Abstract
Background:
Fanconi Anemia (FA) is associated with an increased risk of developing head and neck squamous cell cancer (HNSCC) and presents a treatment dilemma due to concerns of increased toxicities from chemotherapy and radiation therapy (RT).
Methods:
We reviewed the literature on HNSCC in FA patients and report on our experience treating 9 FA patients with HNSCC.
Results:
Surgery was generally well-tolerated and surgery alone resulted in durable local control for two patients. Four patients received adjuvant RT which was tolerable in most cases, although one patient required a treatment break and early cessation of RT. Three of the irradiated patients received concurrent cetuximab.
Conclusions:
In patients with adverse features, adjuvant radiation with concurrent cetuximab may be feasible with careful monitoring, although local disease control is infrequent. Early detection via screening permitting a surgery-alone approach represents the best opportunity for cure in FA patients with HSNCC.
Keywords: Fanconi Anemia, head and neck cancer, radiation therapy, cetuximab
Precis:
Head and neck cancer is common in Fanconi Anemia patients. Case reports and series remain the only source of information about this challenging population. We report on a large patient series and review the literature on head and neck cancer in Fanconi Anemia patients.
Introduction
FA is a rare genetic disorder occurring in 1–2 per 100,000 births(1). Mutations in one of at least 22 genes are responsible, however approximately 90% are caused by mutations in three genes: FANCA, FANCC, and FANCG(1). All genes implicated in the development of FA participate in the FA DNA interstrand crosslink repair pathway, which functions to repair DNA crosslinks by via utilizing elements of the nucleotide excision, homologous recombination, and translesion synthesis repair pathways. Thus, FA patients exhibit an impaired capacity to repair DNA crosslinks, making platinum agents contraindicated due to toxicity risks. The tolerability of radiation therapy is also reported to be significantly impaired.(2)
Patients with FA have a dramatically increased risk of developing SCCs, particularly HNSCC(3). HNSCCs in FA patients present at a young age, frequently at advanced initial stage, and have a high propensity to recur locally(4, 5). Under an institutional review board approved retrospective protocol, we identified 9 patients with FA treated for HNSCC at Memorial Sloan Kettering Cancer Centerr. Each patient is reviewed individually with additional details in Tables 1 and 2. We review the literature on this unique patient population, incorporating our experience.
Table1:
Abbreviations: Flu: Fludarabine, Bu: Busulfan, CP: Cyclophosphamide, TBI: Total body irradiation, NK: natural killer cells,
| Patient | Sex | Complementation Group | BMT Type; Age | BMT Conditioning | HNSCC Site | Stage | Age at HNSCC | Cancer Screening? |
|---|---|---|---|---|---|---|---|---|
| 1 | M | FA-D2 c.355C>T; c.888+157A>G | Matched, unrelated donor; 32 | Flu, Bu, CP | Mandible | pT4aN0 | 35.5 | Yes |
| 2 | M | FA-G | Matched Sibling; 10 | Flu, CP, TBI | Oral Tongue | pT1Nx | 28.0 | Yes |
| 3 | M | Unknown | Matched, unrelated donor; 24 and 31; NK infusion and anti-WT-1 T cells | Flu, CP, TBI | Buccal mucosa | pT4aN0 | 33.9 | Yes |
| 4 | F | Unknown | - | - | Cervical esophagus | T2N0 | 32.6 | No |
| 5 | M | Unknown | - | - | Mandibular gingiva | pT4aN1 | 37.2 | No |
| 6 | F | FA-C (IVS4 homozygous) | Matched, unrelated; 21 | Flu, CP, TBI | Oral tongue | pT2N1 | 29.0 | Yes |
| 7 | M | FA-A (mosaic) | - | - | Maxilla | pT1Nx; rpT0N2b | 33.6 | No |
| 8 | M | FA-C | Matched, unrelated donor; 17 | Flu, CP, TBI | Hypopharynx | pT3N2c | 33.2 | Unknown |
| 9 | F | FA-A | - | - | Oral tongue | 3 × pT1N0; rpTxN1 | 31.7 | Yes |
Table2:
Abbreviations: Flu: NED: no evidence of disease, C. diff: Clostridium difficile, PEG: percutaneous endogastrostomy tube, BID: twice per day
| Patient | Surgery | Surgical Complications | Radiotherapy | Treatment break | RT Complications | Systemic Therapy | Systemic Therapy Complications | Status |
|---|---|---|---|---|---|---|---|---|
| 1 | Marginal mandibulectomy and ipsilateral level I-IV dissection | None | - | - | - | - | Alive, NED, 21 months post diagnosis | |
| 2 | Partial glossectomy | None | - | - | - | - | Alive, NED, 13 months after diagnosis | |
| 3 | Marginal mandibulectomy and left level I-III dissection | Immediate post-op respiratory failure, intensive care, death | - | - | - | - | Dead 7 weeks after surgery | |
| 4 | - | - | - | - | - | Carboplatin and Paclitaxel | Pancytopenia, liver failure, C. diff colitis | Dead from disease progression 18 months after diagnosis |
