In patients undergoing laparoscopic cholecystectomy, replacing intravenous acetaminophen with preoperative oral acetaminophen can easily be implemented in ambulatory surgery centers. This results in substantial cost savings, with few differences in pain or consumption of rescue opioids among patients in post-anesthesia care units.
Keywords: Multimodal analgesia, acetaminophen, ambulatory surgical center, laparoscopic cholecystectomy, postoperative pain, single-dose acetaminophen
Abstract
Study Objective
The primary aim was to compare postoperative pain scores in patients undergoing laparoscopic cholecystectomy and receiving intravenous (IV) or oral (PO) acetaminophen (APAP) as part of a multimodal analgesic regimen to examine whether PO APAP is non-inferior to IV APAP.
Design
Retrospective analysis
Setting
Ambulatory surgical center (ASC) in an academic setting
Patients
579 patients (18–70 years old), American Society of Anesthesiologists physical status I–III, undergoing laparoscopic cholecystectomy
Interventions
Patients received 1,000 mg IV APAP intraoperatively (n = 319) or 1,000 mg PO APAP preoperatively (n = 260).
Measurements
The primary outcome was the median difference in post-anesthesia care unit (PACU) end-pain scores between the groups. Median pain scores were also compared on PACU admission, and at 15, 30, 45, and 60 minutes. Additional measures include PACU rescue-analgesia consumption, time to first PACU rescue analgesia, intraoperative use of opioid and nonopioid analgesics, PACU length of stay, and PACU rescue nausea and vomiting therapy.
Main Results
In both groups, the PACU median end-pain score was 2. The 90% confidence interval (CI) for difference in median pain scores between groups was [0, 0]; the CI upper limit was below the non-inferior margin of 1 pain-score point, indicating PO APAP’s non-inferiority to IV APAP. There were no statistically significant differences in the percentages of patients receiving PACU hydromorphone equivalents between the IV and PO groups (75% vs. 77%, P = 0.72) or in the mean dose received (0.5 mg vs. 0.5 mg, P = 0.66).
Conclusion
Single-dose PO APAP is non-inferior to IV APAP for postoperative analgesia in ASC laparoscopic cholecystectomy patients. The value of single-dose IV APAP in this population should be further explored.
INTRODUCTION
Multimodal analgesia has become an increasingly preferred strategy in managing perioperative pain with the goal of reducing opioid consumption and its accompanying adverse effects while leading patients to a quicker recovery.1,2 Including multimodal pain management as a 2018 qualified and clinical data registry (QCDR) measure for elective surgeries may lead to greater perioperative use of non-narcotic analgesic agents such as acetaminophen (APAP), gabapentinoids, and nonsteroidal anti-inflammatory drugs.3
An ever-growing body of evidence supports APAP’s analgesic and opioid-sparing properties.1,2 Intravenous (IV) APAP reliably achieves peak plasma concentration within 15 minutes compared to oral (PO) APAP, which peaks within 45 to 75 minutes.4,5 Despite possessing a longer and more variable time to peak concentration than IV APAP, 1,000 mg of PO APAP is reported to have an 89% bioavailability.6,7
Compared with placebo, IV APAP has been shown to decrease postoperative opioid requirements after laparoscopic abdominal surgery.8,9 Comparisons between IV and PO APAP, however, are inconclusive about the superiority of the more expensive IV formulation in reducing postoperative pain and opioid consumption.10–13 Because of rising APAP use, our practice restricted IV APAP to patients who were poor candidates for oral dosing, which resulted in substantial cost savings.
The goal of this retrospective analysis was to compare postoperative pain scores in ambulatory surgical center (ASC) patients undergoing laparoscopic cholecystectomy who were receiving either intraoperative IV APAP or preoperative PO APAP as part of a multimodal analgesic regimen. We hypothesized that the pain scores would be similar in patients who received a single-dose of intraoperative IV APAP or preoperative PO APAP and who were discharged home shortly after surgery.
