Abstract
Background
The efficacy of licensed direct-acting antiviral (DAA) regimens is assumed to be the same for hepatitis C virus (HCV)–monoinfected patients (HCV-Mono) and HIV/HCV-coinfected patients (HCV-Co). However, the high sustained viral response (SVR) rates of DAA regimens and the small number of HIV-infected patients included in registration trials have made it difficult to identify predictors of treatment failure, including the presence of HIV.
Methods
We compared treatment outcomes for ledipasvir/sofosbuvir (LDV/SOF) against HCV G1 in treatment-naïve HCV-Mono and HCV-Co without cirrhosis in a prospective registry of individuals receiving DAAs for HCV.
Results
Up to September 2017, a total of 17 269 patients were registered, and 1358 patients (1055 HCV-Mono/303 HCV-Co) met the inclusion criteria. Significant differences between HCV-Mono and HCV-Co were observed for age, gender, and G1 subtype distribution. Among HCV-Co, 99.0% were receiving antiretroviral therapy. SVR rates for LDV/SOF at 8 weeks did not differ significantly between HCV-Mono and HCV-Co (96.9% vs 94.0%; P = .199). However, the SVR rate for LDV/SOF at 12 weeks was significantly higher for HCV-Mono than HCV-Co (97.2% vs 91.8%; P = .001). A multivariable logistic regression model including age, sex, liver stiffness, G1 subtype, HCV-RNA, HIV, and treatment duration showed the factors associated with treatment failure to be male sex (adjusted odds ratio [aOR], 2.49; 95% confidence interval [CI], 1.27–4.91; P = .008) and HIV infection (aOR, 2.23; 95% CI, 1.13–4.38; P = .020).
Conclusions
The results of this large prospective study analyzing outcomes for LDV/SOF against HCV G1 in treatment-naïve noncirrhotic patients suggest that HIV infection is a predictor of treatment failure in patients with chronic hepatitis C.
Keywords: antiviral agents/administration & dosage/*therapeutic use, DAA, hepatitis C, chronic/*complications/*drug therapy, HIV infections/*complications, sustained virologic response
The licensure of direct-acting antiviral agents (DAAs) marked a turning point in the treatment of infection by hepatitis C virus (HCV) [1]. This therapeutic innovation offered new prospects for the treatment of individuals coinfected by HCV and HIV, a difficult-to-treat population in the interferon plus ribavirin (RBV) era, with lower sustained viral response (SVR) rates and higher frequencies of serious adverse events than patients monoinfected with HCV [2–4].
Currently, it is recommended that coinfected persons receive the same treatment as monoinfected individuals, assuming that the efficacy of approved DAA regimens does not differ between the groups [5, 6]; although this is not entirely the case for American Association for the Study of Liver Diseases/Infectious Diseases Society of America guidelines in the United States, in which ledipasvir and sofosbuvir (LDV/SOF) for 8 weeks is not recommended for patients with HIV/HCV coinfection, regardless of baseline HCV RNA level [6]. However, whether HIV infection is a predictor of treatment response to DAA regimens is difficult to assess owing to the high SVR rates associated with most licensed all-oral DAA-based regimens against HCV and the low number of clinical trials with DAA including both monoinfected and coinfected patients [7, 8]. As for real-world case series, some have found an association between HIV coinfection and lower SVR rates [9, 10], whereas in others, SVR rates were not significantly different between monoinfected and coinfected individuals [11–14].
We aimed to assess whether HIV infection is associated with failure of DAA therapy. Therefore, we focused on previously untreated noncirrhotic patients infected with HCV genotype 1, with or without HIV, who were treated with LDV/SOF. We adopted this approach because it provided a large enough sample size of patients and because we could easily control for some predictors of response, including genotype, type of regimen, presence of cirrhosis, previous exposure to anti-HCV therapy, and use of RBV.
METHODS
Design and Patient Selection
Patients were selected for this study from the Madrid Registry of Use of DAA for HCV (RUA-VHC), a compulsory prospective registry of individuals receiving DAAs for HCV infection in the region of Madrid created in November 2014 by the Madrid Regional Health Service (SERMAS). Providing baseline data for this online registry is mandatory for the retrieval of DAAs in hospital pharmacies. Likewise, providing exhaustive follow-up data is a condition for reimbursement. The decision to treat was made and the regimen selected by the treating physician according to current guidelines.
Data collected prospectively in the RUA-VHC have been reported in depth elsewhere [15]. In summary, baseline data included demographics, whether the patient was HIV-infected and receiving antiretroviral therapy (ART), HCV genotype and subtype, HCV RNA load, history of anti-HCV therapy, liver fibrosis stage, and presence or absence of cirrhosis. The date of initiation and type of DAA regimen, use of RBV, and planned treatment duration were also recorded.
In September 2016, a case report form was used to collect the following HIV-related variables offline: HIV transmission category, Centers for Disease Control and Prevention (CDC) clinical category, baseline and nadir CD4+ T-cell counts, and baseline HIV viral load. In March 2017, the online registry was modified to include all the variables related to HIV infection mentioned above. Since then, this information has been registered prospectively. The study protocol was approved by the ethics committee of Hospital Universitario La Paz for the analysis of anonymous routine clinical data without written informed consent for purposes of scientific publication.
In this analysis, patients included in the RUA-VHC were eligible if they were age 18 years or older, were infected with HCV genotype 1, were noncirrhotic and previously untreated, were treated with LDV/SOF without ribavirin for 8 or 12 weeks, and were scheduled to finish treatment on or before September 31, 2017.
Measurements
Fibrosis stage and cirrhosis were determined by liver biopsy or transient elastography (FibroScan, Echo-Sens, Paris, France). Cirrhosis was defined as liver stiffness >12.5 kPa or clinical evidence of liver decompensation. The remaining liver stiffness cutoffs were as follows: ≤7 kPa, the cutoff to rule out null or mild fibrosis; <9.5 kPa, the cutoff to rule out advanced fibrosis–cirrhosis [16].
HCV RNA was measured at baseline, at the end of therapy, and 12 weeks after completion of treatment. Real-time polymerase chain reaction assays for the quantification of HCV RNA included Roche COBAS AmpliPrep/COBAS TaqMan HCV (Roche Molecular Systems, Pleasanton, CA; lower limit of detection [LLOD], 15 IU/mL), Abbott RealTime HCV assay (Abbott Laboratories, Abbott Park, IL; LLOD, 12 IU/mL), and Siemens Versant HCV RNA, version 1.0 (Siemens Healthcare GmbH, Erlangen, Germany; LLOD, 15 IU/mL).
