Abstract
Advances in the treatment of heart failure with a reduced ejection fraction (HFrEF) due to systolic dysfunction are engaging an ever-expanding compendium of molecular signaling targets. Well established approaches modifying hemodynamics and cell biology by neurohumoral receptor blockade are evolving, exploring the role and impact of modulating intracellular signaling pathways with more direct myocardial effects. Even well tread avenues are being reconsidered with new insights into the signaling engaged and thus opportunity to treat underlying myocardial disease. This review explores therapies that have proven successful, those that have not, those that are moving into the clinic but whose utility remains to be confirmed, and those that remain in the experimental realm. The emphasis is on signaling pathways that are tractable for therapeutic manipulation. Of the approaches yet to be tested in humans, we chose those with a well-established experimental history, where clinical translation may be around the corner. The breadth of opportunities bodes well for the next generation of heart failure therapeutics.
Keywords: heart failure, signaling pathways, therapy, pharmacology, epigenetics
Introduction
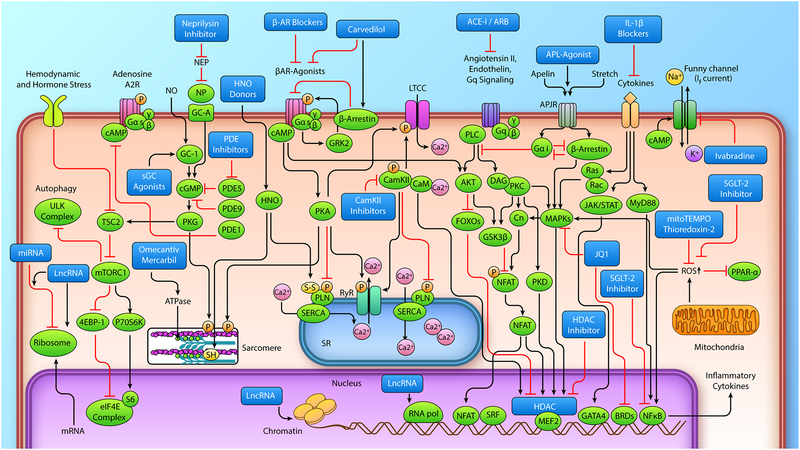
The past few decades have witnessed transformative progress in the treatment of heart failure (HF) with depressed systolic function, but persistent societal and economic burdens from heart failure cry out for even better therapeutic arrows in our quiver. Current therapies encompass small molecules, biologicals, implantable devices, and heart transplantation, as summarized in the clinical stage-specific practice guidelines1, 2 and Supplemental Table 1. In this review, we focus on molecular therapeutic targets for heart failure – past, present, and future. A number of signaling pathways a number of which were. Along the way, we highlight the successes and failures, and discuss approaches making their way from animals to humans. We organized this discussion into four major sections – evidence-based therapies currently in use, new therapies undergoing clinical trials, approaches that have been largely abandoned due to disappointing clinical data, and new avenues being pursued in experimental studies that may launch to humans in the relatively near future. The scope of this effort is encapsulated in Figure 1–which highlights the various signaling pathways engaged, and identifies the therapeutic target in use or currently under study for treating HF. The reader is referred to a review issue from 2011 that covers additional pathways3. We hope the current update offers a succinct survey for both scientist and physician to appreciate what we have achieved and where we need to go.
Figure 1. Myocyte signaling approaches for the treatment of Heart Failure with Reduced Ejection Fraction.
Individual therapeutic approaches are identified in the figure by the dark blue boxes. Abbreviations are: miRNA, micro ribonucleic acid; mRNA, messenger ribonucleic acid; lncRNA, long noncoding ribonucleic acid; PI3K, phosphoinositide 3-kinase; TSC2, tuberous sclerosis complex-2; mTORC1, mechanistic target of rapamycin complex 1; 4EBP-1, eukaryotic translation initiation factor 4E binding protein 1; p70S6K, ribosomal protein S6 kinase; eIF4E, eukaryotic translation initiation factor 4E; S6, ribosomal protein S6; ULK, UNC-51 like kinase; NO, nitric oxide; NP, natriuretic peptide; ANP, atrial natriuretic peptide; sGC, soluble guanylate cyclase; cGMP, cyclic guanosine monophosphate; PDE, phosphodiesterase; PKG, protein kinase G; PKC, protein kinase C; PKD, protein kinase D; SH, thiol modification; S-S, disulfide; DAG, diacylglycerol; HNO, nitroxyl; β-AR, β-adrenergic receptor; cAMP, cyclic adenosine monophosphate; GRK2, G protein-coupled receptor kinase 2; PKA, protein kinase A; PLN, phospholamban; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; FOXO, forkhead box O; SR, sarcoplasmic reticulum; RyR, ryanodine receptor; LTCC, L-type calcium channel; CAMKII, calcium-calmodulin-dependent kinase II; CaM, calmodulin; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; APL-agonist, apelin agonist; IL-1β, interleukin-1β; GSK3-β, glycogen synthase kinase 3-β; NFAT, nuclear factor of activated T cells; MAPKs, mitogen-activated protein kinase; SRF, serum response factor; MEF2, myocyte enhancer factor 2; HDAC, histone deacetylase; GATA4, GATA binding protein 4; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; RNA pol, RNA polymerase; SGL-T2 inhibitors, sodium-glucose transporter 2 inhibitors; PPAR-α, peroxisome proliferator activated receptor-α; MyD88, myeloid differentiation primary response gene 88; JAK, janus kinase; STAT, signal transducer and activator of transcription; Gsα, stimulatory G protein α-subunit, Gqα, G protein-q α-subunit; PLC, Phospholipase C; P, phosphorylation modification; ROS, reactive oxygen species. (Illustration Credit: Ben Smith).
Beta-adrenergic blockers
Heart failure is associated with hyper-activation of the sympathetic nervous system mediated largely by epinephrine and norepinephrine, in an effort to sustain cardiac output.4 While appropriate for acute responses, sustained “hyperadrenergic” drive contributes to myocardial toxicity and HF. Identification of different adrenergic receptors dates back nearly 80 years when Raymond Ahlquist named β-adrenergic receptors as those mediating vasodilation, uterine relaxation, and “excitatory” effects of epinephrine in the heart.5
The β1-adrenergic receptor (β1AR) is the most common in the normal mammalian heart (80%), the remaining being mostly β2-receptors (β2AR). However, β1-receptor density declines in HF by 60% while β2-density is unchanged. β1-receptors primarily couple to the protein Gαs, activating adenylyl cyclase to generate cAMP and thus stimulate protein kinase A (PKA). PKA phosphorylation of L-type Ca channels, myosin binding protein C, phospholamban, troponin I, titin, and other proteins involved with excitation-contraction result in greater contractility, heart rate, diastolic distensibility, and relaxation.6 Genetically induced excess β1AR expression (15 fold over baseline) causes spontaneous heart failure,7 whereas its suppression impedes chronic myocardial toxicity. A widely used beta-blocker - carvedilol (a β1,β2, α1 AR blocker) confers additional properties, in that its binding to the β1AR induces a conformational change favoring coupling to inhibitory G-protein - Gαi rather than stimulatory Gαs. This in turn activates the β-arrestin pathway in a process known as biased agonism, transactivating extracellular growth factor receptor and promoting activation of mitogen activated kinase ERK1/2 as well as Akt kinase to confer cardioprotection.6, 8 The biased agonism features of carvedilol have been demonstrated in vitro and in vivo in mice9.
The β2- receptor primarily engages Gαs but also Gαi through a mechanism dependent on PKA. Gαi activation inhibits adenyl cyclase, counteracting Gαs. While β1AR stimulation results in more diffuse cytosolic cyclic adenosine monophosphate (cAMP) generation, β2AR stimulation induces plasma membrane-localized pools, though this compartmentation is reduced in HF leading to more diffuse β2AR-cAMP.10 Engineered mice are able to tolerate cardiac overexpression of β2AR up to 60 fold (vs. less than 15 fold of β1AR)7 without developing heart failure, with cardiomyopathy manifesting at 100 fold expression level.11 β2AR also exhibits biased agonism, with β-arrestin modification and transactivation signaling.6
HF results in marked desensitization of βARs, particularly β1AR, decreasing myocardial responses to catecholamines, but not to the point of preventing the noxious effects of the hyperadrenergic state. The cause is multifactorial, involving reduced receptor expression, uncoupling of β1AR and β2AR from cAMP-signaling by GRK2 and GRK5 phosphorylation, β-arrestin activation, receptor internalization, and enhanced Gαi coupling.6 βAR blockers counteract the detrimental effects of the hyper-adrenergic state, in spite of the apparent paradox that the β-adrenergic signaling is already downregulated. Even though β1AR are downregulated by around 60% in the failing heart, cardiomyocytes are still under toxic hyper-adrenergic stimulation12, 13. Thus, further gentle inhibition of β1AR signaling with β1 blockers is protective, associated with enhanced downstream signaling, organ responsiveness to acute catecholamines, and increased expression of β1AR. Restoration of plasma membrane β1AR density and β1AR/β2AR ratio can confer additional effects.14 This enhanced signaling means that on-demand catecholamine response can be more sensitively transduced while competing with the AR blocker at the receptor level. Chronic β-blockade also reverses a pathological gene expression program, increasing the expression of α-myosin heavy chain, decreasing β-myosin heavy chain, and increasing SERCA2a expression.15 Moreover, βAR blockers have beneficial hemodynamic effects, decreasing cardiac work, afterload, and oxygen consumption.16
Multiple studies of βAR blockade in HFrEF patients have revealed reduced hospital admission, improved quality of life, slowed disease progression, and reduced mortality. Though initially, concerns were raised that βAR blockade would result in functional decompensation in patients with LV dysfunction,17 studies pioneered by Waagstein and colleagues using the cardioselective β-blocker practolol showed the opposite.18 These agents are now strongly recommended as first line therapy after a myocardial infarction to prevent HF development. Three βAR blockers have reduced mortality in heart failure in large, controlled clinical trials: bisoprolol,19 metoprolol succinate (long acting),20 and carvedilol.21 While meta-analysis has tried to determine if one approach is superior to another, the issue remains unclear. Carvedilol has additional vasodilator effects that could be helpful but also are less well tolerated in patients with low blood pressure. Whether it provides added value from biased agonism is unknown.
Inhibitors of the renin-angiotensin-aldosterone system (RAAS)
Efforts to enhance cardiac performance by reducing systemic vascular resistance started in the 1980s, first using a combination of isosorbide dinitrate and hydralazine, and later angiotensin-converting enzyme-inhibitors (ACE inhibitors). Prior basic research had shown RAAS signaling is upregulated in HFrEF and key to maintaining blood pressure in acute HF and to plasma volume expansion in chronic HF.22 Both plasma renin activity and aldosterone were shown to rise in chronic advanced HF patients.23 Importantly, whereas various vasoactive agents existed to lower cardiac afterload, targeting the RAAS system proved most effective long term. Cardio-protection by RAAS antagonism suppresses this pathway in both general circulation and in the myocardium itself,24 the latter also reducing pathological hypertrophy, fibrosis, and dysfunction25 via effects on both cardiomyocytes and fibroblasts.25 This is primarily linked to Gαq-coupled angiotensin type 1 receptor (AT1R) signaling.26
ACE inhibition is an example of a HF-therapy eureka moment, as the CONSENSUS trial testing enalapril found such a marked decline in absolute 6 month mortality (44% to 26% in severe HF patients) that it was terminated early, after enrolling just 253 patients.27 It took nearly 30 years to move beyond this in a trial involving >8000 patients (discussed next). The ACE inhibitor Enalapril reduces mortality and hospitalizations in patients with symptomatic and asymptomatic LV dysfunction. Similar efficacy is obtained by blocking AT1R28 and by aldosterone receptor29 blockers. Two downstream cleavage products of angiotensin II, angiotensin 1–7 and angiotensin 1–9, have beneficial effects in the heart.30, 31 While this suggested angiotensin receptor blockers (ARBs) might be superior to ACE inhibitors, the data does not support this. Generation of these cleavage products by non ACE-dependent mechanisms as well as lack of ACE2 suppression by current ACE inhibitors may explain this.
