the tight junctions between the retinal pigment epithelium (RPE) and blood–retina barrier, the retina is a relatively immune privileged tissue (5). This means that introduction of a foreign antigen, such as a viral vector, is usually tolerated without risk of stimulating a severe inflammatory immune response (2). The retina also requires only small amounts of the vector to achieve a therapeutic response (2,5). The enclosed nature of the eye, due to the tight blood–ocular barrier, reduces the risk of widespread dissemination of the locally administered vector and therefore unwanted systemic effects (5,6). Furthermore, it is relatively easy to access the eye surgically, which facilitates intravitreal or subretinal administration of the vector to the affected tissue (2,5). A range of noninvasive techniques is available for monitoring the retinal structure and assessing visual function after surgery, facilitating evaluation of treatment efficacy (2,5). Last, because retinal cells are differentiated and nondividing, continuous gene expression can be achieved with nonintegrating vectors (7).
LUXTURNA™ (voretigene neparvovec-rzyl; Spark Therapeutics Inc., USA) was the first Food and Drug Administration-approved directly administered gene therapy for the treatment of a disease caused by mutations in a specific gene (8). LUXTURNA™ is an AAV vector-based gene therapy indicated for patients with genetically confirmed biallelic RPE65 mutation-associated retinal dystrophy (9-11). The RPE65 gene encodes retinal pigment epithelial 65-kDa protein (RPE65), which is involved in phototransduction (conversion of light photons into a neuronal signal); absent or defective RPE65 caused by mutations results in visual impairment (9).
Many other retinal gene therapies are currently in development. These therapies, which are mostly in phase 1/2 trials, employ different vectors for the treatment of a range of IRDs (12,13). Recombinant AAV is the most frequently used vector in retinal gene therapy (2). AAV is a single-stranded DNA parvovirus that is nonpathogenic to humans (5). In addition to the favorable toxicity and immunogenic profile, recombinant AAV vectors are suitable for retinal gene therapy owing to their stable, nonintegrating genetic material and ability to transduce postmitotic retinal target cells (2,7). To date, thirteen wild-type AAV serotypes (AAV1–AAV13) have been isolated; and although these vary in terms of the cell types they infect and their transduction efficiencies, the majority are able to transduce the RPE at low to moderate doses (14,15).
Recombinant lentiviral vectors are also being tested in phase 1/2 of retinal gene therapy (16), although they are a much less common vehicle than AAV. Studies in mice have shown that subretinal use of lentiviral vectors appears to be well tolerated (5,17). Lentiviral vectors have been shown to successfully transduce photoreceptors in the newborn mouse retina, leading to phenotypic improvements in animal models of IRDs, including those for Stargardt disease (18,19) and Usher syndrome (20,21), a form of retinitis pigmentosa (RP) associated with hearing loss. However, lentivirus vectors appear to have a much lower capability than AAV vectors to transduce the adult photoreceptor (16,22).
Lentiviral vectors have a larger packaging capacity (8 kb) than AAV vectors (4.7 kb), meaning they can carry complete gene coding sequences that exceed 5 kb, such as the replacement genes for Stargardt disease, Usher syndrome, and Leber congenital amaurosis type 10 (16,22). The use of dual AAV vectors, each of which contains half of a large transgene expression cassette, has shown promise in matching this packaging capability of lentivirus vectors (16,22). Although dual AAV vector photoreceptor transduction efficiency is currently lower than with a single AAV vector, the dual system has been shown to improve retinal phenotype in the Stargardt disease and Usher syndrome type 1B mouse models (22-24). Lentiviruses are integrating viral vectors, unlike the nonintegrating AAV, meaning they can induce permanent and heritable modifications to transduced cells; however, this can also lead to mutations in the host cells (5). The large size of lentiviral vectors can also affect their distribution in tissu(5).
AAV-based vectors are being employed for the treatment of choroideremia (AAV2-REP1, Nightstar Therapeutics, UK) (25,26), X-linked RP (XLRP; AAV-RPGR, Nightstar Therapeutics, UK; AAV-RPGR, MeiraGTx UK II Ltd., UK; rAAV2tYF-GRK1-RPGR, Applied Genetic Technologies Corp, USA) (27-29), RP due to mutations in PDE6B (HORA-PDE6B, Horama S.A., France) (30), ND4-associated Leber Hereditary Optic Neuropathy (rAAV2/2-ND4 [GS010], GenSight Biologics, France, and Bascom Palmer Eye Institute, USA) (31), X-linked retinoschisis (AAV-RS1, National Eye Institute, USA; rAAV2tYF-CB-hRS1; Applied Genetic Technologies Corp, USA) (12), and achromatopsia (AAV-CNGB3, MeiraGTx UK II Ltd., UK; rAAV2tYF-PR1.7-hCNGB3 and AGTC-402, Applied Genetic Technologies Corp, USA; rAAV.hCNGA3, STZ eyetrial, Germany) (12). Lentiviral vectors are being used for the treatment of patients with neovascular age-related macular degeneration (RetinoStat®, Oxford BioMedica, UK) (32,33), Usher syndrome (UshStat®, Sanofi, France) (34,35) and Stargardt disease (EIAV-ABCA4 [SAR422459], sanofi, France) (12,13).
INHERITED RETINAL DISEASES
Identification of the genetic cause of the patient’s IRD is recommended and is an important part of patient care under the guidance of the treating physician or genetic counselor (36). Genetic testing is available from commercial sources and, in some cases, from non-profit organizations; however, recommendations state that approved laboratories should be used and direct-to-consumer and patient-sought genetic testing should be avoided (36).
Retinitis Pigmentosa: the Most Common IRD
RP is thought to affect 1.5 million people worldwide (37), with a prevalence ranging from 1 in 3000 to 1 in 4000, in certain populations (38,39). As a progressive IRD, RP is characterized by the degeneration of rod photoreceptor cells in the early stages of disease, followed by cone cell death. The RPE subsequently detaches from Bruch’s membrane and migrates to intraretinal perivascular sites. As a result, RP generally presents as night blindness in adolescence with ensuing loss of visual field from early adulthood. Visual acuity (VA) remains relatively well preserved until more advanced disease and the photoreceptor cell loss affects the macula, generally during middle age (37).
The heterogenous nature of patients with RP corresponds, at least in part, to the considerable number of mutations that have been associated with the disease (37). More than 90 genes have been implicated in the development of RP (see http://www.sph.uth.tmc.edu/RetNet/www.sph.uth.tmc.edu/RetNet/) (40), and each defect results in a gene-specific subtype of RP with particular clinical and symptomatic features (37). The severity of RP has been associated with the inheritance pattern; XLRP tends to follow a more severe course than autosomal recessive RP (arRP), while patients with autosomal dominant RP (adRP) generally have the best prognosis in terms of maintaining VA (37). Approximately 20–30% of patients with RP present with the syndromic form, which is associated with additional abnormalities outside of the eye (37).
Many of the genes implicated in RP are involved in the photoreceptor-RPE complex and interphotoreceptor matrix (37). XLRP represents 10–20% of all RP, with mutations in the RP GTPase regulator (RPGR) gene being responsible for approximately 70% of the X-linked form of the disease (1,38). Through binding to RPGR-interacting protein (RPGRIP), RPGR is anchored to the connecting cilium between the photoreceptor cell inner and outer segments, and is involved in the gating mechanism facilitating and restricting protein movement, particularly opsins, between the segments (37,38). Although not yet fully elucidated, it is thought that the photoreceptor degeneration observed in patients with XLRP caused by a mutation in RPGR is a result of defective protein trafficking in the connecting cilia (41). A majority of XLRP-causing mutations in RPGR have been located in the open reading frame 15 (ORF15) exon (38), an alternative splice product that is highly expressed in the retina (42).
