Abstract
Patients with heart failure (HF) syndromes have been categorized as those with reduced ejection fraction (EF; HFrEF) or preserved EF (HFpEF), and ischemia plays a key role in both types. HF remains a major cause of morbidity and mortality worldwide, and with the aging of our population this burden continues to rise, predominantly as a result of hospitalizations for HFpEF. Patients with obstructive coronary artery disease more likely have HFrEF, rather than HFpEF, secondary to acute ischemic injury resulting in myocardial infarction, and large outcomes trials of treatments with neurohumoral inhibition have documented reduced adverse outcomes. In contrast, similar treatments in patients with HFpEF have not proven beneficial. This therapeutic dilemma may be attributed, in part, to heterogeneity in the underlying pathophysiology with different systemic and myocardial signaling pathways, despite similar clinical presentations and findings, in patients with HFpEF. Also, emerging evidence indicates that impaired myocardial perfusion and inflammation secondary to multiple comorbidities are key mechanisms in HFpEF. We will thoroughly review the role of ischemic heart disease in the pathogenesis of HFrEF and HFpEF, and discuss the medical management strategies available for these conditions.
Keywords: Myocardial ischemia, heart failure with preserved ejection fraction (HFpEF), heart failure with reduced ejection fraction (HFrEF)
Subject Terms: Heart Failure, Ischemia, Treatment
Overview
It has been estimated that 6.2 million Americans over the age of 20 years suffer from heart failure (HF). This disorder continues to be a significant cause of morbidity and mortality in developed nations, and ischemic heart disease (IHD) is the common etiology.1, 2 The continuing decline in deaths due to acute myocardial infarction (MI) and the aging of our population are predicted to result in a 46% increase in the prevalence of HF by 2030, which would equate to >8 million adults in the United States.3 Over the last two decades, there has been improvement in cardiovascular medical therapies and the development of devices that have improved overall survival for patients with HF. However, it is important to note that although HF survival ratio has plateaued, the prevalence of HF continues to increase at a worrisome rate.1
Prevention and management of HF remains a major public health concern due to its enormous financial and societal burden.4 HF represents the most common cause of hospitalization in the elderly, with an estimated annual cost of $40 billion that is predicted to increase to almost $69.7 billion by 2030.3 In the United States, there is a 20%–45% lifetime risk of developing HF for adults age 45–95 years.5, 6 The current annual incidence of HF hospitalization in the United States has reached ~1 million.1 Furthermore, the prevalence of HF with preserved ejection fraction (HFpEF) is increasing compared to HF with reduced ejection fraction (HFrEF).7 Traditionally, this classification of HF (i.e., HFrEF and HFpEF) has been based on EF values as estimated with imaging modalities such as echocardiography, radionuclide ventriculography, contrast angiography and cardiac magnetic resonance imaging. For the purpose of this review, we will discuss established and ongoing medical therapies for both types of HF that are driven by evidence using this traditional classification. However, it is important to acknowledge that emerging evidence suggests that using EF for classification of HF might be suboptimal. This concern is because there are other forms of HF that do not necessarily fall into the traditional EF classification such as HF with mid-range EF and HF with recovered EF, as well as subclinical left ventricular (LV) dysfunction, including LV hypertrophy, etc.8 Additionally, other assessment modalities such as global longitudinal strain provide better prognostic information because disease phenotypes have changed since the era when EF was developed. Thus, continued use of a classification based on EF alone may be insufficient.8
Nearly 70% of all HF syndromes can be attributed to underlying IHD.9 Despite efforts to address the key prevention risk factors for IHD, the incidence of HF hospitalizations has not largely changed and is actually projected to rise.4 Ischemia plays a pivotal role in the development and progression of both types of HF. Patients with an obstructive epicardial stenosis (e.g. coronary artery disease [CAD]) are more likely to have HFrEF (rather than HFpEF) as a result of acute ischemic injury causing in myocardial infarction (MI) with scar formation, and large outcomes trials of treatments with neurohumoral inhibition have clearly documented reduced adverse outcomes. In contrast, similar treatments in patients with HFpEF have not proven beneficial, and emerging evidence indicates that impaired myocardial perfusion and inflammation, secondary to multiple systemic comorbidities, are key. This therapeutic dilemma may be attributed to the fact that the pathophysiology of HFpEF is heterogeneous, and individuals with this syndrome may have defects in different systemic and myocardial signaling pathways, despite similar clinical presentations and findings.
Epidemiology of CAD, and more broadly IHD, among patients with HF
Over the past four decades, there have been 26% and 48% increases, among men and women, respectively, in HF prevalence resulting from MI.10 This is in contrast to a decrease of 13% among men and 25% among women in HF attributed to hypertension and ~25% reduction in HF secondary to valvular heart disease in both sexes.9 Epidemiological data show that the role of CAD in HF varies based on geographic region. While only 10% of all HF cases in Sub-Saharan Africa can be attributed to CAD, as high as 50%–70% of all cases in the United States and Europe, and 30%–40% of all cases in Asia and Latin America, are caused by underlying CAD.11 Data from cohorts enrolled in HF treatment trials suggest that approximately two of three HF cases were associated with obstructive CAD.12–16 However, this may underestimate the actual prevalence of CAD or IHD in HF patients, as many of these HF treatment trials did not enroll patients with recent MI, angina, or evidence of ischemia. Findings from epidemiological studies, such as the Framingham Heart Study (FHS) and the Olmstead County Study (OCS), concur with these observations. During the 118,000 person-years follow-up of the FHS cohort, ~25% of patients had a MI prior to developing HF, whereas only 5% of the cohort developed HF without a prior, clinically recognized, ischemic event.10 Compared with these FHS patients, all of those enrolled in the OCS had a history of MI but no history of HF.17 At a mean follow-up of 7 years, 41% had developed new-onset HF. Among patients with HF, ~45% had HFrEF (defined as EF <50%), ~18% had HFpEF (EF ≥50%), and the remainder did not have LV function assessment within 60 days of their HF diagnosis.
Prognosis
Once the diagnosis of HF is made, it is important to distinguish between ischemic and non-ischemic causes. The presence of IHD due to flow-limiting obstructive coronary atherosclerosis (e.g. CAD) often portends a poor prognosis in patients with HF compared to patients with a “so-called” non-ischemic cardiomyopathy-related HF syndrome.9 This poor prognosis link with obstructive CAD has been documented by data from several cohorts enrolled in HF clinical trials. Patients with an LVEF ≤35% enrolled in the Studies of Left Ventricular Dysfunction Treatment (SOLVD) trial with prior MI had a two-fold higher hospitalization rate for decompensated HF and four-fold higher mortality rate versus patients without a prior MI.18 We also observed that these SOLVD patients with severe LV systolic dysfunction had increased risk for recurrent acute coronary syndromes (ACS) that was modified by enalapril, suggesting that activation of the renin–angiotensin converting enzyme (ACE) system was involved.19 These findings were confirmed by the Survival and Ventricular Enlargement (SAVE) trial, which found a 70% increase in risk of cardiovascular-related death and/or LV enlargement in patients with LV systolic dysfunction due to prior MI versus those without prior MI.18, 20 Again, in SAVE these adverse outcomes were modified by ACE inhibition with captopril. Data from several other studies have confirmed that HF on a background of CAD is associated with poor outcomes over follow up.21, 22 Others have also emphasized the critical importance of early heightened inflammation as a predictor of HF and mortality suggesting a potential target for future trials of medical therapy to prevent HF in post-MI patients.23
Pathophysiologic interplay between ischemia and HF
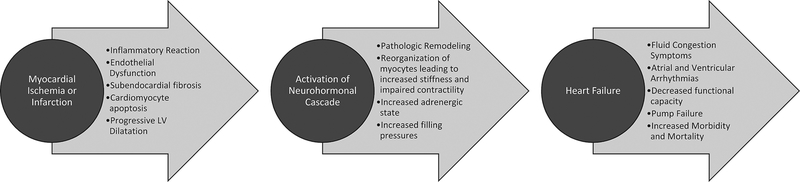
The transition between presence of myocardial ischemia and development of HF is often an abrupt plaque-related event (e.g. erosion or rupture) resulting in thrombotic occlusion of an epicardial coronary artery. Clinically, these patients usually present with an ACS, that may be divided into those with or without ST-segment elevation on the electrocardiogram, and then further subdivided into those with or without evidence of myocardial necrosis based on cardiac troponin efflux (e.g., MI) (Figure 1). Following a MI, the amount of myocardium infarcted, the territory of the infarcted segment, the development of mitral regurgitation, and the presence of certain tachyarrhythmias contribute to the development of clinical HF. In the setting of acute ischemia, there is loss of functioning cardiomyocytes resulting in myocardial stunning and myocardial necrosis, with subsequent myocardial inflammation, hypertrophy, and fibrosis. These changes activate the neurohormonal cascade that results in adverse LV remodeling, thereby ensuing dilation and dysfunction that often also includes the non-infarcted myocardium.24 LV remodeling, dilation, and ischemic mitral regurgitation together act as a substrate for HF development. In the absence of ACS and an acute ischemic event, the integrity of myocardial tissue can be compromised by the deleterious effects of chronic ischemia.25, 26 A subset of patients might develop objective evidence of myocardial ischemia, such as electrocardiographic changes, in the absence of chest discomfort or an angina equivalent symptom (i.e., silent myocardial ischemia). This silent myocardial ischemia syndrome is more often seen in patients with hypertension, diabetes, denervation after heart transplant, or those who have a history of known obstructive CAD. In an analysis from the Atherosclerosis Risk In Communities of ~9,000 subjects free of CVD at baseline, silent myocardial ischemia (defined as electrocardiographic evidence for MI without clinical MI after the baseline visit) was associated with the development of subsequent HF over 13 years (median follow-up) even with adjustment for demographic and risk factors of HF.27 These findings were consistent in subgroups stratified by HF risk predictors, also HF risk associated with silent myocardial ischemia was stronger among the younger (<53 years old) patients vs older patients.
Figure 1:
Schematic diagram of progression of coronary artery disease to heart failure.
