Abstract
PURPOSE
We assessed bone mineral density (BMD) change with aromatase inhibitor (AI) treatment in a contemporary cohort of women with breast cancer treated in Kaiser Permanente Northern California.
METHODS
Percent and estimated annual percent changes in BMD at the total hip and lumbar spine were examined in 676 women receiving AI therapy who had two serial BMD reports available (at least 1 year apart) before and after AI initiation (N=317) or during continued AI therapy (N=359). BMD changes were examined at the total hip and lumbar spine and compared by age and clinical subgroups.
RESULTS
Women experienced BMD declines after AI initiation or continued therapy, with median annual percent change −1.2% (interquartile range, IQR −2.4 to −0.1%) at the hip and −1.0% (IQR −2.3 to 0.1%) at the spine after AI initiation, and −1.1% (IQR −2.4 to 0.1%) at the hip and −0.9% (IQR −2.4 to 0.5%) at the spine during continued therapy. Higher levels of bone loss were observed among younger (<55 years) compared with older (≥75 years) women at the hip (−1.6% vs. −0.8%) and at the spine (−1.5% vs. −0.5%) after AI initiation, and at the hip (−1.4% vs. −1.2%) and at the spine (−2.4% vs. −0.001%) during continued therapy.
CONCLUSIONS
Small but consistent declines in total hip and lumbar spine BMD were present in breast cancer patients following AI therapy initiation or continued AI therapy. Although the overall rates of osteoporosis were low, greater estimated levels of annual bone loss were evident among women <55 years.
INTRODUCTION
In the past decade, aromatase inhibitors (AIs) have become an important component of endocrine therapy for postmenopausal women with hormone-receptor positive (HR-positive) breast cancer. In comparison to tamoxifen, they have been shown to be associated with improved disease-free and recurrence-free survival [1,2]. However, AIs can potentially have detrimental effects on bone, with multiple studies demonstrating accelerated bone loss and increased risk of osteopenia and osteoporosis [3,4]. Compounded with the natural effects of menopause due to ovarian aging and natural estrogen deficiency, women with breast cancer receiving AIs may be at higher risk of fracture secondary to bone loss [5–7].
Current clinical practice guidelines recommend that postmenopausal women with HR-positive breast cancer consider taking AI therapy at some point during adjuvant treatment, either as up-front therapy or as sequential treatment after tamoxifen [1]. Furthermore, adverse effect profiles and patient preferences should be considered in deciding whether and when to incorporate AI therapy. These include bone mineral density (BMD) testing and clinical assessment of risk factors for fracture, given the known adverse effects of AIs on bone. However, fewer studies have examined longitudinal change in BMD of patients receiving AIs in real-world clinical populations outside of clinical trial settings. This current study examines the relative change in BMD at the total hip and lumbar spine in two clinical scenarios: from initiation of AI therapy, or continuation of AI therapy, in one of the largest contemporary cohorts of women with breast cancer patients diagnosed between 2006 and 2013.