MATERIALS AND METHODS
Study Design
We conducted a retrospective non-inferiority study by reviewing the electronic medical records of patients undergoing elective laparoscopic cholecystectomy surgery from June 2015 to June 2017 at Lyndon B. Johnson (LBJ) General Hospital’s ASC. Non-inferior trials are frequently employed when an experimental treatment (PO APAP) is not expected to be better than a control treatment (IV APAP) regarding a primary efficacy endpoint (pain) but may prove less costly.14 The study encompassed time periods of one year before and one year after the change in route of APAP administration. We obtained approval from the University of Texas McGovern Medical School (HSC-MS-17-0840) and Harris Health System (17-10-1767) institutional review boards. Informed consent was deemed not necessary because of the study’s retrospective design.
TRIALS DEFINED.
Superiority trial = statistical tests show one treatment is superior to the other14
Equivalence trial = statistical tests show two treatments are “not too different” in clinical characteristics14
Non-inferiority trial = statistical tests show study treatment is no worse than standard therapy14
Anesthesia and Postoperative Pain Management
At our institution, patients undergoing laparoscopic cholecystectomy receive general endotracheal anesthesia. Anesthesia is induced with IV propofol and lidocaine, and muscle relaxation is achieved with IV rocuronium. Intravenous fentanyl is administered at induction, then at the discretion of the anesthesia team throughout the procedure. General anesthesia is maintained with desflurane or sevoflurane. All patients receive IV ondansetron intraoperatively and have surgical incisions infiltrated with 0.2% ropivacaine during closure. Intravenous ketorolac and dexamethasone administration is strongly encouraged. Full reversal of neuromuscular blockade is facilitated with neostigmine and the muscarinic antagonist glycopyrrolate. Intraoperative administration of long-acting opioids such as hydromorphone and morphine is discouraged.
Postoperatively, rescue analgesia is achieved with IV morphine or hydromorphone (converted to hydromorphone equivalents) 0.2 mg every 15 minutes, with a maximum dose of 1 mg for moderate (4–6) or severe (7–10) pain scores. Oral hydrocodone is usually administered once the patient is tolerating fluids by mouth. A second dose of ondansetron is administered in the event of postoperative nausea and vomiting (PONV), and IV promethazine is administered if PONV persists.
Inclusion and Exclusion
Our analysis included patients aged 18 to 70 years undergoing laparoscopic cholecystectomy between June 2015 and June 2017, who were receiving either 1,000 mg IV APAP intraoperatively or 1,000 mg PO APAP preoperatively. Patients were excluded if they had undergone procedures in addition to laparoscopic cholecystectomy, received < 1,000 mg APAP, or received a transverse abdominal plane block. Patients were also excluded during the three-month transition from IV to PO APAP where overlap between the two drug forms existed.
Endpoints
The primary outcome of the study was the difference in median end-pain scores, recorded before discharge from the post-anesthesia care unit (PACU), between IV APAP and PO APAP patients. This difference was also compared at PACU admission, and at 15, 30, 45, and 60 minutes. Registered nurses documented postoperative pain scores of 0 to 10 using the numeric rating scale (NRS) or Wong-Baker FACES pain scale.
Additional outcomes included intraoperative and PACU opioid and non-opioid analgesic consumption, need for rescue-PONV therapy, and PACU length of stay (LOS). The time from APAP administration to PACU admission was compared in both groups, as was the time to first PACU rescue opioid.
Statistical Methods
In Fenlon et al.’s 2013 randomized, double-blinded trial, mean visual analog scale (VAS) pain scores at one hour after molar-extraction surgery were 5.2 for PO APAP and 4.7 for IV APAP, with a standard deviation (SD) of 2.2.11 Whereas Fenlon et al. used a VAS point of 2 or a 20% margin and proved PO APAP’s non-inferiority to IV APAP,11 our analysis used 1 pain-scale point as the non-inferiority margin. To test the null hypothesis that the difference in pain scores between PO and IV methods is greater than or equal to 1 pain-scale point, we performed the one-sided, two-sample t test. At the 5% significance level, groups of 240 patients would provide 80% power for proving PO APAP’s non-inferiority, given the mean and SD of VAS scores suggested by Fenlon et al. The actual retrospective cohorts consisted of 319 IV APAP patients and 260 PO APAP patients, providing satisfactory power for evaluation purposes.
