Abstract
Four series of total 35 new pyrazolo[4,3-d]pyrimidine compounds were designed, synthesized and evaluated for their inhibitory activity against LPS-induced NO production in RAW264.7 macrophages. Among them, compound 4e was found to be the most potent inhibitor, which decreased the production of cytokines in vitro, such as NO, IL-6 and TNF-α, with IC50 values of 2.64, 4.38 and 5.63 μM, respectively. Further studies showed that compound 4e inhibited cytokines secretion of macrophages through suppressing TLR4/p38 signaling pathway. Additionally, compound 4e showed in vivo anti-inflammatory activity in LPS-induced model of acute lung injury. These data suggested that compound 4e may be a promising lead structure for the treatment of ALI.
Keywords: Pyrazolo[4,3-d]pyrimidine; synthesis; anti-inflammatory activity; acute lung injury
1. Introduction
Acute lung injury (ALI), characterized by increased permeability of endothelium and epithelium as well as loss of vascular integrity, is an acute inflammatory disease with high morbidity and mortality1,2. ALI is directly or indirectly caused by pneumonia, inhalation injury, drowning and so forth2–4, which clinical manifestations include pulmonary edema, dyspnea, hypoxemia5–7. Many therapies for ALI have been conducted, but effective therapeutic agents were not discovered up to now8. Recently, several studies have shown that various inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), play a pivotal role in the development and progrossion of ALI9–11. Increasing evidences showed that suppressing the over-secretion of inflammatory cytokines have been emeraged as a promising strategy for the treatment of ALI12,13.
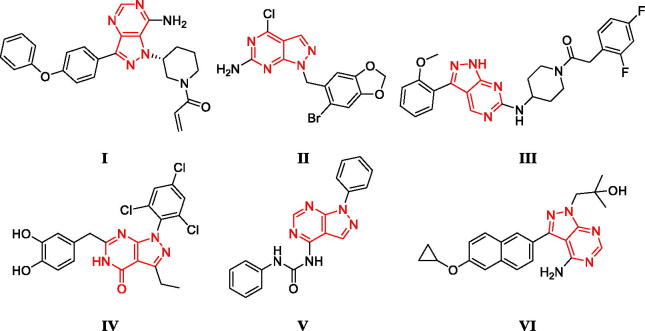
Pyrazolopyrimidine moiety is an important drug-like scaffold14, which have shown a wide range of clinical applications including bruton's tyrosine kinase inhibitor ibrutinib (I)15, tumor necrosis factor receptor-associated protein 1 (TRAP1) inhibitor (II)16, cyclin-dependent kinase (CDK) inhibitors (III, IV)17,18, anti-inflammation (V)19 and bumped kinase inhibitors (VI, Figure 1)20.
Figure 1.
The structures of several pyrazolopyrimidines.
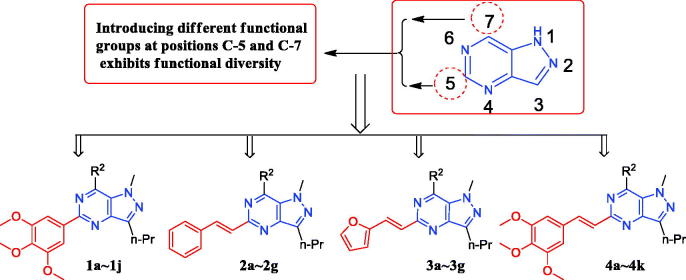
In our previous study, a series of pyrazole-pyrimidine derivatives were synthesized for antitumor evalution21. Further studies, some analogs exhibited anti-inflammatory activity in RAW 264.7 macrophage cells. In order to find potent anti-inflammatory agents, the scaffold was further modified and evaluated for their inhibitory effect against LPS-induced NO production in RAW264.7 macrophages (Figure 2).
Figure 2.
Summary of target derivatives.
2. Experimental section
2.1. Chemistry
Commercial reagents were used without further purification. Thin layer chromatography (TLC) was carried out on pre-coated silica GF254 plates with visualization by UV light at 254 nm in the appropriate solvents system for all reactions. Unless noted otherwise, the purification of all compounds was processed by silica gel column chromatography. Melting points were determined on a XT4MP apparatus (Taike Corp., Beijing, China) without correction. 1H and 13C NMR spectra were recorded on a Bruker AM-300 (1H, 300 MHz; 13C, 75 MHz) or Agilent DD2 600 MHz (1H, 600 MHz; 13C, 151 MHz). The 1H and 13C spectra were recorded in CDCl3 or DMSO-d6 using tetramethylsilane (TMS) as the internal standard. High-resolution electron impact mass spectra (HR-MS) were recorded on a Micro Mass GCT CA 055 instrument under 70 eV electron impact.
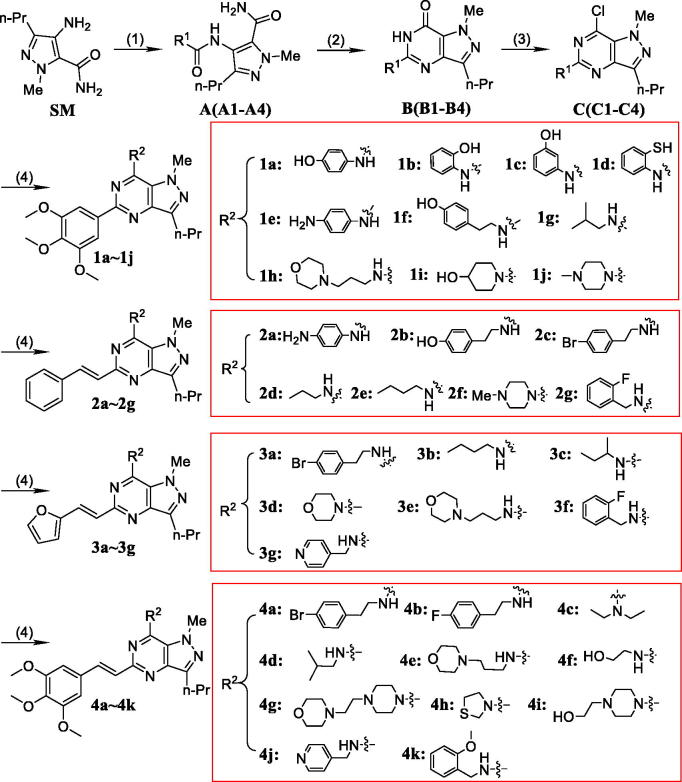
2.2. General procedures for the synthesis of title compounds 1a–1j, 2a–2g, 3a–3g and 4a–4k
A mixture of intermediate C1 (1.88 g, 5 mmol) and 4-aminophenol (0.545 g, 5 mmol) were dissolved in isopropanol (IPA) (20 ml). The reaction mixture was refluxed for 6–10 h monitored by TLC. The solvent was removed under reduced pressure, and the residue was purified by column chromatography (gradient elution of PE/EtOAc 85/15 then 80/20 v/v) to obtain compound 1a.
Compounds 1 b–1j, 2a–2g, 3a–3g and 4a–4k were obtained using the same method (Scheme 1).
Scheme 1.
Synthesis of pyrazolo[4,3-d]pyrimidine derivatives 1a∼1j, 2a∼2g, 3a∼3g and 4a∼4ka. aReaction conditions and reagents: (1) substituted carboxylic acid, EDCI, HOBt, TEA, rt, stirred for 18 h; (2) NaOEt, EtOH, reflux, 8–10 h; (3) POCl3, reflux, 8 h; (4) amine derivatives, IPA, reflux.
4–(1-Methyl-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-ylamino)phenol(1a). Title compound 1a was isolated as a white powder in 79.2% yield (1.78 g, 3.96 mmol), mp 241 – 242 °C. 1HNMR (600 MHz, DMSO-d6): δ 9.34 (d, J = 3.3 Hz, 1H, NH), 8.70 (brs, 1H, OH), 7.65 (d, J = 2.1 Hz, 2H, ArH), 7.58 (d, J = 6.7 Hz, 2H, ArH), 6.84 (d, J = 6.6 Hz, 2H, ArH), 4.31 (d, J = 2.4 Hz, 3H, OCH3), 3.84 (d, J = 2.1 Hz, 6H, 2 × OCH3), 3.72 (d, J = 2.2 Hz, 3H, NCH3), 2.90 (s, 2H, CH2), 1.86 – 1.84 (m, 2H, CH2), 0.99 (t, J = 7.2 Hz, 3H, CH3). 13C NMR (151 MHz, DMSO-d6): δ 155.0,154.1, 152.6, 147.6, 144.0, 143.1, 138.7, 133.9, 130.1, 125.1, 120.8, 114.6, 104.6, 60.1, 55.6, 39.2, 27.2, 21.4, 13.9. HR-MS (ESI): calcd for C24H28N5O4 [M + H]+, 450.2136; found 450.2136.