| 5 | Partial mandibulectomy and left level I-III dissection | Hardware infection requiring removal, ARDS requiring intensive care | 4240 cGy in 20 fractions. | Yes due to infected hardware. Treatment stopped due to Grade 4 dermatitis. | Hardware associated abscess, PEG, Tracheostomy, Grade 3 mucositis and xerostomia, cytopenia, Grade 4 dermatitis | Concurrent cetuximab × 2 doses; cetuximab alone after RT cessation | Pancytopenia, unclear if related to cetuximab. | Dead from pancytopenia, sepsis 20 months after diagnosis, 17 months after surgery, 7 months from RT |
| 6 | Partial glossectomy and ipsilateral level I-IV neck dissection | None | 66 Gy in 33 fractions | No | PEG, Grade 2 dermatitis and mucositis | None | - | Dead from progressive local disease |
| 7 | 1) maxillectomy 2) right modified neck dissection |
None | 70.4 CGE Proton Beam | No | Grade 2 dermatitis, mucositis, xerostomia | Concurrent cetuximab | None | Alive with local recurrence 26 months from diagnosis, 20 months from initial surgery, 16 months from RT |
| 8 | Pharingolaryngocervical esophagectomy and bilateral level II-V dissection | None | 1) 70 Gy in 35 fractions 2) 30 Gy in 10 fractions to cavernous sinus recurrence |
No | PEG, grade 3 dermatitis, grade 2 mucositis, xerostomia, and dysphagia | 1) Concurrent cetuximab 2) Nivolumab |
1) Well tolerated 2) Encephalitis |
Dead from encephalitis and recurrent pneumonia 12 months from diagnosis, 10 months from surgery, and 7 months from first RT. |
| 9 | 1) marginal mandibulectomy and ipsilateral modified neck dissection 2) partial glossectomy 3) partial glossectomy 4) right modified neck dissection |
Gross residual disease | 3.7 Gy BID × 2 days (Quad Shot), three cycles | - | Carotid bleed | 1) Concurrent cetuximab 2) paclitaxel 3) tremelimumab and durvalumab |
Apparently well tolerated, although died 10 days after last immunotherapy | Dead, cause unclear, 77 months from initial diagnosis, 8 months from most recent surgery, 2 months from last palliative RT. |
Patient series:
Patient 1: Diagnosed with FA at age 30 after developing pancytopenia, he underwent successful bone marrow transplant (BMT) at age 32. He underwent oral cancer surveillance annually and was described to have an oral tongue leukoplakia. At age 35 he received a marginal resection of the mandible and ipsilateral modified radical neck dissection (MRND) which revealed moderately differentiated SCC with mandible invasion and carcinoma within 1 mm of the deep margin. Twenty lymph nodes (LNs) were benign. Adjuvant radiation was discussed, however the patient underwent close observation. He is currently NED 20 months after surgery and 21 months after diagnosis.
Patient 2: Diagnosed with FA at age 4 due to pancytopenia, he underwent successful transplant at age 9. At age 22 he developed leukoplakia on the dorsum of his tongue, which waxed and waned, intermittently observed on oral cancer surveillance. At age 28 he underwent a partial glossectomy, which revealed moderately differentiated SCC with negative margins. He is now NED 7 months after surgery and 8 months after diagnosis.
Patient 3: Diagnosed with FA at age 9 with diagnostic diepoxybutane (DEB) test after a sibling was diagnosed with FA. At age 23 he was diagnosed with MDS, and at age 24 he received a bone marrow transplant (BMT) from a 10/10 HLA matched unrelated donor after conditioning with cyclophosphamide, fludarabine, and 450 cGy TBI. His graft failed, and at age 31 we underwent another TCD BMT from the same donor. He was conditioned using busulfan and fludarabine only. His MDS returned at age 32. He subsequently underwent NK-cell infusion from HLA-haploidentical sister and infusion of anti-WT-1 T cells from the same donor as his prior transplants. At age 33 he progressed to acute myelogenous leukemia (AML) which was managed with continuation of the anti-WT-1 T cell protocol, maintenance allopurinol, and transfusion support.
At age 30 he underwent resection of a lesion on the buccal mucosa and the tongue, however pathology was benign. He underwent oral cancer screening intermittently, and returned to the oral surgeon after developing a leukoplakic lesion of the buccal mucosa. At age 33 he underwent resection of the buccal tumor and marginal mandibulectomy with ipsilateral MRND. Pathology revealed poorly differentiated invasive SCC with invasion into skeletal muscle and bone with several positive margins. His postoperative course was complicated by immediate postoperative tachycardia, hypotension requiring intensive care unit (ICU) admission with intubation and vasopressor support. He was maintained on intensive care until withdrawal of life support 7 weeks after surgery. Notably, at the time of his resection his hematologic status was tenuous with neutropenia and peripheral blasts consistent with active AML.