Aldosterone is a key component of the RAAS that is secreted from the adrenal cortex in response to angiotensin II. In addition to augmenting sodium reabsorption in the distal convoluted tubule, aldosterone promotes vascular and myocardial hypertrophy, fibrosis, inflammation, endothelial dysfunction, and oxidative stress. Its receptor is upregulated in cardiomyocytes from HFrEF patients,32 and aldosterone antagonists improve ventricular remodeling, heart failure outcomes, and sudden cardiac death.33 The mechanisms of this antiarrhythmic effect include reduced extracellular matrix deposition, reduced ventricular remodeling, and direct effects on intracellular calcium signaling in cardiomyocytes.34
Combining neprilysin with AT1R inhibition
Neutral endopeptidases (NEP) proteolytically modify and degrade proteins, including those secreted into the bloodstream. One target is the natriuretic peptides, and the concept that blocking NEP might enhance natriuretic activity took shape about 35 years ago.35 Neprilysin is a membrane bound zinc-dependent endopeptidase with >50 substrates, that was first discovered in the proximal renal tubule, and later in the brain where it metabolizes endogenous opioids. It cleaves substrates in multiple organ systems, with cardiovascular effects potentially related to its degradation of natriuretic peptides (atrial natriuretic peptide, ANP>>brain natriuretic peptide, BNP), adrenomedullin, substance P, and bradykinin, which work together to cause vasodilation. Studies in animal models and humans demonstrated an increase in circulating and urinary cyclic guanosine monophosphate (a consequence of augmented natriuretic peptide signaling36) after neprilysin inhibitor treatment.35, 37 Serum BNP levels have also been seen to rise in some trials, whereas NT-proBNP which is not targeted by NEP generally declines, likely reflecting reduced cardiac volumes.38–40 Caution is needed interpreting the BNP change as the assay can also react with its cleavage products41, and ANP which is anticipated to be more impacted by NEP inhibition, remains to be measured. NEP also cleaves angiotensin and bradykinin, and their rise upon NEP inhibition can cause adverse side effects, including angioedema,38 and why AT1R blockers are concomitantly employed. Angiotensin is also cleaved into two subfragments, angiotensin 1–7 and angiotensin 1–9, which have beneficial effects in the heart,30, 31 and NEP inhibition may also increase these to provide further benefit than just a reduction in natriuretic peptide clearance.42 The pivotal trial reported in 2014 testing a combined AT1R blocker (valsartan) and neprilysin inhibitor (sacubitril) versus an ACE inhibitor was conducted in >8000 HF patients with moderate disease. It found a 20% relative reduction and 4.7% absolute reduction in cardiovascular death or heart failure hospitalization.38 Subsequent studies have noted a reduction in arrhythmia burden, though the exact mechanism beyond improved ventricular remodeling is unknown.43 The drug is formed by attaching the component molecules together with an inert linker that is cleaved, resulting in 1:1 stoichiometry. Valsartan is present to suppress potential detrimental effects from elevated angiotensin II levels due to sacubitril. Subsequent studies have confirmed beneficial effects and this combination is rapidly becoming a standard of care.
Combination isosorbide dinitrate and hydralazine: Rationale in a sub-population
Combination therapy with nitrates and hydralazine in chronic heart failure was first developed to achieve the hemodynamic benefits of both preload and afterload reduction seen with sodium nitroprusside in acute heart failure.44 Postulated mechanisms include stimulation of nitric oxide (NO) signaling and lowering oxidative stress by hydralazine; though these hypotheses have not been proven. The Vasodilator-Heart Failure Trial (V-HeFT I) was the first randomized, placebo-controlled study of hydralazine and isosorbide dinitrate (ISDN) compared to prazosin or placebo, and showed a mortality benefit in HF patients.45 V-HeFT II compared enalapril to combination hydralazine-ISDN finding a borderline mortality benefit favoring enalapril (p=0.08).46 However, subgroup analysis of both studies identified self-described black patients as having a greater mortality benefit with hydralazine-ISDN vs enalapril,47 and this was subsequently confirmed in a trial of self-described black HF patients (A-HeFT).48 Why this applies only to this self-described racial cohort remains uncertain. Theories include more reduced NO and greater oxidative/nitrosative stress in these patients49 coupled to a higher prevalence of SNPs affecting endothelial nitric oxide synthase50 and G-protein beta-3 subunit.51 Despite being a class I recommendation in clinical guidelines, hydralazine-ISDN remains markedly underutilized in this population,52 potentially due to poor tolerability, the inconvenience of 3× daily dosing, and even social caution over race-specific therapy.
Ivabradine: Controlling heart rate alone
Elevated heart rate is viewed as a biomarker of HF severity caused by hyper-sympathetic stimulation.53 Thus, much like βAR blockade first did, purposely slowing heart rate would seem imprudent. Development of If (funny channel) blockers such as ivabradine allowed this hypothesis to be tested, and conventional wisdom was wrong - again. If current is composed of K+ and Na+ flux, and the channels are hyperpolarization-activated and cyclic nucleotide-gated (HCN4).54 Ivabradine blocks If intracellularly, reducing diastolic depolarization rate of pacemaker cells to slow the heart rate.54 As it blocks the channel in its open state, it is more potent at higher heart rates.54 Since it has no impact on intrinsic contractility (beyond that regulated by heart rate), it has been considered in HF patients intolerant to βAR-blockers. In clinical trials, ivabradine improved clinical outcome in moderate-severe HFrEF patients with HR ≥70 bpm on standard therapy, reducing cardiovascular death or hospitalization by 18% after ~2 years55. However, only 26% of subjects had received appropriate βAR-blocker doses, so similar outcomes might arise with β-blocker up-titration. Current guidelines recommend ivabradine to reduce HF-related hospital admissions in patients with resting HR>70 bpm, with stable HFrEF on standard medical therapy, or who are intolerant to or contraindicated for βAR-blockade. However, patients intolerant to βAR-blockers are often sicker than those studied in the ivabradine trials, so the precise sweet spot for this drug remains to be clarified.
Sodium-glucose cotransporter 2 (SGLT2) Inhibitors: Oral anti-diabetic drugs with serendipitous cardiovascular effects
SGLT2 belongs to the 6-member SLC5 family of sodium-dependent glucose cotransporters. The low-capacity, high-affinity Na-glucose co-transporter, SGLT1, is mainly expressed in the gastrointestinal tract, whereas the high-capacity, low-affinity transporter, SGLT2, is expressed primarily in the kidney in the brush border of the proximal tubule, where it controls 90% of glucose reabsorption from the urine.56 Inhibition of SGLT2 induces glucosuria, associated with hemoglobin A1c reduction of 0.7–1.0% and weight loss of 2–3 Kg in patients with type-2 diabetes (T2D). Despite a seemingly strong scientific basis for benefits of anti-glycemic agents on the failing heart, SGLT2 inhibitors were the first that indeed also reduced HF hospitalization and cardiovascular death in patients with T2D.57 This discovery followed a series of studies with other T2D therapies that yielded neutral results or posed risk concerns. In what started out as another standard FDA-mandated cardiovascular safety trial – inhibiting SGLT2 conferred very surprising beneficial effects. The EMPA-REG OUTCOME trial58 of 7020 patients with T2D and a high risk of CV events, showed those randomized to empagliflozin versus placebo had a substantial decline in the composite outcome (cardiovascular death, myocardial infarction, or stroke), driven primarily by a decline in cardiovascular death. Emplagliflozin also lowered all-cause mortality and hospital admissions for heart failure, worsening of renal function and need for renal replacement therapy. Systolic blood pressure declined ~5 mmHg. There have been several follow-up trials, notably the 17160 subject DECLARE–TIMI 5859 study of dapagliflozin, another SGLT2 inhibitor, also in T2D patients with increased CVD risk, that found active therapy also reduced the composite outcome of cardiovascular death or hospitalization for heart failure, being driven primarily by the latter.
These data are remarkable not only for the magnitude of the effects observed, but also for the lack of clarity regarding the mechanisms underlying them. SGLT2 inhibitors confer anti-inflammatory effects, reduce oxidative stress, improve endothelial function, and modulate neurohormonal pathways in experimental models.60 They may also reduce plasma volume via natriuresis and lower blood pressure by this and/or direct vasodilation mechanisms. However, such hemodynamic effects should be achievable with existing diuretics and vasodilators. Other mechanisms include shifting cardiac energy substrate to favor fatty acids and ketone bodies. While SGLT2 is not expressed at the protein level in cardiomyocytes, its inhibition is associated with reduced myocyte shortening, L-type Ca2+ channel currents61 and inhibition of the Na+/H+ exchanger-1 in isolated cardiomyocytes.62 These would depress myocyte function, so hard to reconcile with better HF survival. More work is clearly needed.
New approaches under active clinical investigation
Small molecule sarcomere activators to enhance contractility
Current clinically approved positive inotropes (e.g. dobutamine, milrinone) increase intracellular calcium, energy demand, and arrhythmia risk by raising cAMP levels in the myocyte. Levosimendan also elevates cAMP, but has additional effects, impacting troponin C to enhance sarcomere force-calcium responsiveness, and vasodilating by ATP-sensitive potassium channel agonism. For nearly 3 decades, efforts have been made to avoid calcium and cAMP pathways and instead enhance sarcomere function directly, but their story lines are twisty with many dead ends, most due to worsening diastole or poor pharmacological properties. Omecamtiv Mercarbil (OM, Cytokinetics, San Francisco, CA) represents the most recent and to date most promising sarcomere enhancer.63 OM binds specifically to cardiac myosin at the ATP-ase catalytic domain, increasing ATPase activity, and favoring formation of force-generating crossbridges.63 This increases contractility while simultaneously prolonging systole, but does not alter intracellular calcium, and demands less oxygen consumption than with βAR-agonists. Phase 2 studies report improved cardiac function,64 and while some safety concerns have been raised regarding low level increases in circulating troponin-I, the overall safety profile was sufficient to move onto the current pivotal trial (GALACTIC-HF, NCT02929329, Phase 3, Recruiting). This trial should be complete in early 2021. Other myosin activators, e.g. MYK-491 (MyoKardia, Inc., San Francisco, CA) have recently entered Phase 1b/2a studies in stable HFrEF patients (NCT03447990).
Cyclic GMP-targeted Phosphodiesterase (PDE) inhibitors: PDE1 and PDE9
The phosphodiesterases (PDEs) are an 11-member superfamily of enzymes expressed as ~100 isoforms, that hydrolyze cAMP and/or cyclic guanosine monophosphate (cGMP) with varying selectivity.65 Differential expression of each PDE in various cell types provides further specificity for their effects. PDE3 inhibitors were explored for HFrEF (e.g enoximone, milrinone), to elevate cAMP. These inhibitors confer positive inotropic, chronotropic, and vasodilatory effects, but they remain in limited clinical use due to worsened HF mortality with chronic therapy,66 and arrhythmia concerns.
