Abstract
Active cell death, in its many forms, is a fundamental biological process, and its study over the past several decades has provided key insights into the molecular processes, functions, and consequences responsible. Here, we pose five questions, or riddles, that may provide a guide to the next decade of cell death research. Focusing mainly on four types of active cell death (apoptosis, necroptosis, pyroptosis, and ferroptosis) and mainly in mammals, this Perspective explores the possible research directions that might answer these riddles, or at least prompt new ones.
It is a riddle wrapped in mystery inside an enigma
-Winston Churchill
My relationship with death remains the same. I am strongly against it.
-Woody Allen
Introduction
It never really came as a surprise that cells in a complex organism die; every living thing eventually dies. What was perhaps unexpected was that during development cells die in what appears to be a “programmed” manner (Ellis and Horvitz, 1986; Lockshin, 1969; Saunders, 1966). While necrotic cell death was described in the 19th century, pathologists also recognized that another form of cell death occurred during embryogenesis, in response to stress, and as an aspect of tissue homeostasis. Initially based on morphological features of the dying cells, this cell death was defined and given the name “apoptosis” in 1972 (Kerr et al., 1972). By the 1980’s, the first biochemical markers of apoptosis had emerged and mechanisms were sought.
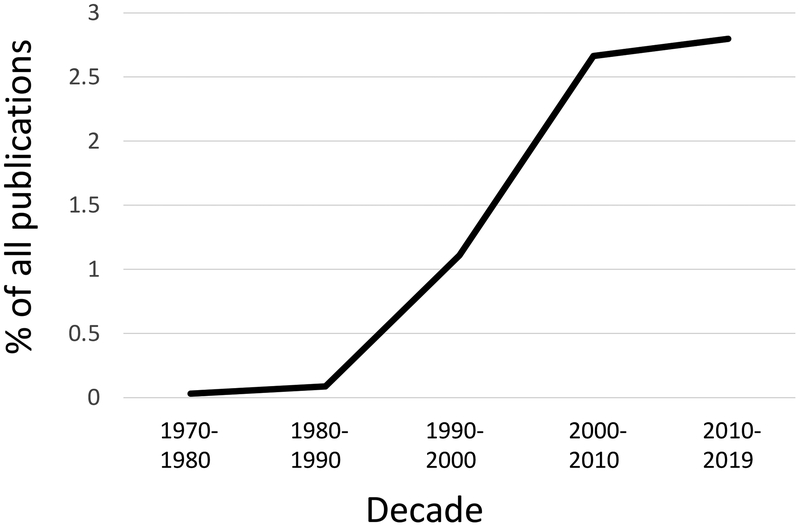
The fact that apoptosis could be induced and/or predicted to occur at defined developmental stages, and the idea that it involved active participation on the part of the dying cell, led to efforts to dissect the process genetically and biochemically. Arguably, technical advances in molecular characterization of identified genes and the success of applying such approaches to fundamental biological processes (e.g. cell cycle) led to the “renaissance” in cell death research that followed. For the twenty years spanning 1990–2010, publications on regulated cell death increased exponentially to occupy a significant part of the scientific literature, a representation that plateaued (but was sustained) over the past decade (Figure 1).
Figure 1.
Publications on cell death by decade. Results of Pubmed Searches using the terms “apoptosis, necroptosis, pyroptosis, ferroptosis” for each time period shown, divided by total Pubmed publications for that time period. The values are undoubtedly an underestimate.
As we anticipate the beginning of another decade of cell death research, it may be useful to take stock of the current state of this mature field and pose some fundamental questions with the potential to shape the advances to come. The author readily acknowledges that the queries that form the basis of the exploration before the reader are based on only one person’s opinion, and if experience is a guide, likely represent at best modest predictors of the future discoveries. That said, questions drive us: a good question is half of knowledge. Herein, are five. There are certainly more.
Before stepping into the unknown, it may be helpful to provide an abbreviated perspective on the state of our understanding of cell death. Here, we focus on “active” cell death (as opposed to “passive”) in which a cell participates via molecular pathways in its own demise. Superficially, it has been useful (at least for this author) to parse these pathways of active cell death into 1) those that involve molecular processes that appear to have evolved to promote a form of cell death (“suicide”) and 2) those that involve processes that preserve cell integrity such that when disrupted, death occurs as a consequence of the cell’s active, physiological state (“sabotage”). The ontology of sabotage is illustrative of the latter idea: removal of a railway tie (sabot) will only “kill” a train that is actively moving. For the most part, this overview focuses on three forms of suicide: apoptosis, necroptosis, and pyroptosis, and one form of cell death that appears to be by sabotage: ferroptosis. These are summarized in Box 1.
Box 1. The ways cells actively die.
The following serves as a primer, or reminder, of the four cell death pathways that form the majority of the discussions herein. For more details, the reader is referred to more extensive discussions elsewhere (Galluzzi et al., 2018; Green, 2018).
Apoptosis
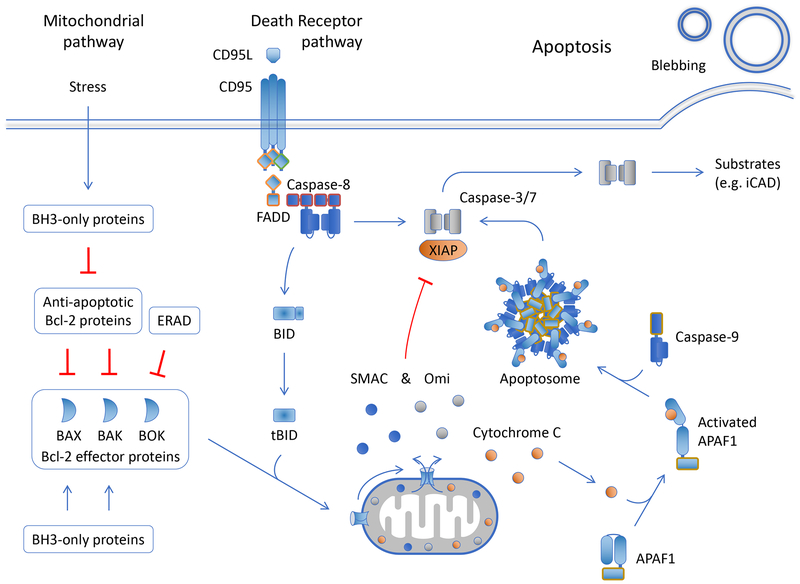
a) The mitochondrial pathway of apoptosis. The mitochondrial (or intrinsic) pathway is engaged by cellular stress or developmental cues that alter the expression and/or function of proteins of the Bcl-2 family (Figure 2). Proteins in this family share one or more Bcl-2 homology (BH) regions and function in the regulation of the integrity of the outer mitochondrial membrane in mammals and several (but not all) metazoan phyla. Three Bcl-2 effector proteins, Bax, Bak, and Bok, directly cause mitochondrial outer membrane permeabilization (MOMP), releasing proteins of the mitochondrial inter-membrane space into the cytosol. Each of these can act independently of the others in effecting MOMP. Active Bax and Bak are inhibited by the anti- apoptotic Bcl-2 proteins, including Bcl-2, Bcl-xL, Mcl-1, and Bfl/A1, among others. Bok appears to be constitutively active, regulated predominantly by degradation via the endoplasmic reticulum associated degradation (ERAD) machinery, and perhaps by other mechanisms independently of the anti-apoptotic Bcl-2 proteins. A third Bcl-2 subfamily, the BH3-only proteins, regulates the other two, inhibiting the anti-apoptotic Bcl-2 proteins and activating Bax and Bak to effect MOMP. Specificities and dynamic interactions among the Bcl-2 proteins in a cell determine whether or not MOMP occurs.
Proteins released to the cytosol upon MOMP include holocytochrome c, which binds to the cytosolic protein, APAF1, inducing the oligomerization of the latter into the apoptosome. The “hub” of the APAF1 oligomer interacts with the initiator caspase endopeptidase, caspase-9, activating it by enforced dimerization. The active caspase-9 cleaves and thereby activates the executioner caspase endopeptidases, caspase-3 and caspase-7.
Caspase-9, −3, and −7 are bound and inhibited by XIAP. Other proteins, released upon MOMP, include Smac, and Omi, which antagonize XIAP, permitting the caspases to function. The executioner caspases cleave hundreds of substrates in the cell. These include iCAD, a chaperone and inhibitor of caspase-activated DNase, and cleavage of iCAD unleashes CAD to cut inter-nucleosomal DNA. Other substrates affect the distribution of phospholipids in the plasma membrane, allowing phosphatidylserine (PS), normally constrained to the inner leaflet, to be exposed on the cell surface. PS acts as a signal to promote phagocytosis of the dying cell prior to loss of plasma membrane integrity. By cleaving substrates, the executioner caspases cause the features of apoptotic cell death, including chromatin condensation and membrane blebbing, but are not strictly required for cell death per se, as disruption of mitochondrial function upon MOMP can condemn the cell to death due to metabolic catastrophe (although such “caspase-independent cell death” does not have the features of apoptosis.
b) The death receptor pathway of apoptosis. The death receptor (or extrinsic) pathway of apoptosis is engaged when a subset of the TNF receptor family (the death receptors) are ligated. A simplified death receptor pathway exemplified by the death receptor CD95 is shown in Figure 2 (as we will see, signaling from other death receptors, such as TNFR1, can be more complex). The intracellular region of the death receptor, upon ligation, recruits the adapter molecule FADD, which in turn recruits the initiator caspase, caspase-8. Bound caspase-8 then recruits additional caspase-8 molecules, which are activated by dimerization. The active caspase-8 cleaves and thereby activates the executioner caspases-3 and −7.
