Abstract
Background
Persons living with human immunodeficiency virus (HIV) (PLWH) with high cytomegalovirus (CMV)–specific interferon (IFN) γ response have increased numbers of endothelium homing receptor (CX3CR1)+–expressing cells that are associated with cardiovascular disease. The current study was performed to investigate the effect of cellular levels of CMV DNA on these markers.
Methods
Eighty paired peripheral blood mononuclear cell samples were collected ≥12 months apart from 40 CMV-seropositive PLWH with suppressed HIV RNA, who started antiretroviral therapy at median of 3-months of infection. The samples were assessed for CMV-specific IFN-γ response by means of enzyme-linked immunospot assay, and participants were classified as low responders (LRs) or high responders (HRs) based on IFN-γ production (≤100 or >100 spot-forming units [SFUs]/105 cells).
Results
Of the 40 participants, 26 (65%) were HRs and 14 (35%) LRs at baseline, which did not change over time or by CMV levels (median at first/second time points, 383/308 SFUs/106 cells for HRs vs 21/41 SFUs/106 for LRs). A decrease in IFN-γ over time was associated with higher CMV DNA levels (P < .01). High CMV response was also associated with increased CD28+CD27−CD4+ T cells expressing CX3CR1 (P < .001). Similarly, increased IFN-γ production was associated with increased CMV-specific CX3CR1+CD28+CD27−CD4+ and CD8+ T cells (P < .001).
Conclusions
These findings demonstrate that levels of CMV-specific IFN-γ response in PLWH are stable over time, and that HRs have increased circulating T cells expressing CX3CR1 that may put them at increased risk of cardiovascular disease and other inflammatory diseases.
Keywords: cardiovascular disease, CX3CR1 memory cells, people living with HIV, sub clinical CMV
Human cytomegalovirus (CMV) establishes a latent infection that can periodically reactivate without overt clinical disease in the immunologically normal host [1, 2]. In immunocompromised individuals including transplant recipients, and persons living with human immunodeficiency virus (HIV) type 1 (PLWH), reactivation of CMV frequently can result in severe disease. Even in coinfected PLWH with preserved immune systems taking antiretroviral therapy (ART), reactivation of CMV is common, but CMV disease rarely occurs [2–4].
Increasingly, the immune response against CMV is being recognized as a potential cause of acute and chronic inflammation [5–8]. For PLWH starting ART, the restoration of immunity associated with an increase in CD4+ cells can result in an acute immune reconstitution inflammatory syndrome that is usually directed against opportunistic organisms, including CMV [9–12]. Numerous studies have shown that CMV-specific cellular immunity mediated by cytokine CD4+ and cytolytic CD8+ T cells is crucial for the control of CMV replication [7, 13–15]. T-helper 1 cytokines, interferon (IFN) γ and tumor necrosis factor (TNF) α, are produced by CMV-specific CD4+ T cells before CMV-specific antibodies and cytolytic CD8+ T cells [13–16]. Paradoxically, although these cytokines are central to an effective immune response, persistent expression of IFN-γ by CMV-specific effector T cells can result in chronic inflammation and immune activation contributing to immune senescence and cardiovascular complications [17–21]. Thus, CMV-specific CD4+ T cells are regulators of protective immunity and immunopathology, in particular related to cardiovascular disease (CVD) [22, 23].
Endothelial cell inflammation and damage play a critical role in the development of CVD and atherosclerosis [24–26]. Atherosclerosis is considered a nonresolving inflammatory disease characterized by migration and infiltration of activated macrophages and T cells within the atherosclerotic lesions and is mediated by the CX3CL1 (fractalkine)–CX3CR1 axis [17, 18, 27–29]. Both human studies and animal models suggest that CMV infection contributes to chronic endothelial cell inflammation and vascular damage [17, 18, 20, 21, 30, 31]. In the setting of HIV infection, coinfection with CMV could potentiate the inflammatory process by expanding the number of CMV-specific CX3CR1+ T cells, with the release of IFN-γ and TNF-α leading to the increased expression of CX3CL1 on endothelial cells [17, 18, 20]. In turn, this increase promotes the migration of CX3CR1 inflammatory cells to the arterial wall, resulting in chronic inflammation and atherosclerosis. Previous studies have shown that (1) CD4+ T cells in the immunocompetent host with prior CMV infection respond to CMV antigen presented by endothelial cells and produce IFN-γ and TNF-α and (2) individuals with high frequencies of CMV-specific T cells have higher levels of CX3CL1 and endothelial cell damage than those with low frequencies [17, 18].
In the current study, we hypothesized that if CMV increases the risk of CVD in PLWH, a subset of individuals can be identified who maintain persistently high levels of IFN-γ–producing CMV-specific CX3CR1-bearing T cells over time. We also posited that increased levels of CMV DNA detected over time would be associated with those individuals identified as having specifically high proportions.
MATERIALS AND METHODS
Study Participants
Paired peripheral blood mononuclear cell (PBMC) samples from 40 men who have sex with men were obtained from a repository of samples collected longitudinally from the San Diego Primary Infection Resource Consortium between November 2001 and January 2013. All participants were CMV and Epstein-Barr virus (EBV) seropositive. The estimated date of HIV infection was calculated using established algorithms [32]. ART was initiated a median of 3 months after this date. In all participants, complete suppression of HIV RNA had been achieved by the time of the first sample, a median of 34 weeks after ART initiation; a second sample was obtained ≥12 months after the first specimen tested. This study was approved by the University of California, San Diego, Office of Human Research Protections Program.
CMV and EBV DNA Quantification
CMV and EBV DNA levels were quantified in 5 × 106 PBMCs by means of digital droplet polymerase chain reaction, as described elsewhere [33]. Copy numbers were calculated as the mean of replicate polymerase chain reaction measurements and normalized to 1 × 106 cells, as determined by ribonuclease P/MRP subunit p30.
Cell Isolation and Culture
Cryopreserved PBMCs were centrifuged, washed, resuspended in R10 media, and incubated overnight at 37oC and 5% carbon dioxide. On day 2, cells were washed with R10 media, assessed for viability using the trypan blue exclusion method, and cultured in Roswell Park Memorial Institute 1640 medium (Gibco) and 10% human serum (MP Biomedicals) in the presence or absence of the immunodominant tegument pp65 peptide pool of CMV (2 µg/mL) (National Institutes of Health AIDS reagent program).