The normality of continuous variables was examined by histogram and the Kolmogorov–Smirnov test, and held for age and body mass index (BMI). For those variables, the summary statistics were the mean and SD, and we used the two-sample t test to conduct a mean comparison. All other continuous variables were summarized as median and interquartile range. Using the Wilcoxon rank-sum test, we compared medians between two samples, and used Pearson’s Chi-squared test to evaluate the association between categorical variables and analgesic method. In contrast to Fenlon et al., our study’s primary outcome of end-pain score exhibited positively skewed distribution; thus, median pain scores and interquartile ranges were reported.11 The 90% confidence interval (CI) for location shift (PO–IV) was calculated with the Hodges–Lehmann estimator. We evaluated the CI upper limit against the non-inferiority margin of 1 point, and non-inferiority would be concluded if the upper limit fell below that point.
RESULTS
Participants
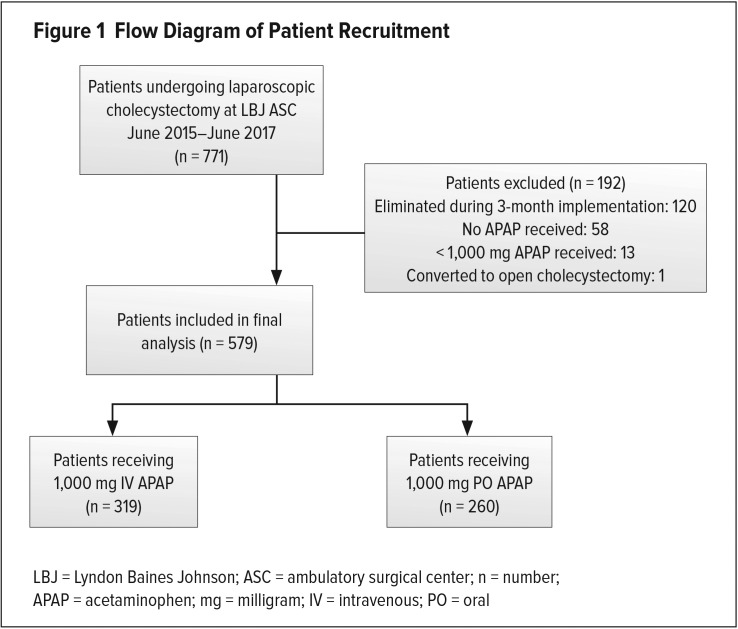
A total of 771 elective laparoscopic cholecystectomies took place during the two-year time period. After our review, 120 patients who had surgery during the three months of implementation were excluded because of overlap between drug routes. Also, 58 patients who received neither form of APAP and 13 patients who received < 1,000 mg APAP were excluded. One patient was eliminated because of conversion to open cholecystectomy. Ultimately, we analyzed data for 579 patients: 319 IV APAP patients and 260 PO APAP patients (see Figure 1).
Figure 1.
Flow Diagram of Patient Recruitment
LBJ = Lyndon Baines Johnson; ASC = ambulatory surgical center; n = number; APAP = acetaminophen; mg = milligram; IV = intravenous; PO = oral
Demographics and Clinical Characteristics
Demographic data (Table 1) were not significantly different between the groups. The oral APAP patients had a slightly higher BMI than IV APAP patients (30.75 vs. 29.43, P = 0.002). All patients had documented end-pain scores; variability existed at other time intervals as some patients were discharged sooner than others and some lacked documentation at specific time points (Table 2). However, all time periods provided satisfactory power with 240 patients per group, except PO APAP at the 60-minute mark (Table 2).
Table 1.