Attention has also turned to cGMP signaling which allosterically activates protein kinase G (PKG) by binding to regulatory domains in its N-terminus, disinhibiting the C-terminus catalytic domain. PKG’s primarily role in CV disease is known as vasodilation, yet many studies have revealed potent intracellular signaling capable of blunting pathological hypertrophy, fibrosis, ischemic disease, and mitochondrial toxicity.65 Signaling is transduced by the phosphorylation and inactivation of the non-selective transient receptor potential cation channel – TRPC6,67 stimulation of regulator of G-protein signaling (RGS2 and RGS4)68 to block Gαq-coupled signaling, enhancing proteosome activity,69 and most recently, TSC2 (tuberin) to potently control activation of the mechanistic target of rapamycin – complex-170. Sarcomere proteins modified by PKG include troponin I to enhance relaxation, myosin binding protein C that impacts adrenergic-stimulated contractile reserve, and titin to increase diastolic distensibility.65
Once generated, cGMP hydrolysis is selectively regulated by PDE5 and PDE9, and may be further impacted by several dual-substrate esterases - PDE1, PDE2, and PDE3 under the right conditions.71 PDE5 has been the most studied cGMP-PDE so far, and its inhibitors (e.g. sildenafil, tadalafil) are used to treat pulmonary hypertension and erectile dysfunction. Despite substantial experimental data supporting PDE5 inhibition for HF indications,71 clinical translation remains uncertain. Multiple small single-center trials in HFrEF patients reported improved exercise physiology, capacity, pulmonary pressures, and associated hemodynamics, from PDE5 inhibition.72 However, there is no large-scale multicenter HFrEF trial, and a ~200 patient study of HFpEF did not support benefit, and has left its fate uncertain73.
The other cGMP-selective phosphodiesterase – PDE9 – has recently moved forward as an inhibitor target in clinical HF trials. This PDE has the highest affinity for cGMP, and is expressed at the mRNA level mostly in the brain and gut, with some in kidney, and low levels in skeletal muscle, fat, and the heart.74 Its potential involvement with HF was first revealed in a 2015 study where myocardial PDE9A expression was found to increase in HFrEF (and HFpEF), and its genetic or pharmacological inhibition improved heart function, reduced hypertrophy and fibrosis in pressure-overloaded mouse hearts.75 This study also revealed PDE9 favors hydrolysis of cGMP generated by natriuretic peptide signaling, in contrast to PDE5 that controls cGMP coupled to nitric oxide signaling. PDE9 inhibition remains an effective means of countering hypertrophic heart disease even when NO synthase is blocked, whereas PDE5 inhibition is ineffective in this setting. While inhibiting either stimulates PKG, the phospho-kinome that is modified contains many different targets.75 More strikingly, there are marked disparities in microRNA changes, with PDE5 inhibition reducing many miRNAs enhanced by pressure-overload, whereas PDE9 inhibition alters essentially none76. This is despite the fact that cardiac improvement is very similar with each treatment – reduced hypertrophy and fibrosis, and enhanced function. The reliance of PDE5 inhibitor efficacy on NO-cGMP signaling may have sex-specific therapy implications, as PDE5 inhibitors fail to counter heart disease in female mice following ovariectomy. Their benefit is restored by estrogen replacement, highlighting a key role of estrogen-dependent NO signaling to PDE5 regulation.77 Clinical trials of a PDE9 selective antagonist (CRD-733) for HFrEF began in late 2018.
Yet another PDE whose inhibition recently entered clinical trials for HFrEF is PDE1. This is a dual-substrate Ca2+-calmodulin activated esterase, expressed by three genes to yield isoforms with different substrate selectivity. The heart expresses PDE1A and PDE1C, the former being >20× more avid for cGMP over cAMP, whereas the latter has equal selectivity. This is likely important for human translation, as mice and rats mostly express PDE1A, whereas larger mammals such as rabbits, dogs, and humans express mostly PDE1C.78 In a recent preclinical study, a highly selective PDE1 inhibitor (ITI-214, Intracellular Therapies, Inc. NY) was tested in conscious dogs and anesthetized rabbits. In both models, the compound produced positive inotropic, lusitropic, chronotropic effects, and arterial vasodilation.78 Similar changes with much less heart rate rise were observed in dogs with HFrEF. PDE1 inhibition appears to impact a cAMP pool different to that coupled to βAR or PDE3 regulation. Unlike cilostamide (PDE3-inhibitor), ITI-214 did not enhance myocyte force or calcium transients in cells pre-stimulated with isoproterenol, but did impact signaling linked to adenosine type 2b receptors. These adenosine receptors are important regulators of ischemia-reperfusion injury as well as linked to cAMP-regulated contraction.78 A link to adenosine receptor signaling is supported by another study of ischemia-induced apoptosis in myocytes.79 Much more work is needed to dissect where PDE1 regulated cAMP signaling occurs, what exactly lies downstream, and what other features differentiate it from other methods to enhance cAMP. As ITI-214 had already passed Phase I human safety studies, a clinical Phase Ib-IIa HFrEF study was started in the fall of 2018 (NCT03387215) to assess safety and cardiac/hemodynamic effects of a single dose.
Nitroxyl (HNO) – combined inotropy, lusitropy, and vasodilation.
Angello Angeli’s salt, the first known nitroxyl (HNO) donor was described in 1896. Unlike Alfred Nobel, whose contemporaneous discovery of nitroglycerin quickly led to a highly remunerative business in explosives, Angeli’s salt had to wait more than a century before it found translational utility. Although HNO is chemically similar to the more famous nitric oxide, its biological effects differ substantially. HNO is considered highly electrophilic and avidly reacts with negative charged thiol groups (thiolates) of proteins, a key feature of its biological effects. It reversibly converts thiol to disulfide residues and/or to sulfinamides (less reversibly). HNO also has antioxidant properties by reacting with oxidizing radicals to generate NO.80 HNO-induced vasorelaxation requires modification of soluble guanylate cyclase81 much like NO; however its augmentation of contractility is cGMP/sGC/PKG and cAMP/PKA independent, and not impacted by βAR blockade. Rather, HNO enhances contractility by increasing Ca2+ cycling through the sarcoplasmic reticulum by generating an intermolecular di-sulfide in phospholamban to disinhibit SERCA2a, increasing the open probability of the ryanodine receptor (RyR2), and enhancing myofilament calcium sensitivity coupled to thiol modification of tropomyosin, actin, and myosin light chain.82–85 Angeli’s salt is very unstable, and the search for room temperature-stable HNO donors that could serve as pharmaceuticals began in earnest in 2005. These molecules were tested in experimental animals and humans, confirming positive inotropy, lusitropy, and vasodilation effects.86, 87 A third generation HNO donor BMS-986231 was tested in acute HF, where the compound rapidly and sustainably lowered pulmonary capillary pressure while improving cardiac index, without altering heart rate, or inducing arrhythmia, hypotension, or other significant adverse events.88 Ongoing Phase 2B trials (NCT03357731, NCT03016325, NCT03730961) are further testing its clinical efficacy.
Soluble guanylate cyclase stimulators/activators
Activation of the NO-cGMP pathway using NO donors, organic or inorganic nitrates or nitrites are being studied for HFrEF and HFpEF. They have limitations due to tolerance or requiring enzymatic conversion for efficacy, as well as oxidant stressors that impair the capacity of NO to generate cGMP and activate PKG. An alternative approach is to stimulate soluble guanylyl cyclase (sGC) directly to generate cGMP. NO interacts with the reduced heme at the proteins central core to catalyze cGMP synthesis from GTP, and cGMP in turn activates PKG. While circumventing inadequate NO-stimulated cGMP, this approach does not mimic other NO effects from S-nitrosation of proteins and anti-oxidant chemistry.89
A class of sGC activators (ataciguat and cinaciguat) or stimulators (riociguat and vericiguat) have been developed, extensively tested in various experimental models, and in initial clinical trials. The activators are heme-independent but also lack synergistic stimulation by NO. The stimulators regulate the cyclase allosterically, allowing for synergistic effects upon NO binding to heme.89 The activators have very potent vasodilator effects and trials of one of these, cinaciguat, in HFrEF patients were terminated early due hypotension and lack of benefit.89, 90 However, the stimulator vericiguat was also tested in HFrEF patients, and while the study missed the primary end-point (reducing N-terminal pro-BNP levels), high doses suggested potential lowering of cardiovascular death and hospitalization rates.91 This spawned the ongoing Phase III VICTORIA trial testing vericiguat in HFrEF (NCT02861534). The other stimulator, riociguat, is approved for chronic thromboembolic pulmonary hypertension.
Apelin
Apelin (APJ)a was first identified in 1993 as an orphan G-protein coupled receptor. Its amino acid sequence is similar to AT1R but does not bind angiotensin.92 Its ligand apelin was discovered in 1998, and is an adipokine synthesized as a 77 amino acids pre-propeptide. Pyr1-apelin-13 has a high affinity for the APJ receptor and is the main isoform in the heart.92 The APJ receptor works as a bifunctional transducer. On one hand, it behaves as a mechanosensor to stimulate hypertrophy, as APJ-null cardiomyocytes display less hypertrophy to stretch stimuli, and whole hearts less hypertrophy following transverse aortic constriction (TAC). The load-induced hypertrophic response is G-protein independent but β-arrestin dependent. On the other hand, apelin binding activates Gαi, blunting stretch-induced hypertrophy initiated by the same receptor. This response is bidirectional as stretch also reduces G-protein signaling triggered by apelin binding the APJ receptor.93 Apelin also blunts hypertrophic responses to other GPCR agonists such as angiotensin II.94 Thus, its regulatory role is rather complex. In addition, apelin stimulates contractility, and this can be blocked by inhibiting phospholipase C (PLC), protein kinase C, Na+/H+ exchanger, and Na+/Ca2+ exchanger,95 much like Gαq-coupled signaling via PLC. Apelin increases force more in isolated failing than normal cardiac muscle due to increased intracellular Ca2+ and not alterations in myofilament Ca2+ sensitivity.96 Several apelin agonists have been used with some success in vitro and in vivo.97 Novartis is currently undergoing a phase-2 trial of CLR325 for HFrEF (NCT02696967), and assessing hemodynamic effects in patients with obesity and diabetes (NCT03449251). Amgen also has developed a small molecule apelin analog AMG 986, and is conducting studies to assess acute and chronic hemodynamic effects (NCT03276728).