If XIAP is present in the cell, this inhibits the executioner caspases (as above). Caspase-8 also cleaves and thereby activates one of the BH3-only proteins, Bid, which antagonizes the anti- apoptotic Bcl-2 proteins and activates Bax and Bak, causing MOMP. Smac and Omi, released upon MOMP, inhibit XIAP, permitting the executioner caspases to promote apoptosis.
A molecule resembling caspase-8, but lacking proteolytic activity, c-FLIP, disrupts the oligomerization of caspase-8. While the caspase-8-c-FLIP dimer is an enzymatically active protease (see below), it does not promote apoptosis. Therefore c-FLIP can prevent apoptosis by the death receptor pathway.
Caspase-8 is not only activated upon ligation of death receptors. A kinase, RIPK1, can bind to FADD to promote caspase-8 activation and apoptosis. RIPK1-dependent caspase-8 activation can occur as a result of engagement of some TLRs, via the adapter molecule TRIF and by interactions with another kinase, RIPK3. RIPK1 and RIPK3 are also involved in the complex interactions that engage another active cell death mechanism, necroptosis, outlined next.
Executioner caspases, engaged by either of the above apoptotic pathways, cleave and thereby activate a pore forming protein, Gasdermin E, thus promoting a “secondary necrosis” which occurs in apoptotic cells that express the protein. Such cells display characteristics of apoptosis (e.g., membrane blebbing, nuclear condensation) as well as features of necrosis (Rogers et al., 2017; Wang et al., 2017).
Necroptosis
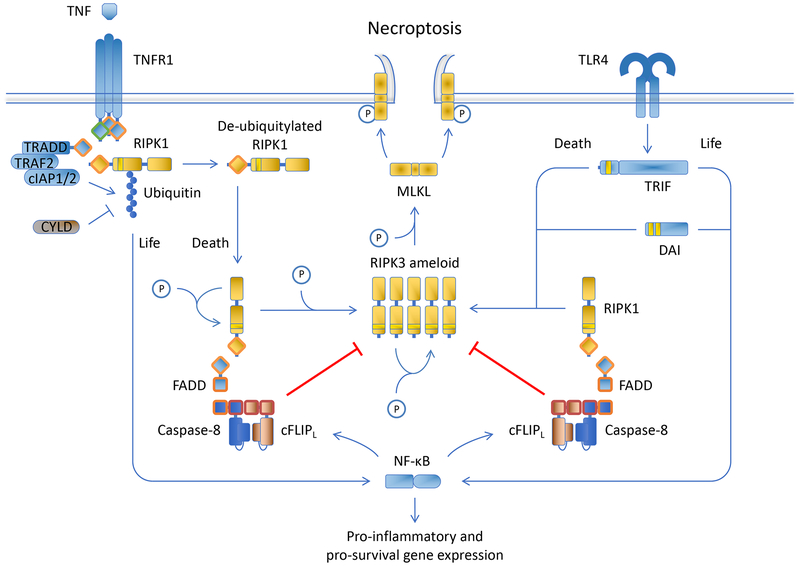
Necroptosis can be engaged by ligation of death receptors, some TLRs (via TRIF), and by an intracellular nucleic acid sensor, ZBP1 (Figure 3). Unlike apoptosis, necroptosis is a form of regulated necrosis.
Ligation of death receptors causes the recruitment of RIPK1 to the intracellular region of the death receptor. The ubiquitin ligases, cIAP1 and cIAP2 ubiquitinylate RIPK1 via non-degradative K63 linkages, and the ubiquitinylated RIPK1 acts in a kinase-independent manner to activate NF-kB via the IKK complex. NF-kB prevents death receptor-induced apoptosis, in part, by inducing the expression of c-FLIP. Deubiquitinases remove the ubiquitin chains, releasing RIPK1 when then undergoes a conformational change due to auto-phosphorylation, exposing a region (RHIM) that self-oligomerizes and recruits RIPK3 (which also contains the RHIM oligomerization domain). RIPK3 thus becomes activated, and this kinase phosphorylates and thereby activates the effector molecule, MLKL. Phosphorylated MLKL oligomerizes and targets the plasma membrane, inducing phospholipid scrambling (exposing PS) and disrupting plasma membrane integrity, resulting in necrotic cell death.
This process, however, is disrupted by the function of the FADD-caspase-8-c-FLIP complex, which as noted above is enzymatically active. The caspase-8-c-FLIP dimer cleaves RIPK1 and RIPK3, preventing necroptosis. Inhibition or disruption of caspase-8-c-FLIP proteolytic activity therefore allows necroptosis to proceed. Inhibition of RIPK1 kinase activity prevents its conformational change, and thereby blocks death receptor-induced necroptosis.
RIPK3 is also directly oligomerized and activated by TRIF (upon ligation of some TLRs) and by ZBP1, promoting necroptosis. The process proceeds somewhat in reverse of that described above, as the oligomerized RIPK3 binds RIPK1, recruiting in turn the FADD-caspase-8-c-FLIP complex, which destroys the RIPK3 oligomer. Again, necroptosis proceeds when the function of the caspase-8-c-FLIP dimer is inhibited or disrupted. In such settings, RIPK1 is required for the inhibition, but not the activation of RIPK3.
The complex of RIPK1, RIPK3, and MLKL, regardless of the pathway, is referred to as the necrosome.
Pyroptosis
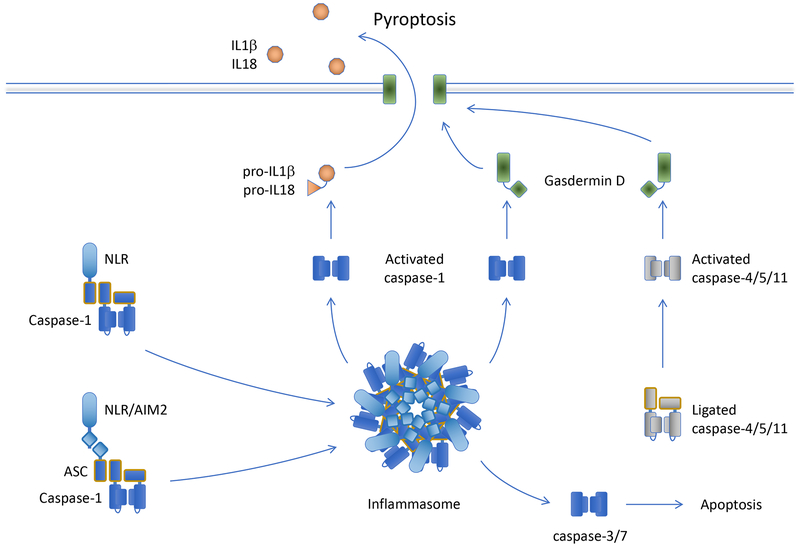
Pyroptosis is a form of regulated necrosis, although in some cases the pathway can engage apoptosis. Pyroptosis occurs when the effector molecule, Gasdermin D (GSDMD), is cleaved, promoting its oligomerization to form large pores in the plasma membrane, leading to cell death. Several caspases are capable of cleaving GSDMD to produce this effect, caspase-1, caspase-4, caspase-5, and caspase-11 (caspase-11 is only found in rodents, which lack caspase-4 and caspase-5). Caspase-4, caspase-5, and caspase-11 are bound by intracellular bacterial lipopolysaccharides and are thus directly activated via oligomerization to cleave GSDMD (Figure 4).
Caspase-1, in contrast, must be engaged by a large caspase activation platform, called the inflammasome, in order to attain its proteolytic function. There are several molecules that can form an inflammasome, but most inflammasomes include an adapter protein, ASC, that when oligomerized binds to caspase-1 and activates it by dimerization. Two types of protein function in ASC oligomerization (or in some cases, ASC-independent, direct caspase-1 binding). These include several of the intracellular NOD-like receptors (NLRs) and the cytosolic DNA sensor AIM1. NLRs that participate in inflammasome formation generally sense different intracellular PAMPs associated with infection, but in at least one case (NLRP3) can also respond to cellular damage induced by inert material such as engulfed asbestos, uric acid crystals, and calcium phosphate crystals, among others. Ligation of these sensors cause the NLRs or AIM1 to oligomerize, recruit ASC, and thereby bind and activate caspase-1 to promote pyroptosis via GSDMD. In addition, intracellular K+ ions inhibit that activation of one of the NLRs, NLRP3, and an efflux of this ion can promote activation of this NLR to form an inflammasome.
