Abstract
Patients with amnestic mild cognitive impairment (MCI) and Alzheimer’s disease (AD) often demonstrate high rates of false memories, leading to stressful and frustrating situations for both patients and caregivers in everyday life. Sometimes these false memories are due to failures in monitoring the source of the information. In the current study, we examined interventions aimed to enhance the use of the metacognitive “recall-to-reject” memory strategy. Such interventions could improve source memory and decrease false memory in patients with MCI. Because the picture superiority effect (better memory for pictures compared to words) has been shown to be present in both patients with MCI and healthy older controls, we investigated whether pictures could help patients with MCI use a recall-to-reject strategy in a simulation of real-world source memory task. In this experiment, patients with MCI and healthy older adults were asked to simulate preparing for and then taking a trip to the market. Subjects first studied 30 pictures of items in their “cupboard,” followed by a list of 30 words of items on their “shopping list.” At test, participants saw 90 pictures (30 cupboard, 30 list, 30 new) organized as they would be if walking down the market aisles, and are provided with either standard or metacognitive instructions. With standard instructions, they were asked if they needed to buy the item. With the metacognitive instructions, they were asked a series of questions to help guide them through a recall-to-reject strategy to highlight the different sources of memories. Results showed that the metacognitive instructions did significantly reduce the false memory rates for patients with MCI. Further studies need to investigate how to best implement these practical strategies into the everyday lives of patients.
Keywords: mild cognitive impairment, Alzheimer’s disease, retrieval monitoring, false recognition
1. Introduction
Episodic memory is impaired early in amnestic mild cognitive impairment (MCI), which has a high rate of conversion to Alzheimer’s disease (AD). Patients with MCI and mild AD dementia show impairments in recognizing and recalling information compared to healthy older adults (Budson et al., 2004; Budson, Wolk, Chong, & Waring, 2006; Budson et al., 2007; Dalla Barba, Nedjam, & Dubois, 1999; Embree, Budson, & Ally, 2012). Additionally, these patients show increased rates of false memories (Balota et al., 1999; Budson, Daffner, Desikan, & Schacter, 2000; Gallo et al., 2006; Hildebrandt, Haldenwanger, & Eling, 2009; O’Connor et al., 2015), which can create stressful and frustrating situations for patients and caregivers, as well as limiting a patient’s ability to live independently. False memories are often due to failures in monitoring the source of the information. Potentially, false memories in patients with MCI could be reduced and overall memory improved if strategies could be identified that might enhance source monitoring abilities. In the current study, we examined the use of metacognitive instructions to encourage the use of source monitoring techniques.
Patients with Alzheimer’s disease (AD) dementia have been shown to exhibit higher rates of false recognition and recall relative to healthy age matched peers (Balota et al., 1999; Budson et al., 2000; Gallo et al., 2006; O’Connor et al., 2015). In false memory studies utilizing study lists of semantically related items (e.g., door, glass, pane, shade, etc.), patients are more likely to falsely endorse strongly related but unstudied lure items in a memory test (e.g., window’) compared to healthy older adults. When individuals study lists of related items, it strengthens gist information, that is, information regarding the common elements of a set of items. Healthy older adults are able to combat gist by using item-specific recollection, or recollection of specific unique details about studied items. In contrast, patients with AD dementia are impaired in their use of item-specific recollection and rely more heavily on gist, causing them to endorse items they have not experienced previously.
Additionally, both patients with MCI and those with mild AD dementia exhibit elevated false memories in more traditional memory tasks that do not use semantically related study items (Ally, Gold, & Budson, 2009a, 2009b; Embree, Budson, & Ally, 2012). It has been suggested that patients with MCI and patients with AD dementia are impaired in their use of recollection and rely more on their sense offamiliarity to inform their recognition memory decisions. Dual process theories of memory suggest that two independent processes contribute to successful recognition memory (Yonelinas, 1994, 2002). Recollection refers to the encoding and retrieval of rich, context filled information of an item or an event. Familiarity refers to the feeling of having encountered something before, but lacking information regarding the context or specific details of where that information was originally encountered. Although familiarity is not entirely spared in patients with MCI and mild AD dementia, it is better preserved than recollection (Ally, Gold, et al., 2009a; Ally, McKeever, Waring, & Budson, 2009; Westerberg et al., 2006). Thus, false memories are, in general, thought to be tied to the overreliance on familiarity (which is directly related to gist).
1.2. Memory Monitoring
Healthy individuals (both young and old) use specific details of an item or event (via recollection) to engage in strategies to enhance retrieval monitoring—detailed information that can be used to strategically regulate the accuracy of responding in a memory test (Dodson & Schacter, 2002; Gallo, Cotel, Moore, & Schacter, 2007; Gallo, Weiss, & Schacter, 2004; Schacter, Israel, & Racine, 1999). For instance, individuals can engage in diagnostic monitoring, whereby they reject an item based on the failure of recollection to conform to a certain expectation (e.g., “I didn’t see this item, because I would have remembered it since it would’ve been a particularly unique picture.”). Additionally, healthy individuals can engage in disqualifying monitoring, whereby they reject an item based on the recall of logically inconsistent information (e.g., “I didn’t see this item as printed word because I specifically remember it studying it as a picture.”)
Patients with mild AD dementia are impaired on the use of these monitoring strategies compared to healthy older adults (Budson, Dodson, Daffner, & Schacter, 2005; Budson, Sitarski, Daffner, & Schacter, 2002; Gallo, Chen, Wiseman, Schacter, & Budson, 2007). In a study examining one type of diagnostic monitoring strategy (the distinctiveness heuristic), patients with AD and healthy older adults studied sets of categorized pictures or words (Budson et al., 2002). During the encoding phase, half the items were presented as a visual word paired with an auditory recording, while the other half were presented as a picture with an auditory recording. In the test phase, items were presented as either as pictures paired with auditory words or as only auditory words. Results showed that when pictures were used in the memory test, healthy older adults had numerically lower false recognition compared to when only auditory words were used. Healthy older adults developed an expectation that test items should elicit vivid perceptual recollection, and if it did not, they would reject the item. In contrast, patients with mild AD dementia were less likely to engage in this strategy, and were not able to reduce their false recognitions. A follow-up study revealed that patients with mild AD dementia are aware of the distinctiveness heuristic as a viable strategy, but are unable to selectively apply it to new items to reduce their false recognitions (Budson et al., 2005). Using a criterial recollection task emphasizing the use of recollection, Gallo, Chen, Wiseman, Schacter, and Budson (2007) also found that patients with AD showed limited use of the distinctiveness heuristic when compared to healthy older adults.