IFN-γ Enzyme-Linked Immunospot Assay
Frequencies of IFN-γ–producing cells were determined by means of enzyme-linked immunospot (ELISPOT) assay. PBMCs were thawed and rested overnight, and 105 viable cells were cultured in ELISPOT plates coated with capture antibody for IFN-γ (BD Biosciences), with or without pp65 peptide pool (2 µg/mL). Cytokine-producing cells were detected using kits for IFN-γ (BD Biosciences), according to the manufacturer’s instructions. Spot-forming units (SFUs) were automatically calculated with ImmunoSpot software for pp65 peptide pool stimulation and medium-only (negative) control wells. Data are presented as mean IFN-γ response in SFUs per 105 cells produced by pp65, minus vehicle control. Participants producing IFN-γ at ≤100 or >100 SFUs/105 cells were classified as low responders (LRs) or high responders (HRs), respectively.
Antibodies and Other Reagents
The following antibodies were used for flow cytometry: Alexa Fluor488-anti-CD45RA, phycoerythrin (PE)–anti-CD57, allophycocyanin/cyanine 7 (Cy7) or PE/Cy7–anti-CD3, Alexa Fluor 647–anti-CX3CR1, Alexa Fluor 700–anti-CD27, Brilliant Violet 510–anti-CD28, PE/Cy7–anti-CD8 and Brilliant Violet 650–anti-CD279 (programmed cell death protein-1-PD-1) (all from Biolegend); LIVE/DEAD fixable violet dye (Molecular Probes); allophycocyanin–eFluor 780–anti-CD4 (both from ebiosciences)
Immunolabeling and Flow Cytometry
Cells were surface stained for surface markers using respective antibodies and cell staining buffer (Biolegend). Flow cytometry was performed using a FACS Canto cytometer, and live cells were analyzed using FlowJo software (Tree Star). Controls for each experiment included unstained cells control, fluorescence-minus-one controls, and isotype-matched antibodies.
Statistical Analysis
Nonparametric tests were first used to determine associations between 2 variables. IFN-γ production was compared between LRs and HRs with nonparametric Mann-Whitney U test and between the 2 time points with Wilcoxon matched-pairs test; comparisons between different parameters were analyzed using Spearman correlation. Subsequently, we developed linear mixed-effects regression models to investigate the relationship of CMV shedding to each of the following outcomes during ART: (1) IFN-γ production; (2) CX3CR1-expressing CD45RA−CD4+ T cells; (3) CX3CR1-expressing CD45RA−CD8+ T cells; (4) CX3CR1 expressed on CMV-specific CD45RA−CD4+ T cells; (5) CX3CR1 expressed on CMV-specific CD45RA−CD8+ T cells; and (6) PD-1 expression on circulating CD45RA−CD28+CD27− effector memory T (TEM) cells.
Because the CMV and EBV data were not collected at the same time as the immunologic data, we estimated mean levels of CMV and EBV shedding for each individual with Bayesian hierarchical models using ≥2 DNA measurements from each participant to estimate the CMV and EBV DNA levels, as described elsewhere [33]. In all models, we tested the association of the following fixed effects with the outcome: (1) time (between visits 1 and 2 in weeks); (2) estimated CMV level; (3) estimated EBV level; and (4) IFN-γ level (when that was not the outcome). In the models of CX3CR1+, we added the cell subtype (CD28−CD27−, CD28+CD27−, CD28−CD27+, and CD28+CD27+) as a fixed and random effect. We tested the interactions of all fixed effects with time and cell subtype. Effects associated with the outcome at the 10% significance level were considered for the final models.
RESULTS
Participants, Clinical Characteristics, and HIV and CMV Status
Eighty blood samples were collected longitudinally from 40 recently HIV-infected participants who were CMV and EBV seropositive. All participants had achieved complete suppression of HIV RNA (defined as <50 copies/mL) with ART during study follow-up and had 2 stored PBMC specimens obtained ≥1 year apart. Clinical and demographic data are summarized in Table 1.
Table 1.
Demographic and Clinical Variables in Study Participants (N = 40)
| Variable | Median Value (IQR)a |
|---|---|
| Male sex, no. (%) | 40 (100) |
| Age, y | 35.00 (26.50–39.50) |
| EDI to ART, mo | 3.00 (2.50–8.50) |
| Baseline values | |
| Log viral load, log10 copies/ml | 11.34 (10.37–13.30) |
| CD4+ cell count, cells/μl | 485.50 (362.25–649.50) |
| CD4+/CD8+ T-cell ratio, copies/µL | 0.47 (0.32–0.60) |
| CMV DNA copies | |
| Time 1 | 5.19 (2.80–9.58) |
| Time 2 | 3.21 (0.00–5.38) |
| EBV DNA, copies/μL | |
| Time 1 | 83.15 (20.67–247.45) |
| Time 2 | 12.10 (2.14–68.59) |
| Circulating CD4+CX3CR1+ cells, %b | |
| Time 1 | 0.60 (0.38–1.06) |
| Time 2 | 0.66 (0.43–1.06) |
| Circulating CD8+CX3CR1+ cells, %b | |
| Time 1 | 0.61 (0.35–1.22) |
| Time 2 | 0.81 (0.30–1.27) |
Abbreviations: ART, antiretroviral therapy; CMV, cytomegalovirus; EBV, Epstein-Barr virus; EDI, estimated date of infection; IQR, interquartile range.
aData represent median (IQR) values unless otherwise specified.
bCirculating CD4+CX3CR1+ and CD8+CX3CR1+ cell values represent means across subtypes.
Response to CMV over time
Previous studies showed that the host CD4+ T-cell response to CMV antigen can produce IFN-γ and TNF-α at levels sufficient to drive the induction of endothelial cell CX3CL1 (fractalkine), a key marker of endothelial cell inflammation [17, 18]. It has also been demonstrated that, according to their IFN-γ response, CMV-seropositive persons can be divided into HRs and LRs [18]. In the current study, we tested the hypothesis that response to CMV in HIV/CMV-coinfected individuals with sustained HIV suppression would remain constant over time (ie, that HRs would remain HRs and LRs would remain LRs).