In addition to cleaving GSDMD, caspase-1 (but not other pyroptotic caspases) processes two cytokines from their inactive pro-forms to their active mature species, interleukin-1b (IL-1b) and interleukin-18 (IL-18). These lack signal sequences for secretion, and can be released through GSDMD pores or upon cell lysis.
In the absence of GSDMD, caspase-1 can cleave and thereby activate the executioner caspases, and can also cleave and activate Bid to engage MOMP. Thus, without GSDMD, apoptosis ensues upon caspase-1 activation.
Ferroptosis
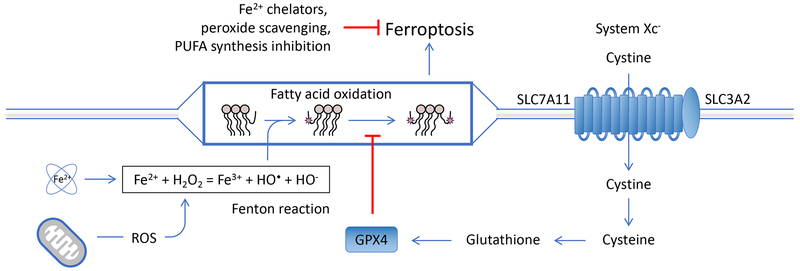
Free intracellular iron atoms react with hydrogen peroxide (e.g., produced by the action of mitochondrial superoxide dismutatase on superoxide radicals in the mitochondria) in the Fenton reaction, which can result in the peroxidation of poly-unsaturated fatty acids (PUFA) (Figure 5). These oxidized lipids produce more oxidized lipids and other toxic products in the presence of oxygen, unless they are neutralized by the action of the only cellular lipid peroxidase, GPX4. The recycling of GPX4 to mediate this protective process requires glutathione, which in turn requires NADPH to recycle. Glutathione is synthesized from cysteine, which is transported into the cell through the system Xc− transporter. Cysteine deprivation, inhibition of the system Xc− transporter, reduction in NADPH, or inhibition of GPX4 therefore promote lipid peroxidation in cells with available free iron, hydrogen peroxide generation, and PUFA. Unconstrained lipid peroxidation results in ferroptosis, a regulated necrosis. Sequestration of iron, scavenging of lipid peroxides and/or hydrogen peroxide, or inhibition of PUFA synthesis can protect cells from ferroptosis.
While other distinct forms of cell death exist, some of which are mentioned in the final section, our focus on the subset listed above will help to constrain our discussion and allow comparisons between them. Further, and again for the most part, these questions are addressed in the context of cell death processes that occur in mammals. While many of the mechanisms are conserved throughout the animal kingdom (and sometimes beyond), differences in the “wiring” of these pathways in other organisms can confuse our considerations and are (largely) avoided herein.
Riddle #1: How deadly is death?
How final is engagement of active, cell death pathways, and are there consequences for survival after such engagement? Where is the “point-of-no-return” of a cell death process, after which, to quote the Bard, “no traveler returns?” To what extent can a dying cell be “saved?”
In the three forms of regulated necrosis considered here (necroptosis, pyroptosis, and ferroptosis), cell death occurs when the relevant effector mechanisms damage the integrity of the plasma membrane beyond repair. It follows, then, that such repair may antagonize the cell death process. In ferroptosis, lethality occurs as a result of peroxidation of poly-unsaturated fatty acids (PUFA) that self-propagates unless halted by the lipid peroxidase, GPX4 (Stockwell et al., 2017). Indeed, ferroptosis only occurs when the function of GPX4, sustained by glutathione, is perturbed, and no other mechanism is known to prevent this death process once initiated (although it is possible that diminished GPX4 activity can be sufficient if upstream hydrogen peroxide generation and/or PUFA synthesis is constrained).
The other two forms of regulated necrosis, pyroptosis and necroptosis, are effected by engagement of pore-forming proteins, Gasdermin D (GSDMD) and MLKL, respectively, that disrupt the plasma membrane (see Box 1). Components of the Endosomal Sorting Complexes Required for Transport (ESCRT), especially those of ESCRT III, function in the repair of laser- or bacterial toxin-induced plasma membrane damage (Jimenez et al., 2014; Scheffer et al., 2014) and this repair is triggered by an influx of Ca++ at the site of damage (Scheffer et al., 2014). ESCRT III, along with other ESCRT components similarly antagonize the necrotic activity of active MLKL (Gong et al., 2017b) and GSDMD (Ruhl et al., 2018) to sustain cell integrity upon engagement of the cell death pathways, and this repair process again appears to be dependent upon influx of Ca++ (Gong et al., 2017b). As a result, ESCRT-mediated plasma membrane repair can preserve cell survival when the activity of the effectors is sufficiently low (Gong et al., 2017b; Ruhl et al., 2018), or the engaged pathway is disrupted prior to lysis (Gong et al., 2017b).
Executioner caspases can induce necrosis upon cleavage of Gasdermin E (also called DFNA5), widely expressed in epithelial and other cell types (Rogers et al., 2017; Wang et al., 2017). It is unknown if ESCRT components similarly repair pores formed by the activated molecule, although this would appear likely. In this case, antagonizing necrosis might be expected to promote conventional caspase-dependent apoptosis in these dying cells.
Signaling events upstream of terminal effector activation in both necroptosis and pyroptosis can result in expression or processing of cytokines and chemokines within the cell, and sustained viability by ESCRT can either antagonize (pyroptosis) or enhance (necroptosis) the release of such mediators. As an example of the latter, engagement of RIPK1 upon induction of necroptosis can activate NF-kB to drive gene expression, and the resulting mediators are important for promoting appropriate antigen presentation by dendritic cells for anti-cancer immunity (Yatim et al., 2015). Disruption of the ESCRT machinery accelerates necroptotic death, reducing the production of such mediators, and thereby compromises the presentation of cell-associated antigens to T cells (Gong et al., 2017b).
In pyroptosis, processed IL1b and IL18, as well as other cellular components are released by the action of GSDMD at the plasma membrane (Kayagaki et al., 2015; Shi et al., 2014), either by diffusion through GSDMD pores (Evavold et al., 2018) or by subsequent lysis of the cell (Shi et al., 2015). The function of ESCRT III to remove the GSDMD pores and preserve plasma membrane integrity thus limits this release (Ruhl et al., 2018).
The plasma membrane damage in necroptosis may itself generate signals that result in elaboration of secreted mediators. In murine cells, activation by dimerization of an N-terminal fragment of human MLKL that is unlikely to interact with other signaling molecules causes plasma membrane damage and induces the expression of CXCL1 and CXCL10 chemokines. The production of these chemokines is curtailed when ESCRT III is disrupted to promote plasma membrane lysis (Gong et al., 2017b). It is possible that MLKL-mediated membrane damage elicits a “plasma membrane stress response” that engages a gene expression signature (that includes these chemokines), the effects of which depend on the delay in lysis effected by ESCRT III (Gong et al., 2017a).
When considering the point of no return in apoptosis, the destruction of the mitochondrial outer membrane upon mitochondrial outer membrane permeabilization (MOMP) can commit a cell to die as a consequence of a metabolic catastrophe even if caspases are not engaged (Lartigue et al., 2009). Such death can be averted after MOMP if glycolysis is enforced and autophagic removal of damaged mitochondria allows expansion of the remaining, intact organelles (Colell et al., 2007; Tait et al., 2010). However, emerging evidence has revealed that limited caspase activation invoked by engagement of death receptors (Lovric and Hawkins, 2010) or when a minority of mitochondria undergo MOMP (Ichim et al., 2015) can result in induction of the caspase-activated DNase (CAD) and DNA damage. As a result, cells that survive the engagement of the apoptotic machinery display increased genomic instability that is dependent on both caspases and CAD activity.
Cell survival despite executioner caspase activation has been documented in several scenarios, a phenomenon that has been named “anastasis” (Ding et al., 2016; Sun et al., 2017; Tang et al., 2015), and may permit other described effects of activated executioner caspases in living cells (Nakajima and Kuranaga, 2017). An example of the latter is the putative role for active caspase- 3 in the pruning of dendritic spines in the brain (Erturk et al., 2014). Changes in gene expression in cells surviving due to anastasis may reflect the effects of the specific stressor used to induce apoptosis, or perhaps by the survival process itself. Intriguingly, cells that survive activation of MLKL in necroptosis, a process called resuscitation (Gong et al., 2017b), similarly display gene expression changes that overlap those of anastasis (Gong et al., 2017a). It is tempting to speculate that these common changes may include a general expression signature of cell survival despite induction of different core death pathways.