Patient 4: Diagnosed with a T2N0 SCC of the cervical esophagus at age 32, she underwent neoadjuvant chemotherapy with two cycles of carboplatin and paclitaxel. Shortly after the second cycle she was hospitalized with Clostridium difficile colitis, pancytopenia, jaundice, and elevated LFTs. Due to the severity of this reaction, the diagnosis of FA was considered, and DEB was diagnostic. She refused surgery or RT and died from local progression 18 months after diagnosis.
Patient 5: Diagnosed with FA at age 6, he had chronic pancytopenia but did not receive any specific therapies for FA. At 34 he had resection of an oral tongue SCC in situ. At 37 he underwent partial mandibulectomy and ipsilateral MRND with SCC invading the mandible and a positive margin. There was a soft tissue deposit and one LN in level IB. His postoperative course was complicated by infection of his mandibular hardware, which had to be removed. This operation was complicated by prolonged ICU admission with acute respiratory distress syndrome (ARDS). Eight months following initial surgery, he recurred in the pretracheal soft tissue with dermal metastases. RT with concurrent cetuximab was planned. After 10 fractions of RT he developed a neck abscess and mucositis prompting a treatment break. He had a percutaneous endoscopic gastrostomy (PEG) tube and tracheostomy placed. He resumed radiation without additional cetuximab. He experienced grade 3 mucositis and xerostomia. He also had progressive cytopenias while on treatment notably with WBCs declining from 3.0 K/mcl pretreatment to 1.4 K/mcl at treatment end. Over the same period, his HgB declined from 12.6 g/dL to 6.7 g/dL and platelets declined from 50 K/mcl to 22 K/mcl. After 42.4 Gy cumulative dose, he was noted to have exposed mandibular hardware and persistent bleeding. Radiation was held, but he resumed weekly cetuximab for 3 months with temporary response in the visible tumors. He died 7 months following cessation of RT.
Patient 6: Diagnosed with FA at age 6 due to pancytopenia and eventually successful transplant after progression to MDS and AML at 21. She underwent regular oral cancer screening with waxing and waning leukoplakia. At 29 she underwent partial glossectomy and ipsilateral MRND. Pathology revealed moderately differentiated invasive SCC with positive margins and invasion of skeletal muscle. One of 18 LN was involved. She underwent 66 Gy to the oral cavity and 50.4 Gy to the bilateral neck. Her radiation side effects were manageable. Seven months after completing RT, she developed recurrence in the oral tongue. She developed seizures and encephalopathy of unknown cause. She entered hospice and died 9 months after completing RT, 12 months after surgery, and 13 months form the date of her diagnosis.
Patient 7: Diagnosed with FA at age 11 after a DEB was diagnostic in workup of anemia. He had a history of oral leukoplakia. At 33 he underwent maxillectomy with buccal mucosa resection which revealed SCC. Four months after initial surgery, he underwent a MRND with multiple metastatic nodes with extranodal extension (ENE). He was treated with proton beam RT with 70.4 CGE to the high-risk volume and 60 CGE to the remaining ipsilateral neck (Figure 1), with concurrent cetuximab. He tolerated treatment remarkably well. He developed an in-field recurrence 16 months after RT, but was alive 26 months from initial diagnosis.
Figure 1.
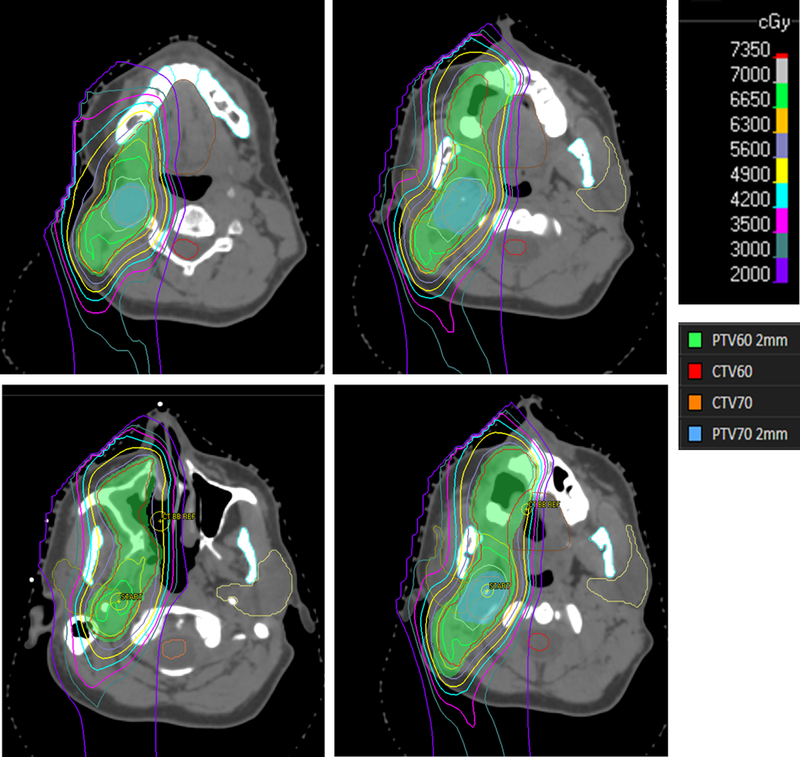
Representative isodose lines of Patients 7’s postoperative proton plan
Patient 8: Diagnosed with FA at age 8 after bleeding post tooth extraction, he underwent successful transplant at age 17. At 33 he underwent pharingolaryngocervical esophagectomy and bilateral MRND for a poorly differentiated SCC in the piriform sinus. Pathology revealed perineural, lymphovascular, and skeletal muscle invasion. There was tumor present less than 1mm from multiple margins and in multiple LNs in the bilateral neck with ENE. He recovered from surgery uneventfully. He was treated post operatively with 70 Gy RT to the high-risk regions, 54 Gy to the high-risk nodal regions, and 45 Gy to the remaining bilateral neck with concurrent weekly cetuximab. He tolerated treatment without interruption. Three months after completing radiation he was found to have recurrence in the cavernous sinus. He completed 30 Gy palliative IMRT without apparent complication. He was then started on nivolumab (3mg/kg). He received 3 doses before being admitted for a prolonged period with presumed nivolumab encephalitis and recurrent aspiration pneumonia and died of these causes 12 months from SCC diagnosis, 10 months from surgery, and 7 months from initial RT.
