Abstract
Hyaluronic acid (HA, also known as hyaluronan), is a non-sulfated linear glycosaminoglycan polymer consisting of repeating disaccharide units of d-glucuronic acid and N-acetyl-d-glucosamine abundantly present in the extracellular matrix. The sizes of hyaluronic acid polymers range from 5000 to 20,000,000 Da in vivo, and the functions of HA are largely dictated by its size. Due to its high biocompatibility, HA has been commonly used as soft tissue filler as well as a major component of biomaterial scaffolds in tissue engineering. Several studies have implicated that HA may promote differentiation of adipose tissue derived stem cells in vitro or in vivo when used as a supporting scaffold. However, whether HA actually promotes adipogenesis in vivo and the subsequent metabolic effects of this process are unclear. This review summarizes some recent publications in the field and discusses the possible directions and approaches for future studies, focusing on the role of HA in the adipose tissue.
Keywords: Hyaluronan, Hyaluronic acid, Adipose tissue, Adipogenesis, Extracellular matrix, Dermal filler
Introduction
Hyaluronan (HA) is a non-sulfated linear glycosaminoglycan polymer consisting of repeating disaccharide units of β−1,4 linked d-glucuronic acid (GlcUA) and β−1,3 linked N-acetyl-d-glucosamine (GlcNAc). HA is secreted to the extracellular matrix in most of mammalian tissues. It is synthesized by three plasma membrane-bound hyaluronan synthases, HAS1, HAS2 and HAS3. During their synthesis, the nascent HA chains are extruded through pore-like structures into the extracellular space [1]. Newly synthesized HA can be processed by hyaluronidases (HYALs) or broken down non-enzymatically by reactive oxygen species [2]. Hyaluronidases hydrolyze the hexosaminidic β(1–4) linkage between GlcNAc and GlcUA of the HA chain and release small HA fragments.
The half-life of HA differs in different organs [3,4]. The turnover of HA is extremely high in circulation. In humans, the plasma half-life of HA is estimated to be about 2–6 min, resulting in a total turnover of 10–100 mg per day [5], The whole body HA turnover in various tissues is estimated to take place within 3 days with about 5 g per day turned over [6]. HA synthesis and degradation is also very dynamic at the cellular level. In cells, normal HA synthesis is activated transiently for cell division or motility, after which HA is rapidly cleared from the site by endocytic uptake and hyaluronidase-catalyzed hydrolysis [7].
Cosmetic use of hyaluronan
HA has high cross-species structural homology, which makes HA synthesized in bacteria or other species non-antigenic and non-immunogenic in humans [8]. This property enables its widespread application for cosmetic uses [9]. In fact, HA plays a central role in the dermal filler industry. By itself, it is the agent of choice for wrinkle fillers, preferred over collagens or other categories of smaller synthetic wrinkle fillers [10]. HA fillers are also useful in repairing scars or in other conditions, such as HIV-associated lipodystrophies [11,12] and steroid atrophy [13]. Most HA-fillers are derived from bacterial culture to ensure very low protein contamination, and multiple cross-linking approaches have been developed to increase its stability. Unwanted side effect of HA fillers are rare, and the effects last from several months to over a year [10,14].
HA fillers are predominantly injected into the subcutaneous adipose tissue, and deeper injections lead to prolonged efficacy [15]. Longevity of the volumizing effect after injection of HA fillers demonstrates high inter-subject variations, no correlation was found between the longevity of volumizing effect and the tissue hyaluronidase level [16]. Skin improvements can be observed even after full biodegradation of the filler. Moreover, the longevity of HA filler effects may be connected with some long-term structural modification of the adipose tissue [17].
Physical properties of hyaluronan solutions that can influence the state of the adipose tissue
HA has a very high affinity for water molecules. At the same time, it is a soluble polymer which is normally rapidly resorbed after injection. Its stability and mechanical properties are improved if HA is cross-linked or bound to collagens when used as a cosmetic dermal filler.
The concentration of HA in different tissues varies significantly, being up to 500 μg/g in human skin, up to 5 mg/g in uterine cervix at late pregnancy, up to 3 mg/mL in synovial fluid, and up to 100 ng/mL in blood serum [18,19]. In hypertrophic adipose tissue, HA was measured in concentration up to 16 pg/cell [20], which for a cell with a diameter of about 100 μm corresponds to a volume concentration of about 30 μg/g. Since HA in adipose tissue is mainly concentrated in the pericellular space around the adipocyte, local concentrations of the HA around hypertrophic adipocytes are likely to be much higher.
The relative concentration of HA is of primary importance for the biophysical properties of an HA solution, since HA molecules behave as highly hydrated random coils, which start to entangle at concentrations of approximately 1 mg/mL [21]. Above the entanglement point, the viscosity of HA solution rapidly increases with increasing HA concentration c (exponentially, as c3.3), and the HA solution becomes gel-like. This behavior can significantly influence the proliferative and differentiative properties of adipose tissue stem cells (ASCs) [22]. The elasticity of the HA gel also increases with increasing molecular weight and concentration of HA.