Anti-inflammatory targets: IL-1β
Anti-inflammatory therapy started in earnest with efforts to block tumor necrosis factor alpha (TNF-α) as its elevation in HFrEF98 was thought to contribute to chamber dysfunction.99 However, the chimeric TNF-α neutralizing antibody (infliximab) provided no clinical benefit despite a modest rise in EF, and at higher doses, increased death or hospitalization from HF.100 Etanercept, a TNF-α receptor-decoy to bind circulating TNF-α also failed to show clinical benefit.101 Blockade of interleukin-6, TGF-β, and interleukin-18, have yielded mixed results, and broad immunosuppression with corticosteroids, intravenous immunoglobulin, cyclosporine, and methotrexate have been unsuccessful.99, 102 Interleukin-1, whose main circulating form is IL-1β, is a major acute and chronic pro-inflammatory chemokine. Following acute myocardial infarction, injured cells release the precursor peptide that is activated by assembly of the inflammasome. IL-1β is directly implicated in impaired systolic function by uncoupling adenyl cyclase from the βAR, reducing L-type Ca2+channel current, and by downregulating phospholamban and SERCA2a, among other mechanisms.103 Multiple animal studies have shown improved outcome after myocardial infarction (acute and more chronic) using various antibody approaches to neutralize the IL-1β stimulus (reviewed in103). In humans a pooled analysis of two small trials showed that blocking the IL-1 pathway with an IL-1 receptor antagonist (anakinra) early after myocardial infarction decreased the incidence of heart failure without a significant change in the risk of recurrent myocardial infarction104. The CANTOS trial reported in 2017 randomized 10061 subjects with stable coronary disease, a history of MI, and elevated high-sensitivity C-reactive protein (hsCRP) as a biomarker for inflammation, testing the role of canakinumab – a monoclonal antibody against IL-1β - versus placebo. Treatment lowered the rate of recurrent MI, though this came with a higher risk of fatal infection.105 While not specifically powered for HF outcomes, a sub-group analysis found subjects hospitalized for HF on active treatment had lower levels of hsCRP that correlated with reduced hospitalization rate, and a composite of re-hospitalization and HF-related mortality.106 This is the first evidence that anti-inflammatory therapy may provide benefit in HF, though in a select group of patients with ischemic heart disease. Sorting out the risk/benefit ratio is needed to move the approach forward. Not surprisingly, the astronomic cost of canakinumab at US market price of $73,000 per year, to be taken life-long after the diagnosis of heart failure is made, represents a major barrier for its widespread use. At the current price canakinumab is not cost-effective to be used for prevention of recurrent cardiovascular events and is estimated to need a price cut of 98% to reach this.107
Promising therapies that fell short in clinical trials
Relaxin peptide is secreted by the corpus luteum in early pregnancy and binds to relaxin family peptide receptor (RXFP1) to upregulate VEGF, anti-fibrotic matrix metalloproteinases, NO-mediated vasodilation, and downregulate TGFβ.108 These effects led to its therapeutic assessment in HFrEF. The RELAX-AHF trial in acute decompensated HF109 studied 1161 patients, and while breathlessness (primary endpoint) did not improve, 6 month mortality following brief exposure fell surprisingly. This triggered the 6545-patient RELAX-AHF-2 trial using a similar design which missed its primary endpoints (180 day mortality and reduction in 5-day worsening of heart failure). While the biology is intriguing, relaxin’s fate remains in doubt.
Endothelin antagonism seemed a promising HF target given the hormone’s role in pathological fibrosis, hypertrophy, hypertension, and upregulation in in HFrEF110. Despite encouraging preclinical data, multiple clinical trials found no benefit in HFrEF, some reporting adverse effects.111 The cause likely involves competing effects in different cell types, receptor subtype selectivity, non-linear dose response signaling, and other factors.
Sarcoplasmic reticulum (SR) calcium cycling is abnormal in HF, contributing to depressed systolic and diastolic function. Limited Ca2+ uptake by the SR-ATPase112 (SERCA2a) and diastolic leakage out of the SR by the ryanodine receptor (RyR2), both contribute to a fall in systolic Ca2+ and higher diastolic levels. SERCA2a expression and function declines in human and experimental heart failure112, and its genetic upregulation benefits experimental HF models113 and human myocardial tissue.114 Translation to humans was less successful. In the CUPID IIb trial,115 the largest HF gene-therapy study to date, intracoronary delivery of recombinant adeno-associated virus expressing SERCA2a in 250 HFrEF patients did not alter primary or secondary outcomes. Inadequate dosing with poor myocardial uptake of the viral cargo is proposed as a potential cause, and interest in the target remains particularly with small molecule approaches.
Inhibition of promiscuous RyR2 Ca2+ release has been effected using binding protein regulators to stabilize receptor open probability. FKBP12.6 has been targeted with small molecules known as Rycals® to reduce its dissociation from the RyR and thus diastolic Ca2+ leakage.116 However, early-phase clinical testing in HFrEF was discontinued.
Biased ligand activation of GPCRs has the potential to affect receptor signaling beyond the primary G-protein, such as transactivating β-arrestin mediated cascades. TRV027 was developed to achieve this for the AT1R. In pre-clinical HF models, TRV027 lowered systemic resistance while improving contractility and renal blood flow.117, 118 Based on this, a ~600 patient Phase-2b acute HF dose-finding study of TRV027 (BLAST-AHF) was performed119, but found no benefit over placebo on the primary composite endpoint of 30-day survival, heart failure hospitalization, worsening heart failure, dyspnea score, or length of hospital stay.
Glucagon-like peptide GLP-1 is an endogenous incretin that enhances glucose-dependent insulin secretion and suppresses glucagon release.120 Preclinical data suggested GLP-1 agonism benefits ischemia-reperfusion,121, 122 and a pilot clinical study tested recombinant GLP-1 in severe HFrEF, finding improved LV function, clinical status and quality of life.123 A subsequent large trial of liraglutide, a long acting GLP-1 derivative, in T2D patients without HF but at high CV risk found less all-cause and CV mortality,124, 125 but non-fatal MI, stroke, and HF-hospitalizations incidence were unchanged. Subsequent trials of HFrEF patients, however, found some adverse effects (pro-arrhythmia and worsened heart failure)126, or were neutral127.
Emerging therapies from experimental models
Epigenetic modifiers: Histone deacetylase inhibitors, bromodomain and extraterminal (BET) bromodomain inhibitors, microRNAs, and long noncoding RNAs
Stress response signaling in HF is prominently affected by transcriptional changes regulated by epigenetic modifiers that facilitate or impede access to particular portions of the genome.128 Genomic DNA is wound tightly around histones, but this changes upon acetylation, methylation, phosphorylation, and other modifications to regulate transcription. Relevant proteins are grouped as “writers” that turn on transcription, “erasers” that turn it off, and “readers” that enhance assembly of transcriptional machinery. Pharmacologic agents that affect each of these processes can profoundly modify the cell’s stress/growth response, and have applications in various diseases including cancer and cardiovascular disease.129 However, given their ubiquitous involvement in signal transduction, and key roles in development and normal physiology, therapeutic epigenetic modification would seem likely to need careful application with target specificity.
Histone deacetylase inhibition (suppressing “writers”) obtained by genetic models or pharmacological tool compounds have generated considerable interest given their anti-hypertrophic, anti-fibrotic, and other HF ameliorating properties, particularly in mice.130 Surprisingly, even broad inhibitors appear tolerated in these animals, though large animal data remain scant. Further, there are some conflicting results based on the mode of inhibition and/or inhibitor used. At present, HDAC inhibitors are FDA-approved for treating lymphoma.131
Bromodomain and extra terminal (BET) proteins are epigenetic “readers” that bind to acetylated histones and augment the assembly of transcriptional machinery. The BET protein bromodomain-containing protein 4 (BRD4) displays increased expression in animal models of heart failure, and BRD4 blockade with the small molecule JQ1132 reduces stress-induced left ventricular hypertrophy and dysfunction. Mechanisms of benefit from BRD4 inhibition in heart failure include inhibition of Nuclear Factor kappa-light-chain-enhancer of B cells (NFκB) and Transforming Growth Factor-β−signaling networks.133 JQ1 improved disease in animal models with pre-existing heart failure and in human induced-pluripotent stem cell-derived cardiomyocytes, and did not suppress physiologic hypertrophy from exercise.133 There are two planned trials testing the BET inhibitor RVX000222 (Resverlogix Corp) in high risk diabetic patients with coronary artery disease (NCT02586155) and apabetalone (Resverlogix) in pulmonary arterial hypertension (NCT03655704). No HF trials have yet been initiated.
Noncoding RNAs, both long (long non-coding RNAs or lncRNAs) and short (microRNAs or miRNAs) are master regulators of signal transduction. LncRNAs modify gene transcription and protein translation by recruiting histone modification complexes, X-chromosome inactivation, and regulating miRNAs among the mechanisms. LncRNAs have a role in cardiac pathologic remodeling to stress,134–136 and activation of fibroblasts following cardiac injury.137 The pre-clinical work has yet to translate to a therapeutic effort in humans. MicroRNAs regulate protein translation of messenger RNA. Though many miRNAs have been implicated in animal models of heart failure, and manipulated to therapeutically improve heart disease, their translational role has so far been more as potential disease biomarkers. Limitations to translation include the complexity of their introduction, particularly for miRNA mimetics, lack of organ/tissue specificity, multiplicity of mRNA targets for each miRNA (~1000 each), and longevity of the response. MiRNA mimetics have been tested in cancer trials, with mixed results.138 MiRagen Therapeutics, Inc has two clinical trials at early stages for MRG201 (a miR-29 analogue), an anti-fibrotic miRNA that is being tested for keloid treatment (NCT03601052, Phase 2) and MRG110 (a miR-92a inhibitor) for wound healing (NCT03603431, Phase 1). No HF or cardiovascular studies are currently in progress.
Calcium-calmodulin kinase II
The serine-threonine kinase Ca2+/Calmodulin activated kinase II (CaMKII) is a multimeric complex consisting of two hexameric assembled rings, with isoform subunits encoded by four different genes. The γ and δ isoforms are present in the heart, with the δ isoform being predominant. Each subunit contains an N-terminal catalytic domain, a C-terminal association domain, and a regulatory segment that functions as the autoinhibitory domain by sterically blocking substrate access to the catalytic site. The autoinhibitory domain contains sites for phosphorylation, O-GlcNAC modifications, oxidation, and for calmodulin recognition. Calcium activated calmodulin binding disrupts autoinhhibition of the catalytic domain and autophosphorylates the kinase to maintain activation independent of Ca2+.139 CamKII is also activated by methionine oxidation, independently of Ca2+-calmodulin signaling.140 Physiological activation of CamKII and in disease states leads to phosphorylation of L-type Ca2+ channels, increasing Ca2+ currents,141–143 which facilitate sarcoplasmic reticulum (SR) Ca2+ release. CamKII also phosphorylates phospholamban, promoting SR Ca2+ uptake,144 and phosphorylation of the ryanodine receptor, which increases both diastolic SR Ca2+ leak as well as systolic release (reviewed in139). As increased intracytoplasmic Ca2+ concentration is a common pathway in cardiac hypertrophy, heart failure, cardiac ischemia, and arrhythmias, it is not surprising that augmented CamKII activity has been associated with all of the these. Inhibition of CamKII activity has demonstrated to improve outcomes in several animal models of heart failure.145 CamKII activity increases in human HFrEF, and its myocyte-specific overexpression induces heart failure and sudden cardiac death in mice. Increased neurohormonal activation, a hallmark of the heart failure syndrome, causes increased CamKII activation by β-adrenergic stimulation145 and CamKII oxidation by angiotensin and aldosterone promotes cardiac injury.140, 146
A variety of CamKII inhibitors including competitive (KN-93 and KN-62), peptide-based (AC3-I, CN19o, CN19β) and ATP binding inhibitors, have been tested in preclinical models. Some have off-target effects and others remain inadequately screened. Rimacalib (SMP-114, Dainippon Sumitomo Pharma) completed phase II trials for treatment of rheumatoid arthritis, and other therapy programs are currently underway.147 One concern over CamKII inhibitors is the risk of cognitive side effects if they penetrate the central nervous system, so must be avoided.
G-protein receptor kinase inhibitors
Upon GPCR agonism, the heterotrimeric G-protein complex dissociates with GTP binding to Gα-subunits to engage primary signaling. There are several mechanisms for reversing this, including removal of GTP to restore the complex and turn off receptor agonism, and phosphorylation of the receptor sub-units by G-protein receptor kinases (GRK) that recruit β-arrestins to reduce signaling and internalize the receptors. The first mechanism is controlled by regulators of G protein signaling (RGS), GTPase activating proteins that catalyze removal of GTP from Gα to turn receptor signaling off.148 Reducing RGS2 removes early compensatory responses against pressure-overload in mice68. Alternatively, increasing RGS3 or RGS2 improves β2AR signaling in failing myocytes, and is observed in vivo when HF with dyssynchronous contraction is treated with biventricular pacing – called cardiac resynchronization therapy149.