METHODS
Study Cohort
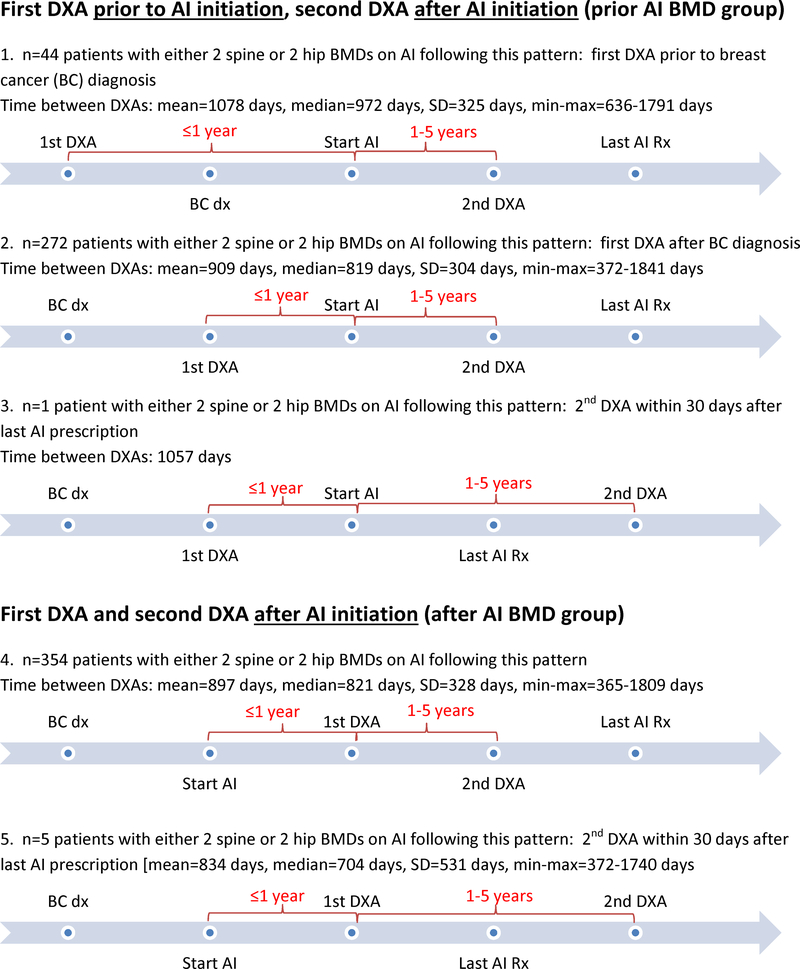
The Pathways Study is a large observational cohort study of 4,505 female members of Kaiser Permanente Northern California recruited from 2006 to 2013 with ongoing prospective follow-up. Within this cohort, 2,157 (47.9%) women initiated treatment with an AI (anastrozole, letrozole and exemestane) for their primary breast cancer [8]. To examine BMD changes in women who received AI therapy, two population subsets were identified (Figure 1). The first group (n=317) included women with baseline BMD measurement within one year prior to initiation of AI therapy and a subsequent BMD measurement between 1–5 years after initiation of AI selecting the last scan during AI therapy available in this time period). The second group (n=359) included women without a baseline BMD test within one year prior to AI initiation, but with two BMD measurements available during AI therapy, separated by 1–5 years. The remaining n=1,481 women were not eligible for this analysis because they had no BMD measurement (n=119), had only one measurement (n=455), had a measurement outside the allowable time period (n=821), or had a measurement taken when not on AI therapy (n=86). This ineligible group was very similar to the eligible group by demographic and clinical characteristics, yet was diagnosed with higher stage breast cancer (Stage III and IV) (data not shown).
Figure 1.
Timeline between breast cancer (BC) diagnosis, 1st and 2nd DXA, and start of aromatase inhibitor (AI) therapy and last AI prescription
Patient Characteristics
Race/ethnicity, calcium and vitamin supplementation before breast cancer diagnosis, and information pertaining to menopause, were collected from self-report during the baseline interview at the time of enrollment. Menopause was defined based on self-report of having no menses for at least twelve consecutive months, prior complete hysterectomy and/or oophorectomy of both ovaries [8]. Height and weight were primarily obtained from the electronic medical record at breast cancer diagnosis, with data from 2% of the cohort based on self-reported measures at the baseline interview. Body mass index (BMI) was calculated using weight (kg) divided by height (m) squared.
Using health plan databases, prior fracture was classified based on outpatient and hospitalization diagnoses of fractures involving the neck, spine, trunk, pelvis, and upper and lower extremities (International Classification of Diseases, 9th edition, ICD-9 805, 807–815, 817–825, 827–829) excluding open fracture codes, fractures involving spinal cord injury, fractures of the face/skull, fingers and toes, and those associated with major trauma (E800–848). Pharmacologic exposures were determined using filled prescriptions tracked in outpatient regional pharmacy databases, including treatment with bisphosphonate drugs (BP) and AIs.
Breast Cancer History
Clinical and diagnostic tumor characteristics were obtained from the KPNC Cancer Registry approximately four months post-diagnosis. Variables included: stage at diagnosis, estrogen/progesterone receptor (ER/PR) positivity, HER2/neu (Her2) status, surgery type, and treatments received.