The osmotic pressure in an HA gel is dependent on its concentration and ionic strength of the solution, J, as Π = Ac9/4J−3/4, where A is about 1.4 × 103 kPa, and c and J are expressed in mole [23]. This behavior corresponds to earlier reported experimental results that the HA solutions with concentrations of 5 mg/mL, 10 mg/mL, and 20 mg/mL have the osmotic pressures of about 1 mm Hg, 4.5 mm Hg and 18 mm Hg, respectively [24]. This osmotic pressure can be further increased if HA is connected with collagen, which is the case in WAT, where the pericellular HA is connected with Col VI. Since the concentration of HA can increase more than twice in WAT of diet-induced and genetically obese mice compared to controls [20], this phenomenon can lead to an approximately 4.75-fold increase of the osmotic pressure in the gel. Such an increase of osmotic pressure can strongly decrease the transcapillary transport in WAT, thus creating conditions similar to those observed in solid tumors [25]. The osmotic pressure in an HA gel can be reduced if the solution contains salts with high ionic strength [23]. For example, in a solution containing 100 mM NaCl + 100 mM CaCl2, the osmotic pressure in an HA gel will be reduced 3.5-fold compared to a solution containing 200 mM NaCl. A recent report indicated that differentiating preadipocytes (which are known to produce high levels of HA during differentiation) demonstrate reduced fat deposition in the presence of higher concentrations of NaCl [26].
Once the cell experiences an increase in the external osmotic pressure, water flows out of the cell, its volume and turgor decreases and the cell shrinks until a new osmotic equilibrium is reached. This can inflict significant damage on cells. To counter this damage, different types of cells rapidly produce and accumulate polyols, which, in the case of adipocytes, are present as glycerol. Glycerol accumulated intracellularly would leak out of the cell if glycerol transports via aquaglyceroporin channels, unless these channels are effectively inactivated. If this process persists, adipocyte would need to continuously synthesize glycerol or undergo lipolysis. This continuous glycerol efflux from adipocytes would enter the circulation and end up in the liver and other organs. This could be the underlying mechanism for body contouring. For example, additional adipose tissue HA is produced at high temperature during the body contouring treatment. The HA binds to a large amount of water that temporally improves the local skin texture while at the same time promotes the adipose tissue lipolysis; afterward, excessively accumulated water and exported glycerol and lipids will be cleared over the time, which leads to a circumference reduction effect.
Hyaluronan in adipogenesis
White adipocyte tissue grows by cellular hyperplasia and volume expansion during development and a calorie surplus. However, the origin of white adipocytes and developmental process, especially in adulthood, are complicated and remain to be completely understood, though much progress has been made in the recent past [27]. The prevailing hypothesis is that a perivascular population of cells which are high in Pdgfrβ and Zfp423 and resemble mural cells (pericytes and vascular smooth muscle cells) give rise to new adipocytes under pro-adipogenic conditions, such as high-fat diet treatment [28]. During maturation of preadipocytes, they progressively change their shape and accumulate lipid droplets, a process that needs to be coordinated with the remodeling of the extracellular matrix (ECM) to accommodate the expanding cellular volume and intercellular space [29]. Vice versa, adipogenesis is spatially and temporally regulated by ECM.
Changes in HA levels have been observed during differentiation of 3T3–L1 preadipocytes in vitro [30], hyperglycemia can even divert dividing osteoblastic precursor cells to a metabolically stressed adipogenic program, while at the same time inducing the synthesis of hyaluronan [34]. Supplementation of HA in culture medium prolonged lifespan, reduced cellular senescence, and enhanced differentiation potential of murine adipose tissue stromal cells [31]. In contrast, adipogenesis in 3T3–L1 cells was inhibited by reducing HA levels via treating them with exogenous hyaluronidase, or by inhibiting HA synthesis via 4-methylumbelliferone treatment or by reducing HAS2 levels [32]. It is important to note that most adipocytes are cultured under high-glucose and high-insulin conditions to promote differentiation and maintain their adipocyte identity [30], an environment that can promote inflammation and the generation of reactive oxygen species (ROS) [33]. These in vitro conditions, therefore, may not reflect physiological adipose tissue differentiation in vivo. Nevertheless, in a high-fat diet induced obesity mouse model, in vivo administration of exogenous hyaluronidase enzymes reduced abdominal fat accumulation and inhibited lipid accumulation in liver and thereby increased insulin sensitivity [32,35], implicating a possible role of HA in adipogenesis in vivo.
HA exerts many different biological functions on adipose tissue via binding to different cell surface proteins, including receptors such as CD44, RHAMM/HMMR, Brevican, TNFIP6, LYVE1 and SHAP [36–38]. CD44 is one of major cell surface binding proteins for HA [39], and the PDGFRɑ + CD44+ subpopulation of preadipocyte is highly proliferative [40]. Activation of RHAMM/HMMR receptor antagonizes the CD44 signaling and suppresses adipogenesis [41]. The adipogenic potential of HA combined with its physical properties makes it the top choice of supporting matrices for in vivo transplantation of preadipocytes or adipocyte stem cells (ASCs) [42–46]. A study that evaluated different scaffolds for human ASC allografts showed that differentiation of hASCs was augmented when the cells were encapsulated in cross-linked hyaluronan scaffolds gels [47]. A similar experiment performed in pigs showed the emergence of islets of mature adipocytes and neovascularization of the fat tissue arising from injected preadipocytes mixed with HA gels; interestingly, the degree of crosslinking by carboxyl groups amidation seems to be an important factor in determining the adipogenic potential of the HA gel [46]. HA scaffolds were also shown to decrease the necrosis of adipocytes during allografting in a rabbit model [48].