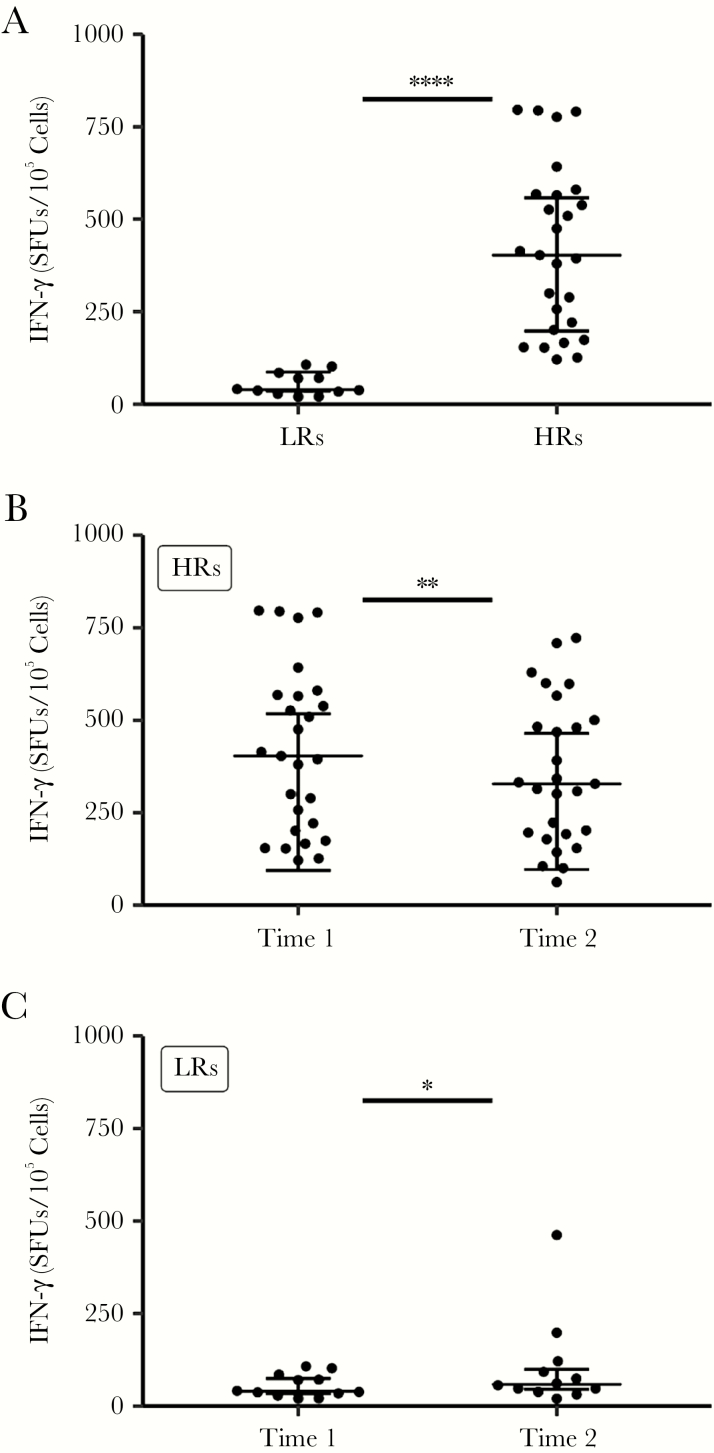
Initially, we quantified the response to CMVpp65 by using ELISPOT to determine the frequency of cells producing IFN-γ. Of the 40 study participants, 26 (65%) were HRs (mean IFN-γ response [standard deviation (SD)], 416.2 [41.7] SFUs/105 cells) and 14 (35%) were LRs (43.9 [10], SFUs/105 cells) (Figure 1A). Next, we evaluated the change in IFN-γ in response to CMVpp65 ≥12 months after the first time point. Although the response to CMVpp65 at the second time point declined in HRs (mean [SD] IFN-γ response, 388.5 [42] vs 310 [38] SFUs/105; P = .007), it did not change significantly in LRs (36 [10] vs 41 [30]; P = .23), and each group of responders, with few exceptions, maintained their response category (Figure 1B and 1C).
Figure 1.
Change in interferon (IFN) γ over time. A, Peripheral blood mononuclear cell (PBMCs) from 40 men coinfected with human immunodeficiency virus and cytomegalovirus (CMV) were collected at 2 time points (times 1 and 2), the first sample a median of 34 weeks after the start of antiretroviral therapy (A) and the second >12 months later (B, C). PBMCs were cultured on enzyme-linked immunospot (ELISPOT) plates coated with capture anti–IFN-γ antibody, with or without CMVpp65 (2 µg/mL). After 18 hours, plates were washed, and biotinylated anti-IFN-γ detection antibody was added for 2 hours, followed by the addition of streptavidin–horseradish peroxidase enzyme conjugate for 1 hour; plates were then washed, and substrate was added using the AEC substrate kit (BD Biosciences). The substrate reaction was stopped by washing wells with water. Spots were air dried, and counted with an automated ELISPOT reader, which calculated the number of spot-forming units (SFUs) per 105 PBMCs. Participants producing IFN-γ at >100 or ≤100 SFUs/105 cells were classified as high responders (HRs) (B) or low responders (LRs) (C), respectively. For all graphs, each dot represents an individual donor, plots include observations from the 25th to 75th percentiles, and horizontal lines represent median values. *P = .27; **P < .005; ****P < .001.
CMV DNA Load and CMV-Specific IFN-γ Response
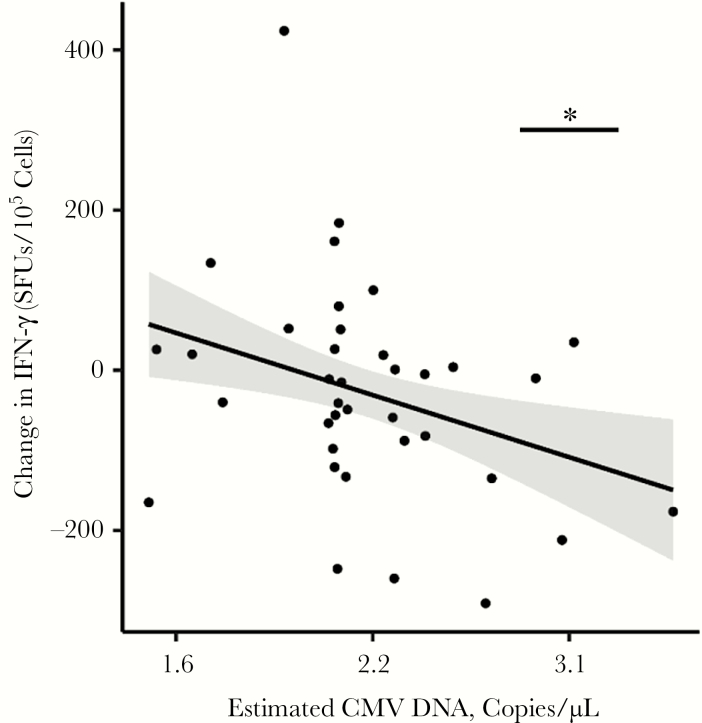
Our next series of analyses was designed to determined whether the CMV load as measured by CMV DNA in peripheral blood cells altered the IFN-γ response. Because 10 (25%) of the 40 participants had CMV DNA measured on the same date as the outcome measures, we used ≥2 CMV DNA measurements from different dates and applied Bayesian hierarchical modeling [33] to all CMV DNA measurements to calculate an estimate of CMV DNA shedding for each person. Interestingly, the amount of CMV DNA was not associated with the magnitude of IFN-γ response at baseline (P > .20). However, the change in IFN-γ levels over time was influenced by CMV DNA levels (CMV × time interaction, −1.85 [0.67]; P < .01). Specifically, a more rapid decline in IFN-γ levels over time was associated with higher amounts of CMV DNA (ie, above the average estimated CMV DNA level).
