Abstract
Background
The recently established association between higher levels of DNA-incorporated thioguanine nucleotides and lower relapse risk in childhood acute lymphoblastic leukaemia (ALL) calls for reassessment of prolonged 6-thioguanine (6TG) treatment, while avoiding the risk of hepatotoxicity.
Objectives
To assess the incidence of hepatotoxicity in patients treated with 6TG, and to explore if a safe dose of continuous 6TG can be established.
Data sources
Databases, conference proceedings, and reference lists of included studies were systematically searched for 6TG and synonyms from 1998–2018.
Methods
We included studies of patients with ALL or inflammatory bowel disorder (IBD) treated with 6TG, excluding studies with 6TG as part of an intensive chemotherapy regimen. We uploaded a protocol to PROSPERO (registration number CRD42018089424). Database and manual searches yielded 1823 unique records. Of these, 395 full-texts were screened for eligibility. Finally, 134 reports representing 42 studies were included.
Results and conclusions
We included data from 42 studies of ALL and IBD patients; four randomised controlled trials (RCTs) including 3,993 patients, 20 observational studies including 796 patients, and 18 case reports including 60 patients. Hepatotoxicity in the form of sinusoidal obstruction syndrome (SOS) occurred in 9–25% of the ALL patients in two of the four included RCTs using 6TG doses of 40–60 mg/m2/day, and long-term hepatotoxicity in the form of nodular regenerative hyperplasia (NRH) was reported in 2.5%. In IBD patients treated with 6TG doses of approximately 23 mg/m2/day, NRH occurred in 14% of patients. At a 6TG dose of approximately 12 mg/m2/day, NRH was reported in 6% of IBD patients, which is similar to the background incidence. According to this review, doses at or below 12 mg/m2/day are rarely associated with notable hepatotoxicity and can probably be considered safe.
Introduction
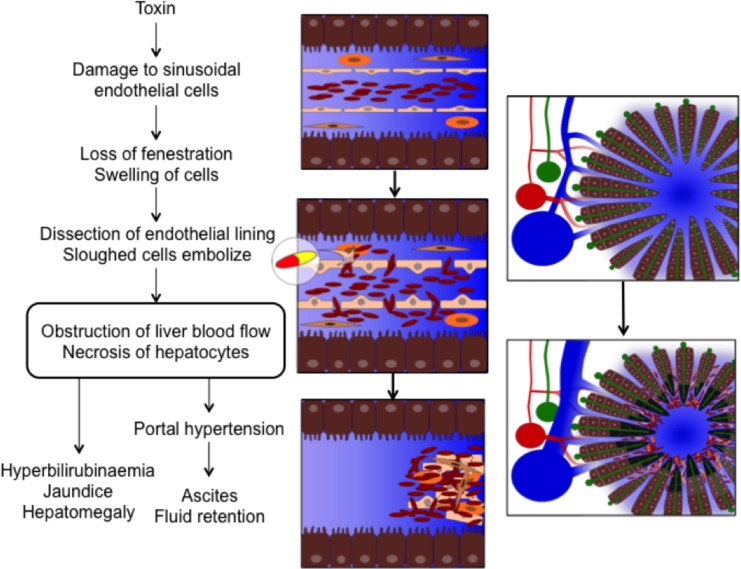
Hepatotoxicity was first noted as a complication of 6-thioguanine (6TG) treatment in the late 1970s [1] and has since been described in the form of sinusoidal obstruction syndrome (SOS) in childhood acute lymphoblastic leukaemia (ALL) [2–4] and nodular regenerative hyperplasia (NRH) in both inflammatory bowel disease (IBD) [5] and childhood ALL. [6] This reflects that SOS and NRH possibly represent a spectrum of related hepatic microvascular disorders spanning acute onset to chronic manifestation, although the underlying cellular mechanisms that connect them remain to be established Fig 1. [7–9]
Fig 1. The pathophysiology of sinusoidal obstruction syndrome.
The presumed pathophysiological mechanism underlying sinusoidal obstruction syndrome is a drug-mediated damage to sinusoidal endothelial cells, causing swelling and loss of fenestration. This allows red blood cells to enter the space of Disse and dissect off the endothelial lining. The sloughed off cells embolise downstream and cause obstruction of the hepatic microcirculation and consequently hepatocellular necrosis. [7] These pathophysiological changes lead to the clinical symptoms: jaundice, tender hepatomegaly, ascites and fluid retention. Figure by Linea Natalie Toksvang.