For HFrEF, key therapeutic targets are obstructive epicardial CAD and, to a lesser extent, coronary microvascular dysfunction in the damaged and remote myocardium, as well as the extent of necrotic and ischemic myocardium. These conditions contribute to worsening of endothelial dysfunction, atheroma formation, and progression to acute plaque-related events (erosion or rupture) resulting in abrupt epicardial vessel occlusion leading to cardiomyocyte injury and necrosis. Prompt percutaneous intervention is highly successful in restoring perfusion with subsequent recovery of damaged cardiomyocytes. When reperfusion is either delayed or ineffective (e.g. no-reflow phenomena), recovery of damaged cardiomyocytes is limited or incomplete leading to myocardial fibrosis with adverse ventricular remodeling, and in some cases aneurysm formation or, less frequently, rupture.28 These conditions, along with continuing ischemic injury in some cases, contribute to HFrEF and are targets for therapy.
In contrast to HFrEF where LV dilation occurs due to myocardial scarring, the pathophysiological mechanisms for development of HFpEF secondary to ischemia are different and more complex. On a background of heightened systemic inflammation, creating vulnerable myocardium and its microvasculature, the ability to augment blood flow during periods of increased demand becomes limited resulting in patchy areas of ischemia. During periods of cardiomyocyte ischemia, passive stiffness of myocardial fibers increases, which results in impaired myocardial relaxation and subsequent elevations in LV filling pressures.29 This further limits myocardial blood flow, increasing ischemia, and leads to pulmonary congestion and shortness of breath, the hallmark symptom of HF. Thus, the HF syndrome occurs even in the setting of reasonably preserved LV systolic function (e.g. ejection fraction, EF ≥50%).
Traditionally, HFpEF had been thought to occur secondary to myocardial overload due to long standing hypertension30 or aortic stenosis; however, considerable recent evidence indicates that microvascular dysfunction involving the smaller coronary vessels in non-infarcted regions contributes to continuing and/or recurring ischemia.31 Endothelial dysfunction increases vascular stiffness and resistance, and decreases tissue perfusion, leading to multi-organ dysfunction (e.g. renal, skeletal muscle, pulmonary vasculature) and an increase in cardiac afterload. The latter may be provoked by activities occurring during daily life such as mental stress. This HF syndrome is highly prevalent among older women and Black patients, and the presence of multiple comorbidities (e.g. diabetes, obesity, inactivity, chronic kidney disease, chronic obstructive pulmonary disease, etc.) with heightened systemic inflammation is the rule. This inflammation results in down-regulation of eNOS expression, uncoupling of eNOS, increased production of reactive oxygen species, and reduction in NO with generalized endothelial dysfunction. Ultimately, a prolonged state of attenuated NO activity and endothelial dysfunction serves to further propagate HF progression.32, 33 Additionally, inflammation with periarteriolar fibrosis contribute to further limiting microvascular function augmenting ischemia and diastolic dysfunction in the absence of obstructive epicardial coronary stenoses.
Interleukin-33 (IL-33) inhibits myocardial fibrosis in the pressure overloaded LV by acting via its receptor, ST2 (encoded by the gene, Il1rl1).34 It is unclear if this cytokine also modulates periarteriolar fibrosis. Thus, loss of ST2 signaling rather than changes in IL-33 expression may contribute to periarteriolar fibrosis during aging or pressure overload, but manipulating this pathway alone may not prevent or reverse fibrosis. In a study of ~1,200 patients with positron emission tomography (PET) myocardial perfusion imaging, those with impaired coronary flow reserve had a higher incidence of cardiac events, driven mainly by HF hospitalizations.35 In another PET study of ~200 patients, impaired coronary flow reserve was independently associated with diastolic dysfunction at a median of 4.1 years.36
In summary, with HFpEF extracardiac comorbidities such as aging, metabolic risk, systemic hypertension, obesity, loss of female sex hormones, renal insufficiency, etc. lead to continuing LV dysfunction and remodeling through systemic inflammation with coronary endothelial and vascular smooth muscle dysfunction. These processes result in LV diastolic dysfunction through macrophage infiltration, leading to interstitial fibrosis. With altered paracrine signaling of cardiomyocytes, they become hypertrophied and stiff related to reduced nitric oxide and cyclic guanosine monophosphate. Other organs are affected (lungs, skeletal muscle, kidneys, etc.) resulting in pulmonary hypertension, skeletal muscle weakness or sarcopenia, with sodium and fluid retention.37
Management of patients with CAD and ischemic cardiomyopathy (HFrEF)
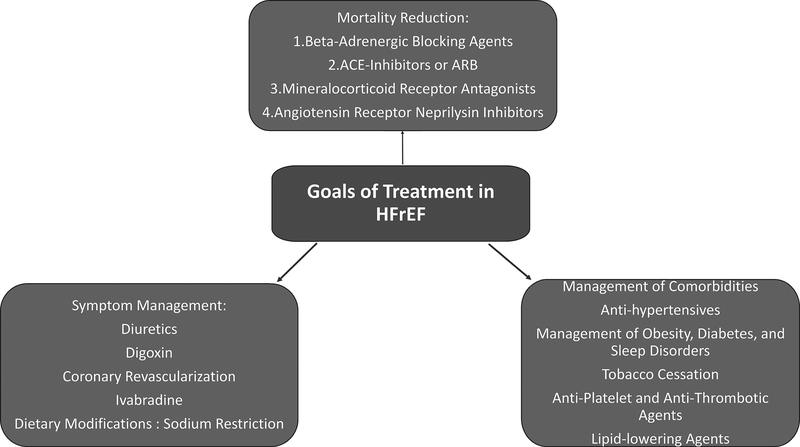
The development and progression of cardiomyopathy and subsequent HFrEF in setting of prior MI or known CAD presents clinicians with multiple management tasks. These areas require optimized management of comorbid conditions and pharmacological therapies to improve survival and symptom control (Figure 2).
Figure 2:
Goals of Treatment in heart failure with reduced ejection fraction (HFrEF). ACE=angiotensin converting enzyme; ARB= angiotensin receptor blockers
Over the past three decades, several landmark clinical trials (Table 1) have provided robust evidence regarding benefit with the use of pharmacological therapies in patients with HFrEF which have been endorsed by the current ACC/AHA/HFSA guidelines (Table 2). Notably, this benefit is observed with long-term adherence to these medical therapies. While data from the TRED-HF has demonstrated that withdrawal of these therapies in patients with dilated cardiomyopathy and recovered EF results in relapse of cardiomyopathy38, it remains unknown whether a similar relationship between medication withdrawal and relapse of EF in patients with recovered ischemic cardiomyopathy exists. Further, there is robust evidence confirming the mortality benefit of primary prevention implantable defibrillators in HFrEF patients with ischemic cardiomyopathy receiving guideline directed therapy13, 39, 40, as well as cardiac resynchronization therapy for select patients with ischemic cardiomyopathy41, and LV assist devices (LVADs) in patients with refractory HFrEF despite optimal medical and device therapies as a bridge to transplant or destination therapy.42 Most recently, the LVAD MPC-II trial documented that intramyocardial injection of allogenic mesenchymal progenitor cells in patients receiving an LVAD improved temporary weaning in IHD patients with HFrEF. Perhaps more important was the observation of a significantly lower risk of gastrointestinal bleeding43, offering a potential therapeutic option for these patients in the future.
Table 1:
Major trials demonstrating mortality benefit with medical therapy in patients with heart failure with reduced ejection fraction (HFrEF)
| Medication (Class) | Trial Name | Number of Patients Enrolled | Primary Outcome |
|---|---|---|---|
| Carvedilol (Beta Blocker) | CAPRICORN | 1,959 | All-cause mortality or hospital admission for cardiovascular problem |
| Metoprolol XL (Beta Blocker) | MERIT-HF | 3,991 | All-cause mortality |
| Trandolapril (ACE-inhibitor) | TRACE | 1,749 | All-cause mortality |
| Captopril (ACE-inhibitor) | SAVE | 2,231 | All-cause mortality, cardiovascular mortality or morbidity |
| Ramipril (ACE-inhibitor) | AIRE | 2,006 | All-cause mortality |
| Losartan (ARB) | OPTIMAAL | 5,477 | All-cause mortality |
| Valsartan (ARB) | VALIANT | 14,703 | All-cause mortality |
| Eplerenone (MRA) | EPHESUS | 6,632 | All-cause mortality, cardiovascular mortality, heart failure hospitalizations, acute MI, stroke, or ventricular arrhythmias |
| Valsartan-Sacubitril (ARNI) | PARADIGM-HF | 8,442 | Composite death from cardiovascular cases or hospitalizations for heart failure |
ACE-inhibitor = Angiotensin Converting Enzyme Inhibitor; ARB = Angiotensin Receptor Blocker; MRA = Mineralocorticoid Receptor Antagonists; ARNI = Angiotensin Receptor Neprilysin Inhibitor
Table 2:
Guideline recommendations and level of evidence for therapies shown to improve mortality
| Medication Class | Recommendation | Recommendation Class/Level of Evidence | Guidelines |
|---|---|---|---|
| Beta-Blockers | Recommended for all patients with current or prior symptoms of HFrEF | Class I; LOE: A | 2013 ACCF/AHA Heart Failure Guideline |
| ACE-Inhibitors | Recommended in all patients with HFrEF and current or prior symptoms | Class I; LOE: A | 2013 ACCF/AHA Heart Failure Guideline 2017 ACC/AHA/HFSA Heart Failure Focused Update |
| Angiotensin Receptor Blockers | Recommended in patients with HFrEF with current or prior symptoms who are intolerant to ACE-inhibitor | Class I; LOE: A | 2013 ACCF/AHA Heart Failure Guideline 2017 ACC/AHA/HFSA Heart Failure Focused Update |
| Mineralocorticoid Receptor Antagonists | Recommended for all patients with NYHA Class II-IV heart failure with LVEF ≤ 35%. Patients with NYHA Class II should have a history of prior heart failure hospitalization or elevated BNP prior to initiation | Class I; LOE: A | 2013 ACCF/AHA Heart Failure Guideline |
| Angiotensin Receptor Neprilysin Inhibitor | Recommended in selected patients with chronic HFrEF in conjunction with beta-blockers and aldosterone antagonists | Class I; LOE: B | 2017 ACC/AHA/HFSA Heart Failure Focused Update |
ACE-inhibitors = Angiotensin Converting Enzyme Inhibitors; LOE = Level of Evidence; ACCF = American College of Cardiology Foundation; AHA = American Heart Association; HFSA = Heart Failure Society of America; NYHA = New York Heart Association; LVEF = Left Ventricular Ejection Fraction; HFrEF = Heart Failure with Reduced Ejection Fraction
Medical therapies for mortality reduction
Beta-adrenergic blocking agents
Beta-blocker therapy has been shown to provide survival benefit in patients with HFrEF secondary to ischemic or non-ischemic cardiomyopathy.44–46 The majority of trials showing benefit of beta blocker therapy excluded patients with prior MI or recent PCI. In a multicenter, randomized, placebo-controlled trial of ~2,000 patients with prior MI and EF ≤40%47, beta-blocker therapy resulted in reduction of all-cause and cardiovascular mortality, as well as recurrent, non-fatal MI. These were incremental benefits in addition to those of therapy with statins and ACE inhibition. Another randomized, placebo-controlled trial analyzed the effects of beta-blocker therapy in patients with ischemic cardiomyopathy.48 At 19 months mean follow-up, beta-blocker therapy was associated with improved EF and LV dimensions, with reductions in death and hospital readmissions. A prespecified subgroup analysis of the Metoprolol CR/XL Randomized Intervention Trial in Congestive HF (MERIT-HF) showed similar findings in patients with EF ≤40% and those with a history of hospitalization for MI.49 On background of aspirin, statins, ACE inhibitors (ACEIs), and revascularization, beta-blocker therapy was associated with reduction in mortality and morbidity. Use of beta-blocker therapy for reduction in mortality in patients with remote history of MI or ACS and HFrEF has a Class I, Level of Evidence A recommendation by ACC/AHA and ESC guidelines for HF management.50, 51 An important consideration is that the doses of these agents tolerated by HF patients are often limited by lower blood pressures, and only a few reach doses that would produce beta blockade in the true pharmacologic sense.