To visualize this association, we computed the change in IFN-γ levels from visit 1 to visit 2 and plotted it against estimated CMV DNA levels (Figure 2). As the significant CMV-time interaction indicates, lower expected CMV DNA levels were associated with increases in IFN-γ over time, and higher CMV levels with decreases in IFN-γ. For instance, expected CMV DNA levels of 1.6 and 3.1 copies/µL (ie, 1 SD below and above the mean) were associated with an IFN-γ increase by 46.6 SFUs/105 (95% confidence interval, −40.1 to 133.3) and a decrease by 108.5 points (17.0–200.1) from visit 1 to visit 2, respectively. This CMV-time interaction was significant even after outliers were removed or the outcome was log-transformed (P < .05).
Figure 2.
Estimated cytomegalovirus (CMV) DNA levels are inversely correlated with the change in interferon (IFN) γ over time. Peripheral blood mononuclear cells from human immunodeficiency virus–infected, CMV-infected individuals (n = 40) were collected at 2 time points >12 months apart (times 1 and 2), and the frequency of cells producing IFN-γ (in spot-forming units [SFUs] per 105 cells) in response to CMVpp65 was determined by means of enzyme-linked immunospot assay, as in Figure 1. Black dots represent change in IFN-γ from visit 1 to visit 2; black line and shaded area, model-estimated values and their 95% confidence intervals. *P < .01.
EBV DNA Load and CMV IFN-γ Response
To assess whether the change in the IFN-γ response to CMV was specific to the estimated CMV DNA load, we examined the association between CMV IFN-γ and the estimated EBV DNA load. We selected EBV to study because it is 100% prevalent among the study cohort and is a herpesvirus that is frequently reactivated in immunosuppressed individuals, including those infected with HIV. Using the same method as for CMV, we found no association between the CMV IFN-γ response and EBV (P > .2).
Association of CMV With CX3CR1+ Memory CD4+ and CD8+ T Cells
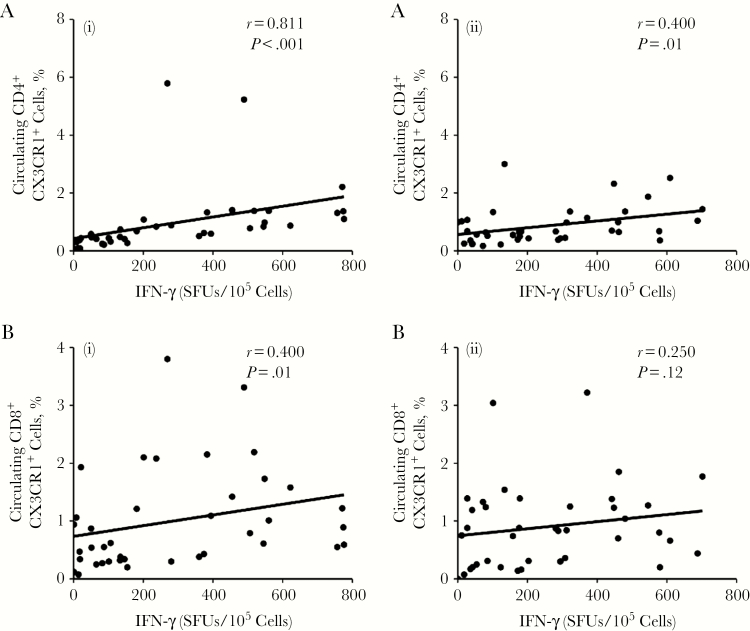
HIV/CMV-coinfected individuals are potentially at increased risk of atherosclerosis, and CMV-specific CX3CR1-bearing CD4+ T cells can promote chronic inflammation [20, 21]. Therefore, we hypothesized that HIV/CMV-coinfected individuals with a high response to CMV would have a higher frequency of memory CD4+CX3CR1+ T cells. To examine this relationship, we determined the correlation between the frequency of IFN-γ–producing cells (in SFUs per 105 cells) in response to CMVpp65 and circulating CD45RA−CD4+CX3CR1+ memory T cells in the 2 samples obtained ≥12 months apart. The IFN-γ response was strongly correlated with CD4+ count at baseline (r = 0.811; P < .001), and this relationship persisted over time (r = 0.4; P = .01) (Figure 3A, left and right). Similarly, IFN-γ was correlated with the frequency of CD45RA−CD8+CX3CR1+ memory T cells at baseline (r = 0.4; P = .01), but this was no longer significant over time (r = 0.25; P = .12) (Figure 3B, left and right).
Figure 3.
Association of cytomegalovirus (CMV) with CX3CR1+ memory CD4+ and CD8+ T cells. To determine whether the interferon (IFN) γ response to CMV correlates with the frequency of circulating CD45RA−CX3CR1+ CD4+ and CD8+ memory cells (CD4+CX3CR1+ and CD8+CX3CR1+ cells), peripheral blood mononuclear cells of donors coinfected with CMV and human immunodeficiency virus were stained with antibodies against CD14, CD3, CD4, CD8, CD45RA, and CX3CR1 and LIVE/DEAD fixable violet dye, and live cells were analyzed as CD14−CD3+ CD45RA−CX3CR1+ CD4+/CD8+by means of flow cytometry. A, Percentages of CD4+CX3CR1+ cells were determined and correlations between the percentage of CD4+CX3CR1+ cells and the frequency of IFN-γ–producing cells at times 1 (left) and 2 (right) were calculated. B, Percentages of CD8+CX3CR1+ cells were determined and correlations between the percentage of CD8+CX3CR1+ cells and the frequency of IFN-γ–producing cells at times 1 (left) and 2 (right) were calculated. For all graphs, each dot represents an individual donor.