The heart expresses multiple GRKs, with GRK2 and GRK5 being the most prominently linked to HF pathophysiology.150 GRK2 has received the most attention to date. Its expression increases in experimental and human heart failure and is linked to desensitization of β-adrenergic receptors. Mice with myocyte-targeted GRK2 overexpression develop worse HF upon exposure to chronic isoproterenol151 and conditional deletion of GRK2 is protective.152
Given the critical interaction of GRK2 with Gβγ dimers for membrane translocation and receptor modification, the first tactic taken was to express a carboxy-terminal GRK2 fragment (βARKct) that competitively binds to Gβγ,153 and expression of this fragment after the onset of infarction is protective.152 Translation using in vivo gene therapy has been reported in rabbit154 and pig MI models.155 Another approach is to inhibit kinase activity, and interestingly, a clinically used antidepressant serotonin reuptake inhibitor, paroxetine, was found to do this, as did a compound that also blocks Rho kinase-1.156 However, the doses required are too high for human translation and more potent selective small molecules based on these structures are under development.156 As with β-blockers, inhibiting GRK2 offsets β-adrenergic pathway desensitization, thereby increasing a signaling pathway thought to be detrimental to HF. However, where modulation is applied in this pathway matters, and the signaling coupled with GRK2 suppression does not mimic that from βAR agonism. For example, suppressing GRK2 also reduces direct signaling from Gβγ subunits, that activate ERK157 and PLCε and are linked to hypertrophy.158
In addition to phosphorylating GPCRs, GRK5 contains a calmodulin-activated nuclear translocation sequence resulting in non-canonical control over gene transcription. In addition to phosphorylating class II HDAC-5 to disinhibit key pro-hypertrophic transcription factors (e.g. MEF2, NFAT, and SRF), GRK5 also directly stimulates NFκB and NFAT transcriptional activity (reviewed in150). This is eliminated if either the calmodulin binding domain is impeded or the nuclear localization sequence is removed. Genetic expression of solely the N-terminus calmodulin binding region as a sort of decoy to blunt GRK5 nuclear translocation reduces pathological hypertrophy both in vivo and in vitro.
Antioxidants: Thioredoxin, mitoTempo, and NO pathway enhancement
Despite the recognized role of oxidative stress in the pathophysiology of HF, antioxidant therapies have been disappointing. However, prior strategies were generally broad ROS scavengers, whereas more targeted intracellular approaches show greater promise. Two approaches, treatment with mitoTEMPO and increasing thioredoxin-2 signaling, both target mitochondrial-specific ROS. MitoTEMPO reduces both cytosolic and mitochondrial ROS, and treatment with mitoTEMPO improved LV function and reduced arrhythmia in a guinea pig HF model.159 Thioredoxin proteins are essential for scavenging ROS and controlling apoptosis in the setting of ROS accumulation. Cardiac-specific deletion of the mitochondrial-specific thioredoxin-2 leads to LV dysfunction in mice,160 and loss of function thioredoxin-2 mutations in humans are associated with dilated cardiomyopathy.161 Augmentation of thioredoxin-2 signaling via inhibition of apoptosis stress kinase-1, improves ventricular function in these mice, suggesting this as a relevant target to rescue heart failure in the setting of reduced thioredoxin-2 activity.162 Gene therapy efforts to enhance thioredoxin and small molecular activators are being pursued.
Antioxidants to augment nitric oxide signaling have also been studied, but the results proved disappointing. Oxidation of NO synthase results in a decline in NO generation and rather generation of O2−. Supplementation with the critical cofactor tetrahydrobiopterin (BH4) to recouple NOS was effective in animal models,163 but translation to humans was unsuccessful. One likely reason was that oral BH4 is first oxidized to BH2, a form that impairs NOS function, and must be first reduced to BH4 to be effective. This was less well achieved in humans than in mice, so levels required for antioxidant signaling were not achieved. Other studies, however, have found BH4 has benefits beyond antioxidant effects, suppressing inflammatory responses164 and impacting T-cell function,165 and so may prove therapeutic by other mechanisms.
Modulating intracellular calcium handling by S100A1
S100A1 is a multifaceted regulator of intracellular calcium handling, linking mitochondrial ATP generation to excitation-contraction coupling via interactions with SERCA2a, RyR2, and myocardial F1-ATPase.166 S100A1 expression declines in human heart failure and experimental overexpression improves ventricular function and remodeling after infarction, and adeno-associated virus gene therapy improved cardiac performance in a porcine ischemic cardiomyopathy model.167 Further development as gene therapy in humans is ongoing.
Summary
The armamentarium for HFrEF treatment has undergone various paradigm transitions, from one that was mostly hemodynamic in orientation, to those aimed at reversing maladaptive signaling pathways and/or enhancing beneficial ones. Advancing these molecular signaling discoveries to clinical therapy must confront the background therapy that we already have achieved, blocking βAR, AT1R, AldoR, restoring chamber synchrony (if contraction is dyssynchronous), and each of these confers many beneficial molecular signaling effects by themselves. Yet the recent positive results for combining neprilysin inhibition with AT1R, and the results of SGLT2 inhibition, indicate that this is not an impossible task, and that significant advances lay ahead. Perhaps the next paradigm will include an integration of existing and novel hemodynamic modulators with more patient-specific targeting of maladaptive molecular pathways, as opposed to the historical one size fits all approach.
Supplementary Material
Table 1.
New pharmacologic signaling approaches for HFrEF under active clinical investigation
| Drug class | Proposed mechanism of action | Name of drug (Company) | Current clinical research phase |
|---|---|---|---|
| Direct sarcomere activators | Directly activates myosin | Omecamtiv mercarbil (Cytokinetics) | Phase 3 |
| Direct sarcomere activators | Directly activates myosin | MYK-491 (MyoKardia, Inc.) | Phase 1b/2a |
| Phosphodiesterase inhibitors | Inhibits PDE9, augments cGMP | CRD-733 (Cardurion Pharmaceuticals) | Phase 1 |
| Phosphodiesterase inhibitors | Inhibits PDE1, augments cGMP and cAMP | ITI-214 (Intracellular Therapies, Inc.) | Phase 1b/2a |
| Nitroxyl donors | Antioxidant, enhances calcium sensitivity and calcium handling, augments NO signaling | BMS-986231 (Bristol-Myers Squibb) | Phase 2b |
| Soluble guanylate cyclase stimulators | Augments cGMP-PKG signaling | Vericiguat (Bayer/Merck) | Phase 3 |
| Apelin | Gαi activation and Inhibition of β-arrestin, ERK activation | CLR325 (Novartis); AMG986 (Amgen) | Phase 3; Phase 1 |
| Anti-inflammatory agents | Blocks inflammatory interleukin 1β | Canakinumab (Novartis) | Phase 3 |
HFrEF, Heart failure with reduced ejection fraction; PDE, phosphodiesterase; cGMP, cyclic guanosine monophosphate; cAMP, cyclic adenosine monophosphate; NO, nitric oxide; PKG, Protein Kinase G; ERK, extracellular signal-regulated kinase
Table 2.
Promising therapies for HFrEF that fell short in clinical trials
| Drug class | Name of drug (Company) | Patient population studied | Reason for failure |
|---|---|---|---|
| Synthetic relaxin | Serelaxin (Novartis) | Acute decompensated heart failure | No benefit |
| Endothelin antagonists | Bosentan (Actelion), macitentan (Actelion), ambrisentan (Gilead) | Chronic HFrEF | No benefit, signal for harm |
| Sarcoplasmic reticulum ATP-ase gene therapy | SERCA2a gene therapy (Celladon Corporation) | Chronic HFrEF | No benefit, possible underdosing, issues with delivery |
| Ryanodine Receptor stabilizers | Rycals (ARMGO) | Chronic HFrEF | No benefit. Early termination |
| Biased G-protein coupled receptor signaling | TRV027 (Trevena) | Acute decompensated heart failure | No benefit |
| Glucagon-like peptide 1 agonism | Liraglutide (Novo Nordisk) | Chronic HFrEF | No benefit, signal for harm |
HFrEF, Heart failure with reduced ejection fraction; ATP, adenosine triphosphate
Table 3.
Emerging therapies for HFrEF that have shown promise in experimental models
| Mechanistic approach | Drug class | Model studied |
|---|---|---|
| Epigenetic modifiers | Histone deacetylase inhibitors | Genetic deletion, ischemia-reperfusion, pressure overload, mice |
| Epigenetic modifiers | Bromodomain and extraterminal bromodomain (BET) inhibitors | Presure overload and ischemic cardiomyopathy, mice; induced pluripotent stem cell cardiomyocytes, human |
| Epigenetic modifiers | Long noncoding RNAs | Pressure overload and ischemic cardiomyopathy, mice; embryonic stem cell-derived cardiomyocytes, human |
| Epigenetic modifiers | microRNAs | |
| Calcium-calmodulin kinase II inhibitors | Calcium-calmodulin kinase II inhibitors | Ischemic cardiomyopathy, chronic catecholamine infusion, mice |
| G-protein receptor kinase inhibitors | G-protein receptor kinase 2 and 5 inhibitors | Chronic catecholamine infusion, mice; ischemic cardiomyopathy, mice, rabbits, and pigs |
| Mitochondrial ROS scavengers | MitoTEMPO | Combined pressure overload and chronic catecholamine infusion, guinea pig |
| Mitochondrial ROS scavengers | Apoptosis stress kinase-1 inhibitors to augment thioredoxin-2 signaling | Spontaneous cardiomyopathy in cardiac-specific thioredoxin-2 genetic deletion, mice |
| Antioxidants | Tetrahydrobiopterin to enhance eNOS-generation of NO, suppress inflammation | Pressure overload, mice |
| Intracellular calcium modulation | S100A1 | Ischemic cardiomyopathy, pigs |
HFrEF, Heart failure with reduced ejection fraction; ROS, reactive oxygen species; eNOS, endothelial nitric oxide synthase; NO, nitric oxide
Sources of funding
Supported by NIH-NHLBI R35–135827, AHA-16SFRN27870000 (DAK), T32–07227 (MPV, VSH).
Disclosures:
David Kass has received research grant support from Intracellular Therapy Inc. and the National Institutes of Health for work pertaining to the use of PDE1 inhibitors and cGMP/PKG regulation strategies to treat heart failure. He is also a member of scientific advisory boards for Amgen, Cytokinetics, and Cardurion, and consultant for Intracellular Therapy Inc, Bristol Myers Squibb, and Janssen Research and Development in the field of heart failure therapeutics. Dr. Kass co-founded Cardioxyl Inc. and is party to a purchase agreement from Bristol Myers Squibb. Drs. Vera and Hahn have nothing to disclose.
