Introduction
With approximately 10 million new cases of traumatic brain injury (TBI) being reported worldwide each year (Hyder et al., 2007) and their long-lasting deficits continually impacting an estimated 2% of the population (Thurman et al., 1999; Langlois et al., 2006), TBIs are a significant health care concern. Unfortunately, due to the inherent complexity of TBI, there are relatively few effective treatment strategies for managing long-term disabilities reported to encompass a multitude of higher-order cognitive deficits that significantly impact patients’ ability to assimilate back into normal life. Cognitive impairments often reflect executive dysfunction, such as the inability to retain new information and blunted behavioral flexibility resulting in a hindered ability to adapt to novel environmental contingencies (Horneman and Emanuelson, 2009; Bondi et al., 2014). To more closely investigate clinically-translatable executive deficits post-TBI, this study used a behavioral assessment known as the attentional set-shifting test (AST), which comprises a series of increasingly difficult perceptual discriminations to obtain a reward, drawing methodological parallels to the Wisconsin Card Sorting Test (WCST) typically employed to evaluate similar executive function deficits in humans (Stuss et al., 2000; Greve et al., 2002; Benge et al., 2007). This task has been used extensively in rodents to describe the behavioral inflexibility that parallels higher-order cognitive deficits reported in the clinic as a result of neuropsychiatric and neurodegenerative disorders such as schizophrenia, Alzheimer’s Disease, and Parkinson’s Disease (Tait et al., 2007; Broberg et al., 2009; Goetghebeur et al., 2010; Rane et al., 2012; Nikiforuk et al., 2016a, 2016b). We previously utilized the AST to describe executive function impairments resulting from an experimental brain trauma induced over the right parietal cortex in a cortical deformation depth dependent manner (Bondi et al., 2014).
Clinical and pre-clinical findings support a cholinergic hypothesis through which disruptions in acetylcholine (ACh) neurotransmission after traumatic brain injury mediate many of the cognitive impairments reported (Arciniegas, 2003). Acetylcholinesterase inhibitors (AChEIs) represent a drug class that has shown improvement in memory and executive function in TBI patients (Khateb et al., 2005; Tenuovo, 2005; Silver et al., 2006; Ballesteros et al., 2008) and mixed results in rodents (Shaw et al., 2013; Yu et al., 2015; de la Tremblaye et al., 2017). Specifically, galantamine (GAL) is an AChEI used as a cognitive enhancer for patients who present mild dementia, a condition generally found in the elderly population (Bickel et al., 1991; Coyle and Kershaw, 2001). In TBI studies, AChEIs have been reported to confer benefits in memory and executive function, however, these findings are somewhat controversial as a lack of effects or even deleterious effects have been reported after the administration of certain AchEIs such as donepezil (Tenovuo et al., 2005; Ballesteros et al., 2008; Shaw et al., 2013; Bondi et al., 2018; Campbell et al., 2018). Given that the elderly are more prone to falls resulting in subsequent brain damage, GAL is of specific interest when evaluating executive function treatments as it not only inhibits the function of acetylcholinesterase, but also acts as a non-specific, positive allosteric modulator at the α4- and α7-nicotinic acetylcholine receptors (nAChR) and increases the overall expression of nAChRs in the frontal cortex (Bickel et al., 1991; Fayuk and Yakel, 2004; Geerts et al., 2005; de la Tremblaye et al., 2017), which has been associated with neuroprotective effects in both neurodegenerative disorders such as Parkinson’s disease and TBI (Akaike et al., 2010; Quik et al., 2012; Koukouli and Maskos, 2015). GAL has been shown to enhance executive function in preclinical studies and has considerable clinical promise. Specifically, Nikiforuk and colleagues (2015, 2016a,b) reported improved cognitive performance on the AST after GAL administration in rats, however these studies only evaluated acute GAL administration. After TBI, chronic GAL dosing has been demonstrated to produce beneficial effects on the Morris water maze (Hernandez et al., 2006; de la Tremblaye et al., 2017), however its prospective beneficial effects on a frontal lobe-mediated behavioral task are also warranted. Hence, the current study sought to further the investigation of GAL as a treatment for executive function deficits prevalent in the TBI population by testing the hypothesis that, when given chronically (i.e. daily from 24 hours post-injury through test day), GAL would normalize the executive function deficits induced by a moderate CCI in adult male rats.
Methods
Animals
Fifty-nine adult male Sprague-Dawley rats (Harlan Laboratories Inc., Indianapolis, IN) were housed in commercially available Plexiglas® cages and maintained in a temperature (21 ± 1 °C) and light (12 hr on/12 hr off; lights on at 7:00 a.m.) controlled environment with food and water available ad libitum. The rats were acclimated to this environment for one week prior to the beginning of experimental manipulations, all of which occurred during the light portion of the cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Every attempt was made to limit the number of rats used and to minimize pain and suffering.
Surgery
All rats weighed 300-325 grams on the day of surgery and were randomly assigned to either the controlled cortical impact (CCI) or SHAM injury group. Surgical procedures were performed as previously described (Shaw et al., 2013; Bondi et al., 2014; de la Tremblaye et al., 2017; Radabaugh et al., 2017). Briefly, surgical anesthesia was induced with 4% isoflurane in 2:1 N2O:O2 and the rats were intubated and secured in a stereotaxic frame. During surgery the rats were maintained at a surgical level with 2% isoflurane and carrier gases. A heating pad was used to maintain core temperature at 37 ± 0.5 °C. Using aseptic procedures, a craniectomy was performed in the right hemisphere with a dental drill, and the bone plate was removed between bregma and lambda in order to provide sufficient clearance for the 6 mm-wide impact tip, which was centered within the opening. A TBI was then produced by impacting the exposed right parietal cortex to a depth of 2.8 mm tissue deformation at 4 m/sec. After the impact, anesthesia was discontinued and the incision was promptly sutured. SHAM injury rats were subjected to the same surgical procedures as the CCI animals except for the cortical impact.
Acute neurological evaluation
Immediately following the termination of anesthesia, hindlimb reflexive ability was evaluated by briefly squeezing the rats’ paw every 5 sec and recording the time to produce a withdrawal response. Return of the righting reflex also was measured by recording the time required to turn from the supine to prone position. These tests are sensitive indicators of injury severity and duration of anesthesia (Dixon et al., 1991; Kline et al., 2010; Bondi et al., 2014). Once the effects of anesthesia had completely worn off, as indicated by spontaneous movement in the holding cage, the rats were returned to the housing facility. Rats were weighed and their general health was monitored daily until sacrifice.