Most of the 62 identified genes linked with arRP (40) account for around 1% of cases, including previously discussed RPE65 (43). A large number of the genes responsible for adRP are known, accounting for around 70% of the families in adRP cohort studies (40). Mutations in the rhodopsin gene (RHO) account for approximately 30% of cases (44). RHO encodes rhodopsin, a G protein–coupled receptor that constitutes more than 90% of the total protein in rod photoreceptor cell outer segments. Rhodopsin absorbs light entering the eye and becomes activated, initiating the visual transduction pathway. Mutations in RHO have been found to impact rhodopsin protein folding, activation and trafficking, thus disrupting the visual cycle and affecting visual function (43).
Gene Therapy for the Treatment of Retinitis Pigmentosa
For the treatment of RP caused by X-linked or autosomal recessive genetic mutations, a one-step gene augmentation therapy approach that replaces the nonfunctioning gene can be employed. Gene therapy for the treatment of adRP, however, is more challenging because one allele carries the mutation and one allele carries the normal gene. The mutated protein that is produced from the mutated allele may itself be toxic or induce a dominant negative effect whereby the mutated protein interferes with the function of the protein produced from the normal allele. Therefore, expression from the mutant allele needs to be silenced to prevent the disease state. Gene therapy for adRP must account for mutational heterogeneity; for example, more than 150 individual mutations in RHO have been implicated in rhodopsin-linked adRP (45). A two-step approach has been developed, whereby the mutant and wild-type (WT) RHO alleles are suppressed using ribonucleic acid interference, and a suppression-resistant functional RHO gene is introduced. Investigation of the suppression-replacement approach in the adRP mouse and canine models demonstrated functional benefit (45,46); however, to date, no in-human trials in gene therapy for RHO RP are yet underway.
Gene therapy for the treatment of patients with XLRP caused by mutations in the RPGR gene is now in early human testing. Preclinical studies highlighted the challenges associated with developing RPGRORF15 gene therapy (47-49). First, the gene has poor sequence stability, likely owing to an unusual sequence of purine-rich repeats that are prone to mutation (47,49,50). Second, the RPGR protein undergoes complex posttranscriptional processing and inadvertent splicing may occur, particularly at the adenine- and guanine-rich mutational hotspot (50). One study used an RPGR-knockout mouse model to demonstrate that a truncated RPGR, produced by RPGR transgene transcript alternative splicing, behaved as a dominant, gain-of-function mutant, resulting in faster photoreceptor degeneration than in the knockout (48). Posttranslational glutamylation is known to be critical for the function of RPGR in photoreceptors; a lack of glutamylation in mice results in a phenotype similar to that observed in RPGR knockout mice (51). In an attempt to overcome these challenges and improve sequence stability, the coding sequence of RPGRORF15 Has been optimized (42,50). In the case of gene therapy with AAV vectors, sequence optimization can provide increased transgene expression that limits the need for regulatory elements such as woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), which use up limited AAV packaging capacity (42).
A variety of parameters were assessed to optimize RPGRORF15 expression efficiency. The nucleotide sequence can be changed without altering the translated amino acid sequence (silent substitutions) of the transgene in order to improve cytosine/guanine content and to remove unwanted repeat sequences and/or restriction sites that may interfere with cloning. These modifications often provide the most important advantages of optimizing codons in difficult sequences such as RPGRORF15 and work to improve sequence fidelity. Changing nucleotides without changing the resulting amino acid sequence carries the potential to make the sequence more stable and less prone to spontaneous mutations during the production of vectors for gene therapy. In addition, codon optimization helps prevent further splicing of the transgene that might occur during the RPGR transcription. Expression and function of the codon-optimized RPGR (coRPGRORF15) was evaluated in knockout mouse models that lack RPGRORF15 expression in the retina. CoRPGRORF15 was found to demonstrate greater sequence stability than WT RPGR, showing no sequence deviation throughout the vector production process compared with numerous mutations observed with the WT variant. Additionally, the coRPGRORF15 construct demonstrated a 4-fold higher expression level and significantly higher protein expression in transfected cells compared with the WT construct. Importantly, the full-length RPGR protein, identical to the WT form in terms of sequence and posttranslational glutamylation, was produced by the coRPGRORF15 construct (50). The safety assessment of coRPGRORF15, performed in a bilateral, masked, controlled trial in 44 WT mice, indicated no toxic effect arising from surgery, the vector or the coRPGRORF15 transgene. Finally, coRPGRORF15 was found to rescue rod and cone function in the knockout mice (50). Based on these data, a phase 1 clinical trial of gene therapy for the treatment of RPGR-linked XLRP using an AAV2/8 vector harboring the coRPGRORF15 gene is now underway (NCT03116113).
Two other studies have been initiated to assess retinal gene therapy for the treatment of XLRP. The first is an open-label phase 1/2 dose escalation study to evaluate the efficacy and safety of AAV2/5.hRKp.RPGR over 18 months in adults and children aged over 5 years with XLRP (NCT03252847; sponsored by MeiraGTx UK Ltd) (27,42). The second is also an open-label phase 1/2 dose escalation study over 36 months, which aims to assess the efficacy and safety of rAAV2tYF-GRK1-RPGR in adults and children aged over 6 years with XLRP (NCT03316560; sponsored by Applied Genetic Technologies Corp.) (28,42).
Natural history studies provide valuable additional characterization of disease progression and help to define outcome measures that will demonstrate clinically significant change in interventional studies. Several are ongoing for XLRP including NCT03314207 (sponsored by Applied Genetic Technologies Corp.) (52), NCT03349242 (sponsored by MeiraGTX UK Ltd) (53), and XOLARIS (sponsored by Nightstar Therapeutics).
Choroideremia: a Rare Inherited Retinal Disease
Choroideremia is a rare, X-linked retinal disorder characterized by progressive degeneration of the RPE, retina and choroid (54,55). The disease is estimated to affect 1 in 50,000 individuals, primarily males (55). However, choroideremia is likely to be underdiagnosed because of its similarities to other retinal dystrophies, especially in the early stages; and as such, it is thought to account for approximately 4% of all blindness (56). Similarly to RP, choroideremia can present in childhood as night blindness; nevertheless, at this stage, patients generally maintain good visual acuity and detailed central vision (54,57). Clinically, in the early stage of disease (Fig. 1a), a mottled pattern representing minor pigmentary changes may be seen at the fundus equator, with focal atrophy and increased visualization of the pericentral choroidal vasculature (54,58). The optical coherence tomography (OCT) image may appear normal or show slight thinning of the outer nuclear layer corresponding to shortening of photoreceptor cell outer segments (58). Progressive constriction of visual fields over time, due to the degeneration of retinal cells impinging onto the fovea, leads to vision loss in early adulthood (59). This may be visualized on fundus photography and OCT as preserved, scallop-edged islands of pigmentation (Fig. 1b) surrounded by choroidal atrophy (58). Microperimetry testing at this point may reveal a less stable fixation area (59). A mean onset of moderate visual impairment occurs in the fifth decade (Lam BL, et al. Presented at: Association for Research in Vision and Ophthalmology [ARVO] Annual Meeting. Honolulu, HI, USA. 2018). Vision loss in patients with choroideremia has been estimated to occur at a rate of −0.5 Early Treatment Diabetic Retinopathy Study (ETDRS) letters per year (Lam BL, et al. Presented at: Association for Research in Vision and Ophthalmology [ARVO] Annual Meeting. Honolulu, HI, USA. 2018). However, best-corrected visual acuity (BCVA) especially can vary widely among patients depending on whether the fovea has been affected by retinal atrophy and how much of the fovea is preserved on a residual island (59). In late-stage choroideremia, a small island of residual RPE may be seen (Fig. 1c), with possible visualization of large choroidal vessels and the sclera in cases with an acutely thinned retina (57,58). Although total blindness is likely in patients with end-stage disease owing to total retinal atrophy (after approximately the sixth decade of life) (54,57,58), preserved photoreceptor cells, even with aberrant outer segments, may provide a small amount of visual acuity, albeit with a very limited visual field (58). While anatomical endpoints remain symmetrical, during mid- to late-stage disease, visual acuity and mean macular threshold sensitivity may become asymmetric between eyes (59). Similarly, the nasal and temporal areas of the central macula have been found to decline asymmetrically, with the area nasal to the fovea degenerating at a faster rate than temporal (59).
