SUMMARY
Chronic alcohol abuse and alcoholism are considered risk factors for prostate cancer (PCa) progression, but the mechanism is unknown. Previously, we found that: (1) fragmentation of the Golgi complex correlates with the progression of PCa; (2) ethanol (EtOH) induces Golgi disorganization, which, in turn, alters intra-Golgi localization of some Golgi proteins. Also, progression of the prostate tumor is associated with activation of N-acetylglucosaminyltransferase-V (MGAT5)-mediated N-glycosylation of pro-metastatic proteins, including matriptase and integrins, followed by their enhanced retention at the cell surface. Here, using high-resolution microscopy, we found that alcohol effect on Golgi in low passage androgen-responsive LNCaP cells mimic the fragmented Golgi phenotype of androgen-refractory high passage LNCaP and PC-3 cells. Next, we detected that transition to androgen unresponsiveness is accompanied by downregulation of N-acetylglucosaminyltransferase-III (MGAT3), the enzyme that competes with MGAT5 for anti-metastatic N-glycan branching. Moreover, in low passage LNCaP cells, alcohol-induced Golgi fragmentation induced translocation of MGAT3 from the Golgi to the cytoplasm, while intra-Golgi localization of MGAT5 appeared unaffected. Then, the relationship between Golgi morphology, MGAT3 intracellular position, and clinicopathologic features was assessed in human PCa patient specimens with and without a history of alcohol dependence. We revealed that within the same clinical stage, the level of Golgi disorganization and the cytoplasmic shift of MGAT3 was more prominent in patients consuming alcohol. In vitro studies suggest that EtOH-induced downregulation of MGAT3 correlates with activation of MGAT5-mediated glycosylation and overexpression of both matriptase and integrins. In sum, we provide a novel insight into the alcohol-mediated tumor promotion.
Keywords: prostate cancer, alcohol, disorganization of Golgi complex, N-glycosylation, metastasis
Prostate cancer (PCa) is the most common cancer among men and the second-leading cause of cancer death [1]. Whether alcohol is a risk factor for PCa has been the longstanding question [2–5]. Various recent population-based, case-control studies have revealed a strong association of PCa incidence with heavier consumption of alcohol [6–10]. Although this conclusion is not fully supported by a few other observations [11, 12], the scientific consensus is that excessive alcohol consumption is linked to advanced PCa and its mortality [6, 8, 13–16]. Moreover, recent observation clearly indicates that post-diagnosis alcohol consumption was associated with increased mortality in PCa patients [17]. Therefore, understanding the cellular and molecular mechanisms underlying alcohol interference with PCa progression will address an important question raised by clinicians: whether alcohol abstinence is an important therapeutic intervention in PCa.
Evidence from the literature indicates that fragmentation of the Golgi apparatus is a hallmark of cancer progression [18–21]. Indeed, our group also recently identified Golgi fragmentation in PCa cells, which was correlated with the progression of PCa [22, 23]. A few years ago, we introduced a novel concept of onco-Golgi: that Golgi disorganization is associated with the activation of various pro-oncogenic and pro-metastatic pathways [23]. Alcohol consumption induces Golgi fragmentation in different organs, including prostate [24–27], which impairs Golgi docking of several Golgi residential enzymes, including glycosyltransferases and kinases [28] and results in their mislocalization, which, in turn, cardinally changes both glycosylation and phosphorylation [22]. We recently reported the important observation that in ethanol (EtOH)-treated androgen-responsive PCa cells, fragmentation of the Golgi was accompanied by the translocation of glycogen synthase kinase β (GSK3β) from the Golgi to the cytoplasm, followed by the phosphorylation of histone deacetylase 6 (HDAC6) and activation of the downstream HSP90-androgen receptor (AR) pathway [29]. EtOH-treated cells also demonstrate increased migration, growth in mice xenograft, proliferation, and secretion of prostate-specific antigen (PSA) [30]. However, it is important to define the impact of alcohol during the transition of PCa from AR-responsive form to the AR-independent aggressive stage, castration-resistant prostate cancer (CRPC), when tumors begin to metastasize.
In primary PCa and metastatic lesions, the level of matriptase and integrins is enhanced and is positively correlated with tumor grade and poor prognosis [31, 32]. The key to understanding this phenomenon is the interesting competition of branching enzymes in N-glycosylation, where the typical biantennary N-linked chain can be transformed in a β1,6-branched structure by the action of N-acetylglucosaminyltransfera se-V (MGAT5). Then, branching is provided by formation of polylactosaminic chains that specifically bind to Galectin-3 (Gal-3) (Fig. 1, left panel). Overexpression of MGAT5 was detected in a different type of tumor and was suggested as a prognostic marker in PCa [33–35]. Conversely, the biantennary N-linked chain can be modified by the addition of a bisecting GlcNAc, the reaction catalyzed by N-acetylglucosaminyltransferase-III (MGAT3) (Fig. 1, right panel). It is known that MGAT5 is antagonized by MGAT3, whose enhanced expression has been found to block MGAT5-mediated glycosylation and suppress metastasis [36–38]. Bisecting GlcNAc structures in certain glycoproteins importantly changed their expression levels and decreased their sorting to the cell surface [39]. However, when the same proteins, for example, matriptase and integrins, are modified by MGAT5, their expression and retention at the cell surface are enhanced via interaction with Gal-3 [36, 40]. Indeed, the transient overexpression of MGAT5 significantly enhanced the activity of matriptase and invasion ability in the LNCaP cells [41]. Here, we hypothesize that alcohol-induced Golgi disorganization results in overexpression of pro-metastatic proteins due to activation of their MGAT5-mediated glycosylation.