Drug administration
GAL (galantamine hydrobromide, TCI Chemicals, Portland, OR) was prepared daily by dissolving in sterile saline, which also served as the VEH. GAL (1 or 2 mg/kg) or a comparable volume of VEH (1 mL/kg) was administered intraperitoneally beginning 24 h after CCI or SHAM injury and once daily for 4 weeks until test day. On the days of behavioral testing, the injections were administered 1 h prior to testing. The doses were selected based on preliminary data from our laboratory showing a narrow therapeutic range. The route of administration is standard protocol in our laboratory (Monaco et al., 2014; Phelps et al., 2015; de la Tremblaye et al., 2017)
Attentional set-shifting test (AST)
The AST task used in our laboratory was adapted from Birrell and Brown (2000) and has been described previously (Bondi et al., 2014). One week prior to behavioral testing (i.e. day 21 post-surgery), rat food intake was limited to 80% of the standard diet (i.e. 14 g a day) with water freely available. We previously reported that injured rats were not more susceptible to losing weight during the mild food restriction process when initiated at 3 weeks following parietal CCI (Bondi et al., 2014). We also recorded weights on a daily basis prior to and after food restriction in order to determine whether food restriction, injury, or drug treatment effected patterns of weight gain prior to testing. Cognitive testing took place in a custom-built rectangular Plexiglas arena (30 × 51 × 25 cm) that is painted gray on the outer side of all surfaces. A removable divider separates one third of the arena forming a start box, which was also used as a holding area between trials to allow the experimenter to clean the arena and change the pots. There is a permanent divider in the opposite one third of the arena that separates the two pots from one another. The separation allows for quick removal of the rat after a response has been recorded. The terracotta digging pots (internal rim diameter, 7 cm; depth, 6 cm) were all defined by a pair of cues (i.e. odor and digging media/pot). Each pot was marked with a single, distinct odor by adding two drops (10 μL /drop) of aromatic oil (NOW Foods, Bloomingdale, IL) to the inner rim 5 days prior to use. Odors were reapplied daily (1–2 μL) to maintain consistent intensity. A different pot was used for each combination of digging medium and odor, and only one odor was ever applied to a given pot. The “reward” was one quarter of a Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN) buried 2 cm below the surface of the digging medium in the “positive” pot. To avoid location by smell rather than learning and discrimination, pots were sprinkled with Cheerio dust prior to each stage. To begin each trial, the removable divider was lifted to allow the rat access to the other two thirds of the arena and explore the pots. A correct choice is recorded when the rat chooses the pot containing the Cheerio reward without digging in the incorrect pot. Digging was defined as a vigorous displacement of the medium to retrieve and consume the reward buried within the pot. Simply investigating or smelling the surface of the pot without displacing the material was not considered a “dig”, thus allowing the rat to be able to assess all characteristics of the pots before making their choice (i.e., digging). The behavioral procedure was performed over the course of three days and consisted of habituation, training, and testing.
Habituation:
Rats were first trained to dig reliably in pots to obtain the food reward. Two unscented pots were placed into clean home cages. Every five minutes, a food reward was buried in the pots with increasing depths of sawdust (i.e. three times at each increment of no sawdust, one third full, one half full, and completely full pots). Once the rat had learned to dig in the pots for a food reward, they were placed into the testing arena and given three trials to retrieve a Cheerio from both sawdust-filled pots.
Training:
Rats were trained on a series of simple discriminations (SD) in order to reach a criterion of six consecutive correct trials. First, they learned to associate the food reward with a medium cue using new unscented pots (felt vs. paper strips). After reaching criterion for the medium discrimination, rats had to learn to discriminate between aromatic odors in sawdust-filled pots (lemon vs. eucalyptus). All rats were trained using the same pairs of stimuli and in the same order. The positive and negative cues for each rat were randomly determined and equally represented. The training exemplars were not used again during testing. Any rat that failed to complete the training procedure was eliminated from subsequent testing.
Testing:
Rats were then tested on a series of increasingly difficult discrimination stages, each also requiring a criterion of six consecutive correct trials, based on the cue progression previously established (Bondi et al., 2014). The first stage was an SD involving only one stimulus dimension (i.e., odor or medium), with groups split in half with regard to initial stimulus dimension (for clarity, subsequent description will consider only the example beginning with an odor discrimination). The second stage was a compound discrimination (CD), in which the same contingency rule was required (e.g., odor), and the second, irrelevant stimulus dimension (e.g., medium) was introduced. As in the SD task, only one odor was associated with reward, but two different digging media were paired randomly with the odors. The third stage was a reversal (R1) of the previous discrimination, in which the same odors and media were used. Odor was maintained as the relevant dimension; however, the negative odor from the previous stage became positive (i.e., was associated with reward), and the positive odor from the previous stage became negative (no reward). The fourth stage was an intradimensional (ID) shift in which all new stimuli (odors and media) were introduced. Again, odor remained the relevant dimension and digging medium was still irrelevant. The fifth stage was a reversal of this discrimination (R2), in which the previously negative odor became positive, similar to R1. The sixth task required an extradimensional (ED) cognitive set-shift, in which all new stimuli were again introduced, but the dimension that had been repeatedly reinforced as the informative, relevant dimension (thus forming a “cognitive set”) was now irrelevant, and the previously distracting dimension (i.e., the digging medium) became the relevant dimension. Finally, the seventh stage was another reversal (R3), where the previously negative stimulus became positive, as in the previous reversals. The assignment of each exemplar in a pair as being positive or negative in a given stage, as well as the left-right positioning of the pots in each trial, were determined randomly in advance. The dependent measures were the number of trials required to reach criterion for each stage of the test, the total number of errors per stage, as well as set-loss errors, which occurred when 50% of the rule contingency had been achieved (i.e., 3,4, or 5 correct responses followed by an incorrect choice)(Stuss, et al., 2000). The rats were allowed 10 min to make a choice on each trial. If a choice was not made within this interval, the trial was scored as an error and the rat was returned to the start box. Rats failing to make a choice on six consecutive trials, or failing to complete a stage within 50 trials, were eliminated from further testing.
Histology
Cortical lesion volume was assessed after the completion of behavioral testing (i.e., 5 weeks post-surgery). Rats were anesthetized using Fatal-Plus (Henry Schein Animal Health, Columbus, OH; 0.25 mL, intraperitoneally) and then perfused transcardially with 200 mL of 0.1 M of phosphate buffered saline (pH 7.4), followed by 300 mL of 4% paraformaldehyde (PFA). Brains were extracted, postfixed in 4% PFA for 1 week, dehydrated with alcohols, and embedded in paraffin. Seven-micrometer-thick coronal sections were cut at 1-mm intervals through the lesion on a rotary microtome and mounted on Superfrost®/Plus glass microscope slides (Fisher Scientific, Pittsburgh, PA). After drying at room temperature, sections were deparaffinized in xylenes, rehydrated, and stained with cresyl violet. Cortical lesion volumes (mm3) were assessed by an observer blinded to experimental conditions using a Nikon Eclipse 90i microscope (Nikon Corporation, Tokyo, Japan). The area of the lesion (mm2) was first calculated by outlining the inferred area of missing cortical tissue for each section (typically 5–7; Nikon NIS-Elements AR 3.22.14 software; Nikon) and then by summing the lesions obtained, as previously reported (Olsen et al., 2012; Monaco et al., 2014; Bondi et al., 2014).