Outcomes
Follow-up data in the online registry included the following: (i) SVR, defined as undetectable plasma HCV RNA at 12 weeks after completion of treatment; (ii) relapse, defined as detectable post-treatment HCV RNA after undetectable HCV RNA at the end of therapy; and (iii) viral breakthrough, defined as detectable HCV RNA at the end of therapy and at week 12. Discontinuations due to adverse events or for reasons other than adverse events, losses to follow-up, and deaths were also registered.
Statistical Analysis
When analyzing treatment effectiveness, any patient who initiated LDV/SOF therapy without confirmed SVR or missing outcome data was considered a treatment failure. Three multivariable logistic regression models were used to identify independent baseline factors associated with treatment failure. The first model included variables that were associated with treatment failure in univariable analysis with P < .05. The second model included variables that were associated with treatment failure in univariable analysis with P < .1. The third model was a fully adjusted model including age, sex, liver stiffness, HCV genotype, HCV RNA load, HIV infection, and treatment duration. Analyses were performed for the entire data set and for subgroups of treatment duration (8 and 12 weeks). Wald tests were used to derive P values. The analyses were performed using Stata, version 14 (Stata Corp, College Station, TX).
RESULTS
Patient Characteristics
Up to September 2017, a total of 17 269 patients (13 720 monoinfected patients and 3549 coinfected patients) initiated all-oral DAAs for treatment of HCV infection in 25 hospitals in the region of Madrid. Of these, 1358 patients (1055 monoinfected patients and 303 coinfected patients) met the inclusion criteria (Supplementary Figure 1). A total of 272 of the 1358 patients (20.0%) included in this study were also included in a paper describing the real-world outcomes of all-oral DAA-based therapy in 2369 HIV/HCV-coinfected patients [15].
The baseline characteristics of the 1358 patients categorized by LDV/SOF treatment duration and by type of patient (monoinfected or coinfected) are shown in Table 1. Overall, 497 patients were treated with LDV/SOF for 8 weeks (36.6%), and 861 patients were treated with LDV/SOF for 12 weeks (63.4%). A higher percentage of monoinfected patients (39.2%) than coinfected patients (27.4%) were treated with LDV/SOF for 8 weeks.
Table 1. .
Baseline Characteristics of 1358 Previously Untreated Noncirrhotic Patients Infected With HCV Genotype 1 and Treated With LDV/SOF
| 8 wk | 12 wk | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Monoinfected Patients | Coinfected Patients | P a | Total | Monoinfected Patients | Coinfected Patients | P a | Monoinfected and Coinfected Patients | |
| Variables | n = 497 | n = 414 | n = 83 | n = 861 | n = 641 | n = 220 | n = 1358 | ||
| Age, median (IQR), y | 56 (49–66) | 59 (50–68) | 50 (46–54) | <.001 | 56 (50–68) | 61 (52–71) | 51 (48–54) | <0.001 | 56 (50–67) |
| Male sex, No. (%) | 258 (51.9) | 192 (46.4) | 66 (79.5) | <.001 | 491 (57.0) | 325 (50.7) | 166 (75.4) | <0.001 | 749 (55.1) |
| HCV genotype/subtype, No. (%) | <.001 | <0.001 | |||||||
| 1a | 160 (32.2) | 95 (22.9) | 65 (78.3) | 395 (45.9) | 224 (34.9) | 171 (77.7) | 555 (40.9) | ||
| 1b | 322 (64.8) | 311 (75.1) | 11 (13.2) | 434 (50.4) | 398 (62.1) | 36 (16.4) | 756 (55.7) | ||
| 1 not subtyped | 15 (3.0) | 8 (1.9) | 7 (8.4) | 32 (3.7) | 19 (3.0) | 13 (5.9) | 47 (3.5) | ||
| HCV RNA, No. (%) | |||||||||
| Log IU/mL, median (IQR) | 5.9 (5.4–6.4) | 5.9 (5.4–6.3) | 6.1 (5.6–6.5) | .033 | 6.4 (6.0–6.8) | 6.4 (5.9–6.8) | 6.5 (6.0–6.8) | 0.070 | 6.2 (5.7–6.6) |
| >6 million IU/mL, No. (%) | 18 (3.6) | 12 (2.9) | 6 (7.2) | .054 | 216 (25.1) | 154 (24.0) | 62 (28.2) | 0.220 | 234 (17.2) |
| Transient elastography, No. (%) | |||||||||
| No | 8 (1.6) | 8 (1.9) | 0 | 30 (3.5) | 30 (4.7) | 0 | 38 (2.8) | ||
| Yes | 489 (98.4) | 406 (98.1) | 83 (100.0) | 831 (96.5) | 611 (95.3) | 220 (100.0) | 1320 (97.2) | ||
| Stiffness kPa, median (IQR) | 8.6 (7.9–9.4) | 8.6 (7.9–9.3) | 8.6 (7.8–10.0) | .582 | 9.1 (8.1–10.4) | 9.1 (8.1–10.4) | 9.0 (8.1–10.3) | 0.534 | 8.8 (8.0–10.1) |
| ≥9.5 kPa, No. (%) | 121 (24.7) | 94 (23.1) | 27 (32.5) | .071 | 373 (44.9) | 284 (46.5) | 89 (40.4) | 0.123 | 494 (7.4) |
Abbreviations: HCV, hepatitis C virus; IQR, interquartile range; LDV/SOF, ledipasvir/sofosbuvir.
a P values were derived from Pearson’s chi-square test or the nonparametric Mann-Whitney test for differences in categorical or continuous variables, respectively.
In brief, 55.1% were men, and the median age was 56 years. The HCV subtype distribution was 1a (40.9%), 1b (55.7%), and 1 not subtyped (3.5%). The median HCV RNA was 6.2 Log IU/mL, and 17.2% of patients had an HCV RNA >6 million IU/mL. A total of 1320 (97.2%) patients underwent transient elastography at baseline. The median liver stiffness value was 8.8 kPa, and 494 (37.4%) patients had a liver stiffness value ≥9.5 kPa (but ≤12.5 kPa), which was indicative of advanced fibrosis. Statistically significant differences between monoinfected patients and coinfected patients were observed at baseline for age, gender, and genotype 1 subtype distribution (Table 1).