Fig. 1.
Optical coherence tomography (left) and fundus photography (right) of (a) early-, (b) mid-, and (c) late-stage choroideremia.
Choroideremia results from deletion or mutation of the CHM gene, resulting in loss of expression (54,60). Sequence variations, translocations, point mutations, small deletions, insertions, nonsense, and frameshift mutations in the CHM gene have all been identified as causative mutations in choroideremia (54). The consequence of this is absence of a functional CHM gene product, Ras-associated binding (Rab) escort protein-1 (REP1) (54,60,61). Rab proteins are guanosine triphosphate hydrolase enzymes whose function is essential for vesicle trafficking processes throughout cells of the body (54,61). These proteins regulate vesicle formation, motility, tethering and fusion to the acceptor membrane, and signaling to other organelles (54,61,62). Rab functionality is dependent first on REP for activation, then followed by prenylation (Fig. 2) (54,64). There are two REP proteins in mammals, REP1 and Rab protein 2 (REP2); and in most tissues of patients with choroideremia, functional REP2 is able to compensate for deficient or nonfunctional REP1 (54,64,65). In the retina, however, REP2 is not able to function as a complete substitute, leaving a portion of Rab proteins inactivated and unprenylated (54,64). Although current understanding is incomplete, the Rab27a isoform may play a key role in the underlying disease; this isoform is highly expressed in the RPE and choroid (64), the REP1-Rab27a complex has a higher affinity for geranylgeranyl transferase type-II (prenylation complex) than REP2-Rab27a, and Rab27a has one of the slowest prenylation rates of all Rab proteins, which are thought to contribute to hypoprenylation in choroideremia (66). It is thought that functional REP1 deficiency and reduced prenylation of Rabs initially cause defects in the RPE, which are followed by photoreceptor cell death as a secondary consequence (67-70). Rab27a is also localized to melanosomes and is involved in their transport to the periphery of melanocytes (54,64); therefore, degeneration of the RPE may be a consequence of defective melanosome transport, leading to insufficient protection against harmful light exposure (61). Currently there is no approved treatment for choroideremia (54); however, maintaining visual acuity and visual fields is a clinically meaningful long-term treatment goal (26,71).
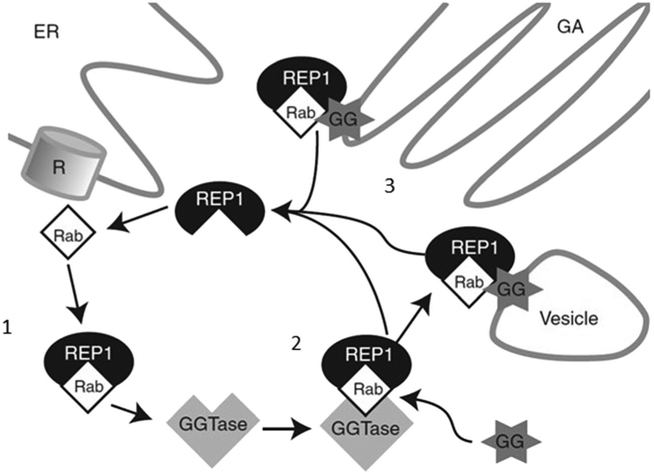
Fig. 2.
The interaction between Ras-associated binding (Rab) protein and the Rab escort protein 1 (REP1). Ribosomes (R), located on the endoplasmic reticulum (ER), generate Rab proteins, which subsequently bind to REP1 (1). REP1 presents the Rabs to a catalytic Rab geranylgeranyltransferase (GGTase) subunit for prenylation with usually two prenyl groups (GG) in a geranylgeranyl transfer reaction (2). Following prenylation, Rabs are transported by REP1 to destination organelles such as the Golgi apparatus (GA), where they attach to the lipid membrane (3) (61,63). Figure reproduced with permission from Cold Spring Harbor Laboratory Press (63)
A NOVEL VECTOR CONSTRUCT FOR CHOROIDEREMIA GENE THERAPY
The Use of Adeno-Associated Virus in Choroideremia Gene Therapy
AAV2 is the most extensively examined serotype for gene therapy vectors and has been well characterized in several animal models (62,72). AAV2-based vectors have been shown to have a good affinity for photoreceptors and the RPE, and successful use of AAV2-mediated gene therapy for the treatment of a number of retinal dystrophies has been demonstrated in clinical trials (10,11,13,73-75). Given its small size of 1.9 kB, the CHM gene fits well within the packaging limits of the AAV2 vector while maintaining the necessary space to accommodate other regulatory elements, such as the gene promoter and poly-A signal sequences (62). AAV2-mediated gene transfer therefore lends itself to the treatment of patients with choroideremia.
Development of the AAV2-REP1 Vector Construct
Through the introduction of the functional CHM gene and enhanced expression of the REP1 protein, gene therapy for choroideremia aims to restore intracellular protein trafficking and reduce the accumulation of waste products in retinal cells, thus slowing or stopping the decline in vision. An AAV2 vector construct encoding REP1 (AAV2-REP1) has been shown to deliver a functional version of the CHM gene into the RPE and photoreceptor cells (7,26). AAV2-REP1 is an AAV2 expression cassette that encodes REP1 and comprises a combined cytomegalovirus enhancer with the chicken β-actin (CBA) promoter and rabbit β-globin splice acceptor site (CAG promoter) upstream and a WPRE downstream of the CHM complementary DNA (1,7,26,76). The CBA promoter has been shown to provide effective transduction of and longterm transgene expression in the RPE in clinical trials (26,63) and is often the promoter of choice for retinal gene therapy (1). The vitelliform macular dystrophy 2 promoter, which is specifically expressed in the RPE, has also been used as an alternative to the CBA promoter in a lentiviral vector. Although gene expression is not as high as with the CBA promotor (1), subretinal delivery of this construct in a mouse model of choroidal neovascularization has been shown to lead to efficient transduction of the RPE with stable expression at 21 days post-injection that persists for at least 9 months (77). AAV2-REP1 is the only retinal gene vector to include the WPRE sequence (63). The WPRE has been shown to enhance AAV transgene expression in the retina and improve vector efficiency, allowing lower doses of vector to be delivered to achieve the desired effect (7). The ability of the WPRE sequence to increase levels within the target cell may be due to its ability to enhance transcript termination (7,78). As well as being used in human retinal gene therapy clinical trials (25,26), the WPRE sequence has also been used in other gene therapy studies and was approved by the Food and Drug Administration for use in a clinical trial of AAV2 in Parkinson’s disease (63,79).
UNDERSTANDING THE MULTIPLICITY OF INFECTION FOR DOSE TARGETING
The multiplicity of infection (MOI), the ratio between the total number of vector particles and the number of cells potentially transduced, is known to be correlated with treatment effect in gene therapy (26). The MOI is an important consideration for treating degenerative conditions such as choroideremia because the volume of the target tissues is constantly shrinking. This means that the MOI of viral particles per cell may vary at different stages of the disease. Because the MOI will increase for a fixed titer of vector as the target area reduces, the dose of the vector per mm2 of target tissue will therefore change as choroideremia disease progresses (26).