Statistical analyses
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statistica 64 version 13 Academic software (Dell Inc., Tulsa, OK). Righting reflex, hindlimb reflexive ability, and weight gain patterns during food restriction were analyzed by two-way analysis of variance (ANOVA) with injury and drug as factors to ensure that no surgery biases were reflected in subsequent drug assignment groups. For analysis of behavior on the AST, trials to criterion on medium and odor training SDs, as well as the number of trials to criterion, total response errors, and set loss errors were recorded for each test stage. These measures were analyzed by three-way multivariate ANOVA (MANOVA, Injury × Drug Treatment × Stage), with repeated measures over Stage. In order to ensure that MANOVA assumptions were met, the Levene’s Test for Homogeneity of Variances and Kolmogorov-Smirnov test for normality were performed. To also alleviate any biases with regards to perceptual stimuli, ED shift type analyses were achieved by means of a two-way ANOVA (Shift x Stage), with repeated measures over Stage. When significant ANOVA or MANOVA main effects or interactions were indicated, post-hoc comparisons to determine specific group differences were performed using Newman-Keuls tests or post-hoc one-way ANOVAs, as appropriate. The effects of chronic GAL treatment specifically on cognitive performance on the R1 stage were then analyzed separately using a priori hypotheses, which dictated that the design of this experiment predicted an isolated single-cell effect to which the MANOVA interaction term can be insensitive when embedded in a complex multi-factorial design in which several of the groups were expected to show no effect (e.g., see Anderson, 1961). Therefore, these a priori hypotheses were tested specifically by planned contrast analyses, first comparing TBI rats chronically injected with VEH to those subjected to SHAM surgery and receiving daily VEH injections, and then comparing TBI-GAL treated rats (1 or 2 mg/kg/day) to the VEH-treated injured group. Total trials to criterion across the test and histological data were analyzed using unpaired t-tests. Results are expressed as the mean ± standard error of the mean (SEM), and significance for all statistical analyses was set at p<0.05.
Results
A total of 15 rats were excluded from all of the statistical analyses. One injured rat was eliminated due to death post-surgery. The other exclusions occurred due to an inability to either complete the training protocol (i.e., 2 TBI and 2 SHAM), an inability to complete testing (i.e. 2 TBI and 1 SHAM), an inability to complete the required criterion within 50 trials per stage (i.e., 2 TBI and 1 SHAM), or displayed scores >2 standard deviations above the group mean after the initial analysis (i.e., 3 TBI and 1 SHAM).
Acute neurological evaluation
There was a statistically significant effect of TBI compared to SHAM with respect to withdrawal latency after a brief paw pinch was administered to either limb (left range = 162±4.55 sec, F1,38= 883.79, p<0.001; right range= 157.55±4.49 sec, F1,38= 910.81, p<0.001) after cessation of anesthesia. Moreover, there were no significant differences observed between groups randomly pre-assigned to commence daily GAL or VEH injections (left paw: F2,38= 0.9, p=0.41; right paw: F2,38= 0.92, p=0.41). A two-way ANOVA also detected significant differences between TBI and SHAM groups for return to righting ability (F1,38= 325.22, p < 0.001; TBI range: 396.68±14.18 sec; SHAM range: 139.91 ±2.92 sec), again with no bias reflective of subsequent drug group pre-assignment (F2,38= 1.422, p=0.25). A one-way ANOVA was then used to assess percentage of body-weight change during the 1 week of mild food restriction as described previously (Bondi et al., 2014). No differences were revealed among groups regardless of whether they were SHAM or TBI, VEH or GAL-treated with regard to percent weight loss (all p’s>0.05). Mean percent weight loss ranged from 2.29% of average body weight at initiation of food restriction for SHAM rats to 3.68% for TBI rats. Both of these values were well within the expected range of weight loss following a week of mild food restriction paradigm consisting of 80% of the rat’s normal diet (Bondi et al., 2008, 2010).
Attentional set-shifting test
Overall MANOVA and AST training
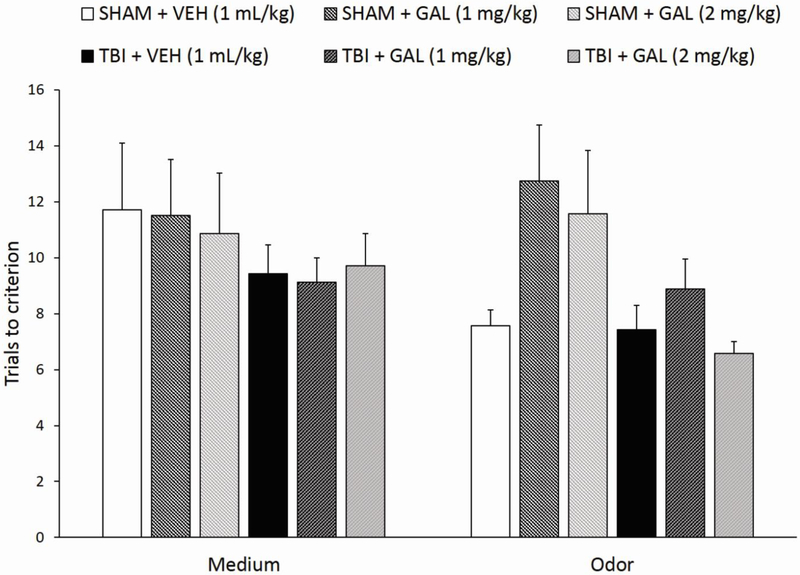
A three-way MANOVA test (Injury x Drug Treatment x Training stage) revealed a significant effect of Injury (F1,38= 7.47, p<0.01) for the total number of trials to reach criterion for the SD tasks during the training day, with no other significant factors or interaction. Regardless of drug treatment, TBI rats completed the medium and odor simple discriminations overall slightly faster than SHAM rats (Fig. 1).
Fig. 1.
Mean ± standard error of the mean (SEM) trials to reach criterion on simple discriminations tasks (medium and odor) on the training day preceding testing. Overall, regardless of drug treatment, TBI rats completed the medium and odor simple discriminations slightly faster than SHAM rats. No individual group comparisons were significant (n=7-8/group; overall effect of Injury, p<0.01).
AST – ED set-shift comparison
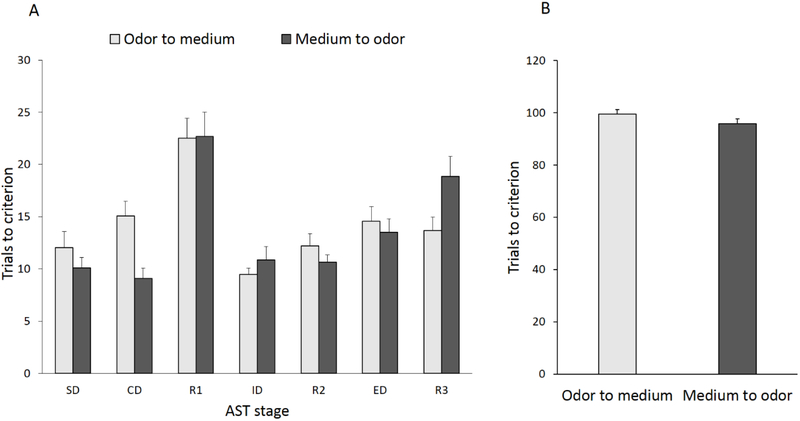
Figure 2 shows a comparison between rats subjected to an odor-to-medium versus medium-to-odor ED set-shift on the trials to reach the criterion for each task of the AST regardless of injury or treatment group. There was no significant effect of ED shift type (F1,42 = 0.02, p=0.88) on AST performance across stages, however there was a significant ED shift type x Stage interaction (F6,252=2.24, p<0.05; Fig. 2A). Newman Keuls post hoc analyses revealed a significant difference on R3 (p<0.05), suggesting that overall, rats subjected to a medium-to-odor relevant dimension switch performed worse that rats subjected to an odor-to-medium switch. No other stage comparisons were significantly different regardless of the dimension to which the contingency belonged, such as odor or medium. When a t-test was subsequently performed to compare total trials across the test for rats assigned to either of the two perceptual stimuli set-shifts, no differences were unveiled by means of an independent t-test (t42= 0.88, p=0.31, Fig. 2B).