Table of Abbreviations:
- HFrEF, HFpEF
heart failure with a (reduced, preserved) ejection fraction
- β1AR, β2AR
beta-1 or beta-2 adrenergic receptor
- PKA
protein kinase A
- ERK1/2
extracellular response kinase ½
- Gai
inhibitory G protein alpha subunit
- Gas
stimulatory G protein alpha subunit
- cAMP
cyclic adensine monophosphate
- cGMP
cyclic guanosine monophosphate
- GRK2, GRK5
G-receptor kinase 2; 5
- SERCA2a
sarcoplasmic reticular calcium ATPase 2a
- RAAS
renin angiotensin aldosterone system
- AT1R
angiotensin type 2 receptor
- AldoR
aldosterone receptor
- ACE
angiotensin converting enzyme
- ARB
angiotensin receptor blocker
- NEP
neprilysin
- ANP
A-type natriuretic peptide
- BNP
B-type natriuretic peptide
- NO
nitric oxide
- SGLT2
sodium-glucose co-transporter 2
- SLC5
sodium-glucose co-transporter family
- T2D
type 2 diabetes mellitus
- CVD
cardiovascular disease
- OM
omecamtiv mercarbiil
- PDE
phosphodiesterase
- PKG
protein kinase G
- TRPC6
transient receptor potential canonical channel subtype 6
- RGS2/4
regulator of G-protein signaling type 2 or type 4
- sGC
soluble guanylate cyclase
- APJ
Apelin
- PLC
phospholipase C
- TGFb
tissue growth factor beta
- IL-1b
interleukin 1-beta
- hsCRP
high sensitivity C reactive protein
- RyR2
ryanodine receptor type 2
- GPCR
G protein coupled receptor
- GLP-1
glucogon like peptide – 1
- BRD4
bromodomain-containing protein 4
- BET
bromodomain and extra terminal
- miRNA
microRNA
- lncRNA
long non-coding RNA
- CaMKII
calcium calmodulin activated kinase II
- MEF2
myocyte enhancer factor 2
- NFAT
nuclear factor of activated T cells
- SRF
serum response factor
- ROS
reactive oxygen species
- BH4
tetrahydrobiopterin
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology F and American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 3.Simpson PC. Where are the new drugs to treat heart failure? Introduction to the special issue on “key signaling molecules in hypertrophy and heart failure”. J Mol Cell Cardiol. 2011;51:435–7. [DOI] [PubMed] [Google Scholar]
- 4.Rundqvist B, Elam M, Bergmann-Sverrisdottir Y, Eisenhofer G and Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95:169–75. [DOI] [PubMed] [Google Scholar]
- 5.Ahlquist RP. A study of the adrenotropic receptors. The American journal of physiology. 1948;153:586–600. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Gareri C and Rockman HA. G-Protein-Coupled Receptors in Heart Disease. Circulation research. 2018;123:716–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelhardt S, Hein L, Wiesmann F and Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ and Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. The Journal of clinical investigation. 2007;117:2445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Hanada K, Staus DP, Makara MA, Dahal GR, Chen Q, Ahles A, Engelhardt S and Rockman HA. Galphai is required for carvedilol-induced beta1 adrenergic receptor beta-arrestin biased signaling. Nature communications. 2017;8:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE and Gorelik J. Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327:1653–7. [DOI] [PubMed] [Google Scholar]
- 11.Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, Yatani A and Dorn GW 2nd. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–14. [DOI] [PubMed] [Google Scholar]
- 12.Bristow MR. Treatment of chronic heart failure with beta-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circulation research. 2011;109:1176–94. [DOI] [PubMed] [Google Scholar]
- 13.Swedberg K, Viquerat C, Rouleau JL, Roizen M, Atherton B, Parmley WW and Chatterjee K. Comparison of myocardial catecholamine balance in chronic congestive heart failure and in angina pectoris without failure. The American journal of cardiology. 1984;54:783–6. [DOI] [PubMed] [Google Scholar]
- 14.Heilbrunn SM, Shah P, Bristow MR, Valantine HA, Ginsburg R and Fowler MB. Increased beta-receptor density and improved hemodynamic response to catecholamine stimulation during long-term metoprolol therapy in heart failure from dilated cardiomyopathy. Circulation. 1989;79:483–490. [DOI] [PubMed] [Google Scholar]
- 15.Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, Quaife RA and Bristow MR. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. The New England journal of medicine. 2002;346:1357–65. [DOI] [PubMed] [Google Scholar]
- 16.Hjalmarson A and Waagstein F. New therapeutic strategies in chronic heart failure: challenge of long-term beta-blockade. European heart journal. 1991;12 Suppl F:63–9. [DOI] [PubMed] [Google Scholar]
- 17.Stephen SA. Unwanted effects of propranolol. The American journal of cardiology. 1966;18:463–72. [DOI] [PubMed] [Google Scholar]
- 18.Waagstein F, Hjalmarson A, Varnauskas E and Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. British heart journal. 1975;37:1022–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet (London, England). 1999;353:9–13. [PubMed] [Google Scholar]
- 20.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet (London, England). 1999;353:2001–7. [PubMed] [Google Scholar]
- 21.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK and DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. The New England journal of medicine. 2001;344:1651–8. [DOI] [PubMed] [Google Scholar]
- 22.Watkins L Jr., Burton JA, Haber E, Cant JR, Smith FW and Barger AC. The renin-angiotensin-aldosterone system in congestive failure in conscious dogs. J Clin Invest. 1976;57:1606–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dzau VJ, Colucci WS, Hollenberg NK and Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation. 1981;63:645–51. [DOI] [PubMed] [Google Scholar]
- 24.Re RN. Mechanisms of disease: local renin-angiotensin-aldosterone systems and the pathogenesis and treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2004;1:42–7. [DOI] [PubMed] [Google Scholar]
- 25.Gray MO, Long CS, Kalinyak JE, Li HT and Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352–63. [DOI] [PubMed] [Google Scholar]
- 26.Sadoshima J and Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–23. [DOI] [PubMed] [Google Scholar]
- 27.Group CTS. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–35. [DOI] [PubMed] [Google Scholar]
- 28.Cohn JN, Tognoni G and Valsartan Heart Failure Trial I. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. [DOI] [PubMed] [Google Scholar]
- 29.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B and Group E-HS. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.21073363 [Google Scholar]
- 30.Fattah C, Nather K, McCarroll CS, Hortigon-Vinagre MP, Zamora V, Flores-Munoz M, McArthur L, Zentilin L, Giacca M, Touyz RM, Smith GL, Loughrey CM and Nicklin SA. Gene Therapy With Angiotensin-(1–9) Preserves Left Ventricular Systolic Function After Myocardial Infarction. J Am Coll Cardiol. 2016;68:2652–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM and Reudelhuber TL. Angiotensin(1–7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–26. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida M, Ma J, Tomita T, Morikawa N, Tanaka N, Masamura K, Kawai Y and Miyamori I. Mineralocorticoid receptor is overexpressed in cardiomyocytes of patients with congestive heart failure. Congest Heart Fail. 2005;11:12–6. [DOI] [PubMed] [Google Scholar]
- 33.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Eplerenone Post-Acute Myocardial Infarction Heart Failure E and Survival Study I. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. [DOI] [PubMed] [Google Scholar]
- 34.Benitah JP, Perrier E, Gomez AM and Vassort G. Effects of aldosterone on transient outward K+ current density in rat ventricular myocytes. J Physiol. 2001;537:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavero PG, Margulies KB, Winaver J, Seymour AA, Delaney NG and Burnett JC Jr. Cardiorenal actions of neutral endopeptidase inhibition in experimental congestive heart failure. Circulation. 1990;82:196–201. [DOI] [PubMed] [Google Scholar]
- 36.Gerzer R, Witzgall H, Tremblay J, Gutkowska J and Hamet P. Rapid increase in plasma and urinary cyclic GMP after bolus injection of atrial natriuretic factor in man. J Clin Endocrinol Metab. 1985;61:1217–9. [DOI] [PubMed] [Google Scholar]
- 37.Kromer EP, Elsner D, Kahles HW and Riegger GA. Effects of atriopeptidase inhibitor UK 79300 on left ventricular hydraulic load in patients with congestive heart failure. Am J Hypertens. 1991;4:460–3. [DOI] [PubMed] [Google Scholar]
- 38.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P-H and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 39.Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Belohlavek J, Bohm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzalez-Medina A, Hagege AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan O, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz-Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr., Silva-Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC, Investigators P-H and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- 40.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E and Investigators P-H. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 41.Luckenbill KN, Christenson RH, Jaffe AS, Mair J, Ordonez-Llanos J, Pagani F, Tate J, Wu AH, Ler R and Apple FS. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem. 2008;54:619–21. [DOI] [PubMed] [Google Scholar]
- 42.Bayes-Genis A, Barallat J and Richards AM. A Test in Context: Neprilysin: Function, Inhibition, and Biomarker. J Am Coll Cardiol. 2016;68:639–653. [DOI] [PubMed] [Google Scholar]
- 43.Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, Finn PV, Hartley LH, Liu J, Lefkowitz M, Shi V, Zile MR and Solomon SD. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–7. [DOI] [PubMed] [Google Scholar]
- 44.Massie B, Chatterjee K, Werner J, Greenberg B, Hart R and Parmley WW. Hemodynamic advantage of combined administration of hydralazine orally and nitrates nonparenterally in the vasodilator therapy of chronic heart failure. Am J Cardiol. 1977;40:794–801. [DOI] [PubMed] [Google Scholar]
- 45.Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH and et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;314:1547–52. [DOI] [PubMed] [Google Scholar]
- 46.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M and et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–10. [DOI] [PubMed] [Google Scholar]
- 47.Carson P, Ziesche S, Johnson G and Cohn JN. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group. J Card Fail. 1999;5:178–87. [DOI] [PubMed] [Google Scholar]
- 48.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R Jr., Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN and African-American Heart Failure Trial I. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. [DOI] [PubMed] [Google Scholar]
- 49.Kalinowski L, Dobrucki IT and Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–7. [DOI] [PubMed] [Google Scholar]
- 50.McNamara DM, Tam SW, Sabolinski ML, Tobelmann P, Janosko K, Venkitachalam L, Ofili E, Yancy C, Feldman AM, Ghali JK, Taylor AL, Cohn JN and Worcel M. Endothelial nitric oxide synthase (NOS3) polymorphisms in African Americans with heart failure: results from the A-HeFT trial. J Card Fail. 2009;15:191–8. [DOI] [PubMed] [Google Scholar]
- 51.McNamara DM, Taylor AL, Tam SW, Worcel M, Yancy CW, Hanley-Yanez K, Cohn JN and Feldman AM. G-protein beta-3 subunit genotype predicts enhanced benefit of fixed-dose isosorbide dinitrate and hydralazine: results of A-HeFT. JACC Heart Fail. 2014;2:551–7. [DOI] [PubMed] [Google Scholar]
- 52.Ferdinand KC, Elkayam U, Mancini D, Ofili E, Pina I, Anand I, Feldman AM, McNamara D and Leggett C. Use of isosorbide dinitrate and hydralazine in African-Americans with heart failure 9 years after the African-American Heart Failure Trial. Am J Cardiol. 2014;114:151–9. [DOI] [PubMed] [Google Scholar]
- 53.Koruth JS, Lala A, Pinney S, Reddy VY and Dukkipati SR. The Clinical Use of Ivabradine. Journal of the American College of Cardiology. 2017;70:1777–1784. [DOI] [PubMed] [Google Scholar]
- 54.DiFrancesco D The role of the funny current in pacemaker activity. Circulation research. 2010;106:434–46. [DOI] [PubMed] [Google Scholar]
- 55.Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G and Tavazzi L. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet (London, England). 2010;376:875–85. [DOI] [PubMed] [Google Scholar]