Patients with AD dementia have also demonstrated impairments in using a disqualifying monitoring strategy. Gallo, Sullivan, Daffner, Schacter, and Budson (2004) examined patients with mild AD dementia and healthy older adults on an associative recognition task. In their experiment, participants studied pairs of words, some of which were presented only once, others three times. In the subsequent test phase, participants were presented with pairs of words: intact pairs, rearranged pairs, and new pairs. Of particular interest were the rearranged pairs, in this condition both words are familiar and so to correctly reject these pairs, participants need to be able to recall the specific associations made during the study phase. Repetition increased false recognition of these rearranged pairs for patients with AD, but it did not have an effect on healthy older adults who were able to successfully use a recollection-based recall-to-reject monitoring strategy.
1.3. Source Monitoring and Source Memory
Source monitoring and its effect on false memory has also been examined in patients with MCI and mild AD dementia. Monitoring frameworks used to explain the reduction of false memory have been derived from the more general source-monitoring framework (Johnson, Hashtroudi, & Lindsay, 1993; Mitchell & Johnson, 2009). The source monitoring framework provides an explanation of how individuals are able to identify the origin (or “source”) of information. Details may include perceptual (e.g., color), temporal, or spatial (e.g., location) characteristics. In turn, these details allow an individual to more easily differentiate between potentially similar memories. Thus, source memory can be thought to be closely related to memorial recollection.
Similar to recollection, source memory has been shown to be impaired in both patients with MCI and patients with mild AD dementia. In general, patients are less able to determine the source of memories. Relative to healthy older adults, patients are less likely to remember if they read or self-generated a sentence they were supposed to remember (Multhaup & Balota, 1997), what room they studied an item in (Pierce, Waring, Schacter, & Budson, 2008), whether they performed an action or if they only imagined performing an action (Dalla Barba et al., 1999; O’Connor et al., 2015), or whether or not they intentionally remembered or intentionally forgot a study item (El Haj, Fasotti, & Allain, 2014).
Given the nature of their cognitive impairment, it is not surprising that patients with mild AD dementia perform poorly on tasks of source memory. Accurate source memory retrieval relies on several cortical areas (Mitchell & Johnson, 2009). Areas of the medial temporal lobes (e.g., the hippocampus) are responsible for the binding of item-specific and contextual episodic information. The anterior prefrontal cortices are thought to help monitor and differentiate between sources of information, while the dorsolateral prefrontal cortices (DLPFC) is involved with more organizational processes. The AD pathophysiological process is thought to initially cause destruction to the medial temporal lobes along with other areas of the temporo-parietal region. As AD patients progress from mild cognitive impairment to very mild, mild, and moderate dementia stages, the disease causes increased destruction to lateral temporal, parietal, and later, frontal lobes (Apostolova et al., 2006; Braskie, Toga, & Thompson, 2013; Scahill, Schott, Stevens, Rossor, & Fox, 2002; Whitwell et al., 2007; Whitwell, 2010). Based on this pathological progression and prior findings that executive functioning is less impaired in MCI compared to AD (Carter, Caine, Burns, Herholz, & Lambon Ralph, 2012), patients with MCI may be more able to use strategies to boost source memory compared to individuals with more advanced disease.
Although patients with MCI and AD dementia have significant memory impairments, evidence has shown that these patients may have relatively intact meta-memory abilities, and can use explicit instructional information to modify their performance in memory tasks. For example, patients changed the nature of their responding when told the proportions of old and new items in a memory test. In a study by Waring, Chong, Wolk, and Budson (2008), participants were presented with a recognition memory test composed of 50% old and 50% new words, but participants were told that either 30% or 70% of the words were old. Patients and healthy older adults became more conservative in their responding and also reduced their rate of false recognition in the 30% condition. Other studies have shown that patients with AD and controls perform similarly on various measure of meta-memory (Bäckman & Lipinska, 1993; Gallo, Cramer, Wong, & Bennett, 2012; Moulin, Perfect, & Jones, 2000; Schmitter-Edgecombe & Seelye, 2011). Thus, leveraging meta-memory may provide an avenue to help patients improve their performance in memory tasks.
In the current study, we were interested in examining how meta-memorial/meta-cognitive processes can help patients with MCI improve memory monitoring and, in turn, improve their overall memory. In the current experiment, healthy older adults and patients with MCI went through two study-test phases in which they were to imagine themselves preparing and then going on a trip to the grocery store. We wanted to create an experimental task that reflected a real world activity of daily living for older adults in order to examine how the introduction of a strategy might be beneficial. In both study phases, participants viewed a set of words (their grocery list) and a set of pictures (items already in their kitchen). In the subsequent test phase, they were to imagine going to the store to buy the items on their list. Participants made simple yes/no decisions about whether to buy an item in the standard test instructions, whereas in the metacognitive instruction condition participants were asked a series of questions that served to highlight and encourage the use of metacognitive retrieval monitoring strategies. In particular, the first question, “Is this item familiar to you?” should result in an initial memory retrieval attempt. An item should be familiar both in the list and the cupboard condition. The goal of the second question, “Was this item in your cupboard?” is to try to get the participants to use salient information from pictures to engage in additional monitoring, effectively to help them successfully reject items that they do not need to buy. Prior evidence in patients with MCI has shown that memory for pictures is generally more robust than memory for words, and we believe this may facilitate the use of additional memory monitoring mechanisms (Ally, 2012; Ally, Gold, et al., 2009b; Deason, Hussey, Budson, & Ally, 2012; Deason, Flannery, Hussey, & Ally, 2015). We predicted that both groups would benefit from the metacognitive instructions and would show a reduction in false memories compared to the standard instruction condition.