Bone Mineral Density (BMD) Assessment
Baseline BMD findings prior to or around the time of breast cancer diagnosis have previously been reported [9]. For these analyses examining change in BMD with AI therapy, clinical BMD reports based on findings from Hologic bone densitometers were used to extract BMD values for the total hip and lumbar spine using key text string search algorithms. These algorithms were previously validated in a subset of 239 women with 532 BMD values prior to 2008, where a concordance rate of 96% was demonstrated when comparing the BMD values derived from the algorithms to the electronic BMD values extracted directly from Hologic densitometers (unpublished data). Using Hologic and Hologic NHANES Caucasian reference data for peak BMD values, T-scores were also calculated using the following formula: T-score = (observed BMD – peak BMD) / standard deviation of peak BMD [10,11]. According to established criteria [12], osteoporosis was defined by a BMD T-score of −2.5 or lower, osteopenia by BMD T-score between −1 and −2.5, and normal BMD by BMD T-score of −1 or greater.
We calculated percent BMD change by ((2nd BMD - 1st BMD) / 1st BMD) x 100. We also annualized the change measure by dividing by time between measurements, in years. Two scenarios were examined. First, for those with baseline BMD obtained within one year prior to initiating AI (median 49.0 days prior to AI initiation, interquartile range, IQR 9.0–114.0 days), the assumption was made that BMD values largely reflected baseline BMD just prior to AI initiation, and estimated annual percent change in BMD was calculated from the point of AI initiation until the follow-up BMD measurement. Second, for women without a BMD within one year prior to initiating AI, two subsequent BMD measurements during AI therapy were used (separated by a median of 2.2 years, IQR 1.9–3.0 years), with the estimated annual percent change in BMD calculated between these two measurements.
RESULTS
Among 676 women who initiated AI therapy and had sequential measurement of BMD, 317 had a baseline BMD within one year prior to AI initiation and during ongoing treatment (“prior AI BMD” group), and the remaining 359 women had at least two BMD measurements during ongoing AI exposure (“after AI BMD” group) (Figure 1). Most women were age 55 years and older (90.4%) and postmenopausal (96.8%) at the time of cancer diagnosis; had Stage I or II breast cancer (91.0%) and started AI therapy on average 4.7 months after initial diagnosis. The cohort was racially and ethnically diverse with 72.2% white, 6.1% African American, 11.1% Hispanic, 8.9% Asian and 1.8% other race. About two-thirds of the women were overweight or obese at baseline (71.0%). More than one third (38.8%) of the women were taking calcium and/or vitamin D supplements before breast cancer diagnosis, with 43.8% who had their baseline BMD prior to AI initiation, compared with 34.4% who had their baseline BMD measurement during AI treatment. There were 102 women (15.1%) with a history of fracture before breast cancer diagnosis, and 56 women (8.3%) had a prior history of BP use before breast cancer diagnosis (Table 1).
Table 1.