Clinical Characteristics
| IV APAP (n = 319) | Oral APAP (n = 260) | P-value | |
|---|---|---|---|
| Age (years) | 41.06 ± 10.60 | 41.73 ± 11.51 | 0.46 |
| Gender | 0.81 | ||
| Male | 25 (8%) | 19 (7%) | |
| Female | 294 (92%) | 241 (93%) | |
| BMI | 29.43 ± 4.91 | 30.75 ± 5.25 | 0.002 |
| Hispanic | 294 (92%) | 237 (91%) | 0.66 |
| ASA physical status | 0.07 | ||
| 1 | 74 (23%) | 43 (16%) | |
| 2 | 217 (68%) | 184 (71%) | |
| 3 | 28 (9%) | 33 (13%) | |
| PACU LOS (min) | 79 (66, 95) | 80 (66, 94.5) | 0.89 |
| APAP to PACU admission (min) | 62 (51, 82) | 150.5 (114, 188.5) | < 0.001 |
| APAP to PACU admission (hr) | < 0.001 | ||
| ≤ 1 | 148 (46%) | 1 (0.5%) | |
| 1–2 | 163 (51%) | 79 (30.5%) | |
| > 2 | 8 (3%) | 180 (69%) |
IV = intravenous; PO = oral; APAP = acetaminophen; n = number; BMI = body mass index; ASA = American Society of Anesthesiologists; PACU = post-anesthesia care unit; LOS = length of stay; min = minutes; hr = hour
Mean and standard deviations are shown for age and BMI. Two-sample t test was used to compare age and BMI between groups. Median and interquartile ranges are shown for LOS and time between APAP and PACU admission. These variables were compared between two samples by Wilcoxon rank-sum test. Pearson’s chi-square test was used to evaluate the association between categorical characteristics and analgesia method.
Table 2.
Outcomes and Equivalence Evaluation
| IV APAP | PO APAP | 90% CI for Location Shift | ||||
|---|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | (Oral–IV) | NI margin, 1 point | |
| Initial pain score | 318 | 0 (0, 0) | 260 | 0 (0, 0) | (0, 0) | Upper limit of CI ≤ 1 |
| Pain score at 15 min | 292 | 4 (0, 7) | 244 | 5 (0, 7) | (0, 0) | Upper limit of CI ≤ 1 |
| Pain score at 30 min | 290 | 5 (2, 7) | 250 | 5 (2, 7) | (0, 0) | Upper limit of CI ≤ 1 |
| Pain score at 45 min | 250 | 3 (0, 5) | 240 | 4 (0, 5) | (0, 1) | Upper limit of CI ≤ 1 |
| Pain score at 60 min | 241 | 2 (0, 4) | 195 | 2 (0, 4) | (0, 0) | Upper limit of CI ≤ 1 |
| End pain score | 319 | 2 (0, 3) | 260 | 2 (0, 3) | (0, 0) | Upper limit of CI ≤ 1 |
IV = intravenous, PO = oral; APAP = acetaminophen; CI - confidence interval; n = number; IQR = interquartile range; NI = non-inferiority; min = minutes
Median and interquartile ranges are shown for pain scores. The Hodges-Lehmann CI was calculated for location shift (Oral–IV). If the upper limit does not go beyond the 1 point-NI margin, non-inferiority of the PO to IV method can be concluded for the NI margin of 1 point.
Primary Outcome Data
Throughout all time points, median pain scores were similar for both analgesic methods (Table 2). At the termination of monitoring, the median end-pain score was 2 for both methods, indicating the similar performance of both forms. At this time point, the 90% CI for difference in pain scores (PO–IV) was [0, 0] and the CI upper limit was below the 1-point non-inferiority margin. Therefore, PO APAP’s non-inferiority to IV APAP can be statistically concluded, as can the noninferiority of PO APAP at all other time points measured (Table 2).