2–(1-Methyl-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-ylamino)phenol(1 b). Title compound 1 b was isolated as a white powder in 66.7% yield (1.50 g, 3.34 mmol), mp 207 – 208 °C.1H NMR (600 MHz, CDCl3 + DMSO-d6): δ 7.95 (s, 1H, OH), 7.68 (s, 2H, ArH), 7.12 (t, J = 7.5 Hz, 1H, ArH), 7.07 (d, J = 8.0 Hz, 2H, ArH), 6.90 (t, J = 7.5 Hz, 1H, ArH), 4.43 (s, 3H, OCH3), 3.90 (s, 6H, 2 × OCH3), 3.83 (s, 3H, NCH3), 3.13 (t, J = 7.2 Hz, 2H, CH2), 1.89 – 1.80 (m, 2H, CH2), 1.05 (t, J = 7.3 Hz, 3H, CH3). HR-MS (ESI): calcd for C24H28N5O4 [M + H]+, 450.2136; found 450.2134.
3–(1-Methyl-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-ylamino)phenol(1c). Title compound 1c was isolated as a white powder in 72.0% yield (1.62 g, 3.6 mmol), mp 221 – 222 °C. 1H NMR (600 MHz, DMSO-d6): δ 9.76 (s, 1H, OH), 7.74 (s, 2H, ArH), 7.31 (s, 1H, ArH), 7.25 (t, J = 8.0 Hz, 1H, ArH), 7.18 (d, J = 8.0 Hz, 1H, ArH), 6.71 (d, J = 8.0 Hz, 1H, ArH), 4.37 (s, 3H, OCH3), 3.87(s, 6H, 2 × OCH3), 3.74 (s, 3H, NCH3), 3.08 (t, J = 7.5 Hz, 2H, CH2), 1.84 – 1.75 (m, 2H, CH2), 0.99 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (151 MHz, DMSO-d6): δ 157.7, 154.0, 152.8, 148.3, 141.7, 140.3, 138.7, 129.1, 121.3, 114.8, 112.5, 111.3, 107.0, 106.2, 99.3, 60.2, 56.0, 27.4, 25.5, 21.9, 13.8. HR-MS (ESI): calcd for C24H28N5O4 [M + H]+, 450.2136; found 450.2137.
2–(1-Methyl-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-ylamino)benzenethiol(1d). Title compound 1d was isolated as a yellow solid in 47.4% yield (1.10 g, 2.37 mmol), mp 269 – 271 °C. 1H NMR (600 MHz, DMSO-d6): δ 11.85 (s, 1H), 10.22 (s, 1H, SH), 9.17 (s, 1H, NH), 8.23 (d, J = 8.1 Hz, 1H, ArH), 8.15 (d, J = 8.2 Hz, 1H, ArH), 7.62 (t, J = 7.7 Hz, 1H, ArH), 7.53 (m, 3H, ArH), 4.34 (s, 3H, OCH3), 3.96 (s, 6H, 2 × OCH3), 3.81 (s, 3H, NCH3), 2.57 (m, 2H, CH2), 1.71 – 1.67(m, 2H, CH2), 0.97 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (151 MHz, DMSO-d6): δ 164.5, 154.1, 153.0, 152.1, 147.8, 142.3, 133.8, 132.0, 127.2, 126.3, 123.3, 122.6, 121.8, 114.7, 106.7, 60.4, 56.5, 40.6, 27.2, 21.4, 14.0. HR-MS (ESI): calcd for C24H28N5O3S [M + H]+, 466.1907; found 466.1910.
N1-(1-Methyl-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-yl)benzene-1,4-diamine(1e). Title compound 1e was isolated as a yellow solid in 78.4% yield (1.76 g, 3.92 mmol), mp240 – 241 °C. 1H NMR (600 MHz, DMSO-d6): δ 10.42 (brs, 2H, NH2), 9.51 (brs, 1H, NH), 7.92 (d, J = 7.9 Hz, 2H, ArH), 7.66 (s, 2H, ArH), 7.47 (d, J = 7.6 Hz, 2H, ArH), 4.36 (s, 3H, OCH3), 3.85 (s, 6H, 2 × OCH3), 3.73 (s, 3H, NCH3), 2.98 (s, 2H, CH2), 1.86 – 1.82 (m, 2H, CH2), 0.99 (t, J = 7.3 Hz, 3H, CH3). HR-MS (ESI): calcd for C24H29N6O3 [M + H]+, 449.2296; found 449.2302.
4–(2-(1-Methyl-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-ylamino)ethyl)phenol(1f). Title compound 1f was isolated as a white solid in 64.7% yield (1.54 g, 3.24 mmol), mp 197 – 199 °C. 1H NMR (300 MHz, DMSO-d6): δ 9.24 (s, 1H, OH), 7.77 (s, 2H), 7.44 (t, J = 5.6 Hz, 1H, NH), 7.13 (d, J = 8.3 Hz, 2H), 6.72 (d, J = 8.3 Hz, 2H), 4.18 (s, 3H), 3.88 (s, 6H), 3.85 – 3.76 (m, 2H), 3.74 (s, 3H), 2.99 – 2.90 (m, 2H), 2.87 (t, J = 7.4 Hz, 2H), 1.89 – 1.77 (m, 2H), 0.98 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, DMSO-d6): δ 155.7, 155.4, 152.6, 149.2, 143.7, 142.1, 138.8, 134.4, 129.6, 129.4, 120.8, 115.1, 104.7, 60.1, 55.7, 42.4, 39.0, 33.9, 27.2, 21.4, 13.9. HR-MS (ESI): calcd for C26H32N5O4 [M + H]+, 478.2449; found 478.2452.
N-Isobutyl-1-methyl-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(1 g). Title compound 1 g was isolated as a white solid in 88.3% yield (1.83 g, 4.41 mmol), mp 138 – 139 °C. 1H NMR (300 MHz, CDCl3): δ 7.79 (s, 2H, ArH), 5.24 (t, J = 5.5 Hz, 1H, NH), 4.25 (s, 3H, OCH3), 3.99 (s, 6H, 2 × OCH3), 3.90 (s, 3H, NCH3), 3.58 (t, J = 6.2 Hz, 2H, NCH2), 2.98 (t, J = 7.6 Hz, 2H, CH2), 2.19 (m, 1H, CH), 1.94 – 1.84 (m, 2H, CH2), 1.07 – 1.02 (m, 9H, CH3). HR-MS (ESI): calcd for C22H32N5O3 [M + H]+, 414.2500; found 414.2505.
1-Methyl-N-(3-morpholinopropyl)-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(1 h). Title compound 1 h was isolated as a white solid in 68.0% yield (1.65 g, 3.40 mmol), mp 167 – 168 °C. 1H NMR (600 MHz, CDCl3) δ: 7.78 (s, 2H, ArH), 6.20 (s, 1H, NH), 4.27 (s, 3H, OCH3), 3.99 (s, 6H, 2 × OCH3), 3.90 (s, 3H, NCH3), 3.84 (dd, J = 11.6, 5.9 Hz, 2H, CH2), 3.77 – 3.68 (m, 4H, 2 × CH2), 2.98 (t, J = 7.6 Hz, 2H, CH2), 2.59 (dd, J = 12.6, 6.4 Hz, 2H, CH2), 2.52 (s, 4H, 2 × CH2), 2.02 – 1.96 (m, 2H, CH2), 1.93 – 1.88 (m, 2H, CH2), 1.04 (t, J = 7.4 Hz, 3H, CH3). 13C NMR (151 MHz, CDCl3): δ 156.9, 153.1, 149.7, 146.0, 143.3, 139.5, 134.9, 121.3, 105.3, 66.7, 61.0, 58.4, 56.2, 54.2, 41.1, 39.4, 27.9, 24.6, 22.2, 14.2. HR-MS (ESI): calcd for C25H37N6O4 [M + H]+, 485.2871; found 485.2873.
1–(1-Methyl-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidin-7-yl)piperidin-4-ol(1i). Title compound 1i was isolated as a white powder in 55.3% yield (1.22 g, 2.77 mmol), mp 143 – 144 °C. 1H NMR (600 MHz, DMSO-d6): δ 7.73 (s, 2H, ArH), 4.84 (s, 1H, OH), 4.07 (s, 3H, OCH3), 3.88 (m, 8H, 2 × OCH3 + CH2), 3.81 (s, 1H, OCH), 3.74 (s, 3H, NCH3), 3.30 (s, 2H, CH2), 2.91 (d, J = 3.1 Hz, 2H, CH2), 1.95 (s, 2H, CH2), 1.85 (s, 2H, NCH2), 1.63 (d, J = 8.2 Hz, 2H, NCH2), 1.02 – 0.96 (m, 3H, CH3). 13C NMR (151 MHz, DMSO-d6): δ 154.9, 153.4, 152.8, 145.3, 144.2, 139.1, 133.7, 123.6, 104.8, 65.6, 60.1, 55.8, 46.7, 38.7, 33.7, 27.3, 21.3, 14.0. HR-MS (ESI): calcd for C23H32N5O4 [M + H]+, 442.2449; found 442.2447.
1-Methyl-7–(4-methylpiperazin-1-yl)-3-propyl-5–(3,4,5-trimethoxyphenyl)-1H-pyrazolo[4,3-d]pyrimidine(1j). Title compound 1j was isolated as a white solid in 82.9% yield (1.83 g, 4.15 mmol), mp 116 – 117 °C. 1H NMR (600 MHz, CDCl3): δ 7.79 (s, 2H, ArH), 4.11 (s, 3H, OCH3), 3.99 (s, 6H, 2 × OCH3), 3.91 (s, 3H, NCH3), 3.65 (s, 4H, 2 × NCH2), 3.03 (t, J = 7.6 Hz, 2H, CH2), 2.69 (s, 4H, 2 × NCH2), 2.41 (s, 3H, NCH3), 1.94 – 1.91 (m, 2H, CH2), 1.06 (t, J = 7.4 Hz, 3H, CH3). 13C NMR (151 MHz, CDCl3): δ 156.4, 153.9, 153.2, 147.6, 145.6, 139.8, 134.3, 124.5, 105.4, 61.0, 56.3, 54.6, 49.4, 46.3, 38.6, 28.0, 22.2, 14.2. HR-MS (ESI): calcd for C23H33N6O3 [M + H]+, 441.2609; found 441.2613.