CD4+CX3CR1+ T Cells Consist of CD28+CD27− Memory Subsets in HIV/CMV-Coinfected Individuals
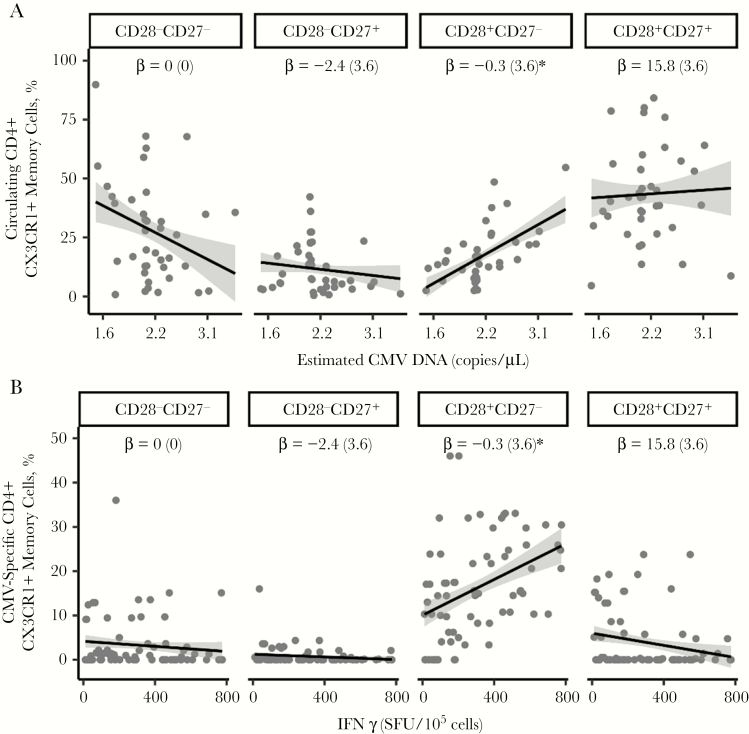
Because TEM cells have the ability to produce effector cytokines, IFN-γ and TNF-α, and our findings showed that IFN-γ is associated with circulating frequency of CD45RA−CD4+CX3CR1+ cells (Figure 3A), we next determined the frequency of the TEM cell subset in the CD45RA−CD4+CX3CR1+ memory cell population. Initially, we measured the relationship between the estimated CMV DNA level and CD28+CD27+, CD28−CD27+, CD28+CD27−, and CD28−CD27− subsets in CD45RA−CD4+CX3CR1+ memory cells present in PBMCs of HIV/CMV-coinfected participants.
After the significant IFN-γ by subset interaction effects were controlled for (P < .001), CMV was negatively associated with the CD28−CD27− subset (−12.3 [4.4]; P < .01) and positively associated with the CD28+CD27− subset (11.7 [4.4]; P < .01) but not with CD28+CD27+ (2.8 [4.4]; P = .56) or CD28−CD27+ (−2.3 [4.4]; P = .60; Figure 4A). Unlike with CD45RA−CD4+CX3CR1+ T cells, the relationship between CD45RA−CD8+CX3CR1+ and the estimated CMV DNA levels was neither significant (P > .2) nor dependent on T-cell subsets (P > .2), even though it was associated with IFN-γ in the CD28+CD27+ subset (−21.8 [11.0]; P < .05). Neither outcome was associated with time or EBV, with or without interacting with other variables (P > .2). These findings suggest that CMV shedders have a higher frequency of circulating CD4+ TEM cells that coexpress CX3CR1.
Figure 4.
CD4+CX3CR1+ T cells consist of CD28+CD27− memory subsets in individuals coinfected with human immunodeficiency virus and cytomegalovirus (CMV). Peripheral blood mononuclear cells (PBMCs) from such coinfected donors (n = 40) taking antiretroviral therapy (ART) were at >12 months apart (first sample obtained a median of 34 weeks after ART initiation). PBMCs from both time points (n = 80) were either stained (A) or first cultured (B) with or without CMVpp65 (2 µg/mL) for 48 hours and then stained with antibodies against CD14, CD3, CD4, CD45RA, CX3CR1, CD27, and CD28 and LIVE/DEAD fixable stain. Live cells were analyzed as CD14−CD3+CD4+CD45RA−CX3CR1+, and percentages of CD28−CD27−, CD28−CD27+, CD28+CD27−, and CD28+CD27+ cells were determined using flow cytometry. A, Circulating frequency of T-cell subsets were analyzed with estimated CMV DNA levels. B, CMV-specific T-cell subsets were analyzed with the frequency of cells producing interferon (IFN) γ. Gray dots represent the means of measures from visits 1 and 2; black lines, regression lines; and gray shading, 95% confidence intervals. Regression (β) coefficient with standard error. *P < .01.
Next, we evaluated whether the expansion of TEM cells defined by CD27 and CD28 is specific to CMV antigen. PBMCs were cultured with or without a CMVpp65 peptide pool; the frequency of memory subsets was determined by means of flow cytometry. The frequency of IFN-γ–producing cells (in SFUs per 105 cells) significantly changed over time (P = .02) and was associated with CMV-specific CD45RA−CD4+CX3CR1+ levels in a subtype-dependent manner (P < .001). Specifically, this association was significantly positive with the CD28+CD27− subset (13.4 [3.6]; P < .01) (Figure 4B) but not with CD28+CD27+ (−3.0 [3.6]; P = .40), CD28−CD27+ (−0.9 [3.6]; P = .91), or CD28−CD27− (−4.7 [3.6]; P = .38). This outcome was not associated with EBV with or without interaction with subsets (P > .2). CMV-specific CD45RA−CD8+CX3CR1+ levels were associated with IFN-γ in the CD28+CD27− subtype (28.8 [5.6]; P < .001), but not with any other factors (P > .2). Collectively, these findings indicate that HIV/CMV-coinfected persons with suppressed HIV RNA have a high frequency of CMV-specific CD28+CD27− memory T cells that coexpress CX3CR1 and IFN-γ.