Vector delivery sites should also be carefully considered when evaluating MOI. The two main locations for vector delivery are the vitreous body of the eye, when the vector solution is delivered via intravitreal injection, and the subretinal space, when the vector solution is injected between the photoreceptors and RPE (80). In advanced choroideremia, in which the area of intact, treatable retina, choroid and RPE is limited, subretinal delivery may offer the most targeted approach currently available. In this case, the MOI is maximized, off-target exposure is minimal, and the potential for an inflammatory response is reduced because the vector is delivered directly to the target cells within an immune-privileged environment (80,81). Conversely, in early-stage choroideremia, before considerable retinal degeneration has occurred, an intravitreal approach could be considered because a larger area of the retina will be exposed to the vector. However, to treat a broader area of the retina, a higher concentration of vector would be required to achieve an effective MOI over the larger treatment area. This circumstance in turn potentially raises additional safety considerations with regard to toxicity of the viral vector and inflammation; the higher total vector dose may increase the risk of shedding and biodistribution, which may be more likely to provoke a potentially harmful immune response (80,81). One option could be the use of multiple subretinal blebs instead of one single bleb; the concentration of vector would not need to be increased and there would be no increased risk of systemic exposure due to the vector being delivered directly to the target cells, as with a single bleb. However, multiple blebs may increase the risk for macular hole formation and complications such as retinal detachment (81,82).
IMPORTANT INSIGHTS FROM PRECLINICAL MODELS
Restoration of REP1 expression and function has been demonstrated in cell and animal models of choroideremia following delivery of CHM via a recombinant AAV (76,83,84). The aims of these studies were functional activation of Rab proteins; reestablishment of Rab membrane trafficking; restoration of prenylation activity; and, in the animal models, a positive effect on retinal function.
The ability to restore functional REP1 expression by AAV2-mediated delivery of REP1 to affected cells was first investigated in in vitro models of choroideremia (83-85). Defective lymphocytes and fibroblasts devoid of REP1 were isolated from patients with choroideremia and used to test the ability of the AAV-REP1 vector to rescue the expression and function of the REP1 protein (84). Results demonstrated the effective delivery of REP1 to the affected cells, as well as restoration of REP1 protein expression and function (84). In addition, in vitro reproduction of prenylation has been used to demonstrate the activity of REP1 delivered by an AAV2 vector (85).
In a second study, personalized in vitro models of choroideremia were generated using lymphoblasts or induced pluripotent stems cells from patients with loss of function CHM mutations (83). The proviral plasmid, pAAV2-CBAe-hCHM (WT human CHM cDNA [hCHM] under the control of a hybrid, cytomegalovirus enhancer-CBA promoter), delivered the CHM gene in a dose-dependent manner. A 50-fold increase in REP1 expression was observed in AAV2-hCHM treated cells compared with uninfected cells (MOI of 2×105 viral genomes/cell). Additionally, a significant 2–3-fold increase in the prenylation of RAB27a (substrate of REP1) was observed in cells transduced with AAV2-hCHM compared with the untreated controls, and localization of the RAB27 protein at the cell membrane was restored following AAV2-mediated delivery of REP1. Overall, the transduction of affected cells with the CHM transgene restored REP1 enzymatic activity and corrected the protein trafficking defect caused by loss of REP1 function. The gene transfer process was efficient, and preliminary safety data were encouraging (83).
In animal models, the AAV2 vector expressing REP1 under the control of a cytomegalovirus-enhanced CBA promoter augmented by a WPRE (AAV2/2-CBA-WPRE) has been shown to hold promise as a suitable vector for CHM gene therapy (7,76). First, Tolmachova et al. delivered the AAV2/2-CBA-REP1 vector subretinally in CHMnull/WT heterozygous female carrier mice, which have the choroideremia phenotype (76). REP1 expression was subsequently observed in both photoreceptor cells and the RPE. No impairment in retinal function was observed following high- or low-dose AAV2/2-CBA-REP1 in control wild-type mice containing normal levels of REP1, confirming that overexpression of REP1 does not result in any obvious toxic effects on retinal function. Heterozygous female carriers (CHMnull/WT) showed progressive retinal degeneration with an obvious reduction in dark-adapted electroretinogram amplitude. Following treatment with high-dose AAV2/2-CBA-REP1, dark-adapted retinal function seemed to improve, a result that was not seen at the low dose, suggesting a dose response. Overall, this study demonstrated that the AAV2/2-CBA-REP1 vector provided strong and functional transgene expression (76). More recently, Patricio et al. delivered pAAV2-REP1-WPRE via subretinal injection to C57BL/6 J mice and observed extensive expression of human REP1 throughout the retinal layers: the photoreceptors, outer nuclear layer, and RPE (7).
SURGICAL APPROACH
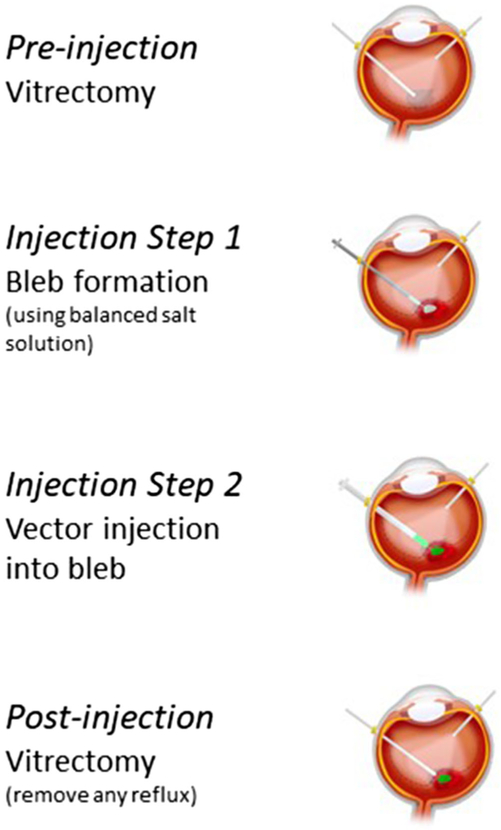
AAV2-REP1 is administered using a three-step surgical procedure (Fig. 3) (82). First, core vitrectomy and posterior vitreous detachment are performed to allow visualization of the injection site and to reduce vitreous traction during vector injection. The procedure involves a three-port small-gauge (23 or 25 G) pars plana vitrectomy followed by induction of posterior vitreous detachment and core and peripheral vitrectomy. Second, macular detachment is induced using a 41 G blunt-tipped subretinal cannula and subretinal injection of a balanced salt solution (BSS). The site of the retinotomy is selected close to upper temporal vascular arcade, avoiding the blood vessel and facilitating the correct localization and optimal delivery of the vector to the target area (82). The BSS subretinal bleb propagating toward the fovea may be visualized using intraoperative, real-time cross-sectional OCT imaging (82,86). In the final step, the AAV2 vector is slowly infused into the subretinal BSS bleb. Slow infusion is essential in patients with choroideremia because the targeted subretinal space, where the retina is preserved, is often less than the 1-mL volume of vector injected, and the retina can become overstretched. Once the vector has been delivered, irrigation and aspiration of the vitreous cavity is performed to help to remove vector refluxed into the vitreous cavity. Care needs to be taken to avoid injecting an air bubble into the subretinal space to minimize the risk of acute retinal stretch and possible development of macular hole. Additionally, gas tamponade should be avoided to reduce the risk of vector reflux and cataract formation; following vitreous cavity irrigation, the eye is often left fluid filled (82).
Fig. 3.