HA is also used in in vivo differentiation of “beige” or “brite” adipocyte, the third kind of adipocytes that are similar to brown adipocytes, rich in mitochondria and uncoupling protein-1 (UCP1) [49,50]. Beige adipocytes can emerge through transdifferentiation from white adipocytes controlled by sympathetic neuronal signals [51–53], or through de novo beige adipogenesis, also mediated by sympathetic input [54,55]. Expansion of UCP1 positive beige adipocytes that uncouple mitochondrial respiration from ATP synthesis has repeatedly been shown to be physiologically beneficial by reducing circulating glucose and lipid levels. However, in human adults, brown or beige adipose tissues are scarce, and cold-induction remains the most efficient method to induce beige adipose tissue thus far. It is therefore important to engineer large quantities of UCP1-positive beige adipocytes in vivo. Recent developments in formulating hyaluronic acid-based scaffolds have enabled functional tissue allografts [56], that support the in vivo differentiation of transplanted ASCs to beige adipose tissue with successful vascularization in the host [57].
Despite many positive results in vitro or in animal models, HA’s pro-adipogenic effects have been questioned in some studies [58]. The challenge that these aforementioned studies face is to go back to the exact injection site to dissect the transplant tissue out for histological analysis. Many times the histological pictures are inconclusive in distinguishing adipocytes from other types of cells in the matrix, especially among cells loaded with lipid droplets and bona fide adipocytes.
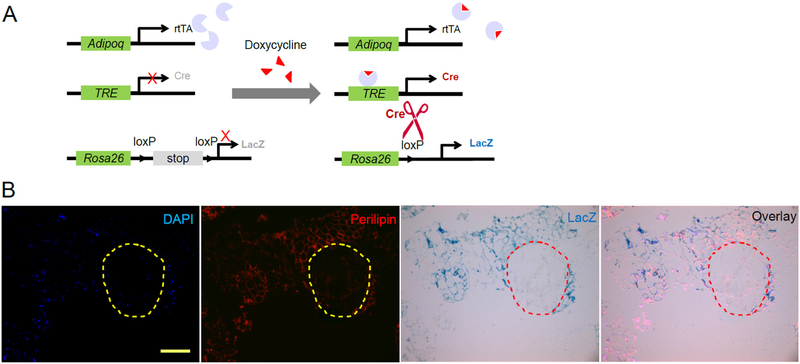
One approach to more carefully dissect the role of HA in adipogenesis in vivo is utilizing the AdipoChaser mouse model we previously developed (Fig. 1A) [54]. After supplementing doxycycline in the diet for 4–5 days, all existing adipocytes are labeled in blue (after reacting with the substrate X-gal), then doxycycline is withdrawn, any new adipocytes emerging from that point forward remain unlabeled. To test the effects of the HA-based dermal filler Juvederm Ultra XC (Allergan, Santa Barbara) on adipogenesis in vivo, we used this mouse model and pre-labeled all adipocytes blue using doxycycline, and after complete doxycycline withdrawal, we injected Juvederm into the inguinal fat pad and switched mice to a high-fat diet to promote adipogenesis. 6 weeks later we dissected the fat pads injected with Juvederm and performed LacZ staining, and performed immunofluorescent staining for Perilipin1 (the slides were also counterstained with DAPI). We can clearly visualize the blue staining overlapping with the red Perilipin1 staining, indicating pre-existing, pre-labeled adipocytes. Within the Juvederm gel area, multiple nuclei are stained with DAPI, suggesting infiltration of cells into the Juvederm gel. However, none of those cells have positive Perilipin1 staining, suggesting none of them are mature adipocytes (Fig. 1B).
Fig. 1.
Detection of de novo adipogenesis using AdipoChaser mice. (A) Schematic graph of the AdipoChaser system. Adiponectin-rtTA (Adipoq-rtTA), TRE-Cre and Rosa26-loxP-stop-loxP-lacZ triple transgenic mouse is hereby called the AdipoChaser mouse. It constitutively expresses rtTA in mature adipocytes but only expresses Cre when doxycycline (dox) is supplemented. The Cre will subsequently recombine the loxP sites and remove the stop cassette to allow expression of LacZ. The LacZ expression will persist even after removal of dox. But new adipocytes emerging from non-adiponectin expressing progenitor or stem cells after doxycycline removal will not express LacZ. LacZ reacts with X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and develops a dark blue color. So existing mature adipocytes will be labeled blue after dox supplementation, and any new adipocytes emerging after removal of dox will not be labeled. (B) Representative β-gal (blue) and Perilipin1 (red) staining of subcutaneous white adipose tissue in AdipoChaser mouse 6 weeks after Juvederm injection. Nuclei are counterstained with DAPI (blue). The circle indicates the boundary of Juvederm and adipose tissue. Scale bar: 250 μm.