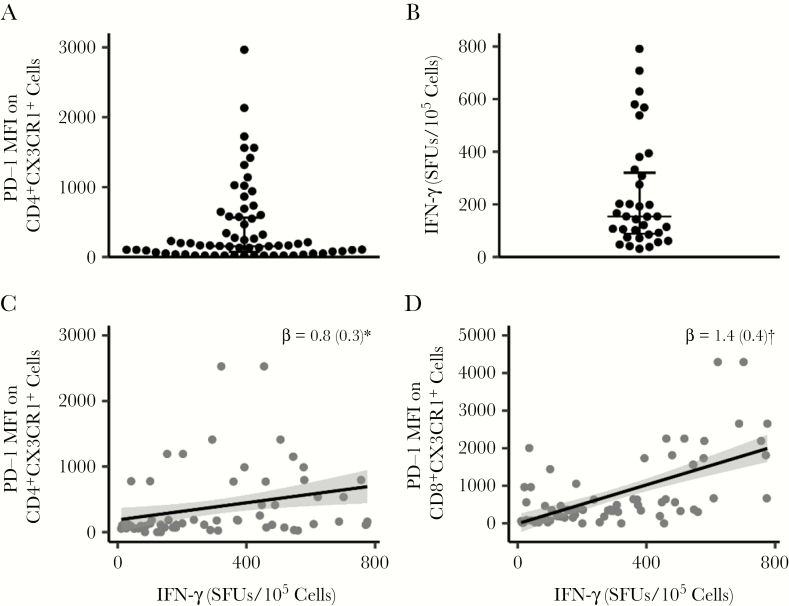
In an earlier study, we showed that men who have sex with men and who are living with HIV and have detectable CMV in their semen had higher levels of PD-1 expression on circulating CD45RA−CD28+CD27− TEM cells than those without detectable genital CMV DNA [34]. Therefore, we sought to assess whether PD-1 expression is associated with CMV-specific CD45RA−CD4+CX3CR1+ memory T cells in PLWH with detectable CMV DNA in blood cells. For these studies, PBMCs were cultured with or without CMVpp65, and the mean fluorescence intensity of PD-1 was determined on CD45RA−CD4+CX3CR1+ cells using flow cytometry (mean [SD], 416.7 [76]; median, 142.5) (Figure 5A).
Figure 5.
PD-1 is associated with interferon (IFN) γ but not with CX3CR1+ memory cells or levels of cytomegalovirus (CMV) DNA. Peripheral blood mononuclear cells (PBMCs) from donors coinfected with human immunodeficiency virus and CMV (n = 40) and taking antiretroviral therapy (ART) were obtained >12 months apart (first sample obtained a median of 34 weeks after ART initiation). PBMCs from both time points (n = 80) were cultured with or without CMVpp65 (2 µg/mL) for 48 hours and then stained with antibodies against CD14, CD3, CD4, CD45RA, CX3CR1, and PD-1 and LIVE/DEAD fixable stain; expression of PD-1 (mean fluorescence intensity [MFI]) on live CD14−CD3+CD4+CD45RA−CX3CR1+ cells was then determined by means of flow cytometry. PD-1 MFI was calculated as value for control PBMC minus that for PBMC treated with CMVpp65. A, PD-1 MFI on CD4+CX3CR1+ memory cells. B, Frequency of IFN-γ–producing cells (determined in Figure 1) in donors with PD-1 MFI less than the median value; each dot in the plots represents an individual donor, and plots include observations from the 25th to 75th percentiles; horizontal lines represent median values. C, D, PD-1 MFI on CD4+ and CD8+ cells was correlated with the frequency of cells producing IFN-γ. Each black dot in the plots represents data from an individual donor. C–E, Gray dots represent means of measures from visits 1 and 2; black lines and gray shadowing, regression lines and their 95% confidence intervals, respectively. Note that dots are overlapping because some individuals have the same PD-1 values (0). Regression (β) coefficients are shown with standard error. *P < .05; †P < .01.
We identified a subset of PLWH with very low to undetectable levels of PD-1 expression. However, these individuals were still capable of producing IFN-γ, as determined by CMV ELISPOT (mean [SD] IFN-γ response, 238.5 [34.24] SFUs/105 cells; median, 174 SFUs/105 cells) (Figure 5B). Overall, PD-1 expression on CD4+ T cells was associated with IFN-γ expression (626.3 [254.3]; P = .02) (Figure 5C) and time (P = .04) but was not associated with CMV DNA level, with or without interaction with time (P > .2). PD-1 expression on CD4+ T cells was not associated with CMV DNA in CD4+CX3CR1+ T-cell subsets (P > .2). PD-1 expression on CD8+ T cells was associated with IFN-γ expression (1035 [331]; P < .01) (Figure 5D) but not with any other factors (P > .2). Thus, we identified a subset of HIV-CMV-coinfected individuals with a memory T-cell phenotype of CD4+CX3CR1+PD-1− that produces IFN-γ.
DISCUSSION
Since the 1970s, the role of microbial organisms in CVD has been intensely investigated. In particular, CMV has been implicated as a risk factor for atherosclerosis in many epidemiologic, clinical, animal, and in vitro studies [30, 31, 35–37]. To promote the atherogenic process, a pathogen must be able to infect endothelial cells, establish persistent infection, and elicit a robust immunologic response that can persist over decades. Moreover, to have a broad effect on a population basis, the pathogen should infect a large proportion of persons. Thus, although other pathogens may demonstrate some of these properties, CMV, more than any other infectious agent, meets all of these criteria in HIV-uninfected and HIV-infected populations. Consistent with observations in HIV-uninfected individuals and transplant recipients, CMV has constantly emerged in PLWH as the most common pathogen associated with early signs of cardiac disease [20, 38]. However, because most of the human studies associating CMV humoral and cell-mediated responses have been cross-sectional, few longitudinal data exist indicating that a subgroup of persons can be identified as being at increased risk for CVD based on their immunologic response to CMV. This information is of particular importance in PLWH, almost 100% of whom are coinfected with CMV.
Previous studies have shown that CMV-seropositive HIV-uninfected individuals with high frequencies of CMV-specific T cells, when compared with donors with low levels of such cells, have higher quantities of CX3CL1 in endothelial cells, which is associated with damage and loss of endothelial cells [18, 39]. In the current study, we show that HIV/CMV-coinfected individuals with high CMV frequencies maintain their response status irrespective of levels of detectable CMV DNA over time. Thus, they are potentially at increased risk of CMV-associated inflammatory conditions, including CVD.