2. Material and methods
2.1. Participants
Twenty-one healthy older adults (OCs; 6 male) and 19 patients with MCI (15 male) were recruited for this study. Healthy older adults were recruited through online postings and community postings in the Boston community. Patients with MCI met criteria described by the National Institute on Aging and Alzheimer’s Association (NIA-AA) workgroup criteria (Albert et al., 2011) and were recruited from the Boston University Alzheimer’s Disease Center (BU ADC). These patients were each assessed and diagnosed by a neurologist and neuropsychologist according to national ADC criteria and were otherwise healthy. Participants were screened for clinically significant depression, alcohol or drug use, past stroke, traumatic brain injury, or other neurologic disorder. All participants were native English speakers and had normal or corrected to normal vision. The study was approved by the human studies committees of VA Boston Healthcare System, Boston, MA, the Edith Nourse Rogers Memorial Veterans Hospital, Bedford, MA, and Boston University, Boston, MA. Written informed consents were obtained from all participants. Participants were paid $10/hour for their participation.
Healthy older adults and patients were administered a battery of neuropsychological tests in order to determine their eligibility for participation in the study. This battery included the MMSE (Folstein, Folstein, & McHugh, 1975), CERAD Word List Memory Test (Morris et al., 1989), Trail Making Test Part B (Adjutant General’s Office, 1944), Verbal fluency to letters and categories (Monsch et al., 1992), and the short form Boston Naming Test (Mack, Freed, Williams, & Henderson, 1992). Table 1 presents demographic and neuropsychological data for the participants.
Table 1.
Demographic and Neuropsychological data for participant groups
| Test | Older Adults Mean (SD) n=21 |
Patients with MCI Mean (SD) n=19 |
|---|---|---|
| Age | 75.62 (8.67) | 77.89 (6.1) |
| Years of Education | 16.01 (2.15) | 15.37 (2.97) |
| MMSE | 29.38 (.74) | 27.68 (1.57) * |
| CERAD Word List | ||
| Immediate | 20.71 (4.95) | 13.89 (3.13) * |
| Delayed | 7.43 (1.75) | 3.32 (1.77) * |
| Recognition | 9.86 (0.48) | 8.42 (1.22) * |
| Trails-B | 77.81 (27.19) | 136.16 (77.87) * |
| Trails-A | 33.7 (12.6) | 44.0 (18.07) * |
| FAS | 49.05 (11.26) | 37.16 (13.45) * |
| CAT | 46.10 (12.94) | 33.11 (10.9) * |
| BNT-15 | ||
| No cue | 14.71 (0.64) | 13.21 (2.44)* |
| Semantic cue | 0.05 (0.22) | 0.0 (0) |
| Phonemic cue | 0.29 (0.64) | 0.95 (1.47) ~ |
significant difference between the two groups at p < 0.05
marginal difference between the two groups at p = 0.069
2.2. Materials and Design
The stimuli were 180 common objects that could be found in a grocery store. The stimuli were selected from the updated version of the Battig and Montague (1969) norms (Van Overschelde, Rawson, & Dunlosky, 2004). The stimuli were divided into 6 lists of 30 items, which were matched on word length and word frequency (Kučera & Francis, 1967). Assignment of lists to experimental conditions was counterbalanced across participants. The study was programmed using E-Prime 2.0 Professional software (Psychology Software Tools, Pittsburgh, PA) and run on PC laptops. The stimuli were presented in the center of the screen on a white background. Color pictures were all resized to 450 pixels by 450 pixels and words were presented in 32 point black Courier font.
2.3. Procedure
Each participant was administered two separate study/test phases. During each study section, participants were asked to imagine a scenario where they were preparing to go grocery shopping. In this scenario, participants were instructed that before they went to the store, they were going to prepare by examining their grocery list as well as items they already had in their cupboards. Participants were then presented with 30 items in their ‘cupboard’ (pictures) followed by 30 items on their ‘grocery list’ (words), with each item presented for 3 seconds. They were told to remember the items for later as they would need to buy the items on their grocery list. In the subsequent test phase, participants were to imagine they were at the grocery store going through the aisles. As they viewed each picture of an item on the grocery aisle, participants needed to decide whether they needed to buy the item or not. They were also explicitly told not to make any new/impulse buys, to discourage buying unnecessary items. There were a total of 90 pictures. These pictures included 30 pictures of items that had been on the grocery list (need to buy), 30 pictures of items that had been in the cupboard (do not need to buy), and 30 pictures of items not encountered in the study phase (do not need to buy). All test items were presented as pictures and appeared on the screen until the participant made a yes/no buy response. Each individual study/test phase corresponded to two different testing conditions, the Standard Instruction test condition, and the Metacognitive Instructions test condition.
In the Standard Instructions test condition, participants were only asked one question after each of the 90 pictures was presented: “Do you need to buy this item?” At the conclusion of the first study-test session, the subjects had a short break and were told that the next study-test session would use novel items and explicitly told to not recall any objects from the first study-test session. No items were repeated between the two study-test sessions. The order of the two study-test sessions was counterbalanced between participants.
In the Metacognitive Instruction test condition, participants were again told to simulate going through the grocery store aisles and were discouraged from making impulse/new item purchases. However, the participants were now presented with three questions for every item. When each picture was shown, the participants first answered the question, “Is this item familiar to you?” Once they answered this question, the next question appeared on the screen, “Was this item in your cupboard?” Finally, they answered the same question asked in the simple test session, “Do you need to buy this item?” The participant made responses to these three questions for each of the 90 pictures presented during the test.