Clearly, this result should not be interpreted as evidence suggesting HA cannot promote adipogenesis in vivo. The Juvederm gel used in the study is a cross-linked HA with a very high HA concentration of 24 mg/mL, which should produce high osmotic pressure within the gel area and may pose a restrictive environment for adipose progenitor cells to expand and further develop into mature adipocytes. Future studies will have to test whether alternative HA mixtures are better, or test the effects in transgenic mouse models that overproduce HA locally. It is also important to point out that the inguinal fat pad is known to have very low adipocyte proliferation rates in vivo. There may be a very high barrier for adipogenesis to take place in this inguinal adipose depot. Pretreatment of mice with tamoxifen, which stimulates de novo adipogenesis following transient lipoatrophy [59] may lower this barrier for HA to stimulate adipogenesis in the inguinal fat pad.
Furthermore, we cannot exclude that the cells infiltrating the HA gel are the immature adipocytes. Low molecular weight HA generated during degradation of HA gel is shown to induce chemokine expression in macrophages [60] and endothelial cells [61] through induction of CD44 and CXC1/GRO1 proteins. CXC1 is one of the dominant chemokines in preadipocytes [62], and it is recently shown that the immature adipocytes undergo chemotaxis through the activation of CXCL1 and CXCL8 [63]. From these observations, we can infer that the degradation of the HA gel and the production of low molecular weight fragments may induce the chemotaxis of immature adipocytes leading to their penetration into the HA gel.
HA effects on the proliferation of ASCs have also been demonstrated to be dose-dependent, with a maximal proliferation of these cells at an HA concentration corresponding to the HA entanglement point of about 1 mg/mL [22]. Further increase of HA concentrations above this point reduces the proliferation of ASCs which can be connected with rapidly increasing viscosity and osmotic pressure under such high HA concentrations. This can also explain some of the contradictory experimental results reported.
Hyaluronan in adipose tissue and systemic metabolism
Adipocyte hyperplasia and hypertrophy are hall-marks of obesity, which precedes the development of many metabolic diseases, including diabetes [64,65]. Accumulating evidence suggests a role of HA in this process. For example, the HA receptors RHAMM/HMMR and CD44 have been implicated in the development of diabetes. Especially, a genome-wide association study links the major HA receptor CD44 with type 2 diabetes [66]. Injection of an anti-CD44 monoclonal antibody makes mice more resistant to insulin-dependent diabetes mellitus [67], suggesting that the HA activated CD44 signaling is involved in the development of the disease. Beyond the mechanisms that we previously discussed as to how HA may alternate physical properties of adipose tissue and subsequently its metabolism, HA is also implicated in the modulation of extracellular matrix and inflammative states of adipose tissue and many other metabolic organs. Importantly, HA is one of the most reliable bio-markers for non-alcoholic fatty liver disease [68], with an especially strong predicting power in liver fibrosis when combined with other serum biomarkers such as procollagen III N-terminal peptide and TIMP1 [69]. Also, high levels of HA and inflammatory cells accumulate around diabetic pancreatic islets suggesting a role of HA in type 1 diabetes [70].
In diabetic patients, serum and tissue HA is elevated [71] as excess glucose in circulation and tissue enters hexosamine biosynthetic pathway to produce UDP-GlcNAc, an intermediate metabolite for HA synthesis [72]. The elevated intracellular UDP-GlcNAc levels also affect protein modification to affect cellular signaling such as PKC signaling [73] which leads to the increase of HAS2 expression [74,75], serving as a feed-forward mechanism to synergistically promote HA synthesis.
Treatment of HFD-induced insulin resistant mice with recombinant hyaluronidase PH20 (PEGPH20) reduced HA accumulation in muscle and improved whole-body insulin sensitivity [35]. Interestingly, treatment with PEGPH20 also resulted in up to 35% reduction in adipose tissue mass and a simultaneous reduction in adipocyte size [35]. The improvement of insulin sensitivity was attributed to better blood perfusion in skeletal muscle [35]. Whether the reduction in adipose tissue weight contributed to the improvement of insulin sensitivity is unknown, nor the direct effect of reducing HA in the adipose tissue.
Importantly, the metabolic outcome of HA is also a function of its size [38], and the lower molecular weight HA fragments have very different functions in contrast to the high molecular weight HA on adipose tissue. Small HA fragments produced by hyaluronidases can induce angiogenesis, an important component of adipose tissue healthy expansion. However, a recent study showed medium molecular weight (approx. 50 kDa) HA inhibits adipogenesis in cultured 3 T3–L1 cells [76], complicating the view on the role of different molecular weight HAs in adipogenesis. In a separate report from the same research group, the 50 kDa HA fragments have been shown to decrease adipogenic differentiation in vitro and in vivo. Oral administration of these HA fragments decreased body weight, adipose tissues, serum lipid (low-density lipoprotein cholesterol, triglyceride), and leptin levels in mice fed on a high-fat diet. HA fragments also decreased the hypertrophy of adipose tissue and ameliorated liver steatosis, showing a strong anti-obesity and anti-diabetic effect, possibly through enhancing PPARα and suppressing PPARγ expression [77]. It is important to note that the orally administrated HA may not enter circulation, so the site of action for the HA used in this study may be the intestinal digestive track.