Fig. 2.
A) Progression through the attentional set-shifting protocol stages renders comparable cognitive performance patterns for trials to reach criterion of six consecutive correct responses regardless of the initial discriminative dimension (odor or medium), on the first six stages of the AST. A significant ED shift type x Stage interaction rendered rats subjected to a medium-to-odor relevant dimension switch performing worse that rats subjected to an odor-to-medium switch on R3 only. However, there was no injury-related bias against a sensory modality necessary for contingency rule acquisition (i.e., forming a cognitive set) and extradimensional set-shifting. B) No differences were reported when comparing total trials across the complete test for rats assigned to either of the two perceptual stimuli set-shifts. Data are expressed as mean trials to criterion ± SEM (n=22/group). SD, simple discrimination; CD, compound discrimination; ID, intradimensional set-shift; ED, extradimensional set-shift; R1, first reversal; R2, second reversal; R3, third reversal.
AST – Total Trials to Criterion
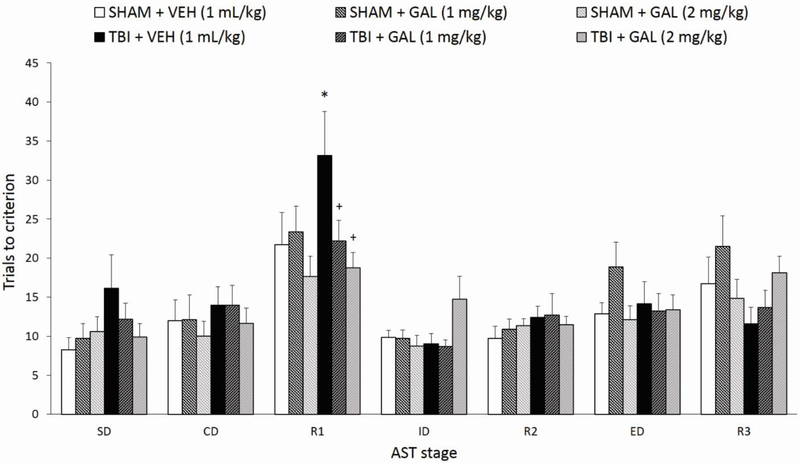
Figure 3 shows the effects of parietal CCI injury and chronic systemic GAL administration (1 or 2 mg/kg/day, i.p.) on the trials to criterion (TTC) for each stage of the AST at 4 weeks post-surgery. A three-way repeated measures MANOVA (Injury x Drug Treatment x Stage), with repeated measures over Stage, revealed a significant effect of Stage (F6,228= 23.01, p<0.001), a significant Injury x Drug interaction (F2,38=3.33, p<0.05), while the Drug x Stage interaction neared significance (F12,228= 1.62, p=0.08), but no effects of Injury (F1,38=0.4, p=0.53) or Drug (F2,38=0.79, p=0.46) alone, nor an Injury x Drug Treatment x Stage interaction (F12,228=1.32, p=0.21). In order to ensure that MANOVA assumptions were met, the Levene’s Test for Homogeneity of Variances rendered equal error variances on all stages analyzed [R1: F5,38=1.4, p=0.24; ED: F5,38= 1.95, p=0.11; R3: F5,38= 1.29, p=0.29]. Moreover, the Kolmogorov-Smirnov test for normality also rendered data distribution as normal on all stages [R1: d=1.6; ED: d=0.11; R3: d=0.14, all p’s not significant). For the main effect of Stage, post-hoc comparisons across all groups showed that significantly more trials were required to reach criterion during R1 (compared to all other tasks, p’s<0.001), ED (compared to SD, p<0.05 and R2, p<0.01), and R3 (compared to SD, CD, ID, and R2; p’s<0.005). As previously reported, this effect validates the inherent difficulty of stimulus reversals and the ED shift stage compared to other stages of the AST (Bondi et al., 2008, 2010, 2014). Subsequent post-hoc ANOVAs for individual AST stages did not render significant effects based on Injury or Drug factors (p’s>0.05), including on the first stage, a SD, which was a repetition of previous training stages but with all new stimuli, thus inferring the injury-related advantage during training was not transferred to testing (Fig. 3). On the R1, ED, and R3 stages, where higher-order behavioral flexibility governs the ability to unlearn the previous stimulus while learning a new rule, one-way ANOVAs rendered a trend for the Drug factor in R1 (F2,38= 2.91, p=0.07), but no other factor effects on either stage (p’s>0.05). Post-hoc analyses also determined that on the R1 stage, the relevant comparisons [TBI + VEH versus SHAM + VEH, TBI + GAL (1mg/kg) versus TBI + VEH, and TBI + GAL (2 mg/kg) versus TBI +VEH] neared significance (all p’s <0.09) but was, as predicted, compromised by being embedded in a complex multi-factorial design in which several of the groups were expected to show no effect (i.e., drug effects in SHAM rats). These three relevant group comparisons were all p >0.2 for the ED and R3 stages. However, planned contrasts analyses for the three pre-determined relevant comparisons on R1 rendered a significant overall effect (F3,38=3.01, p<0.05), with TBI + VEH rats performing significantly worse than SHAM + VEH rats, and both GAL treatment regimens significantly reversing the TBI-induced cognitive deficit on reversal learning (all p’s <0.05, Fig. 3). Planned contrasts for ED and R3 were not significant (ED: F3,38=0.16, p=0.92, contrast p’s >0.6; R3: F3,38=0.8, p=0.5, contrast p’s>0.16).
Fig. 3.
Controlled cortical impact injury impaired reversal learning on the AST compared to SHAM + VEH, manifested as an overall significantly higher number of trials to criterion, while chronic GAL treatment (1 and 2 mg/kg/day) restored cognitive performance after brain trauma. Significantly more trials were required to reach criterion during R1 (compared to all other tasks), ED (compared to SD and R2), and R3 (compared to SD, CD, ID, and R2) when rats were collapsed across groups (p’s<0.05). *p<0.05 versus SHAM + VEH group, +p<0.05 versus TBI + VEH group. Data are expressed as mean trials to criterion ± SEM (n=7-8/group). TBI, traumatic brain injury; AST, attentional set-shifting test.