The development and severity of 6TG-induced hepatotoxicity appear to be dose-related, and rarely occurs at low cumulative doses. [10,11] Hence, a non-systematic review from 2013 focusing on IBD patients reported NRH in 0–53% of patients at 6TG doses of 12–58 mg/m2/day (20–100 mg/day) with no cases reported with 6TG doses below 12 mg/m2/day. [5] A meta-analysis from 2011 analysed 6TG treatment in childhood ALL and reported a seven-fold increased incidence of SOS in two of three randomised controlled trials (RCTs), in which patients received 6TG at doses of 40–60 mg/m2/day instead of 6-mercaptopurine (6MP) during maintenance treatment. [12]
Overall survival for childhood ALL now exceeds 90%; [13] however the poor prognosis following relapse still remains a major challenge. [14] Thus, although a number of novel anti-leukaemic treatment options, including immunotherapy, have emerged in recent years, their role in first line therapy remains uncertain. [13] Therefore, it is important to explore possible improvements with the use of the traditional anti-leukaemic agents, including thiopurines. The theoretical pharmacological advantages of 6TG over 6MP include a more direct intracellular activation pathway, higher potency, and shorter duration of exposure necessary for cytotoxicity. The cytotoxic effect of 6TG and 6MP is ascribed to the metabolite thioguanine nucleotides (TGN), which are incorporated into DNA and RNA as false purine bases as well as inhibiting de novo synthesis of purines. [3,15] Red blood cell (RBC) levels of TGN (ery-TGN) have been assumed to reflect treatment intensity and adherence. An ery-TGN level of more than 230–260 pmol/8·108 RBC has been associated with therapeutical response in IBD patients. However, the association between metabolite levels, efficacy, and adverse events remains uncertain. [16] It was recently demonstrated that ery-TGN correlate with TGN incorporated into leucocyte DNA (DNA-TGN). Furthermore, higher levels of DNA-TGN correlate with a lower relapse risk in childhood ALL. [17] This finding calls for a reassessment of the feasibility of prolonged 6TG treatment for childhood ALL, while avoiding the risk of severe hepatotoxicity.
The primary objective of this systematic review was to assess the incidence of hepatotoxicity in IBD and childhood ALL patients treated with 6TG compared to 6MP or standard of care. The secondary objective was to explore if a safe dose of 6TG can be established.
Methods
We conducted this systematic review in accordance with the Cochrane Handbook [18] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. [19,20] The systematic review protocol was uploaded to the International Prospective Register of Systematic Reviews (PROSPERO) before the literature search was conducted (registration number CRD42018089424), and is presented in S1 Appendix. Furthermore the protocol has been published on protocols.io (dx.doi.org/10.17504/protocols.io.zjnf4me). The review authors were not blinded to the journal titles, study authors or institutions. In case of insufficient reporting, study authors were contacted for additional information. We sought to avoid double counting by juxtaposing author names, location, setting, and sample sizes.
Eligibility criteria
We included RCTs, controlled clinical trials including quasi-randomised trials, observational studies (e.g. prospective and retrospective cohort studies, case-control, and cross-sectional studies), case series, and case reports in which patients at any age were treated with 6TG. We excluded studies investigating 6TG as part of an intensive chemotherapy regimen. Since most studies using 6TG for an extended period of time either as monotherapy or as part of maintenance treatment are within the field of IBD and ALL, we chose to limit the review to these two disease populations. We compared 6TG treatment to 6MP or standard of care (other non-6TG treatment regimens). We included studies published between 1998 and March 2018. We made no restrictions regarding timing, type of setting or language.
Information sources
We systematically searched PubMed/MEDLINE, Embase/Ovid, Scopus/Elsevier, Web of Science Core Collection/Clarivate Analytics, and The Cochrane Central Register of Controlled Trials (CENTRAL)/Wiley (The Cochrane Library, issue 2, 2018). The search strategy included synonyms of 6TG in title, author keywords or thesaurus, and the time frame 1998 to 2018, and was conferred with a health care librarian. The specific search strategies for each database are presented in S2 Appendix.