(E)-N1-(1-Methyl-3-propyl-5-styryl-1H-pyrazolo[4,3-d]pyrimidin-7-yl)benzene-1,4-diamine(2a). Title compound 2a was isolated as a white solid in 54.6% yield (1.05 g, 2.73 mmol), mp 163 – 164 °C. 1H NMR (300 MHz, DMSO-d6): δ 9.40 (s, 2H), 7.90 – 7.78 (m, 2H, ArH), 7.73 – 7.58 (m, 3H, ArH), 7.46 – 7.26 (m, 6H, ArH), 4.34 (s, 3H, NCH3), 2.94 – 2.87 (m, 2H, CH2), 1.86 – 1.69 (m, 2H, CH2), 1.02 – 0.92 (m, 3H, CH3). HR-MS (ESI): calcd for C23H25N6 [M + H]+, 385.2135; found 385.2131.
(E)-4–(2-(1-Methyl-3-propyl-5-styryl-1H-pyrazolo[4,3-d]pyrimidin-7-ylamino)ethyl)phenol(2 b). Title compound 2 b was isolated as a white solid in 73.2% yield (1.51 g, 3.66 mmol), mp 163 – 164 °C. 1H NMR (300 MHz, DMSO-d6): δ 9.24 (s, 1H), 7.81 (d, J = 15.9 Hz, 1H), 7.67 (d, J = 7.3 Hz, 2H), 7.43 (t, J = 7.4 Hz, 2H), 7.33 (t, J = 7.0 Hz, 2H), 7.16 (dd, J = 12.1, 3.7 Hz, 3H), 6.75 (d, J = 8.3 Hz, 2H), 4.16 (s, 3H), 3.76 (dd, J = 14.7, 5.8 Hz, 2H), 2.99 – 2.86 (m, 2H), 2.80 (t, J = 7.5 Hz, 2H), 1.83 – 1.70 (m, 2H), 0.95 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 156.6, 155.7, 149.0, 143.8, 141.9, 136.3, 134.2, 129.7, 129.6, 129.3, 128.9, 128.3, 127.1, 120.8, 115.2, 42.6, 39.0, 33.87, 27.3, 21.7, 13.9. HR-MS (ESI): calcd for C25H28N5O [M + H]+, 414.2288; found 414.2291.
(E)-N-(4-Bromophenethyl)-1-methyl-3-propyl-5-styryl-1H-pyrazolo[4,3-d]pyrimidin-7-amine(2c). Title compound 2c was isolated as a yellow solid in 81.1% yield (1.93 g, 4.06 mmol), mp 209 – 210 °C. 1H NMR (600 MHz, CDCl3): δ 7.88 (d, J = 15.8 Hz, 1H), 7.62 (d, J = 7.5 Hz, 2H), 7.48 (d, J = 8.2 Hz, 2H), 7.38 (t, J = 7.6 Hz, 2H), 7.30 (t, J = 7.3 Hz, 1H), 7.23 (d, J = 15.8 Hz, 1H), 7.18 (d, J = 8.1 Hz, 2H), 5.05 (s, 1H), 4.07 (s, 3H), 3.98 (dd, J = 12.7, 6.7 Hz, 2H), 3.06 (t, J = 6.9 Hz, 2H), 2.94 (t, J = 7.7 Hz, 2H), 1.90 – 1.82 (m, 2H), 1.03 (t, J = 7.3 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.7, 149.0, 145.7, 143.1, 137.9, 136.8, 135.4, 131.9, 130.6, 129.0, 128.7, 128.2, 127.3, 121.0, 120.6, 41.8, 38.9, 34.7, 27.7, 22.3, 14.1. HR-MS (ESI): calcd for C25H27N5Br [M + H]+, 476.1444; found 476.1448.
(E)-1-Methyl-N,3-dipropyl-5-styryl-1H-pyrazolo[4,3-d]pyrimidin-7-amine(2d). Title compound 2d was isolated as a white solid in 84.3% yield (1.41 g, 4.22 mmol), mp 139 – 140 °C. 1H NMR (600 MHz, CDCl3 + DMSO-d6): δ 9.03 (s, 1H, NH), 8.04 (d, J = 15.6 Hz, 1H, ArH), 7.72 (d, J = 15.6 Hz, 1H, ArH), 7.67 (d, J = 3.8 Hz, 2H, ArH), 7.43 (s, 3H, ArH), 4.42 (s, 3H, NCH3), 3.86 (dd, J = 13.4, 6.6 Hz, 2H, NCH2), 3.07 (t, J = 7.5 Hz, 2H, CH2), 1.89 (dt, J = 14.6, 7.3 Hz, 2H, CH2), 1.86 – 1.77 (m, 2H, CH2), 1.08 (t, J = 7.4 Hz, 3H, CH3), 1.04 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (151 MHz, CDCl3 + DMSO-d6): δ 152.8, 149.8, 142.4, 139.5, 133.8, 129.8, 128.3, 128.3, 127.7, 120.3, 118.2, 43.1, 39.6, 27.0, 21.7, 21.4, 13.1, 11.0. HR-MS (ESI): calcd for C20H26N5 [M + H]+, 336.2183; found 336.2184.
(E)-N-Butyl-1-methyl-3-propyl-5-styryl-1H-pyrazolo[4,3-d]pyrimidin-7-amine(2e). Title compound 2e was isolated as a white solid in 91.9% yield (1.61 g, 4.60 mmol), mp 156 – 157 °C. 1H NMR (300 MHz, DMSO-d6): δ 8.71 (brs, 1H, NH), 8.06 (d, J = 15.7 Hz, 1H, =CH), 7.70 (d, J = 6.6 Hz, 2H, ArH), 7.59 – 7.42 (m, 4H, ArH), 4.28 (s, 3H, NCH3), 3.81 (dd, J = 13.2, 6.4 Hz, 2H, CH2), 2.92 (t, J = 7.5 Hz, 2H, CH2), 1.76 – 1.67 (m, 4H, 2 × CH2), 1.50 – 1.42 (m, 2H, CH2), 1.01 – 0.93 (m, 6H, 2 × CH3). HR-MS (ESI): calcd for C21H28N5 [M + H]+, 350.2339; found 350.2338.
(E)-1-Methyl-7–(4-methylpiperazin-1-yl)-3-propyl-5-styryl-1H-pyrazolo[4,3-d]pyrimidine(2f). Title compound 2f was isolated as a white solid in 56.8% yield (1.07 g, 2.84 mmol), mp 156 – 157 °C. 1H NMR (300 MHz, DMSO-d6): δ 11.86 (brs, 1H), 8.02 (d, J = 15.8 Hz, 1H), 7.74 (d, J = 7.1 Hz, 2H), 7.47 (dt, J = 17.9, 9.1 Hz, 4H), 4.42 (d, J = 13.1 Hz, 2H), 4.14 (s, 3H), 3.79 (t, 2H), 3.55 (d, J = 12.0 Hz, 2H), 3.30 (d, J = 9.7 Hz, 2H), 2.96 (t, J = 7.4 Hz, 2H), 2.83 (s, 3H), 1.80 – 1.73 (m, 2H), 0.98 (t, J = 7.3 Hz, 1H). HR-MS (ESI): calcd for C22H29N6 [M + H]+, 377.2448; found 377.2447.
(E)-N-(2-Fluorobenzyl)-1-methyl-3-propyl-5-styryl-1H-pyrazolo[4,3-d]pyrimidin-7-amine(2 g). Title compound 2 g was isolated as a yellow solid in 77.9% yield (1.56 g, 3.90 mmol), mp 175 – 176 °C. 1H NMR (600 MHz, CDCl3): δ 7.88 (d, J = 15.8 Hz, 1H), 7.62 (d, J = 7.7 Hz, 2H), 7.55 (t, J = 7.5 Hz, 1H), 7.38 (t, J = 7.6 Hz, 2H), 7.29 (dd, J = 12.1, 6.3 Hz, 2H), 7.22 (d, J = 15.8 Hz, 1H), 7.16 – 7.08 (m, 2H), 5.52 (s, 1H, NH), 5.01 (d, J = 5.7 Hz, 2H, CH2), 4.22 (s, 3H), 2.94 (t, J = 7.7 Hz, 2H), 1.87 – 1.83 (m, 2H), 1.02 (t, J = 7.3 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 161.4 (d, J = 245.5 Hz), 157.6, 149.0, 145.7, 143.2, 136.9, 135.5, 130.6 (d, J = 4.4 Hz), 129.5 (d, J = 8.3 Hz), 128.9, 128.6, 128.2, 127.3, 125.5 (d, J = 14.2 Hz), 124.4 (d, J = 3.5 Hz), 121.1, 115.5 (d, J = 21.4 Hz), 39.0, 39.0, 27.7, 22.2, 14.0. HR-MS (ESI): calcd for C24H25N5F [M + H]+, 402.2089; found 402.2084.