AST – Total Errors
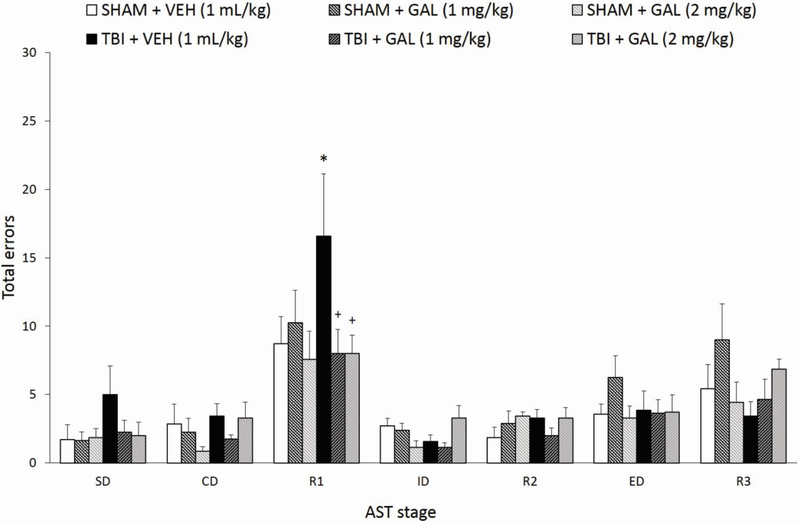
Figure 4 depicts the effects of CCI and chronic GAL treatment (1 or 2 mg/kg/day, i.p.) of the total errors (TE) for each test stage at 4 weeks post-surgery. A three-way repeated measures MANOVA with repeated measures over Stage, revealed a significant effect of Stage (F6,228=26.06, p<0.001), a significant Injury x Drug interaction (F2,38=3.66, p<0.05), but no effects of Injury (F1,38=0.4, p=0.53), Drug (F2,38=0.73, p=0.49), Drug x Stage interaction (F12,228=1.56, p=0.1), nor an Injury x Drug Treatment x Stage interaction (F12,228=1.47, p=0.14). For the main effect of Stage, post-hoc comparisons across all groups showed that significantly more errors occurred in order to reach criterion during R1 and R3, compared to all other tasks (R1 versus all other stages: p’s<0.001; R3 versus SD-R2, p’s<0.001, R3 versus ED, p<0.05). Subsequent post-hoc ANOVAs for individual AST stages did not render significant effects based on Injury or Drug factors (p’s>0.05), except on ID, where there was a significant Injury x Drug Treatment interaction (F2,38= 5.75, p<0.01), which was not accompanied by any significant post-hoc comparisons (p’s>0.05). In parallel with TTC analyses, planned contrasts analyses for the three pre-determined relevant total error comparisons on each of the three stages of interest (R1, ED, and R3) rendered a near significance overall omnibus F effect for R1 (F3,38=2.66, p=0.06), with individual contrasts showing TBI + VEH rats to perform significantly worse than SHAM + VEH rats (p<0.05), and both GAL treatment regimens significantly reversing the TBI-induced cognitive deficit on reversal learning (both p’s <0.05, Fig. 4). Planned contrasts for ED and R3 were not significant (ED: F3,38=0.01, p=0.99, contrast p’s>0.87; R3: F3,38=0.69, p=0.56, contrast p’s>0.17).
Fig. 4.
Controlled cortical impact injury impaired reversal learning on the AST compared to SHAM + VEH, manifested as significantly higher total response error rates in the AST, while chronic GAL treatment (1 and 2 mg/kg/day) restored cognitive performance after brain trauma. When rats were collapsed across groups, significantly more errors occurred in order to reach criterion during R1 and R3, compared to all other tasks (p’s<0.05). *p<0.05 versus SHAM + VEH group, +p<0.05 versus TBI + VEH group. Data are expressed as mean total errors ± SEM (n=7-8/group).
AST – Set Loss Errors
Set loss errors for each rat on every stage were calculated as the errors occurring after 50% or more of contingency acquisition has been achieved (i.e., an error after three, four, or five correct responses), parallel to the WCST (Stuss et al., 2000). Repeated-measures MANOVA found a significant effect of Stage only (F6,228,= 4.57, p<0.001), but no other group effects or interactions. For the main effect of Stage, post-hoc comparisons across all groups, regardless of injury or drug treatment, showed that significantly more trials were required to reach criterion during R1, compared to all other tasks (R1 versus: SD, p<0.005; CD, p<0.01; ID, p<0.001; R2, p<0.001; ED, p<0.05; R3, p<0.005), however additional injury- or drug-related post hoc analyses were not further performed for this measure (data not shown).
Histology: Cortical lesion volume
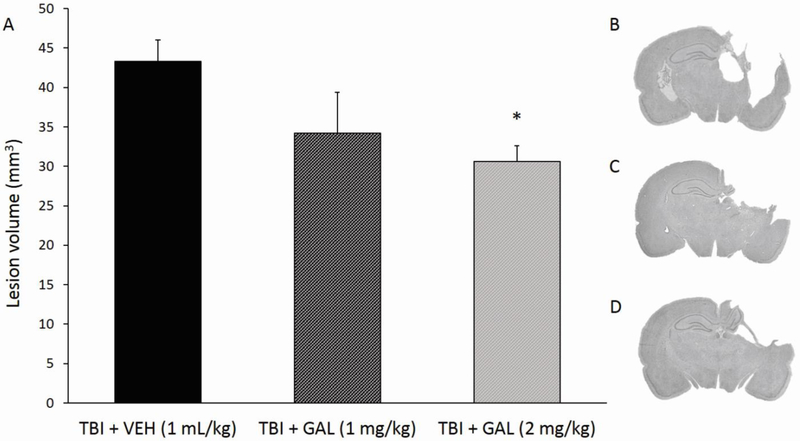
At 5 weeks post-surgery, the mean cortical lesion volume in the TBI-VEH group was 43.31 mm3, in line with previous reports (Bondi et al., 2014), while mean cortical lesion volume for the TBI + GAL (1mg/kg) group was 34.22 mm3, and the TBI + GAL (2mg/kg) group had a mean cortical lesion volume of 30.64 mm3 Individual t-test analyses revealed that the TBI + GAL (2 mg/kg) group exhibited statistically smaller cortical lesions than TBI + VEH group (t7 = 3.85, p<0.01, Fig. 5). No other group comparisons were significant.
Fig. 5.
A) Mean (± SEM) cortical lesion volume (mm3) 5 weeks after controlled cortical impact injury at 2.8 cortical deformation depth. Left to right: TBI + VEH group represents rats that were injured and did not receive drug treatment; TBI + GAL (1 mg/kg) and TBI + GAL (2 mg/kg) groups represent rats receiving one of the two doses of GAL administration. CCI injury produced the largest cortical lesion volume, with GAL dose-dependently reducing the extent of cavity formation (*p<0.01 versus TBI + VEH group). B-D) Panels are mean depictions of the lesion within each group at the level of the dorsal hippocampus (TBI + VEH at top, TBI + GAL (1mg/kg) in middle, TBI + GAL (2 mg/kg) at bottom).