Additional manual searches included reference lists of included studies, conference proceedings from the American Society of Hematology (ASH), European Hematology Association (EHA), American College of Gastroenterology (ACG), European Crohn’s and Colitis Organization (ECCO), American Association for the Study of Liver Diseases (AASLD), International Union of Basic & Clinical Pharmacology (IUPHAR), and European Association for Clinical Pharmacology and Therapeutics (EACPT). The ClinicalTrials.gov registry (www.clinicaltrials.gov), the International Standard Randomized Controlled Trial Number (ISRCTN) registry (www.controlled-trials.com), and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en) were searched for research protocols, and PROSPERO was searched for on-going systematic reviews. We conducted the latest search on March 7, 2018.
Study selection
We imported the 3,377 records from the database search into the reference manager software EndNote (Clarivate Analytics, Boston, MA, USA), and made a gross removal of duplicates. We then used Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) to remove additional duplicates and conduct the screening. Two review authors independently screened titles and abstracts and subsequently evaluated eligible reports in full text. We recorded reasons for exclusion. The 134 included records were downloaded in full text and added to a shared group in the reference manager software Mendeley (Elsevier Inc, New York, NY, USA).
Data collection process
Two review authors independently extracted data from included studies in duplicate using a standardised extraction form in the software Excel Microsoft Office 2010 (Microsoft, Redmond, WA, USA), which was approved by all authors and pilot tested on ten reports. A third author checked data extraction. A simplified sheet was used for articles in Polish, Russian, and Chinese–with consultant help for data extraction. Extracted data items included authors and year of publication, study characteristics (design, blinding, setting, duration, inclusion and exclusion criteria, source of funding, conflict of interest, ethical approvals, key conclusions), patient characteristics (number of patients, age, sex, ethnicity, disease, comorbidity, concomitant therapy), details of the interventions (duration of 6TG, dose of 6TG, cumulative dose of 6TG, maximum dose of 6TG, route of administration, ery-TGN levels), comparator (comparator drug, duration of 6MP or other standard of care drug, dose of 6MP or other standard of care drug, cumulative dose of 6MP or other standard of care drug, maximum dose of 6MP or other drug, route of administration), follow-up, and outcomes as described below.
Primary outcome
Incidence of any hepatotoxicity reported as SOS, veno-occlusive disease (VOD), NRH, discordant thrombocytopenia (transfusion-resistant and/or unexplained by treatment), drug-induced liver injury or non-specified hepatotoxicity. Due to the lack of standardised definitions of hepatotoxicity, authors of the included studies may not have used the above-mentioned terms. To assess additional hepatotoxicity we therefore report any pathological findings of liver biopsies and use the Ponte di Legno (PdL) toxicity working group consensus criteria for SOS, which entail fulfilment of at least three out of the following five criteria: (i) hyperbilirubinaemia; (ii) hepatomegaly; (iii) ascites; (iv) weight gain of 5% or more; (v) discordant thrombocytopenia.[21] Furthermore, we considered an increase in alanine transaminase, aspartate transaminase, alkaline phosphatase, conjugated bilirubin or total bilirubin of more than two times upper normal limit as evidence of hepatotoxicity. [22]
Secondary outcomes
Diagnostic methods (number of patients who had a liver biopsy, indication for liver biopsy, other diagnostic methods, study conclusions about diagnostic methods); dose reduction or truncation of 6TG due to hepatotoxicity (how many patients had 6TG truncated or the dose reduced; doses before and after dose reduction).
Risk of bias of individual studies
Two review authors independently evaluated risk of bias of the included reports using the Cochrane Collaboration tool for RCTs. [18] The study quality assessment tools of the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) for quality assessment of Observational Cohort and Cross-Sectional Studies and Controlled Intervention Studies [23] were used to assess the risk of bias of observational studies. Case reports and case series that were in English and not a subset of a RCT or observational study were evaluated through the “Methodological quality and synthesis of case series and case reports” approach. [24] A graphic illustration of potential bias of RCTs was made using the Review Manager (RevMan [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Studies with low risk of bias were ascribed more weight in the overall findings of the review compared to studies with higher risk of bias.
Assessment of heterogeneity
We will visualise the findings of the included studies and heterogeneity between the studies in a figure. Meta-regression analyses were not performed.
Data synthesis
We present a systematic narrative synthesis of the characteristics and findings of the included studies, since the studies were too heterogeneous in terms of design and comparator in order to perform a quantitative data synthesis. We calculated doses in mg/m2 with the assumption that an average adult is 1.73 m2, and that 30 kg correspond to one m2. Data are presented as median (range) unless otherwise stated.