3. Results
3.1. Hit and False Alarm rates
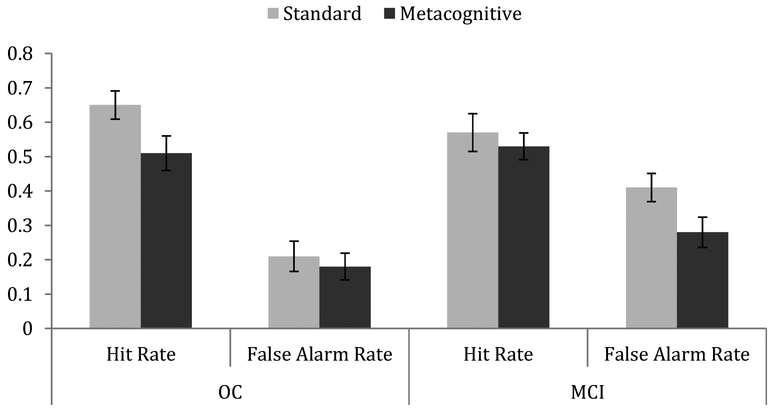
A repeated-measures Analysis of Variance (ANOVA) was conducted using a between-subjects factor of Group (healthy older adults vs. patients with MCI) and Gender (male, female) and the within-subjects factor of Instruction Type (standard, metacognitive) and Item Type (hits, false alarms) for hit rate and false alarm rate. In this analysis, the total false alarm rate was used, which included both false alarms to new items as well as “buy” responses to items that had been in the cupboard. No main effects of Group or Gender were observed (Group: F(1, 36) = 1.123, p =.30, ηp2 = .030; Gender: F(1, 36) = .54, p =.47, ηp2 = .015). There was a main effect of Instruction with higher “buy” response rates for the Standard compared to the Metacognitive Instruction condition (F(1, 36) = 4.48, p < 0.05, ηp2 = .111) as well as a main effect of Item Type with higher rates for hits than false alarms (F(1, 36) = 36.92, p < 0.01, ηp2 = .506, see Figure 1). There was an interaction between Group and Item Type (F(1, 36) = 4.31, p < 0.05, ηp2 = .107), as there was no difference between groups in overall hit rates (t(39) < 1), but MCI patients had increased false alarm rates compared to healthy older adults (t(39) = 7.99, p < 0.01). There was also a three-way interaction between Instruction Type, Item Type, and Group (F(1, 36) = 4.83, p < 0.05, ηp2 = .118).
Figure 1.
Hit rates and false alarm rates for instruction conditions for healthy older adults and patients with MCI. Error bars are standard errors.
To follow-up on the three-way interaction, repeated-measures ANOVAs were conducted separately on hit rates and false alarm rates. There were no significant main effects or interactions found with hit rates. Similarly, a repeated-measures ANOVA was also conducted on false alarm rates, also including type of false alarm (cupboard vs. new) as an additional within subjects factor. There was a main effect of Instruction Type (F(1, 36) = 7.03, p < .05, ηp2 = .163). Total false alarm rates were higher when participants had been given the standard instruction compared to the metacognitive instruction (Standard: M = .31, Metacognitive: M = .23). There was also a main effect of Group with MCI patients making more false alarms than healthy older adults (F(1, 36) = 4.65, p < .05, ηp2 = .114; OC: M = .20, MCI: M = .35). There was a marginal interaction between Instruction Type and Group (F(1, 36) = 3.52, p =.069, ηp2 = .085). Follow-up t-tests showed that there was no significant difference between instruction conditions for total false alarm rates in healthy older adults (t(20) = 1.25, p =.23), but there was a significant difference for total false alarm rates between the Standard (M = 41%) and Metacognitive (M = 28%) Instructions in patients with MCI (t(18) = 2.61, p < .05). There was also a main effect of false alarm type with both groups having increased false alarm rates for the new items compared to cupboard items (F(1, 36) = 5.98, p < 0.05, ηp2 = .142).
3.2. Response Bias (Br)
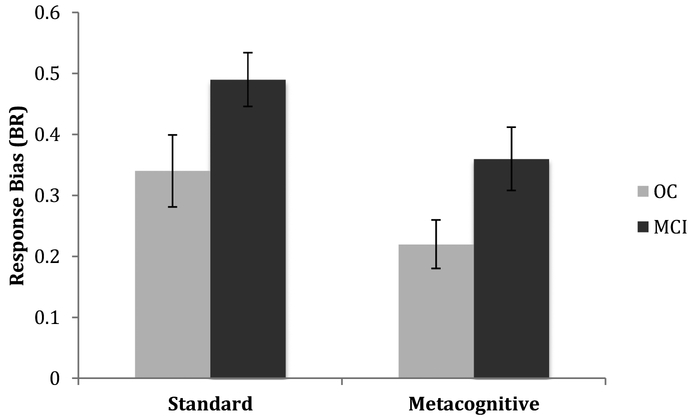
We also examined response bias using the traditional signal detection theory parameter, C (Snodgrass & Corwin, 1988). Response bias, measured by C, can be either conservative (less likely to respond old; indicated by positive values) or liberal (more likely to respond old; indicated by negative values). To examine response bias performance, a repeated-measures ANOVA was conducted using a between-subjects factors of Group (healthy older adults vs. patients with MCI) and Gender (male, female) and the within-subjects factor of Instruction type (standard, metacognitive) for Br (False Alarm Rate/(1 – (Hit Rate – False Alarm Rate)); Snodgrass & Corwin, 1988). Both participant groups demonstrated a more conservative response bias when presented with the Metacognitive Instructions than the Standard Instructions (F(1, 36) = 5.05, p < .05, ηp2 = .14; see Table 2 and Figure 2). Healthy older adults showed a trend toward a more conservative response bias than patients with MCI (F(1, 36) = 3.38, p = .074, ηp2 = .086).
Table 2.
Mean hit rate, false alarm rate, discrimination, and response bias for each participant group (standard deviation in parenthesis).
| Instruction Type | Healthy Older Adults | Patients with MCI | |
|---|---|---|---|
| Hit Rate | Standard | .65 (.19) | .57 (.24) |
| Metacognitive | .51 (.23) | .53 (.17) | |
| False Alarm Rate | Standard | ||
| Cupboard | .13 (.21) | .37 (.39) | |
| New | .30 (.36) | .45 (.36) | |
| Total | .21 (.20) | .41 (.18) | |
| Metacognitive | |||
| Cupboard | .19 (.24) | .20 (.23) | |
| New | .17 (.23) | .37 (.29) | |
| Total | .18 (.18) | .28 (.19) | |
| Discrimination (Hit Rate – False Alarm Rate) | Standard | .44 (.25) | .16 (.33) |
| Metacognitive | .32 (.33) | .24 (.20) | |
| Response Bias (Br) | Standard | .34 (.27) | .49 (.19) |
| Metacognitive | .22 (.18) | .36 (.23) |
Figure 2.
Response bias (Br) for both groups. Error bars are standard errors.