These seemingly contradictory studies reveal the complexity of the function of HA. Previous studies largely rely on in vitro experiments. Even when in vivo experiments were performed, most of the time, it involved whole body genetic manipulations or drug treatments that affect simultaneously multiple organs in the research subjects. Tissue-specific and inducible systems will be more useful for a careful assessment of the functions of HA in various metabolic organs and the potential roles in multiple organ crosstalks. Doxycycline-inducible and tissue-specific HA synthase or hyaluronidase overexpression systems should serve this purpose and will be of great value to the studies of functions of HA in metabolic tissues, including the adipose tissue.
Concluding remarks
Decades of HA studies started from chemical structure, physical properties to the roles of HA in mediating immune response, with a good portion of the effects devoted to bioengineering of the HA as a scaffold for many biological applications. Recent advancements in genetics and the development of serum stable hyaluronidase enzymes have advanced our understanding of HA in the metabolism, especially the role of HA in adipogenesis and adipose tissue metabolism. Future studies should leverage the advancement in genetically engineered animal models that tissue specifically overexpress an HA synthase or a hyaluronidase to carefully dissect the roles of HA in the adipose tissue and other metabolic organs.
Acknowledgments
The original research was supported by NIH Grants R01-DK55758, R01-DK099110, P01-DK088761, and P01-AG051459 as well as a grant from the Cancer Prevention and Research Institute of Texas (CPRIT RP140412) to P.E.S., NIH Grant K99-DK114498 to Y.Z. I.L.K is the managing partner of Wellcomet GmbH. Wellcomet GmbH provided support in the form of salaries for I.L.K, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The commercial affiliation of I.L.K with Wellcomet GmbH does not alter our adherence to all Matrix Biology policies on sharing data and materials.
The authors apologize for the inability, due to the enormous amount of literature on hyaluronan studies, to reference all studies relevant to this review.
Abbreviations used:
- HA
hyaluronic acid
- ECM
extracellular matrix
- HYAL
hyaluronidase
- ASCs
adipose tissue stem cells
- UCP1
uncoupling protein-1
- GlcUA
d-glucuronic acid
- GlcNAc
N-acetyl-d-glucosamine
- UDP
Uridine diphosphate.
References
- [1].Prehm P, Release of hyaluronate from eukaryotic cells, Biochem. J 267 (1) (1990) 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anderegg U, Simon JC, Averbeck M, More than just a filler - the role of hyaluronan for skin homeostasis, Exp. Dermatol 23(5) (2014) 295–303. [DOI] [PubMed] [Google Scholar]
- [3].Schiller S, Dorfman A, The metabolism of mucopolysaccharides in animals. IV. The influence of insulin, J. Biol. Chem 227 (2) (1957) 625–632. [PubMed] [Google Scholar]
- [4].Fraser JR, Laurent TC, Turnover and metabolism of hyaluronan, CIBA Found. Symp 143 (1989) 41–53 (discussion 53–9, 281–5). [PubMed] [Google Scholar]
- [5].Fraser JR, Laurent TC, Engstrom-Laurent A, Laurent UG, Elimination of hyaluronic acid from the blood stream in the human, Clin. Exp. Pharmacol. Physiol 11 (1) (1984) 17–25. [DOI] [PubMed] [Google Scholar]
- [6].Stern R, Kogan G, Jedrzejas MJ, Soltes L, The many ways to cleave hyaluronan, Biotechnol. Adv 25 (6) (2007) 537–557. [DOI] [PubMed] [Google Scholar]
- [7].McAtee CO, Barycki JJ, Simpson MA, Emerging roles for hyaluronidase in cancer metastasis and therapy, Adv. Cancer Res 123 (2014) 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Amarnath LP, Srinivas A, Ramamurthi A, In vitro hemo-compatibility testing of UV-modified hyaluronan hydrogels, Biomaterials 27 (8) (2006) 1416–1424. [DOI] [PubMed] [Google Scholar]
- [9].Glogau RG, Fillers: from the past to the future, Semin. Cutan. Med. Surg 31 (2) (2012) 78–87. [DOI] [PubMed] [Google Scholar]
- [10].Goodman GJ, Bekhor P, Rich M, Rosen RH, Halstead MB, Rogers JD, A comparison of the efficacy, safety, and longevity of two different hyaluronic acid dermal fillers in the treatment of severe nasolabial folds: a multicenter, prospective, randomized, controlled, single-blind, within-subject study, Clin. Cosmet. Investig. Dermatol 4 (2011) 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bechara FG, Gambichler T, Brockmeyer NH, Sand M, Altmeyer P, Hoffmann K, Hyaluronic acid new formulation: experience in HIV-associated facial lipoatrophy, Dermatology 217 (3) (2008) 244–249. [DOI] [PubMed] [Google Scholar]
- [12].Pavicic T, Ruzicka T, Korting HC, Gauglitz G, Monophasic, cohesive-polydensified-matrix crosslinking-technology-based hyaluronic acid filler for the treatment of facial lipoatrophy in HIV-infected patients, J. Drugs Dermatol 9 (6) (2010) 690–695. [PubMed] [Google Scholar]