ACEIs and angiotensin receptor blocking agents
Symptomatic improvement and mortality benefit with ACE-inhibition has been documented in trials dating to the 1980s, however these trials did not exclusively enroll patients with ischemic cardiomyopathy.52, 53 Post-MI patients with LVEF <35% were evaluated in the Trandolapril Cardiac Evaluation (TRACE)54, which randomized patients to trandolapril or placebo. The trial showed that long-term use of ACE-inhibition in patients with reduced LV function post MI reduced risk of overall mortality, mortality from cardiovascular causes, sudden death, and HF development. Similar findings were shown in the SAVE trial with a 22% relative risk reduction in HF hospitalization and 25% reduction in recurrent MI with captopril.20 Patients randomized to ramipril in the Acute Infarction Ramipril Efficacy (AIRE) study experienced a 27% reduction in mortality compared with those randomized to placebo.55 Although SAVE and AIRE enrolled post-MI patients with LV systolic dysfunction, the key difference was presence of clinical HF in patients enrolled in AIRE while patients in SAVE were asymptomatic from a HF standpoint. Nonetheless, the results from these randomized trials make it clearly evident that patients with CAD or post-MI and with ischemic cardiomyopathy receive benefit from ACE-inhibition. The ACC/AHA and ESC guidelines endorse the use of ACEIs in setting of history of MI or ACS with reduced LVEF, a Class I Level of Evidence A recommendation.50, 51
An alternative approach to block the renin-angiotensin system is through AT1 receptor angiotensin receptor blockers (ARBs). Clearly these agents work at a final common pathway, blocking angiotensin-II effects. The OPTIMAAL trial56 randomized ~5,000 patients with acute MI and HFrEF to either losartan or captopril. At a mean follow up of 2.7 years, there was a non-significant difference in all-cause mortality. The Valsartan in Acute Myocardial Infarction (VALIANT) Trial57 randomized ~15,000 post-MI patients, with either clinical evidence of HF and/or ischemic cardiomyopathy (EF ≤40%), to either captopril, valsartan, or combination valsartan and captopril. Although results showed an increased incidence of adverse events in the combination group, there were no significant mortality differences comparing captopril alone with valsartan alone. Thus, both trials support a strategy targeting angiotensin receptor blockade in post-MI patients with HFrEF. The HF guidelines recommend use of ARB therapy in patients with MI or ACS and subsequent ischemic cardiomyopathy or HFrEF, who are intolerant to ACEIs for reduction in morbidity and mortality.50, 51
Mineralocorticoid receptor antagonists
Evidence supports use of mineralocorticoid receptor antagonism in patients with New York Heart Association (NYHA) class II-IV HFrEF to reduce morbidity and mortality58–60, including the subset of patients with ischemic cardiomyopathy. The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) randomized ~6,000 patients with HFrEF to eplerenone or placebo.61 At 16 months, eplerenone was associated with a 15% reduction in all-cause mortality.
The 35 percent reduction in the risk of hospitalization for worsening heart failure may be attributable to the ability of spironolactone to reduce myocardial and vascular fibrosis. Blockade of aldosterone receptors by spironolactone, in addition to standard HF therapy, should be considered for the treatment of patients with severe HF. To gain insight on the benefit of mineralocorticoid receptor antagonism early after MI and development of HF symptoms, EPHESUS evaluated outcomes at 30-days follow-up.62 When eplerenone was initiated about 1-week post-MI in patients with HFrEF, there was reduction in 30-day all-cause mortality. These data have been extrapolated for use of other mineralocorticoid receptor antagonists such as spironolactone given similar efficacy of both agents.63 In patients with HFrEF due to ischemic cardiomyopathy, use of spironolactone has been associated with reversal of negative cardiac remodeling64, as well as decreased ventricular arrhythmias65, thereby reducing morbidity risk. Reduction in mortality and morbidity with spironolactone was also seen in a large, randomized placebo-control trial where the majority of the patients had HFrEF secondary to ischemic cardiomyopathy.58 The HF guidelines give a Class I, Level of Evidence A recommendation for use of mineralocorticoid receptor antagonists in patients with LVEF ≤35% and NYHA class II-IV HF, unless otherwise contraindicated.50, 51
Angiotensin receptor/neprilysin inhibitors
Angiotensin receptor/neprilysin inhibitors (ARNI) consist of an ARB and neprilysin inhibitor. Neprilysin contributes to the degradation of the biologically active natriuretic peptides and several other vasoactive compounds, including bradykinin. With inhibition of neprilysin, circulating levels of these compounds rise which counteract increased atrial and ventricular pressures occurring with HF, thereby decreasing preload and afterload and augmenting natriuresis.66 The PARADIGM-HF trial67 evaluated ARNI versus enalapril in HFrEF patients, and was terminated early due to a 21% reduction in cardiovascular mortality and HF hospitalizations in patients randomized to valsartan/sacubitril. Both the ACC/AHA and ESC guidelines recommend replacing ACEI with ARNI when possible.50, 51 More recently, the PIONEER-HF trial showed that initiation of ARNI in patients with HFrEF and acutely decompensated HF led to a greater reduction in NT-proBNP concentration and was not associated with a higher risk of side effects such as renal dysfunction and hypotension compared with enalapril.68 Although ARNI have shown mortality benefit in HFrEF patients, there is currently a lack of data regarding efficacy of these agents in post-MI patients, patients with prior MI or known CAD, and patients specifically with ischemic cardiomyopathy. However, it is important to note that almost 60% of patients enrolled in both arms of PARADIGM-HF had HFrEF secondary to ischemic cardiomyopathy. The benefit of ARNI during post-MI period has been suggested in animal studies69, while a large prospective clinical trial in post-MI patients with new LV systolic dysfunction is ongoing (PARADISE-MI; NCT02924727).
Medical therapies for symptom management
Diuretics
By inhibiting sodium reabsorption at various sites in renal tubules, diuretics result in increased urinary sodium excretion. This translates in decreased fluid retention in patients with HF, improved symptom management, and increased exercise tolerance.70 Initiation of diuretic therapy is based on clinical signs of vascular congestion such as jugular venous distention, peripheral edema, or shortness of breath. Hence, diuretics are a mainstay of symptom management in HF patients. Unless contraindicated, the use of diuretics in patients with HFrEF with evidence of fluid retention carries a class I, level of evidence C recommendation per current HF guidelines.6
Coronary revascularization
Revascularization in patients with HFrEF and flow-limiting CAD has been the topic of multiple investigations. The Surgical Treatment for Ischemic HF (STICH) was a trial of ~1,200 patients, with LVEF ≤35% and CAD appropriate for surgical revascularization, randomized to either optimal medical therapy alone or coronary artery bypass grafting (CABG) in addition to optimal medical therapy.71 Compared with optimal medical therapy alone, addition of CABG resulted in improvement in cardiovascular mortality and HF hospitalizations over long-term follow-up. Data regarding PCI on a background of optimal medical therapy in patients with LVEF <35% are not as robust. However, pre-specified sub-group analysis of the Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial showed similar outcomes with PCI using drug-eluting stents versus CABG in patients with LVEF <40%.72 In accordance with these data, revascularization via surgical or percutaneous approach should be considered in patients with obstructive CAD and HFrEF, as it offers improved survival and quality of life.
If channel blockeade (Ivabradine)
One newer agent in the realm of HF pharmacological therapies is ivabradine, which acts on the funny (If) ion channels that play an important role in activity of myocardial pacemaker cells. The primary advantage of ivabradine over beta-blocker therapy is a lack of negative inotropism.73 The Ivabradine and Outcomes in Chronic HF (SHIFT) trial evaluated its efficacy in HF patients, majority were due to IHD.74 During the mean follow-up of 22.9 months, the results from this randomized, double-blind, placebo-controlled trial showed a significant reduction in cardiovascular death or HF hospitalization, and this was similar in those with IHD vs those without IHD. The mortality benefit was primarily related to reduction in resting heart rate. Then, the Ivabradine for patients with stable CAD and left-ventricular systolic dysfunction (BEAUTIFUL) trial randomized CAD patients with LV dysfunction (LVEF ≤40%) to either ivabradine or placebo.75 The primary outcome, cardiovascular death and hospitalization for HF or acute MI, was not improved, however, in the subgroup with baseline resting heart rate >70 beats per minute, ivabradine reduced hospitalization for fatal or non-fatal MI or coronary revascularization. A sub-analysis of SHIFT showed that in patients on no beta-blocker therapy or <50% of target dose, addition of ivabradine resulted in reduced primary endpoint while in patients who were on >50% of target beta-blocker dose, addition of ivabradine only reduced HF hospitalization.76 This may explain, in part, the differences between findings from the SHIFT and BEAUTIFUL trials. Based on these data, the 2017 ACC/AHA Update on HF guidelines have given ivabradine a IIa recommendation for patients with NYHA class II-III HFrEF to reduce HF hospitalizations, who are already receiving guideline-directed medical therapy for HF, including maximally tolerated beta-blocker therapy, and a resting heart rate >70 beats per minute while in sinus rhythm.50
Management of comorbid conditions
Antihypertensive therapy
Control of blood pressure remains a key component of HF and IHD/CAD management since hypertension is a highly prevalent, modifiable risk factor in the development and perpetuation of HF, as well as atherosclerosis progression. Current guidelines recommend (Class I, Level of Evidence C) titration of guideline-directed medical therapy to achieve systolic blood pressure <130 mmHg.50 No evidence from randomized trials is available to support superiority of one agent over the other. Hence it is recommended that a combination of beta-blockers, ACEIs, or ARB or ARNI, mineralocorticoid receptor antagonists, and diuretics be used as first-line antihypertensives in patients with HFrEF.77 Given negative inotropic effects, avoidance of non-dihydropyridine calcium channel blockers is recommended in patients with LV dysfunction and HFrEF.