4. Discussion
Patients with MCI showed improved memory performance in this experiment when given instructions encouraging the use of retrieval monitoring strategies. The current results revealed that patients with MCI were able to reduce their false alarm rates when provided with guiding metacognitive instructions compared to when they received standard recognition memory instructions. Patients with MCI demonstrated a higher rate of false alarms than healthy older adults in general, although there were no group differences in hit rates. Unlike the patient group, healthy older adults did not show a significant reduction in false alarms with the Metacognitive Instructions. Hit rates were also not significantly impacted by instruction condition in either group. Response bias was influenced by instruction condition as both groups showed more conservative response biases in the Metacognitive Instruction condition than in the Standard condition. Overall, patients with MCI seemed to benefit from explicit metacognitive instructions, particularly reflected in their reduced false alarm rates and more conservative response bias.
In the current study, patients had to be able to separate Grocery List items, Cupboard items, and new items. This task essentially required efficient source monitoring for accurate performance. These findings indicate that patients with MCI benefited from the Metacognitive Instruction condition. One possible explanation was that they were more likely to engage in memory monitoring strategies (such as recall to reject) to reduce false alarms when they were provided with a series of guiding questions. The reduction of false alarms may be due to the combination of two factors: 1) the use of pictures during study for Cupboard items may have increased the saliency of these items; and 2) the increased saliency afforded by pictures may have enabled the use of different monitoring strategies, such as the distinctiveness heuristic and recall-to-reject.
An alternate explanation of the reduced false alarms in the Metacognitive Instruction condition is that these instructions encouraged adoption of a stricter criterion for responding “buy” to an item. Along with the reduction in false alarms, the Metacognitive Instruction condition led both healthy older adults and patients with MCI to adopt a more conservative response bias. Patients with MCI and AD dementia typically demonstrate an abnormally liberal response bias, or increased tendency to respond “old” in an old/new recognition memory test, when compared to healthy older adults (Balota et al., 2002; Bartok et al., 1997; Budson et al., 2006; Deason et al., 2012; Snodgrass & Corwin, 1988). Potentially, patients with AD could improve their memory and reduce false recognition by shifting to a more conservative response bias. Waring et al. (2008) showed that it was possible for patients to shift their response bias upon explicit instruction about the ratio of old to new items in the test phase. In Waring et al. (2008), patients were given incorrect information about the distribution of old versus new items that altered their response bias. Providing false information is not a practical strategy to implement in real life situations to alter patients’ response bias. The current experiment successfully shifted patients with MCI to a more conservative response bias by encouraging a different type of strategy by the use of guided questions highlighting important differences in source information in the Metacognitive Instruction condition.
Although healthy older adults also showed a shift to a more conservative response bias in the Metacognitive Instruction condition, they did not show the corresponding significant reduction in their false alarm rates. This lack of change in either hit rates or false alarm rates between instruction conditions suggests that the strategy encouraged by the Metacognitive Instruction condition may not be useful for healthy older adults. We initially predicted that both healthy older adults and patients with MCI would have shown a reduction in false alarm rates. One explanation for this finding might be that healthy older adults were already employing retrieval monitoring strategies in the standard instruction condition and the explicit questions in the Metacognitive Instruction condition made no difference or even complicated their memory retrieval process. Potentially, healthy older adults’ shift to a more conservative response bias resulted in the requirement for a strong recollected experience to endorse an item as one needed to buy. They may have ended up requiring a stronger memory to be retrieved than in the Standard Instruction condition as a result of the pattern of questions.
Since healthy older adults showed a similar shift to a conservative response bias but not a reduction in false memories, this suggests potentially that patients with MCI benefited more from the change in instructions because they started out with a more liberal response bias or because the patients with MCI began utilizing retrieval-monitoring strategies that they had not been using in the Standard Instruction condition. Future studies will be necessary to examine the separate contribution of these two factors.
4.1. Neural Correlates Associated with Monitoring
Efficient retrieval monitoring is thought to be reliant on intact frontal lobe functioning.As reviewed earlier, the AD pathophysiological process can cause the degeneration of the frontal lobes, especially towards the later stages of the disease. Several studies have linked impaired retrieval monitoring with impaired frontal functioning in patients with focal frontal lesions (Curran, Schacter, Norman, & Galluccio, 1997; Parkin, Bindschaedler, Harsent, & Metzler, 1996) or with activity in the frontal regions in young adults (Gallo, McDonough, & Scimeca, 2009). Not surprisingly, patients with lesions in these areas are less able to accurately perform source memory tasks (for a review, see Mitchell & Johnson, 2009). Degeneration to these areas may explain poor source memory performance in patients with mild AD dementia. These frontal regions may be more intact in patients with MCI compared to patients with mild AD dementia.
Electrophysiological evidence has been used to show that patients with MCI may have some preservation of frontal memorial functioning, at least for certain stimuli. Ally, McKeever, Waring, & Budson (2009) examined the neural correlates of successful recognition memory in patients with MCI using event-related potentials (ERP). Their results suggested that for picture recognition, the old/new ERP effect related to recollection was diminished in patients with MCI compared to healthy older adults, but there was no difference in the FN400 familiarity old/new effect or in a later frontal component potentially related to executive retrieval monitoring (Allan & Rugg, 1997; Ranganath, Heller, & Wilding, 2007; Wilding & Rugg, 1996). This late right frontal ERP effect has been shown to be increased when memory retrieval is more difficult (Wolk, Gold, Signoff, & Budson, 2009) and may potentially serve as a compensatory mechanism when memory is impaired (Ally et al., 2009). These findings suggest that the neural correlates of familiarity for pictures as well as some monitoring abilities might be more preserved in these patients and thus, a promising avenue for rehabilitative techniques. As these regions are more affected as Alzheimer’s disease progresses, one might predict that strategies relying on preserved monitoring abilities may be less effective in patients with more advanced Alzheimer’s disease.