Other Analysis
There were no statistically significant differences between groups in the percentage of patients receiving rescue opioids in PACU (Table 3) or in the median dose received (Table 4). Nonopioid analgesic use was also similar between groups (Table 3). Intraoperative hydromorphone equivalents were administered to 9% of IV subjects compared to 4% of PO subjects (P = 0.03), but this could be due to concurrent departmental implementation of enhanced recovery and opioid-reducing strategies during the study period (Table 3). Similarly, the 200-mcg median dose of intraoperative fentanyl in IV patients was greater than the 150-mcg median dose in PO patients (P < 0.001, Table 4). A larger percentage of IV patients than PO patients received rescue antiemetic agents in PACU, but this did not reach statistical significance (Table 3).
Table 3.
Analgesic and Antiemetic Consumption
| IV APAP (n = 319) | PO APAP (n = 260) | P-value | |
|---|---|---|---|
| Intraoperative dexamethasone | 252 (79%) | 206 (79%) | 0.95 |
| Intraoperative ketorolac | 212 (66%) | 188 (72%) | 0.13 |
| Intraoperative fentanyl | 319 (100%) | 260 (100%) | N/A |
| Intraoperative hydromorphone equivalents | 28 (9%) | 11 (4%) | 0.030 |
| PACU hydromorphone equivalent | 240 (75%) | 199 (77%) | 0.72 |
| PACU hydrocodone | 305 (96%) | 248 (95%) | 0.90 |
| PACU ondansetron | 84 (26%) | 51 (20%) | 0.06 |
| PACU promethazine | 2 (1%) | 3 (1%) | 0.50 |
| Scopolamine | 157 (49%) | 103 (40%) | 0.021 |
IV = intravenous; PO = oral; APAP = acetaminophen; n = number; PACU = post-anesthesia care unit Chi-square test count and percentage was used to evaluate the association between analgesic agent consumption and analgesia methods.
Table 4.
Amount of Analgesic and Antiemetic Agents in Recipients
| Agent | IV APAP | PO APAP | P-value |
|---|---|---|---|
| Intraoperative dexamethasone (mg) | 0.47 | ||
| 4 | 172 (68%) | 134 (65%) | |
| 8 | 80 (32%) | 72 (35%) | |
| Intraoperative ketorolac (mg) | 0.048 | ||
| 15 | 13 (6%) | 4 (2%) | |
| 30 | 199 (94%) | 184 (98%) | |
| Intraoperative fentanyl (mcg) | 200 (100, 250) | 150 (100, 200) | < 0.001 |
| Intraoperative hydromorphone equiv (mg) | 0.5 (0.5, 1.25) | 1.0 (0.5, 1.5) | 0.24 |
| PACU hydromorphone equiv (mg) | 0.5 (0.35, 0.8) | 0.5 (0.4, 0.9) | 0.66 |
| PACU hydrocodone (mg) | < 0.001 | ||
| 5 | 197 (65%) | 188 (76%) | |
| 7.5 | 59 (19%) | 48 (19%) | |
| 10 | 49 (16%) | 12 (5%) | |
| PACU ondansetron (mg) | 0.92 | ||
| 4 | 81 (96%) | 49 (96%) | |
| 8 | 3 (4%) | 2 (4%) | |
| PACU promethazine (mg) | 0.17 | ||
| 6.25 | 1 (50%) | 3 (100%) | |
| 12.5 | 1 (50%) | 0 (0%) |
IV = intravenous; PO = oral; APAP = acetaminophen; mg = milligram; mcg = microgram; equiv = equivalent; PACU = post-anesthesia care unit
Continuous variables are summarized as median and interquartile range and compared by Wilcoxon rank-sum test. The association between categorical variable and analgesia method was evaluated by Pearson’s chi-square test.
The time to first PACU rescue opioid was 21 minutes in IV patients and 23 minutes in PO patients, reaching a statistical significance of P = 0.014 (Table 5). The difference in PACU LOS from admission to departure was not statistically significant between IV and PO groups (79 minutes (vs. 80 minutes, P = 0.89). Sixty-nine percent of PO patients received APAP more than two hours before PACU admission, which allowed adequate time to achieve peak plasma concentration (Table 1).