Antiplatelet and antithrombotic therapies
The role of antiplatelet agents is well established in patients with prior MI and HFrEF. The main role of antiplatelet agents here has been for secondary prevention of atherosclerotic vascular events. However, a paucity of data exists regarding whether use of antiplatelet agents result in improved outcomes from a HF standpoint. This lack of data is, in part, due to most of the randomized trials enrolling patients with concomitant use of anticoagulants and antiplatelet agents. An earlier analysis from the Studies of Left Ventricular Dysfunction Treatment (SOLVD) trial showed that although the use of antiplatelet agents (primarily aspirin) was associated with 19% reduction in mortality and HF readmissions, this difference was entirely attributed to randomization to the enalapril therapy.78
Apart from the well-known cardioembolic protective benefits of anticoagulation in patients with atrial dysrhythmias or LV mural thrombus, improvement in clinical outcomes with oral anticoagulant agents for patients in sinus rhythm and ischemic cardiomyopathy has been a topic of multiple investigations. The Warfarin/Aspirin Study in HF (WASH) Trial evaluated antithrombotic therapy in HF, of which ~60% had ischemic cardiomyopathy, randomized to placebo, warfarin, or 300 mg/day aspirin. At a mean follow-up of 27 months, there were no significant differences between the three arms in terms of death, nonfatal MI, or nonfatal stroke.79 This trial was followed by two other randomized trials that showed similar findings: anticoagulants such as warfarin in patients with sinus rhythm and HF due to ischemic cardiomyopathy did not improve cardiovascular outcomes compared with placebo or antiplatelet agents.80, 81 Lastly, the Warfarin and Aspirin in Patients with HF and Sinus Rhythm (WARCEF) trial also failed to show improvement in the first occurrence of death, ischemic stroke, or intracerebral hemorrhage with warfarin or aspirin.82 More recently, the COMMANDER HF trial, which randomized ~5,000 patients mostly with HFrEF due to CAD and sinus rhythm to low dose rivaroxaban (2.5 mg twice daily) versus placebo, showed that rivaroxaban was not associated with a lower rate of death, myocardial infarction, or stroke compared with placebo.83 However, a post-hoc analysis of this trial showed that low dose rivaroxaban might be associated with a lower risk of thromboembolic events, namely the composite of myocardial infarction or stroke.84 These findings are in line with the HF subgroups of the COMPASS and ATLAS ACS 2-TIMI 51 (the data regarding EF were not reported in both trials) which also suggested that low dose rivaroxaban might be of benefit.85, 86 In light of these findings, routine antiplatelet agents or anticoagulants are not recommended for improvement in ischemic cardiomyopathy or HF outcomes, but future studies are encouraged to identify the subset of ischemic cardiomyopathy patients that would potentially benefit from low dose anticoagulant therapy in light of the encouraging findings from these post-hoc analyses. Nevertheless, antiplatelet agents, specifically aspirin, are indicated in patients with CAD and HF for secondary prevention of ischemic events.
Lipid-lowering agents
The 3-hydroxy-3methylglutaryl-coenzyme A reductase inhibitors, or statins, are the cornerstone for primary and secondary prevention of atherosclerotic cardiovascular events such as MI and stroke.87–89 Although benefit in preventing adverse cardiovascular events is apparent, the earlier studies demonstrating this benefit did not extend to patients with HF. The incremental benefit of using statins in patients with HF has been evaluated by several trials since the 1990s. A subgroup analysis from the Scandinavian multicenter study showed that over a 5-year follow-up, simvastatin (compared with placebo) was associated with reduction in HF (8.3% vs. 10.3%, p < 0.015).90 Data from retrospective analyses and observational studies continued to suggest a beneficial role of statins in HF patients.91 However, the Controlled Rosuvastatin Multinational Trial in HF (CORONA) compared rosuvastatin vs placebo in patients with ischemic cardiomyopathy and HFrEF.92 At 33 months (median follow up), rosuvastatin, compared with placebo, did not reduce the primary outcome, a composite of non-fatal MI, non-fatal stroke, and death from cardiovascular causes. The neutral effects of statin therapy in improving HF outcomes was again demonstrated the randomized, double-blind, placebo-controlled trial—the GISSI-HF Trial.93 Since CORONA and GISSI-HF, several post-hoc analyses have evaluated the impact of statins in a subset of ischemic cardiomyopathy patients with elevated biomarkers, such as C-reactive-protein, reflecting heightened inflammatory characteristics that may receive benefit with statin therapy.94, 95 Although these post-hoc analyses from CORONA have suggested positive results, given the lack of confirmative data from randomized control trials, statin therapy for the sole purpose of improvement in ischemic HF outcomes is not currently recommended.
Management of other comorbid conditions
Obesity and associated insulin resistance have been associated with adverse outcomes in patients with HFrEF.96, 97 Optimal management of diabetes, obesity, and metabolic syndrome is key in improving clinical outcomes in patients with established HFrEF. Additionally, the guidelines also recommend management of iron deficiency anemia as well as sleep disorders in patients with NYHA class II-IV HF.50 Treatment with iron supplementation in patients with iron deficiency anemia has been shown to improve the functional capacity, quality of life, and might be associated with reduction in heart failure hospitalizations.98, 99 In addition, several small randomized trials have shown that compliance with continuous positive airway pressure in patients with obstructive sleep apnea and HF is recommended to improve daytime sleepiness, nocturnal oxygenation, and functional status in patients with HF100, 101 however; these trials failed to show an improvement on objective outcomes, similar to trials of continuous positive airway pressure in those without HF at baseline.102, 103 The hypothesis of the impact of continuous positive airway pressure on HF hospitalizations and mortality in patients with obstructive sleep and HF is being tested in an ongoing randomized trial (ADVENT-HF, NCT01128816).
Medical Therapy for HFpEF
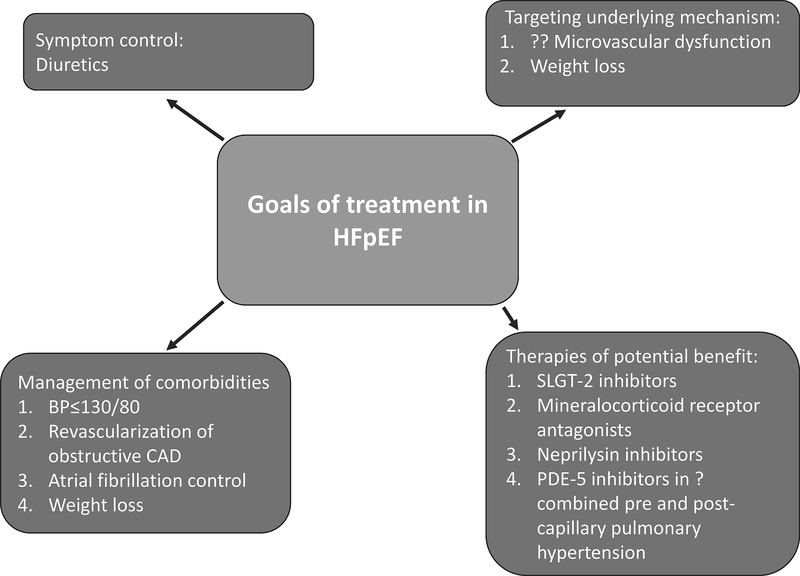
As discussed earlier, emerging evidence suggests that HFpEF is not only related to long-standing hypertension but is associated with multiple comorbid conditions and microvascular dysfunction (which is highly prevalent in patients with non-obstructive CAD) secondary to a heightened systemic inflammatory state. Unfortunately, to date there has been no therapy proven to improve adverse outcomes in patients with HFpEF, unlike HFrEF. In this section, we will discuss the goals of therapy and potential pharmacological therapies in patients with HFpEF (Figure 3).
Figure 3:
Goals of Treatment in heart failure with preserved ejection fraction (HFpEF) BP= blood pressure; CAD= coronary artery disease; PDE-5 inhibitors= phosphodiestrase-5 inhibitors; SLGT-2 inhibitors= Sodium-glucose cotransporter-2 inhibitors
Symptom control
Similar to congestion relieve with diuretics in patients with HFrEF, diuretics are used to control the symptoms of congestion in patients with HFpEF, however; there is limited evidence to support the benefit of diuretics in these patients.
Management of concomitant comorbidities
Although the hypothesis that long standing hypertension is a predisposing factor for HFpEF33, excellent blood pressure control remains of utmost importance in these patients to achieve a blood pressure ≤130/80 mmHg77, but some observational analyses have cautioned against excessively lowering the systolic blood pressure to levels <120 mmHg.104 In addition, the presence of concomitant obstructive CAD in some patients with HFpEF has been linked with increased mortality, and revascularization of obstructive CAD has been shown to improve the outcomes.105 In these patients, treatment with antiplatelet therapy is recommended. Atrial fibrillation is highly prevalent with HFpEF (up to 40%), and has been linked to increased morbidity and mortality.106 Yet, the optimal management of atrial fibrillation in this setting remains unclear. An interesting knowledge gap, for example, is whether a rhythm control strategy in these patients might be superior to a rate control strategy.107 Long-term anticoagulation to reduce the risk of systemic thromboembolic complications is recommended similar to the general population. The ongoing OPTIMIZE-HFPEF trial is testing the hypothesis whether management of concomitant comorbidities might improve clinical status in patients with HFpEF.108
Obesity is one of the most prevalent comorbidities among patients with HFpEF. Epidemiological studies have estimated that >80% of older patients with HFpEF are obese or overweight.109 Excess body weight plays an important role in the pathogenesis of HFpEF through expanding the plasma volume and systemic inflammation.110 Obese individuals are prone to enhanced renal tubular sodium reabsorption resulting in an expanded plasma volume.111 Adipocytes exert an inflammatory response by producing cytokines, which in turn contribute to microvascular dysfunction.112 Therefore, counseling patients about the importance of weight loss through calorie restriction, exercise and bariatric surgery is recommended. However, these options might not always be feasible in this population due to poor functional capacity and/or concomitant comorbidities.
