Abstract
The dopamine (DA) system is critical for various forms of learning about salient environmental stimuli. Prior work has shown that deletion of the obligatory NR1 subunit of the N-methyl-d-aspartate (NMDA) receptor on neurons expressing the DA transporter (DAT) in mice results in reduced phasic release from DA-containing neurons. To further investigate the contribution of phasic DA release to reward-related learning and cognitive flexibility, the current study evaluated DAT-NR1 null mutant mice in a touchscreen-based pairwise visual discrimination and reversal learning paradigm. Results showed that these mutants were slower to attain a high level of choice accuracy on the discrimination task, but showed improved late reversal performance on sessions where correct choice was above chance. A number of possible interpretations are offered for this pattern of effects, including the opposing possibilities that discrimination memory was either stronger by the completion of training (overtraining effect) or weaker (learning deficit), both of which could potentially produce faster reversal. These data add to the extensive literature ascribing a critical role for DAergic neurotransmission in cognitive functions and the regulation of reward-related behaviors of relevance to addictions.
Keywords: dopamine, NMDA receptor, NR1 subunit, cognitive flexibility, behavioral flexibility, discrimination, reversal, touchscreen, learning, mutant mouse
Graphical abstract
Introduction
The dopamine (DA) system is critical for various forms of learning about salient environmental stimuli. Pacemaker-like firing of midbrain DA neurons results in tonic release of low concentrations. Burst-firing of DA neurons produces phasic DA release of higher concentrations and has recently been shown to result in sustained, post-burst elevations of DA as well [1,2]. Phasic DA release is a neural substrate of reward-related behaviors, but understanding of how this signal contributes to behavior is still incomplete.
Deletion of the obligatory NR1 subunit of the N-methyl-d-aspartate receptor (NMDAR) on neurons expressing the DA transporter (DAT) results in reduced phasic DA release from DAergic containing neurons in response to both unconditioned and conditioned stimuli [3–6]. These mice (DAT-NR1 null mutant) have been found to have deficits in behaviors such as formation of a conditioned place preference, cue-dependent spatial navigation (water maze and T-maze), cued fear conditioning, and delays in learning to perform an operant response for food reward [3,5].
Previous studies of the role of the DA system in behavioral flexibility support the notion that DA facilitates reversal learning [7–15] (Del Guidice et al. 2014). To further investigate how phasic DA release contributes to reward learning and cognitive flexibility, the current study tested mice lacking NR1 on DA neurons in a pairwise visual discrimination and reversal learning task recently shown to engage VTA → NAc DAergic signaling [16].
Materials and methods
Subjects
Deletion of NR1 on DAergic neurons was achieved, as previously described [2], by crossing Grin1loxP/loxP mice with Slc6a3+/Cre, Grin1Δ/+ mice expressing Cre under control of the DAT gene (Slc6a3). This cross resulted in DAT-NR1 null mutant mice (Slc6a3+/Cre, Grin1Δ/loxp) which lack functional NMDAR on DAergic neurons and their littermate controls (Slc6a3+/Cre, Grin1+/loxp) (Figure 1A). While the controls did lack one functional copy of the NR1 gene, previous studies have demonstrated that current through the NMDA receptor and behavioral outcomes are equivalent to wild type animals [3,4,17]. Mice were bred on a C57BL/6J genetic background; we have previously demonstrated robust performance on touchscreen-based tasks in this strain [17,18]. Male and female DAT-NR1 null mutants (n=3 males and n=4 females) and littermate controls (n=4 males and n=4 females) were bred at the University of Washington and shipped to NIAAA after weaning. Mice were 240 days old at the time of testing and genotypes were matched for age and free-feeding weight at the start of testing. All experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the local NIAAA Animal Care and Use Committee.