CX3CR1 is the receptor for chemokine CX3CL1 and also serves as the homing receptor expressed on memory T cells [40–42]. The CX3CR1-CX3CL1 axis has emerged as a key regulator of inflammation and vascular injury [20, 27, 29]. In HIV-uninfected individuals, CX3CL1 is up-regulated on endothelial cells in response to CMV antigen stimulation of PBMCs from CMV-seropositive individuals, and this response is markedly increased in persons with a high frequency of CMV [17, 18]. Moreover, CX3CR1-bearing cells have been identified as the major IFN-γ–producing cells, and cells expressing CX3CR1, including T cells, macrophages and natural killer cells, are the major cell type recruited to endothelial sites of inflammation [39, 43]. Our current data confirm and expand on these findings, and they demonstrate that the increased number of CX3CR1-bearing cells is significantly correlated with CMV-specific IFN-γ production. The link to CVD is further supported by the induction of CX3CL1 production in endothelial cells when exposed to TNF-α and IFN-γ, and in HIV/CMV-coinfected individuals, there is a positive correlation between circulating CD4+CX3CR1+ T cells and intima-media thickness [20].
The data presented here further establish that the frequency of cells producing IFN-γ in response to CMVpp65 is directly correlated with the frequency of circulating CD45RA−CD4+CX3CR1+ memory T cells in HIV-CMV-coinfected individuals. Importantly, this correlation is maintained after prolonged HIV suppression with ART. During chronic CMV infection, repeated antigenic stimulation causes memory CD8+ T cells to accumulate at high frequencies, reaching up to 20% of total CD8+ T cells, called “inflated memory” CD8+ T cells [44–47]. Earlier studies have established that memory CD4+ T cells specific for CMV glycoprotein B and pp65 can also expand, reaching frequencies comparable to those of CD8+ T cells [48]. Of interest, CD8+ T-cell inflation during latent murine CMV infection is strongly dependent on CD4+ T-cell helper function [49]. Our findings that individuals with a high frequency of CMV maintain their response status suggests that those with high CMV frequencies undergo T-cell memory inflation that is sustained over time.
Similar to CD8+ T cells, inflated memory CD4+ T cells have a phenotype of CD45RA− TEM, and RA+ revertants that lack CD27−CD28−PD-1− cell markers are also positive for CX3CR1+ [48]. In the current study, we show that in a subset of PLWH, CMV-specific CD45RA−CD4+ inflated TEM cells occur in HIV/CMV-coinfected individuals who have sustained viral suppression on ART. Similar to the findings of Abana et al [48] and Pachnio et al [50], we found that the CD27−CD28+ TEM cells expand at the highest frequency in response to CMV, and these are CX3CR1+CD4+ T cells [48, 50]. Of interest, the CMVpp65-specific TEM cell frequency in our study, which ranged from 0% to 60%, was lower than that found by Abana et al [48] using tetramer specific for glycoprotein B. This difference is largely due to the difference in response to the CMVpp65 peptide pool used in our studies, which unlike glycoprotein B, tends to elicit less memory inflation. Our findings that CD4+ T-cell inflation was CMV specific and not due to a bystander effect of inflammation or the presence of another herpesvirus (EBV) further supports the idea that these findings are CMV specific. CMV-directed inflated CD4+ T cells are proatherogenic, producing IFN-γ and TNF-α (discriminating them from exhausted T cells), and expressing CX3CR1, perforin, and granzyme [48, 51].
In conclusion, HIV/CMV-coinfected individuals maintain their CMV response state independent of the level of CMV DNA present in PBMCs. Individuals with high CMV frequencies harbor high frequencies of CD4+CX3CR1+ T cells that are associated with CVD, which probably places these IFN-γ HRs at increased risk for CVD and other CMV-associated inflammatory conditions. Drugs designed to interrupt the CX3CR1-CX3CL1 interaction may prove beneficial in decreasing the effects of chronic inflammation associated with CMV and other drivers of inflammation associated with CVD.
Acknowledgments
Author contributions. A. G., S. G., and S. A. S. conceived and designed the experiments; A. G. and R. T. performed the experiments; and A. G., S. G., M. N., and S. A. S. analyzed the data and wrote the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This research was supported in part by (National Institute of Health grants R56 HL125028 and R24 AI106039 to the Primary Infection Resource Consortium, R21 AI127132 to A. G.; AI68636 to SGW, and AI027763 [To SGW]); and the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (impaactnetwork.org). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network was provided by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants UM1AI068632 [IMPAACT Leadership and Operations Center Grant], UM1AI068616 [IMPAACT SDMC], and UM1AI106716 [IMPAACT Laboratory Center Grant]), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health. Portions of this research were performed with support of the Flow Cytometry Core at the University of California, San Diego, Center for AIDS Research (National Institute of Health grant P30 AI036214), the VA San Diego Health Care System, and the San Diego Veterans Medical Research Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bronke C, Palmer NM, Jansen CA, et al. Dynamics of cytomegalovirus (CMV)-specific T cells in HIV-1-infected individuals progressing to AIDS with CMV end-organ disease. J Infect Dis 2005; 191:873–80. [DOI] [PubMed] [Google Scholar]
- 2. Saharia KK, Koup RA. T cell susceptibility to HIV influences outcome of opportunistic infections. Cell 2013; 155:505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anglaret X, Minga A, Gabillard D, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin Infect Dis 2012; 54:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Springer KL, Weinberg A. Cytomegalovirus infection in the era of HAART: fewer reactivations and more immunity. J Antimicrob Chemother 2004; 54:582–6. [DOI] [PubMed] [Google Scholar]
- 5. Compton T, Kurt-Jones EA, Boehme KW, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 2003; 77:4588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freeman ML, Mudd JC, Shive CL, et al. CD8 T-cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin Infect Dis 2016; 62:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. La Rosa C, Diamond DJ. The immune response to human CMV. Future Virol 2012; 7:279–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van de Berg PJ, Heutinck KM, Raabe R, et al. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J Infect Dis 2010; 202:690–9. [DOI] [PubMed] [Google Scholar]
- 9. Anderson AM, Fountain JA, Green SB, et al. Human immunodeficiency virus-associated cytomegalovirus infection with multiple small vessel cerebral infarcts in the setting of early immune reconstitution. J Neurovirol 2010; 16:179–84. [DOI] [PubMed] [Google Scholar]