Pharmacological therapies of uncertain benefit
Beta-blockers
Despite the strong evidence that demonstrated the survival benefit of beta-blockers therapy in the HFrEF, most of which have CAD, studies in HFpEF patients have been less encouraging.113 In a patient-level meta-analysis of 11 randomized trials with 14,262 patients with HF in sinus rhythm, beta-blocker therapy reduced the risk of all-cause and cardiovascular mortality over a median 1.3 years follow up in those with reduced or borderline ejection fraction (i.e., 40–49%), but not in those with ejection fraction ≥50%.114 Although some observational data suggest that HFpEF patients with elevated heart rate (i.e., >70 bpm) might benefit from high doses of beta-blocker therapy115, this hypothesis has not been evaluated in randomized trials.
ACEIs/ARBs
Similar to beta-blocker therapy, several randomized trials have proven the survival benefit of ACEI/ARBs in patients with HFrEF, but the evidence for these therapies in HFpEF has been less convincing. In the I-PRESERVE trial of 4,128 patients with HFpEF, irebsartan failed to reduce cardiovascular death or hospitalization for a cardiovascular cause compared with placebo.116 These findings were consistent in CHARM-Preserved that showed no benefit for candesartan, compared with placebo, in reducing cardiovascular death or HF hospitalizations.117
A recent Cochrane Database report provides the most comprehensive evidence available for beta-blocker therapy and renin-angiotensin aldosterone system inhibition on morbidity and mortality in HFpEF.118. They included 37 randomized, parallel group trials, over 18,000 adults with HFpEF, defined as LVEF >40%. Among 10 studies (3087 subjects) investigating beta-blocker therapy, the pooled analysis indicated reduction in cardiovascular mortality (15% beta-blocker therapy vs 19% control; RR 0.78; 95% CI 0.62 to 0.99; number needed to treat to benefit [NNTB] 25). However, quality of evidence was low with no effect on cardiovascular mortality when limited to studies with low risk of bias (RR 0.81; 95% CI 0.50 to 1.29) and no effect on all-cause mortality, HF hospitalization or quality of life. For ACEI, 8 studies (2,061 subjects) were included with moderate overall quality of evidence. ACEI likely had little or no effect on cardiovascular mortality, all-cause mortality, HF hospitalization, or quality of life. ARB was assessed in 8 studies (8,755 subjects) with high overall quality of evidence suggesting that ARB had little or no effect on cardiovascular mortality, all-cause mortality, HF hospitalization, or quality of life but increased hyperkalemia (0.9% ARB vs 0.5% control; RR 1.88; 95% CI 1.07 to 3.33).
Available evidence for beta blockers, ACEIs, ARBs and ARNIs is limited and uncertainty exists whether these treatments have a role in HFpEF without an alternative indication (e.g. hypertension, chronic kidney disease, etc.). This comprehensive review highlights a persistent and critically important gap in available evidence.
Therapies to target microvascular dysfunction
As discussed previously, microvascular dysfunction due to the heightened systemic inflammatory state is an important pathophysiological component of HFpEF and has been considered a recent therapeutic target for investigations. For example, statin therapy is known to have an anti-inflammatory and anti-oxidant effect, however a subgroup analysis from GISSI-HF demonstrated no benefit with rosuvastatin in patients with ejection fraction >40%.93 But previous studies results are inconsistent due to limited power with small sample sizes and/or lack of adjustment for known prognostic factors and differences in baseline characteristics between patients treated with and without statins. A recent meta-analysis of prospective observational studies examining statins and mortality in HFpEF patients used propensity score analysis.119 Four studies with 5,536 patients (2,768 patients on statins; mean age, 65–77 years; male, 43–66%; CAD, 42–64%; hypertension, 61–82%; diabetes, 20–29%; follow-up duration, 12–36 months) were included. Pooled analysis found statin therapy was associated with reduced mortality (OR [95% CI] = 0.690 [0.493–0.965]; P=0.030). Future randomized trials in HFpEF are warranted to confirm this potential survival benefit of statins.
Another targeted pathway is interleukin-1 receptor blockade with anakinra (an IL-1 receptor blocker used for rheumatological disorders). In a randomized pilot trial of 28 patients with HFpEF, anakinra failed to improve exercise capacity at 12 weeks, but patients reported better quality of life.120 Similarly, the nitric oxide pathway, another mediator for vasodilation, was evaluated through use of isosorbide mononitrate in the NEAT-HFpEF trial, a double blind cross over trial of 110 patients with HFpEF. Isosorbide mononitrate failed to improve exercise capacity and quality of life compared with placebo.121 Collectively, these findings suggest that while microvascular dysfunction is an important component of the pathophysiology of HFpEF, it does not represent the unifying etiology, and HFpEF is likely to be a multifactorial condition.
Pharmacological therapies of potential benefit
Sodium-glucose cotransporter-2 inhibitors
Evolving evidence demonstrates that sodium-glucose cotransporter-2 (SGLT-2) inhibitors reduce the risk of HF hospitalizations among subjects with type 2 diabetes and at high risk for cardiovascular events.122, 123 This benefit was observed irrespective of whether the patients were considered at low or high risk for HF.124 In these trials, data regarding the EF were not reported, but it is reasonable to consider that this was related to HFpEF since most patients did not have cardiovascular disease at baseline. This beneficial effect in reducing the risk of HF hospitalizations has been attributed to the osmotic diuretic effect that this class of medications exerts through inhibition of glucose reabsorption in the proximal renal tubules, and hence reduction of the plasma volume.125, 126 An ongoing trial (EMPEROR-Preserved, NCT03057951) is evaluating the impact of empagliflozin on the composite of cardiac mortality or HF hospitalizations in patients with HFpEF without type 2 diabetes, whereas another trial, PRESERVED-HF NCT03030235, is evaluating the impact of another SGLT-2, dapagliflozin, on NP levels. It would be of interest to learn the impact of these agents on the symptoms of congestion in patients with HFpEF.
Mineralocorticoid receptor antagonists
Encouraged by strong evidence supporting mineralocorticoid receptor antagonists and benefit of reducing plasma volume in patients with HFrEF, spironolactone was evaluated in patients with HFpEF in the Treatment of Preserved Cardiac Function HF with an Aldosterone Antagonist (TOPCAT), a placebo-controlled trial of 3,445 patients.127 The main trial showed a non-significant reduction in cardiovascular mortality, aborted cardiac arrest, and HF hospitalizations, but the risk of HF hospitalizations was reduced with spironolactone. Interestingly, on further investigation it appears that patients enrolled from Russia and Georgia were unlikely to have HF at entry, and there were also concerns that patients from these countries likely did not take study medication.128 Some argue that TOPCAT could have been a positive trial in terms of the primary endpoint if these patients who did not have HF at baseline were not enrolled. In the recent Cochrane analysis evaluating the different therapies for HFpEF118, 12 studies (4,408 subjects) investigated mineralocorticoid receptor antagonists. The mineralocorticoid receptor antagonists reduced HF hospitalization (11% mineralocorticoid receptor antagonists vs 14% control; RR 0.82; 95% CI 0.69 to 0.98; NNTB 41; moderate-quality evidence), but had limited or no effect on all-cause and cardiovascular mortality and quality of life measures with higher hyperkalemia risk (16% mineralocorticoid receptor antagonists vs 8% control; RR 2.11; 95% CI 1.77 to 2.51). Taken these findings collectively, the ACC/AHA guidelines recommend spironolactone in patients with refractory HFpEF.50 An ongoing trial (SPIRRIT, NCT02901184) will enroll 3,335 patients with HFpEF to test the hypothesis that spironolactone reduces risk of all-cause mortality.
Neprilysin Inhibitors
As mentioned earlier, neprilysin inhibitors prevent breakdown of several vasoactive NPs that have an anti-inflammatory role and exert a lipolytic effect.129 Neprilysin inhibitors also act by lowering aldosterone levels and blocking its action.130 These beneficial effects would theoretically reduce plasma volume and improve microvascular function in patients with HFpEF. ARNI (sacubitril/valsartan) has shown encouraging results in a phase II randomized trial of 149 patients with HFpEF by reducing circulating N-terminal pro-brain NPs.131 Whether this benefit could be translated into reduction in cardiac mortality or HF hospitalizations is eagerly awaited when PARAGON-HF is completed.132 In addition, data from the ongoing PARALLAX trial, NCT03066804, will provide important details on the impact of ARNI on quality of life in patients with HFpEF.
Phosphodiestrase-5 inhibitors
Phosphodiestrase-5 inhibitors, in particular sildenafil, are widely used in the management of pulmonary hypertension133 and have been an area of investigations in patients with HFpEF, as these agents would hypothetically improve right-ventricular function. In one small randomized trial of HFpEF patients with pulmonary hypertension, sildenafil improved pulmonary pressure and LV diastolic dysfunction.134 By contrast, a large multicenter, double-masked, randomized trial (RELAX) of 216 patients failed to improve exercise capacity or clinical status with sildenafil in patients with HFpEF.135 However, it is important to note that pulmonary hypertension was not confirmed in patients enrolled in the RELAX trial, which might have contributed to the lack of benefit from sildenafil in that trial. Studies have demonstrated patients with HFpEF and pulmonary hypertension might not only develop isolated post-capillary pulmonary hypertension. In a subset of patients that developed combined pre- and post-capillary pulmonary hypertension, in one prospective registry, sildenafil was shown to improve symptoms and exercise capacity.136 An ongoing trial is evaluating this hypothesis in a randomized fashion (PASSION trial, German Registry for Clinical Studies DRKS00014595).