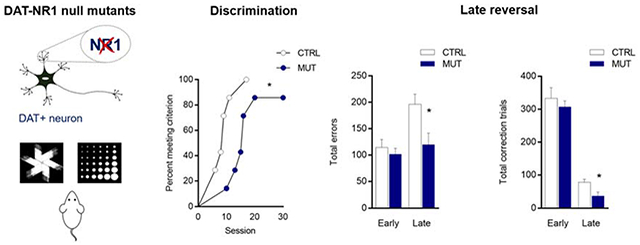
Figure 1: Impaired discrmination learning in mutant mice lacking NR1 on DAT neurons.
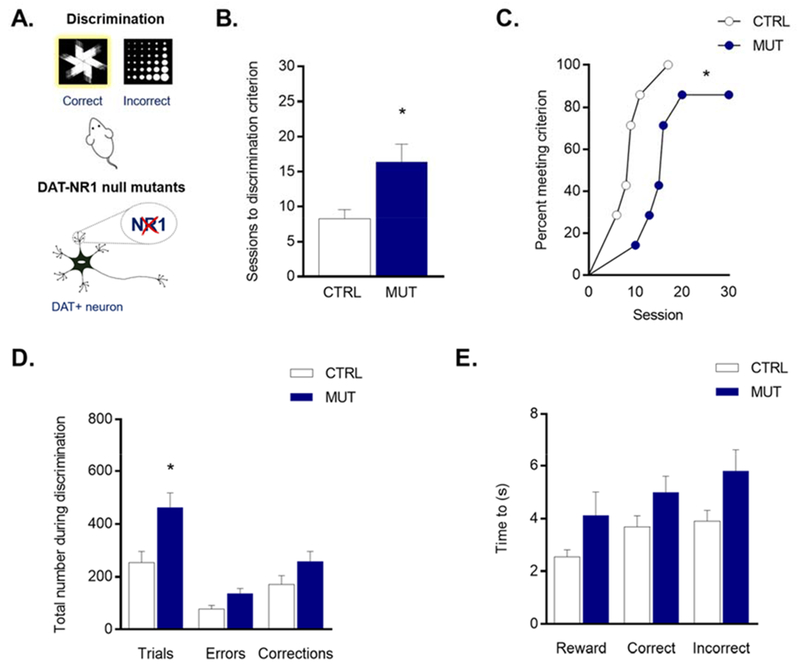
(A) Mice with genetic deletion of the NR1 subunit on DAT+ neurons were trained on a pairwise visual discrimination task. (B) Number of sessions to reach discrimination criterion in control mice (white) and null mutant (blue) mice and (C) survival analysis of sessions to discrimination criterion suggests impaired performance in null mutant mice. (D) Total trials, errors, and correction trials completed during all discrimination sessions. (E) Latency to respond for a correct choice, incorrect choice, and reward. Data are expressed as mean ± SEM. *P < 0.05 versus control.
Behavioral testing
Apparatus and pre-training
Testing procedures were based on those previously reported [9,19,20] using the Bussey-Saksida Touch Screen System (model 80614, Lafayette Instruments, Uafayette, IN, USA). Prior to testing, body weight was reduced and maintained at 85% free-feeding weight throughout testing to motivate responding. Reward was a 14 mg food pellet (#F05684, BioServ, Frenchtown, NJ, USA), provided first in the home cage and then in the test chamber for 30 minutes to acclimate mice to the training environment and the reward (~10 reward pellets/mouse). Prior to discrimination, mice were trained to associate the dispensing of reward with presentation of a 2-second, 65-dB tone and illumination of the magazine light, initiate each trial with a head entry into the food magazine upon illumination, touch 1 of 2 touchscreen windows with a 6.5 cm2 stimulus (selected randomly from a catalogue) to receive a reward, and avoid indiscriminate touchscreen responding (i.e., touches at a blank window).
Discrimination
Two novel 6.5 cm2 stimuli (‘fan’ and ‘marbles’) (Figure 1A) were presented simultaneously: responses at the ‘fan’ stimulus produced a food reward at a continuous rate of reinforcement, responses at the ‘marble’ stimulus (=‘errors’) produced no food reward and a 15-second ‘timeout’ period. Each error was followed by a correction trial (“correction”) in which the 2 stimuli were presented in the same spatial configuration. The next trial proper could not begin until a correct response was made on a correction trial. Mice were given 30 trials (excluding any correction trials) per session (1 session per day) until they attained a performance criterion of >85% correct responses on two consecutive sessions. Dependent measures included sessions to criterion, total trials, percent correct responses (=100*(correct choices/total choice)), total errors, total correction trials (=corrections), and latency to choice and reward.