(E)-N-(4-Bromophenethyl)-5–(2-(furan-2-yl)vinyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-7-amine(3a). Title compound 3a was isolated as a yellow solid in 66.6% yield (1.55 g, 3.33 mmol), mp 128 – 129 °C. 1H NMR (600 MHz, CDCl3): δ 7.65 (d, J = 15.7 Hz, 1H), 7.47 (d, J = 8.2 Hz, 2H), 7.45 (s, 1H), 7.15 (d, J = 8.2 Hz, 2H), 7.12 (d, J = 15.7 Hz, 1H), 6.50 (d, J = 3.2 Hz, 1H), 6.45 (dd, J = 3.0, 1.7 Hz, 1H), 5.03 (s, 1H), 4.05 (s, 3H), 3.94 (q, J = 6.7 Hz, 2H), 3.03 (t, J = 6.9 Hz, 2H), 2.95 – 2.90 (m, 2H), 1.86 – 1.82(m, 2H), 1.01 (t, J = 7.3 Hz, 3H). 13C NMR (151 MHz, CDCl3); δ 157.5, 153.2, 149.0, 145.7, 143.1, 142.9, 138.0, 131.8, 130.5, 127.3, 122.9, 121.0, 120.6, 111.7, 110.1, 41.8, 38.8, 34.7, 27.7, 22.2, 14.0. HR-MS (ESI): calcd for C23H25BrN5O [M + H]+, 466.1237; found 466.1238.
(E)-N-Butyl-5–(2-(furan-2-yl)vinyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-7-amine(3 b). Title compound 3 b was isolated as a yellow solid in 87.4% yield (1.48 g, 4.37 mmol), mp 101 – 102 °C. 1H NMR (600 MHz, CDCl3): δ 7.61 (d, J = 15.7 Hz, 1H), 7.42 (s, 1H), 7.09 (d, J = 15.7 Hz, 1H), 6.47 (d, J = 3.2 Hz, 1H), 6.42 (dd, J = 3.1, 1.7 Hz, 1H), 5.07 (s, 1H), 4.18 (s, 3H), 3.68 (q, J = 7.0 Hz, 2H), 2.94 – 2.87 (m, 2H), 1.85 – 1.81 (m, 2H), 1.74 – 1.67 (m, 2H), 1.49 – 1.45(m, 2H), 1.00 (t, J = 7.3 Hz, 6H). 13C NMR (151 MHz, CDCl3): δ 157.7, 153.4, 149.5, 145.6, 143.0, 142.9, 127.5, 122.9, 121.2, 111.8, 110.2, 40.7, 39.1, 31.6, 27.8, 22.3, 20.4, 14.2, 14.0. HR-MS (ESI): calcd for C19H26N5O [M + H]+, 340.2132; found 340.2137.
(E)-N-sec-Butyl-5–(2-(furan-2-yl)vinyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-7-amine(3c). Title compound 3c was isolated as a white solid in 89.4% yield (1.51 g, 4.47 mmol), mp 115 – 116 °C. 1H NMR (600 MHz, CDCl3): δ 7.60 (d, J = 15.7 Hz, 1H), 7.43 (s, 1H), 7.10 (d, J = 15.7 Hz, 1H), 6.49 (d, J = 3.1 Hz, 1H), 6.43 (dd, J = 3.2, 1.7 Hz, 1H), 4.80 (d, J = 7.5 Hz, 1H), 4.52 – 4.45 (m, 1H), 4.21 (s, 3H), 2.95 – 2.90 (m, 2H), 1.88 – 1.82 (m, 2H), 1.77 – 1.63 (m, 2H), 1.34 (d, J = 6.5 Hz, 3H), 1.05 – 1.02 (m, 3H), 1.01 (t, J = 5.8 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.8, 153.4, 149.1, 145.7, 143.1, 142.9, 127.7, 122.9, 121.1, 111.8, 110.1, 47.8, 39.1, 29.7, 27.8, 22.4, 20.4, 14.2, 10.6. HR-MS (ESI): calcd for C19H26N5O [M + H]+, 340.2132; found 340.2126.
(E)-4–(5–(2-(Furan-2-yl)vinyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-7-yl)morpholine(3d). Title compound 3d was isolated as a white solid in 73.9% yield (1.31 g, 3.70 mmol), mp 108 – 109 °C. 1H NMR (600 MHz, CDCl3): δ 7.63 (d, J = 15.7 Hz, 1H), 7.44 (s, 1H), 7.15 (d, J = 15.7 Hz, 1H), 6.50 (d, J = 3.2 Hz, 1H), 6.44 (dd, J = 3.2, 1.7 Hz, 1H), 4.08 (s, 3H), 3.94 – 3.90 (m, 4H), 3.57 – 3.54 (m, 4H), 2.96 (t, 2H), 1.89 – 1.85 (m, 2H), 1.02 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.0, 153.5, 153.0, 147.4, 145.4, 142.9, 126.8, 124.2, 123.1, 111.8, 110.4, 66.4, 49.9, 38.4, 27.8, 22.1, 14.0. HR-MS (ESI): calcd for C19H24N5O2 [M + H]+, 354.1925; found 354.1925.
(E)-5–(2-(Furan-2-yl)vinyl)-1-methyl-N-(3-morpholinopropyl)-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-7-amine(3e). Title compound 3e was isolated as a white solid in 56.4% yield (1.16 g, 2.82 mmol), mp 98 – 99 °C. 1H NMR (600 MHz, CDCl3): δ 7.62 (d, J = 15.7 Hz, 1H), 7.43 (s, 1H), 7.10 (d, J = 15.7 Hz, 1H), 6.48 (d, J = 3.1 Hz, 1H), 6.43 (dd, J = 3.1, 1.7 Hz, 1H), 6.07 (s, 1H), 4.24 (s, 3H), 3.80 (q, J = 6.1 Hz, 2H), 3.72 (t, J = 4.4 Hz, 4H), 2.94 – 2.91 (m, 2H), 2.57 (t, J = 6.2 Hz, 2H), 2.52 (s, 4H), 1.97 – 1.89 (m, 2H), 1.87 – 1.83 (m, 2H), 1.01 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.7, 153.4, 149.5, 145.8, 143.0, 142.9, 127.7, 122.8, 121.3, 111.9, 110.1, 66.8, 58.4, 54.2, 40.9, 39.3, 27.9 24.7 22.4 14.2. HR-MS (ESI): calcd for C22H31N6O2 [M + H]+, 411.2503; found 411.2510.
(E)-N-(2-Fluorobenzyl)-5–(2-(furan-2-yl)vinyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-7-amine(3f). Title compound 3f was isolated as a white solid in 79.4% yield (1.55 g, 3.97 mmol), mp132 – 133 °C. 1H NMR (300 MHz, DMSO-d6): δ 7.87 (t, J = 5.6 Hz, 1H, ArH), 7.73 (s, 1H, NH), 7.52 (t, J = 7.6 Hz, 1H, ArH), 7.43 (d, J = 15.8 Hz, 1H, =CH), 7.33 – 7.18 (m, 2H, ArH), 7.13 (t, J = 7.2 Hz, 1H, ArH), 6.79 (d, J = 15.8 Hz, 1H, =CH), 6.69 (d, J = 3.3 Hz, 1H, ArH), 6.60 – 6.54 (m, 1H, ArH), 4.84 (d, J = 5.5 Hz, 2H, CH2), 4.24 (s, 3H, NCH3), 2.78 (t, J = 7.5 Hz, 2H, CH2), 1.78 – 1.71 (m, 2H, CH2), 0.93 (t, J = 7.4 Hz, 3H, CH3). HR-MS (ESI): calcd for C22H23FN5O [M + H]+, 392.1881; found 392.1885.
(E)-5-(2-(Furan-2-yl)vinyl)-1-methyl-3-propyl-N-(pyridin-4-ylmethyl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(3 g). Title compound 3 g was isolated as a yellow solid in 82.2% yield (1.54 g, 4.11 mmol), mp 117 – 118 °C. 1H NMR (600 MHz, CDCl3): δ 8.60 – 8.55 (m, 2H), 7.47 (d, J = 15.7 Hz, 1H), 7.41 (d, J = 1.7 Hz, 1H), 7.34 – 7.32 (m, 2H), 7.07 (d, J = 15.7 Hz, 1H), 6.44 (d, J = 3.3 Hz, 1H), 6.42 (dd, J = 3.3, 1.8 Hz, 1H), 5.56 (t, J = 5.8 Hz, 1H), 4.94 (d, J = 5.7 Hz, 2H), 4.24 (s, 3H), 2.93 (t, J = 7.7 Hz, 2H), 1.87 – 1.83 (m, 2H), 1.01 (t, J = 7.3 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.6, 153.1, 150.2, 149.0, 148.1, 146.0, 143.7, 143.1, 127.0, 123.2, 122.6, 121.0, 111.9, 110.5, 43.8, 39.2, 27.9, 22.4, 14.2. HR-MS (ESI): calcd for C21H23N6O [M + H]+, 375.1928; found 375.1928.