At baseline, 99.0% of coinfected patients were on ART. Full data on HIV-related characteristics were available for analysis from approximately half of the coinfected patients. No statistically significant differences were found between patients with complete HIV data and patients with incomplete HIV data for age, HCV genotype distribution, and HCV RNA load. However, patients with incomplete HIV data were more frequently male and had a lower liver stiffness value (Supplementary Table 1). Differences in mechanism of acquisition of HIV were found between coinfected patients treated for 8 or 12 weeks, with a lower proportion of injection drug use and a higher frequency of men who have sex with men among the former group (Table 2).
Table 2. .
Baseline HIV-Related Characteristics of 303 Previously Untreated Noncirrhotic HIV/HCV-Coinfected Patients With HCV Genotype 1 Who Were Treated With LDV/SOF
| Variable | 8 wk | 12 wk | Total | P a |
|---|---|---|---|---|
| Variables | n = 83 | n = 220 | n = 303 | |
| HIV risk factor, No. (%) | .021 | |||
| Injection drug use | 39 (47.0) | 120 (54.5) | 159 (52.5) | |
| Men who have sex with men | 8 (9.6) | 4 (1.8) | 12 (4.0) | |
| Heterosexual relations | 5 (6.0) | 8 (3.6) | 13 (4.3) | |
| Transfusions | 0 | 2 (0.9) | 2 (0.7) | |
| Unknown | 31 (37.3) | 86 (39.1) | 117 (38.6) | |
| CDC clinical category, No. (%) | .305 | |||
| A | 21 (25.3) | 38 (17.3) | 59 (19.5) | |
| B | 9 (10.8) | 38 (17.3) | 47 (15.5) | |
| C | 22 (26.5) | 60 (27.3) | 82 (27.1) | |
| Unknown | 31 (37.3) | 84 (38.2) | 115 (37.9) | |
| Nadir CD4+/mm3, No. (%) | .622 | |||
| >500 | 8 (9.6) | 14 (6.4) | 22 (7.3) | |
| 200–499 | 17 (20.5) | 38 (17.3) | 55 (18.1) | |
| <200 | 27 (32.5) | 84 (38.2) | 111 (36.6) | |
| Unknown | 31 (37.3) | 84 (38.2) | 115 (38.0) | |
| Baseline CD4+/mm3, No. (%) | ||||
| >500 | 22 (26.5) | 75 (34.1) | 97 (32.0) | .057 |
| 200–499 | 9 (10.8) | 43 (19.5) | 52 (17.2) | |
| <200 | 2 (2.4) | 7 (3.2) | 9 (3.0) | |
| Unknown | 50 (60.2) | 95 (43.2) | 145 (47.9) | |
| Known | 33 (39.8) | 125 (56.8) | 158 (52.1) | .008 |
| Median (IQR) | 632 (474–847) | 595 (372–819) | 607 (389–822) | .474 |
| ART, No. (%) | .285 | |||
| No | 0 | 3 (1.4) | 3 (1.0) | |
| Yes | 83 (100.0) | 217 (98.6) | 300 (99.0) | |
| HIV-RNA, No. (%) | ||||
| Unknown | 31 (37.3) | 81 (36.8) | 112 (37.0) | .932 |
| Known | 52 (62.6) | 139 (63.2) | 191 (63.0) | |
| Detectable | 5 (9.6) | 7 (5.0) | 12 (6.3) | .246 |
| Undetectable | 47 (90.4) | 132 (95.0) | 179 (93.7) |
Abbreviations: ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; HCV, hepatitis C virus.
a P values derived from Pearson’s chi-square test or the nonparametric Mann-Whitney test for differences in categorical or continuous variables, respectively.
Treatment Response
LDV/SOF for 8 Weeks
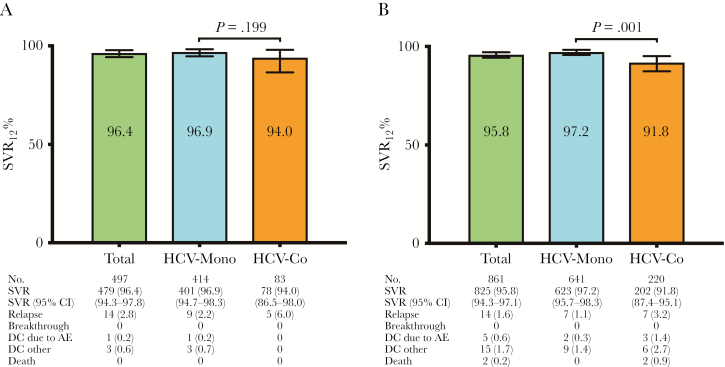
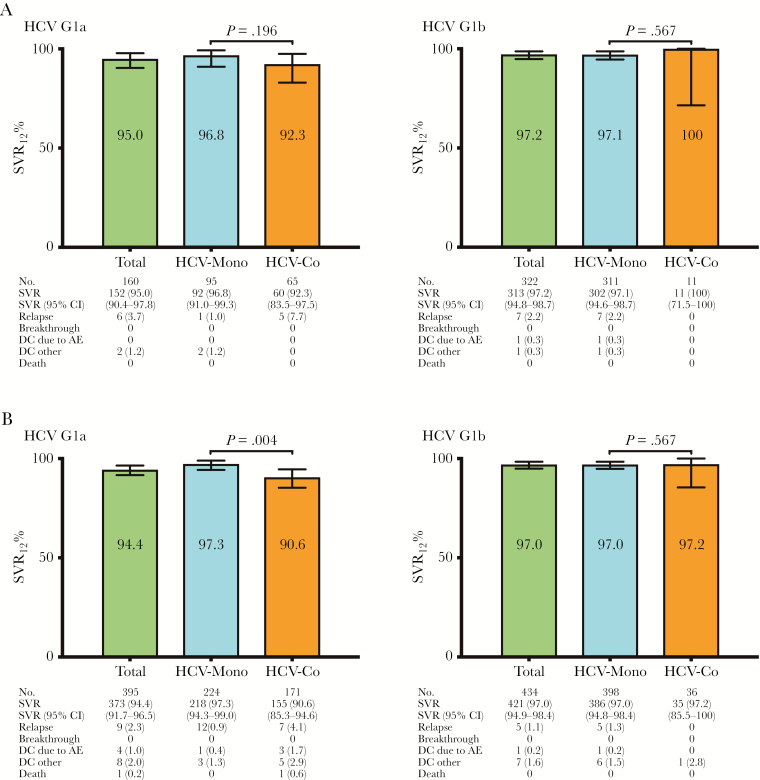
Treatment responses to LDV/SOF for 8 weeks are shown in Figure 1A. A total of 497 patients (414 monoinfected patients and 83 coinfected patients) received SOF/LDV without RBV for 8 weeks. Overall, the SVR rate of 8 weeks of therapy with SOF/LDV was 96.4%, without significant differences in SVR rates found between monoinfected patients and coinfected patients (96.9% vs 94.0%; P = .199). There were no viral breakthroughs. The overall relapse rate was 2.8%, and no statistically significant differences in relapse rates were found between monoinfected patients and coinfected patients (2.2% vs 6.0%; P = .06). Only 1 patient discontinued SOF/LDV owing to adverse events, and 3 patients discontinued therapy for reasons not related to adverse events. All 4 were monoinfected patients. Outcomes for 8 weeks’ treatment with LDV/SOF without RBV for HCV genotype 1a and 1b analysis are shown in Figure 2A.