Reversal
On the session following attainment of discrimination criterion, the designation of stimuli as correct versus incorrect was reversed for each mouse (correct = marbles, incorrect = fan). Mice were trained on 30-trial daily sessions to a criterion of ≥85% correct responding (excluding correction trials) over 2 consecutive sessions. Dependent measures included sessions to criterion, total trials, percent correct responses (=100*(correct choices/total choice)), total errors, total correction trials (=corrections), and latency to choice and reward. Because reversal (unlike discrimination) is characterized by early perseveration at the previously rewarded stimulus, whereas late reversal is dominated by learning about the newly rewarded stimulus [18–20], data were also split into early and late reversal sessions (Figure 2A). This was accomplished for each mouse by averaging all sessions on which performance was <50% correct (=early) and ≥50% correct (=late). Additionally, the number of discrimination sessions was correlated with the number of trials, errors, and corrections trials in late reversal across all mice.
Figure 2: Improved late reversal learning in mutant mice lacking NR1 on DAT neurons.
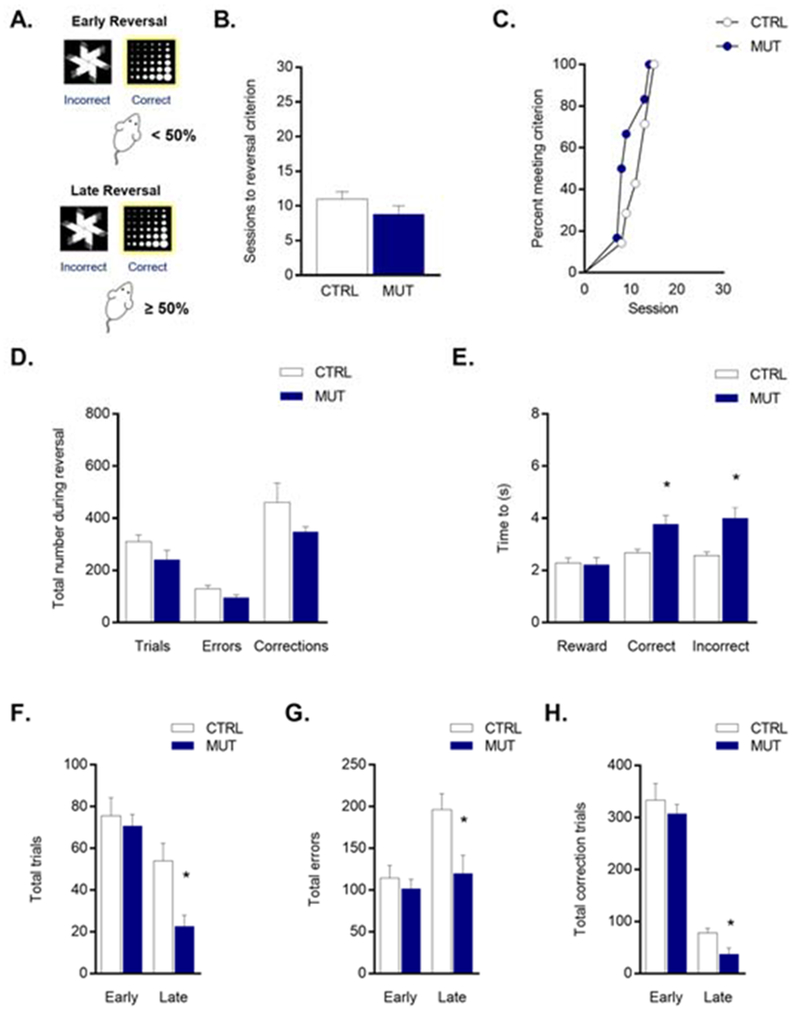
(A) Early reversal was defined as sessions with < 50% correct responding and late reversal as sessions with ≥50% correct responding. (B) Number of sessions to reach reversal criterion in control (white) and null mutant (blue) mice and (C) survival analysis of sessions to reversal criterion suggests similar performance between genotypes. (D) Total trials, errors, and correction trials completed during all reversal sessions were similar between genotypes. (E) Latency to respond for a correct choice, incorrect choice, and reward during all reversal sessions suggest null mutant mice were slower to make a response, but not to collect a reward. (F) Total trials during early and late reversal, (G) total errors during early and late reversal, and (H) total corrections trials during early and late reversal suggest improved performance in null mutant mice. Data expressed as mean ± SEM. *P <0.05 versus control
Statistical analysis
Data were analyzed with t-tests corrected for multiple comparisons with the Holm-Sidak method and Pearson correlation, as appropriate, using GraphPad Prism v7.