Baseline characteristics of women with invasive breast cancer at the time of aromatase inhibitor (AI) initiation
| All Women | Women who had first BMD in the year prior to AI therapy initiation (Prior AI BMD Group) | Women who had first BMD after AI therapy initiation (After AI BMD Group) | |
|---|---|---|---|
| N=676 | N=317 | N=359 | |
| N (%) | N (%) | N (%) | |
| Age at AI Initiation | |||
| <55 years | 65 (9.6) | 27 (8.5) | 38 (10.6) |
| 55–64 years | 303 (44.9) | 136 (42.9) | 167 (46.5) |
| 65–74 years | 233 (34.4) | 112 (35.3) | 121 (33.7) |
| ≥75 years | 75 (11.1) | 42 (13.3) | 33 (9.2) |
| mean (SD) | 64.4 (7.8) | 65.0 (8.0) | 63.8 (7.6) |
| Race/Ethnicity | |||
| White | 488 (72.2) | 219 (69.1) | 269 (74.9) |
| African American | 41 (6.1) | 23 (7.3) | 18 (5.0) |
| Hispanic | 75 (11.1) | 35 (11.0) | 40 (11.1) |
| Asian | 60 (8.9) | 33 (10.4) | 27 (7.5) |
| Other | 12 (1.8) | 7 (2.2) | 5 (1.4) |
| AJCC Stage | |||
| I | 396 (58.6) | 194 (61.2) | 202 (56.3) |
| II | 219 (32.4) | 100 (31.6) | 119 (33.2) |
| III | 58 (8.6) | 22 (6.9) | 36 (10.0) |
| IV | 3 (0.4) | 1 (0.3) | 2 (0.6) |
| Menopausal Status at Baselinea | |||
| Premenopausal | 22 (3.3) | 11 (3.5) | 11 (3.1) |
| Postmenopausal | 654 (96.8) | 306 (96.5) | 348 (96.9) |
| BMI at Baselinea (kg/m2) | |||
| <18.5 | 6 (0.9) | 1 (0.3) | 5 (1.4) |
| 18.5–24.9 | 190 (28.1) | 91 (28.7) | 99 (27.6) |
| 25–29.9 | 209 (30.9) | 106 (33.4) | 103 (28.7) |
| ≥30 | 271 (40.1) | 119 (37.5) | 152 (42.3) |
| mean (SD) | 28.7 (6.4) | 28.6 (6.5) | 28.8 (6.3) |
| Any Fracture Before Breast Cancer Diagnosis | |||
| No | 574 (84.9) | 271 (85.5) | 303 (84.4) |
| Yes | 102 (15.1) | 46 (14.5) | 56 (15.6) |
| Bisphosphonate Use Before Breast Cancer Diagnosis | |||
| No | 620 (91.7) | 290 (91.5) | 330 (91.9) |
| Yes | 56 (8.3) | 27 (8.5) | 29 (8.1) |
| Vitamin Supplement Use Before Breast Cancer Diagnosis | |||
| None | 410 (61.2) | 176 (56.2) | 234 (65.6) |
| Calcium | 104 (15.5) | 51 (16.3) | 53 (14.8) |
| Vitamin D | 92 (13.7) | 53 (16.9) | 39 (10.9) |
| Both | 64 (9.6) | 33 (10.5) | 31 (8.7) |
Baseline refers to information collected at the time of the baseline interview, which is on average 2 months after breast cancer diagnosis
Table 2 shows baseline BMD measured prior to AI initiation, or during AI therapy, and classification by BMD T-score in the total hip and lumbar spine. Approximately two-thirds had normal BMD in the hip and half had normal BMD in the spine at baseline (T score −1.0 or higher); the remainder had osteopenia (T score between −1.0 and −2.5) and few women had osteoporosis (T score −2.5 or below). Less than 10% received treatment with BP between the two scans in the prior BMD group, as well as between the two scans in the after AI BMD group. Less than 4% and 3% of cases had a fracture diagnosis (incident or prevalent) during the BMD follow-up in each group, respectively.
Table 2.
Bone mineral density (BMD) and bisphosphonate use of women with invasive breast cancer on aromatase inhibitor (AI) therapy
| Women who had first BMD in the year prior to AI therapy initiation (Prior AI BMD Group) | Women who had first BMD after AI therapy initiation (After AI BMD Group) | |
|---|---|---|
| N=317 | N=359 | |
| N (%) | N (%) | |
| Baseline or First Total Hip BMD T-scorea | ||
| ≤ −2.5 (osteoporosis) | 2 (0.7) | 9 (2.6) |
| −2.5 < T < −1.0 (osteopenia) | 83 (27.4) | 94 (27.5) |
| ≥ −1.0 (normal) | 218 (72.0) | 239 (69.9) |
| Baseline or First Lumbar Spine BMD T-scorea | ||
| ≤ −2.5 (osteoporosis) | 20 (6.5) | 28 (8.1) |
| −2.5 < T < −1.0 (osteopenia) | 123 (40.2) | 118 (34.2) |
| ≥ −1.0 (normal) | 163 (53.3) | 199 (57.7) |
| Bisphosphonate Prescription/Refill Between 1st and 2nd DEXA Scan | ||
| No | 291 (91.8) | 326 (90.8) |
| Yes | 26 (8.2) | 33 (9.2) |
| <55 years at first refill | 6 (22.2) | 5 (15.1) |
| 55–64 years at first refill | 10 (37.1) | 18 (54.6) |
| 65–74 years at first refill | 6 (22.2) | 9 (27.3) |
| ≥75 years at first refill | 4 (18.5) | 1 (3.0) |
| Fracture Between 1st and 2nd DEXA Scan | ||
| No | 306 (96.5) | 351 (97.8) |
| Yes | 11 (3.5) | 8 (2.2) |
Baseline BMD was either <1 year prior to AI initiation or the first BMD after AI initiation.