Figure 1. .
A, Outcomes for 8 weeks of treatment with sofosbuvir/ledipasvir without ribavirin for HCV genotype 1 in treatment-naïve, noncirrhotic patients. B, Outcomes for 12 weeks of treatment with sofosbuvir/ledipasvir without ribavirin for HCV genotype 1 in treatment-naïve, noncirrhotic patients. P values are derived from Pearson’s chi-square test. Abbreviations: AE, adverse event; CI, confidence interval; DC, treatment discontinuations (number [%]); HCV, hepatitis C virus; HCV-Co, HIV/HCV-coinfected patients; HCV-Mono, HCV-monoinfected patients; SVR, sustained viral response (number [%]).
Figure 2. .
A, Treatment outcomes for 8 weeks of treatment with sofosbuvir/ledipasvir without ribavirin for HCV genotype 1a and 1b in treatment-naïve, noncirrhotic patients. B, Treatment outcomes for 12 weeks of treatment with sofosbuvir/ledipasvir without ribavirin for HCV genotype 1a and 1b in treatment-naïve, noncirrhotic patients. P values are derived from Pearson’s chi-square test. Abbreviations: AE, adverse event; CI, confidence interval; Coinfected patients, HIV/HCV-coinfected patients; DC, treatment discontinuations (number [%]); HCV, hepatitis C virus; MoP, HCV-monoinfected patients.
LDV/SOF for 12 Weeks
Treatment response to LDV/SOF for 12 weeks is shown in Figure 1B. A total of 861 patients (641 monoinfected patients and 220 coinfected patients) received SOF/LDV without RBV for 12 weeks. Overall, the SVR rate of 12 weeks of therapy with SOF/LDV was 95.8%. Statistically significant differences in SVR rates were found between monoinfected patients and coinfected patients (97.2% vs 91.8%; P = .001). Again, there were no viral breakthroughs. The overall relapse rate was 1.6%, and no statistically significant differences in relapse rates were found between monoinfected patients and coinfected patients (1.1% vs 3.2%; P = .06). Five patients (0.6%) discontinued SOF/LDV owing to adverse events (2 monoinfected patients [0.3%] and 3 coinfected patients [1.4%]; P = .11). Fifteen patients (1.7%) discontinued therapy for reasons not related to adverse events (9 monoinfected patients [1.4%] and 6 coinfected patients [2.7%]; P = .23). Two coinfected patients died before SVR could be assessed: both died from complications of acute exacerbation of chronic obstructive pulmonary disease. Outcomes for 12 weeks of treatment with LDV/SOF without RBV for HCV genotype 1a and 1b are shown in Figure 2B.
Variables Associated With Treatment Failure
The results of the univariable and multivariable logistic regression models to identify baseline variables associated with treatment failure for the full data set are shown in Table 3. In the univariable analysis, male sex and the presence of HIV infection were the only variables significantly associated with increased odds of treatment failure. In all 3 multivariable models, male sex and HIV infection were the only variables significantly associated with increased odds of treatment failure. In the fully adjusted model, the adjusted odds ratio (aOR) of treatment failure for males in comparison with females was 2.49 (95% CI, 1.27–4.91; P = .008). Likewise, the aOR of treatment failure for coinfected patients in comparison with monoinfected patients was 2.23 (95% CI, 1.13–4.38; P = .020). We also performed separate analyses to identify baseline variables associated with treatment failure according to treatment duration. Among patients treated for 8 weeks, male sex was the only variable independently associated with treatment failure (Supplementary Table 2). Among patients treated for 12 weeks, HIV infection was the only variable independently associated with treatment failure (Supplementary Table 3).
Table 3. .
Results From Univariable and Multivariable Logistic Regression Models to Identify Independent Baseline Factors Predictive of Treatment Failure Considering the Whole Data Set (Monoinfected, Coinfected, 8 and 12 Weeks), 1358
| Variable | Treatment Failures | Univariable | Multivariable Model 1a | Multivariable Model 2b | Multivariable Model 3c | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age | .473 | .441 | |||||||
| <45 | 4 (2.9) | 1.00 | 1 | ||||||
| 45–54 | 23 (4.8) | 1.69 (0.58–4.98) | 1.64 (0.55–4.89) | ||||||
| ≥55 | 27 (3.6) | 1.27 (0.44–3.67) | 2.03 (0.67–6.15) | ||||||
| Sex | .001 | .008 | .010 | .008 | |||||
| Female | 12 (2.0) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Male | 42 (5.6) | 2.96 (1.54–5.67) | 2.47 (1.27–4.82) | 2.43 (1.24–4.77) | 2.49 (1.27–4.91) | ||||
| Liver stiffness, kPaa | .300 | .399 | |||||||
| <9.5 | 28 (3.4) | 1.00 | 1 | ||||||
| ≥9.5 | 25 (5.1) | 1.52 (0.88–2.64) | 1.48 (0.84–2.61) | ||||||
| Unknown | 1 (2.6) | 0.77 (0.10–5.82) | 1.19 (0.15–9.27) | ||||||
| HCV genotype | .079 | .879 | .722 | ||||||
| 1b | 22 (2.9) | 1.00 | 1.00 | 1 | |||||
| 1a | 30 (5.4) | 1.91 (1.09–3.34) | 1.15 (0.60–2.19) | 1.27 (0.65–2.49) | |||||
| 1 not subtyped | 2 (4.3) | 1.48 (0.34–6.50) | 0.89 (0.19–4.07) | 0.89 (0.19–4.10) | |||||
| HCV RNA IU/mL | .911 | .676 | |||||||
| <6 000 000 | 45 (4.0) | 1.00 | 1 | ||||||
| ≥6 000 000 | 9 (3.8) | 0.96 (0.46–1.99) | 0.85 (0.40–1.82) | ||||||
| HIV infection | <.001 | .007 | .024 | .020 | |||||
| No | 31 (2.9) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 23 (7.6) | 2.71 (1.56–4.73) | 2.20 (1.24–3.89) | 2.08 (1.10–3.94) | 2.23 (1.13–4.38) | ||||
| Treatment duration | .612 | .850 | |||||||
| 12 wk | 36 (4.2) | 1.00 | 1 | ||||||
| 8 wk | 18 (3.6) | 0.86 (0.48–1.53) | 1.06 (0.57–1.96) |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; OR, odds ratio.