Figure 1.

Schema of MGAT3 and MGAT5-mediated N-glycosylation. Bisecting GlcNAc blocks glycans branching via MGAT5.
MATERIAL AND METHODS
Antibodies.
The primary antibodies used were: a) rabbit polyclonal – MGAT3 (Abcam, ab103427), MGAT5 (Abcam, ab68595), and ST14 (Matriptase) (Abcam, ab28266); b) mouse monoclonal – E-cadherin (Abcam, ab1416), β-actin (Sigma, A2228), integrin alpha V + beta 6 (Abcam, ab77906), and giantin (Abcam, ab37266); c) mouse polyclonal – GM130 (Abcam, ab169276). The secondary antibodies (Jackson ImmunoResearch) were: a) HRP-conjugated donkey anti-rabbit and donkey anti-mouse for Western-blotting (W-B) (711–035-152 and 715–035-151, respectively); b) donkey anti-mouse Alexa Fluor 488 and anti-rabbit Alexa Fluor 594 (115–546-003 and 711–585-152, respectively) for immunofluorescence (IF).
Cell culture and alcohol treatment.
LNCaP and PC-3 cells are purchased from ATCC. Cells were grown in phenol red-free RPMI medium with 11 mM glucose, 10% FBS, 2mM glutamine, non-essential amino acids and 100U/ml of Penicillin plus Streptomycin. Given their androgen responsiveness, cells were cultured under treatment with 10 nM dihydrotestosterone (DHT). Twenty-four hours after seeding cells (at 75% confluence), culture media were changed for one containing 35 mM EtOH for another 96 h. The medium was replaced every 48 h to maintain a constant EtOH concentration. Control cells were seeded at the same time as treated cells and maintained in the same medium; EtOH was replaced by the appropriate volume of medium to maintain similar caloric content.
Immunoprecipitation (IP) and transfection.
For the identification of proteins in the complexes pulled down by IP, confluent cells grown in a T75 flask were washed three times with 6 ml PBS each, harvested by trypsinization, and neutralized with the FBS-containing medium. IP steps were performed using antibodies that are covalently coupled to Dynabeads M-270 Epoxy beads (Pierce) according to the manufacturer’s instructions. All cell lysate samples for IP experiments were normalized by appropriate proteins. To determine whether the target protein was loaded evenly, the input samples were preliminarily run on a separate gel with different dilutions of control samples vs. treated, then probed with anti-target protein Abs. The intensity of obtained bands was analyzed by ImageJ software, and samples with identical intensity were subjected to IP. Mouse or rabbit non-specific IgG was used as non-specific controls. Proteins were separated on SDS PAGE on mini-gels with various acrylamide % specified for each experiment (Bio-Rad). MGAT5 and scrambled ON-TARGET plus smartpool siRNAs were purchased from Santa Cruz Biotechnology. All products consisted of pools of three target-specific siRNAs. Cells were transfected with 100–150 nM siRNAs using Lipofectamine RNAi MAX reagent (Life science technologies).
Confocal immunofluorescence microscopy.
Staining of cells was performed by methods described previously [42]. Normal human prostate and prostate adenocarcinoma tissue arrays were purchased from US Biomax and Novus Biologicals. After deparaffinization and dehydration, tissues were blocked in 1% donkey serum for 1 hour at RT° and incubated with the primary antibodies for 3 hours at RT°. After washing with PBST three times the slides were incubated with Alexa Fluor secondary Abs for 1 hour at RT°. The nuclei of the tissues were counterstained with DAPI (Invitrogen). Slides were examined under an LSM 800 Zeiss Airyscan microscope performed at the Advanced Microscopy Core Facility of the University of Nebraska Medical Center. Images were analyzed using ZEN 2.1 software and IMARIS versions 7.2.2–7.6.0 (Bitplane Scientific). For some figures, image analysis was performed using Adobe Photoshop and ImageJ. Statistical analysis of colocalization was performed by ImageJ, calculating the Pearson correlation coefficient.
Three-dimensional structured illumination (3D-SIM) microscopy and image analysis.
SIM imaging of Golgi ribbons was performed on a Zeiss ELYRA PS.1 superresolution scope (Carl Zeiss Microscopy, Germany) using a PCO.Edge 5.5 camera equipped with a Plan-Apochromat 63×1.4 oil objective. Optimal grid sizes for each wavelength were chosen according to the recommendations of the manufacturer. For 3D-SIM, stacks with a step size of 110 nm were acquired sequentially for each fluorophore, and each fluorescent channel was imaged with three pattern rotations with 3 translational shifts. The final SIM image was created using modules built into the Zen Black software suite that accompanies the imaging setup. Analyses were undertaken on 3D-SIM data sets in 3D using IMARIS versions 7.2.2–7.6.0 (Bitplane Scientific). The 3D mask was obtained by applying a Gaussian filter to merged channels, thresholding to remove low-intensity signals, and converting the obtained stack into a binary file that mapped all voxels of interest for coefficient calculation. For colocalization studies, IMARIS ‘Colocalization Module’ was used. To avoid subjectivity, all thresholds were automatically determined using algorithms, based on the exclusion of intensity pairs that exhibit no correlation [43].
Miscellaneous.
Protein concentrations were determined with the Coomassie Plus Protein Assay (Pierce Chemical Co). The results shown are representative of three independent experiments. Data are expressed as mean ± SEM. The analysis was performed using a 2-sided t-test. A value of p < 0.05 was considered statistically significant.