(E)-N-(4-Bromophenethyl)-1-methyl-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(4a). Title compound 4a was isolated as a yellow solid in 87.2% yield (2.47 g, 4.36 mmol), mp 215 – 217 °C. 1H NMR (600 MHz, CDCl3): δ 7.79 (d, J = 15.7 Hz, 1H), 7.48 (d, J = 8.4 Hz, 2H), 7.21 – 7.17 (m, 2H), 7.15 (d, J = 15.7 Hz, 1H), 6.85 (s, 2H), 5.08 (t, J = 5.7 Hz, 1H), 4.08 (s, 3H), 4.03 – 3.96 (m, 2H), 3.92 (s, 6H), 3.89 (s, 3H), 3.07 (t, J = 6.9 Hz, 2H), 2.96 – 2.87 (m, 2H), 1.88 – 1.84 (m, 2H), 1.02 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.8, 153.5, 149.2, 145.7, 143.2, 138.5, 138.1, 135.4, 132.6, 132.0, 130.7, 128.6, 121.2, 120.8, 104.4, 61.1, 56.2, 41.9, 39.0, 34.8, 27.8, 22.4, 14.2. HR-MS (ESI): calcd for C28H32BrN5O3 [M + H]+, 566.1761; found 566.1760.
(E)-N-(4-Fluorophenethyl)-1-methyl-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(4b). Title compound 4b was isolated as a yellow solid in 67.7% yield (1.71 g, 3.39 mmol), mp 186 – 187 °C. 1H NMR (300 MHz, CDCl3): δ 7.80 (d, J = 15.7 Hz, 1H), 7.31 – 7.23 (m, 2H), 7.15 (d, J = 15.7 Hz, 1H), 7.05 (t, J = 8.6 Hz, 2H), 6.86 (s, 2H), 5.07 (t, J = 5.6 Hz, 1H), 4.06 (s, 3H), 3.99 (t, J = 6.4 Hz, 2H), 3.92 (s, 6H), 3.89 (s, 3H), 3.08 (t, J = 6.8 Hz, 2H), 2.94 (t, J = 7.7 Hz, 2H), 1.89 – 1.82 (m, 2H),1.02 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 161.9 (d, J = 245.4 Hz), 157.8, 153.5, 149.3, 145.8, 143.2, 138.6, 135.4, 134.7 (d, J = 3.4 Hz), 132.7, 130.4 (d, J = 7.9 Hz), 128.7, 121.2, 115.8 (d, J = 21.3 Hz), 104.4, 61.1, 56.3, 42.1, 39.0, 34.6, 27.8, 22.4, 14.2. HR-MS (ESI): calcd for C28H32FN5O3 [M + H]+, 506.2562; found 506.2560.
(E)-N,N-Diethyl-1-methyl-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(4c). Title compound 4c was isolated as a yellow solid in 81.3% yield (1.79 g, 4.07 mmol), mp 140 – 141 °C. 1H NMR (600 MHz, CDCl3): δ 7.75 (d, J = 15.7 Hz, 1H), 7.16 (d, J = 15.7 Hz, 1H), 6.85 (s, 2H), 4.08 (s, 3H), 3.92 (s, 6H), 3.88 (s, 3H), 3.62 (q, J = 7.1 Hz, 4H), 2.99 – 2.96 (m, 2H), 1.91 – 1.85 (m, 2H), 1.26 (t, J = 7.1 Hz, 6H), 1.04 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.0, 153.6, 153.4, 147.2, 145.1, 138.5, 135.3, 132.7, 128.5, 125.1, 104.5, 61.1, 56.3, 43.9, 38.9, 28.0, 22.3, 14.2, 12.6. HR-MS (ESI): calcd for C24H33N5O3 [M + H]+, 440.2656; found 440.2657.
(E)-N-Isobutyl-1-methyl-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(4d). Title compound 4d was isolated as a yellow solid in 92.3% yield (2.03 g, 4.62 mmol), mp 171 – 172 °C. 1H NMR (600 MHz, CDCl3): δ 7.75 (d, J = 15.7 Hz, 1H, =CH), 7.12 (d, J = 15.7 Hz, 1H, =CH), 6.85 (s, 2H, ArH), 5.15 (s, 1H, NH), 4.24 (s, 3H, OCH3), 3.92 (s, 6H, 2 × OCH3), 3.88 (s, 3H, NCH3), 3.59 (t, J = 6.1 Hz, 2H, NCH2), 2.94 (t, J = 7.7 Hz, 2H, CH2), 2.12 – 2.08 (m, 1H, CH), 1.91 – 1.82 (m, 2H, CH2), 1.08 (d, J = 6.7 Hz, 6H, 2 × CH3), 1.03 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (151 MHz, DMSO-d6): δ 157.8, 153.4, 149.8, 145.6, 143.0, 138.4, 135.2, 132.7, 128.7, 121.2, 104.4, 61.1, 56.2, 48.3, 39.1, 28.4, 27.8, 22.4, 20.6, 14.1. HR-MS (ESI): calcd for C24H34N5O3 [M + H]+, 440.2656; found 440.2651.
(E)-1-Methyl-N-(3-morpholinopropyl)-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(4e). Title compound 4e was isolated as a white solid in 62.8% yield (1.74 g, 3.42 mmol), mp 144 – 145 °C. 1H NMR (600 MHz, CDCl3): δ 7.75 (d, J = 15.7 Hz, 1H), 7.13 (d, J = 15.6 Hz, 1H), 6.85 (s, 2H), 6.18 (t, J = 5.0 Hz, 1H), 4.28 (s, 3H), 3.92 (s, 6H), 3.88 (s, 3H), 3.87 – 3.84 (m, 2H), 3.73 (t, J = 4.7 Hz, 4H), 2.97 – 2.92 (m, 2H), 2.61 (t, J = 6.2 Hz, 2H), 2.54 (s, 4H), 1.97 (t, J = 6.3 Hz, 2H), 1.88 – 1.84(m, 2H), 1.03 (t, J = 7.3 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.8, 153.4, 149.6, 145.8, 142.9, 138.4, 135.2, 132.7, 128.8, 121.4, 104.4, 66.8, 61.1, 58.6, 56.2, 54.3, 41.2, 39.4, 27.9, 24.6, 22.4, 14.2. HR-MS (ESI): calcd for C27H38N6O4 [M + H]+, 511.3027; found 511.3028.
(E)-2–(1-Methyl-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-ylamino)ethanol(4f). Title compound 4f was isolated as a white solid in 77.3% yield (1.65 g, 3.87 mmol), mp 127 – 128 °C. 1H NMR (600 MHz, DMSO-d6): δ 7.66 (d, J = 15.8 Hz, 1H), 7.10 (d, J = 15.8 Hz, 1H), 7.06 (s, 1H), 6.97 (s, 2H), 4.83 (t, J = 5.1 Hz, 1H), 4.18 (s, 3H), 3.86 (s, 6H), 3.72 (t, J = 7.3 Hz, 4H), 3.69 (s, 3H), 2.78 (t, J = 7.5 Hz, 2H), 1.77 – 1.73 (m, 2H), 0.94 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3 + DMSO-d): δ 156.6, 153.1, 149.3, 143.6, 141.9, 137.7, 134.3, 132.1, 128.7, 120.8, 104.4, 60.0, 59.3, 55.9, 42.9, 39.0, 27.3, 21.8, 13.9. HR-MS (ESI): calcd for C22H30N5O4 [M + H]+, 42.2292; found 428.2293.
(E)-4–(2-(4–(1-Methyl-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-yl)piperazin-1-yl)ethyl)morpholine(4 g). Title compound 4 g was isolated as a white solid in 54.7% yield (1.55 g, 2.74 mmol), mp 167 – 169 °C. 1H NMR (300 MHz, CDCl3): δ 7.77 (d, J = 15.7 Hz, 1H), 7.16 (d, J = 15.7 Hz, 1H), 6.85 (s, 2H), 4.09 (s, 3H), 3.92 (s, 6H), 3.88 (s, 3H), 3.73 (t, J = 4.7 Hz, 4H), 3.60 (s, 4H), 2.98 (t, J = 7.7 Hz, 2H), 2.74 (s, 4H), 2.64 – 2.56 (m, 4H), 2.52 (t, J = 4.5 Hz, 4H), 1.91 – 1.83 (m, 2H), 1.03 (t, J = 7.3 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.2, 153.8, 153.4, 147.3, 145.0, 138.5, 135.7, 132.5, 128.1, 124.5, 104.4, 67.0, 61.1, 56.4, 56.2, 55.8, 54.3, 53.2, 49.5, 38.7, 28.0, 22.3, 14.2. HR-MS (ESI): calcd for C30H43N7O4 [M + H]+, 566.3449; found 566.3449.
(E)-3–(1-Methyl-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-yl)thiazolidine(4 h). Title compound 4 h was isolated as a yellow solid in 68.2% yield (1.55 g, 3.41 mmol), mp 145 – 146 °C. 1H NMR (600 MHz, CDCl3): δ 7.74 (d, J = 15.7 Hz, 1H), 7.16 (d, J = 15.7 Hz, 1H), 6.85 (s, 2H), 4.86 (s, 2H), 4.19 (s, 3H), 4.12 (t, J = 6.3 Hz, 2H), 3.93 (s, 6H), 3.89 (s, 3H), 3.21 (t, J = 6.3 Hz, 2H), 3.02 – 2.97 (m, 2H), 1.90 – 1.87 (m, 2H), 1.04 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 156.8, 153.4, 151.8, 146.9, 145.6, 138.7, 135.7, 132.3, 127.8, 124.0, 104.5, 60.9, 60.9, 56.2, 56.2, 55.1, 52.8, 38.9, 38.9, 30.6, 27.8, 22.1, 14.0. HR-MS (ESI): calcd for C23H30N5O3S [M + H]+, 456.2064; found 456.2065.