Table 3 shows the change in BMD measurement after AI initiation or during continued AI therapy. Overall, both groups experienced some degree of bone loss over time. In the prior AI BMD group, the overall percent change in hip BMD was −3.2% (IQR −5.7 to −0.3%), with an estimated annual hip BMD loss of −1.2% (IQR −2.4 to −0.1%). This was similar to the after AI BMD group, which experienced an overall decline in hip BMD of −2.6% (IQR −5.4 to 0.3%), with an estimated annual hip BMD loss of −1.1% (IQR −2.4 to 0.1%). The bone loss in the spine was also similar between the two groups, with an estimated annual percent loss of −1.0% (IQR −2.3 to 0.1%) and −0.9% (IQR −2.4 to 0.5%) for the prior and after AI groups, respectively. Somewhat higher rates of bone loss were evident among younger women compared with older women. Specifically, in the prior AI BMD group, the estimated annual percent change in hip BMD was −1.6% (IQR −3.2 to −0.3%) in the <55 year group vs. −0.8% (IQR −2.1 to 0.4%) in the ≥75 year group. Similarly, the estimated annual percent change in spine BMD was −1.5% (IQR −2.7 to −0.8%) vs. −0.5% (IQR −1.6 to 1.4%). In the after AI BMD group, the estimated annual percent change in hip BMD was −1.4% (IQR −2.9 to 0.4%) in the <55 year group vs. −1.2% (IQR −2.3 to 0.1%) in the ≥75 year group, and the estimated annual percent change in spine BMD was −2.4% (IQR −4.0 to −0.1%) for the <55 group vs. −0.001% (IQR −1.5 to 1.0%) for the ≥75 year group.
Table 3.
Change in bone mineral density (BMD) for total hip and lumbar spine of women with invasive breast cancer on aromatase inhibitor (AI) therapy
| Women who had first BMD in the year prior to AI therapy initiation (Prior AI BMD Group) | Women who had first BMD after AI therapy initiation (After AI BMD Group) |
|||||
|---|---|---|---|---|---|---|
| N=317 | N=359 | |||||
| N (%) | N (%) | |||||
| TOTAL HIP | N (%) | Median (IQ range Q1, Q3) | Mean (SD) | N (%) | Median (IQ range Q1, Q3) | Mean (SD) |
| Percent Changeain Hip BMD (%) | 303 (95.6) | −3.2 (−5.7, −0.3) | −3.0 (4.9) | 342 (95.3) | −2.6 (−5.4, 0.3) | −2.8 (4.8) |
| Estimated Annual Percent Change* in Hip BMD (%) | 303 (95.6) | −1.2 (−2.4, −0.1) | −1.2 (2.2) | 342 (95.3) | −1.1 (−2.4, 0.1) | −1.1 (2.3) |
| Percent Changea in Hip BMD by Age (%) | ||||||
| <55 years | 26 (8.2) | −4.4 (−7.3, −0.7) | −4.0 (4.4) | 36 (10.0) | −2.6 (−6.6, 0.9) | −3.1 (5.6) |
| 55–64 years | 126 (39.7) | −3.4 (−5.5, −0.1) | −2.6 (5.0) | 158 (44.0) | −2.7 (−5.9, 0) | −2.9 (5.0) |