Summary
HF remains a leading cause of mortality and morbidity worldwide, and the prevalence of the condition continues to rise as our population ages and deaths due to MI continue to decrease, with HFpEF becoming the predominant type. IHD is a prevalent factor for both HFrEF and HFpEF. For therapy with either HFrEF or HFpEF, some individual steps in signaling cascades can be targeted by specific interventions: atherosclerosis risk by diet, physical activity, smoking cessation, and lipid lowering, systemic hypertension by blood pressure reduction, metabolic risk by caloric restriction, systemic inflammation by statins, pulmonary hypertension by phosphodiesterase-5 inhibitors, muscle weakness by exercise training, sodium and fluid retention by diuretics and monitoring devices, myocardial nitric oxide bioavailability by inorganic nitrate-nitrite, myocardial cyclic guanosine monophosphate content by neprilysin or phosphodiesterase-9 inhibition, and myocardial fibrosis by aldosterone inhibition. Because of the heterogeneity in both HF syndromes, personalized therapeutic strategies are proposed. Multiple established pharmacological therapies with proven survival benefit are available in patients with HFrEF as beta-blockers, ACEIs/ARBs, ARNI, and mineralocorticoid receptor antagonists. The evidence for therapies in HFpEF remains less convincing largely due to knowledge gaps in understanding the underlying mechanisms for this condition. Growing evidence suggests that HFpEF is a multifactorial condition with coronary microvascular dysfunction secondary to systemic inflammation, obesity, inactivity, and plasma volume expansion as potential underlying culprits. Similar to HFrEF management, comorbidities and risk factors such as blood pressure and weight control are key. Some therapies appear to have an emerging role in the management of HFpEF, such as SLGT-2 inhibitors, mineralocorticoid receptor antagonists, and ARNI. Ongoing trials (Table 3) will help to clarify the role of these pharmacological therapies in patients with HFpEF. The role for statins in HFpEF needs further evaluation.
Table 3:
Ongoing trials for potential therapies for heart failure with preserved ejection fraction (HFpEF)
| Intervention | Trial name | Clinical trial registration number | Primary outcome |
|---|---|---|---|
| Management of comorbidities | OPTIMIZE-HFPEF | NCT02425371 | Clinical score status |
| Empagliflozin (SLGT-2 inhibitor) | EMPEROR-Preserved | NCT03057951 | Composite of cardiac mortality or HF hospitalizations |
| Dapagliflozin (SLGT-2 inhibitor) | PRESERVED-HF | NCT03030235 | Natriuretic peptide levels |
| Spironolactone | SPIRRIT | NCT02901184 | All-cause mortality |
| Neprilysin inhibitors | PARAGON-HF | NCT01920711 | Composite of cardiac mortality or HF hospitalizations |
| Neprilysin inhibitors | PARALLAX | NCT03066804 | Quality of life |
| Sildenafil | PASSION | DRKS00014595 | Composite of all-cause mortality or HF hospitalizations |
HF=heart failure; SLGT-2 inhibitor= sodium-glucose cotransporter-2 inhibitors
Sources of Funding
Dr. Pepine receives support from the NIH (HL087366; HL132448; HL033610; HL130163); the US Department of Defense (PR161603—WARRIOR Trial); the Gatorade Trust through funds distributed by the University of Florida Department of Medicine; NIH NCATS—University of Florida Clinical and Translational Science UL1TR001427; and PCORnet-OneFlorida Clinical Research Consortium CDRN-1501–26692.
Disclosures
Dr Pepine reports grant support (significant) from Adelphi Values (Qualitative MVA), Amorcyte (PRESERVE), Athersys (MI-NSTEMI), BioCardia (CardiAMP), Brigham and Women’s Hospital (INVESTED), Capricor (ALLSTAR), Cytori Therapeutics (ATHENA), Duke Univ. (ADAPTABLE), Gilead Sciences Inc. (RWISE, Univ. FL site), Merck & Co. Inc. (VICTORIA), Mesoblast (TEVA, Univ. FL site), NIH/NHLBI (CONCERT), US Dept. of Defense (WARRIOR), Ventrix (CV-201); educational support (modest) for the Vascular Biology Working Group from Amgen Inc., AstraZeneca Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals Inc., Daiichi Sankyo, Ionis, Relypsa; Consultant fees/honoraria (modest) from Amgen Inc., AstraZeneca Pharmaceuticals, Bayer Healthcare Pharmaceuticals, Gilead, Merck and (significant) from Ironwood Pharmaceuticals Inc. and SLACK Inc.; Task force member (no compensation) FACT—Foundation for the Accreditation of Cellular Therapy. The other authors have no disclosures.
Non-standard Abbreviations and Acronyms
- ACE
angiotensin-converting enzyme
- ACEI
ACE inhibitor
- ACS
acute coronary syndrome
- ADVENT-HF
Effect of Adaptive Servo Ventilation (ASV) on Survival and Hospital Admissions in Heart Failure
- AIRE
Acute Infarction Ramipril Efficacy
- ARB
angiotensin receptor blocker
- ARNI
angiotensin receptor/neprilysin inhibitors
- ATLAS ACS-2, TIMI 51
Trial of Rivaroxaban in Patients with Recent Acute Coronary Syndrome
- BEAUTIFUL
Ivabradine for Patients with Stable Coronary Artery Disease and Left-Ventricular Systolic Dysfunction trial
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- COMMANDER HF
A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants with Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure
- COMPASS HF
Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure
- CORONA
Controlled Rosuvastatin Multinational Trial in HF
- EF
ejection fraction
- EMPEROR-Preserved
Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction
- EPHESUS
Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study
- FHS
Framingham Heart Study
- FREEDOM
Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease
- GISSI-HF
Effect of Rosuvastatin in Patients with Chronic Heart Failure trial
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- I PRESERVE
Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction trial
- IHD
ischemic heart disease
- IL-33
interleukin-33
- LV
left ventricular
- LVAD
left ventricular assist device
- MI
myocardial infarction
- NYHA
New York Heart Association
- OCS
Olmstead County Study
- OPTIMAAL
Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan
- OPTIMIZE-HFPEF
Optimizing the Management of Heart Failure With Preserved Ejection Fraction in the Elderly by Targeting Comorbidities trial
- PARADIGM-HF
Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure
- PARADISE-MI
Prospective ARNI vs ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI
- PARAGON-HF
Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients with Preserved Ejection Fraction
- PARALLAX
A Randomized, Double-blind Controlled Study Comparing LCZ696 to Medical Therapy for Comorbidities in HFpEF Patients
- PASSION
Paclitaxel Eluting Stent Versus Conventional Stent in ST-segment Elevation Myocardial Infarction
- PET
positron emission tomography
- PIONEER-HF
Comparison of Sacubitril/Valsartan versus Enalapril on Effect on nt-pRo-bnp in Patients Stabilized from an Acute Heart Failure Episode
- PRESERVED-HF
Dapagliflozin in PRESERVED Ejection Fraction Heart Failure
- RELAX
Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure with Preserved Ejection Fraction trial
- SAVE
Survival and Ventricular Enlargement trial
- SHIFT
Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial
- SOLVD
Studies of Left Ventricular Dysfunction Treatment trial
- SPIRRIT
Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure with Preserved Ejection Fraction
- STICH
Surgical Treatment for Ischemic HF trial
- TOPCAT
Treatment of Preserved Cardiac Function HF with an Aldosterone Antagonist
- TRACE
Trandolapril Cardiac Evaluation
- VALIANT
Valsartan in Acute Myocardial Infarction
- WARCEF
Warfarin and Aspirin in Patients with HF and Sinus Rhythm
- WASH
Warfarin/Aspirin Study in HF
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E Heart failure. JACC Heart Fail. 2013;1:1–20. 10.1016/j.jchf.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention, Stroke C. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JS, Wall HK, Ritchey MD. Million Hearts 2022: small steps are needed for cardiovascular disease prevention. JAMA. 2018. 10.1001/jama.2018.13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. 10.1001/jama.2009.1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology F, American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 7.Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–685. 10.1016/j.jchf.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marwick TH. Ejection Fraction Pros and Cons: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2360–2379. 10.1016/j.jacc.2018.08.2162 [DOI] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D, Framingham Heart S. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF, Sadowski Z, Golba KS, Prior DL, Rouleau JL, Bonow RO. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202–1213. 10.1161/CIRCULATIONAHA.106.623199 [DOI] [PubMed] [Google Scholar]
- 11.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168:1186–1194. 10.1016/j.ijcard.2012.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J, Evaluation MSGMIRC. Miracle Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. 10.1056/NEJMoa013168 [DOI] [PubMed] [Google Scholar]
- 13.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial I. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. 10.1056/NEJMoa043399 [DOI] [PubMed] [Google Scholar]
- 14.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. 10.1056/NEJMoa032423 [DOI] [PubMed] [Google Scholar]
- 15.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. 10.1056/NEJMoa050496 [DOI] [PubMed] [Google Scholar]
- 16.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R Jr., Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. 10.1056/NEJMoa042934 [DOI] [PubMed] [Google Scholar]
- 17.Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail. 2005;7:119–125. 10.1016/j.ejheart.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. 10.1056/NEJM199108013250501 [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Pepine CJ, Garces C, Pouleur H, Salem D, Kostis J, Benedict C, Rousseau M, Bourassa M, Pitt B. Effect of enalapril on myocardial infarction and unstable angina in patients with low ejection fractions. Lancet. 1992;340:1173–1178. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ Jr., Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. 10.1056/NEJM199209033271001 [DOI] [PubMed] [Google Scholar]
- 21.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. 10.1056/NEJM200004133421502 [DOI] [PubMed] [Google Scholar]
- 22.Steg PG, Dabbous OH, Feldman LJ, Cohen-Solal A, Aumont MC, Lopez-Sendon J, Budaj A, Goldberg RJ, Klein W, Anderson FA Jr. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation. 2004;109:494–499. 10.1161/01.CIR.0000109691.16944.DA [DOI] [PubMed] [Google Scholar]
- 23.Suleiman M, Khatib R, Agmon Y, Mahamid R, Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H, Aronson D. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J Am Coll Cardiol. 2006;47:962–968. 10.1016/j.jacc.2005.10.055 [DOI] [PubMed] [Google Scholar]
- 24.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. [DOI] [PubMed] [Google Scholar]
- 25.Scarabelli T, Stephanou A, Rayment N, Pasini E, Comini L, Curello S, Ferrari R, Knight R, Latchman D. Apoptosis of endothelial cells precedes myocyte cell apoptosis in ischemia/reperfusion injury. Circulation. 2001;104:253–256. [DOI] [PubMed] [Google Scholar]