- [13].Elliott L, Rashid RM, Colome M, Hyaluronic acid filler for steroid atrophy, J. Cosmet. Dermatol 9 (3) (2010) 253–255. [DOI] [PubMed] [Google Scholar]
- [14].Philipp-Dormston WG, Bergfeld D, Sommer BM, Sattler G, Cotofana S, Snozzi P, Wollina U, Hoffmann KPJ, Salavastru C, Fritz K, Consensus statement on prevention and management of adverse effects following rejuvenation procedures with hyaluronic acid-based fillers, J. Eur. Acad. Dermatol. Venereol 31 (7) (2017) 1088–1095. [DOI] [PubMed] [Google Scholar]
- [15].Wollina U, Midfacial rejuvenation by hyaluronic acid fillers and subcutaneous adipose tissue—a new concept, Med. Hypotheses 84 (4) (2015) 327–330. [DOI] [PubMed] [Google Scholar]
- [16].Bertossi D, Sbarbati A, Cerini R, Barillari M, Favero V, Picozzi V, Ruzzenente O, Salvagno G, Guidi GC, Nocini P, Hyaluronic acid: in vitro and in vivo analysis, biochemical properties and histological and morphological evaluation of injected filler, Eur. J. Dermatol 23 (4) (2013) 449–455. [DOI] [PubMed] [Google Scholar]
- [17].Kruglikov IL, Wollina U, Soft tissue fillers as non-specific modulators of adipogenesis: change of the paradigm? Exp. Dermatol 24 (12) (2015) 912–915. [DOI] [PubMed] [Google Scholar]
- [18].Cowman MK, Lee HG, Schwertfeger KL, McCarthy JB, Turley EA, The content and size of hyaluronan in biological fluids and tissues, Front. Immunol 6 (2015) 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Akgul Y, Holt R, Mummert M, Word A, Mahendroo M, Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth, Endocrinology 153(7) (2012) 3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Han CY, Subramanian S, Chan CK, Omer M, Chiba T, Wight TN, Chait A, Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion, Diabetes 56 (9) (2007) 2260–2273. [DOI] [PubMed] [Google Scholar]
- [21].Laurent TC, Laurent UB, Fraser JR, The structure and function of hyaluronan: an overview, Immunol. Cell Biol 74(2) (1996) A1–7. [DOI] [PubMed] [Google Scholar]
- [22].Succar P, Medynskyj M, Breen EJ, Batterham T, Molloy MP, Herbert BR, Priming adipose-derived mesenchymal stem cells with hyaluronan alters growth kinetics and increases attachment to articular cartilage, Stem Cells Int 2016 (2016), 9364213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Horkay F, Basser PJ, Londono DJ, Hecht AM, Geissler E, Ions in hyaluronic acid solutions, J. Chem. Phys 131 (18) (2009), 184902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guyton AC, Granger HJ, Taylor AE, Interstitial fluid pressure, Physiol. Rev 51 (3) (1971) 527–563. [DOI] [PubMed] [Google Scholar]
- [25].Heldin CH, Rubin K, Pietras K, Ostman A, High interstitial fluid pressure - an obstacle in cancer therapy, Nat. Rev. Cancer 4 (10) (2004) 806–813. [DOI] [PubMed] [Google Scholar]
- [26].Cui H, Yang S, Zheng M, Liu R, Zhao G, Wen J, High-salt intake negatively regulates fat deposition in mouse, Sci. Rep 7 (1) (2017) 2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hepler C, Vishvanath L, Gupta RK, Sorting out adipocyte precursors and their role in physiology and disease, Genes Dev 31 (2) (2017) 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, Gupta RK, Pdgfrbeta+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice, Cell Metab 23 (2) (2016) 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mariman EC, Wang P, Adipocyte extracellular matrix composition, dynamics and role in obesity, Cell. Mol. Life Sci 67 (8) (2010) 1277–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Calvo JC, Gandjbakhche AH, Nossal R, Hascall VC, Yanagishita M, Rheological effects of the presence of hyaluronic acid in the extracellular media of differentiated 3T3–L1 preadipocyte cultures, Arch. Biochem. Biophys 302 (2) (1993) 468–475. [DOI] [PubMed] [Google Scholar]
- [31].Chen PY, Huang LL, Hsieh HJ, Hyaluronan preserves the proliferation and differentiation potentials of long-term cultured murine adipose-derived stromal cells, Biochem. Biophys. Res. Commun 360 (1) (2007) 1–6. [DOI] [PubMed] [Google Scholar]
- [32].Ji E, Jung MY, Park JH, Kim S, Seo CR, Park KW, Lee EK, Yeom CH, Lee S, Inhibition of adipogenesis in 3T3–L1 cells and suppression of abdominal fat accumulation in high-fat diet-feeding C57BL/6J mice after downregulation of hyaluronic acid, Int. J. Obes 38 (8) (2014) 1035–1043. [DOI] [PubMed] [Google Scholar]
- [33].Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M,Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE, The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species, J. Biol. Chem 280 (6) (2005) 4617–4626. [DOI] [PubMed] [Google Scholar]