Table 5.
Time to First PACU Rescue Opioid
| IV APAP (n = 319) | PO APAP (n = 260) | P-value | |
|---|---|---|---|
| Patients receiving PACU hydromorphone | 240 | 199 | |
| Time to first rescue opioid (min) | 21 (14.5, 26) | 23 (16, 30) | 0.014 |
IV = intravenous; PO = oral; APAP = acetaminophen; n = number; PACU = post-anesthesia care unit; min = minutes
Time values are summarized as median and interquartile range. Wilcoxon rank-sum test was used to compare median times between the two analgesia methods.
DISCUSSION
As the economic pressure to contain health care spending grows, cost-effective selection of drugs becomes more important for health care providers.15 By examining the discrepancy in cost between IV and PO APAP––$35.00 and $0.05, respectively, at our institution––we sought to address one question: Do ASC laparoscopic cholecystectomy patients who receive PO APAP have similar postoperative pain scores to patients who receive the more expensive IV APAP, as part of a multimodal analgesic regimen?
Comparing median pain scores in 579 such patients at our facility showed that preoperative PO APAP was non-inferior to intraoperative IV APAP in reducing postoperative pain (Table 2). These results were similar to Fenlon et al.11 When retrospectively comparing IV and PO APAP in 181,640 patients with open colectomies, Wasserman et al. demonstrated the superiority of opioid reduction on postoperative day 1 in patients receiving PO APAP, and questioned the routine use of IV APAP from a value perspective.12 Despite the greater use of intraoperative opioids among IV patients than PO patients in our analysis, there was no statistically significant difference in PACU rescue-opioid use between the groups (Table 3). Our study did reveal with statistical significance that IV patients requested PACU rescue opioids two minutes earlier than PO patients, although the clinical significance of two minutes is questionable.
Among the limitations of our study are its retrospective design, lack of strict treatment protocols, and lack of a control group. Health care providers from both the anesthesia and PACU teams were not blinded to which APAP form subjects received. Nothwithstanding the lack of randomization, demographic data between the groups were similar. There was variability in provider dosing of intraoperative opioids and PACU hydrocodone. Also, there was no examination of the breakthrough pain scores that occurred between the studied time periods.
While conducting this analysis, we discovered that documentation of the “recovery care complete” phase in the electronic medical record was entered at varying time points, as opposed to the documented time point of when patients met discharge criteria. In response, PACU LOS was calculated from PACU admission to departure, subjecting it to potential logistical delays. Nevertheless, LOS comparison between the groups did not reach statistical significance. Another possible limitation is our use of both NRS and FACES pain scales, but each scale has proven valid and sensitive in assessing changes in pain intensity.16 We examined ASC patients scheduled for laparoscopic cholecystectomy who were optimized for surgery; thus, the results may not be generalizable to an inpatient population with acute biliary disease. Also, the study did not adjust for patients who consumed opioids preoperatively or who had a history of opioid abuse.
In summary, replacing single-dose IV APAP with preoperative PO APAP in patients undergoing laparoscopic cholecystectomy is an intervention that can easily be replicated in ASCs, and one that results in substantial cost savings with minimal differences in PACU pain scores or consumption of rescue opioids. The value of routine use of single-dose IV APAP in this patient population should be explored further. Also, further randomized, controlled, head-to-head trials comparing PO and IV APAP in different surgical populations are needed to determine whether the more expensive IV APAP is indeed superior to PO APAP in reducing pain scores and opioid consumption.
Footnotes
Disclosure: The authors report no commercial or financial interest in regard to this article.