- 26.Tomai F, Crea F, Chiariello L, Gioffre PA. Ischemic preconditioning in humans: models, mediators, and clinical relevance. Circulation. 1999;100:559–563. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi WT, Zhang ZM, Chang PP, Rosamond WD, Kitzman DW, Wagenknecht LE, Soliman EZ. Silent Myocardial Infarction and Long-Term Risk of Heart Failure: The ARIC Study. J Am Coll Cardiol. 2018;71:1–8. 10.1016/j.jacc.2017.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM, Antoniucci D. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–2357. [DOI] [PubMed] [Google Scholar]
- 29.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002;105:1503–1508. [DOI] [PubMed] [Google Scholar]
- 30.Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2016;375:1868–1877. 10.1056/NEJMcp1511175 [DOI] [PubMed] [Google Scholar]
- 31.Elgendy IY, Pepine CJ. Heart failure with preserved ejection fraction: is ischemia due to coronary microvascular dysfunction a mechanistic factor? Am J Med. 2019. 10.1016/j.amjmed.2018.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J, Health ABCSI. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. 10.1016/j.jacc.2009.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 34.Altara R, Ghali R, Mallat Z, Cataliotti A, Booz GW, Zouein FA. Conflicting vascular and metabolic impact of the IL-33/sST2 axis. Cardiovasc Res. 2018;114:1578–1594. 10.1093/cvr/cvy166 [DOI] [PubMed] [Google Scholar]
- 35.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. 10.1161/CIRCULATIONAHA.113.008507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. 10.1093/eurheartj/ehx721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G, Voors AA. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18:588–598. 10.1002/ejhf.497 [DOI] [PubMed] [Google Scholar]
- 38.Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, Venneri L, Tayal U, Auger D, Midwinter W, Whiffin N, Rajani R, Dungu JN, Pantazis A, Cook SA, Ware JS, Baksi AJ, Pennell DJ, Rosen SD, Cowie MR, Cleland JGF, Prasad SK. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet. 2018. 10.1016/S0140-6736(18)32484-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barakat AF, Mahmoud AN, Elgendy IY. Primary prevention implantable cardioverter defibrillator in patients with reduced ejection fraction: for ischemic or non-ischemic cardiomyopathy or both? J Thorac Dis. 2017;9:2749–2751. 10.21037/jtd.2017.08.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial III. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. 10.1056/NEJMoa013474 [DOI] [PubMed] [Google Scholar]
- 41.McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, Page RL, Hlatky MA, Rowe BH. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297:2502–2514. 10.1001/jama.297.22.2502 [DOI] [PubMed] [Google Scholar]
- 42.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH, HeartMate II I. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. 10.1056/NEJMoa0909938 [DOI] [PubMed] [Google Scholar]
- 43.Pagani FD. Intramyocardial injection of mesenchymal precursor cells in left ventricular assist device recipients: impact on myocardial recovery and morbidity Late Breaking Clinical Trial presented at the AHA Scientific Sessions 2018, Chicago, IL, 11 November 2018. Available at: https://professional.heart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_502946.pdf [Google Scholar]
- 44.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 45.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 46.Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. [DOI] [PubMed] [Google Scholar]
- 47.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. [DOI] [PubMed] [Google Scholar]
- 48.Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Australia/New Zealand Heart Failure Research Collaborative Group. Lancet. 1997;349:375–380. [PubMed] [Google Scholar]
- 49.Janosi A, Ghali JK, Herlitz J, Czuriga I, Klibaner M, Wikstrand J, Hjalmarson A, Group M-HS. Metoprolol CR/XL in postmyocardial infarction patients with chronic heart failure: experiences from MERIT-HF. Am Heart J. 2003;146:721–728. 10.1016/S0002-8703(03)00163-7 [DOI] [PubMed] [Google Scholar]
- 50.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 51.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 52.A placebo-controlled trial of captopril in refractory chronic congestive heart failure. Captopril Multicenter Research Group. J Am Coll Cardiol. 1983;2:755–763. [DOI] [PubMed] [Google Scholar]
- 53.Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol. 1988;62:60A–66A. [DOI] [PubMed] [Google Scholar]
- 54.Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–1676. 10.1056/NEJM199512213332503 [DOI] [PubMed] [Google Scholar]
- 55.Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993;342:821–828. [PubMed] [Google Scholar]
- 56.Dickstein K, Kjekshus J. Optimaal Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet. 2002;360:752–760. [DOI] [PubMed] [Google Scholar]
- 57.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM, Valsartan in Acute Myocardial Infarction Trial I. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. 10.1056/NEJMoa032292 [DOI] [PubMed] [Google Scholar]
- 58.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 59.Vizzardi E, D’Aloia A, Giubbini R, Bordonali T, Bugatti S, Pezzali N, Romeo A, Dei Cas A, Metra M, Dei Cas L. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure. Am J Cardiol. 2010;106:1292–1296. 10.1016/j.amjcard.2010.06.052 [DOI] [PubMed] [Google Scholar]
- 60.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Emphasis-Hf Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
- 61.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. 10.1056/NEJMoa030207 [DOI] [PubMed] [Google Scholar]
- 62.Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, van Veldhuisen DJ, Zannad F, Krum H, Mukherjee R, Vincent J, Investigators E. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46:425–431. 10.1016/j.jacc.2005.04.038 [DOI] [PubMed] [Google Scholar]
- 63.Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol. 2008;31:153–158. 10.1002/clc.20324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li MJ, Huang CX, Okello E, Yanhong T, Mohamed S. Treatment with spironolactone for 24 weeks decreases the level of matrix metalloproteinases and improves cardiac function in patients with chronic heart failure of ischemic etiology. Can J Cardiol. 2009;25:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramires FJ, Mansur A, Coelho O, Maranhao M, Gruppi CJ, Mady C, Ramires JA. Effect of spironolactone on ventricular arrhythmias in congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. Am J Cardiol. 2000;85:1207–1211. [DOI] [PubMed] [Google Scholar]
- 66.Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail. 2014;2:663–670. 10.1016/j.jchf.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 67.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 68.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, Investigators P-H. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med. 2018. 10.1056/NEJMoa1812851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, Atar D, Krum H. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8:71–78. 10.1161/CIRCHEARTFAILURE.114.001785 [DOI] [PubMed] [Google Scholar]
- 70.Patterson JH, Adams KF Jr., Applefeld MM, Corder CN, Masse BR. Oral torsemide in patients with chronic congestive heart failure: effects on body weight, edema, and electrolyte excretion. Torsemide Investigators Group. Pharmacotherapy. 1994;14:514–521. [PubMed] [Google Scholar]
- 71.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne-Nickens P, Sopko G, Rouleau JL, Investigators S. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med. 2016;374:1511–1520. 10.1056/NEJMoa1602001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bansilal S, Farkouh ME, Hueb W, Ogdie M, Dangas G, Lansky AJ, Cohen DJ, Magnuson EA, Ramanathan K, Tanguay JF, Muratov V, Sleeper LA, Domanski M, Bertrand ME, Fuster V. The Future REvascularization Evaluation in patients with Diabetes mellitus: optimal management of Multivessel disease (FREEDOM) trial: clinical and angiographic profile at study entry. Am Heart J. 2012;164:591–599. 10.1016/j.ahj.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs. 2007;67 Suppl 2:15–24. [DOI] [PubMed] [Google Scholar]
- 74.Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, Investigators S. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. 10.1016/S0140-6736(10)61198-1 [DOI] [PubMed] [Google Scholar]
- 75.Fox K, Ford I, Steg PG, Tendera M, Ferrari R, Investigators B. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. 10.1016/S0140-6736(08)61170-8 [DOI] [PubMed] [Google Scholar]
- 76.Swedberg K, Komajda M, Bohm M, Borer J, Robertson M, Tavazzi L, Ford I, Investigators S. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol. 2012;59:1938–1945. 10.1016/j.jacc.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 77.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 78.Al-Khadra AS, Salem DN, Rand WM, Udelson JE, Smith JJ, Konstam MA. Antiplatelet agents and survival: a cohort analysis from the Studies of Left Ventricular Dysfunction (SOLVD) trial. J Am Coll Cardiol. 1998;31:419–425. [DOI] [PubMed] [Google Scholar]
- 79.Cleland JG, Findlay I, Jafri S, Sutton G, Falk R, Bulpitt C, Prentice C, Ford I, Trainer A, Poole-Wilson PA. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J. 2004;148:157–164. 10.1016/j.ahj.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 80.Cokkinos DV, Haralabopoulos GC, Kostis JB, Toutouzas PK. Efficacy of antithrombotic therapy in chronic heart failure: the HELAS study. Eur J Heart Fail. 2006;8:428–432. 10.1016/j.ejheart.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 81.Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M, Jafri SM, Krol WF, O’Connor CM, Schulman KA, Teo K, Warren SR. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616–1624. 10.1161/CIRCULATIONAHA.108.801753 [DOI] [PubMed] [Google Scholar]
- 82.Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R, Investigators W. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. 10.1056/NEJMoa1202299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M, Lam CSP, Mehra MR, Neaton JD, Nessel CC, Spiro TE, van Veldhuisen DJ, Greenberg B, Investigators CH. Rivaroxaban in Patients with Heart Failure, Sinus Rhythm, and Coronary Disease. N Engl J Med. 2018;379:1332–1342. 10.1056/NEJMoa1808848 [DOI] [PubMed] [Google Scholar]
- 84.Greenberg BH. Effects of rivaroxaban on thrombotic events in heart failure patients with coronary disease and sinus rhythm Late Breaking Clinical Trial presented at the American Heart Association Scientific Sessions 2018, Chicago, November 11, 2018. Available at: http://www.abstractsonline.com/pp8/#!/4682/presentation/59523. [Google Scholar]
- 85.Connolly SJ, Eikelboom JW, Bosch J, Dagenais G, Dyal L, Lanas F, Metsarinne K, O’Donnell M, Dans AL, Ha JW, Parkhomenko AN, Avezum AA, Lonn E, Lisheng L, Torp-Pedersen C, Widimsky P, Maggioni AP, Felix C, Keltai K, Hori M, Yusoff K, Guzik TJ, Bhatt DL, Branch KRH, Cook Bruns N, Berkowitz SD, Anand SS, Varigos JD, Fox KAA, Yusuf S, investigators C. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:205–218. 10.1016/S0140-6736(17)32458-3 [DOI] [PubMed] [Google Scholar]