RESULTS
We recently showed that alcohol dehydrogenase (ADH)-generated metabolites of EtOH, notably acetaldehyde (Ach), are the major contributors to Golgi fragmentation in prostate cells [30]. Moreover, Ach was responsible for enhanced: a) anchorage-independent growth of these cells, b) their migration, c) adhesion to the endothelial cells, and c) PSA secretion [30]. Our important finding in the same publication was that EtOH accelerates LNCaP tumor xenograft in athymic male mice. Therefore, these cells are an excellent model to study the EtOH effect on the progression of PCa. Also, previously we found that contrary to compact and perinuclear Golgi in low passage androgen-responsive LNCaP cells, androgen-restrictive PC-3, and DU145 cells demonstrate fragmented Golgi [22]. Here, using 3D SIM imaging, we found identical disorganized Golgi structure in high passage (c-85) LNCaP cells, which also represent androgen-refractory PCa cells [44]. Predictably, we found that treatment of low passage (c-26) LNCaP cells with 35 mM EtOH for 96 h results in disorganization of the Golgi, resembling the Golgi in the non-treated high passage (c-85) LNCaP and PC-3 cells (Fig. 2). Thus, the effect of EtOH on low-aggressive PCa cells seems to mimic the phenotype of highly aggressive PCa cells.
Figure 2.

3D SIM imaging of Golgi (giantin) in low passage LNCaP (c-26) cells before and after EtOH treatment, and in non-treated high passage LNCaP (c-85) and PC-3 cells. Nuclei were counterstained with DAPI (blue); bars, 5 µm.
Next, we examined whether the transition to androgen-unresponsiveness is associated with alteration of MGAT3-mediated glycosylation. First, we detected by W-B that level of MGAT3 in low passage LNCaP (c-26) cells are higher than that in high passage c-86. Conversely, in LNCaP (c-86), the expression of MGAT5 is higher than that in LNCaP (c-26) cells (Fig. 3A). Further, using the W-B of the plasma membrane (PM) fractions, we detected that the LNCaP (c-86) cells express more matriptase at the cell surface than LNCaP (c-26) cells (Fig. 3B). Next, we performed matriptase IP and probed with L-PHA lectin, which binds preferentially to GlcNAc residues on β1–6 branches of tri- or tetra-antennary sugar chains, the product of MGAT5 [45]. As shown in Fig. 3C, matriptase from LNCaP (c-86) cells bears a higher amount of MGAT5-mediated glycans than that from LNCaP (c-26) cells. Further, we performed the siRNA-induced knockdown (KD) of MGAT5 gene in PC-3 cells (Fig. 4A) and observed that the lack of MGAT5 is associated with a reduction of matriptase expression at the PM fraction, indicating the intimate relationship between the activity of MGAT5 and retention of matriptase at the cell surface (Fig. 4B). Overall, these results indicate that the progression of PCa is associated with downregulation of MGAT3 with concomitant activation of MGAT5-mediated glycosylation.
Figure 3.
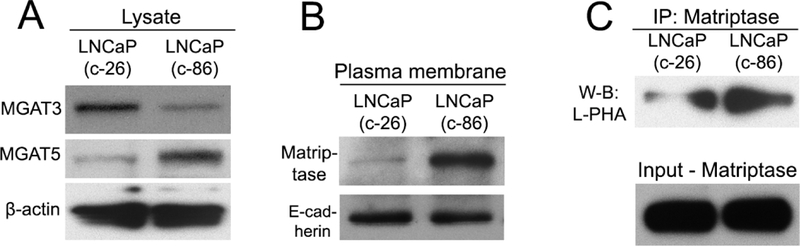
(A) MGAT3 and MGAT5 W-B of the cell lysate from LNCaP (c-26) and (c-86) cells; β-actin was used as a loading control. (B) Matriptase W-B of the plasma membrane (PM) fractions from LNCaP (c-26) and (c-86) cells; the PM samples were normalized by E-cadherin. (C) The L-PHA lectin W-B of Matriptase IP from LNCaP (c-26) and (c-86) cells. The bottom panel represents the input normalized for the Matriptase.
Figure 4.
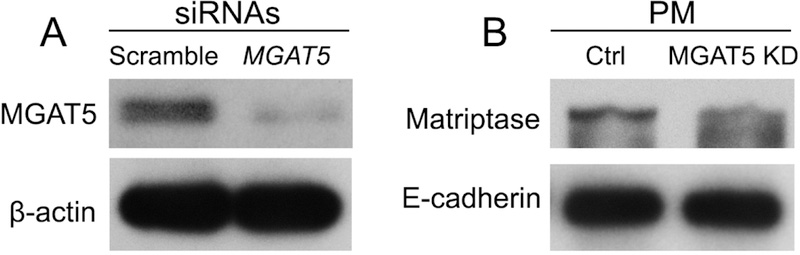
(A) MGAT5 W-B of the cell lysate from LNCaP (c-26) treated with a scramble of MGAT5 siRNAs; β-actin was used as a loading control. (B) Matriptase W-B of the plasma membrane (PM) fraction from cells in A; the PM samples were normalized by E-cadherin.