We do not know to what extent anastasis in apoptosis or resuscitation in necroptosis occurs in natural or therapeutic situations in mammals in vivo. In Drosophila, a probe for caspase activity suggests that anastasis may be extensive, with a majority of cells engaging caspase activation during development without coincident cell death (Ding et al., 2016; Tang et al., 2015). With respect to resuscitation, kidneys from live human donors display evidence of activated, phospho-MLKL in endothelial cells following, but not prior to transplantation, without any sign of necroptosis in the healthy tissue (Gong et al., 2017b). Intriguingly, this is accompanied by induction of the expression of ESCRT components, important for preserving survival despite MLKL activation. This expression might occur as a consequence of HIF1 activation by hypoxia during the transplant (Gong et al., 2017a).
Ultimately, an analysis of cell survival despite engagement of cell death pathways will require the use of systems that “mark” cells that have withstood the cell death process. One approach would be the development of sensors that activate CRE to remove a lox-stop-lox and permit expression of a fluorescent protein. Such sensors can be readily envisioned for executioner and inflammatory caspases, and perhaps for activated RIPK3 (although activated MLKL may prove more challenging).
Riddle #2. When is a toxin not toxic?
When a clonal population of cells are faced with a death-inducing stimulus, why do some cells survive when their clonemates die, and are there consequences for this state of survival? A failure to kill less than 100% of cells that are exposed to a death inducer is almost always viewed as the relative susceptibility to undergo cell death, and not the ability of some cells to withstand the death inducing signal.
It is axiomatic that if a mutation affecting expression or function of a gene can render a cell resistant to a death-inducing treatment, such mutations will be positively selected upon application of that treatment. This idea underlies much of the thinking about relapse in cancer. More recently, however, we have come to realize that cells can survive a toxic treatment, such as a chemotherapeutic agent, by attaining a transient state called “persistence” without mutation. The surviving cells are “persisters or “persister cells”. In contrast to the positive selection of resistance mutations, progeny of such persisters are as sensitive to the stimulus as were the original cells (Hangauer et al., 2017; Viswanathan et al., 2017). One way to think about this is the concept of the lethal dose (LD) at which some proportion of a clone of cells die, e.g., LD50, at which 50% die. This simple concept has important implications beyond cell biology; indeed, survival of individuals at intermediate LD of infectious microbes has revealed fundamental host-microbe interactions (Sanchez et al., 2018). Applying this to cells may similarly reveal novel mechanisms at the cellular level; we understand a great deal about the way in which cells die, but relatively little about those that survive. What are the stochastic differences between otherwise identical cells that define the persistent state, and is this state induced or does it exist prior to treatment?
Persistence is likely to be distinct from the processes outlined above for the first riddle. While cells that engage a cell death pathway and survive (with subsequent consequences) certainly persist, there is no evidence that persistence, per se, involves engagement of core cell death processes. Instead, the “decision” to persist appears to occur upstream of such core processes. As an example, the induction of p53 by DNA damage can elicit the expression of molecules (such as BH3-only proteins, see Box 1) that promote MOMP and apoptosis, but p53 also induces cell cycle arrest and DNA repair that can preserve cell survival. Elegant single cell dynamic studies have shown that these contrasting effects of p53, that control a decision point upstream of the cell death pathway, are predicted by the nature of p53 protein stability. Cells that display oscillating p53 protein expression repair and survive, while those with persistent, elevated p53 engage apoptosis (Paek et al., 2016). While informative, these observations do not themselves explain persistence; why some but not other cells in a population display a particular pattern of p53 stability remains unclear. Further, p53-deficient cells nevertheless display the persistence phenomenon.
Other stresses that can induce apoptosis also engage stress responses that can antagonize cell death, perhaps best illustrated by the unfolded protein response (UPR) during endoplasmic reticulum-induced stress (Hetz and Papa, 2018) Many cells function at the limit of their capacity for secretion, and a wide variety of conditions can cause dysfunction in this process, eliciting a UPR. These include but are not limited to nutrient deprivation, hypoxia, generation of reactive oxygen species, and disruption of calcium homeostasis. The UPR engages three distinct pathways affecting transcription of genes involved in resolving the stress, but also genes that promote apoptosis, such that cells that do not manage the stress ultimately die. Those cells that survive thus display a transient resistance to treatments that induce a UPR. While responses such as the UPR may help to explain persistence under some circumstances, it again remains unclear why some cells but not others succeed in resolving the stress.
Persistence has been studied in some detail in the apoptotic response to the death receptor, TRAIL, at the single cell level (Flusberg et al., 2013; Spencer et al., 2009). It appears that a threshold of caspase-8 activation exists such that cells that do not achieve this threshold survive and display a transient resistance to subsequent treatment with the ligand, or with ligands for other death receptors, such as Fas/CD95. Whether this persistent state is stochastic (that is, produced as a consequence of pre-existing levels of the relevant pathway molecules) or is induced by signals elicited by the pathway is not fully resolved. However, evidence in support of the latter exists, in that this persistence can be sustained by periodic engagement of the death receptor in the presence of caspase inhibitors, and that the persistent cell state includes a signature of NF-kB-induced inflammation (Flusberg et al., 2013). It is possible that a scaffolding function for caspase-8, independent of its catalytic activity, participates in the generation of the persistent state (Henry and Martin, 2017), although experiments to test this (which would require inducible expression of the caspase) have not been described to date.
Some of the best evidence supporting the idea of a general persistent state comes from studies of emergent sensitivities of cells that survive treatments with chemotherapeutic agents (Hangauer et al., 2017; Viswanathan et al., 2017). A variety of clonal cell lines were treated with a number of different agents and surviving cells were expanded. Remarkably, in nearly every case the persistent cells were now sensitive to cell death induction by an inhibitor of GPX4, the lipid peroxidase that antagonizes ferroptosis. In contrast, the parental lines were usually resistant to GPX4 inhibition, and importantly, treatment with the GPX4 inhibitor to remove sensitive cells prior to treatment with the chemotherapeutic agent did not influence the numbers of persisters, nor their emergent dependence on GPX4 (Hangauer et al., 2017; Viswanathan et al., 2017). These results strongly suggest that persistence is not selection of cells with a pre-existing (if transient) state of resistance, but instead, is acquired in some cells in response to the stressor that engages cell death mechanisms in their non-persistent clone mates. Further, there is something about the persistent state that creates a dependence on GPX4.
Ferroptosis that occurs upon GPX4 inhibition or glutathione depletion requires iron, hydrogen peroxide (which together produce superoxide as a consequence of the Fenton reaction) and polyunsaturated fatty acids (PUFA), the targets of lipid peroxidation (Dixon et al., 2012). It is likely that the hydrogen peroxide that fuels ferroptosis is produced by mitochondria (Krainz et al., 2016) Therefore, sensitivity to GPX4 inhibition in persisters may be due to changes in metabolism (mitochondrial and/or PUFA production), induced by pro-apoptotic agents, that impart a survival advantage for these cells that achieve such changes. Alternatively, the dependence on GPX4 in persisters may arise as a by-product of the persistent state, e.g., as a result of one or more transcription factors that drive both transient resistance to cell death and the metabolic state that creates GPX4 dependence. As another example, the induction of autophagy by cellular stress improves cell survival, and can also sensitize cells to ferroptosis via the degradation of ferritin (Hou et al., 2016). If persister cells sustain a state of autophagic flux, this might explain the emergent dependence on GPX4.
The importance of persistence as a phenomenon depends on several factors. As noted above, we often assume that selection of pre-existing cells harboring mutations that render them resistant to a therapeutic treatment is the mechanism of relapse in tumor therapy. However, it is possible that transient persistence provides a population of surviving cells in which such mutations may arise following treatment, especially if the treatment promotes mutagenesis. The synergistic effects of combined chemotherapy and GPX4 inhibition in in vivo cancer models (Hangauer et al., 2017) may reflect this kind of response. Careful studies of numbers of resistant clones prior to and following treatments (essentially the “Luria Delbruck experiment” applied to tumor cells) may help to determine if and when persistence provides an opportunity for resistance mutations to arise. An understanding of persistence and its link to GPX4 dependence may therefore have profound implications for cancer therapy.
Riddle #3. How dispensable is something that is essential?
Many papers and reviews assert that cell death, especially apoptosis, is crucial for development and tissue homeostasis. A superficial search on Google Scholar provides over 50 papers with the phrase “apoptosis is essential for development,” and over 3500 that include “apoptosis is essential.” It is indisputable that apoptosis and other forms of cell death occur in metazoan development, and indeed, apoptosis is required for a specific event in Drosophila development (White et al., 1994). In nematodes, “normal development” requires apoptosis, in that without it, extra cells appear, but animals nevertheless mature (Ellis and Horvitz, 1986). In mammals, defective apoptosis is often lethal to embryonic development. But is it essential?