- 56.Wright EM, Loo DD and Hirayama BA. Biology of human sodium glucose transporters. Physiological reviews. 2011;91:733–94. [DOI] [PubMed] [Google Scholar]
- 57.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA and Cherney DZI. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation. 2017;136:1643–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC and Inzucchi SE. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 59.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM and Sabatine MS. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2018. [Google Scholar]
- 60.Nassif M and Kosiborod M. Effect of glucose-lowering therapies on heart failure. Nature reviews Cardiology. 2018;15:282–291. [DOI] [PubMed] [Google Scholar]
- 61.Hamouda NN, Sydorenko V, Qureshi MA, Alkaabi JM, Oz M and Howarth FC. Dapagliflozin reduces the amplitude of shortening and Ca(2+) transient in ventricular myocytes from streptozotocin-induced diabetic rats. Molecular and cellular biochemistry. 2015;400:57–68. [DOI] [PubMed] [Google Scholar]
- 62.Baartscheer A, Schumacher CA, Wust RC, Fiolet JW, Stienen GJ, Coronel R and Zuurbier CJ. Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia. 2017;60:568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan BP, Muci A, Lu PP, Qian X, Tochimoto T, Smith WW, Garard M, Kraynack E, Collibee S, Suehiro I, Tomasi A, Valdez SC, Wang W, Jiang H, Hartman J, Rodriguez HM, Kawas R, Sylvester S, Elias KA, Godinez G, Lee K, Anderson R, Sueoka S, Xu D, Wang Z, Djordjevic N, Malik FI and Morgans DJ Jr. Discovery of omecamtiv mecarbil the first, selective, small molecule activator of cardiac Myosin. ACS Med Chem Lett. 2010;1:472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA and Malik FI. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–83. [DOI] [PubMed] [Google Scholar]
- 65.Kokkonen K and Kass DA. Nanodomain Regulation of Cardiac Cyclic Nucleotide Signaling by Phosphodiesterases. Annu Rev Pharmacol Toxicol. 2017;57:455–479. [DOI] [PubMed] [Google Scholar]
- 66.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, Mallis GI, Sollano JA, Shannon J, Tandon PK and deMets DL. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325:1468–1475. [DOI] [PubMed] [Google Scholar]
- 67.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, Tomaselli GF and Kass DA. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol. 2010;48:713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Nagayama T, Bedja D, Gabrielson K, Blanton R, Siderovski DP, Mendelsohn ME and Kass DA. RGS2 mediates cardiac compensation to pressure-overload and anti-hypertrophic effects of PDE5 inhibition. J Clin Invest. 2009;119:408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ranek MJ, Terpstra EJ, Li J, Kass DA and Wang X. Protein kinase g positively regulates proteasome-mediated degradation of misfolded proteins. Circulation. 2013;128:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ranek MJ, Kokkonen-Simon KM, Chen A, Dunkerly-Eyring BL, Vera MP, Oeing CU, Patel CH, Nakamura T, Zhu G, Bedja D, Sasaki M, Holewinski RJ, Van Eyk JE, Powell JD, Lee DI and Kass DA. PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress. Nature. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim GE and Kass DA. Cardiac Phosphodiesterases and Their Modulation for Treating Heart Disease. Handb Exp Pharmacol. 2017;243:249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD and Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–62. [DOI] [PubMed] [Google Scholar]
- 73.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E and Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisher DA, Smith JF, Pillar JS, St Denis SH and Cheng JB. Isolation and characterization of PDE9A, a novel human cGMP-specific phosphodiesterase. J Biol Chem. 1998;273:15559–64. [DOI] [PubMed] [Google Scholar]
- 75.Lee DI, Zhu G, Sasaki T, Cho GS, Hamdani N, Holewinski R, Jo SH, Danner T, Zhang M, Rainer PP, Bedja D, Kirk JA, Ranek MJ, Dostmann WR, Kwon C, Margulies KB, Van Eyk JE, Paulus WJ, Takimoto E and Kass DA. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519:472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kokkonen-Simon KM, Saberi A, Nakamura T, Ranek MJ, Zhu G, Bedja D, Kuhn M, Halushka MK, Lee DI and Kass DA. Marked disparity of microRNA modulation by cGMP-selective PDE5 versus PDE9 inhibitors in heart disease. JCI insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasaki H, Nagayama T, Blanton RM, Seo K, Zhang M, Zhu G, Lee DI, Bedja D, Hsu S, Tsukamoto O, Takashima S, Kitakaze M, Mendelsohn ME, Karas RH, Kass DA and Takimoto E. PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Invest. 2014;124:2464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hashimoto T, Kim GE, Tunin RS, Adesiyun T, Hsu S, Nakagawa R, Zhu G, O’Brien JJ, Hendrick JP, Davis RE, Yao W, Beard D, Hoxie HR, Wennogle LP, Lee DI and Kass DA. Acute Enhancement of Cardiac Function by Phosphodiesterase Type 1 Inhibition. Circulation. 2018;138:1974–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Knight W, Chen S, Mohan A and Yan C. A Multi-Protein Complex with TRPC, PDE1C, and A2R Plays a Critical Role in Regulating Cardiomyocyte cAMP and Survival. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fukuto JM. A recent history of nitroxyl chemistry, pharmacology and therapeutic potential. British journal of pharmacology. 2019;176:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu G, Groneberg D, Sikka G, Hori D, Ranek MJ, Nakamura T, Takimoto E, Paolocci N, Berkowitz DE, Friebe A and Kass DA. Soluble guanylate cyclase is required for systemic vasodilation but not positive inotropy induced by nitroxyl in the mouse. Hypertension. 2015;65:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sivakumaran V, Stanley BA, Tocchetti CG, Ballin JD, Caceres V, Zhou L, Keceli G, Rainer PP, Lee DI, Huke S, Ziolo MT, Kranias EG, Toscano JP, Wilson GM, O’Rourke B, Kass DA, Mahaney JE and Paolocci N. HNO enhances SERCA2a activity and cardiomyocyte function by promoting redox-dependent phospholamban oligomerization. Antioxid Redox Signal. 2013;19:1185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di Benedetto G, O’Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng H, Kass DA and Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res. 2007;100:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Froehlich JP, Mahaney JE, Keceli G, Pavlos CM, Goldstein R, Redwood AJ, Sumbilla C, Lee DI, Tocchetti CG, Kass DA, Paolocci N and Toscano JP. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–2. [DOI] [PubMed] [Google Scholar]
- 85.Gao WD, Murray CI, Tian Y, Zhong X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, Van Eyk JE and Paolocci N. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res. 2012;111:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sabbah HN, Tocchetti CG, Wang M, Daya S, Gupta RC, Tunin RS, Mazhari R, Takimoto E, Paolocci N, Cowart D, Colucci WS and Kass DA. Nitroxyl (HNO): A novel approach for the acute treatment of heart failure. Circ Heart Fail. 2013;6:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paolocci N, Katori T, Champion HC, St John ME, Miranda KM, Fukuto JM, Wink DA and Kass DA. Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from beta-adrenergic signaling. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tita C, Gilbert EM, Van Bakel AB, Grzybowski J, Haas GJ, Jarrah M, Dunlap SH, Gottlieb SS, Klapholz M, Patel PC, Pfister R, Seidler T, Shah KB, Zielinski T, Venuti RP, Cowart D, Foo SY, Vishnevsky A and Mitrovic V. A Phase 2a dose-escalation study of the safety, tolerability, pharmacokinetics and haemodynamic effects of BMS-986231 in hospitalized patients with heart failure with reduced ejection fraction. European journal of heart failure. 2017;19:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farah C, Michel LYM and Balligand JL. Nitric oxide signalling in cardiovascular health and disease. Nature reviews Cardiology. 2018;15:292–316. [DOI] [PubMed] [Google Scholar]
- 90.Kraehling JR and Sessa WC. Contemporary Approaches to Modulating the Nitric Oxide-cGMP Pathway in Cardiovascular Disease. Circulation research. 2017;120:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Muller K, Roessig L, Pieske B, Investigators S-R and Coordinators. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA. 2015;314:2251–62. [DOI] [PubMed] [Google Scholar]
- 92.Lu L, Wu D, Li L and Chen L. Apelin/APJ system: A bifunctional target for cardiac hypertrophy. International journal of cardiology. 2017;230:164–170. [DOI] [PubMed] [Google Scholar]
- 93.Scimia MC, Hurtado C, Ray S, Metzler S, Wei K, Wang J, Woods CE, Purcell NH, Catalucci D, Akasaka T, Bueno OF, Vlasuk GP, Kaliman P, Bodmer R, Smith LH, Ashley E, Mercola M, Brown JH and Ruiz-Lozano P. APJ acts as a dual receptor in cardiac hypertrophy. Nature. 2012;488:394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang ZZ, Wang W, Jin HY, Chen X, Cheng YW, Xu YL, Song B, Penninger JM, Oudit GY and Zhong JC. Apelin Is a Negative Regulator of Angiotensin II-Mediated Adverse Myocardial Remodeling and Dysfunction. Hypertension (Dallas, Tex : 1979). 2017;70:1165–1175. [DOI] [PubMed] [Google Scholar]
- 95.Szokodi I, Tavi P, Foldes G, Voutilainen-Myllyla S, Ilves M, Tokola H, Pikkarainen S, Piuhola J, Rysa J, Toth M and Ruskoaho H. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res. 2002;91:434–40. [DOI] [PubMed] [Google Scholar]
- 96.Dai T, Ramirez-Correa G and Gao WD. Apelin increases contractility in failing cardiac muscle. Eur J Pharmacol. 2006;553:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mughal A and O’Rourke ST. Vascular effects of apelin: Mechanisms and therapeutic potential. Pharmacology & therapeutics. 2018;190:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levine BKJ, Mayer L, et al. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. New England Journal of Medicine. 1990. 223:236–241. [DOI] [PubMed] [Google Scholar]
- 99.Bartekova M, Radosinska J, Jelemensky M and Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 2018;23:733–758. [DOI] [PubMed] [Google Scholar]
- 100.Chung ES, Packer M, Lo KH, Fasanmade AA and Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40. [DOI] [PubMed] [Google Scholar]
- 101.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F and Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–602. [DOI] [PubMed] [Google Scholar]
- 102.Panahi M, Papanikolaou A, Torabi A, Zhang JG, Khan H, Vazir A, Hasham MG, Cleland JGF, Rosenthal NA, Harding SE and Sattler S. Immunomodulatory interventions in myocardial infarction and heart failure: a systematic review of clinical trials and meta-analysis of IL-1 inhibition. Cardiovascular research. 2018;114:1445–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Tassell BW, Toldo S, Mezzaroma E and Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128:1910–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW, Oddi C, Carbone S, Trankle CR, Roberts CS, Mueller GH, Gambill ML, Christopher S, Markley R, Vetrovec GW, Dinarello CA and Biondi-Zoccai G. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). The American journal of cardiology. 2015;115:288–92. [DOI] [PubMed] [Google Scholar]
- 105.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P and Glynn RJ. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 106.Everett BM, Cornel J, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ and Ridker PM. Anti-Inflammatory Therapy with Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation. 2018. [DOI] [PubMed] [Google Scholar]
- 107.Sehested TSG, Bjerre J, Ku S, Chang A, Jahansouz A, Owens DK, Hlatky MA and Goldhaber-Fiebert JD. Cost-effectiveness of Canakinumab for Prevention of Recurrent Cardiovascular Events. JAMA cardiology. 2019;4:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Du XJ, Bathgate RA, Samuel CS, Dart AM and Summers RJ. Cardiovascular effects of relaxin: from basic science to clinical therapy. Nat Rev Cardiol. 2010;7:48–58. [DOI] [PubMed] [Google Scholar]
- 109.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr., Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M and Investigators REiAHF. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. [DOI] [PubMed] [Google Scholar]
- 110.Jankowich MD, Wu WC and Choudhary G. Association of Elevated Plasma Endothelin-1 Levels With Pulmonary Hypertension, Mortality, and Heart Failure in African American Individuals: The Jackson Heart Study. JAMA cardiology. 2016;1:461–9. [DOI] [PubMed] [Google Scholar]