(E)-2–(4-(1-Methyl-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-yl)piperazin-1-yl)ethanol(4i). Title compound 4i was isolated as a yellow solid in 75.4% yield (1.87 g, 3.77 mmol), mp 141 – 142 °C. 1H NMR (600 MHz, CDCl3): δ 7.78 (d, J = 15.7 Hz, 1H), 7.17 (d, J = 15.7 Hz, 1H), 6.86 (s, 2H), 4.11 (s, 3H), 3.93 (s, 6H), 3.89 (s, 3H), 3.70 (t, J = 4.8 Hz, 2H), 3.62 (s, 4H), 2.99 (t, J = 7.7 Hz, 2H), 2.79 (s, 4H), 2.68 (t, J = 4.8 Hz, 2H), 1.90 – 1.86 (m, 2H), 1.04 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.2, 153.8, 153.4, 147.3, 145.1, 138.5, 135.7, 132.5, 128.0, 124.4, 104.4, 61.1, 59.5, 57.9, 56.2, 52.5, 49.6, 38.6, 27.9, 22.3, 14.2. HR-MS (ESI): calcd for C26H37N6O4 [M + H]+, 492.2871; found 492.2871.
(E)-1-Methyl-3-propyl-N-(pyridin-4-ylmethyl)-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(4j). Title compound 4j was isolated as a yellow solid in 86.3% yield (2.05 g, 4.32 mmol), mp 171 – 173 °C. 1H NMR (600 MHz, CDCl3): δ 8.62 – 8.56 (m, 2H), 7.56 (d, J = 15.7 Hz, 1H), 7.39 – 7.33 (m, 2H), 7.09 (d, J = 15.7 Hz, 1H), 6.77 (s, 2H), 5.57 (t, J = 5.7 Hz, 1H), 4.98 (d, J = 5.6 Hz, 2H), 4.26 (s, 3H), 3.89 (s, 6H), 3.86 (s, 3H), 2.95 (t, J = 7.7 Hz, 2H), 1.88 – 1.84 (m, 2H), 1.02 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 157.7, 153.4, 150.2, 149.0, 148.1, 145.9, 143.5, 138.5, 135.7, 132.5, 128.2, 122.6, 121.0, 104.3, 61.1, 56.2, 43.9, 39.3, 27.8, 22.4,14.2. HR-MS (ESI): calcd for C26H30N6O3 [M + H]+, 475.2452; found 475.2452.
(E)-N-(2-Methoxybenzyl)-1-methyl-3-propyl-5–(3,4,5-trimethoxystyryl)-1H-pyrazolo[4,3-d]pyrimidin-7-amine(4k). Title compound 4k was isolated as a yellow solid in 79.8% yield (2.01 g, 3.99 mmol), mp 211 – 213 °C. 1H NMR (600 MHz, CDCl3): δ 7.83 (d, J = 15.7 Hz, 1H), 7.50 – 7.44 (m, 1H), 7.31 (td, J = 8.0, 1.5 Hz, 1H), 7.14 (d, J = 15.7 Hz, 1H), 6.97 (t, J = 7.9 Hz, 2H), 6.88 (s, 2H), 5.87 (t, J = 5.6 Hz, 1H), 4.96 (d, J = 5.6 Hz, 2H), 4.20 (s, 3H), 3.95 (s, 3H), 3.93 (s, 6H), 3.89 (s, 3H), 2.97 – 2.90 (m, 2H), 1.86 – 1.83 (m, 2H), 1.01 (t, J = 7.4 Hz, 3H). HR-MS (ESI): calcd for C28H34N5O4 [M + H]+, 504.2065; found 504.2067.
3. Biological evaluation
3.1. Cell culture
Mouse peritoneal RAW264.7 macrophages were obtained from BeNa Culture Collection Company. Cells were cultured in DMEM (Hyclone, USA) supplemented with 10% FBS (Biological Industries, Israel), 100 U/ml penicillin and 100 μg/ml streptomycin (Beyotime, China) at 37 °C in a humidified atmosphere containing 5% CO2.
3.2. Determination of NO, TNF-α and IL-6
RAW264.7 cells were seeded into 48-well plate with 6 × 104 cells per well and incubated for 24 h. Cells were pretreated with the title compounds (10 μM) for 1 h, followed by exposure to LPS (0.5 μg/ml) for 24 h. The supernatants were collected and examined for NO production using Griess reagent (Beyotime, China). The levels of TNF-α and IL-6 in the supernatant were determined using the ELISA kit, according to the manufacturer's instructions (eBioScience, San Diego, CA).
3.3. Cell viability assay
RAW264.7 cells (6 × 103 cells/per) were seeded into 96-well plate and incubated for 24 h. Cells were pretreated with the title compounds (20 μM) for 1 h and incubated with LPS (0.5 μg/ml) for 24 h. MTT solution (5 mg/ml in PBS) was added to each well and incubated for 4 h at 37 °C. The MTT containing media was removed and then 150 μL of DMSO was added. The absorbance was detected at 490 nm by a microplate reader (MQX200, Bio-Tek, USA).
3.4. Western blotting
RAW264.7 cells were seeded into a 6-well plate at a density of 3 × 105 cells per well and then cultured for 24 h. Cells were pretreated with compound 4e (2, 1, 0.5 μM) for 1 h, followed by exposure to LPS (0.5 μg/ml) for 0.5 h.
Cells were lysed with RIPA lysis buffer (Beyotime, China) containing PMSF and phosphatase inhibitors, and then incubated on ice for 30 min. The protein lysates were separated by 12% SDS-PAGE and subsequently transferred onto PVDF membranes (GE Healthcare, UK). The blocked membranes were incubated with the indicated primary antibodies at 4 °C overnight (All of the antibodies were purchased from Cell Signaling Technology, USA). After washing three times with TBST (Beyotime, China), the membranes were incubated with HRP-conjugated secondary antibody (Beyotime, China) for 1 h at room temperature.
3.5. In vivo experiment
Male C57BL/6 mice weighing 18 – 22 g were purchased from Animal Department of Anhui Medical University. Mice were randomly divided into five groups (n = 8): physiological saline as negative control group, LPS (20 mg/kg) stimulated group, compound 4e high dose (20 mg/kg) group, compound 4e low dose (10 mg/kg) group and celecoxib (15 mg/kg) as positive control group. Compound 4e or celecoxib was administrated intraperitoneally (i.p.) 0.5 h before LPS injection via tail vein. Mice were anesthetized and sacrificed 48 h after LPS injection. Lung tissues were collected and fixed in 4% paraformaldehyde, followed by embedded in paraffin. After dehydration, sections were stained with Hematoxylin and Eosin (H&E) staining.
4. Results and discussion
4.1. Chemistry
4-Amino-1-methyl-3-propyl-4,5-dihydro-1H-pyrazole-5-carboxamide (SM) was prepared according to previous protocol21. SM was treated with substituted carboxylic acid in the presence of EDCI and HOBt to yield A1–A4. Compounds B1–B4 were synthesized from compounds A1–A4via cyclization in the presence of sodium ethoxide. Key intermediates C1–C4 were carried out under N2 atmosphere with POCl3 at 95 °C for 8 h (Scheme 1). (General procedures see Supporting Information).
4.2. Inhibitory activity against LPS-induced NO release
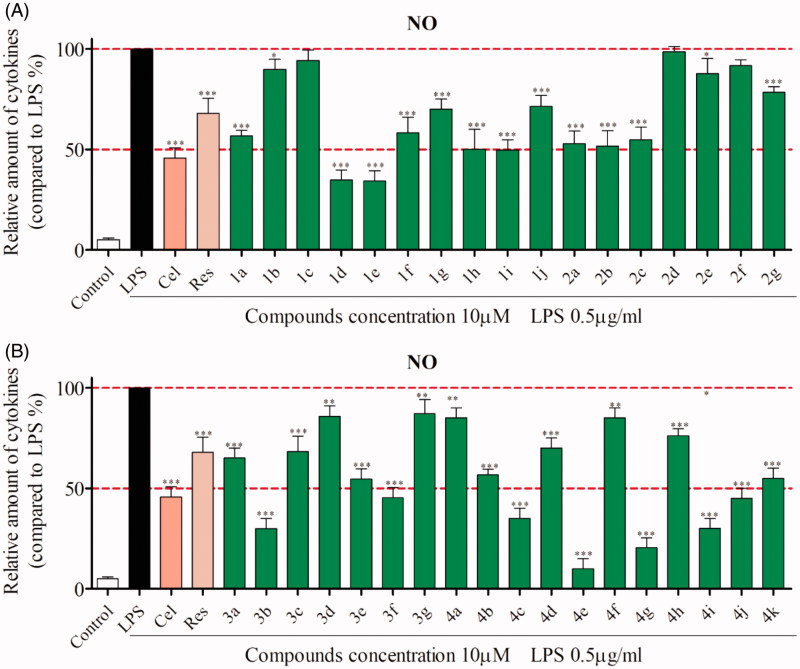
Nitric oxide (NO) is an important pro-inflammatory mediator, relating to several inflammatory diseases, such as rheumatoid arthritis, chronic hepatitis and ALI22,23. Therefore, inhibition of its overproduction may provide a useful therapy for inflammatory diseases24. Treatment of RAW 264.7 cells with LPS stimulated NF-κB signaling pathway, resulting in the production of cytokines including NO, IL-6 and TNF-α. Briefly, RAW 264.7 cells were pre-incubated with tested compounds, followed by incubated with LPS. The supernatants were collected and then nitrite levels were determined. The results indicated that almost all tested compounds were able to inhibit NO production at 10 µM (Figure 3). Being the most potent, compound 4e reduced NO release more intensely than both celecoxib and resveratrol. Accordingly, the introduction of 3-morpholinopropan-1-amine group at C-7 of pyrazolo[4,3-d]pyrimidine scaffold can improve the inhibitory activity.