aMultivariable model 1 includes variables with a P value <.05 in the univariable analysis.
bMultivariable model 2 includes variables with a P value <.1 in the univariable analysis.
cMultivariable model 3 is a fully adjusted one, including every variable detailed in the first column.
DISCUSSION
Whether HIV infection is associated with treatment failure of DAA therapy is a controversial issue. The C-WORTHY and C-EDGE CO-STAR studies, 2 of the few clinical trials with DAA that included both monoinfected and coinfected patients, revealed similar rates of sustained virological response to grazoprevir and elbasvir in both groups [7, 8]. These results are not easy to interpret owing to issues of study design in the C-WORTHY study (different treatment durations, different doses of elbasvir, and the addition or not of ribavirin) [7] and the low number of coinfected patients included in the C-EDGE CO-STAR study [8]. Some real-world practice studies have found lower SVR rates with all-oral DAA-based therapy in coinfected patients than in monoinfected patients [9, 10], whereas in other studies, SVR rates were not significantly different between monoinfected and coinfected individuals [11–14]. Again, the results of these studies are difficult to interpret owing to the lack of homogeneity in essential variables such as HCV genotype, liver disease severity, prior exposure to anti-HCV therapy, and DAA regimen used.
We answered our research question by analyzing a large and homogeneous group of HCV genotype 1–infected treatment-naïve noncirrhotic patients treated with LDV/SOF. We found lower SVR rates for LDV/SOF in coinfected patients than in monoinfected patients, with most of the treatment failures in both groups being viral relapses. The independent association between HIV and treatment failure was found after adjustment for age, sex, liver stiffness, HCV subtype, HCV RNA load, and treatment duration.
Interestingly, when we carried out separate analyses according to treatment duration, we found an association between HIV and treatment failure among patients treated for 12 weeks but not among patients treated for 8 weeks. This could be due to a type 2 error, as the number of HIV-infected patients treated for 8 weeks was substantially lower than the number treated for 12 weeks (83 vs 220). However, it must be considered that in comparison with patients treated for 8 weeks, those treated for 12 weeks, irrespective of whether they were HIV-infected, had a significantly higher frequency of both HCV-RNA >6 million IU/mL and a liver stiffness value >9.5 kPa, which is indicative of advanced fibrosis. Moreover, among HIV-infected individuals, the proportion of MSM was higher in those treated for 8 weeks than in those treated for 12 weeks. Given the recent ongoing outbreak of HCV among HIV-infected MSM, it is conceivable that more recent HCV infections were included in the former group than in the latter. This, in turn, could have influenced SVR rates, as treatment of acute or early HCV infection has been associated with very high SVR rates [17]. For these reasons, any potentially disadvantageous influence of HIV infection on treatment response would be more easily detected in patients treated for 12 weeks than in patients treated for 8 weeks.
The reason why HIV infection influenced treatment response could not be ascertained from our data. However, this observation could be explained by some characteristics of coinfected patients, such as a CD4+ count <200 cells/mm3 and prior clinical AIDS, which are indicative of immunosuppression and have been associated with failure of all-oral DAA-based therapy in coinfected patients [15]. Of note, 82 of the 188 (43.6%) coinfected patients for which the CDC clinical category was known had a prior diagnosis of AIDS. Likewise, 9 of the 158 (5.7%) coinfected patients with known CD4+ cell counts at baseline had <200 CD4+ cells/mm3. There is some evidence that the host immune response may play a role in HCV clearance even during DAA-based therapies, possibly through the recognition and elimination by T cells of viral variants with resistance-associated substitutions (RAS) [18]. Of note, in a recent analysis of clinical trials of patients with genotype 1 HCV infection treated with LDV/SOF, NS5A RAS were present in 8%–16% of patients before treatment and had a negative impact on treatment outcome [19]. If less effective clearance of resistant viral variants by the immune system is the reason why HIV infection negatively affected treatment response, then the newer pan-genotypic drug regimens that include NS5A inhibitors with a high barrier to resistance (eg, sofosbuvir/velpatasvir or glecaprevir/pibrentasvir) could be a preferred option over other recommended regimens in coinfected patients with a prior diagnosis of AIDS and/or a CD4+ cell count <200 CD4+ cells/mm3.
Our study is limited by the lack of data on adherence and on concomitant medication, including proton pump inhibitors [20]. Another limitation is the absence of information on preexisting viral variants with RAS, which prevented us from analyzing their prevalence among groups and assessing their impact on treatment outcomes. Our study is also limited by the fact that full data on HIV-related characteristics at the time of the data analysis were available for only half of the patients. This precluded the performance of additional comparative analyses between monoinfected patients and coinfected patients with a baseline CD4+ cell count below and above 200 cells/mm3, or with and without a prior diagnosis of clinical AIDS, for better assessment of the effect of immunosuppression on treatment response among coinfected individuals. Finally, another limitation is the absence of information about the mechanism of transmission of HCV in HCV-monoinfected persons.
Nevertheless, to our knowledge, ours is the largest real-world study comparing treatment outcomes between monoinfected and coinfected patients in which there was no variability according to HCV genotype, liver disease severity, exposure to previous anti-HCV therapy, or DAA-based regimen.
In conclusion, the results of this study analyzing treatment outcomes for LDV/SOF against HCV genotype 1 in treatment-naïve noncirrhotic patients suggest that HIV infection is a predictor of treatment failure in patients with chronic hepatitis C. This finding cannot be used to support the notion that HIV/HCV-coinfected individuals are a difficult-to-treat population in the era of all-oral DAA-based therapy. However, it does argue in favor of prioritizing the use of newer simplified and pan-genotypic anti-HCV regimens among coinfected patients to lessen the possibility of treatment failure due to suboptimal adherence or presence of resistant viral variants.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank Thomas O’Boyle for writing assistance.