| 65–74 years | 110 (34.7) | −3.1 (−5.9, −0.9) | −3.6 (4.7) | 116 (32.3) | −2.6 (−5.1, 0.4) | −2.4 (4.3) |
| ≥75 years | 41 (12.9) | −2.4 (−5.3, 0.8) | −2.3 (5.0) | 32 (8.9) | −2.4 (−5.1, 0.1) | −3.0 (5.1) |
| Estimated Annual Percent Changea in Hip BMD by Age (%) | ||||||
| <55 years | 26 (8.2) | −1.6 (−3.2, −0.3) | −1.9 (2.2) | 36 (10.0) | −1.4 (−2.9, 0.4) | −1.2 (2.8) |
| 55–64 years | 126 (39.7) | −1.2 (−2.3, 0) | −1.1 (2.3) | 158 (44.0) | −1.1 (−2.5, 0) | −1.1 (2.3) |
| 65–74 years | 110 (34.7) | −1.1 (−2.2, −0.4) | −1.5 (2.1) | 116 (32.3) | −0.9 (−2.3, 0.1) | −1.1 (2.1) |
| ≥75 years | 41 (12.9) | −0.8 (−2.1, 0.4) | −0.8 (2.1) | 32 (8.9) | −1.2 (−2.3, 0.1) | −1.2 (2.0) |
| LUMBAR SPINE | N (%) | Median (IQ range Q1, Q3) | Mean (SD) | N (%) | Median (IQ range Q1, Q3) | Mean (SD) |
| Percent Changea in Spine BMD (%) | 306 (96.5) | −2.4 (−5.6, 0.3) | −2.2 (5.6) | 345 (96.1) | −2.1 (−5.4, 1.0) | −2.1 (5.4) |
| Estimated Annual Percent Changea in Spine BMD (%) | 306 (96.5) | −1.0 (−2.3, 0.1) | −1.0 (2.3) | 345 (96.1) | −0.9 (−2.4, 0.5) | −0.9 (2.5) |
| Percent Changea in Spine BMD by Age (%) | ||||||
| <55 years | 27 (8.5) | −3.1 (−7.1, −1.4) | −3.7 (5.7) | 38 (10.6) | −5.0 (−9.1, −0.2) | −4.6 (6.6) |
| 55–64 years | 133 (41.9) | −2.5 (−5.8, 0.1) | −2.4 (5.8) | 164 (45.7) | −2.4 (−5.5, 1.1) | −2.3 (4.9) |
| 65–74 years | 105 (33.1) | −2.4 (−5.7, 0.1) | −2.4 (5.2) | 115 (32.0) | −1.8 (−5.1, 0.9) | −1.5 (5.6) |
| ≥75 years | 41 (12.9) | −1.3 (−3.9, 2.1) | −0.2 (5.2) | 28 (7.8) | 0.01 (−2.9, 2.4) | −0.4 (3.8) |
| Estimated Annual Percent Changea in Spine BMD by Age (%) | ||||||
| <55 years | 27 (8.5) | −1.5 (−2.7, −0.8) | −1.8 (2.7) | 38 (10.6) | −2.4 (−4.0, −0.1) | −2.0 (3.3) |
| 55–64 years | 133 (41.9) | −1.1 (−2.3, 0) | −1.1 (2.3) | 164 (45.7) | −0.9 (−2.0, 0.4) | −0.8 (2.3) |
| 65–74 years | 105 (33.1) | −0.9 (−2.3, 0) | −1.0 (2.1) | 115 (32.0) | −0.9 (−2.4, 0.4) | −0.7 (2.3) |
| ≥75 years | 41 (12.9) | −0.5 (−1.6, 1.4) | −0.1 (2.3) | 28 (7.8) | −0.001 (−1.5, 1.0) | −0.1 (2.0) |
Second BMD was within two years of AI initiation for those with a baseline BMD value, or within 1–5 years of the first BMD on AI therapy. Percent change was calculated between first and second BMD on AI therapy or between the interval from AI initiation to second BMD. Annual percent change was calculated by dividing by time between measurements, in years. A (−) indicates percent reduction.