4.2. Improving Source Memory in Patients with AD dementia
In the current study, the picture superiority effect was leveraged to improve the encoding of contextual and perceptual details for each item in an effort to reduce false memories at retrieval. This idea has been supported in other studies in healthy younger and older adults that have examined the utility of encoding strategies that emphasize the storage of detailed, item-specific information (termed item-specific encoding). In these studies, individuals are asked to name a unique characteristic for each study item they encounter. Item-specific encoding is thought to improve source differentiation and has been show to improve overall memory discrimination in healthy younger and older adults (Huff & Bodner, 2013; Thomas & McDaniel, 2013). Improvement in source differentiation, in turn, can facilitate the usage of retrieval strategies, such as the recall-to-reject or the distinctiveness heuristic. Specifically, enhanced perceptual or contextual information is more readily accessible for individuals to engage in retrieval strategies. In a recent study in patients with MCI and AD (Tat et al., 2016), healthy older adults and patients with MCI were able to improve their overall memory discrimination when engaging in an item-specific encoding strategy, relative to a more semantically-based, relational strategy. Patients with AD were less likely to improve memory discrimination using an item-specific strategy. This suggests that enhancing perceptual and/or conceptual details serves to improve source differentiation in patients that are less cognitively impaired (e.g., patients with MCI). Several other studies have examined potential explanations and also compensation mechanisms for impaired source memory in patients with AD (Rosa, Deason, Budson, & Gutchess, 2015, 2016). In Rosa et al. (2016), they examined how the relation to self might improve source memory performance in patients with MCI due to AD. Patients were asked to pack items in a basket or suitcase while relating each item to the self or to another individual. Source memory data revealed that although self-referencing did not improve overall source memory accuracy, it did reduce source misattributions they made to themselves, and they were more likely to attribute the source of information to other individuals. When retrieval monitoring strategies, such as the one used in the current study, are paired with manipulations that enhance detail oriented information (e.g., item-specific encoding or the picture superiority effect) or relation to self, memory may be further improved. Future studies should aim to examine the use of encoding strategies, reference to self, and retrieval instruction manipulations in conjunction with each other.
The results of this study provide additional information regarding interventions that can be used to facilitate retrieval monitoring. The current data revealed when patients are provided with step-by-step metacognitive instructions, they are able to better engage in retrieval monitoring, and in turn, reduce false memories. Further examination of retrieval monitoring strategies, especially in the context of real life scenarios, is important for translating these strategies into successful interventions. These interventions would enable patients to live more independent and fulfilling lives.
Highlights.
We examined whether guided metacognitive instructions could improve source memory.
Healthy older adults and patients with MCI were tested.
MCI patients reduced false recognition in the metacognitive instruction condition.
Metacognitive instructions potentially encouraged retrieval monitoring strategies.
Acknowledgments
This research was supported by a Veteran’s Administration Clinical Science, Research & and Development Merit Review Award ICX000736A (AEB), National Institute on Aging grants R01 AG025815 (AEB), K23 AG031925 (BAA), P30 AG13846 (AEB), and a Department of Veterans Affairs, Veterans Health Administration, VISN 1 Early Career Development Award to RGD. This material is also the result of work supported with resources and the use of facilities at the Bedford VA Hospital in Bedford, MA and the VA Boston Healthcare System, Boston, MA.
Appendix
A repeated-measures ANOVA was conducted using a between-subjects factors of Group (healthy older adults vs. patients with MCI) and Gender (male, female) and the within-subjects factor of Instruction type (standard, metacognitive) for Pr (Hit Rate – False Alarm Rate; Snodgrass & Corwin, 1988). In this analysis, the total false alarm rate used included both false alarms to new items as well as “buy “ responses to items that had been in the cupboard. There was a main effect of Group (F(1, 36) = 4.31, p < . 05, ηp2 = . 107) as well as an interaction between Instruction type X Group (F(1, 36) = 4.83, p < .05, ηp2 = .118, see Figure 2a). Healthy older adults showed a higher discrimination rate than patients with MCI (OC: M = .38, MCI: M = .20). Follow-up t-tests showed that there was a marginal difference between standard (M = .44) and metacognitive instruction conditions for healthy older adults (M = .32; t(20) = 1.92, p = 0.069). For the patients with MCI, the Pr values went the opposite direction numerically even though this difference was not significant (Standard: M = .16, Metacognitive: M = .24; t(18) = 1.15, p = 0.27).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjutant General’s Office. (1944). Army Individual Test Battery. Washington, DC: War Department. article. [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association, 7(3), 270–9. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan K, & Rugg M . (1997). An event-related potential study of explicit memory on tests of cued recall and recognition. Neuropsychologia, 35(4), 387–397. 10.1016/S0028-3932(96)00094-2 [DOI] [PubMed] [Google Scholar]
- Ally BA, Gold CA, & Budson AE (2009a). An evaluation of recollection and familiarity in Alzheimer’s disease and mild cognitive impairment using receiver operating characteristics. Brain and Cognition, 69(3), 504–13. 10.1016/j.bandc.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, & Budson AE (2009b). The picture superiority effect in patients with Alzheimer’s disease and mild cognitive impairment. Neuropsychologia, 47(2), 595–8. 10.1016/j.neuropsychologia.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, McKeever JD, Waring JD, & Budson AE (2009). Preserved frontal memorial processing for pictures in patients with mild cognitive impairment. Neuropsychologia, 47(10), 2044–2055. 10.1016/j.neuropsychologia.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, & Thompson PM (2006). 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer’s disease. Brain, 129(11), 2867–2873. 10.1093/brain/awl274 [DOI] [PubMed] [Google Scholar]