- [34].Wang A, Midura RJ, Vasanji A, Wang AJ, Hascall VC, Hyperglycemia diverts dividing osteoblastic precursor cells to an adipogenic pathway and induces synthesis of a hyaluronan matrix that is adhesive for monocytes, J. Biol. Chem 289 (16) (2014) 11410–11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kang L, Lantier L, Kennedy A, Bonner JS, Mayes WH, Bracy DP, Bookbinder LH, Hasty AH, Thompson CB, Wasserman DH, Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance, Diabetes 62 (6) (2013) 1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhu Y, Crewe C, Scherer PE, Hyaluronan in adipose tissue: beyond dermal filler and therapeutic carrier, Sci. Transl. Med 8 (323) (2016), 323ps4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Day AJ, Prestwich GD, Hyaluronan-binding proteins: tying up the giant, J. Biol. Chem 277 (7) (2002) 4585–4588. [DOI] [PubMed] [Google Scholar]
- [38].Jiang D, Liang J, Noble PW, Hyaluronan as an immune regulator in human diseases, Physiol. Rev 91 (1) (2011) 221–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B, CD44 is the principal cell surface receptor for hyaluronate, Cell 61 (7) (1990) 1303–1313. [DOI] [PubMed] [Google Scholar]
- [40].Lee YH, Petkova AP, Granneman JG, Identification of an adipogenic niche for adipose tissue remodeling and restoration, Cell Metab 18 (3) (2013) 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bahrami SB, Tolg C, Peart T, Symonette C, Veiseh M, Umoh JU, Holdsworth DW, McCarthy JB, Luyt LG, Bissell MJ,Yazdani A, Turley EA, Receptor for hyaluronan mediated motility (RHAMM/HMMR) is a novel target for promoting subcutaneous adipogenesis, Integr. Biol. (Camb) 9 (3) (2017) 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Collins MN, Birkinshaw C, Hyaluronic acid based scaffolds for tissue engineering–a review, Carbohydr. Polym 92 (2) (2013) 1262–1279. [DOI] [PubMed] [Google Scholar]
- [43].Allison DD, Grande-Allen KJ, Review. Hyaluronan: a powerful tissue engineering tool, Tissue Eng 12 (8) (2006) 2131–2140. [DOI] [PubMed] [Google Scholar]
- [44].Davidenko N, Campbell JJ, Thian ES, Watson CJ, Cameron RE, Collagen-hyaluronic acid scaffolds for adipose tissue engineering, Acta Biomater 6 (10) (2010) 3957–3968. [DOI] [PubMed] [Google Scholar]
- [45].Borzacchiello A, Mayol L, Ramires PA, Pastorello A, Di Bartolo C, Ambrosio L, Milella E, Structural and rheological characterization of hyaluronic acid-based scaffolds for adipose tissue engineering, Biomaterials 28 (30) (2007) 4399–4408. [DOI] [PubMed] [Google Scholar]
- [46].Hemmrich K, Van de Sijpe K, Rhodes NP, Hunt JA, Di Bartolo C, Pallua N, Blondeel P, von Heimburg D, Autologous in vivo adipose tissue engineering in hyaluronan-based gels—a pilot study, J. Surg. Res 144 (1) (2008) 82–88. [DOI] [PubMed] [Google Scholar]
- [47].Flynn LE, Prestwich GD, Semple JL, Woodhouse KA, Proliferation and differentiation of adipose-derived stem cells on naturally derived scaffolds, Biomaterials 29 (12) (2008) 1862–1871. [DOI] [PubMed] [Google Scholar]
- [48].Piccinno MS, Veronesi E, Loschi P, Pignatti M, Murgia A,Grisendi G, Castelli I, Bernabei D, Candini O, Conte P, Paolucci P, Horwitz EM, De Santis G, Iughetti L, Dominici M, Adipose stromal/stem cells assist fat transplantation reducing necrosis and increasing graft performance, Apoptosis 18 (10) (2013) 1274–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH,Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM, Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human, Cell 150 (2) (2012) 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J, Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes, J. Biol. Chem 285 (10) (2010) 7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, Yang X, O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat, Cell 159 (2) (2014) 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, Neel BG, Bence KK, Andrews ZB, Cowley MA, Tiganis T, Leptin and insulin act on POMC neurons to promote the browning of white fat, Cell 160 (1–2) (2015) 88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhu Y, Gao Y, Tao C, Shao M, Zhao S, Huang W, Yao T, Johnson JA, Liu T, Cypess AM, Gupta O, Holland WL, Gupta RK, Spray DC, Tanowitz HB, Cao L, Lynes MD, Tseng YH, Elmquist JK, Williams KW, Lin HV, Scherer PE, Connexin 43 mediates white adipose tissue Beiging by facilitating the propagation of sympathetic neuronal signals, Cell Metab 24 (3) (2016) 420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang QA, Tao C, Gupta RK, Scherer PE, Tracking adipogenesis during white adipose tissue development, expansion and regeneration, Nat. Med 19 (10) (2013) 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shao M, Ishibashi J, Kusminski CM, Wang QA, Hepler C,Vishvanath L, MacPherson KA, Spurgin SB, Sun K, Holland WL, Seale P, Gupta RK, Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program, Cell Metab 23 (6) (2016) 1167–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tharp KM, Stahl A, Bioengineering beige adipose tissue therapeutics, Front. Endocrinol. (Lausanne) 6 (2015) 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tharp KM, Jha AK, Kraiczy J, Yesian A, Karateev G, Sinisi R, Dubikovskaya EA, Healy KE, Stahl A, Matrix-assisted transplantation of functional beige adipose tissue, Diabetes 64 (11) (2015) 3713–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Stillaert FB, Di Bartolo C, Hunt JA, Rhodes NP, Tognana E, Monstrey S, Blondeel PN, Human clinical experience with adipose precursor cells seeded on hyaluronic acid-based spongy scaffolds, Biomaterials 29 (29) (2008) 3953–3959. [DOI] [PubMed] [Google Scholar]