Animals lacking components of the mitochondrial pathway of apoptosis, including APAF1, caspase-9, caspase-3, or carrying a mutation in cytochrome c that permits electron transport but not efficient APAF1 activation, frequently die during embryogenesis, displaying forebrain outgrowth and excess neurons. This would therefore appear to be a clear case where apoptosis is essential to remove cells in development. However, upon closer inspection, this conclusion is suspect. Properly timed closure of the neural tube arrests proliferation of some neurons, and a delay in timing or efficiency of this closure by disruption of rapid apoptotic cell death allows this proliferation to continue, producing the observed effects (Yamaguchi et al., 2011). In some genetic backgrounds, such disruption of mitochondrial apoptosis has, at best, relatively mild effects in development (Leonard et al., 2002).
Recent studies have raised additional issues. While animals lacking the mitochondrial pathway of apoptosis, owing to the ablation of the MOMP effectors Bax, Bak, and Bok (see Box 1), usually fail to survive embryogenesis (due to a failure in neural tube closure and multiple midline defects) or early life post-birth (due to cleft palate defects), a small number survive to adulthood (Ke et al., 2018). These animals, while displaying excessive accumulation of lymphocytes and other cells, nevertheless appear to have mostly normal tissue and organ architecture in many tissues previously thought to depend on apoptosis for development. No compensation by other forms of cell death (such as necroptosis or pyroptosis) were observed.
Animals lacking caspase-8 or its adapter FADD die in early embryogenesis, an effect that is dependent on RIPK3 and the necroptosis effector, MLKL (Weinlich et al., 2017). Thus, caspase- 8- or FADD-deficient animals that also lack either RIPK3 or MLKL develop and mature at Mendelian frequencies but eventually succumb to the expansion of an unusual T cell population and autoimmunity (Autoimmune Lymphoproliferative Syndrome). These animals are deficient in all caspase-8-dependent apoptotic pathways, such as the death receptor pathways. Therefore, while apoptosis is undoubtedly important for the normal, efficient development of many mammalian tissues, it is not universally essential for development or homeostasis.
One prominent idea is that while necrosis induces inflammation, apoptosis (and perhaps other regulated cell death modes) evolved as a strategy to prevent inflammatory responses to cells that die as a consequence of developmental or homeostatic cues (Kearney and Martin, 2017; Kerr et al., 1972; Martin et al., 2012). Thus, complex organisms control inflammation by controlling the mode of cell death. While attractive in many ways (and discussed in more detail in Riddle #4), there may be a problem with this idea. Compelling evidence exists that a functional death receptor pathway of apoptosis arose at least as early as the common progenitor of the cnidaria (corals) and the chordates (such as ourselves) (Quistad et al., 2014). Similarly, a functional mitochondrial pathway of apoptosis is shared by the platyhelminths (planaria) (Bender et al., 2012). While molecules that function in apoptotic pathways are found throughout the animal phyla, these studies provide evidence that they function in highly conserved ways to promote apoptosis in animals that do not have (as far as we know) inflammatory cell responses. Of course, it remains possible that such responses exist and are elicited by other modes of cell death (such as necrosis) in such organisms, compelling evidence is lacking.
What, then, is cell death “for?” Or more succinctly, when is cell suicide essential? From an evolutionary standpoint, active cell suicide, even at the level of single-celled organisms, is a stable strategy when it operates to restrict the spread of obligate intracellular parasites to genetically related cells (James and Green, 2002). In contrast, “altruistic suicide,” say in response to limiting nutrients, is prone to defector strategies that select for suicide-resistant individuals (and hence, not stable).
That regulated cell suicide can be essential for combatting infection seems to be especially clear in the case of pyroptosis. Mice lacking caspase-1 and caspase-11, and thus lacking all pyroptotic inflammasomes, are sensitive to a variety of bacterial and viral infections, as are animals lacking specific pyroptosis-inducing sensors such as NLRs or AIM2 (Xia et al., 2018).
Indeed, caspase-11 in rodents, and caspases-4 and −5 in other mammals, including humans, are direct sensors of bacterial lipopolysaccharides (Shi et al., 2014).
In keeping with this role, caspase-11 is widely expressed in murine cells. In contrast, the expression of caspase-1 is more restricted. Many invertebrates have large numbers of caspases (e.g., 31 have been annotated in sea urchins) and it is possible that many of these serve as direct sensors of intracellular pathogens.
But is it the cell death, itself, that controls infection? Caspase-1 processes IL1b and IL18 to promote immune responses, and the release of these and other mediators depends on the activation and function of GSDMD (which is also required for pyroptosis) (Kayagaki et al., 2015; Shi et al., 2014). Animals lacking only caspase-11 are more sensitive to some bacterial infections (Jorgensen et al., 2017), but caspase-11-induced GSDMD activity can also promote caspase-1 activation via the NLRP3 inflammasome, likely due to K+ efflux (Ruhl and Broz, 2015), and the extent to which this mechanism of caspase-1 activation participates in innate defense is unexplored. At this stage, we simply do not know to what extent pyroptotic death, itself, restricts infection. To conclude this, it would require identification of microbes that show increased virulence in GSDMD-deficient mice, but not those lacking IL-1b and IL18.
Necroptosis is also thought to function predominantly in the control of infection, an idea supported by the phyletic distribution of necrosome components among the vertebrates (Dondelinger et al., 2016). Animals lacking RIPK3 (and hence, necroptosis) often show enhanced sensitivity to some viral infections, but at least in some cases, this is independent of its role in activating MLKL to promote cell death (Daniels et al., 2017; Moriwaki and Chan, 2017). As RIPK3 can trigger both necroptosis and apoptosis (via caspase-8), studies have sought to identify infections with enhanced virulence in animals lacking caspase-8 (or FADD) and MLKL to ablate both cell death pathways. While at least one example of this exists, involving infection with an influenza strain (Nogusa et al., 2016), more extensive studies are needed to explore the roles of these cell death pathways in infection. In addition to its roles in cell death, RIPK3 can also promote inflammation, and in at least one example (West Nile Virus) it is this function of RIPK3 that appears to be important in controlling infection (Daniels et al., 2017).
An alternative way to probe the role of cell death in infection is to examine molecules produced by microbes that function to block cell death pathways. Viral and bacterial proteins that interfere with caspase-1 activation or function are described (Man et al., 2017), but as noted above, it is difficult to conclude that these evolved to limit cell death, as opposed to other important functions of the inflammasome. With respect to apoptosis, several different viruses produce bona fide anti-apoptotic Bcl-2 family proteins, or in some cases non-Bcl-2 proteins that function to inhibit Bax and/or Bak, effectively blocking MOMP and apoptosis (Galluzzi et al., 2008) Similarly, some viruses produce proteins that interfere with death receptor signaling to caspase-8 or activation of RIPK3 (Kaiser et al., 2013). In insects, the baculoviruses express two inhibitors of caspase that function to maintain cell survival until the lytic phase of viral replication (Clem, 2001). It is tempting to conclude that these mechanisms evolved to thwart cell death pathways that would otherwise eliminate virally infected cells.
Another approach to addressing the essential nature of cell death in the control of infection is to identify organisms that only effectively infect animals that are deficient in cell death mechanisms. For example, mice deficient in caspase-1 and caspase-11 can be infected by a soil bacterium that does not infect wild-type animals (Maltez et al., 2015). It will be interesting to know if animals lacking specific effector molecules, such as GSDMD (pyroptosis), MLKL (necroptosis), or Bax and Bak (apoptosis) harbor viruses or bacteria that are not found in their wild-type littermates. Such studies are in their infancy.
Riddle #4. If a cell dies in the forest of the body, does it make a sound?
How does cell death affect the physiology of surrounding cells, and of the body in general? Do dead cells in the body have an “afterlife?” The death of a cell can have two general types of impact on the organism: it can induce or inhibit immune responses (innate and/or adaptive) and it can promote the proliferation of surviving cells (“compensatory proliferation”). The former has been most extensively studied in mammalian systems, while the latter is best described in Drosophila (although also observed in mammals).
It is likely that both represent aspects of wound healing in which damaged cells are removed and, in the process, the generative and resolution phases of repair are coordinated. Indeed, engulfment of dying cells has important roles in wound healing in flies (Weavers et al., 2016) and mammals (Bosurgi et al., 2017).
In general, necrotic cell death (whether regulated or not) elicits an inflammatory response, while apoptosis does not, and can be actively anti-inflammatory (Davidovich et al., 2014). Indeed, this is one feature that helped to define the process of apoptosis as distinct from necrosis (Kerr et al., 1972). With respect to adaptive immunity (especially the presentation of corpse-associated proteins to T lymphocytes), this simple dichotomy is replaced with another: immunogenic versus non-immunogenic cell death. The latter can be simply “silent” with respect to adaptive immunity or actively tolerogenic (preventing subsequent responses to proteins associated with the corpse). These distinctions do not simply sort with the mode of cell death; both apoptotic and necroptotic cells can promote antigen presentation to T cells, depending on the death-inducing stimulus and other environmental factors (Galluzzi et al., 2017). Understanding immunogenic cell death at a mechanistic level has clear consequences for cancer immunotherapy and vaccine development for infectious diseases.