Patient 9: This patient had a sibling severely affected with FA. The patient herself was found to be a FA carrier, although the details of this are unclear. She did not receive transplant or transfusions.
She had a history of oral leukoplakia. Between the ages of 31 and 35, she had 3 oral tongue primary cancers, each removed surgically with negative margins including one right neck dissection. At 36 she developed cervical adenopathy and underwent repeat posterior and lateral neck dissection which revealed metastasis in a single LN with ENE. Surgical resection of an involved retropharyngeal node was deemed futile after observing extensive tumor in the retropharyngeal space penetrating the paravertebral fossa and base of skull (BOS). She underwent palliative quad shot radiotherapy (QS-RT, 3.7 Gy BID × 2 days) to the retropharyngeal and BOS tumor with concurrent weekly cetuximab. One month after her second cycle of QS-RT, she developed a carotid sentinel bleed. It was unclear whether the bleed was related to tumor infiltration of the carotid or an adverse effect of radiation. She ultimately underwent a third cycle of QS-RT combined with low dose paclitaxel (20mg/m2). She continued weekly cetuximab and did have two further doses of paclitaxel: 60 mg/m2 and then 80mg/m2. She also received a single dose of tremelimumab (1mg/kg) and durvalumab (20mg/kg). On follow-up imaging she was noted to have reduction in retropharyngeal mass treated with RT, but her adenopathy increased on cetuximab and paclitaxel.
She passed away 10 days after these immunotherapies.
Discussion and Review of Literature
Cancer Susceptibility in Fanconi Anemia
Patients with FA are at risk for development of several malignancies, most frequently hematologic, particularly MDS and AML, which occur in 10–15% of patients by the age of 50, with most cases diagnosed between ages 15 and 35(6). The most effective treatment for hematologic manifestations of FA, including treatment and prevention of hematologic malignancies, is hematopoietic stem cell transplant. In the International Fanconi Anemia Registry (IFAR) 20-year report, 36% of patients had undergone hematopoietic stem cell transplant (HSCT) for hematologic malignancy which occurred in 16% of patients, or bone marrow failure, an eventual occurrence in 80% of patients(3). Of our 9 patients, 5 received transplant, however hematologic manifestations were minor in others. Two of our patients never had a transplant or received transfusions, suggesting that the propensity to develop HNSCC may be unrelated to the severity of hematologic manifestations of FA.
FA patients are at high risk for developing SCC. The IFAR study observed a 20% risk of SCC by age 40(3). The increased risk of SCC is thought to be related to chromosomal instability inherent to the pathology of FA. In a study of solid tumors that arose prior to BMT, Rosenberg et al estimate a risk of 33% of developing a solid malignancy by the age of 48(7). Receipt of HSCT is associated with further increased risk of solid tumors. A postulated explanation for increased SCC incidence in transplanted patients is the chemotherapy and RT used in conditioning of patients for transplant(8). Efforts to eliminate radiation in HSCT conditioning have been increasingly successful even in mismatched or unrelated donor transplants(9).
While chemotherapy and radiation in conditioning likely contribute to risk of SCC, they are probably not the largest risks associated with transplant. Rosenberg et al investigated the risks of SCC in FA, comparing 145 patients who did not receive transplant with 117 who did(10). Transplant was associated with a 4.4-fold age-specific hazard of development of SCC. Fifteen years after transplant, patients had a 10.1% annual rate of development of SCC, dramatically higher than the general population. The primary risk identified was high-grade GVHD. There was a 32.8-fold hazard ratio of developing SCC for patients who had grade III/IV acute GVHD. Radiation, either TBI or total lymphoid irradiation (TLI) was part of conditioning in 85% of patients, but was not associated with developing SCC. Four of our 5 transplanted patients were conditioned with cyclophosphamide, fludarabine, and TBI. All tolerated conditioning well, and none experienced GVHD.