The surgical procedure for adeno-associated virus–Ras-associated binding (Rab) escort protein 1 vector delivery.
PROMISING CLINICAL OUTCOMES FOR PATIENTS WITH CHOROIDEREMIA
In 2014, the first patient outcomes were reported in a phase 1 study following retinal gene therapy with an AAV2 vector encoding REP1 (AAV2-REP1) (26). A total of six patients representing different stages of choroideremia and foveal degeneration underwent treatment in one eye, with the untreated fellow eye acting as a control; outcomes were assessed at 6 months and 3.5 years post treatment (26,71). At baseline, two patients had advanced disease and a low baseline BCVA, and four patients had near-normal BCVA (26). Six months after treatment, the patients with low baseline BCVA gained 21 and 11 letters in their treated eyes, compared with a gain of 11 letters and loss of 1 letter in the control eye, respectively (26). Importantly, these improvements were maintained at 3.5 years post-treatment, whereas BCVA continued to decrease in the untreated control eyes (71). The four patients who had near-normal BCVA at baseline had less scope for an improvement to be observed (26,71). In these patients, at 6 months post treatment, BCVA in the study eyes returned to baseline (recovered to within one to three letters) in three patients, and this recovery was maintained at the 3.5-year follow-up for two of these individuals (26,71). The remaining patient experienced a decrease in visual acuity in both the treated eye and the untreated eye at the 3.5-year time point (29 and 18 letters, respectively) (71). Because this patient received a lower dose of injected vector than the other patients due to surgical complication, it is speculated that this lower dose contributed to the lack of treatment effect, and the loss of visual acuity was due to continued foveal degeneration (71). The point of maximal retinal sensitivity, which represents the minimal detectable light stimulus, and mean retinal sensitivity increased in all five eyes treated with full dose of the vector by means of +2.2 dB, (standard error [SE] 0.8) and +1.7 dB (SE 1.0), respectively, compared with −0.8 dB (SE 1.5) and −1.6 (SE 0.9) in control eyes, respectively. Mean retinal sensitivity was correlated with vector dose over the 6 months (r=0.82, p=0.04) (26). Retinal thinning has been observed following subfoveal gene delivery in other gene therapy trials (26,87); however, this was not observed in the patients who received full dose vector in this study – mean retina thickness 6 months after surgery was similar to that of baseline (169 μm [SE 26 μm] vs 175 μm [SE 27 μm], respectively) (26). Retinal thickness was not reported at 3.5 years. No clinically significant detrimental effects were observed following foveal detachment (26), indicating that the surgical procedure for vector delivery is viable in patients with choroideremia.
Additional studies have been initiated following these positive data. A large program of phase ½ studies is now underway in the USA (NCT02553135) (88), Canada (NCT02077361)(25), Germany (NCT02671539; Fischer et al., Retina [accepted]) and the UK (NCT02407678) to further assess the safety, tolerability and efficacy of the AAV2-REP1 vector in the treatment of choroideremia. Data obtained from a meta-analysis of the four studies are equally promising; among the 32 patients treated, microperimetry and anatomical outcomes were preserved and 16% of patients demonstrated a clinically meaningful ≥15-letter gain in visual acuity over 1 year of follow up (analysis supported by Nightstar Therapeutics; Fischer MD, et al. Presented at: American Academy of Ophthalmology. Chicago, IL, USA. 2018). In parallel, the REGENERATE phase 2 study (NCT02407678), based in the UK, aims to assess the efficacy and safety over 2 years of follow up following a single subretinal injection of AAV-REP1 in patients (N = 30) with genetic or molecular confirmed choroideremia, active disease clinically visible within the macular region, and a BCVA of >6/60 (logMAR 1.0) in the study eye (89). The primary outcome measure is change in BCVA from baseline over 2 years; secondary outcome measures are change from baseline in central visual field and area of surviving RPE over 2 years (89).
Overall, the data obtained so far demonstrate that subretinal delivery of a functional CHM gene into patients with choroideremia facilitates expression of REP1, thereby slowing or stopping the progression of choroideremia and the decline in vision (26,71). Maintenance of visual acuity was achieved by the majority of patients in these studies (71). AAV2-REP1 is well tolerated, with reported adverse events consistent with those associated with vitrectomy (26). In each of these phase 1 and 2 clinical studies, the untreated contralateral eye served as the control (26,71). However, there are two important implications for this selection that should be considered in future clinical studies. First, an asymmetry in visual acuity between eyes is often observed in later stages of choroideremia, likely owing to differences in foveal involvement between eyes (59). Second, visual acuity improvements in the control eye have been observed following gene therapy for the treatment of choroideremia and Leber congenital amaurosis, possibly due to reduced nystagmus or intertest variability (26,90).
An additional phase 2 trial is now underway to assess the safety of bilateral treatment with AAV2-REP1 (91). GEMINI (NCT03507686) is a multicenter, open-label, prospective, two-period, interventional safety study of participants with genetically confirmed choroideremia and active disease clinically visible within the macular region of both eyes, treated sequentially in both eyes with AAV-REP1. The primary endpoint is the incidence of treatment-emergent adverse events from baseline over 2 years. Secondary outcome measures are change in BCVA, autofluorescence, extent of ellipsoid zone, and sensitivity from baseline over 2 years (91).
FUTURE DIRECTIONS FOR GENE THERAPY IN THE TREATMENT OF CHOROIDEREMIA
Over the past 30 years, since the first in vivo retinal gene transfer study (92), gene therapy for the treatment of retinal disorders has advanced considerably. Encouraging efficacy and safety results from preclinical models of choroideremia (76,83,84) paved the way for the first human trials in 2014 (26), and the recent phase 1/2 studies further support these data findings (Fischer MD, et al. Presented at: American Academy of Ophthalmology. Chicago, IL, USA. 2018).
A large, prospective, global, observational study is underway to address the knowledge gap in the natural history of choroideremia. The NIGHT study (NCT03359551) aims to characterize choroideremia disease progression longitudinally, with the view to helping identification of outcome measures and development of appropriate interventional study designs (Lam BL, et al. Presented at: Association for Research in Vision and Ophthalmology [ARVO] Annual Meeting. Honolulu, HI, USA. 2018).
Building on early-phase data, a large phase 3 study of AAV2-REP1 for the treatment of patients with choroideremia has now been initiated (93). The STAR trial (NCT03496012) is a randomized, parallel-arm study that aims to enroll approximately 160 patients from sites in the USA, Europe, Canada and South America (93). Patients with a genetically confirmed diagnosis of choroideremia and active disease clinically visible within the macular region of the study eye are randomized to receive high-dose AAV2-REP1 (1.0 × 1011 genome particles), low-dose AAV2-REP1 (1.0 × 1010 genome particles), or neither (observation arm) (93). Because it is known that visual acuity can decline asymmetrically in choroideremia, with the greatest intereye difference observed in patients with the most advanced disease (59), an observation arm is included to avoid the challenges associated with using the fellow eye as the control. The primary endpoint of the STAR trial is the proportion of treated patients with a 15-letter gain in visual acuity from baseline over 12 months. Secondary endpoints are change in autofluorescence, extent of ellipsoid zone, sensitivity, contrast sensitivity, and color vision from baseline over 12 months (93).
These studies are predicated on the assumption that maintenance or gain of visual acuity is a clinically meaningful longterm treatment goal for patients with an otherwise persistently degenerative retinal disease.