Recently, we have found the same phenomenon in O-glycan branching; in advanced PCa cells, downregulation of core 2 N-acetylglucosaminyltrans ferase-L/1 (C2GnT-L) was correlated with activation of glycosylation through Galβ1–3GalNAcαSer/Thr:α2–3sialyltransferase 1 (ST3Gal1), the enzyme that competes with C2GnT-L for core-1 substrates [22]. Consequently, the expression level of polylactosamine was reduced, while pro-metastatic sialyl‐T antigen was enhanced, allowing cells to evade Gal-1-induced apoptosis [22]. Further analysis revealed that Golgi disorganization in androgen-refractory cells is associated with mislocalization of C2GnT-L from Golgi to the cytoplasm. In the meantime, the intra-Golgi position of ST3Gal1 was unaltered. Thus, we deduced that the most possible explanation of the shift to MGAT5-mediated glycosylation is mislocalization of MGAT3. To validate this hypothesis, we performed an analysis of post-alcohol intracellular localization for both MGAT3 and MGAT5. Our preliminary screening revealed that MGAT3 is localized in earlier Golgi compartments than MGAT5 (data have not shown). Thus, to study intra-Golgi position of both MGAT3 and MGAT5, we used GM130 and giantin as the cis- and medial-Golgi markers, respectively [28, 46]. In EtOH-treated LNCaP (c-26) cells, we detected that the scattering of Golgi membranes was accompanied by the redistribution of MGAT3 from Golgi to the cytoplasm, according to the Pearson colocalization coefficient between MGAT3 and GM130 (Fig. 5 A, C); however, the intra-Golgi position for MGAT5 remained intact based on the unchangeable rate of its colocalization with giantin (Fig. 5 B, C). Interestingly, we noticed the decrease of the IF signal of MGAT3 in EtOH-treated LNCaP cells, implying its partial degradation. This observation was validated by the W-B analysis, confirming that alcohol treatment results in a reduction of the level of MGAT3 and upregulation of MGAT5 (Fig. 6A). Next, we observed that in these cells, EtOH treatment enhances the expression of matriptase and integrin α5 in the PM fraction (Fig. 6B). These data echo observations of others which show that expression of integrins (α5+β3) and (α5+β6) is associated with the androgen-negative status of PCa cells [47, 48], which demonstrate a fragmented Golgi phenotype [30].
Figure 5.
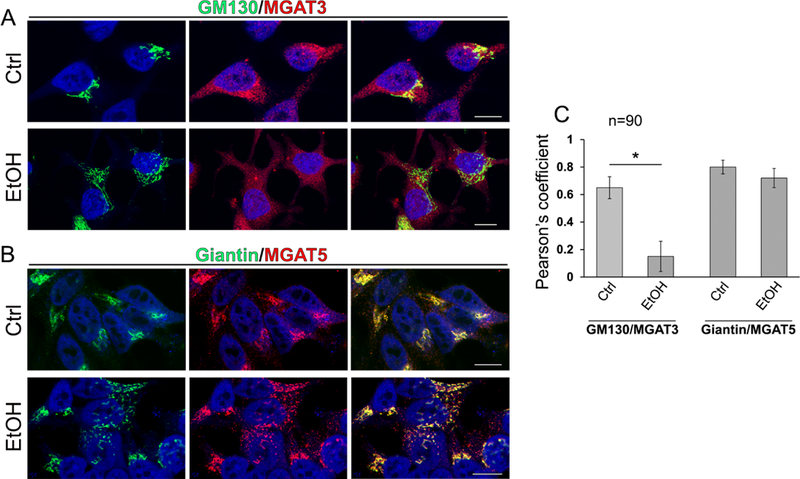
Immunostaining of GM130 (green) and MGAT3 (red) (A), and giantin (green) and MGAT5 (red) (B) in the LNCaP cells: control and treated with 35 mM EtOH for 96 h. Nuclei were counterstained with DAPI (blue). All confocal images were acquired with the same imaging parameters; bars, 10 μm. (C) Quantification of Pearson’s overlap coefficient for indicated proteins for cells from A and B; means ± SD; * - p<0.001.
Figure 6.

(A) MGAT3 and MGAT5 W-B of the lysate from LNCaP cells: control and treated with 35 mM EtOH for 96 h. (B) Integrin α5 W-B of the plasma membrane (PM) fraction from the cells presented in A; the PM samples were normalized by E-cadherin
Previously, we found that the fragmented Golgi phenotype was significantly higher in PCa cells of drinkers than in non-drinkers, suggesting that alcohol consumption may be a critical factor contributing to Golgi disorganization [30]. Herein, we analyzed tissue sections obtained from the primary prostate tumor foci of PCa patients, both drinkers and non-drinkers to examine whether the level of drinking is correlated with the loss of the MGAT3 intra-Golgi position. Questionnaires contained questions on a) preferred alcoholic beverage; b) an average number of drinks consumed in a week within last 5 years; c) frequency of heavy episodic drinking and d) duration of heavy drinking occasions. Sections were provided through the Department of Pathology and Microbiology (IRB protocol # 304–16-EP) at the University of Nebraska Medical Center and the Johns Hopkins University School of Medicine (Prostate Cancer Biorepository Network). We monitored the intracellular position of MGAT3 in normal prostate and in PCa cells from patients with the same grade and Gleason score: non-drinking patients (who do not drink or less that one per month) versus patients who regularly consume alcohol at the moderate level (from 5–6 times per week – 12 oz. beer; 3–5 times per week - 4 oz. glass of wine; 2–3 times – 3.4 oz. strong liquor) or high level (from 2–3 times and more per day - 12 oz. beer; from 1–2 times and more - 4 oz. glass of wine; from one times and more – 3.4 oz. strong liquor). We found that in PCa patients consuming alcohol at the moderate or high level, the segregation of MGAT3 from Golgi membranes is more than in non-alcoholic patients (Fig. 7A). Statistical analysis demonstrates that the frequency of alcohol consumption correlates with not only Golgi fragmentation but also with the loss of MGAT3 position in the Golgi (Fig. 7B).
Figure 7.