Results
Impaired discrimination in DAT-NR1 null mutant mice
The number of sessions necessary to reach criterion during discrimination was significantly greater in DAT-NR1 null mutant mice, as compared to non-mutant littermate controls (t12 = 2.889, P = 0.013) (Figure 1B). The impairment in the mutant mice was further demonstrated by a difference in the session-to-criterion survival curves in the genotypes (Log-rank test, χ2(1, n = 14) = 5.062, P = 0.025) (Figure 1C). There was also a significant increase in total trials in the mutant mice (t12 = 3.088, P = 0.028), but no differences in errors (t12 = 2.480, P = 0.057) or correction trials (t12 = 1.696, P = 0.116) (Figure 1D) (it should be noted that these measures may have reached the threshold for statistical significance given a larger sample size). The mutant mice performed (measured as percent correct responses) as well on the task as controls for the first 5 sessions, at which point performance of the mutants plateaued, while controls progressed to criterion. One mutant failed to meet criterion even after 30 sessions and was consequently not advanced to reversal testing. There were no statistical differences in the latency to respond (correct: t12 = 1.724, P = 0.209; incorrect: t12 = 2.031, P = 0.183) or collect the reward (t12 = 1.719, P = 0.209) between genotypes, though there was a trend for longer latencies in the mutant mice (Figure 1E).
Improved late reversal learning in DAT-NR1 null mutant mice
Genotypes did not differ in sessions (t11 = 1.373, P = 0.197) (Figure 2B), survival curves (Log-rank test, χ2(1, n = 13) = 1.947, P = 0.163) (Figure 2C), total trials (t11 = 1.620, P = 0.249), errors (t11 = 1.944, P = 0.216), or correction trials (t11 = 1.396, P = 0.249) to reversal criterion (Figure 2D). Latency to collect the reward was unchanged (t11 = 0.192, P = 0.851) but the null mutant mice were slower to make both correct (t11= 3.288, P = 0.014) and incorrect (t11= 3.714, P = 0.010) responses than controls (Figure 2E). Splitting data into early (<50% correct) and late (≥50% correct) reversal sessions, revealed that the mutant mice made fewer trials (t11= 3.110, P = 0.029) (Figure 2F) and errors (t11= 2.672, P = 0.043) (Figure 2G) than controls during late reversal, but not early reversal (trials: t11 = 0.465, P = 0.651; errors: t11 = 0.686, P = 0.507). Though the number of correction trials at late reversal were not statistically different between genotypes, this became significant (t10= 2.878, P = 0.033) on removal of 1 outlying value from the control group (Grubbs test, P < 0.05) (Figure 2H). With this outlier removed, the number of discrimination sessions completed also correlated significantly with multiple measures of performance during late reversal, including trials (r = −0.761, P = 0.004), errors (r = −0.835, P = 0.001), and correction trials (r = −0.880, P < 0.001).