The link between SCC in FA patients and traditional risk factors has been partially reported in the literature. Twenty-six percent of the IFAR patients had exposure to tobacco or alcohol, but the extent of exposure is unclear(4). Patient 7 had a history of heavy alcohol use, but otherwise there were only minor exposures to tobacco and alcohol in our patients. HPV is a leading risk factor for SCC of the head and neck, anus, and cervix particularly in younger patients(11). Kutler et al found that 21 of 25 (84%) SCC of the vulva or HN in patients with FA contained quantifiable HPV DNA compared with 36% in non-FA controls(12). This observation has been challenged by a similar study in which HPV DNA was found in only 2 of 21 FA tumors, both of anogenital origin(13). Sufficient tissue for p16 staining and HPV-ISH was obtainable for 3 of our patients, and all 3 were negative.
Head and Neck Squamous Cell Carcinoma in in Fanconi Anemia patients:
The IFAR reported in 2003 that 3% of patients had developed HNSCC, a 500-fold increased risk compared to the general population(14). These cancers were frequently of the oral cavity (68%), followed by larynx and oropharynx, each with 11%. The median age of HNSCC diagnosis was 31 years compared with a median age of 60 for the general population(15). Tumors were typically advanced at diagnosis, with Stage IV disease presenting in 63%. They estimated a cumulative risk of HNSCC of 14% by age 40. In an update in 2016, patient characteristics and treatment information was reported on 35 cases of HNSCC. Treatment included surgery in 30 patients and RT in 16. Tumor recurrence was remarkably common, with 49% of patients overall experiencing recurrence at a median 22 months after definitive therapy. Recurrence was high even among early stage tumors with 10 of 11 stage I and II patients recurring, and six of the 11 stage I and II patients died of local recurrence. The 5-year cause-specific, disease free, and overall survivals were 47%, 43%, and 39%.
In a smaller series from Saint Louis Hospital (SLH) in France, 13 FA patients with HNSCC were identified. Likewise, most cancers were of the oral cavity (85%) and frequently associated with the presence of premalignant dyskeratosis. Of the 9 patients with premalignant lesions, 6 underwent oral surveillance including biopsies to monitor progression. Those under surveillance were typically diagnosed with early stage lesions with 5 of 6 stage I or II at diagnosis. Patients not under surveillance presented with more advanced tumors (5 of 7 were stage III or IV). The numbers are small, but routine oral screening appears to have increased detection of early stage tumors, although it is unclear if this improved outcomes in these patients.
Seven of our patients had oral cavity tumors. The median age of presentation was young at 33.4 years. Three patients presented originally with stage I cancers, although two of these recurred in the neck after margin negative surgery. One patient had a stage II cervical esophageal cancer. The remainder of our patients were stage III or IV. Five of our patients had at least annual head and neck cancer screening, which may have permitted early detection in two patients diagnosed with T1 tumors. The other 3 patients under surveillance presented with advanced disease. While oral screening may not detect all malignant lesions at an early stage, routine screening seems prudent for all FA patients. Of our 9 patients, the only two alive without evidence of disease underwent annual screening and were diagnosed at an early stage and treated with surgery alone.
Treatment challenges:
While surgery is sometimes sufficient for cure of HNSCC, current paradigms strongly support the roles of RT alone or with concurrent chemotherapy (CCRT) in the definitive and adjuvant management of HNSCC. Due to the defects in DNA interstrand crosslink repair in FA, these patients are at increased risk of toxicity with standard of care treatments, which presents a significant dilemma in a disease where locally advanced disease is inadequately managed with surgical resection alone. Additionally, even after successful surgery with clear margins, rapid local recurrence is common in FA patients making minimizing treatment related morbidity and providing optimal disease management a delicate balancing act.
Surgery:
Surgery in FA patients has been well tolerated in historical series. In the IFAR report, 30 patients underwent surgery of their primary tumors including 21 neck dissections(4). Seven patients had postoperative complications including 2 hardware-associated wound infections. The SLH series reported surgery in 10 of 13 patients, but did not elaborate on complications(5). With limited reports of unexpected complications from surgery, it seems that extensive head and neck surgery is feasible in FA patients.
Eight of our patients had surgery, which was well tolerated with two notable exceptions. Patient 3 developed immediate postoperative tachycardia and respiratory distress which ultimately prompted ICU admission. This patient had significant competing medical issues including relapsed, active AML after multiple BMT and investigational transplant strategies. While deemed fit for surgery, his postsurgical complications and inability to recover from them may have been related to his competing sources of morbidity. Patient 5 had wound healing and infectious complications related to his mandibular hardware. This patient was pancytopenic at the time of surgery, which possibly contributed to his poor wound healing and recurrent infections.