- 111.Wei A, Gu Z, Li J, Liu X, Wu X, Han Y and Pu J. Clinical Adverse Effects of Endothelin Receptor Antagonists: Insights From the Meta-Analysis of 4894 Patients From 24 Randomized Double-Blind Placebo-Controlled Clinical Trials. Journal of the American Heart Association. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luo M and Anderson ME. Mechanisms of altered Ca(2)(+) handling in heart failure. Circ Res. 2013;113:690–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cutler MJ, Wan X, Plummer BN, Liu H, Deschenes I, Laurita KR, Hajjar RJ and Rosenbaum DS. Targeted sarcoplasmic reticulum Ca2+ ATPase 2a gene delivery to restore electrical stability in the failing heart. Circulation. 2012;126:2095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, Gwathmey JK, Rosenzweig A and Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, Pogoda JM, Rudy JJ and Zsebo KM. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet (London, England). 2016;387:1178–86. [DOI] [PubMed] [Google Scholar]
- 116.Santulli G, Nakashima R, Yuan Q and Marks AR. Intracellular calcium release channels: an update. The Journal of physiology. 2017;595:3041–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boerrigter G, Lark MW, Whalen EJ, Soergel DG, Violin JD and Burnett JC Jr. Cardiorenal actions of TRV120027, a novel ss-arrestin-biased ligand at the angiotensin II type I receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure. Circ Heart Fail. 2011;4:770–8. [DOI] [PubMed] [Google Scholar]
- 118.Boerrigter G, Soergel DG, Violin JD, Lark MW and Burnett JC Jr. TRV120027, a novel beta-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circulation Heart failure. 2012;5:627–34. [DOI] [PubMed] [Google Scholar]
- 119.Pang PS, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA, Filippatos G, Levy PD, Metra M, Ponikowski P, Teerlink JR, Voors AA, Bharucha D, Goin K, Soergel DG and Felker GM. Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF). Eur Heart J. 2017;38:2364–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–42. [DOI] [PubMed] [Google Scholar]
- 121.Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M and Drucker DJ. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ and Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–50. [DOI] [PubMed] [Google Scholar]
- 123.Sokos GG, Nikolaidis LA, Mankad S, Elahi D and Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9. [DOI] [PubMed] [Google Scholar]
- 124.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, Committee LS and Investigators LT. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lepore JJ, Olson E, Demopoulos L, Haws T, Fang Z, Barbour AM, Fossler M, Davila-Roman VG, Russell SD and Gropler RJ. Effects of the Novel Long-Acting GLP-1 Agonist, Albiglutide, on Cardiac Function, Cardiac Metabolism, and Exercise Capacity in Patients With Chronic Heart Failure and Reduced Ejection Fraction. JACC Heart Fail. 2016;4:559–566. [DOI] [PubMed] [Google Scholar]
- 126.Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hanselmann A, Nilsson B, Moller JE, Hjort J, Rasmussen J, Boesgaard TW, Schou M, Videbaek L, Gustafsson I, Flyvbjerg A, Wiggers H and Tarnow L. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77. [DOI] [PubMed] [Google Scholar]
- 127.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, McNulty SE, Anstrom KJ, Shah MR, Braunwald E, Cappola TP and Network NHFCR. Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;316:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee TI and Young RA. Transcriptional regulation and its misregulation in disease. Cell. 2013;152:1237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gillette TG and Hill JA. Readers, writers, and erasers: chromatin as the whiteboard of heart disease. Circ Res. 2015;116:1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abend A and Kehat I. Histone deacetylases as therapeutic targets--from cancer to cardiac disease. Pharmacology & therapeutics. 2015;147:55–62. [DOI] [PubMed] [Google Scholar]
- 131.McClure JJ, Li X and Chou CJ. Advances and Challenges of HDAC Inhibitors in Cancer Therapeutics. Adv Cancer Res. 2018;138:183–211. [DOI] [PubMed] [Google Scholar]
- 132.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S and Bradner JE. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Duan Q, McMahon S, Anand P, Shah H, Thomas S, Salunga HT, Huang Y, Zhang R, Sahadevan A, Lemieux ME, Brown JD, Srivastava D, Bradner JE, McKinsey TA and Haldar SM. BET bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J, Just A, Fendrich J, Scherf K, Bolesani E, Schambach A, Weidemann F, Zweigerdt R, de Windt LJ, Engelhardt S, Dandekar T, Batkai S and Thum T. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med. 2016;8:326ra22. [DOI] [PubMed] [Google Scholar]
- 135.Han P, Li W, Lin CH, Yang J, Shang C, Nuernberg ST, Jin KK, Xu W, Lin CY, Lin CJ, Xiong Y, Chien H, Zhou B, Ashley E, Bernstein D, Chen PS, Chen HV, Quertermous T and Chang CP. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Z, Zhang XJ, Ji YX, Zhang P, Deng KQ, Gong J, Ren S, Wang X, Chen I, Wang H, Gao C, Yokota T, Ang YS, Li S, Cass A, Vondriska TM, Li G, Deb A, Srivastava D, Yang HT, Xiao X, Li H and Wang Y. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med. 2016;22:1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Micheletti R, Plaisance I, Abraham BJ, Sarre A, Ting CC, Alexanian M, Maric D, Maison D, Nemir M, Young RA, Schroen B, Gonzalez A, Ounzain S and Pedrazzini T. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith CIE and Zain R. Therapeutic Oligonucleotides: State of the Art. Annu Rev Pharmacol Toxicol. 2019;59:605–630. [DOI] [PubMed] [Google Scholar]
- 139.Hegyi B, Bers DM and Bossuyt J. CaMKII signaling in heart diseases: Emerging role in diabetic cardiomyopathy. J Mol Cell Cardiol. 2019;127:246–259. [DOI] [PubMed] [Google Scholar]
- 140.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ and Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yuan W and Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. The American journal of physiology. 1994;267:H982–93. [DOI] [PubMed] [Google Scholar]
- 142.Wu Y, MacMillan LB, McNeill RB, Colbran RJ and Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. The American journal of physiology. 1999;276:H2168–78. [DOI] [PubMed] [Google Scholar]
- 143.Koval OM, Guan X, Wu Y, Joiner ML, Gao Z, Chen B, Grumbach IM, Luczak ED, Colbran RJ, Song LS, Hund TJ, Mohler PJ and Anderson ME. CaV1.2 beta-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4996–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mattiazzi A and Kranias EG. The role of CaMKII regulation of phospholamban activity in heart disease. Frontiers in pharmacology. 2014;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE Jr., Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ and Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nature medicine. 2005;11:409–17. [DOI] [PubMed] [Google Scholar]
- 146.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ and Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nature medicine. 2011;17:1610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pellicena P and Schulman H. CaMKII inhibitors: from research tools to therapeutic agents. Frontiers in pharmacology. 2014;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang P and Mende U. Functional role, mechanisms of regulation, and therapeutic potential of regulator of G protein signaling 2 in the heart. Trends in cardiovascular medicine. 2014;24:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chakir K, Depry C, Dimaano VL, Zhu WZ, Vanderheyden M, Bartunek J, Abraham TP, Tomaselli GF, Liu SB, Xiang YK, Zhang M, Takimoto E, Dulin N, Xiao RP, Zhang J and Kass DA. Galphas-biased beta2-adrenergic receptor signaling from restoring synchronous contraction in the failing heart. Science translational medicine. 2011;3:100ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lieu M and Koch WJ. GRK2 and GRK5 as therapeutic targets and their role in maladaptive and pathological cardiac hypertrophy. Expert opinion on therapeutic targets. 2019. [DOI] [PubMed] [Google Scholar]
- 151.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA and Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–3. [DOI] [PubMed] [Google Scholar]
- 152.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR Jr., Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW 2nd and Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circulation research. 2008;103:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koch WJ, Hawes BE, Inglese J, Luttrell LM and Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. The Journal of biological chemistry. 1994;269:6193–7. [PubMed] [Google Scholar]
- 154.White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ and Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, Katus HA, Most P and Muller OJ. AAV6.betaARKct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. European heart journal. 2013;34:1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Waldschmidt HV, Homan KT, Cato MC, Cruz-Rodriguez O, Cannavo A, Wilson MW, Song J, Cheung JY, Koch WJ, Tesmer JJ and Larsen SD. Structure-Based Design of Highly Selective and Potent G Protein-Coupled Receptor Kinase 2 Inhibitors Based on Paroxetine. Journal of medicinal chemistry. 2017;60:3052–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lorenz K, Schmitt JP, Schmitteckert EM and Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nature medicine. 2009;15:75–83. [DOI] [PubMed] [Google Scholar]
- 158.Campbell AP and Smrcka AV. Targeting G protein-coupled receptor signalling by blocking G proteins. Nature reviews Drug discovery. 2018;17:789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dey S, DeMazumder D, Sidor A, Foster DB and O’Rourke B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ Res. 2018;123:356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, Beck H, Hatzopoulos AK, Just U, Sinowatz F, Schmahl W, Chien KR, Wurst W, Bornkamm GW and Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sibbing D, Pfeufer A, Perisic T, Mannes AM, Fritz-Wolf K, Unwin S, Sinner MF, Gieger C, Gloeckner CJ, Wichmann HE, Kremmer E, Schafer Z, Walch A, Hinterseer M, Nabauer M, Kaab S, Kastrati A, Schomig A, Meitinger T, Bornkamm GW, Conrad M and von Beckerath N. Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy. Eur Heart J. 2011;32:1121–33. [DOI] [PubMed] [Google Scholar]
- 162.Huang Q, Zhou HJ, Zhang H, Huang Y, Hinojosa-Kirschenbaum F, Fan P, Yao L, Belardinelli L, Tellides G, Giordano FJ, Budas GR and Min W. Thioredoxin-2 inhibits mitochondrial reactive oxygen species generation and apoptosis stress kinase-1 activity to maintain cardiac function. Circulation. 2015;131:1082–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, Ketner EA, Majmudar M, Gabrielson K, Halushka MK, Mitchell JB, Biswal S, Channon KM, Wolin MS, Alp NJ, Paolocci N, Champion HC and Kass DA. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hashimoto T, Sivakumaran V, Carnicer R, Zhu G, Hahn VS, Bedja D, Recalde A, Duglan D, Channon KM, Casadei B and Kass DA. Tetrahydrobiopterin Protects Against Hypertrophic Heart Disease Independent of Myocardial Nitric Oxide Synthase Coupling. Journal of the American Heart Association. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cronin SJF, Seehus C, Weidinger A, Talbot S, Reissig S, Seifert M, Pierson Y, McNeill E, Longhi MS, Turnes BL, Kreslavsky T, Kogler M, Hoffmann D, Ticevic M, da Luz Scheffer D, Tortola L, Cikes D, Jais A, Rangachari M, Rao S, Paolino M, Novatchkova M, Aichinger M, Barrett L, Latremoliere A, Wirnsberger G, Lametschwandtner G, Busslinger M, Zicha S, Latini A, Robson SC, Waisman A, Andrews N, Costigan M, Channon KM, Weiss G, Kozlov AV, Tebbe M, Johnsson K, Woolf CJ and Penninger JM. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature. 2018;563:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rohde D, Busch M, Volkert A, Ritterhoff J, Katus HA, Peppel K and Most P. Cardiomyocytes, endothelial cells and cardiac fibroblasts: S100A1’s triple action in cardiovascular pathophysiology. Future cardiology. 2015;11:309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Muller OJ and Most P. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3:92ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.