- [59].Ye R, Wang QA, Tao C, Vishvanath L, Shao M, McDonald JG, Gupta RK, Scherer PE, Impact of tamoxifen on adipocyte lineage tracing: inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase, Mol. Metab 4 (11) (2015) 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW, Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macro-phages. The role of HA size and CD44, J. Clin. Invest 98(10) (1996) 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Takahashi Y, Li L, Kamiryo M, Asteriou T, Moustakas A,Yamashita H, Heldin P, Hyaluronan fragments induce endothelial cell differentiation in a CD44- and CXCL1/GRO1-dependent manner, J. Biol. Chem 280 (25) (2005) 24195–24204. [DOI] [PubMed] [Google Scholar]
- [62].Kabir SM, Lee ES, Son DS, Chemokine network during adipogenesis in 3T3–L1 cells: differential response between growth and proinflammatory factor in preadipocytes vs. adipocytes, Adipocytes 3 (2) (2014) 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-Ning-Tapia EM, Troncoso P, Davis J, Pettaway C, Ward J, Frazier ML, Logothetis C, Kolonin MG, CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment, Nat. Commun 7 (2016), 11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V, Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth, PLoS Comput. Biol 5 (3) (2009), e1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sun K, Kusminski CM, Scherer PE, Adipose tissue remodeling and obesity, J. Clin. Invest 121 (6) (2011) 2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kodama K, Horikoshi M, Toda K, Yamada S, Hara K, Irie J, Sirota M, Morgan AA, Chen R, Ohtsu H, Maeda S, Kadowaki T, Butte AJ, Expression-based genome-wide association study links the receptor CD44 in adipose tissue with type 2 diabetes, Proc. Natl. Acad. Sci. U. S. A 109 (18) (2012) 7049–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Weiss L, Slavin S, Reich S, Cohen P, Shuster S, Stern R,Kaganovsky E, Okon E, Rubinstein AM, Naor D, Induction of resistance to diabetes in non-obese diabetic mice by targeting CD44 with a specific monoclonal antibody, Proc. Natl. Acad. Sci. U. S. A 97 (1) (2000) 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K, Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease, Liver Int 25 (4) (2005) 779–786. [DOI] [PubMed] [Google Scholar]
- [69].Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ, Serum markers detect the presence of liver fibrosis: a cohort study, Gastroenterology 127 (6) (2004) 1704–1713. [DOI] [PubMed] [Google Scholar]
- [70].Bogdani M, Johnson PY, Potter-Perigo S, Nagy N, Day AJ, Bollyky PL, Wight TN, Hyaluronan and hyaluronan-binding proteins accumulate in both human type 1 diabetic islets and lymphoid tissues and associate with inflammatory cells in insulitis, Diabetes 63 (8) (2014) 2727–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Morita M, Yano S, Ishibashi Y, Nakata N, Kurioka S, Sugimoto T, Close relationship between serum hyaluronan levels and vascular function in patients with type 2 diabetes, Biomarkers 19 (6) (2014) 493–497. [DOI] [PubMed] [Google Scholar]
- [72].Buse MG, Hexosamines, insulin resistance, and the complications of diabetes: current status, Am. J. Physiol. Endocrinol. Metab 290 (1) (2006) E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang HS, Tung WH, Tang KT, Wong YK, Huang GJ, Wu JC, Guo YJ, Chen CC, TGF-beta induced hyaluronan synthesis in orbital fibroblasts involves protein kinase C betaII activation in vitro, J. Cell. Biochem 95 (2) (2005) 256–267. [DOI] [PubMed] [Google Scholar]
- [74].Vigetti D, Deleonibus S, Moretto P, Karousou E, Viola M,Bartolini B, Hascall VC, Tammi M, De Luca G, Passi A, Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis, J. Biol. Chem 287 (42) (2012) 35544–35555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vigetti D, Deleonibus S, Moretto P, Bowen T, Fischer JW,Grandoch M, Oberhuber A, Love DC, Hanover JA, Cinquetti R, Karousou E, Viola M, D’Angelo ML, Hascall VC, De Luca G, Passi A, Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcNAcylation, J. Biol. Chem 289 (42) (2014) 28816–28826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Park BG, Lee CW, Park JW, Cui Y, Park YS, Shin WS, Enzymatic fragments of hyaluronan inhibit adipocyte differentiation in 3T3–L1 pre-adipocytes, Biochem. Biophys. Res. Commun 467 (4) (2015) 623–628. [DOI] [PubMed] [Google Scholar]
- [77].Park BG, Park YS, Park JW, Shin E, Shin WS, Anti-obesity potential of enzymatic fragments of hyaluronan on high-fat diet-induced obesity in C57BL/6 mice, Biochem. Biophys. Res. Commun 473 (1) (2016) 290–295. [DOI] [PubMed] [Google Scholar]