REFERENCES
- 1.Prabhakar A, Cefalu JN, Rowe JS, et al. Techniques to optimize multimodal analgesia in ambulatory surgery. Curr Pain Headache Rep. 2017;21(5):24. doi: 10.1007/s11916-017-0622-z. [DOI] [PubMed] [Google Scholar]
- 2.Elvir-Lazo OL, White PF. The role of multimodal analgesia in pain management after ambulatory surgery. Curr Opin Anaethesiol. 2010;23(6):697–703. doi: 10.1097/ACO.0b013e32833fad0a. [DOI] [PubMed] [Google Scholar]
- 3.Anesthesia Quality Institute. QCDR measure specifications–National Anesthesia Clinical Outcomes Registry. 2018. [Accessed April 22, 2019]. p. 70. Published February 23, 2018. Available at: http://www.aqihq.org/files/MIPS/2018/2018_QCDR_Measure_Book.pdf.
- 4.Brett CN, Barnett SG, Pearson J. Postoperative plasma paracetamol levels following oral or intravenous paracetamol administration: a double-blind randomized controlled trial. Anaesth Intensive Care. 2012;40(1):166–171. doi: 10.1177/0310057X1204000121. [DOI] [PubMed] [Google Scholar]
- 5.Singla NK, Parulan C, Samson R, et al. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12(7):523–532. doi: 10.1111/j.1533-2500.2012.00556.x. [DOI] [PubMed] [Google Scholar]
- 6.Ameer B, Divoll M, Abernethy DR, et al. Absolute and relative bioavailability of oral acetaminophen preparations. J Pharm Sci. 1983;72(8):955–958. doi: 10.1002/jps.2600720832. [DOI] [PubMed] [Google Scholar]
- 7.Rawlins MD, Henderson DB, Hijab AR. Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol. 1977;11(4):283–286. doi: 10.1007/BF00607678. [DOI] [PubMed] [Google Scholar]
- 8.Wininger SJ, Miller H, Minkowitz HS, et al. A randomized, double-blind, placebo-controlled, multicenter, repeat-dose study of two intravenous acetaminophen dosing regimens for the treatment of pain after abdominal laparoscopic surgery. Clin Ther. 2010;32(14):2348–2369. doi: 10.1016/j.clinthera.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Salihoglu Z, Yildirim M, Demiroluk S, et al. Evaluation of intravenous paracetamol administration on postoperative pain and recovery characteristics in patients undergoing laparoscopic cholecystetomy. Surg Laparosc Endosc Percutan Tech. 2009;19(4):321–323. doi: 10.1097/SLE.0b013e3181b13933. [DOI] [PubMed] [Google Scholar]
- 10.Yeh YC, Reddy P. Clinical and economic evidence for intravenous acetaminophen. Pharmacotherapy. 2012;32(6):559–579. doi: 10.1002/j.1875-9114.2011.01085.x. [DOI] [PubMed] [Google Scholar]
- 11.Fenlon S, Collyer J, Giles J, et al. Oral vs intravenous paracetamol for lower third molar extractions under general anesthesia; is oral administration inferior [published online December 6, 2012]? Br J Anaesth. 2013;110(3):432–437. doi: 10.1093/bja/aes387. [DOI] [PubMed] [Google Scholar]
- 12.Wasserman I, Poeran J, Zubizaretta N, et al. Impact of intravenous acetaminophen on perioperative opioid utilization and outcomes in open colectomies: a claims database analysis. Anesthesiology. 2018;129(1):77–88. doi: 10.1097/ALN.0000000000002227. [DOI] [PubMed] [Google Scholar]
- 13.Jibril F, Sharaby S, Mohamed A, Wilby KJ. Intravenous versus oral acetaminophen for pain: systematic review of current evidence to support clinical decision-making. Can J Hosp Pharm. 2015;68(3):238–247. doi: 10.4212/cjhp.v68i3.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesaffre E. Superiority, equivalence, and non-inferiority trials. Bull NYU Hosp Jt Dis. 2008;66(2):150–154. [PubMed] [Google Scholar]
- 15.Kass-Bartelmes BL, Bosco L. Research in Action. 8. Agency for Healthcare Research and Quality; Sep, 2002. [Accessed April 22, 2019]. Reducing costs and improving outcomes. Available at: http://archive.ahrq.gov/research/findings/factsheets/pharmaceutical/rxtherapies/rxria.html. [Google Scholar]
- 16.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]