The percent change and estimated annual percent change in BMD was unchanged after excluding the 119 women who had a history of using a BP before the first bone densitometer scan or between the first and second scans (data not shown).
DISCUSSION
In this cohort of 676 women with breast cancer who initiated AI therapy and had sequential measurement of BMD, we observed an average overall 3% decrease in total hip BMD with an estimated average annual BMD loss of 1%, along with an average overall 2% decrease in lumbar spine BMD with an estimated average annual BMD loss of 1%. Higher rates of bone loss were noted in younger (<55 year) compared with older (≥75 year) women at the hip and especially the spine after initiating or continuing AI therapy.
Other studies have reported bone loss in women receiving AI therapy in the range of −4% (total hip) and −2% (lumbar spine) per year [13], somewhat higher than that observed in our study. However, the literature reporting change in BMD over the course of AI therapy has largely been limited to bone sub-studies from the major clinical trials [14]. Our study is one of the first studies to examine BMD findings in a real-world population of women receiving care, including comparison of findings and rate of BMD change in older (or postmenopausal) and younger (or premenopausal) patients. One other observational study examined the natural history of bone loss over six years among premenopausal and early postmenopausal women [15]. In addition, the bone sub-study of the ABCSG-12 clinical trial of premenopausal patients on hormonal therapy with and without the bisphosphonate zoledronic acid measured long-term BMD change at 0, 6, 12, 36, and 60 months [16].
The greater degree of bone loss observed in younger women receiving AI therapy may reflect the expected findings with ovarian aging, where BMD loss is greatest during the first 5–7 years following menopause. These findings may also reflect the added detriment of AI therapy in younger postmenopausal women and those who may still be premenopause or in perimenopause transition.
Our study has some limitations to consider. First, clinical BMD reports and text algorithms were used to derive BMD results from women with baseline or follow-up clinical BMD scans that were not necessarily obtained at the same intervals between patients, relative to AI initiation or continuation. This may have resulted in under- or over-estimation of bone loss with AI therapy. Second, while we observed some differences in bone loss in the younger compared with the older women, younger women comprised a small subset within this cohort (9.6%). Third, a limitation of the dictated clinical BMD report is that BMD was reported to two rather than three decimal points, which may have reduced the precision of calculated BMD change. However, this limitation affected less than 2% of reports used in our study. Fourth, we did not have a control group of patients not on AI for comparison, such as on tamoxifen, to provide a broader context of bone loss related to AI therapy since age-related bone loss is also expected among postmenopausal women.
Overall, this study describes the trajectory of bone loss among women with breast cancer initiating and continuing AI therapy in a large, carefully characterized prospective cohort of breast cancer patients being treated in a real-world clinical setting. We found declining trends in hip and spine BMD among breast cancer patients who initiated AI therapy that were similar for women with BMD measured during continued AI therapy. Higher rates of bone loss were evident among younger women under age 55 years, particularly in the spine after AI initiation. These findings have implications for clinical management and suggest that clinicians caring for breast cancer patients should be aware that younger women may experience greater bone loss compared to older women when on an aromatase inhibitor, and appropriate screening and preventive measures should be implemented prior to and during treatment.
Acknowledgements
We thank Jean Lee for her medical record reviews. This study was funded by the National Cancer Institute R01 CA166701, R01 CA105274, and U01 CA195565.
Funding: R01 CA166701, R01 CA105274, U01 CA195565
Conflict of Interest
Dr. Lo or a household member has received research funding from Amgen, Sanofi, Novartis, GlaxoSmithKline or AstraZeneca unrelated to this study. The other authors declare that they have no conflict of interest.