What is it about a dying cell that triggers inflammation and immune responses? When microbes are present, pathogen-associated molecular patterns (PAMPs) engage pattern recognition receptors (PRRs, such as Toll-like receptors) and these induce inflammation and promote T cell immunity to proteins associated with the dying cells (Blander, 2016). The concept of PAMPs led to the idea that dying cells release damage- (or “danger-“) associated molecular patterns (DAMPs), that again are both pro-inflammatory and promote adaptive T cell immunity (Amarante-Mendes et al., 2018). It is important, however, not to equate these immune outcomes. The most commonly invoked DAMPs are HMGB1 and ATP, although many others have been described, including uric acid, heat shock proteins, histones, mitochondrial proteins and nucleotides, and more.
With respect to inflammation, there is a strong case that the majority of these DAMPs do not play major roles in the inflammatory response to necrotic cells (Martin, 2016). This is based on the idea that signals that engage inflammation are generally antagonized by physiological mediators that modulate the response, thus preventing excessive damage. In a sense, this argument suggests that the existence of physiological mechanisms that counter a specific, putative DAMP can act as a guide to which DAMPs are most important. Of all generally described DAMPs, only extracellular ATP is antagonized in this way, in this case by the extracellular ATPases, CD39 and CD73. While animals lacking these ecto-ATPases show some abnormalities in lymphocyte trafficking, induction of experimental autoimmunity, and response to infectious agents (Antonioli et al., 2013), they are otherwise generally healthy, unlike the examples below. In contrast, members of the IL-1 family all have features that make them ideal candidates for DAMPs. They are processed intracellularly (by caspases or other intracellular proteases, an added regulatory mechanism that is usually required for subsequent interaction with their receptors) and all lack signal peptides for conventional secretion and thus are only released upon cell lysis or via plasma membrane pores. Many are ubiquitously expressed and responses to them are modulated by specific receptor antagonists that are required to prevent spontaneous inflammatory disease. For example, humans and mice lacking the IL-36 receptor antagonist (IL-36RA) present with catastrophic inflammation in response to normally mild tissue damage. Studies in the coming years will likely address the relative importance of DAMPs of the IL-1 family in the context of other putative DAMPs.
Similarly, the signals and mechanisms by which dying cells promote T cell immunity deserve further exploration. Immunogenic, apoptotic cell death has been described to require the release of HMGB1, ATP, and exposure of calreticulin on the cell surface (Galluzzi et al., 2017). In contrast, immunogenic, necroptotic cell death (which also releases HMGB1 and ATP, and likely presents calreticulin due to cell lysis) requires NF-kB activation in the dying cell (Yatim et al., 2015). Apoptotic cells induce T cell immunity if PAMPs are present to engage TLR signaling (Torchinsky et al., 2010). If, as has been suggested, HMGB1 and other DAMPs engage TLRs, why is necroptosis, which releases such DAMPs, insufficient to promote T cell immunity when NF-kB is not engaged in the dying cell? In necroptotic cells, engagement of RIPK1 can promote NF-kB activation (Yatim et al., 2015). Is NF-kB required in dying apoptotic cells for effective antigen presentation following engulfment? And if so, how is it induced? Engagement of death receptors leading to apoptosis can similarly engage NF-kB (Cullen et al., 2013), and following MOMP upon engagement of the mitochondrial pathway released IAP-antagonists (such as Smac) can trigger NF-kB activation (Giampazolias et al., 2017). Whether the NF-kB-induced mediators are important in subsequent antigen presentation (or other consequences of cell death, see below) is unknown.
Dying cells can also produce or induce the production of type I interferons (IFNs) as a consequence of either the cell death process or effects in the cells that engulf them. This involves activation of the cytosolic DNA sensor, STING. In tumors, the activation of STING to produce type I IFNs can promote anti-cancer T cell immunity (Corrales et al., 2016). The induction of MOMP during apoptosis leads to disruption of the inner mitochondrial membrane (by an unknown mechanism) and the release of mitochondrial DNA (mtDNA) from some mitochondria to the cytosol (McArthur et al., 2018; Riley et al., 2018). This mtDNA engages STING, but only when caspase activation is disrupted or inhibited (Rongvaux et al., 2014; White et al., 2014). This may help to explain previously paradoxical findings that inhibition of caspases can enhance effects of cancer therapies, although additional effects of caspases, such as CAD- induced mutagenesis and compensatory proliferation (see below) probably also contribute to this effect (Cao and Tait, 2018).
In contrast, the engulfment of apoptotic cells does not induce STING or type I IFN production in the engulfing cells. However, perturbation of processes involved in the degradation of the engulfed corpse can promote STING-dependent type I IFN production and anti-tumor immunity (Cunha et al., 2018). It is possible that lysosomal DNase II functions to prevent such STING activation, and DNase II-deficient animals present with lethal, STING-dependent interferonopathy (Ahn et al., 2012). An understanding of how corpse-containing phagosomes possibly “leak” DNA to the cytosols of engulfing cells would clearly have implications for anti- cancer immunotherapy.
Compensatory proliferation, another consequence of apoptosis, has been documented in flies and mammals (Fogarty and Bergmann, 2017). An eicosanoid, PGE2, produced by dying or engulfing cells has been implicated in the compensatory proliferation of tissue stem cells (Li et al., 2010). Executioner caspases cleave iPLA2, resulting in production of PGE2 which in turn can promote tumor growth (Huang et al., 2011). However, this effect appears to depend on the caspase-independent activation of NF-kB and COX2 production, and as noted above, a link between NF-kB activity and apoptosis remains somewhat unclear.
Intriguingly, production of another eicosanoid, LTb4, by dying cells has been shown to be required for the initial neutrophil “swarm” that initiates wound repair (Lammermann et al., 2013). How any cell death pathway engages LTb4 production remains unexplored.
Compensatory proliferation of stem cells has direct consequences for oncogenesis. Irradiation induces both thymocyte apoptosis and the generation of thymomas (thymomagenesis), but if apoptosis is curtailed by ablation of the BH3-only protein, p53 upregulated mediator of apoptosis (PUMA), radiation-induced thymomagenesis does not occur (Labi et al., 2010; Michalak et al., 2010). Similarly, PUMA is required for chemically-induced generation of hepatocellular carcinoma (Qiu et al., 2011). Induction of PUMA-independent thymocyte apoptosis with glucocorticoids permits radiation-induced thymomagenesis in PUMA-deficient mice (Michalak et al., 2010). Clearly, the mechanisms and consequences of apoptosis-induced compensatory proliferation in oncogenic processes will be an important area for continued research, and virtually nothing is known about compensatory proliferation in response to other modes of cell death.
Riddle #5. Distressed doctors document directed deaths by dastardly deeds in droves. How many dastardly deeds can documenting doctors dictate?
How many ways can a cell actively die? Thinking about this question is aided by our earlier consideration of the concepts of cell suicide and cell sabotage. Disruption of any process, essential for cell survival, will result in cell death (by definition) and this will be active cell death if a) the requirement for the process depends on the state of the cell (e.g., metabolic, proliferative, differentiation, etc.) and/or b) the disruption is sensed by a suicide pathway to trigger death. Disruption of non-essential processes can also alter that process to become lethal (e.g., by reactive oxygen species or other toxic metabolites). In many cases, the resulting cell death may have unusual features and invite a new “osis.” These are unlikely to be useful unless they can be generalized to several conditions, or if the disruption is due to agents or conditions deemed to be of particular importance.
One example that may meet these criteria is active cell death by parthanatos (notably not an “osis”) which is dependent on the DNA repair enzyme poly-ADP ribose polymerase Others include cell death that involves lysosomal permeabilization (lysosomal cell death, LCD), and netosis, a form of cell death restricted to neutrophils that functions to eject “nets” of DNA from the nucleus to the extracellular space (Vanden Berghe et al., 2014). The latter is thought to function in host defense.
Here, though, we consider another form of cell death that is frequently active on the part of the dying cell, a form that could be classified as “assisted suicide.” Cytotoxic lymphocytes kill target cells, in part, by the introduction of proteases (granzymes) into cells via a perforin-mediated process. Different granzymes engage apoptosis or regulated necrosis in the target cell (Martinez-Lostao et al., 2015), processes that are important for removal of infected or transformed cells. There are several granzymes, no one of which is essential for host defense, and these perform several immunological and physiological functions that are independent of cell death mechanisms (Arias et al., 2017).