Financial support. This work was supported by the Spanish AIDS Research Network (RD16/0025/0017), which is included in the Spanish I+D+I Plan and is co-financed by ISCIII-Subdirección General de Evaluacion and European Funding for Regional Development (FEDER), and the Fondo de Investigación de Sanidad en España (FIS)/Instituto de Salud Carlos III (Spanish Health Research Funds; PI17/00657).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX. PHYSICIANS AND HOSPITAL PHARMACISTS OF THE MADRID REGISTRY OF USE OF DAA FOR HCV (RUA-VHC)
Hospital General Universitario Gregorio Marañón: Ahumada, Adriana; Ais Larisgoitia, Arantza; Aldámiz Echevarría Lois, María Teresa; Bañares Cañizares, Rafael; Berenguer Berenguer, Juan; Blanco Sánchez, Germán; Chamorro De Vega, Esther; Clemente Ricote, Gerardo; Collado Borell, Roberto; Díaz Fontenla, Fernando; Escudero Vilaplana, Vicente; Eworo Ndongo, Alia; Fernández Cruz, Ana; Flores Fernandez, Virginia; García González, Xandra; García Sánchez, Raquel; Gijón Vidaurreta, Paloma; Giménez Manzorro, Álvaro; Herranz Alonso, Ana; Ibáñez García, Sara; Lallana Sainz, Elena; Lobato Matilla, María Elena; López Bernaldo De Quirós, Juan Carlos; Manrique Rodríguez, Silvia; Martínez Fernandez-Llamazares, Cecilia; Martínez Ortega, Pilar; Miralles Martin, Pilar; Montilla De Mora, Pedro; Padilla Ortega, Belén; Parras Vázquez, Francisco; Pérez Valderas, María Dolores; Revuelta Herrero, José Luis; Ribed Sánchez, Almudena; Rincón Rodríguez, Diego; Rodríguez González, Carmen; Romero Cristóbal, Mario; Romero Jiménez, Rosa; Ruiz Martínez, Cristina; Salcedo Plaza, Magdalena; Sánchez Somolinos, Mar; Sarobe González, Camino; Valerio Minero, Maricela. Hospital Universitario La Paz: Baladé Martinez, Laura; Barreiro García, Pablo; Benítez García, Beatriz; Bernardino De La Serna, José; Castillo Grau, Pilar; De Sebastián Rueda, María; Erdozain Sosa, José Carlos; Freire González, Mercedes; Gabaldón Garnica, Paloma; García Sánchez, Araceli; García-Samaniego Rey, Francisco; González Del Valle, Luis; González Fernández, María Ángeles; González García, Juan J.; Jiménez Nacher, Inmaculada; Jiménez Vicente, Carlos; Lucena Martinez; Patricia; Martín Carbonero, Luz; Molina Cabezuelo, Marta; Montes Ramírez, Mª Luisa; Moreno Celda, Victoria; Moreno Palomino, Marta; Moreno Ramos, Francisco; Olveira Martín, Antonio; Parrilla, Mercedes; Pérez Valero, Ignacio; Rodríguez Martín, Elena; Rodríguez Nava, José Luis; Romero Portales, Miriam; Rossignoli Montero, Ana; Ruiz De Hoyos, Marta; Sobrino Jiménez, Carmen; Soriano Vázquez, Vicente; Valencia Ortega, Eulalia; Varela Fernandez, Hugo Adolfo. Hospital Ramón y Cajal: Albillos Martinez, Agustín; Anguita Ruiz, Eva; Arocena Aranguren, Carlos; Casado Osorio, José; Díaz De Santiago, Alberto; Fortún Abete, Jesús; García González, Miguel; Gea Rodríguez, Francisco; Gómez De Salazar López De Silanes, María Esther; Gramage Caro, Teresa; Graus Morales, Javier; Moreno Guillen, Santiago; Moreno Zamora, Ana María; Navas Elorza, Enrique; Pérez Elías, María Jesús; Pérez Molina, José Antonio; Quereda Rodríguez-Navarro, Carmen; Rodríguez Sagrado, Miguel Ángel; Serrano Villar, Sergio Daniel; Vélez Díaz-Palleres, Manuel; Vivancos Gallego, María Jesús. Hospital Doce de Octubre: Campo Angora, Mercedes; Castellano Tortajada, Gregorio; De Lagarde Sebastián, María; Fernández Vázquez, Inmaculada; Gómez Valbuena, Isabel; Manzano Alonso, María Luisa; Martin Algíbez, Ana María; Matarranz Del Amo, Mariano; Muñoz Gómez, Raquel; Pinar López, Oscar; Pulido Ortega, Federico; Rubio García, Rafael; Serrano Garrote, Olga. Hospital Clínico de San Carlos: Benítez Giménez, María Teresa; Cabello Clotet, Noemí; Cuenca Alarcón, Francisca; Devesa Medina, María José; Estrada Pérez, Vicente; Izquierdo Rubio, Sonia; Maroto Castellanos, Maite; Núñez Orantos, María José; Rodríguez Del Rio, Elena; Sáenz De Tejada López, Marta; Sánchez-Pobre Bejarano, Pilar; Santiago Pérez, Alejandro; Téllez Molina, María Jesús; Vergas García, Jorge. Hospital Fundación Jiménez Díaz: Álvarez Álvarez, Beatriz; Arias Moya, María Ángeles; Becares Martínez, Francisco Javier; Bonilla Porras, Macarena; Cabello Úbeda, Alfonso; Calvo Hernández, Rocío; González Guirado, Agustina; Górgolas Hernández, Miguel De; Hernández Segurado, Marta; Moran Ortiz-Desolorzano, Marta; Morón Merchante, Francisco Javier; Polo Lorduy, Benjamín Arturo; Porres Cubero, Juan Carlos; Tortajada Esteban, Elena Victoria; Varela Silva, Andrés L. Hospital Infanta Leonor: Barrio Antoranz, José; Barrueco Fernandez, Nélida; Cañamares Orbis, Irene; Carrión Alonso, Gemma; Cuevas Tascón, Guillermo; Escobar Rodríguez, Ismael; Esteban Alba, Concepción; Izquierdo García, Elsa; Liras Medina, Ángel; Lozano Maya, María Del Mar; Marino Martínez, Carolina; Ryan Murúa, Pablo; Sáez De La Fuente, Javier; Sánchez Rubio, Luis; Such Díaz, Ana; Tejedor Prado, Pilar; Troya García, Jesús; Villa Poza, José Carlos. Hospital de Puerta de Hierro: Arias Milla, Ana; Baños Pérez, Isolina; Benítez Gutiérrez, Laura María; Calleja Panero, José Luis; Cuervas Mons Martínez, Valentín; Duca, Ana María; Folguera Olías, Carlos; Menchén Viso, Belén; De La Revilla Negro, Juan; Ruiz Gutiérrez, Julia; Sánchez Guerrero, Amelia; Santiago Prieto, María Elvira. Hospital La Princesa: Alañón Plaza, Estefanía; Gallego Aranda, Tomás; García Buey, Luisa Consuelo; González Moreno, Leticia; Ibáñez Zurriaga, Amparo; Marinero Martinez-Lázaro, Almudena; Martinez Nieto, Concepción; Morell