Chemotherapy:
Sensitivity to conventional chemotherapy was first observed after patients had unexpectedly high toxicities to conventional BMT conditioning regimens including alkylating agents such as cyclophosphamide(16). Improved survival was associated with reducing the dose of cyclophosphamide. As such, BMT conditioning regimens have evolved over the years focusing on reduced doses of alkylating agents and use of alternative chemotherapeutics(17).
There are few reports in the literature of FA patients with HNSCC receiving conventional chemotherapy. In the IFAR study, one patient received 56 Gy RT with cisplatin, bleomycin, and methotrexate(4). Severe toxicity was reported including high-grade mucositis, cytopenia, tracheal stenosis, radiation pneumonitis, recurrent pneumonia, persistent myelosuppresion, and hemorrhage. Another patient received methotrexate with RT. High-grade mucositis and dysphagia were reported. The SLH series reported chemotherapy in 3 patients(5). One patient was treated with low dose chemotherapy: cisplatin 8mg/m2 and 5-FU 60mg/m2 without response and severe toxicity. The case of a 24-year-old man with unresectable oral cavity SCC receiving definitive intent CCRT with carboplatin during a course of hyperfractionated RT was reported(18). After 2 weeks, he developed grade 3 pancytopenia and mucositis requiring total parenteral nutrition. After 38.4 Gy, his regimen was halted for two weeks. He then finished with daily RT to a total dose of 67 Gy. After a complete response at the primary site, he had residual LNs dissected and was apparently cured of this malignancy. Interestingly, he was not diagnosed with FA until subsequent development of an anal SCC.
We report one patient who received full dose neoadjuvant-intent chemotherapy with carboplatin and paclitaxel. She was previously unknown to have FA and was diagnosed after a precipitous onset of pancytopenia and liver failure. While she did recover and survive 18 months after diagnosis without additional therapy, her experience with chemotherapy exemplifies the chemosensitivity of FA patients even without clinical manifestations of FA. Patient 9 received low doses of paclitaxel with her last cycle of QS-RT, and eventually received 3 doses up to 80mg/m2. She did not apparently have any difficulties with cautious administration of paclitaxel.
Cetuximab is an alternative for CCRT in HNSCC(19). In the IFAR report, three patients received CCRT with cetuximab rather than conventional chemotherapy. No complications were reported in 2 patients. The third developed toxicities including high-grade mucositis, dysphagia, cytopenia, and did not complete the prescribed course of RT. Wong et al provided a detailed report of a patient treated with postoperative RT with concurrent cetuximab(20). The patient developed grade 3 dermatitis after 45 Gy and grade 3 mucositis after 50 Gy of treatment, but was able to finish the prescribed 70.2 Gy without interruption. After initial loading dose of cetuximab (400mg/m2) he developed neutropenia prompting reduction of subsequent doses (200mg/m2). Due to his brisk skin reaction, the final 2 administrations of cetuximab were held. The acute treatment effects were manageable, but he suffered in-field recurrence rapidly following treatment and died from local disease 10 weeks after RT concluded.
Four of our patients received cetuximab and tolerated it well. Patient 5 had worsening cytopenias while on RT with cetuximab, and brisk dermatitis in the radiation field, although it’s unclear to what extent these effects were related to cetuximab, radiation, or the combination.
Radiotherapy:
Radiosensitivity in FA was first described in the context of BMT conditioning. Evidence that RT as a part of conditioning causes severe adverse events is limited. An RT dose escalation study of BMT conditioning using TBI with 450 cGy vs 600 cGy reported no grade III/IV toxicities with 600 cGy, despite 5 Grade III/IV toxicities with 450 cGy suggesting that the acute toxicity of conditioning in FA patients may be unrelated to dose of RT. Gluckman et al did report that limiting the field of RT improved survival in FA BMT patients in an analysis of the International Bone Marrow Transplant Registry(16). It is unclear whether the increased survival was related to reduced acute adverse events or subsequent complications. The sensitivity of FA patients to developing solid tumors and the potential secondary malignancy risk due to RT in the context of clinical reports of radiosensitivity has been sufficient to promote regimens for conditioning that omit RT, however there are no data that omission of RT reduces cancer risk(22).
In the IFAR report, 16 (45.7%) patients received RT: 13 patients received adjuvant RT after surgery, one received neoadjuvant, and 2 received palliative RT. The median RT dose was 50.5 Gy (25–70.2 Gy). Treatment was completed in 11 patients. Complications were common including high-grade mucositis in 9, high-grade dysphagia in 8, and high-grade cytopenias in 8. Five patients required a treatment break or cessation of RT due to toxicity. Four patients died during RT: three from sepsis and one from cardiac arrest.