Discussion
The current findings demonstrate that reductions in phasic DA release, as produced by selective deletion of the NMDA-NR1 subunit on DAergic neurons, produces an impairment in pairwise visual discrimination learning, but a facilitation of the ability to acquire a reversal of stimulus-reward contingencies. DAT-NR1 null mutant mice and their non-mutant control littermates performed equally during discrimination until performance reached about 70% correct responses (5 sessions). At this point, the mutants plateaued in their performance and were slow to attain the a priori criterion of 85% correct. These data show that NMDA receptor-mediated modulation of phasic DA is not necessary for early discrimination learning, but is important for the establishment of a high level of discrimination performance. The finding that null mutant mice can learn the basic task structure but are impaired at acquiring the discrimination is consistent with previous studies using this model of reduced DA release [3,17] as well as theories suggesting a critical role for DA in reinforcement learning [22,23].
Earlier studies have concluded that striatal DAergic signaling plays a facilitatory role in reversal learning [7–15]. Surprisingly however, although DAT-NR1 null mutant mice completed nearly twice as many trials as the controls during discrimination, there were no genotype differences in behavior during early reversal, when performance was below 50% correct. This could suggest that the strength of the discrimination memory was equivalent across the genotypes, because a weaker memory for the old contingencies would presumably have produced less perseveration to the old CS+ after their reversal [24–26].
During late reversal sessions, when correct choice was greater than 50% (likely reflecting low perseveration and new learning) null mutant mice made fewer errors and completed fewer correction trials than controls, indicative of superior performance at the late reversal stage. This result suggests facilitation of learning about the newly rewarded stimulus in mice with reduced phasic DA release and is consistent with prior reports observing impaired performance in mice with increased striatal DA levels due to genetic knockdown of the dopamine transporter (DAT) [27–29] or deletion of D2 autoreceptors [30] (but see [16]). Finally, while we did observe a longer latency to make a choice during reversal in null mutant mice, reward collection latency was unchanged, suggesting motor behaviors were preserved despite reduced phasic DA release.
There are a number of potential interpretations for the apparently contradictory pattern of impaired discrimination but improved reversal. One is that the more extensive training the mutants received during discrimination produced an overtraining effect that has been associated with faster reversal [31–33]. This possibility is supported by the observation that the number of discrimination sessions correlated negatively with the number of trials, errors, and corrections trials in late reversal. Conversely, improved reversal could be read as evidence that the discrimination memory was indeed weaker but that, for reasons that are unclear, this did not manifest as reduced perseveration earlier in testing. Another, non-exclusive, possibility is that it could stem from attenuated detection of the expectancy violations present during reversal, such that that mutants are in effect able to learn the reversed contingencies with less interference from the prior outcome expectancies. In this context, VTA DA neurons are known to be responsive (increase or decrease their firing) to aversive events, including the absence of reward [34–41], and we recently reported DA release in the VTA → NAc pathway when rewards are unexpectedly rewarded during reversal in this same task [16]. DAT-NR1 null mutant mice also exhibit reduced DA release to tail pinch and impairments when learning requires avoiding (Morris water maze) or responding to (Pavlovian fear conditioning) aversive stimuli [3,5,6], suggesting the loss of phasic DA neuronal firing might blunt the detection of negative events more generally.
This interpretation remains speculative in the absence of further studies and there are a number of other noteworthy caveats to the current dataset. First, NR1 deletion on DAT neurons reduces, but does not eliminate, phasic DA release [5] and thus any residual phasic DA signaling could obscure effects on behavior in the discrimination and reversal task. Related to this, because no measurements of altered DA release were made in the mutants as they were performing this task, we have no direct insight into the precise DAergic correlates of the behavioral abnormalities observed. Further, the mutant model studied here is not restricted to any particular DAergic neuronal pathway, which limits any inferences regarding the importance of specific circuits (e.g., cortical versus striatal) known to play dissociable roles in this task [20,42]. We also cannot rule out the possibility that reduced DA signaling in the retina could have caused visual impairments in the mutant mice that affected their performance on the task [43]. Finally, because our study was not sufficiently powered to detect sex differences in the results and there is little data regarding sex differences in reversal learning, we can discount the possibility that the effects of NR1 deletion on reversal are influenced by sex.