Discussion
Traumatic brain injuries are known to induce a wide array of cognitive disabilities, including deficits in executive functioning and behavioral inflexibility, and yet preclinical behavioral studies rarely evaluate these higher order impairments (Horneman and Emanuelson, 2009; Bondi et al., 2014). There is a large and constantly expanding collection of TBI studies that report deficits in spatial learning and working memory using behavioral tasks such as the Morris water maze and the Barnes maze (Hernandez et al., 2006; Fedor et al., 2010; Kline et al., 2010; Nudi et al., 2015; de la Tremblaye et al., 2017; Radabaugh et al., 2017), however, tasks more sensitive to executive function deficits are rarely utilized. A previous study from our laboratory implemented a well-validated behavioral assessment known as the AST (Bondi et al., 2014), which incorporates increasingly difficult rule shifts to obtain a food reward and draws conceptual parallels to the Wisconsin Card Sorting Test (WCST) typically employed to evaluate similar executive function deficits in humans (Birrell and Brown, 2000; Stuss et al., 2000; Greve et al., 2002; Benge et al., 2007). Our initial study reported executive function impairments resulting from an experimental brain trauma induced over the right parietal cortex in a cortical deformation depth dependent manner. In order to expand the pharmacological armamentarium suitable to resolve cognitive impairments post-injury, the current study sought to determine if the TBI-induced deficits in executive function and behavioral inflexibility can be ameliorated by chronically administering GAL, an acetylcholine-enhancing pharmacotherapy previously shown to confer benefits in several conditions characterized by cognitive deficits, such as dementia and Alzheimer’s disease (Coyle and Kershaw, 2001). As the elderly represent an at-risk population for TBIs, the goals were to assess effects of chronic GAL on higher-order cognitive function in rats: 1) initiate at 24 hrs post-injury in order to preserve experimental design parameters previously shown to be beneficial on motor tasks and spatial learning, as well as to commence prior to secondary cascade damage taking place; 2) mimic chronic administration as in the clinic (Nagakawa et al., 2017), to be followed in future experiments with aged rats subjected to TBI.
Prior to AST, rats were placed on a week-long mild food restriction to encourage motivation during testing days. Weights were monitored during this time and no differences were detected among groups regardless of whether they were SHAM or TBI, VEH or GAL-treated with regard to percent weight loss (all p’s>0.05). Mean percent weight loss ranged from 2.29% of average body weight for SHAM rats to 3.68% for TBI rats, which is within the expected range of weight loss without affecting behavior (Bondi et al., 2008; Bondi et al., 2010). Interestingly, a modest in magnitude but significant effect of injury was revealed during training, such that injured rats learned the simple discriminations faster, regardless of drug treatment, if anything further strengthening the injury-related deficits observed the next day during complex rule reversal. It is thus feasible that GAL treatment in TBI rats maintained performance at optimal levels during both training and testing sessions. During AST, rats subjected to TBI performed significantly worse on R1, manifested as higher total trials and total errors prior to reaching criterion of six consecutive correct responses. These findings replicate our previous study that revealed impairments in reversal learning post-TBI (Bondi et al., 2014). There is typically little room for improvement in simpler, initial stages (SD, CD) or the intra-dimensional set-shift (ID) and its reversal (R2), where rats are reinforcing the previous stages (CD and R1) (Tait et al., 2018). Herein, we did not observe ED shift or R3 deficits post-injury, however ED stage and reversal learning stages, particularly R1 and R3 which occur for the first time in a new dimension, may exhibit inherent variability with regard to the same manipulation such that it is not imperative to obtain the exact deficits across multiple studies in order to acknowledge their existence (Naegeli et al., 2013; Jett et al., 2015). This behavioral flexibility deficit, however, was normalized by daily administration of GAL regardless of dose, 1 or 2 mg/kg/day, suggesting that this pharmacotherapy can ameliorate deficits in executive function impairments post-TBI. Benefits of acute GAL administration have been previously reported in AST alone (Nikiforuk et al., 2015) or sub-acute administration (4 days) following CCI positively impacting blood brain barrier integrity, hippocampal cell survival, spatial learning, and declarative memory, however it was not employed in a clinically-relevant chronic treatment regimen (Zhao et al., 2018).
Olfactory dysfunction is a commonly reported symptom post-TBI and could potentially impact the findings of the AST (Callahan et al., 1999; Bondi et al., 2014). To account for this possible dimensional bias, half of the rats begin their testing with odor and switched to medium during the ED shift stage and the other half begin with medium and switched to odor. When collapsing rats across all groups, there was a significant ED shift type x Stage interaction, such that rats subjected to a medium-to-odor relevant dimension performed worse on R3 only, where there were no injury or drug-related group differences. Moreover, there was no contingency shift difference on R1, where TBI-related cognitive deficits and drug-mediated benefits were revealed for both total trials and total errors, suggesting that olfactory dysfunction did not play a significant role in the current findings. Although the CCI injury is often coined as a “focal” model of TBI, the damage is not contained to the area of actual impact (Hall et al., 2005; Osier and Dixon, 2014). Due to the detrimental pathophysiologic cascades initiated by the mechanical, shearing forces of the impactor tip compressing the brain tissue, areas outside the range of the actual impact are also subjected to extensive damage. The deficits observed in the AST are likely due to interrupted functioning and interconnectivity of brain areas such as the hippocampus, frontal cortices, and striatum (reviewed in McAllister, 2011 and Bondi et al., 2014). This hypothesis is further corroborated by the observation of damage extending beyond the area of impact when analyzing the cortical lesion volumes. However, it is interesting to note that only the 2 mg/kg dose of GAL resulted in significantly smaller lesions. It is not uncommon, however, for studies to report both a narrow dose range of GAL in TBI as well as a lack of correlation between functional and histological recovery demonstrating the complex and heterogenous nature of TBI (Clausen and Hillered 2005; Marklund et al., 2009; Kline et al., 2010; de la Tremblaye et al., 2017).
Impairments in the reversal stages of AST may be attributed to damage in specific brain regions. In fact, previous studies using targeted chemical lesions have evaluated this hypothesis. McAlonan et al. (2003) demonstrated that selective damage to the orbital prefrontal cortex (OPFC) using ibotenic acid results in reversal learning deficits such as a difficulty in overcoming learning when a stimulus has become irrelevant. This is consistent with the clinical understanding that damage to the OPFC often results in deficits in decision making associated with reward expectation. Additionally, Tait et al. (2017) reported that a 6-hydroxydopamine lesion to the dorsomedial striatum, a downstream projection zone of the PFC, also results in reversal deficits supporting the theory that the frontal lobe and striatum are heavily implicated in the ability to adapt to novel environmental contingencies.
Acetylcholine (ACh) acts as both a neurotransmitter and neuromodulator in the striatum, prefrontal, and frontal cortices and is thus thought to be strongly associated with executive functioning, being implicated in both age related cognitive decline (Quik et al., 2012; Wallace and Bertrand, 2013; Chauhan et al., 2016; de la Tremblaye et al., 2017) and nootropic effects of both nicotine and AChEIs (Wallace and Bertrand, 2013; Nikiforuk et al., 2015). Notably in the striatum, ACh exerts a large influence by altering the transmission of medium spiny neurons, the main output of this brain region (Lim et al., 2014; Liu et al., 2007). This alteration has been implicated in many conditions that result in cognitive deficits such as Parkinson’s disease (Lim et al., 2014; Tait et al., 2017). On the other hand, the prefrontal cortex is involved in the coordination of cortical and subcortical inputs converging to execute higher order cognitive functions (Wallace and Bertrand, 2013), thus indirectly affecting AST performance albeit not being directly injured following CCI. A notable brain region that could also be partially implicated in executive function deficits is the hippocampus due to the complex hippocampal–neocortical connectivity (Bondi et al., 2014). Altering hippocampal levels of ACh is believed to play a vital role in learning and memory (Hasselmo, 2006) given that cholinergic input from the medial septum may influence changes in the CA1 region interneuron membrane potentials following ACh release suggesting a potential mechanism behind the spatial learning benefits reported after administering AChEI’s (McQuiston, 2014; de la Tremblaye et al., 2017). Additionally, degradation of the medial PFC results in memory consolidation impairment associated with decreased task-related hippocampal- PFC connectivity and is often implicated in age-related cognitive decline (Rattenborg et al., 2010; Wang et al., 2011; Mander et al., 2013).