Another mechanism of assisted suicide is entosis, which occurs when a cell actively “burrows” via actin-mediated locomotion into a neighboring cell. The now engulfed cell dies due to nutrient deprivation and/or is killed upon fusion of lysosomes with the engulfed compartment (Fais and Overholtzer, 2018). Entosis occurs in epithelial cancers and in primary epithelial cells that lose matrix attachment, and other cells as well. Entosis introduces a fundamental role for cell death that has not been extensively considered in our thinking of other cell death modalities; that of cellular competition. That cells compete for resources (growth factors, nutrients, niches) is not surprising, but the finding that such competition has evolved as an active, molecular process may be. It has been proposed that competition within and between cell lineages was an important driver of development in multicellular organisms (Buss, 1987). Studies of cellular competition has shown that “winners” and “losers” of such competition are distinct at a molecular level and drive the fates (proliferation, cell death) of the opposing class (Bondar and Medzhitov, 2010; Bove et al., 2017).
Studies of cellular competition by entosis provide new insights into this fundamental battle of the cells. For example, upon glucose deprivation, primary cells engage entosis with winners and losers determined by a bimodal distribution of AMPK activation (induced upon reduction of ATP levels) and membrane deformability (stiffness) (Hamann et al., 2017). Cells with high AMPK activation show decreased deformability, and burrow into winners to die. This provides the winners with a proliferative advantage under low nutrient conditions, perhaps also providing a source of nutrients. \ If such competition-associated entosis is a general feature of cells in multicellular organisms, effectively representing an altruistic suicide to preserve cells in a nutrient deprived (or otherwise challenged) tissue, this will have major consequences for our understanding of developmental and homeostatic processes.
Conclusion
The riddles presented here are perhaps silly, but the underlying questions and the forthcoming answers are certainly not. Cell death is a fundamental biological process, and as such, its study has and will continue to have important implications for physiological and pathological processes. In an 1897 letter, Mark Twain quipped, “The report of my death was an exaggeration.” The same might be said of those who suggest that the study of cell death is dying. As the author hopes is apparent, investigations into the mechanisms and functions of cell death are alive, well, and thriving.
Figure 2.
Apoptosis The mitochondrial and death receptor pathways of apoptosis are illustrated. Roles for the engagement of caspase-8 and apoptosis are shown in Figure 3.
Figure 3.
Necroptosis Death receptors, TLRs (that engage TRIF), and ZBP1 bind directly or via RIPK1 to activate RIPK3 and, in turn, MLKL, to execute necroptosis. RIPK3 activation is antagonized by the enzymatic activity of the FADD-caspase-8-c-FLIP complex.
Figure 4.
Pyroptosis Several NLRs and AIM2, when activated, directly or indirectly (via ASC) bind to and activate caspase-1, which cleaves GSDMD to effect pyroptosis, and also processes the cytokines IL-1b and IL-18. Caspase-4, caspase-5, and caspase-11 are directly activated by cytosolic LPS and cleave GSDMD, but not the cytokines.
Figure 5.
Ferroptosis The Fenton reaction causes peroxidation of poly-unsaturated fatty acids (PUFA), which in turn catalyze further lipid oxidation in the presence of O2, disrupting membranes (and generating additional toxic derivatives), leading to ferroptosis. The lipid peroxidase, GPX4, neutralizes oxidized lipids, but requires glutathione (GSH) for recycling. Disruption of GSH synthesis (by cysteine/cystine deprivation or inhibition of the System Xc− transporter) or recycling (requiring NADPH), or inhibition of GPX4 induces ferroptosis in some cells. Ferroptosis is inhibited by sequestration of free iron, inhibition of PUFA synthesis (by ACSL4), or scavenging of reactive oxygen species. Ferroptosis is also inhibited by glutamine deprivation, perhaps due to reduction of PUFA synthesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn J, Gutman D, Saijo S, and Barber GN (2012). STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A 109, 19386–19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, and Bortoluci KR (2018). Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front Immunol 9, 2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonioli L, Pacher P, Vizi ES, and Hasko G (2013). CD39 and CD73 in immunity and inflammation. Trends Mol Med 19, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias M, Martinez-Lostao L, Santiago L, Ferrandez A, Granville DJ, and Pardo J (2017). The Untold Story of Granzymes in Oncoimmunology: Novel Opportunities with Old Acquaintances. Trends Cancer 3, 407–422. [DOI] [PubMed] [Google Scholar]
- Bender CE, Fitzgerald P, Tait SW, Llambi F, McStay GP, Tupper DO, Pellettieri J, Sanchez Alvarado A, Salvesen GS, and Green DR (2012). Mitochondrial pathway of apoptosis is ancestral in metazoans. Proc Natl Acad Sci U S A 109, 4904–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM (2016). The comings and goings of MHC class I molecules herald a new dawn in cross-presentation. Immunol Rev 272, 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondar T, and Medzhitov R (2010). p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 6, 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, et al. (2017). Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove A, Gradeci D, Fujita Y, Banerjee S, Charras G, and Lowe AR (2017). Local cellular neighborhood controls proliferation in cell competition. Mol Biol Cell 28, 3215–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss L (1987). The Evolution of Individuality, (Princeton: Princeton University Press; ). [Google Scholar]
- Cao K, and Tait SWG (2018). Apoptosis and Cancer: Force Awakens, Phantom Menace, or Both? Int Rev Cell Mol Biol 337, 135–152. [DOI] [PubMed] [Google Scholar]
- Clem RJ (2001). Baculoviruses and apoptosis: the good, the bad, and the ugly. Cell Death Differ 8, 137–143. [DOI] [PubMed] [Google Scholar]
- Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, Fitzgerald P, Guio- Carrion A, Waterhouse NJ, Li CW, et al. (2007). GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 129, 983–997. [DOI] [PubMed] [Google Scholar]
- Corrales L, McWhirter SM, Dubensky TW Jr., and Gajewski TF (2016). The host STING pathway at the interface of cancer and immunity. J Clin Invest 126, 2404–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, Lavelle EC, Leverkus M, and Martin SJ (2013). Fas/CD95-induced chemokines can serve as “find-me” signals for apoptotic cells. Mol Cell 49, 1034–1048. [DOI] [PubMed] [Google Scholar]
- Cunha LD, Yang M, Carter R, Guy C, Harris L, Crawford JC, Quarato G, Boada-Romero E, Kalkavan H, Johnson MDL, et al. (2018). LC3-Associated Phagocytosis in Myeloid Cells Promotes Tumor Immune Tolerance. Cell 175, 429–441 e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels BP, Snyder AG, Olsen TM, Orozco S, Oguin TH 3rd, Tait SWG, Martinez J, Gale M Jr., Loo YM, and Oberst A (2017). RIPK3 Restricts Viral Pathogenesis via Cell Death- Independent Neuroinflammation. Cell 169, 301–313 e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovich P, Kearney CJ, and Martin SJ (2014). Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol Chem 395, 1163–1171. [DOI] [PubMed] [Google Scholar]
- Ding AX, Sun G, Argaw YG, Wong JO, Easwaran S, and Montell DJ (2016). CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondelinger Y, Hulpiau P, Saeys Y, Bertrand MJM, and Vandenabeele P (2016). An evolutionary perspective on the necroptotic pathway. Trends Cell Biol 26, 721–732. [DOI] [PubMed] [Google Scholar]
- Ellis HM, and Horvitz HR (1986). Genetic control of programmed cell death in the nematode C. elegans. Cell 44, 817–829. [DOI] [PubMed] [Google Scholar]
- Erturk A, Wang Y, and Sheng M (2014). Local pruning of dendrites and spines by caspase-3- dependent and proteasome-limited mechanisms. J Neurosci 34, 1672–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, and Kagan JC (2018). The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48, 35–44 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S, and Overholtzer M (2018). Cell-in-cell phenomena in cancer. Nat Rev Cancer 18, 758–766. [DOI] [PubMed] [Google Scholar]
- Flusberg DA, Roux J, Spencer SL, and Sorger PK (2013). Cells surviving fractional killing by TRAIL exhibit transient but sustainable resistance and inflammatory phenotypes. Mol Biol Cell 24, 2186–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty CE, and Bergmann A (2017). Killers creating new life: caspases drive apoptosis- induced proliferation in tissue repair and disease. Cell Death Differ 24, 1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Brenner C, Morselli E, Touat Z, and Kroemer G (2008). Viral control of mitochondrial apoptosis. PLoS Pathog 4, e1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Buque A, Kepp O, Zitvogel L, and Kroemer G (2017). Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17, 97–111. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. (2018). Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25, 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampazolias E, Zunino B, Dhayade S, Bock F, Cloix C, Cao K, Roca A, Lopez J, Ichim G, Proics E, et al. (2017). Mitochondrial permeabilization engages NF-kappaB-dependent anti- tumour activity under caspase deficiency. Nat Cell Biol 19, 1116–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YN, Guy C, Crawford JC, and Green DR (2017a). Biological events and molecular signaling following MLKL activation during necroptosis. Cell Cycle 16, 1748–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, Linkermann A, and Green DR (2017b). ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 169, 286–300 e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR (2018). Cell Death: Apoptosis and other means to an end, (Cold Spring Harbor: Cold Spring Harbor Press; ). [Google Scholar]