Figure 3.
Inhibition of LPS-induced NO releasing by compounds 1a∼4k in RAW264.7 cellsa. aRAW264.7 cells were pretreated with compounds (10 μM) for 1 h, and incubated with LPS (0.5 μg/ml) for 24 h. The levels of NO releasing were measured using Griess Reagent assay. (A) Effect of compounds 1a∼2g on NO secretion. (B) Effect of compounds 3a∼4k on NO secretion. Celecoxib (Cel) and resveratrol (Res) were chosen as positive controls. ***p < .001, **p < .01, *p < .05 versus LPS group.
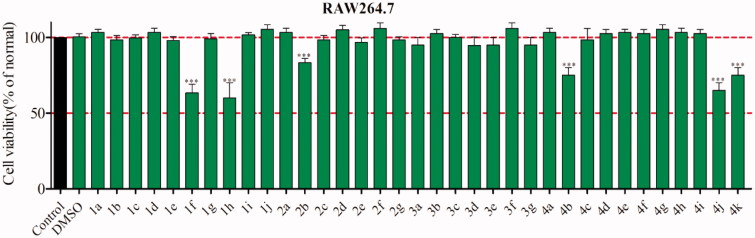
4.3. Assessment of toxicity
To discard that the suppressive effects on NO release was related to cell viability, MTT assay was adopted. As observed in Figure 4, cell viability was not affected by most of compounds at 20 µM, excepting of compounds 1f, 1h, 2b, 4b, 4j and 4k with weak cytotoxicity. The results indicated that their anti-inflammatory activity is not mediated by cytotoxic effect. Therefore, these compounds were worth of further evaluation.
Figure 4.
The cytotoxicity of compounds 1a∼4k in RAW264.7 cellsa. aThe cell viability was evaluated by the MTT assay. ***p < .001 compare with control group.
4.4. Inhibition of LPS-induced release of cytokines
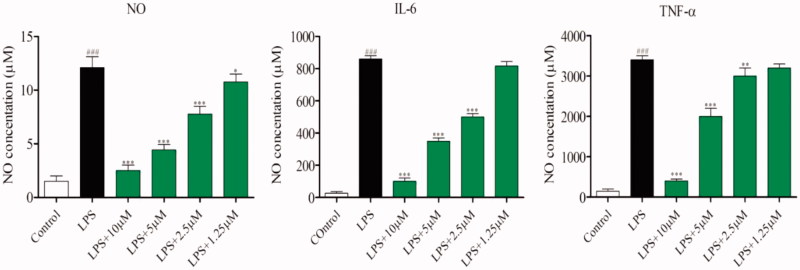
Increasing evidences have showed that the two important cytokines IL-6 and TNF-α play important roles in the pathogenesis of ALI through a series of cytokine signaling pathways25. Therefore, the most potent compound 4e was chosen to further evaluate for inhibition of LPS-induced NO, TNF-α and IL-6 releasing. As shown in Figure 5, compound 4e significantly decreased NO, IL-6 and TNF-α secretion in a concentration-dependent manner, with IC50 values of 2.64, 4.38 and 5.63 μM, respectively. On the basis of above findings, the anti-inflammatory mechanisms of compound 4e were further explored.
Figure 5.
Inhibition of the cytokines productiona. aRAW264.7 cells were pretreated with compound 4e in a series of concentrations (10, 5, 2.5, 1.25 μM) for 1 h, incubated with LPS (0.5 μg/ml) for 24 h. NO releasing was measured using Griess Reagent assay. The levels of TNF-α and IL-6 in the culture medium were measured by ELISA. *** p< .001, **p < .01, *p < .05 versus LPS group.
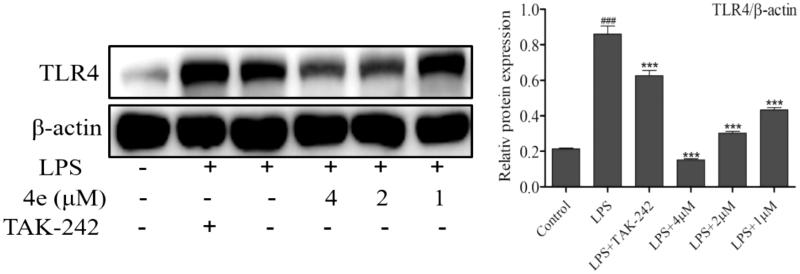
4.5. Inhibition of LPS-induced TLR4 expression
As a key factor in LPS-induced inflammation, TLR4 activates series of cellular signaling pathways, including NF-ĸB and mitogen-activated protein kinases (MAPKs), leading to the secretion of pro-inflammatory cytokines, such as NO, IL-6, TNF-α and IL-1β26–28. Therefore, we investigated whether the expression of TLR4 was down-regulated by compound 4e. As shown in Figure 6, LPS-induced TLR4 overexpression was attenuated by pretreatment of compound 4e in a concentration-dependent manner.
Figure 6.
Inhibit expression of LPS-induced TLR4a. aRAW264.7 cells were pretreated with compound 4e (1 – 4 µM) for 1 h, then stimulated with LPS (0.5 μg/ml) for 24 h. The expression of TLR4 was analyzed by Western blot. The results were showed as means ± SD (n = 3) of at least three independent experiments. TAK-242 was the TLR4 inhibitor. ###p < .001 compared with LPS un-stimulated cells; *p < .05, **p < .01, ***p < .001 compare with LPS-stimulated cells.
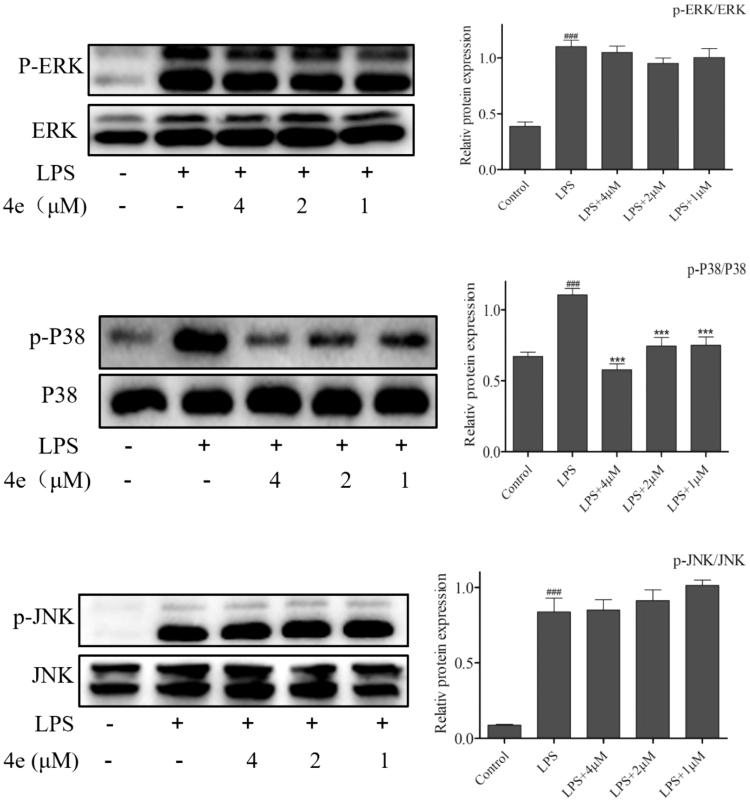
4.6. Inhibition of LPS-induced p38 signaling pathway
MAPKs, including ERK, p38 and JNK, were quite significant in the regulation of inflammation29. Therefore, we detected the effects of compound 4e on LPS-mediated MAPKs signaling activation. As shown in Figure 7, the phosphorylation of p38 but not JNK or ERK was blocked by compound 4e treatment in a concentration-dependent manner. And the total levels of ERK, JNK and p38 were not affected.
Figure 7.
Suppressed LPS-induced p38 activationa. aRAW264.7 cells were pretreated with compound 4e (1–4 µM) for 1 h, then stimulated with LPS (0.5 μg/ml) for 30min. The phosphorylation and total expression of ERK, JNK and p38 were analyzed by Western blot. The results were showed as means ± SD (n = 3) of at least three independent experiments. ###p < .001 compared with LPS un-stimulated cells; *p < .05, **p < .01, ***p < .001 compare with LPS-stimulated cells.