In addition to the cholinergic system playing a direct role in a plethora of brain areas associated with higher-order cognitive functions, specific ACh receptor subtypes are implicated in modulating other neurotransmitter systems. In respect to dopamine, striatal D1 receptors that contribute to positive reinforcement learning, a larger component of both the WCST and the AST, are indirectly activated via the α7-nAChR subtype (Hamada et al., 2004; Hong and Hikosaka, 2007). Additionally, recent studies have emphasized the role of α7-nAChRs in several different neuropsychiatric conditions due to its involvement in the interaction between the cholinergic and glutamatergic systems (Koukouli and Maskos, 2015). Glutamate is the main modulatory neurotransmitter in the brain, however, increased levels of this neurotransmitter following TBI results in wide spread neurotoxicity (O’Neil et al., 2018). Recent studies have shown that positive allosteric modulators of both the α7-nAChRs and the α4-nAChRs result in neuroprotection, a benefit attributed to the effects these receptors have on glutamatergic cells (Akaike et al., 2010; Quik et al., 2012), which may also support the mechanism of action of GAL. The ever-expanding preclinical literature on functional recovery post-TBI is leaning towards investigating common executive function impairments that plague the patient population. This study further supports the notion that the AST can be used as a measure for impairments in higher-order cognitive functions and behavioral flexibility, and demonstrates that performance deficits can be normalized by GAL. Additionally, these findings support the theory that ACh is profoundly involved in brain areas that modulate complex attention and provide evidence as grounds for further testing this therapy in aged subjects, alone or in combination with rehabilitation exposure. If similar results are found, the use of GAL could be become more frequently used in the clinic as the elderly population have an increased incidence of TBI.
Acknowledgments
Supported in part by NIH Grants NS095950, NS099683, UPP/UPMC Academic Foundation, Univ. Pitt Rehabilitation Institute (COB), NS060005, HD069620 and NS084967 (AEK), and T32 AG021885 (IN; PI: Dr. Susan Greenspan)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike A, Takada-Takatori Y, Kume T, Izumi Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of α4 and α7 receptors in neuroprotection. J. Mol. Neurosci 2010; 40:211–216. [DOI] [PubMed] [Google Scholar]
- Anderson NH. Scales and statistics: Parametric and nonparametric. Psychological Bull. 1961;58:305–316. [DOI] [PubMed] [Google Scholar]
- Arciniegas D The cholinergic hypothesis of cognitive impairment caused by traumatic brain injury. Cur. Psych. Reports 2003; 5:391–399. [DOI] [PubMed] [Google Scholar]
- Ballesteros J, Guemes I, Ibarra N, Quemada JI. The effectiveness of donepezil for cognitive rehabilitation after traumatic brain injury: a systematic review. J. Head Trauma Rehabil 2008; 23:171–180. [DOI] [PubMed] [Google Scholar]
- Benge JF, Caroselli JS, Temple RO. Wisconsin Card Sorting Test: factor structure and relationship to productivity and supervision needs following severe traumatic brain injury. Brain Inj. 2007; 21:395–400. [DOI] [PubMed] [Google Scholar]
- Bickel U, Thomsen T, Fischer JP, Weber W, Kewitz H. Galantamine: pharmacokinetics, tissue distribution and cholinesterase inhibition in brain of mice. Neuropharm. 1991; 30:447–454. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci 2000; 20:4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 2008; 33:320–331. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010; 34:913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Cheng JP, Tennant HM, Monaco CM, Kline AE. Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. J. Neurotrauma 2014; 31:926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg BV, Glenthoj BY, Dias R, Larsen DB, Olsen CK. Reversal of cognitive deficits by an ampakine (CX516) and sertindole in two animal models of schizophrenia- sub-chronic and early postnatal PCP treatment in attentional set-shifting. Psychopharmacol. (Berl.) 2009; 206:631–640. [DOI] [PubMed] [Google Scholar]
- Callahan CD, Hinkebein J. Neuropsychological significance of anosmia following traumatic brain injury. J. Head Trauma Rehabil 1999; 14:581–587. [DOI] [PubMed] [Google Scholar]
- Campbell KA, Kennedy RE, Brunner RC, Hollis SD, Lumsden RA, Novack TA. The effect of donepezil on the cognitive ability early in the course of recovery from traumatic brain injury. Brain Inj. 2018; 32(8):972–979. [DOI] [PubMed] [Google Scholar]
- Chauhan PS, Misra UK, Kalita J, Chandravanshi LP, Khanna VK. Memory and learning seems to be related to cholinergic dysfunction in the JE rat model. Physiol. Behav 2016; 156:148–155. [DOI] [PubMed] [Google Scholar]
- Clausen F, Hillered L. Intracranial pressure changes during fluid percussion, controlled cortical impact and weight drop injury in rats. Acta. Neurochir. (Wien) 2005; 147:775–780. [DOI] [PubMed] [Google Scholar]
- Coyle J, Kershaw P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: Effects on the course of Alzheimer’s Disease. Biol. Psychiatry 2001; 49:289–299. [DOI] [PubMed] [Google Scholar]
- De la Tremblaye PB, Bondi CO, Lajud N, Cheng JP, Radabaugh HL, Kline AE. Galantamine and environmental enrichment enhance cognitive recovery after experimental traumatic brain injury but do not confer additional benefits when combined. J. Neurotrauma 2017; 34:1610–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 1991; 39:253–262. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol. Pharmacol 2004; 66:658–666. [DOI] [PubMed] [Google Scholar]
- Fedor M, Berman RF, Muizelaar JP, Lyeth BG. Hippocampal theta dysfunction after lateral fluid percussion injury. J. Neurotrauma 2010; 27:1605–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts H, Guillaumat PO, Grantham C, Bode W, Anciaux K, Sachak S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005; 1033:186–193. [DOI] [PubMed] [Google Scholar]
- Goetghebeur PJ, Lerdrup L, Sylvest A, Dias R. Erythropoiten reverses the attentional set-shifting impairment in a rodent schizophrenia disease-like model. Psychopharmacol. (Berl.) 2010; 212:635–642. [DOI] [PubMed] [Google Scholar]
- Greve KW, Love JM, Sherwin E, Mathias CW, Ramzinski P, Levy J. Wisconsin Card Sorting Test in chronic severe traumatic brain injury: factor structure and performance subgroups. Brain Inj. 2002; 16:29–40. [DOI] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 2005; 22:252–265. [DOI] [PubMed] [Google Scholar]
- Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A. Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J. Neurochem 2004; 90:1094–1103. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol 2006; 16:710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Gearhart DA, Parikh V, Hohnadel EJ, Davis LW, Middlemore ML, Warsi SP, Waller JL, Terry AV. Comparison of galantamine and donepezil for effects on nerve growth factor, cholinergic markers, and memory performance in aged rats. J. Pharmacol. Exp. Ther 2006; 316:679–694. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Dopamine-mediated learning and switching in cortico-striatal circuit explain behavioral changes in reinforcement learning. Front. Behav. Neurosci 2007; 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneman G, Emanuelson I. Cognitive outcome in children and young adults who sustained a severe and moderate traumatic brain injury 10 years earlier. Brain Inj. 2009; 23:907–914. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 2007; 22:341–355. [PubMed] [Google Scholar]
- Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA. Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacol. (Berl) 2015; 232(17):3123–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khateb A, Ammann J, Annoni JM, Diserens K. Cognition-enhancing effects of donepezil in traumatic brain injury. Eur. Neurol 2005; 54:39–45. [DOI] [PubMed] [Google Scholar]
- Kline AE, McAloon RL, Henderson KA, Bansal UK, Ganti BM, Ahmed RH, Gibbs RB, Sozda CN. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma 2010; 27:2021–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukouli F, Maskos U. The multiple roles of the α7 nicotinic acetylcholine receptor in modulating the glutamatergic systems in the normal and diseased nervous system. Biochem. Pharmacol 2015; 97:378–387. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury. J. Head Trauma Rehabil 2006; 21:375–378. [DOI] [PubMed] [Google Scholar]
- Lim SAO, Kang UN, McGehee DS. Striatal cholinergic interneuron regulation and circuit effects. Front. Synaptic Neurosci 2014; 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Otsu Y, Vasuta C, Nawa H, Murphy TH. Action-potential-independent GABAergic tone mediated by nicotinic stimulation of immature striatal miniature synaptic transmission. J. Neurophysiol 2007; 98:581–593. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci 2013; 16:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund N, Morales D, Clausen F, Hanell A, Kiwanuka O, Pitkanen A, Gimbel DA, Philipson O, Lannfelt L, Hillered L, Strittmatter SM, McIntosh TK. Functional outcome is impaired following traumatic brain injury in aging Nogo-A/B-deficient mice. Neurosci. 2009; 163:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci 2011; 13(3):287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res 2003; 146:97–103. [DOI] [PubMed] [Google Scholar]
- McQuiston AR. Acetylcholine release and inhibitory interneuron activity in the hippocampal CA1. Front. Synaptic Neurosci 2014; 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco CM, Gebhardt KM, Chlebowski SM, Shaw KE, Cheng JP, Henchir JJ, Zupa MF, Kline AE. A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. J. Neurotrauma 2014; 31:1934–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naegeli KJ, O’Connor JA, Banerjee P, Morilak DA. Effects of milnacipran on cognitive flexibility following chronic stress in rats. Eur. J. Pharmacol 2013; 703(1-3):62–66. [DOI] [PubMed] [Google Scholar]
- Nagakawa R, Ohnishi T, Kobayashi H, Yamaoka T, Yaima T, Tanimura A, Kato T, Yoshizawa K. Long-term effect of galantamine on cognitive function in patients with Alzheimer's disease versus a simulated disease trajectory: an observational study in the clinical setting. Neuropsychiatr. Dis. Treat 2017; 13:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk A, Kos T, Potasiewicz A, Popik P. Positive allosteric modulation of the alpha 7 nicotinic acetylcholine receptors enhances recognition memory and cognitive flexibility in rats. Eur. Neuropsychopharmacol 2015; 25:1300–1313. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Kos T, Holug M, Potasiewicz A, Popik P. Positive allosteric modulators of the alpha 7 nicotinic acetylcholine receptors reverse ketamine-induced schizophrenia-like deficits in rats. Neuropharmacol. 2016a; 101:389–400. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Potasiewicz A, Kos T, Popik P. The combination of memantine, and galantamine improves cognition in rats: the synergistic role of the α7 nicotinic acetylcholine and NMDA receptors. Beh. Brain Res 2016b; 313:214–218. [DOI] [PubMed] [Google Scholar]
- Nudi ET, Jacqmain J, Dubbs K, Geeck K, Salois G, Searles MA, Smith JS. Combining environmental enrichment, progesterone, and embryonic neural stem cell therapy improves recovery after brain injury. J. Neurotrauma 2015; 32:1117–1129. [DOI] [PubMed] [Google Scholar]
- Olsen AS, Sozda CN, Cheng JP, Hoffman AN, Kline AE. Traumatic brain injury induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A receptor agonist buspirone. J. Neurotrauma 2012; 29:1898–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil DA, Nicholas MA, Lajud N, Kline AE, Bondi CO. Preclinical models of traumatic brain injury: emerging role of glutamate in the pathophysiology of depression. Front. Pharmacol 2018; 9:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osier ND, Dixon CE. The controlled cortical impact model: applications, considerations for researchers, and future directions. Front. Neurol 2014; 7: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps TI, Bondi CO, Ahmed RH, Olugbade YT, Kline AE. Divergent long-term consequences of chronic treatment with haloperidol, risperidone, and bromocriptine on traumatic brain injury-induced cognitive deficits. J. Neurotrauma 2015; 32:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Perez X, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s Disease. Mov. Disord 2012; 27:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radabaugh HL, LaPorte MJ, Greene AM, Bondi CO, Lajud N, Kline AE. Refining environmental enrichment to advance rehabilitation based research after experimental traumatic brain injury. Exp. Neurol 2017; 294:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane P, Shields J, Heffernan M, Guo Y, Akbarian S, King JA. The histone deacetylase inhibitor, sodium butyrate, alleviates cognitive deficits in pre-motor stage PD. Neuropharmacol. 2012; 62:2409–2412. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC, Martinez-Gonzales D, Roth TC, Pravosudov VV. Hippocampal memory consolidation during sleep: a comparison of mammals and birds. Biol. Rev. Camb. Phiolos. Soc 2010; 86:658–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KE, Bondi CO, Light SH, Massimino LA, McAloon RL, Monaco CM, Kline AE. Donepezil is ineffective in promoting motor and cognitive benefits after controlled cortical impact injury in male rats. J. Neurotrauma 2013; 30:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JM, Koumaras B, Chen M, Mirski D, Potkin SG, Reyes P, Gunay I. Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology 2006; 67:748–755. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000; 38(4):388–402. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Difficulty overcoming learned non-reward during reversal learning in rats with ibotenic acid lesions of orbital prefrontal cortex. Ann. N.Y. Acad. Sci 2007; 1121:407–420. [DOI] [PubMed] [Google Scholar]
- Tait DS, Phillips JM, Blackwell AD, Brown VJ. Effects of lesions of the subthalamic nucleus/zona incerta area and dorsomedial striatum on attentional set-shifting in the rat. Neurosci. 2017; 345:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait DS, Bowman EM, Neuwirth LS, Brown VJ. Assessment of intradimensional/extradimensional attentional set-shifting in rats. Neurosci. Biobehav. Res 2018; 89:72–84. [DOI] [PubMed] [Google Scholar]
- Tenovuo O Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury-clinical experience in 111 patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005; 29:61–67. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerro J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil 1999; 14:602–615. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Bertrand D. Importance of the nicotinic acetylcholine receptor system in the prefrontal cortex. Biochem. Pharmacol 2013; 85:1713–1720. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature 2011; 476:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Kim A, Kernie SG. Donepezil rescues spatial learning and memory deficits following traumatic brain injury independent of its effects on neurogenesis. PLoS One 2015; 10:e0118793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Hylin MJ, Kobori N, Hood KN, Moore AN, Dash PK. Post-Injury administration of galantamine reduces traumatic brain injury pathology and improves outcome. J. Neurotrauma 2018; 35(2): 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]