- Backman L, & Lipinska B (1993). Monitoring of general knowledge: Evidence for preservation in early Alzheimer’s disease. Neuropsychologia, 31(4), 335–345. [DOI] [PubMed] [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, McDermott KB, & Yerys BE (1999). Veridical and false memories in healthy older adults and in dementia of the Alzheimer’s type. Cognitive Neuropsychology, 16(3–5), 361–384. 10.1080/026432999380834 [DOI] [Google Scholar]
- Braskie MN, Toga AW, & Thompson PM (2013). Recent advances in imaging Alzheimer's disease. Journal of Alzheimer's Disease, 33, 313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Daffner KR, Desikan R, & Schacter DL (2000). When false recognition is unopposed by true recognition: Gist-based memory distortion in Alzheimer’s disease. Neuropsychology, 14(2), 277–287. 10.1037//0894-4105.14.2.277 [DOI] [PubMed] [Google Scholar]
- Budson AE, Sitarski J, Daffner KR, & Schacter DL (2002). False recognition of pictures versus words in Alzheimer’s disease: The distinctiveness heuristic. Neuropsychology, 16(2), 163–173. 10.1037//0894-4105.16.2.163 [DOI] [PubMed] [Google Scholar]
- Budson AE, Simons JS, Sullivan AL, Beier JS, Solomon PR, Scinto LF, Daffner KR, & Schacter DL (2004). Memory and emotions for the September 11, 2001 terrorist attacks in patients with Alzheimer's disease, patients with mild cognitive impairment, and healthy older adults. Neuropsychology, 18, 315–327. [DOI] [PubMed] [Google Scholar]
- Budson AE, Dodson CS, Daffner KR, & Schacter DL (2005). Metacognition and false recognition in Alzheimer’s disease: further exploration of the distinctiveness heuristic. Neuropsychology, 19(2), 253–8. http://doi.Org/10.1037/0894-4105.19.2.253 [DOI] [PubMed] [Google Scholar]
- Budson AE, Simons JS, Waring JD, Sullivan AL, Hussoin T, & Schacter DL (2007). Memory for the September 11, 2001 terrorist attachks one year later in patients with Alzheimer's disease, patients with mild cognitive impairment, and healthy older adults. Cortex, 43, 875–888. [DOI] [PubMed] [Google Scholar]
- Carter SF, Caine D, Burns A, Herholz K, & Lambon Ralph MA (2012). Staging of the cognitive decline in Alzheimer’s disease: insights from a detailed neuropsychological investigation of mild cognitive impairment and mild Alzheimer’s disease. International Journal of Geriatric Psychiatry, 27, 423–432. [DOI] [PubMed] [Google Scholar]
- Curran T, Schacter DL, Norman KA, & Galluccio L (1997). False recognition after a right frontal lobe infarction: memory for general and specific information. Neuropsychologia, 35(7), 1035–49. 10.1016/S0028-3932(97)00029-8 [DOI] [PubMed] [Google Scholar]
- Dalla Barba G, Nedjam Z, & Dubois B (1999). Confabulation, executive functions, and source memory in Alzheimer’s disease. Cognitive Neuropsychology, 16(3–5), 385–398. 10.1080/026432999380843 [DOI] [Google Scholar]
- Deason RG, Hussey EP, Budson AE, & Ally B. a. (2012). Gist-based conceptual processing of pictures remains intact in patients with amnestic mild cognitive impairment. Neuropsychology, 26, 202–208. 10.1037/a0026958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deason RG, Hussey EP, Flannery S, & Ally BA (2015). Preserved conceptual implicit memory for pictures in patients with Alzheimer’s disease. Brain and Cognition, 99, 112–117. 10.1016/j.bandc.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson CS, & Schacter DL (2002). When False Recognition Meets Metacognition: The Distinctiveness Heuristic. Journal of Memory and Language, 46(4), 782–803. 10.1006/jmla.2001.2822 [DOI] [Google Scholar]
- El Haj M, Fasotti L, & Allain P (2014). Directed forgetting of source memory in normal aging and Alzheimer’s disease. Aging Clinical and Experimental Research, 27(3), 329–336. article. 10.1007/s40520-014-0276-1 [DOI] [PubMed] [Google Scholar]
- Embree LM, Budson AE, & Ally BA (2012). Memorial familiarity remains intact for pictures but not for words in patients with amnestic mild cognitive impairment. Neuropsychologia, 50(9), 2333–2340. 10.1016/j.neuropsychologia.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). Mini-Mental State - Practical Method for Grading Cognitive State of Patients for Clinicians. Journal of Psychiatric Research, 12(3), 189–198. article. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gallo DA, Chen JM, Wiseman AL, Schacter DL, & Budson AE (2007). Retrieval monitoring and anosognosia in Alzheimer’s disease. Neuropsychology, 21(5), 559–68. 10.1037/0894-4105.21.5.559 [DOI] [PubMed] [Google Scholar]
- Gallo DA, Cotel SC, Moore CD, & Schacter DL (2007). Aging can spare recollection-based retrieval monitoring: The importance of event distinctiveness. Psychology and Aging, 22(1), 209–213. 10.1037/0882-7974.22.L209 [DOI] [PubMed] [Google Scholar]
- Gallo DA, Cramer SJ, Wong JT, & Bennett DA (2012). Alzheimer’s disease can spare local metacognition despite global anosognosia: Revisiting the confidence–accuracy relationship in episodic memory. Neuropsychologia, 50(9), 2356–2364. 10.1016/j.neuropsychologia.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA, Mcdonough IM, & Scimeca J (2009). Dissociating Source Memory Decisions in the Prefrontal Cortex : fMRI of Diagnostic and Disqualifying Monitoring, 955–969. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Shahid KR, Olson MA, Solomon TM, Schacter DL, & Budson AE (2006). Overdependence on degraded gist memory in Alzheimer’s disease. Neuropsychology, 20(6), 625–632. 10.1037/0894-4105.20.6.625 [DOI] [PubMed] [Google Scholar]
- Gallo DA, Sullivan AL, Daffner KR, Schacter DL, & Budson AE (2004). Associative recognition in Alzheimer’s disease: evidence for impaired recall-to-reject. Neuropsychology, 18(3), 556–63. 10.1037/0894-4105.18.3.556 [DOI] [PubMed] [Google Scholar]
- Gallo DA, Weiss JA, & Schacter DL (2004). Reducing false recognition with criterial recollection tests: Distinctiveness heuristic versus criterion shifts. Journal of Memory and Language, 51(3), 473–493. 10.1016/j.jml.2004.06.002 [DOI] [Google Scholar]
- Huff MJ, & Bodner GE (2013). When does memory monitoring succeed versus fail? Comparing item-specific and relational encoding in the DRM paradigm. Journal of Experimental Psychology. Learning, Memory, and Cognition, 39(4), 1246–56. 10.1037/a0031338 [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, & Lindsay DS (1993). Source monitoring. Psychological Bulletin, 114(1), 3–28. article. 10.1037//0033-2909.114.1.3 [DOI] [PubMed] [Google Scholar]