In sum, the current study found that reductions of phasic DA release caused by deletion of the obligatory NMDA receptor NR1 subunit on DAT-expressing cells produced significant and complex disturbances in visual discrimination and reversal. In view of previous, sometimes discrepant reports concerning the role of NMDARs in behavioral flexibility [20,26,44–55], these findings highlight the potentially distinct contributions of NMDARs in different brain regions and neuronal populations to flexible behavior and add to the literature implicating DA in a wide-range of cognitive and reward-related behavioral functions.
Highlights.
NMDARs on DAT+ neurons were genetically deleted in mice.
Mice were tested in a pairwise discrimination and reversal learning paradigm.
DAT-NR1 null mutants were impaired at discrimination compared to controls.
Mutants performed better than controls during late reversal (performance ≥ 50%).
These data add to evidence that DA is involved in a range of reward-related behaviors.
Funding sources and acknowledgements
Research supported by the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program and R01MH094536 to LSZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wanat MJ, Willuhn I, Clark JJ, Phillips PEM, Phasic dopamine release in appetitive behaviors and drug addiction, Curr. Drug Abuse Rev 2 (2009) 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lohani S, Martig AK, Underhill SM, DeFrancesco A, Roberts MJ, Rinaman L, Amara S, Moghaddam B, Burst activation of dopamine neurons produces prolonged post-burst availability of actively released dopamine, Neuropsychopharmacology. (2018). doi: 10.1038/s41386-018-0088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJY, Paladini CA, Phillips PEM, Palmiter RD, Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Parker JG, Zweifel LS, Clark JJ, Evans SB, Phillips PEM, Palmiter RD, Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 13491–13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TMK, Allen JM, Mizumori SJY, Bonci A, Palmiter RD, Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety, Nat. Neurosci 14 (2011) 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jones GL, Soden ME, Knakal CR, Lee H, Chung AS, Merriam EB, Zweifel LS, A genetic link between discriminative fear coding by the lateral amygdala, dopamine, and fear generalization, Elife. 4 (2015). doi: 10.7554/eLife.08969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ragozzino ME, The effects of dopamine D1 receptor blockade in the prelimbic–infralimbic areas on behavioral flexibility, Learn. Mem 9 (2002) 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MTL, Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting, Neuropsychopharmacology. 31 (2006) 297–309. [DOI] [PubMed] [Google Scholar]
- [9].Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A, Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice, Behav. Brain Res 171 (2006) 181–188. [DOI] [PubMed] [Google Scholar]
- [10].Lee B, Groman S, London ED, Jentsch JD, Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys, Neuropsychopharmacology. 32 (2007) 2125–2134. [DOI] [PubMed] [Google Scholar]
- [11].Haluk DM, Floresco SB, Ventral striatal dopamine modulation of different forms of behavioral flexibility, Neuropsychopharmacology. 34 (2009) 2041–2052. [DOI] [PubMed] [Google Scholar]
- [12].Laughlin RE, Grant TL, Williams RW, Jentsch JD, Genetic dissection of behavioral flexibility: reversal learning in mice, Biol. Psychiatry. 69 (2011) 1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Clarke HF, Hill GJ, Robbins TW, Roberts AC, Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus, J. Neurosci 31 (2011) 4290–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Klanker M, Sandberg T, Joosten R, Willuhn I, Feenstra M, Denys D, Phasic dopamine release induced by positive feedback predicts individual differences in reversal learning, Neurobiol. Learn. Mem 125 (2015) 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A, The neural basis of reversal learning: An updated perspective, Neuroscience. 