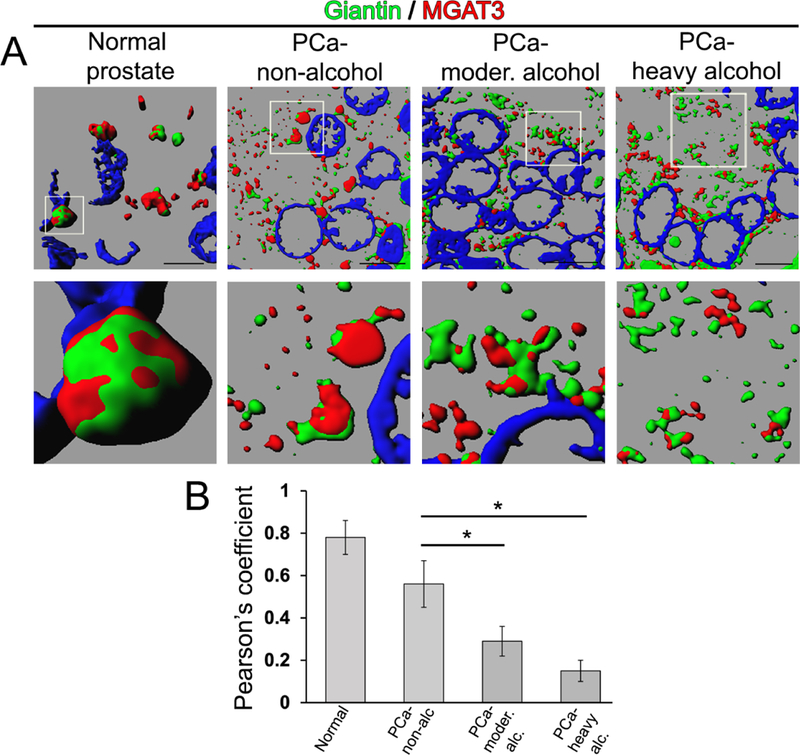
(A) 3D SIM reconstruction of giantin (green) and MGAT3 (red) IF in normal prostate and tissue section of PCa patients (Gleason 7); bars, 5 µm. White boxes indicate area enlarged below. (B) Quantification of colocalization of giantin and MGAT3 from the sections in A; 10 patients for each group were observed; means ± SD; * - p<0.001.
DISCUSSION
Here, for the first time, we provide the mechanism of cancer-specific MGAT5-mediated glycosylation: the transition of PCa from androgen-responsive stage to the androgen-negative is associated with Golgi fragmentation and downregulation of MGAT3. Our both in vitro and in vivo data clearly indicate that alcohol-induced Golgi fragmentation in PCa cells is correlated with the translocation of MGAT3 from Golgi to the cytoplasm, thus facilitating N-glycosylation of pro-metastatic proteins, matriptase and integrins. Therefore, alcohol mimics the effect of PCa progression, and can potentially contribute to the manifestation of tumor growth, its intravasation, adhesion, and extravasation (Fig. 8).
Figure 8.
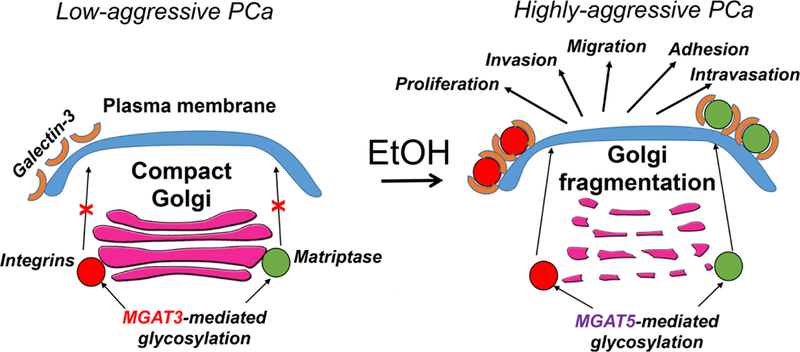
The working model of alcohol effect on N-glycosylation in PCa. In low aggressive PCa cells, pro-metastatic proteins are modified by MGAT3, which reduces their binding to the Gal-3, thus blocking their stabilization and retention at the cell surface. In alcohol-treated PCa cells, Golgi is fragmented and domination of MGAT5-mediated glycosylation promotes plasma membrane expression of proteins via their strong interaction with Gal-3. This, in turn, facilitates PCa progression.
The key question, then, is the mechanism that underlies the Golgi targeting of MGAT3, which seems distinct from the one that utilized by MGAT5, since the intra-Golgi position of MGAT5 is unaffected despite Golgi disorganization. We recently identified that Golgi residential proteins use neither COPI nor COPII vesicles for trafficking to the Golgi and suggest that the specific vesicular complexes carrying these proteins employ two different docking sites at the Golgi: giantin and the complex of GM130 and GRASP65 [28, 30]. We and others have shown that different Golgi glycosyltransferases and kinases are giantin-sensitive because they relocate to the cytoplasm in cells lacking this golgin [22, 28, 30, 49, 50]. This, for instance, occurs in response to endoplasmic reticulum (ER) stress, including one induced by alcohol treatment, when Golgi undergoes reversible remodeling, characterized by varying degrees of scattering and unstacking [51]. In the meantime, the localization of enzymes that employ GM130-GRASP65 docking mechanism seems unaffected, since ER stress has no significant impact on the structure and content of these proteins [46, 52]. Given that EtOH impairs giantin dimeric structure and reduces its content [30, 52], our data indicate that MGAT3 highly likely employs giantin-dependent Golgi docking mechanism. However, this assumption needs further validation. On the other hand, our current results indicate that MGAT5 uses giantin-independent Golgi docking strategy. Thus, Golgi partner of MGAT5 remains to be identified. Different possibilities can be envisaged here. First and most simple is that MGAT5 is docked at the cis-Golgi via GM130-GRASP65 and then transported to the medial-Golgi. The second scenario is that MGAT5 uses medial-Golgi golgin. The potential candidates are the following Golgi matrix proteins: CASP, Golgin-84, Golgin-160, and TMF [53].