Three patients receiving RT were reported in the SLH series(5). One patient received 70 Gy to the primary site and cervical nodes and experienced grade III mucositis and dehydration requiring PEG. Another patient required cessation of therapy after 25 Gy due to progression of disease and severe mucositis. A patient with very locally advanced oropharyngeal SCC received a subtotal resection and underwent adjuvant RT. After 8 Gy in 7 treatment days the patient developed severe thrombocytopenia, which proved fatal despite intensive supportive care and platelet transfusions(18). Wong et al reviewed the literature and found 34 cases of patients with FA receiving RT(20). Complications varied widely, but high-grade mucositis, dermatitis, and myelosuppression have been common sometimes necessitating treatment break or cessation. Four of our patients received adjuvant RT. In these patients, disease control without adjuvant radiation was deemed unlikely, warranting the increased risk of toxicity. Three patients completed their course as prescribed with reasonable side effect profiles.
Patient 7 is the first reported case of proton RT for HNSCC in a FA patient. He was treated with 70 CGE to the high-risk region in the right oral cavity and neck and 60 Gy to the remaining right neck with concurrent cetuximab (Figure 1). He tolerated treatment well with expected grade 2 toxicities. Of note, he is a complementation group A mosaic in the lymphocyte compartment. Mosaicism in FA occurs in approximately 25% of patients and when, by a number of described and proposed mechanisms including back mutations, intragenic crossover, gene conversion, and compensatory mutations, a population of hematopoietic stem cells undergo a phenotypic reversion to wild type, partially compensating for the FA anomaly in the blood (23, 24). Notably, while we do not have specific information about mosaicism in our other patients, it may explain why patients 4, 5, and 9 were able to avoid BMT. While a milder phenotype in the blood is described for FA mosaic patients, whether this protects against additional toxicities of genotoxic therapies is unknown. Patient 4 also had no prior manifestations of FA, but tolerated chemotherapy exceedingly poorly. Using prior HSCT as a surrogate for severity of FA, it does not appear that transplanted patients in the IFAR report were more likely to experience high-grade toxicities(4). Whether or not this patient was less susceptible to treatment related side effects or not, he tolerated proton radiotherapy well. Given the increased toxicities described with RT in FA patients, the reduction of integral dose achievable with protons may potentially be advantageous in this high-risk population(25), however caution must be exercised extrapolating from a single patient experience.
Patient 5 had a complicated course of adjuvant RT. His initial surgery was complicated by hardware infection in the setting of cytopenia presumably complicating his wound healing and ability to fight off infection postoperatively. His subsequent RT after recurrence was complicated by recurrent hardware infection. During that admission he required tracheostomy and PEG placement. He resumed RT to a cumulative dose of 42 Gy, but treatment was stopped again due to bleeding dermatitis overlying his mandibular hardware. This case highlights the challenge of surgery and RT in an FA patient without optimized hematopoiesis.
Patient 9 experienced a carotid sentinel bleed one month after her second cycle of QS-RT RT which was embolized. We cannot confidently state whether the bleed was caused by the tumor infiltrating her carotid artery, an unexpected side effect of RT, or a combination thereof. She eventually completed a third cycle of QS-RT with disease response on imaging and no further complications attributable to RT.
Given the potentially increased toxicity of RT and CCRT in FA patients, it is notable that reports in the literature describe poor treatment outcomes. Survival is limited, with many patients dying within 12 months of completing RT(20). Progression has been reported both during and at the conclusion of RT(4). Similarly poor local control was observed in our cohort, with two in field recurrences at 7 and 16 months, respectively. Patient 8 completed a full adjuvant course of RT and failed out of field in the cavernous sinus. In our series, the only patients who survive without evidence of disease underwent surgery alone for early tumors (2 patients). While we do describe that carefully monitored patients may tolerate full course adjuvant RT, both local control and overall survival are still limited and thus adjuvant RT should only be used if deemed absolutely necessary and the risks and outcomes are carefully discussed with patients.
Summary:
Our institutional experience treating 9 FA patients with HNSCC confirms prior reports in the literature that HNSCC presents at a young age and is frequently aggressive at diagnosis. Surgery was well tolerated in optimized patients. Two patients achieved disease control with surgery alone after screen-detection of small tumors. Adjuvant radiation with concurrent cetuximab is feasible with careful monitoring of acute effects. Patients with poor hematopoiesis and surgical complications may tolerate radiation poorly. Proton therapy may be considered in these patients to minimize integral dose to normal structures, although caution is warranted given the potential for increased rates and severity of dermatitis with protons. Even when full course adjuvant RT can be completed, local control remains elusive, and thus the risks and outcomes of adjuvant treatment should be discussed.
Acknowledgments
Funding Sources: Supported by NIH/NCI core grant P30 CA008748
Footnotes
There are no conflicts of interest.
Thomas Beckham wrote the manuscript. The remaining authors contributed to editing and clinical perspective.