345 (2017) 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Radke AK, Kocharian AK, Covey DP, Lovinger DM, Cheer JF, Mateo Y, Holmes A, Contributions of nucleus accumbens dopamine to cognitive flexibility, Eur. J. Neurosci (2018) 10.1111/ejn.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].James AS, Pennington ZT, Tran P, Jentsch JD, Compromised NMDA/glutamate receptor expression in dopaminergic neurons impairs instrumental learning, but not pavlovian goal tracking or sign tracking, eNeuro. 2 (2015). doi: 10.1523/ENEURO.0040-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lederle L, Weber S, Wright T, Feyder M, Brigman JL, Crombag HS, Saksida LM, Bussey TJ, Holmes A, Reward-related behavioral paradigms for addiction research in the mouse: performance of common inbred strains, PLoS One. 6 (2011) e15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Graybeal C, Bachu M, Mozhui K, Saksida LM, Bussey TJ, Sagalyn E, Williams RW, Holmes A, Strains and stressors: an analysis of touchscreen learning in genetically diverse mouse strains, PLoS One. 9 (2014) e87745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A, GluN2B in corticostriatal circuits governs choice learning and choice shifting, Nat. Neurosci 16 (2013) 1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bergstrom HC, Lipkin AM, Lieberman AG, Pinard CR, Gunduz-Cinar O, Brockway ET, Taylor WW, Nonaka M, Bukalo O, Wills TA, Rubio FJ, Li X, Pickens CL, Winder DG, Holmes A, Dorsolateral striatum engagement interferes with early discrimination learning, Cell Rep 23 (2018)2264–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wise RA, Dopamine, learning and motivation, Nat. Rev. Neurosci 5 (2004) 483–494. [DOI] [PubMed] [Google Scholar]
- [23].Berke JD, What does dopamine mean?, Nat. Neurosci 21 (2018) 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jones B, Mishkin M, Limbic lesions and the problem of stimulus—Reinforcement associations, Exp. Neurol 36 (1972) 362–377. [DOI] [PubMed] [Google Scholar]
- [25].Chudasama Y, Robbins TW, Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional …, J. Neurosci 23 (2003) 8771–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A, Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit, Learn. Mem 15 (2008)50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Del’Guidice T, Lemasson M, Etiévant A, Manta S, Magno LAV, Escoffier G, Roman FS, Beaulieu J-M, Dissociations between cognitive and motor effects of psychostimulants and atomoxetine in hyperactive DAT-KO mice, Psychopharmacology. 231 (2014) 109–122. [DOI] [PubMed] [Google Scholar]
- [28].Cybulska-Klosowicz A, Laczkowska M, Zakrzewska R, Kaliszewska A, Attentional deficits and altered neuronal activation in medial prefrontal and posterior parietal cortices in mice with reduced dopamine transporter levels, Mol. Cell. Neurosci 85 (2017) 82–92. [DOI] [PubMed] [Google Scholar]
- [29].Cybulska-Klosowicz A, Dabrowska J, Niedzielec S, Zakrzewska R, Rozycka A, Potential role of dopamine transporter in behavioral flexibility, Acta Neurobiol. Exp 77 (2017) 176–189. [DOI] [PubMed] [Google Scholar]
- [30].Linden J, James AS, McDaniel C, Jentsch JD, Dopamine D2 receptors in dopaminergic neurons modulate performance in a reversal learning task in mice, eNeuro. 5 (2018). doi: 10.1523/ENEURO.0229-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Reid LS, The development of noncontinuity behavior through continuity learning, J. Exp. Psychol 46 (1953) 107–112. [DOI] [PubMed] [Google Scholar]
- [32].Mackintosh NJ, Further analysis of the overtraining reversal effect, J. Comp. Physiol. Psychol 67 (1969) Suppl:1–18. [DOI] [PubMed] [Google Scholar]
- [33].Van Golf Racht-Delatour B, Massioui NE, Alleviation of overtraining reversal effect by transient inactivation of the dorsal striatum: ORE and dorsal striatum, Eur. J. Neurosci 12 (2000) 3343–3350. [DOI] [PubMed] [Google Scholar]
- [34].Schultz W, Dayan P, Montague PR, A neural substrate of prediction and reward, Science. 275 (1997) 1593–1599. [DOI] [PubMed] [Google Scholar]
- [35].Horvitz JC, Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events, Neuroscience. 96 (2000) 651–656. [DOI] [PubMed] [Google Scholar]