- Hamann JC, Surcel A, Chen R, Teragawa C, Albeck JG, Robinson DN, and Overholtzer M (2017). Entosis Is Induced by Glucose Starvation. Cell Rep 20, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, et al. (2017). Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CM, and Martin SJ (2017). Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory “FADDosome” Complex upon TRAIL Stimulation. Mol Cell 65, 715–729 e715. [DOI] [PubMed] [Google Scholar]
- Hetz C, and Papa FR (2018). The Unfolded Protein Response and Cell Fate Control. Mol Cell 69, 169–181. [DOI] [PubMed] [Google Scholar]
- Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, and Tang D (2016). Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, O’Sullivan B, He Z, Peng Y, Tan AC, et al. (2011). Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med 17, 860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, Haller M, Riley JS, Mason SM, Athineos D, et al. (2015). Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol Cell 57, 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ER, and Green DR (2002). Infection and the origins of apoptosis. Cell Death Differ 9, 355–357. [DOI] [PubMed] [Google Scholar]
- Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, and Perez F (2014). ESCRT machinery is required for plasma membrane repair. Science 343, 1247136. [DOI] [PubMed] [Google Scholar]
- Jorgensen I, Rayamajhi M, and Miao EA (2017). Programmed cell death as a defence against infection. Nat Rev Immunol 17, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, and Mocarski ES (2013). Viral modulation of programmed necrosis. Curr Opin Virol 3, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. (2015). Caspase-11 cleaves gasdermin D for non- canonical inflammasome signalling. Nature 526, 666–671. [DOI] [PubMed] [Google Scholar]
- Ke FFS, Vanyai HK, Cowan AD, Delbridge ARD, Whitehead L, Grabow S, Czabotar PE, Voss AK, and Strasser A (2018). Embryogenesis and Adult Life in the Absence of Intrinsic Apoptosis Effectors BAX, BAK, and BOK. Cell 173, 1217–1230 e1217. [DOI] [PubMed] [Google Scholar]
- Kearney CJ, and Martin SJ (2017). An Inflammatory Perspective on Necroptosis. Mol Cell 65, 965–973. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, and Currie AR (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainz T, Gaschler MM, Lim C, Sacher JR, Stockwell BR, and Wipf P (2016). A Mitochondrial-Targeted Nitroxide Is a Potent Inhibitor of Ferroptosis. ACS Cent Sci 2, 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Krumschnabel G, Manzl C, Tzankov A, Pinon J, Egle A, and Villunger A (2010). Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev 24, 1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, and Germain RN (2013). Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartigue L, Kushnareva Y, Seong Y, Lin H, Faustin B, and Newmeyer DD (2009). Caspase- independent mitochondrial cell death results from loss of respiration, not cytotoxic protein release. Mol Biol Cell 20, 4871–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JR, Klocke BJ, D’Sa C, Flavell RA, and Roth KA (2002). Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J Neuropathol Exp Neurol 61, 673–677. [DOI] [PubMed] [Google Scholar]
- Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, and Li CY (2010). Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal 3, ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockshin RA (1969). Programmed cell death. Activation of lysis by a mechanism involving the synthesis of protein. J Insect Physiol 15, 1505–1516. [DOI] [PubMed] [Google Scholar]
- Lovric MM, and Hawkins CJ (2010). TRAIL treatment provokes mutations in surviving cells. Oncogene 29, 5048–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltez VI, Tubbs AL, Cook KD, Aachoui Y, Falcone EL, Holland SM, Whitmire JK, and Miao EA (2015). Inflammasomes Coordinate Pyroptosis and Natural Killer Cell Cytotoxicity to Clear Infection by a Ubiquitous Environmental Bacterium. Immunity 43, 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Karki R, and Kanneganti TD (2017). Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ (2016). Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J 283, 2599–2615. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Henry CM, and Cullen SP (2012). A perspective on mammalian caspases as positive and negative regulators of inflammation. Mol Cell 46, 387–397. [DOI] [PubMed] [Google Scholar]
- Martinez-Lostao L, Anel A, and Pardo J (2015). How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin Cancer Res 21, 5047–5056. [DOI] [PubMed] [Google Scholar]
- McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, Geoghegan ND, Chappaz S, Davidson S, San Chin H, et al. (2018). BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359. [DOI] [PubMed] [Google Scholar]
- Michalak EM, Vandenberg CJ, Delbridge AR, Wu L, Scott CL, Adams JM, and Strasser A (2010). Apoptosis-promoted tumorigenesis: gamma-irradiation-induced thymic lymphomagenesis requires Puma-driven leukocyte death. Genes Dev 24, 1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, and Chan FK (2017). The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis. Int Rev Cell Mol Biol 328, 253–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima YI, and Kuranaga E (2017). Caspase-dependent non-apoptotic processes in development. Cell Death Differ 24, 1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH 3rd, Ingram JP, Rodriguez DA, Kosoff R, Sharma S, Sturm O, et al. (2016). RIPK3 Activates Parallel Pathways of MLKL- Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell Host Microbe 20, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek AL, Liu JC, Loewer A, Forrester WC, and Lahav G (2016). Cell-to-Cell Variation in p53 Dynamics Leads to Fractional Killing. Cell 165, 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W, Wang X, Leibowitz B, Yang W, Zhang L, and Yu J (2011). PUMA-mediated apoptosis drives chemical hepatocarcinogenesis in mice. Hepatology 54, 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad SD, Stotland A, Barott KL, Smurthwaite CA, Hilton BJ, Grasis JA, Wolkowicz R, and Rohwer FL (2014). Evolution of TNF-induced apoptosis reveals 550 My of functional conservation. Proc Natl Acad Sci U S A 111, 9567–9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JS, Quarato G, Cloix C, Lopez J, O’Prey J, Pearson M, Chapman J, Sesaki H, Carlin LM, Passos JF, et al. (2018). Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, and Alnemri ES (2017). Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8, 14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Jackson R, Harman CC, Li T, West AP, de Zoete MR, Wu Y, Yordy B, Lakhani SA, Kuan CY, et al. (2014). Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S, and Broz P (2015). Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol 45, 2927–2936. [DOI] [PubMed] [Google Scholar]
- Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, and Broz P (2018). ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960. [DOI] [PubMed] [Google Scholar]
- Sanchez KK, Chen GY, Schieber AMP, Redford SE, Shokhirev MN, Leblanc M, Lee YM, and Ayres JS (2018). Cooperative Metabolic Adaptations in the Host Can Favor Asymptomatic Infection and Select for Attenuated Virulence in an Enteric Pathogen. Cell 175, 146–158 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JW Jr. (1966). Death in embryonic systems. Science 154, 604–612. [DOI] [PubMed] [Google Scholar]
- Scheffer LL, Sreetama SC, Sharma N, Medikayala S, Brown KJ, Defour A, and Jaiswal JK (2014). Mechanism of Ca(2)(+)-triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun 5, 5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, and Shao F (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, and Shao F (2014). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192. [DOI] [PubMed] [Google Scholar]
- Spencer SL, Gaudet S, Albeck JG, Burke JM, and Sorger PK (2009). Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature 459, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, et al. (2017). Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Guzman E, Balasanyan V, Conner CM, Wong K, Zhou HR, Kosik KS, and Montell DJ (2017). A molecular signature for anastasis, recovery from the brink of apoptotic cell death. J Cell Biol 216, 3355–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, Parsons MJ, Llambi F, Bouchier-Hayes L, Connell S, Munoz-Pinedo C, and Green DR (2010). Resistance to caspase-independent cell death requires persistence of intact mitochondria. Dev Cell 18, 802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HL, Tang HM, Fung MC, and Hardwick JM (2015). In vivo CaspaseTracker biosensor system for detecting anastasis and non-apoptotic caspase activity. Sci Rep 5, 9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchinsky MB, Garaude J, and Blander JM (2010). Infection and apoptosis as a combined inflammatory trigger. Curr Opin Immunol 22, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, and Vandenabeele P (2014). Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15, 135–147. [DOI] [PubMed] [Google Scholar]
- Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, et al. (2017). Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, and Shao F (2017). Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103. [DOI] [PubMed] [Google Scholar]
- Weavers H, Evans IR, Martin P, and Wood W (2016). Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell 165, 1658–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinlich R, Oberst A, Beere HM, and Green DR (2017). Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol 18, 127–136. [DOI] [PubMed] [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, and Steller H (1994). Genetic control of programmed cell death in Drosophila. Science 264, 677–683. [DOI] [PubMed] [Google Scholar]
- White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, et al. (2014). Apoptotic caspases suppress mtDNA- induced STING-mediated type I IFN production. Cell 159, 1549–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Wang X, Zheng Y, Jiang J, and Hu J (2018). What role does pyroptosis play in microbial infection? J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Shinotsuka N, Nonomura K, Takemoto K, Kuida K, Yosida H, and Miura M (2011). Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J Cell Biol 195, 1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, Green DR, Oberst A, and Albert ML (2015). RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science 350, 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]