Abbreviations
- AI
Aromatase inhibitor
- BMD
Bone mineral density
- BMI
Body mass index
- BP
Bisphosphonate
- HR
Hormone receptor
- IQR
Interquartile range
Footnotes
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
REFERENCES
- 1.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ, American Society of Clinical O (2010) American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28 (23):3784–3796. doi: 10.1200/JCO.2009.26.3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28 (3):509–518. doi: 10.1200/JCO.2009.23.1274 [DOI] [PubMed] [Google Scholar]
- 3.Hadji P (2009) Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol 69 (1):73–82. doi: 10.1016/j.critrevonc.2008.07.013 [DOI] [PubMed] [Google Scholar]
- 4.Chien AJ, Goss PE (2006) Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol 24 (33):5305–5312. doi: 10.1200/JCO.2006.07.5382 [DOI] [PubMed] [Google Scholar]
- 5.Liu M, Goss PE, Ingle JN, Kubo M, Furukawa Y, Batzler A, Jenkins GD, Carlson EE, Nakamura Y, Schaid DJ, Chapman JA, Shepherd LE, Ellis MJ, Khosla S, Wang L, Weinshilboum RM (2014) Aromatase inhibitor-associated bone fractures: a case-cohort GWAS and functional genomics. Mol Endocrinol 28 (10):1740–1751. doi: 10.1210/me.2014-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S, Sturtz K, Wolff AC, Winer E, Hudis C, Stopeck A, Beck JT, Kaur JS, Whelan K, Tu D, Parulekar WR (2016) Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med 375 (3):209–219. doi: 10.1056/NEJMoa1604700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartsch R, Bergen E (2016) ASCO 2016: highlights in breast cancer. Memo 9 (4):211–214. doi: 10.1007/s12254-016-0300-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwan ML, Lo JC, Tang L, Laurent CA, Roh JM, Chandra M, Hahn TE, Hong CC, Sucheston-Campbell L, Hershman DL, Quesenberry CP Jr., Ambrosone CB, Kushi LH, Yao S (2014) Bone health history in breast cancer patients on aromatase inhibitors. PLoS One 9 (10):e111477. doi: 10.1371/journal.pone.0111477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao S, Zhang Y, Tang L, Roh JM, Laurent CA, Hong CC, Hahn T, Lo JC, Ambrosone CB, Kushi LH, Kwan ML (2017) Bone remodeling and regulating biomarkers in women at the time of breast cancer diagnosis. Breast Cancer Res Treat 161 (3):501–513. doi: 10.1007/s10549-016-4068-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo JC, Kim S, Chandra M, Ettinger B (2016) Applying ethnic-specific bone mineral density T-scores to Chinese women in the USA. Osteoporos Int 27 (12):3477–3484. doi: 10.1007/s00198-016-3673-9 [DOI] [PubMed] [Google Scholar]
- 11.Bonnick S (2010) Bone Densitometry in Clinical Practice. Humana Press, New York. doi: 10.1007/978-1-60327-499-9 [DOI] [Google Scholar]
- 12.Schousboe JT, Shepherd JA, Bilezikian JP, Baim S (2013) Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom 16 (4):455–466. doi: 10.1016/j.jocd.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 13.Hadji P, Aapro MS, Body JJ, Bundred NJ, Brufsky A, Coleman RE, Gnant M, Guise T, Lipton A (2011) Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol 22 (12):2546–2555. doi: 10.1093/annonc/mdr017 [DOI] [PubMed] [Google Scholar]
- 14.Bruyere O, Bergmann P, Cavalier E, Gielen E, Goemaere S, Kaufman JM, Rozenberg S, Body JJ (2017) Skeletal health in breast cancer survivors. Maturitas. doi: 10.1016/j.maturitas.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge KE, Sowers MF, Crutchfield M, Lin X, Jannausch M, Harlow SD (2002) Natural history of bone loss over 6 years among premenopausal and early postmenopausal women. Am J Epidemiol 156 (5):410–417 [DOI] [PubMed] [Google Scholar]
- 16.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kassmann H, Piswanger-Solkner JC, Seifert M, Ploner F, Menzel C, Dubsky P, Fitzal F, Bjelic-Radisic V, Steger G, Greil R, Marth C, Kubista E, Samonigg H, Wohlmuth P, Mittlbock M, Jakesz R, Austrian B, Colorectal Cancer Study G (2008) Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol 9 (9):840–849. doi: 10.1016/S1470-2045(08)70204-3 [DOI] [PubMed] [Google Scholar]