References
- 1.Mehta PA, Tolar J. Fanconi Anemia. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al. , editors. GeneReviews(R) Seattle (WA)1993. [PubMed] [Google Scholar]
- 2.Dextraze ME, Gantchev T, Girouard S, Hunting D. DNA interstrand cross-links induced by ionizing radiation: an unsung lesion. Mutat Res 2010. Apr-Jun;704(1–3):101–7. [DOI] [PubMed] [Google Scholar]
- 3.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood 2003. February 15;101(4):1249–56. [DOI] [PubMed] [Google Scholar]
- 4.Kutler DI, Patel KR, Auerbach AD, Kennedy J, Lach FP, Sanborn E, et al. Natural history and management of Fanconi anemia patients with head and neck cancer: A 10-year follow-up. Laryngoscope 2016. April;126(4):870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masserot C, Peffault de Latour R, Rocha V, Leblanc T, Rigolet A, Pascal F, et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer 2008. December 15;113(12):3315–22. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica 2008. April;93(4):511–7. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood 2003. February 01;101(3):822–6. [DOI] [PubMed] [Google Scholar]
- 8.Deeg HJ, Socie G. Malignancies after hematopoietic stem cell transplantation: many questions, some answers. Blood 1998. March 15;91(6):1833–44. [PubMed] [Google Scholar]
- 9.Chao MM, Kuehl JS, Strauss G, Hanenberg H, Schindler D, Neitzel H, et al. Outcomes of mismatched and unrelated donor hematopoietic stem cell transplantation in Fanconi anemia conditioned with chemotherapy only. Ann Hematol 2015. August;94(8):1311–8. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood 2005. January 01;105(1):67–73. [DOI] [PubMed] [Google Scholar]
- 11.Scudellari M HPV: Sex, cancer and a virus. Nature 2013. November 21;503(7476):330–2. [DOI] [PubMed] [Google Scholar]
- 12.Kutler DI, Wreesmann VB, Goberdhan A, Ben-Porat L, Satagopan J, Ngai I, et al. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst 2003. November 19;95(22):1718–21. [DOI] [PubMed] [Google Scholar]
- 13.van Zeeburg HJ, Snijders PJ, Wu T, Gluckman E, Soulier J, Surralles J, et al. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst 2008. November 19;100(22):1649–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg 2003. January;129(1):106–12. [DOI] [PubMed] [Google Scholar]
- 15.Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer 2014. May 15;120(10):1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gluckman E, Auerbach AD, Horowitz MM, Sobocinski KA, Ash RC, Bortin MM, et al. Bone marrow transplantation for Fanconi anemia. Blood 1995. October 01;86(7):2856–62. [PubMed] [Google Scholar]
- 17.Bitan M, Or R, Shapira MY, Aker M, Resnick IB, Ackerstein A, et al. Fludarabine-based reduced intensity conditioning for stem cell transplantation of Fanconi anemia patients from fully matched related and unrelated donors. Biol Blood Marrow Transplant 2006. July;12(7):712–8. [DOI] [PubMed] [Google Scholar]
- 18.Bremer M, Schindler D, Gross M, Dork T, Morlot S, Karstens JH. Fanconi’s anemia and clinical radiosensitivity report on two adult patients with locally advanced solid tumors treated by radiotherapy. Strahlenther Onkol 2003. November;179(11):748–53. [DOI] [PubMed] [Google Scholar]
- 19.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006. February 09;354(6):567–78. [DOI] [PubMed] [Google Scholar]
- 20.Wong WM, Parvathaneni U, Jewell PD, Martins RG, Futran ND, Laramore GE, et al. Squamous cell carcinoma of the oral tongue in a patient with Fanconi anemia treated with radiotherapy and concurrent cetuximab: a case report and review of the literature. Head Neck 2013. October;35(10):E292–8. [DOI] [PubMed] [Google Scholar]
- 21.Rainbow AJ, Howes M. Defective repair of ultraviolet- and gamma-ray-damaged DNA in Fanconi’s anaemia. Int J Radiat Biol Relat Stud Phys Chem Med 1977. February;31(2):191–5. [DOI] [PubMed] [Google Scholar]
- 22.Mehta PA, Davies SM, Leemhuis T, Myers K, Kernan NA, Prockop SE, et al. Radiation-free, alternative-donor HCT for Fanconi anemia patients: results from a prospective multi-institutional study. Blood 2017. April 20;129(16):2308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross M, Hanenberg H, Lobitz S, Friedl R, Herterich S, Dietrich R, et al. Reverse mosaicism in Fanconi anemia: natural gene therapy via molecular self-correction. Cytogenet Genome Res 2002;98(2–3):126–35. [DOI] [PubMed] [Google Scholar]
- 24.Gregory JJ Jr., Wagner JE, Verlander PC, Levran O, Batish SD, Eide CR, et al. Somatic mosaicism in Fanconi anemia: evidence of genotypic reversion in lymphohematopoietic stem cells. Proc Natl Acad Sci U S A 2001. February 27;98(5):2532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leeman JE, Romesser PB, Zhou Y, McBride S, Riaz N, Sherman E, et al. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol 2017. May;18(5):e254–e65. [DOI] [PubMed] [Google Scholar]
- 26.Romesser PB, Cahlon O, Scher E, Zhou Y, Berry SL, Rybkin A, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol 2016. February;118(2):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