- [36].Matsumoto M, Hikosaka O, Two types of dopamine neuron distinctly convey positive and negative motivational signals, Nature. 459 (2009) 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brischoux F, Chakraborty S, Brierley DI, Ungless MA, Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Radke AK, Rothwell PE, Gewirtz JC, An anatomical basis for opponent process mechanisms of opiate withdrawal, J. Neurosci 31 (2011) 7533–7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C, GABA neurons of the VTA drive conditioned place aversion, Neuron. 73 (2012) 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N, Neuron-type-specific signals for reward and punishment in the ventral tegmental area, Nature. 482 (2012) 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lammel S, Lim BK, Malenka RC, Reward and aversion in a heterogeneous midbrain dopamine system, Neuropharmacology. 76B (2014) 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A, Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF, Nat. Neurosci 14 (2011) 1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jackson CR, Ruan G, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG. Retinal dopamine mediates multiple dimensions of light-adapted vision, J. Neurosci 32 (2012) 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Palencia CA, Ragozzino ME, The influence of NMDA receptors in the dorsomedial striatum on response reversal learning, Neurobiol. Learn. Mem 82 (2004) 81–89. [DOI] [PubMed] [Google Scholar]
- [45].Brigman JL, Ihne J, Saksida LM, Bussey TJ, Holmes A, Effects of Subchronic Phencyclidine (PCP) Treatment on Social Behaviors, and Operant Discrimination and Reversal Learning in C57BL/6J Mice, Front. Behav. Neurosci 3 (2009) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dix S, Gilmour G, Potts S, Smith JW, Tricklebank M, A within-subject cognitive battery in the rat: differential effects of NMDA receptor antagonists, Psychopharmacology. 212 (2010) 227–242. [DOI] [PubMed] [Google Scholar]
- [47].Dalton GL, Ma LM, Phillips AG, Floresco SB, Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning, Psychopharmacology. 216 (2011) 525–535. [DOI] [PubMed] [Google Scholar]
- [48].Ding X, Qiao Y, Piao C, Zheng X, Liu Z, Liang J, N-methyl-D-aspartate receptor-mediated glutamate transmission in nucleus accumbens plays a more important role than that in dorsal striatum in cognitive flexibility, Front. Behav. Neurosci 8 (2014) 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Svoboda J, Stankova A, Entlerova M, Stuchlik A, Acute administration of MK-801 in an animal model of psychosis in rats interferes with cognitively demanding forms of behavioral flexibility on a rotating arena, Front. Behav. Neurosci 9 (2015) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Radke AK, Nakazawa K, Holmes A, Cortical GluN2B deletion attenuates punished suppression of food reward-seeking, Psychopharmacology. 232 (2015) 3753–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].McAllister KAL, Mar AC, Theobald DE, Saksida LM, Bussey TJ, Comparing the effects of subchronic phencyclidine and medial prefrontal cortex dysfunction on cognitive tests relevant to schizophrenia, Psychopharmacology. 232 (2015) 3883–3897. [DOI] [PubMed] [Google Scholar]
- [52].Thompson SM, Josey M, Holmes A, Brigman JL, Conditional loss of GluN2B in cortex and hippocampus impairs attentional set formation, Behav. Neurosci 129 (2015) 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Radke AK, Jury NJ, Delpire E, Nakazawa K, Holmes A, Reduced ethanol drinking following selective cortical interneuron deletion of the GluN2B NMDA receptors subunit, Alcohol. 58 (2017) 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, Holmes A, Chronic EtOH effects on putative measures of compulsive behavior in mice, Addict. Biol 22 (2017) 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jury NJ, Radke AK, Pati D, Kocharian A, Mishina M, Kash TL, Holmes A, NMDA receptor GluN2A subunit deletion protects against dependence-like ethanol drinking, Behav. Brain Res (2018). doi: 10.1016/j.bbr.2018.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