Retinal gene therapy still faces challenges, but current research holds promise for overcoming these challenges in the future. To lessen the risk of irreversible damage to fragile degenerating retina, intravitreal administration would be preferable to subretinal administration; but the majority of AAV vectors do not readily cross from the vitreous to the photoreceptor layer (16). In vivo directed evolution has been used to enrich for AAV variants capable of transducing the mouse outer retina after intravitreal administration, isolating the promising vector variant AAV7M8, which is currently undergoing further testing in other species (16,94). As mentioned previously, many IRDs are caused by toxic gain-of-function mutations and results from preliminary studies using AAV vector constructs designed for expression silencing or knockdown of such mutations are encouraging (16,45,46). Genome-editing approaches may also be an alternative method to replace defective gene sequences (16); and where complete loss of photoreceptors has occurred, AAV-based optogenetic gene therapy to convert light-insensitive retinal neurons into artificial photoreceptors may become a viable technique for restoring vision (95). For autosomal dominant IRDs, such as Best macular dystrophy or adRP, Clustered Regularly Interspersed Short Palindromic Repeats (CRISPR)-associated protein 9 (CRISPR-Cas9) genome surgery or gene editing might pave the way for therapeutic intervention (96,97).
ACKNOWLEDGMENTS AND DISCLOSURES
Figure 1 images courtesy of DGB. Editorial support was provided by Rebecca Franklin of Fishawack Communications Ltd. and funded by Nightstar Therapeutics. Tuyen Ong is an employee and equity holder of Nightstar Therapeutics. Mark E. Pennesi is a consultant for AGTC, Astellas, Biogen, Editas, FFB, Gensight, Horama, Ionis, Nacuity, Nightstar Therapeutics, Ophthotech, ProQR Therapeutics, RegenexBio, Sanofi, and Spark Therapeutics, and has received clinical trial support from AGTC and Nightstar Therapeutics. His institution has received support through grant P30 EY010572 from the National Institutes of Health (Bethesda, MD), and by unrestricted departmental funding from Research to Prevent Blindness (New York, NY). David G. Birch is a consultant for Acucela, AGTC, Editas, Genentech, Ionis, Nacuity, and Nightstar Therapeutics, and has received clinical trial support from AGTC, Nightstar, Ionis, and 4D Therapeutics, and grant support through EY09076 from the National Institutes of Health (Bethesda, MD, USA) and from the Foundation Fighting Blindness. Byron L. Lam declares he has no conflict of interest. Stephen H. Tsang declares he has no conflict of interest.
ABBREVIATIONS
- AAV
Adeno-associated virus
- adRP
Autosomal dominant retinitis pigmentosa
- arRP
Autosomal recessive retinitis pigmentosa
- BCVA
Best-corrected visual acuity
- BSS
Balanced salt solution
- CAG
CMV enhancer-CBA promoter-rabbit β-globin splice acceptor site
- CBA
Chicken β-actin
- CRISPR
Clustered Regularly Interspersed Short Palindromic Repeats
- DNA
Deoxyribonucleic acid
- ER
Endoplasmic reticulum
- ETDRS
Early Treatment Diabetic Retinopathy Study
- GA
Golgi apparatus
- GG
Geranylgeranyl
- GGTase
Geranylgeranyltransferase
- hCHM
Wild-type human CHM cDNA
- IRDs
Inherited retinal diseases
- MOI
Multiplicity of infection
- OCT
Optical coherence tomography
- ORF15
Open reading frame 15
- R
Ribosome
- Rab
Ras-associated binding
- REP1
Rab escort protein 1
- REP2
Rab escort protein 2
- RP
Retinitis pigmentosa
- RPE
Retinal pigment epithelium
- RPE65
Retinal pigment epithelial 65-kDa protein
- RPGR
Retinitis pigmentosa guanosine triphosphate hydrolase regulator
- RPGRIP
RPGR-interacting protein
- SE
Standard error
- VA
Visual acuity
- WPRE
Woodchuck hepatitis virus posttranscriptional regulatory element
- WT
Wild-type
- XLRP
X-linked retinitis pigmentosa
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.McClements ME, MacLaren RE. Gene therapy for retinal disease. Transl Res. 2013;161(4):241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta PR, Huckfeldt RM. Gene therapy for inherited retinal degenerations: initial successes and future challenges. J Neural Eng. 2017;14(5):051002. [DOI] [PubMed] [Google Scholar]
- 3.Campa C, Gallenga CE, Bolletta E, Perri P. The role of gene therapy in the treatment of retinal diseases: a review. Curr Gene Ther. 2017;17(3): 194–213. [DOI] [PubMed] [Google Scholar]
- 4.Khan NW, Falsini B, Kondo M, Robson AG. Inherited retinal degeneration: genetics, disease characterization, and outcome measures. J Ophthalmol. 2017;2017:2109014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oner A Recent advancements in gene therapy for hereditary retinal dystrophies. Turk J Ophthalmol. 2017;47(6):338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samiy N Gene therapy for retinal diseases. J Ophthalmic Vis Res. 2014;9(4):506–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patricio MI, Barnard AR, Orlans HO, McClements ME, MacLaren RE. Inclusion of the woodchuck hepatitis virus posttranscriptional regulatory element enhances AAV2-driven transduction of mouse and human retina. Mol Ther Nucleic Acids. 2017;6:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. 2017. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm589467.htm. Accessed 18 May 2018.
- 9.Spark Therapeutics Inc. LUXTURNA™ US Prescribing Information. Available from: https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM589541.pdf. Accessed 20 April 2018.
- 10.Bennett J, Wellman J, Marshall KA, McCague S, Ashtari M, DiStefano-Pappas J, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388(10045):661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu X, Huu VAN, Duan Y, Kermany DS, Valentim CCS, Zhang R, et al. Clinical applications of retinal gene therapies. Precision Clinical Medicine. 2018;1(1):5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengillo JD, Justus S, Cabral T, Tsang SH. Correction of monogenic and common retinal disorders with gene therapy. Genes (Basel). 2017;8(2). 10.3390/genes8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava A In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol. 2016;21:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenberghe LH, Bell P, Maguire AM, Xiao R, Hopkins TB, Grant R, et al. AAV9 targets cone photoreceptors in the nonhuman primate retina. PLoS One. 2013;8(1):e53463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auricchio A, Smith AJ, Ali RR. The future looks brighter after 25 years of retinal gene therapy. Hum Gene Ther. 2017;28(11):982–7. [DOI] [PubMed] [Google Scholar]
- 17.Bartholomae CC, Arens A, Balaggan KS, Yanez-Munoz RJ, Montini E, Howe SJ, et al. Lentiviral vector integration profiles differ in rodent postmitotic tissues. Mol Ther. 2011;19(4):703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008; 15(19):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binley K, Widdowson P, Loader J, Kelleher M, Iqball S, Ferrige G, et al. Transduction of photoreceptors with equine infectious anemia virus lentiviral vectors: safety and biodistribution of StarGen for Stargardt disease. Invest Ophthalmol Vis Sci. 2013;54(6):4061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto T, Gibbs D, Lillo C, Azarian SM, Legacki E, Zhang XM, et al. Lentiviral gene replacement therapy of retinas in a mouse model for usher syndrome type 1B. Gene Ther. 2007;14(7):584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zallocchi M, Binley K, Lad Y, Ellis S, Widdowson P, Iqball S, et al. EIAV-based retinal gene therapy in the shaker1 mouse model for Usher syndrome type 1B: development of UshStat. PLoS One. 2014;9(4):e94272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapani I, Banfi S, Simonelli F, Surace EM, Auricchio A. Gene therapy of inherited retinal degenerations: prospects and challenges. Hum Gene Ther. 2015;26(4): 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trapani I, Colella P, Sommella A, Iodice C, Cesi G, de Simone S, et al. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol Med. 2014;6(2):194–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClements ME, Barnard AR, Singh MS, Charbel Issa P, Jiang Z, Radu RA, et al. An AAV dual vector strategy ameliorates the Stargardt phenotype in adult Abca4−/− mice.Hum Gene Ther. 2018. 10.1089/hum.2018.156 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimopoulos IS, Hoang SC, Radziwon A, Binczyk NM, Seabra MC, MacLaren RE, et al. Two-year results after AAV2-mediated gene therapy for choroideremia: the Alberta experience. Am J Ophthalmol. 2018;193:130–42. [DOI] [PubMed] [Google Scholar]
- 26.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical Trials. Gene therapy for X-linked retinitis pigmentosa (XLRP) retinitis pigmentosa GTPase regulator (RPGR). Available from: https://clinicaltrials.gov/ct2/show/NCT03252847?term=NCT03252847&rank=1. Accessed 7 September 2018.