Intriguingly, here we observed that disposition of MGAT3 results in the reduction of its level, suggesting the degradation of this protein, given that we could not detect any changes in mRNA expression of MGAT3 (data have not presented). We have shown previously that Golgi residential enzymes are recycling via proteasome-mediated degradation [54]. It appears that the Golgi content of these proteins is determined inter alia by a dynamic equilibrium between their constant trafficking from ER to the Golgi and retrograde flow from Golgi to the proteasome for degradation [55]. However, the loss of Golgi retention, induced by different stress conditions, accelerates latter, resulting in downregulation of the enzymes that are not able to contribute to glycosylation anymore [42, 56–58]. In light of these, we assume that MGAT3 employs proteasomes for degradation, though this hypothesis requires additional experiments.
Alcohol is a physiological inducer of ER stress, a condition under which unfolded/misfolded proteins accumulate in the ER, contributing to the alcoholic disorders of major organs such as liver, pancreas, heart, and brain [59]. The ER stress launches the unfolded protein response (UPR), which attenuates protein translation, increases protein folding capacity, and facilitates degradation of unfolded proteins, helping to recover ER homeostasis [60]. In addition, Golgi has a remarkable self-organizing mechanism [61], and it is known that ethanol-induced Golgi disorganization in hepatocytes is reversible upon alcohol withdrawal. We have shown recently that Golgi recovery requires several players, including giantin, whose re-dimerization facilitates fusion of the nascent Golgi membranes [62]. In the meantime, the growing evidence of literature indicates that fragmentation of the Golgi complex in cancer cells is also a consequence of prolonged and sub-lethal ER stress [18–20, 22, 23]. Moreover, a number of recent studies have implicated ER stress and UPR in the progression of PCa and development of CRPC [63, 64]. It is logical to assume that in case of the PCa cells, alcohol may contribute to the maintenance of the fragmented Golgi phenotype via augmentation of ER stress. Therefore, the future studies in this field should provide an answer to the important question, how exactly alcohol facilitates UPR, the important pathway of cancer survival [65, 66].
Acknowledgments
Funding: This research was supported by the K01AA022979-01 award from the National Institute on Alcohol and Alcohol Abuse (to A.P.), the Nebraska Center for Integrated Biomolecular Communication Systems Biology Core (NIGMS P20-GM113126) (to A.P.).
Footnotes
Conflicts of Interest. The authors declare no potential conflicts of interest.
REFERENCES
- 1.Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr 2012;2012(45):152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellison LF. Tea and other beverage consumption and prostate cancer risk: a Canadian retrospective cohort study. Eur J Cancer Prev 2000;9(2):125–30. [DOI] [PubMed] [Google Scholar]
- 3.Schoonen WM, Salinas CA, Kiemeney LA, Stanford JL. Alcohol consumption and risk of prostate cancer in middle-aged men. Int J Cancer 2005;113(1):133–40. [DOI] [PubMed] [Google Scholar]
- 4.Hirayama T. Life-style and cancer: from epidemiological evidence to public behavior change to mortality reduction of target cancers. J Natl Cancer Inst Monogr 1992(12):65–74. [PubMed] [Google Scholar]
- 5.Tonnesen H, Moller H, Andersen JR, Jensen E, Juel K. Cancer morbidity in alcohol abusers. Br J Cancer 1994;69(2):327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michael J, Howard LE, Markt SC, De Hoedt A, Bailey C, Mucci LA, et al. Early-Life Alcohol Intake and High-Grade Prostate Cancer: Results from an Equal-Access, Racially Diverse Biopsy Cohort. Cancer Prev Res (Phila) 2018. [DOI] [PubMed]
- 7.McGregor SE, Courneya KS, Kopciuk KA, Tosevski C, Friedenreich CM. Case-control study of lifetime alcohol intake and prostate cancer risk. Cancer Causes Control 2013;24(3):451–61. [DOI] [PubMed] [Google Scholar]
- 8.Dickerman BA, Markt SC, Koskenvuo M, Pukkala E, Mucci LA, Kaprio J. Alcohol intake, drinking patterns, and prostate cancer risk and mortality: a 30-year prospective cohort study of Finnish twins. Cancer Causes Control 2016;27(9):1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunzmann AT, Coleman HG, Huang WY, Berndt SI. The association of lifetime alcohol use with mortality and cancer risk in older adults: A cohort study. PLoS Med 2018;15(6):e1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J, Stockwell T, Roemer A, Chikritzhs T. Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysis. BMC Cancer 2016;16(1):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demoury C, Karakiewicz P, Parent ME. Association between lifetime alcohol consumption and prostate cancer risk: A case-control study in Montreal, Canada. Cancer Epidemiol 2016;45:11–7. [DOI] [PubMed] [Google Scholar]