- 10. Hartigan-O’Connor DJ, Jacobson MA, Tan QX, Sinclair E; Studies of Ocular Complications of AIDS Research Group Development of cytomegalovirus (CMV) immune recovery uveitis is associated with Th17 cell depletion and poor systemic CMV-specific T cell responses. Clin Infect Dis 2011; 52:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Müller M, Wandel S, Colebunders R, et al. ; IeDEA Southern and Central Africa Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Price P, Murdoch DM, Agarwal U, et al. Immune restoration diseases reflect diverse immunopathological mechanisms. Clin Microbiol Rev 2009; 22:651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med 2006; 203:2865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gamadia LE, Remmerswaal EB, Weel JF, et al. Primary immune responses to human CMV: a critical role for IFN-γ-producing CD4+ T cells in protection against CMV disease. Blood 2003; 101:2686–92. [DOI] [PubMed] [Google Scholar]
- 15. Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202:673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waldrop SL, Pitcher CJ, Peterson DM, et al. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest 1997; 99:1739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bolovan-Fritts CA, Trout RN, Spector SA. Human cytomegalovirus-specific CD4+-T-cell cytokine response induces fractalkine in endothelial cells. J Virol 2004; 78:13173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolovan-Fritts CA, Trout RN, Spector SA. High T-cell response to human cytomegalovirus induces chemokine-mediated endothelial cell damage. Blood 2007; 110:1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu DC, Kerr SJ, Iampornsin T, et al. Restoration of CMV-specific-CD4 T cells with ART occurs early and is greater in those with more advanced immunodeficiency. PLoS One 2013; 8:e77479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sacre K, Hunt PW, Hsue PY, et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS 2012; 26:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van de Berg PJ, Yong SL, Remmerswaal EB, et al. Cytomegalovirus-induced effector T cells cause endothelial cell damage. Clin Vaccine Immunol 2012; 19:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith MZ, Bastidas S, Karrer U, Oxenius A. Impact of antigen specificity on CD4+ T cell activation in chronic HIV-1 infection. BMC Infect Dis 2013; 13:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–95. [DOI] [PubMed] [Google Scholar]
- 25. Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol 2006; 1:297–329. [DOI] [PubMed] [Google Scholar]
- 26. Libby P. Inflammation in atherosclerosis. Nature 2002; 420:868–74. [DOI] [PubMed] [Google Scholar]
- 27. Apostolakis S, Spandidos D. Chemokines and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol Sin 2013; 34:1251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frostegård J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 1999; 145:33–43. [DOI] [PubMed] [Google Scholar]
- 29. Moatti D, Faure S, Fumeron F, et al. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood 2001; 97:1925–8. [DOI] [PubMed] [Google Scholar]
- 30. Bruning JH, Persoons M, Lemström K, et al. Enhancement of transplantation-associated atherosclerosis by CMV, which can be prevented by antiviral therapy in the form of HPMPC. Transpl Int 1994; 7(suppl 1):S365–70. [DOI] [PubMed] [Google Scholar]
- 31. Zhou YF, Leon MB, Waclawiw MA, et al. Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherectomy. N Engl J Med 1996; 335:624–30. [DOI] [PubMed] [Google Scholar]
- 32. Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith DM, Nakazawa M, Freeman ML, et al. Asymptomatic CMV replication during early human immunodeficiency virus (HIV) infection is associated with lower CD4/CD8 ratio during HIV treatment. Clin Infect Dis 2016; 63:1517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dan JM, Massanella M, Smith DM, et al. Brief report: effect of CMV and HIV transcription on CD57 and PD-1 T-cell expression during suppressive ART. J Acquir Immune Defic Syndr 2016; 72:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lemström K, Koskinen P, Krogerus L, et al. Cytomegalovirus antigen expression, endothelial cell proliferation, and intimal thickening in rat cardiac allografts after cytomegalovirus infection. Circulation 1995; 92:2594–604. [DOI] [PubMed] [Google Scholar]
- 36. Valantine HA, Gao SZ, Menon SG, et al. Impact of prophylactic immediate posttransplant ganciclovir on development of transplant atherosclerosis: a post hoc analysis of a randomized, placebo-controlled study. Circulation 1999; 100:61–6. [DOI] [PubMed] [Google Scholar]
- 37. Bentz GL, Yurochko AD. Human CMV infection of endothelial cells induces an angiogenic response through viral binding to EGF receptor and beta1 and beta3 integrins. Proc Natl Acad Sci U S A 2008; 105:5531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 2006; 20:2275–83. [DOI] [PubMed] [Google Scholar]
- 39. Bolovan-Fritts CA, Spector SA. Endothelial damage from cytomegalovirus-specific host immune response can be prevented by targeted disruption of fractalkine-CX3CR1 interaction. Blood 2008; 111:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Böttcher JP, Beyer M, Meissner F, et al. Functional classification of memory CD8+ T cells by CX3CR1 expression. Nat Commun 2015; 6:8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gerlach C, Moseman EA, Loughhead SM, et al. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 2016; 45:1270–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hikono H, Kohlmeier JE, Takamura S, et al. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med 2007; 204:1625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen CN, Chang SF, Lee PL, et al. Neutrophils, lymphocytes, and monocytes exhibit diverse behaviors in transendothelial and subendothelial migrations under coculture with smooth muscle cells in disturbed flow. Blood 2006; 107:1933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J Virol 2000; 74:8140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan N, Shariff N, Cobbold M, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 2002; 169:1984–92. [DOI] [PubMed] [Google Scholar]
- 46. O’Hara GA, Welten SP, Klenerman P, Arens R. Memory T cell inflation: understanding cause and effect. Trends Immunol 2012; 33:84–90. [DOI] [PubMed] [Google Scholar]
- 47. Snyder CM, Cho KS, Bonnett EL, et al. Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS Pathog 2011; 7:e1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abana CO, Pilkinton MA, Gaudieri S, et al. Cytomegalovirus (CMV) epitope-specific CD4+ T cells are inflated in HIV+ CMV+ subjects. J Immunol 2017; 199:3187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walton SM, Torti N, Mandaric S, Oxenius A. T-cell help permits memory CD8+ T-cell inflation during cytomegalovirus latency. Eur J Immunol 2011; 41:2248–59. [DOI] [PubMed] [Google Scholar]
- 50. Pachnio A, Ciaurriz M, Begum J, et al. Cytomegalovirus infection leads to development of high frequencies of cytotoxic virus-specific CD4+ T cells targeted to vascular endothelium. PLoS Pathog 2016; 12:e1005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garg A, Trout R, Spector S. Human immunodeficiency virus type-1 myeloid derived suppressor cells inhibit cytomegalovirus inflammation through interleukin-27 and B7-H4. Sci Rep 2017; 7:44485. [DOI] [PMC free article] [PubMed] [Google Scholar]