Baladrón, Alberto; Pérez Abanades, María; Ramírez Herráiz, Esther; Real Martinez, Yolanda; Santos Gil, Ignacio De Los. Hospital U. Príncipe de Asturias: Arranz Caso, José Alberto; Beceiro Pedreño, Inmaculada; Borrego Rodríguez, Gloria; Casas García, Esperanza; Costero Pastor, Ana Belén; De Miguel Prieto, Julio; Del Pozo Prieto, David; Fernández Pacheco Garcia-Valdecasas, María; Ginés Palomares, Ana; Hernández Gutiérrez, Cristina; Herranz Muñoz, Clara; Herrero Fernández, Marta; Lebrero García, Alberto; Novella Mena, María; Ortiz Campos, María; Pérez Pérez, Diana; Poves Martinez, Elvira; Santolaya Perrín, María Rosario; Sanz Moreno, José; Víctor Palomares, Virginia. Hospital Fundación de Alcorcón: Alonso López, Sonia; Bartolomé García, Emma; Fernández Rodríguez, Conrado; Gutiérrez García, Mª Luisa; Henríquez Camacho, Cesar Augusto; Hervás Gómez, Rafael; Losa García, Juan Emilio; Moreno Núñez, Leonor; Pérez Encinas, Montserrat; Polanco Paz, María Del Mar; Sanmartín Fenollera, Patricia; Velasco Arribas, María. Hospital Infanta Sofía: Albertos Rubio, Sonia; Beltrán Castaño, Rocío; Comas Redondo, Carmen; García Yubero, Cristina; González-Ruano Pérez, Patricia; Hidalgo Aguirre, Lorena; Malmierca Corral, Eduardo; Martinez Hernández, Alicia; Pérez Álvarez, Mónica; Portillo Horcajada, Laura; Suárez García, Inés María. Hospital de Fuenlabrada: Andrés Rosado, Ana; Canalejo Castrillero, Eduardo; Candel García, Beatriz; Carrasco Torrents, América; De La Poza Gómez, Gema; García Rebolledo, Eva María; Hernández Muniesa, Belén; Hinojosa Mena-Bernal, Juan; Mondejar Gutiérrez, Gemma María; Ontanon Nasarre, Ana; Piqueras Alcohol, Belén; Ruiz Giardin, José Manuel; Ruiz, Justo; San Martin López, Juan Víctor; Valer López-Fando, María Paz. Hospital Universitario de Getafe: Diez Fernandez, Raúl; Esteban Fernandez, Francisco Javier; Gaspar Alonso-Vega, Gabriel; Gil Ares, Fernando; Gómez Rubio, Mariano; Molina García, Teresa; Negro Vega, Eva; Pérez Caballero, Gloria; Sánchez Ayuso, Javier; Sánchez-Rubio Ferrández, Javier. Hospital Severo Ochoa: Agud Aparicio, José Luis; Aguirre Losada, Alberto Ángel; Castro Urda, José Luis; Cervero Jiménez, Miguel; Díaz Gómez, Estrella; Domínguez Gozalo, Andrea; García Benayas, Elena; Jusdado Ruiz-Capillas, Juan José; Muñoz Romero, Javier; Torres Perea, Rafael. Hospital de Móstoles: Barros Aguado, Carlos Antonio; Corrales Pérez, Laura; Crespo Robledo, Paloma; González Alonso, Raquel; Mañes Sevilla, Mireya; Merino Morales, Francisco; Moreno Sánchez, Diego; Moriel Sánchez, Carmen; Rubio Cebrián, Beatriz. Hospital del Henares: Campos Fernández De Sevilla, María De Los Ángeles; De Lorenzo Pinto, Ana; Delgado Téllez De Cepeda, Laura; Egües Lugea, Amaia; Gallego Úbeda, Marta; Ibáñez Pinto, Alberto; Sánchez Gómez, Argeme; Sanz Rojas, Patricia; Serrano Herranz, Regino; Tovar Pozo, María; Valbuena González, Marta. Hospital Infanta Cristina: Botella Mateu, Belén; Cuenca Morón, Beatriz De; De Guzmán García-Monge, María Teresa; Domingo Senra, Daniel; Domínguez García, Nuria; Esteban Fernandez-Zarza, Carlos; Martínez Consuegra, José Antonio; Matilla García, Elena; Melero Bermejo, José Antonio; Moreno Díaz, Raquel; Ortiz Duran, María; Pérez Quero, José Luis; Rodríguez Vargas, Blanca. Hospital del Sureste: Buendía Bravo, Silvia; Calvo Ramos, Irina; Capilla Montes, Cristina; Cruz Cruz, María Teresa; Díaz Sánchez, Antonio; Fernández Amago, María Teresa; García Benayas, María Teresa; González Alonso, María Rosario; Marzal Alfaro, Belén; Moya Valverde, Eloísa; Peñalver Cifuentes, Rafael; Rivero Fernández, Miguel. Hospital Rey Juan Carlos: García Barquero, Margarita; García García, Almudena; Gotuzzo Altez, Luis Ricardo; Huertas Velasco, María Antonia; Marcos Rojas Rodríguez De Quesada, Jorge; Nistal Juncos, Sara; Pérez Rial, Gabriel. Hospital Infanta Elena: Alarcón Del Amo, Cristina; Alcalde Rodríguez, Daniel; Barranco Cao, Raquel; Calvache Rodríguez, Almudena; Chico Álvarez, Inmaculada; Collados Arroyo, Virginia; Del Portillo Rubí, Álvaro; Delgado Galán, María; López Martin, María Del Carmen; Rodríguez Rodríguez, Raquel; Tejedor Bravo, Marta; Vegas Serrano, Ana. Hospital de Torrejón: Achécar Justo, Linette María; Arponen, Sari; Blasco Guerrero, Marta; Del Rio Izquierdo, María; Esteva Jiménez, Laura; Gimeno García, Alejandra; González Pino, Andrea; Montero Hernández, María Carmen; Morales Martínez, Lorena; Sáez Bertrand, Catalina. Hospital del Tajo: Alonso Grandes, Elena; Fernández Esteban, Eva; Lo Iacono, Oreste; Monsalvo Arroyo, Raquel; Pedraza Cezón, Luis Antonio; Soto Fernández, Susana; Terrancle Juan, Ignacio. Hospital Gómez Ulla: García Mayor, María De Los Ángeles; López Honduvilla, Francisco José; Menéndez Martínez, María Antonia; Pérez Moran, María José; Prats Olivan, Pilar. Hospital Collado Villalba: Abad Guerra, Javier; Arias Rivera, María Luisa; Bejerano Domínguez, Alicia; Correa Abanto, Lizbeth Milagros; Ferrere González, Federico; Gómez Pérez, Marta; Olivares Valles, Ana. Hospital El Escorial: Aguilar Guisado, Carolina; Belda Bilbao, Luis Miguel; Calvo Fernández, Santiago; García De Aguinaga, Mª Luisa; García Gimeno, María Mercedes; Gongorra López, Andrea; Jaime Sánchez, Belén; Montero Jiménez, Francisco Javier; Sánchez Suárez, Susana. Hospital Central de La Cruz Roja: Fuentes Irigoyen, Raquel; Iborra Herrera, Jerónimo; Navarrete García, Elena; Tejada González, Pilar. Hospital Infantil Niño Jesús: García Rodríguez, María Del Pilar; Muñoz Codoceo, Rosa Ana.