- 12.Vartolomei MD, Kimura S, Ferro M, Foerster B, Abufaraj M, Briganti A, et al. The impact of moderate wine consumption on the risk of developing prostate cancer. Clin Epidemiol 2018;10:431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunner C, Davies NM, Martin RM, Eeles R, Easton D, Kote-Jarai Z, et al. Alcohol consumption and prostate cancer incidence and progression: A Mendelian randomisation study. Int J Cancer 2017;140(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuccolo L, Lewis SJ, Donovan JL, Hamdy FC, Neal DE, Smith GD. Alcohol consumption and PSA-detected prostate cancer risk--a case-control nested in the ProtecT study. Int J Cancer 2013;132(9):2176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowke JH, Howard L, Andriole GL, Freedland SJ. Alcohol intake increases high-grade prostate cancer risk among men taking dutasteride in the REDUCE trial. Eur Urol 2014;66(6):1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawada N, Inoue M, Iwasaki M, Sasazuki S, Yamaji T, Shimazu T, et al. Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: the Japan Public Health Center-based prospective study. Int J Cancer 2014;134(4):971–8. [DOI] [PubMed] [Google Scholar]
- 17.Farris MS, Courneya KS, Kopciuk KA, McGregor SE, Friedenreich CM. Post-diagnosis alcohol intake and prostate cancer survival: A population-based cohort study. Int J Cancer 2018;143(2):253–62. [DOI] [PubMed] [Google Scholar]
- 18.Egea G, Franci C, Gambus G, Lesuffleur T, Zweibaum A, Real FX. cis-Golgi resident proteins and O-glycans are abnormally compartmentalized in the RER of colon cancer cells. J Cell Sci 1993;105 ( Pt 3):819–30. [DOI] [PubMed] [Google Scholar]
- 19.Kellokumpu S, Sormunen R, Kellokumpu I. Abnormal glycosylation and altered Golgi structure in colorectal cancer: dependence on intra-Golgi pH. FEBS Lett 2002;516(1–3):217–24. [DOI] [PubMed] [Google Scholar]
- 20.McKinnon CM, Mellor H. The tumor suppressor RhoBTB1 controls Golgi integrity and breast cancer cell invasion through METTL7B. BMC Cancer 2017;17(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan X, Banerjee P, Guo HF, Ireland S, Pankova D, Ahn YH, et al. Epithelial-to-mesenchymal transition drives a pro-metastatic Golgi compaction process through scaffolding protein PAQR11. J Clin Invest 2017;127(1):117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrosyan A, Holzapfel MS, Muirhead DE, Cheng PW. Restoration of compact Golgi morphology in advanced prostate cancer enhances susceptibility to galectin-1-induced apoptosis by modifying mucin O-glycan synthesis. Mol Cancer Res 2014;12(12):1704–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrosyan A Onco-Golgi: Is Fragmentation a Gate to Cancer Progression? Biochem Mol Biol J 2015;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez FE, Garcia PJ, Padovani CR, Cagnon VH, Martinez M. Ultrastructural study of the ventral lobe of the prostate of rats submitted to experimental chronic alcoholism. Prostate 1993;22(4):317–24. [DOI] [PubMed] [Google Scholar]
- 25.Candido EM, Carvalho CA, Martinez FE, Cagnon VH. Experimental alcoholism and pathogenesis of prostatic diseases in UChB rats. Cell Biol Int 2007;31(5):459–72. [DOI] [PubMed] [Google Scholar]
- 26.Cagnon VH, Tomazini FM, Garcia PJ, Martinez M, Padovani CR, Martinez FE. Structure and ultrastructure of the ventral prostate of isogenic mice (C57B1/6J) submitted to chronic alcohol ingestion. Tissue Cell 2001;33(4):354–60. [DOI] [PubMed] [Google Scholar]
- 27.Gumus E, Solakoglu S, Mutus R, Altay B, Kiziler AR, Miroglu C. Effect of acute alcohol intake on prostate tissue and serum PSA-like protein levels in rats. Urol Int 2005;75(1):50–6. [DOI] [PubMed] [Google Scholar]
- 28.Petrosyan A, Ali MF, Cheng PW. Glycosyltransferase-specific Golgi-targeting mechanisms. J Biol Chem 2012;287(45):37621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manca S, Frisbie CP, LaGrange CA, Casey CA, Riethoven JM, Petrosyan A. The Role of Alcohol-Induced Golgi Fragmentation for Androgen Receptor Signaling in Prostate Cancer. Mol Cancer Res 2019;17(1):225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manca S, Frisbie CP, LaGrange CA, Casey CA, Riethoven JM, Petrosyan A. The Role of Alcohol-induced Golgi Fragmentation for Androgen Receptor Signaling in Prostate Cancer. Mol Cancer Res 2018. [DOI] [PMC free article] [PubMed]
- 31.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer 2008;15(3):657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saleem M, Adhami VM, Zhong W, Longley BJ, Lin CY, Dickson RB, et al. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev 2006;15(2):217–27. [DOI] [PubMed] [Google Scholar]
- 33.Li D, Li Y, Wu X, Li Q, Yu J, Gen J, et al. Knockdown of Mgat5 inhibits breast cancer cell growth with activation of CD4+ T cells and macrophages. J Immunol 2008;180(5):3158–65. [DOI] [PubMed] [Google Scholar]
- 34.Murata K, Miyoshi E, Kameyama M, Ishikawa O, Kabuto T, Sasaki Y, et al. Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin Cancer Res 2000;6(5):1772–7. [PubMed] [Google Scholar]
- 35.Kang R, Saito H, Ihara Y, Miyoshi E, Koyama N, Sheng Y, et al. Transcriptional regulation of the N-acetylglucosaminyltransferase V gene in human bile duct carcinoma cells (HuCC-T1) is mediated by Ets-1. J Biol Chem 1996;271(43):26706–12. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi M, Kuroki Y, Ohtsubo K, Taniguchi N. Core fucose and bisecting GlcNAc, the direct modifiers of the N-glycan core: their functions and target proteins. Carbohydr Res 2009;344(12):1387–90. [DOI] [PubMed] [Google Scholar]
- 37.Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, et al. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 2007;129(1):123–34. [DOI] [PubMed] [Google Scholar]