- Kučera H, & Francis WN (1967). Computational analysis of present-day American English. book, Dartmouth Publishing Group. [Google Scholar]
- Mack WJ, Freed DM, Williams BW, & Henderson VW (1992). Boston Naming Test: Shortened Versions for Use in Alzheimer’s Disease. Journal of Gerontology, 47(3), P154–P158. 10.1093/geronj/47.3.P154 [DOI] [PubMed] [Google Scholar]
- McCabe DP, & Smith AD (2002). The effect of warnings on false memories in young and older adults. Memory & Cognition, 30(7), 1065–1077. 10.3758/BF03194324 [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, & Johnson MK (2009). Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin, 135(4), 638–77. 10.1037/a0015849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, & Thal LJ (1992). Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology, 49(12), 1253–1258. 10.1001/archneur.1992.00530360051017 [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G,… Clark C (1989). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology, 39(9), 1159–65. article. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2771064 [DOI] [PubMed] [Google Scholar]
- Moulin CJ, Perfect TJ, & Jones RW (2000). Evidence for intact memory monitoring in Alzheimer’s disease: metamemory sensitivity at encoding. Neuropsychologia, 38(9), 1242–1250. 10.1016/S0028-3932(00)00037-3 [DOI] [PubMed] [Google Scholar]
- Multhaup KS, & Balota DA (1997). Generation effects and source memory in healthy older adults and in adults with dementia of the Alzheimer type. Neuropsychology, 11(3), 382–391. 10.1037/0894-4105.11.3.382 [DOI] [PubMed] [Google Scholar]
- O’Connor MK, Deason RG, Reynolds E, Tat MJ, Flannery S, Solomon PR,… Budson AE (2015). The imagination inflation effect in healthy older adults and patients with mild Alzheimer’s disease. Neuropsychology, 29(4), 550–560. 10.1037/neu0000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ, Bindschaedler C, Harsent L, & Metzler C (1996). Pathological False Alarm Rates Following Damage to the Left Frontal Cortex. Brain and Cognition, 32(1), 14–27. 10.1006/brcg.1996.0055 [DOI] [PubMed] [Google Scholar]
- Pierce BH, Waring JD, Schacter DL, & Budson AE (2008). Effects of Distinctive Encoding on Source-based False Recognition and Alzheimer Disease, 21(3), 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Heller AS, & Wilding EL (2007). Dissociable correlates of two classes of retrieval processing in prefrontal cortex. NeuroImage, 35(4), 1663–1673. 10.1016/j.neuroimage.2007.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa NM, Deason RG, Budson AE, & Gutchess AH (2015). Self-referencing and false memory in mild cognitive impairment due to Alzheimer’s disease. Neuropsychology, 29(5), 799–805. 10.1037/neu0000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa NM, Deason RG, Budson AE, & Gutchess AH (2016). Source Memory for Self and Other in Patients With Mild Cognitive Impairment due to Alzheimer’s Disease. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 71(1), 59–65. 10.1093/geronb/gbu062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, & Fox NC (2002). Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proceedings of the National Academy of Sciences, 99(7), 4703–4707. 10.1073/pnas.052587399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Israel L, & Racine C (1999). Suppressing False Recognition in Younger and Older Adults: The Distinctiveness Heuristic. Journal of Memory and Language, 40(1), 1–24. 10.1006/jmla.1998.2611 [DOI] [Google Scholar]
- Schmitter-Edgecombe M, & Seelye AM (2011). Predictions of verbal episodic memory in persons with Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology, 33(2), 218–225. 10.1080/13803395.2010.507184 [DOI] [PubMed] [Google Scholar]
- Schmitz R, Dehon H, & Peigneux P (2013). Lateralized processing of false memories and pseudoneglect in aging. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 49(5), 1314–24. http://doi.Org/10.1016/j.cortex.2012.06.005 [DOI] [PubMed] [Google Scholar]
- Snodgrass J, & Corwin J (1988). Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General, 117, 34–50. [DOI] [PubMed] [Google Scholar]
- Tat MJ, Soonsawat A, Nagle CB, Deason RG, O’Connor MK, & Budson AE (2016). The influence of strategic encoding on false memory in patients with mild cognitive impairment and Alzheimer’s disease dementia. Brain and Cognition, 109, 50–58. 10.1016/j.bandc.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AK, & McDaniel MA (2013). The interaction between frontal functioning and encoding processes in reducing false memories. Aging, Neuropsychology, and Cognition, 20(4), 443–470. 10.1080/13825585.2012.736468 [DOI] [PubMed] [Google Scholar]
- Van Overschelde JP, Rawson KA, & Dunlosky J (2004). Category norms: An updated and expanded version of the Battig and Montague (1969) norms. Journal of Memory and Language, 50(3), 289–335. 10.1016/j.jml.2003.10.003 [DOI] [Google Scholar]
- Watson JM, Mcdermott KB, & Balota DA (2004). Attempting to avoid false memories in the Deese/Roediger—McDermott paradigm: Assessing the combined influence of practice and warnings in young and old adults. Memory & Cognition, 32(1), 135–141. 10.3758/BF03195826 [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam M-M, Holdstock JS, Mayes AR, & Reber PJ (2006). When memory does not fail: familiarity-based recognition in mild cognitive impairment and Alzheimer’s disease. Neuropsychology, 20(2), 193–205. 10.1037/0894-4105.20.2.193 [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR (2007). 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 130, 1777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL (2010). Progression of atrophy in Alzheimer's disease and related disorders. Neurotoxicology Research, 18, 339–46. http://doi: 10.1007/s12640-010-9175-1 [DOI] [PubMed] [Google Scholar]
- Wilding EL, & Rugg MD (1996). An event-related potential study of recognition memory with and without retrieval of source. Brain, 119(3), 889–905. 10.1093/brain/119.3.889 [DOI] [PubMed] [Google Scholar]
- Wolk DA, Gold CA, Signoff ED, & Budson AE (2009). Discrimination and reliance on conceptual fluency cues are inversely related in patients with mild Alzheimer’s disease. Neuropsychologia, 47(8–9), 1865–72. 10.1016/j.neuropsychologia.2009.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP (1994). Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20(6), 1341–1354. 10.1037/0278-7393.20.6.1341 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP (2002). The Nature of Recollection and Familiarity: A Review of 30 Years of Research. Journal of Memory and Language, 46(3), 441–517. 10.1006/jmla.2002.2864 [DOI] [Google Scholar]