- 86.Korjian S, Braunwald E, Daaboul Y, Mi M, Bhatt DL, Verheugt FWA, Cohen M, Bode C, Burton P, Plotnikov AN, Gibson CM. Usefulness of Rivaroxaban for Secondary Prevention of Acute Coronary Syndrome in Patients With History of Congestive Heart Failure (from the ATLAS-ACS-2 TIMI-51 Trial). Am J Cardiol. 2018;122:1896–1901. 10.1016/j.amjcard.2018.08.034 [DOI] [PubMed] [Google Scholar]
- 87.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 88.Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. 10.1056/NEJM199811053391902 [DOI] [PubMed] [Google Scholar]
- 89.Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., Garcia FA, Gillman MW, Kemper AR, Krist AH, Kurth AE, Landefeld CS, LeFevre ML, Mangione CM, Phillips WR, Owens DK, Phipps MG, Pignone MP. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:1997–2007. 10.1001/jama.2016.15450 [DOI] [PubMed] [Google Scholar]
- 90.Kjekshus J, Pedersen TR, Olsson AG, Faergeman O, Pyorala K. The effects of simvastatin on the incidence of heart failure in patients with coronary heart disease. J Card Fail. 1997;3:249–254. [DOI] [PubMed] [Google Scholar]
- 91.Raina A, Pickering T, Shimbo D. Statin use in heart failure: a cause for concern? Am Heart J. 2006;152:39–49. 10.1016/j.ahj.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 92.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Janosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J, Group C. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. 10.1056/NEJMoa0706201 [DOI] [PubMed] [Google Scholar]
- 93.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, Gissi HFI. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. 10.1016/S0140-6736(08)61240-4 [DOI] [PubMed] [Google Scholar]
- 94.Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, Adourian A, Bohm M, van Veldhuisen DJ, Komajda M, Cleland JG, Wikstrand J, McMurray JJ, Aukrust P, Group CS. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Eur Heart J. 2012;33:2290–2296. 10.1093/eurheartj/ehs077 [DOI] [PubMed] [Google Scholar]
- 95.McMurray JJ, Kjekshus J, Gullestad L, Dunselman P, Hjalmarson A, Wedel H, Lindberg M, Waagstein F, Grande P, Hradec J, Kamensky G, Korewicki J, Kuusi T, Mach F, Ranjith N, Wikstrand J, Group CS. Effects of statin therapy according to plasma high-sensitivity C-reactive protein concentration in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): a retrospective analysis. Circulation. 2009;120:2188–2196. 10.1161/CIRCULATIONAHA.109.849117 [DOI] [PubMed] [Google Scholar]
- 96.Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139:72–77. [DOI] [PubMed] [Google Scholar]
- 97.Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, Pinkett T, Ghali JK, Wilson AC. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol. 1996;77:1017–1020. [DOI] [PubMed] [Google Scholar]
- 98.Kapoor M, Schleinitz MD, Gemignani A, Wu WC. Outcomes of patients with chronic heart failure and iron deficiency treated with intravenous iron: a meta-analysis. Cardiovasc Hematol Disord Drug Targets. 2013;13:35–44. [DOI] [PubMed] [Google Scholar]
- 99.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, Investigators C-H. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J. 2015;36:657–668. 10.1093/eurheartj/ehu385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bradley TD, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS, Investigators C. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. 10.1056/NEJMoa051001 [DOI] [PubMed] [Google Scholar]
- 101.Smith LA, Vennelle M, Gardner RS, McDonagh TA, Denvir MA, Douglas NJ, Newby DE. Auto-titrating continuous positive airway pressure therapy in patients with chronic heart failure and obstructive sleep apnoea: a randomized placebo-controlled trial. Eur Heart J. 2007;28:1221–1227. 10.1093/eurheartj/ehm131 [DOI] [PubMed] [Google Scholar]
- 102.Abuzaid AS, Al Ashry HS, Elbadawi A, Ld H, Saad M, Elgendy IY, Elgendy A, Mahmoud AN, Mentias A, Barakat A, Lal C. Meta-Analysis of Cardiovascular Outcomes With Continuous Positive Airway Pressure Therapy in Patients With Obstructive Sleep Apnea. Am J Cardiol. 2017;120:693–699. 10.1016/j.amjcard.2017.05.042 [DOI] [PubMed] [Google Scholar]
- 103.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS, Investigators S, Coordinators. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 2016;375:919–931. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 104.Tsimploulis A, Lam PH, Arundel C, Singh SN, Morgan CJ, Faselis C, Deedwania P, Butler J, Aronow WS, Yancy CW, Fonarow GC, Ahmed A. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2018;3:288–297. 10.1001/jamacardio.2017.5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. 10.1016/j.jacc.2014.03.034 [DOI] [PubMed] [Google Scholar]
- 106.Cikes M, Claggett B, Shah AM, Desai AS, Lewis EF, Shah SJ, Anand IS, O’Meara E, Rouleau JL, Sweitzer NK, Fang JC, Saksena S, Pitt B, Pfeffer MA, Solomon SD. Atrial fibrillation in heart failure with preserved ejection fraction: the TOPCAT trial. JACC Heart Fail. 2018;6:689–697. 10.1016/j.jchf.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68:2217–2228. 10.1016/j.jacc.2016.08.048 [DOI] [PubMed] [Google Scholar]
- 108.Fu M, Zhou J, Thunstrom E, Almgren T, Grote L, Bollano E, Schaufelberger M, Johansson MC, Petzold M, Swedberg K, Andersson B. Optimizing the Management of Heart Failure With Preserved Ejection Fraction in the Elderly by Targeting Comorbidities (OPTIMIZE-HFPEF). J Card Fail. 2016;22:539–544. 10.1016/j.cardfail.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 109.Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol. 2016;68:200–203. 10.1016/j.jacc.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 110.Packer M, Kitzman DW. Obesity-Related Heart Failure With a Preserved Ejection Fraction: The Mechanistic Rationale for Combining Inhibitors of Aldosterone, Neprilysin, and Sodium-Glucose Cotransporter-2. JACC Heart Fail. 2018;6:633–639. 10.1016/j.jchf.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 111.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation. 2017;136:6–19. 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;302:H2148–2165. 10.1152/ajpheart.00907.2011 [DOI] [PubMed] [Google Scholar]
- 113.Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. 10.1016/j.jacc.2008.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Bohm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson A, Wikstrand J, Kotecha D, Beta-blockers in Heart Failure Collaborative G. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39:26–35. 10.1093/eurheartj/ehx564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lam PH, Gupta N, Dooley DJ, Singh S, Deedwania P, Zile MR, Bhatt DL, Morgan CJ, Pitt B, Fonarow GC, Ahmed A. Role of high-dose beta-blockers in patients with heart failure with preserved ejection fraction and elevated heart rate. Am J Med. 2018. 10.1016/j.amjmed.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. 10.1056/NEJMoa0805450 [DOI] [PubMed] [Google Scholar]
- 117.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. 10.1016/S0140-6736(03)14285-7 [DOI] [PubMed] [Google Scholar]
- 118.Martin N, Manoharan K, Thomas J, Davies C, Lumbers RT. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 2018;6:CD012721 10.1002/14651858.CD012721.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fukuta H, Goto T, Wakami K, Ohte N. The effect of statins on mortality in heart failure with preserved ejection fraction: a meta-analysis of propensity score analyses. Int J Cardiol. 2016;214:301–306. 10.1016/j.ijcard.2016.03.186 [DOI] [PubMed] [Google Scholar]
- 120.Van Tassell BW, Buckley LF, Carbone S, Trankle CR, Canada JM, Dixon DL, Abouzaki N, Oddi-Erdle C, Biondi-Zoccai G, Arena R, Abbate A. Interleukin-1 blockade in heart failure with preserved ejection fraction: rationale and design of the Diastolic Heart Failure Anakinra Response Trial 2 (D-HART2). Clin Cardiol. 2017;40:626–632. 10.1002/clc.22719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, LeWinter MM, Joseph SM, Shah SJ, Semigran MJ, Felker GM, Cole RT, Reeves GR, Tedford RJ, Tang WH, McNulty SE, Velazquez EJ, Shah MR, Braunwald E. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373:2314–2324. 10.1056/NEJMoa1510774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 123.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 124.Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, Woerle HJ, Hantel S, George JT, Johansen OE, Inzucchi SE. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2018;39:363–370. 10.1093/eurheartj/ehx511 [DOI] [PubMed] [Google Scholar]
- 125.Saad M, Mahmoud AN, Elgendy IY, Abuzaid A, Barakat AF, Elgendy AY, Al-Ani M, Mentias A, Nairooz R, Bavry AA, Mukherjee D. Cardiovascular outcomes with sodium-glucose cotransporter-2 inhibitors in patients with type II diabetes mellitus: A meta-analysis of placebo-controlled randomized trials. Int J Cardiol. 2017;228:352–358. 10.1016/j.ijcard.2016.11.181 [DOI] [PubMed] [Google Scholar]
- 126.Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2018. 10.1016/j.jacc.2018.06.040 [DOI] [PubMed] [Google Scholar]
- 127.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 128.de Denus S, O’Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL. Spironolactone metabolites in TOPCAT - new insights into regional variation. N Engl J Med. 2017;376:1690–1692. 10.1056/NEJMc1612601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Polak J, Kotrc M, Wedellova Z, Jabor A, Malek I, Kautzner J, Kazdova L, Melenovsky V. Lipolytic effects of B-type natriuretic peptide 1–32 in adipose tissue of heart failure patients compared with healthy controls. J Am Coll Cardiol. 2011;58:1119–1125. 10.1016/j.jacc.2011.05.042 [DOI] [PubMed] [Google Scholar]
- 130.Nakagawa H, Oberwinkler H, Nikolaev VO, Gassner B, Umbenhauer S, Wagner H, Saito Y, Baba HA, Frantz S, Kuhn M. Atrial natriuretic peptide locally counteracts the deleterious effects of cardiomyocyte mineralocorticoid receptor activation. Circ Heart Fail. 2014;7:814–821. 10.1161/CIRCHEARTFAILURE.113.000885 [DOI] [PubMed] [Google Scholar]
- 131.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. 10.1016/S0140-6736(12)61227-6 [DOI] [PubMed] [Google Scholar]
- 132.Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Shi VC, Lefkowitz MP, McMurray JJV. Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction: Rationale and Design of the PARAGON-HF Trial. JACC Heart Fail. 2017;5:471–482. 10.1016/j.jchf.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 133.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. 10.1056/NEJMoa050010 [DOI] [PubMed] [Google Scholar]
- 134.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. 10.1161/CIRCULATIONAHA.110.983866 [DOI] [PubMed] [Google Scholar]
- 135.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. 10.1001/jama.2013.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Opitz CF, Hoeper MM, Gibbs JS, Kaemmerer H, Pepke-Zaba J, Coghlan JG, Scelsi L, D’Alto M, Olsson KM, Ulrich S, Scholtz W, Schulz U, Grunig E, Vizza CD, Staehler G, Bruch L, Huscher D, Pittrow D, Rosenkranz S. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol. 2016;68:368–378. 10.1016/j.jacc.2016.05.047 [DOI] [PubMed] [Google Scholar]