4.7. In vivo experiment
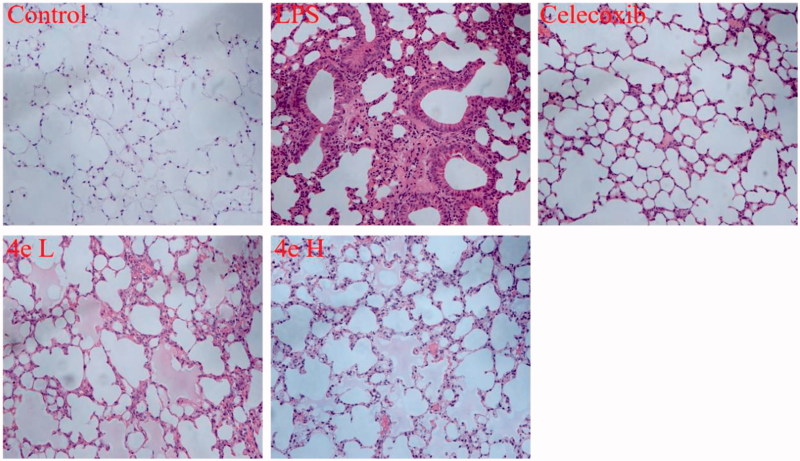
Next, compound 4e was evaluated in LPS-induced ALI mice model. Mice were pretreated with compound 4e by intraperitoneal injection 0.5 h before LPS challenge. LPS stimulation leads to significant pro-inflammatory alterations, including lung edema, inflammatory cell infiltration and destruction of alveolar structure. Pretreatment of mice with compound 4e effectively reduced airspace inflammation and amended the tissue structure of pulmonary lobules (Figure 8). These results indicated the protective effects of compound 4e against LPS-induced ALI in mice model.
Figure 8.
Compound 4e protected LPS-induced acute lung injurya. aC57/BL6 mice were treated with compound 4e (10 mg/kg, 20 mg/kg), and after 30 min, were challenged with 20 mg/kg LPS by tail vein injection. After Hematoxylin and Eosin staining, histological examination was performed by light microscopy (magnification ×200). Celecoxib (15 mg/kg) was a positive drug. ###p < .001 compared with control group; *p < .05, **p < .01, ***p < .001 compare with LPS group.
5. Conclusions
In the present studies, 35 novel pyrazolo[4,3-d]pyrimidine derivatives were designed, synthesized and evaluated for their anti-inflammatory activities in RAW264.7 cells. The preliminary SAR studies show that the introduction of 3-morpholinopropan-1-amine group into pyrazolo[4,3-d]pyrimidine could increase anti-inflammatory activity. Specifically, the most potent compound 4e was selected to further study the mechanism. The results showed that compound 4e concentration-dependently inhibited LPS-induced NO, IL-6 and TNF-α secretion through suppressing TLR4/p38 signaling pathway. In vivo studies in LPS-challenged mice showed that compound 4e effectively normalized pulmonary histopathological changes. Taken together, compound 4e could be potential therapeutics for ALI.
Supplementary Material
Funding Statement
We gratefully acknowledge financial support from Natural Science Foundation of Anhui Provincial Education Department [KJ2017A831] and National Natural Science Funding of China [21572003].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Griffith B, Pendyala S, Hecker L, et al. . NOX enzymes and pulmonary disease. Antioxid Redox Signal 2009;11:2505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu L, Li Y, Wan S, et al. . Protective effects of apocynin nitrone on acute lung injury induced by lipopolysaccharide in rats. Int Immunopharmacol 2014;20:377–82. [DOI] [PubMed] [Google Scholar]
- 3.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI). Br J Haematol 2007;136:788–99. [DOI] [PubMed] [Google Scholar]
- 4.Force ADT, Ranieri VM, Rubenfeld GD, et al. . Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. [DOI] [PubMed] [Google Scholar]
- 5.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. [DOI] [PubMed] [Google Scholar]
- 6.Neudecker V, Brodsky KS, Clambey ET, et al. . Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci Transl Med 2017;9:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler AP, Bernard GR, Thompson BT, et al. . Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006;354:2213–24. [DOI] [PubMed] [Google Scholar]
- 8.Richeldi L, Du Bois RM, Raghu G, et al. . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071–82. [DOI] [PubMed] [Google Scholar]
- 9.Shi JB, Chen LZ, Wang BS, et al. . Novel pyrazolo[4,3-d]pyrimidine as potent and orally active inducible nitric oxide synthase (iNOS) dimerization inhibitor with efficacy in rheumatoid arthritis mouse model. J Med Chem 2019; 62:4013–31. [DOI] [PubMed] [Google Scholar]
- 10.Xing Z, Han J, Hao X, et al. . Immature monocytes contribute to cardiopulmonary bypass-induced acute lung injury by generating inflammatory descendants. Thorax 2017;72:245–55. [DOI] [PubMed] [Google Scholar]
- 11.Imai Y, Kuba K, Neely GG, et al. . Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008;133:235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol 2011;11:213–20. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Tang L, Zhu H, et al. . Design, synthesis, and structure-activity relationship study of novel indole-2-carboxamide derivatives as anti-inflammatory agents for the treatment of sepsis. J Med Chem 2016;59:4637–50. [DOI] [PubMed] [Google Scholar]
- 14.Schenone S, Radi M, Musumeci F, et al. . Biologically driven synthesis of pyrazolo[3,4-d]pyrimidines as protein kinase inhibitors: an old scaffold as a new tool for medicinal chemistry and chemical biology studies. Chem Rev 2014;114:7189–238. [DOI] [PubMed] [Google Scholar]
- 15.Advani RH, Buggy JJ, Sharman JP, et al. . Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013;31:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park H-K, Jeong H, Ko E, et al. . Paralog specificity determines subcellular distribution, action mechanism, and anticancer activity of TRAP1 inhibitors. J Med Chem 2017;60:7569–78. [DOI] [PubMed] [Google Scholar]
- 17.Wyllie S, Thomas M, Patterson S, et al. . Cyclin-dependent kinase 12 is a drug target for visceral leishmaniasis. Nature 2018;560:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markwalder JA, Arnone MR, Benfield PA, et al. . Synthesis and biological evaluation of 1-aryl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidin-4-one inhibitors of cyclin-dependent kinases. J Med Chem 2004;47:5894–911. [DOI] [PubMed] [Google Scholar]
- 19.Abdelazeem AH, Abdelatef SA, El-Saadi MT, et al. . Novel pyrazolopyrimidine derivatives targeting COXs and iNOS enzymes; design, synthesis and biological evaluation as potential anti-inflammatory agents. Eur J Pharm Sci 2014;62:197–211. [DOI] [PubMed] [Google Scholar]
- 20.Hulverson MA, Bruzual I, McConnell EV, et al. . Pharmacokinetics and in vivo efficacy of pyrazolopyrimidine, pyrrolopyrimidine and 5-aminopyrazole-4-carboxamide bumped kinase inhibitors against toxoplasmosis. J Infect Dis 2019;219:1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi JB, Tang WJ, Qi XB, et al. . Novel pyrazole-5-carboxamide and pyrazole-pyrimidine derivatives: synthesis and anticancer activity. Eur J Med Chem 2015;90:889–96. [DOI] [PubMed] [Google Scholar]
- 22.Tham CL, Lam KW, Rajajendram R, et al. . The effects of a synthetic curcuminoid analogue, 2,6-bis-(4-hydroxyl-3-methoxybenzylidine)cyclohexanone on proinflammatory signaling pathways and CLP-induced lethal sepsis in mice. Eur J Pharmacol 2011;652:136–44. [DOI] [PubMed] [Google Scholar]
- 23.Bukhari SN, Lauro G, Jantan I, et al. . Pharmacological evaluation and docking studies of alpha,beta-unsaturated carbonyl based synthetic compounds as inhibitors of secretory phospholipase A(2), cyclooxygenases, lipoxygenase and proinflammatory cytokines. Bioorg Med Chem 2014;22:4151–61. [DOI] [PubMed] [Google Scholar]
- 24.Mohd Aluwi MFF, Rullah K, Yamin BM, et al. . Synthesis of unsymmetrical monocarbonyl curcumin analogues with potent inhibition on prostaglandin E2 production in LPS-induced murine and human macrophages cell lines. Bioorg Med Chem Lett 2016;26:2531–8. [DOI] [PubMed] [Google Scholar]
- 25.Wang JL, Carter J, Kiefer JR, et al. . The novel benzopyran class of selective cyclooxygenase-2 inhibitors-part I: the first clinical candidate. Bioorg Med Chem Lett 2010;20:7155–8. [DOI] [PubMed] [Google Scholar]
- 26.Nagy G, Clark JM, Buzas EI, et al. . Nitric oxide, chronic inflammation and autoimmunity. Immunol Lett 2007;111:1–5. [DOI] [PubMed] [Google Scholar]
- 27.Pan J, Xu T, Xu F, et al. . Development of resveratrol-curcumin hybrids as potential therapeutic agents for inflammatory lung diseases. Eur J Med Chem 2017;125:478–91. [DOI] [PubMed] [Google Scholar]
- 28.Yang HZ, Wang JP, Mi S, et al. . TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol 2012;180:275–92. [DOI] [PubMed] [Google Scholar]
- 29.Chen LZ, Sun WW, Bo L, et al. . New arylpyrazoline-coumarins: synthesis and anti-inflammatory activity. Eur J Med Chem 2017;138:170–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.