- 28.Clinical Trials. Safety and efficacy of rAAV2tYF-GRK1-RPGR in subjects with X-linked retinitis pigmentosa caused by RPGR-ORF15 mutations. Available from: https://clinicaltrials.gov/ct2/show/NCT03316560?term=NCT03316560&rank=1. Accessed 6 September 2018.
- 29.Clinical Trials. A clinical trial of retinal gene therapy for X-linked retinitis pigmentosa (XIRIUS). Available from: https://clinicaltrials.gov/ct2/show/NCT03116113?term=XIRIUS&rank=1. Accessed 13 September 2018.
- 30.Clinical Trials. Safety and efficacy study in patients with retinitis pigmentosa due to mutations in PDE6B gene. Available from: https://clinicaltrials.gov/ct2/show/NCT03328130. Accessed 21 September 2018.
- 31.Clinical Trials. Efficacy study of GS010 for the treatment of vision loss up to 6 months from onset in LHON due to the ND4 mutation (RESCUE). Available from: https://clinicaltrials.gov/ct2/show/NCT02652767?term=NCT02652767&rank=1. Accessed 13 September 2018.
- 32.Campochiaro PA, Lauer AK, Sohn EH, Mir TA, Naylor S, Anderton MC, et al. Lentiviral vector gene transfer of Endostatin/angiostatin for macular degeneration (GEM) study. Hum Gene Ther. 2017;28(1):99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical Trials. A follow-up study to evaluate the safety of RetinoStat® in patients with age-related macular degeneration. Available from: https://clinicaltrials.gov/ct2/show/NCT01678872. Accessed 21 November 2018.
- 34.Clinical Trials. A study to determine the long-term safety, tolerability and biological activity of UshStat® in patients with Usher syndrome type 1B. Available from: https://clinicaltrials.gov/ct2/show/NCT02065011. Accessed 21 November 2018.
- 35.Clinical Trials. Study of UshStat in patients with retinitis pigmentosa associated with Usher syndrome type 1B. Available from: https://clinicaltrials.gov/ct2/show/NCT01505062. Accessed 21 November 2018.
- 36.Stone EM, Aldave AJ, Drack AV, Maccumber MW, Sheffield VC, Traboulsi E, et al. Recommendations for genetic testing of inherited eye diseases: report of the American Academy of ophthalmology task force on genetic testing. Ophthalmology. 2012;119(11):2408–10. [DOI] [PubMed] [Google Scholar]
- 37.Verbakel SK, van Huet RAC, Boon CJF, den Hollander AI, Collin RWJ, Klaver CCW, et al. Non-syndromic retinitis pigmentosa. Prog Retin Eye Res. 2018;66:157–86. [DOI] [PubMed] [Google Scholar]
- 38.Megaw RD, Soares DC, Wright AF. RPGR: its role in photoreceptor physiology, human disease, and future therapies. Exp Eye Res. 2015;138:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Silva SR, Barnard AR, Hughes S, Tam SKE, Martin C, Singh MS, et al. Long-term restoration of visual function in end-stage retinal degeneration using subretinal human melanopsin gene therapy. Proc Natl Acad Sci U S A. 2017;114(42): 11211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daiger SP, Rossiter BJF, Greenberg J, Christoffels A, Hide W. Data services and software for identifying genes and mutations causing retinal degeneration. Invest Ophthalmol Vis Sci. 1998;39:S295. [Google Scholar]
- 41.Khanna H, Hurd TW, Lillo C, Shu X, Parapuram SK, He S, et al. RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J Biol Chem. 2005;280(39):33580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez-Fernandez De la Camara C, Nanda A, Salvetti AP, Fischer MD, MacLaren RE. Gene therapy for the treatment of X-linked retinitis pigmentosa. Expert Opin Orphan Drugs. 2018;6(3):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. 2011;12(4):238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daiger SP, Bowne SJ, Sullivan LS. Genes and mutations causing autosomal dominant retinitis pigmentosa. Cold Spring Harb Perspect Med. 2014;5(10):a017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Millington-Ward S, Chadderton N, O'Reilly M, Palfi A, Goldmann T, Kilty C, et al. Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol Ther. 2011;19(4):642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cideciyan AV, Sudharsan R, Dufour VL, Massengill MT, Iwabe S, Swider M, et al. Mutation-independent rhodopsin gene therapy by knockdown and replacement with a single AAV vector. Proc Natl Acad Sci U S A. 2018;115(36):E8547–E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng WT, Dyka FM, Dinculescu A, Li J, Zhu P, Chiodo VA, et al. Stability and safety of an AAV vector for treating RPGR-ORF15 X-linked retinitis pigmentosa. Hum Gene Ther. 2015;26(9):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong DH, Pawlyk BS, Adamian M, Li T. Dominant, gain-of-function mutant produced by truncation of RPGR. Invest Ophthalmol Vis Sci. 2004;45(1):36–41. [DOI] [PubMed] [Google Scholar]
- 49.Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A, et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci U S A. 2012;109(6):2132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer MD, McClements ME, Martinez-Fernandez De la Camara C, Bellingrath JS, Dauletbekov D, Ramsden SC, et al. Codon-optimized RPGR improves stability and efficacy of AAV8 gene therapy in two mouse models of X-linked retinitis pigmentosa. Mol Ther. 2017;25(8):1854–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun X, Park JH, Gumerson J, Wu Z, Swaroop A, Qian H, et al. Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations. Proc Natl Acad Sci U S A. 2016;113(21):E2925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clinical Trials. Clinical evaluation of patients with X-linked retinitis pigmentosa (XLRP). Available from: https://clinicaltrials.gov/ct2/show/NCT03314207. Accessed 24 September 2018.
- 53.Clinical Trials. Natural history study of patients with X-linked retinal dystrophy associated with mutations in retinitis pigmentosa GTPase regulator (RPGR). Available from: https://clinicaltrials.gov/ct2/show/NCT03349242. Accessed 24 September 2018.
- 54.Coussa RG, Traboulsi EI. Choroideremia: a review of general findings and pathogenesis. Ophthalmic Genet. 2012;33(2):57–65. [DOI] [PubMed] [Google Scholar]
- 55.Kalatzis V, Hamel CP, MacDonald IM. First international choroideremia research symposium. Choroideremia: towards a therapy. Am J Ophthalmol. 2013;156(3):433–7. [DOI] [PubMed] [Google Scholar]
- 56.US National Library of Medicine. Choroideremia. Available from: https://ghr.nlm.nih.gov/condition/choroideremia. Accessed 9 March 2018.