- 38.Nagae M, Kizuka Y, Mihara E, Kitago Y, Hanashima S, Ito Y, et al. Structure and mechanism of cancer-associated N-acetylglucosaminyltransferase-V. Nat Commun 2018;9(1):3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sultan AS, Miyoshi E, Ihara Y, Nishikawa A, Tsukada Y, Taniguchi N. Bisecting GlcNAc structures act as negative sorting signals for cell surface glycoproteins in forskolin-treated rat hepatoma cells. J Biol Chem 1997;272(5):2866–72. [DOI] [PubMed] [Google Scholar]
- 40.Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol 2006;26(8):3181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsui KH, Chang PL, Feng TH, Chung LC, Sung HC, Juang HH. Evaluating the function of matriptase and N-acetylglucosaminyltransferase V in prostate cancer metastasis. Anticancer Res 2008;28(4A):1993–9. [PubMed] [Google Scholar]
- 42.Petrosyan A, Cheng PW. A non-enzymatic function of Golgi glycosyltransferases: mediation of Golgi fragmentation by interaction with non-muscle myosin IIA. Glycobiology 2013;23(6):690–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J 2004;86(6):3993–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothermund CA, Kondrikov D, Lin MF, Vishwanatha JK. Regulation of Bcl-2 during androgen-unresponsive progression of prostate cancer. Prostate Cancer Prostatic Dis 2002;5(3):236–45. [DOI] [PubMed] [Google Scholar]
- 45.Cummings RD, Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem 1982;257(19):11230–4. [PubMed] [Google Scholar]
- 46.Casey CA, Bhat G, Holzapfel MS, Petrosyan A. Study of Ethanol-Induced Golgi Disorganization Reveals the Potential Mechanism of Alcohol-Impaired N-Glycosylation. Alcohol Clin Exp Res 2016;40(12):2573–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper CR, Chay CH, Pienta KJ. The role of alpha(v)beta(3) in prostate cancer progression. Neoplasia 2002;4(3):191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dutta A, Li J, Lu H, Akech J, Pratap J, Wang T, et al. Integrin alphavbeta6 promotes an osteolytic program in cancer cells by upregulating MMP2. Cancer Res 2014;74(5):1598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevenson NL, Bergen DJM, Skinner REH, Kague E, Martin-Silverstone E, Robson Brown KA, et al. Giantin-knockout models reveal a feedback loop between Golgi function and glycosyltransferase expression. J Cell Sci 2017;130(24):4132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lan Y, Zhang N, Liu H, Xu J, Jiang R. Golgb1 regulates protein glycosylation and is crucial for mammalian palate development. Development 2016;143(13):2344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Machamer CE. The Golgi complex in stress and death. Front Neurosci 2015;9:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrosyan A, Cheng PW, Clemens DL, Casey CA. Downregulation of the small GTPase SAR1A: a key event underlying alcohol-induced Golgi fragmentation in hepatocytes. Sci Rep 2015;5:17127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witkos TM, Lowe M. The Golgin Family of Coiled-Coil Tethering Proteins. Front Cell Dev Biol 2015;3:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrosyan A, Ali MF, Verma SK, Cheng H, Cheng PW. Non-muscle myosin IIA transports a Golgi glycosyltransferase to the endoplasmic reticulum by binding to its cytoplasmic tail. Int J Biochem Cell Biol 2012;44(7):1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson CL. Mechanisms of transport through the Golgi complex. J Cell Sci 2009;122(Pt 4):443–52. [DOI] [PubMed] [Google Scholar]
- 56.Petrosyan A, Ali MF, Cheng PW. Keratin 1 plays a critical role in golgi localization of core 2 N-acetylglucosaminyltransferase M via interaction with its cytoplasmic tail. J Biol Chem 2015;290(10):6256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrosyan A, Casey CA, Cheng PW. The role of Rab6a and phosphorylation of non-muscle myosin IIA tailpiece in alcohol-induced Golgi disorganization. Sci Rep 2016;6:31962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrosyan A, Cheng PW. Golgi fragmentation induced by heat shock or inhibition of heat shock proteins is mediated by non-muscle myosin IIA via its interaction with glycosyltransferases. Cell Stress Chaperones 2014;19(2):241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji C Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int 2012;2012:216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji C New Insights into the Pathogenesis of Alcohol-Induced ER Stress and Liver Diseases. Int J Hepatol 2014;2014:513787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Misteli T The concept of self-organization in cellular architecture. J Cell Biol 2001;155(2):181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Casey CA, Thomes P, Manca S, Petrosyan A. Giantin Is Required for Post-Alcohol Recovery of Golgi in Liver Cells. Biomolecules 2018;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storm M, Sheng X, Arnoldussen YJ, Saatcioglu F. Prostate cancer and the unfolded protein response. Oncotarget 2016;7(33):54051–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guan M, Su L, Yuan YC, Li H, Chow WA. Nelfinavir and nelfinavir analogs block site-2 protease cleavage to inhibit castration-resistant prostate cancer. Sci Rep 2015;5:9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yadav RK, Chae SW, Kim HR, Chae HJ. Endoplasmic reticulum stress and cancer. J Cancer Prev 2014;19(2):75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galmiche A, Sauzay C, Chevet E, Pluquet O. Role of the unfolded protein response in tumor cell characteristics and cancer outcome. Curr Opin Oncol 2017;29(1):41–7. [DOI] [PubMed] [Google Scholar]