Prior presentation. Initial data from this analysis were presented at the Conference on Retroviruses and Opportunistic Infections (CROI); March 4–7, 2018; Boston, MA (Presentation #607).
References
- 1. Liang TJ, Ghany MG. Therapy of hepatitis C—back to the future. N Engl J Med 2014; 370:2043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. APRICOT Study Group Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004; 351:438–50. [DOI] [PubMed] [Google Scholar]
- 3. Carrat F, Bani-Sadr F, Pol S, et al. ANRS HCO2 RIBAVIC Study Team Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA 2004; 292:2839–48. [DOI] [PubMed] [Google Scholar]
- 4. McHutchison JG, Lawitz EJ, Shiffman ML, et al. IDEAL Study Team Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361:580–93. [DOI] [PubMed] [Google Scholar]
- 5. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2017; 66:153–94. [DOI] [PubMed] [Google Scholar]
- 6.American Association for the Study of Liver Diseases/Infectious Diseases Society of America. HCV guidance: Recommendations for testing, managing, and treating hepatitis C http://www.hcvguidelines.org. Accessed 8 April 2019. [DOI] [PMC free article] [PubMed]
- 7. Sulkowski M, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet 2015; 385:1087–97. [DOI] [PubMed] [Google Scholar]
- 8. Dore GJ, Altice F, Litwin AH, et al. C-EDGE CO-STAR Study Group Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016; 165:625–34. [DOI] [PubMed] [Google Scholar]
- 9. Arias A, Aguilera A, Soriano V, et al. Rate and predictors of treatment failure to all-oral HCV regimens outside clinical trials. Antivir Ther 2017; 22:307–12. [DOI] [PubMed] [Google Scholar]
- 10. Neukam K, Morano-Amado LE, Rivero-Juárez A, et al. HIV-coinfected patients respond worse to direct-acting antiviral-based therapy against chronic hepatitis C in real life than HCV-monoinfected individuals: a prospective cohort study. HIV Clin Trials 2017; 18:126–34. [DOI] [PubMed] [Google Scholar]
- 11. Ingiliz P, Christensen S, Kimhofer T, et al. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C virus (HCV) infection in HCV-monoinfected and HIV-HCV-coinfected individuals: results from the German Hepatitis C Cohort (GECCO-01). Clin Infect Dis 2016; 63:1320–4. [DOI] [PubMed] [Google Scholar]
- 12. Montes ML, Olveira A, Ahumada A, et al. HULP-HUGM Study Group Investigators Similar effectiveness of direct-acting antiviral against hepatitis C virus in patients with and without HIV infection. AIDS 2017; 31:1253–60. [DOI] [PubMed] [Google Scholar]
- 13. Bruno G, Saracino A, Scudeller L, et al. HCV mono-infected and HIV/HCV co-infected individuals treated with direct-acting antivirals: to what extent do they differ? Int J Infect Dis 2017; 62:64–71. [DOI] [PubMed] [Google Scholar]
- 14. Bischoff J, Mauss S, Cordes C, et al. Rates of sustained virological response 12 weeks after the scheduled end of direct-acting antiviral (DAA)-based hepatitis C virus (HCV) therapy from the National German HCV Registry: does HIV coinfection impair the response to DAA combination therapy? HIV Med 2018; 19:299–307. [DOI] [PubMed] [Google Scholar]
- 15. Berenguer J, Gil-Martin Á, Jarrin I, et al. All-oral direct-acting antiviral therapy against hepatitis C virus (HCV) in human immunodeficiency virus/HCV-coinfected subjects in real-world practice: Madrid Coinfection Registry findings. Hepatology 2018; 68:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology 2017; 152:1544–77. [DOI] [PubMed] [Google Scholar]
- 17. Rockstroh JK, Bhagani S, Hyland RH, et al. Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial. Lancet Gastroenterol Hepatol 2017; 2:347–53. [DOI] [PubMed] [Google Scholar]
- 18. Ahlen G, Frelin L, Brenndorfer ED, et al. Containing “The Great Houdini” of viruses: combining direct acting antivirals with the host immune response for the treatment of chronic hepatitis C. Drug Resist Updat 2013; 16:60–7. [DOI] [PubMed] [Google Scholar]
- 19. Zeuzem S, Mizokami M, Pianko S, et al. NS5A resistance-associated substitutions in patients with genotype 1 hepatitis C virus: prevalence and effect on treatment outcome. J Hepatol 2017; 66:910–8. [DOI] [PubMed] [Google Scholar]
- 20. Tapper EB, Bacon BR, Curry MP, et al. Evaluation of proton pump inhibitor use on treatment outcomes with ledipasvir and sofosbuvir in a real-world cohort study. Hepatology 2016; 64:1893–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.