- 57.Jacobson SG, Cideciyan AV, Sumaroka A, Aleman TS, Schwartz SB, Windsor EA, et al. Remodeling of the human retina in choroideremia: Rab escort protein 1 (REP-1) mutations. Invest Ophthalmol Vis Sci. 2006;47(9):4113–20. [DOI] [PubMed] [Google Scholar]
- 58.Aleman TS, Han G, Serrano LW, Fuerst NM, Charlson ES, Pearson DJ, et al. Natural history of the central structural abnormalities in choroideremia: a prospective cross-sectional study. Ophthalmology. 2017;124(3):359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jolly JK, Xue K, Edwards TL, Groppe M, MacLaren RE. Characterizing the natural history of visual function in choroideremia using microperimetry and multimodal retinal imaging. Invest Ophthalmol Vis Sci. 2017;58(12):5575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seabra MC, Brown MS, Goldstein JL. Retinal degeneration in choroideremia: deficiency of Rab geranylgeranyl transferase.Science. 1993;259(5093):377–81. [DOI] [PubMed] [Google Scholar]
- 61.Corbeel L, Freson K. Rab proteins and Rab-associated proteins: major actors in the mechanism of protein-trafficking disorders. Eur J Pediatr. 2008;167(7):723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zinkernagel MS, MacLaren RE. Recent advances and future prospects in choroideremia. Clin Ophthalmol. 2015;9:2195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnard AR, Groppe M, MacLaren RE. Gene therapy for choroideremia using an adeno-associated viral (AAV) vector. Cold Spring Harb Perspect Med. 2015;5:a017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pereira-Leal JB, Hume AN, Seabra MC. Prenylation of Rab GTPases: molecular mechanisms and involvement in genetic disease. FEBS Lett. 2001;498(2–3):197–200. [DOI] [PubMed] [Google Scholar]
- 65.Seabra MC, Ho YK, Anant JS. Deficient geranylgeranylation of ram/Rab27 in choroideremia. J Biol Chem. 1995;270(41):24420–7. [DOI] [PubMed] [Google Scholar]
- 66.Patricio MI, Barnard AR, Xue K, MacLaren RE. Choroideremia: molecular mechanisms and development of AAV gene therapy. Expert Opin Biol Ther. 2018;18(7):807–20. [DOI] [PubMed] [Google Scholar]
- 67.Krock BL, Bilotta J, Perkins BD. Noncell-autonomous photoreceptor degeneration in a zebrafish model of choroideremia. Proc Natl Acad Sci U S A. 2007;104(11):4600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xue K, Oldani M, Jolly JK, Edwards TL, Groppe M, Downes SM, et al. Correlation of optical coherence tomography and autofluorescence in the outer retina and choroid of patients with choroideremia. Invest Ophthalmol Vis Sci. 2016;57(8):3674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hariri AH, Velaga SB, Girach A, Ip MS, Le PV, Lam BL, et al. Measurement and reproducibility of preserved ellipsoid zone area and preserved retinal pigment epithelium area in eyes with choroideremia. Am J Ophthalmol. 2017;179:110–7. [DOI] [PubMed] [Google Scholar]
- 70.Morgan JI, Han G, Klinman E, Maguire WM, Chung DC, Maguire AM, et al. High-resolution adaptive optics retinal imaging of cellular structure in choroideremia. Invest Ophthalmol Vis Sci. 2014;55(10):6381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edwards TL, Jolly JK, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, et al. Visual acuity after retinal gene therapy for choroideremia. N Engl J Med. 2016;374(20):1996–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3(88):88ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weleber RG, Pennesi ME, Wilson DJ, Kaushal S, Erker LR, Jensen L, et al. Results at 2 years after gene therapy for RPE65- deficient Leber congenital amaurosis and severe early-childhood-onset retinal dystrophy. Ophthalmology. 2016; 123(7):1606–20. [DOI] [PubMed] [Google Scholar]
- 74.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2231–9. [DOI] [PubMed] [Google Scholar]
- 75.Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med. 2015;372(20):1887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tolmachova T, Tolmachov OE, Barnard AR, de Silva SR, Lipinski DM, Walker NJ, et al. Functional expression of Rab escort protein 1 following AAV2-mediated gene delivery in the retina of choroideremia mice and human cells ex vivo. J Mol Med (Berl). 2013;91(7):825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Askou AL, Benckendorff JNE, Holmgaard A, Storm T, Aagaard L, Bek T, et al. Suppression of choroidal neovascularization in mice by subretinal delivery of multigenic lentiviral vectors encoding anti-angiogenic microRNAs. Hum Gene Ther Methods. 2017. 10.1089/hum.2017.079 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 78.Higashimoto T, Urbinati F, Perumbeti A, Jiang G, Zarzuela A, Chang LJ, et al. The woodchuck hepatitis virus posttranscriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007;14(17):1298–304. [DOI] [PubMed] [Google Scholar]
- 79.LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, et al. AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10(4):309–19. [DOI] [PubMed] [Google Scholar]
- 80.Ochakovski GA, Bartz-Schmidt KU, Fischer MD. Retinal gene therapy: surgical vector delivery in the translation to clinical trials. Front Neurosci. 2017;11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng Y, Tang L, Zhou Y. Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58(4):217–26. [DOI] [PubMed] [Google Scholar]
- 82.Xue K, Groppe M, Salvetti AP, MacLaren RE. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond). 2017;31(9):1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vasireddy V, Mills JA, Gaddameedi R, Basner-Tschakarjan E, Kohnke M, Black AD, et al. AAV-mediated gene therapy for choroideremia: preclinical studies in personalized models. PLoS One. 2013;8(5):e61396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anand V, Barral DC, Zeng Y, Brunsmann F, Maguire AM, Seabra MC, et al. Gene therapy for choroideremia: in vitro rescue mediated by recombinant adenovirus. Vis Res. 2003;43(8):919–26. [DOI] [PubMed] [Google Scholar]
- 85.Patricio MI, Barnard AR, Cox CI, Blue C, MacLaren RE. The biological activity of AAV vectors for choroideremia gene therapy can be measured by in vitro prenylation of RAB6A. Mol Ther Methods Clin Dev. 2018;9:288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gregori NZ, Lam BL, Davis JL. Intraoperative use of microscope-integrated optical coherence tomography for subretinal gene therapy delivery. Retina. 2017:1 10.1097/IAE.0000000000001646. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 87.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130(1):9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lam BL, Davis JL, Gregori NZ, MacLaren RE, Girach A, Verriotto JD, et al. Choroideremia gene therapy phase 2 clinical trial: 24-month results. Am J Ophthalmol. 2018;197:65–73. 10.1016/j.ajo.2018.09.012. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 89.Clinical Trials. REP1 gene replacement therapy for choroideremia (REGENERATE). Available from: https://clinicaltrials.gov/ct2/show/NCT02407678?cond=Choroideremia&rank=9. Accessed 17 May 2018.
- 90.Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clinical Trials. A safety study of retinal gene therapy for choroideremia (GEMINI). Available from: https://clinicaltrials.gov/ct2/show/NCT03507686?cond=Choroideremia&rank=3. Accessed 17 May 2018.
- 92.Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328(6126):131–6. [DOI] [PubMed] [Google Scholar]
- 93.Clinical Trials. Efficacy and safety of AAV2-REP1 for the treatment of choroideremia (STAR). Available from: https://clinicaltrials.gov/ct2/show/NCT03496012?cond=Choroideremia&rank=4. Accessed 16 May 2018.
- 94.Ramachandran PS, Lee V, Wei Z, Song JY, Casal G, Cronin T, et al. Evaluation of dose and safety of AAV7m8 and AAV8BP2 in the non-human primate retina. Hum Gene Ther. 2017;28(2):154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duebel J, Marazova K, Sahel JA. Optogenetics. Curr Opin Ophthalmol. 2015;26(3):226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang T, Justus S, Li Y, Tsang SH. BEST1: the BEST target for gene and cell therapies. Mol Ther. 2015;23(12):1805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DiCarlo JE, Mahajan VB, Tsang SH. Gene therapy and genome surgery in the retina. J Clin Invest. 2018;128(6):2177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]