Meta-biases
We addressed publication bias by searching for and including grey literature, such as conference abstracts not published in journals or books. To address selective outcome bias, we compared outcomes between protocols and published reports. If protocols were unavailable, we compared outcomes reported in the methods and results sections. We did not quantitate the impact of meta-biases.
Confidence in cumulative evidence
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach [25] was used to assess the confidence in cumulative evidence. Two review authors independently evaluated the quality of evidence in each of four domains: (1) study design, (2) study quality, (3) consistency, and (4) directness. The evidence on each outcome was graded as ‘very low’, ‘low’, ‘moderate’ or ‘high’. Evidence based on RCTs was initially graded as high, whereas evidence based on observational studies was graded as low. If the studies had serious limitations, important inconsistencies, or uncertainty about the directness of evidence, the overall quality estimate was downgraded.
Results
Study selection
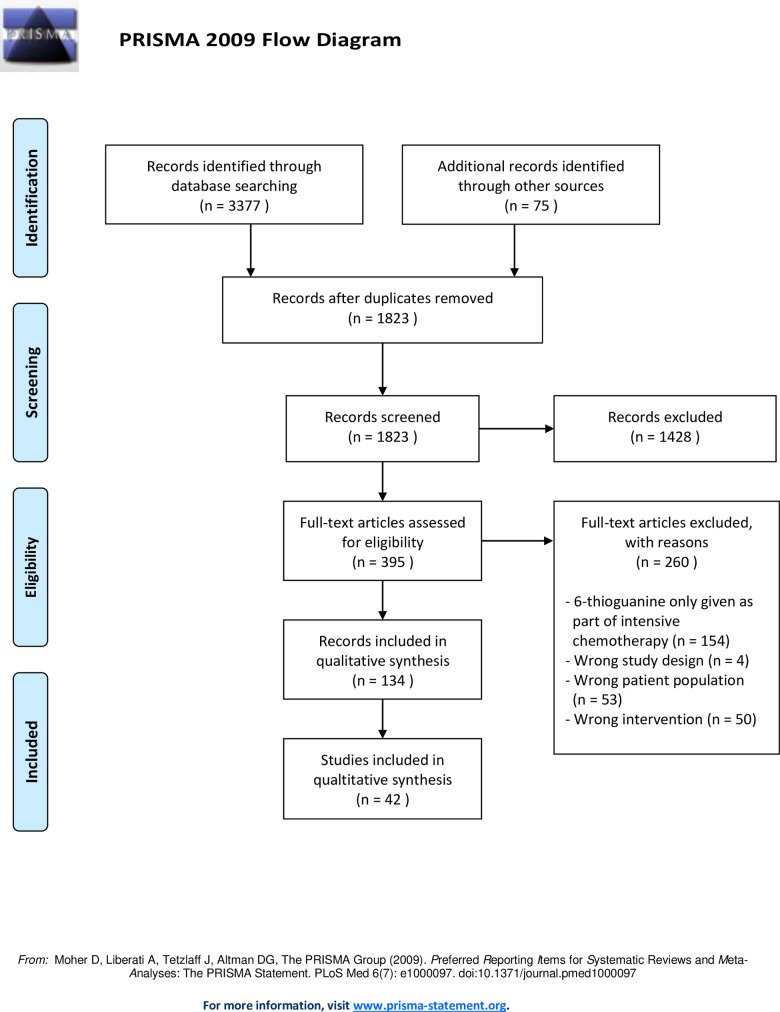
A flow diagram of study selection is presented in Fig 2. The database and manual searches yielded 1823 unique records. Of these, we screened 395 in full-text for eligibility. Finally, 134 reports representing 42 studies were included in the review. Of these, four were RCTs [2,3,26,27], 20 were observational studies [6,27–45], and 18 were case reports [11,46–62]. No similar reviews were found in the database search or on PROSPERO.
Fig 2. PRISMA flow diagram of study selection.
Study characteristics
Characteristics of the individual studies are presented in S3 Appendix.
Risk of bias within studies
Judgements of each risk of bias item for included RCTs are illustrated in S4 Appendix. None of the RCTs were blinded in respect to participants and personnel or outcome assessment. Risk of bias evaluations of observational studies are included in Table B in S3 Appendix. No serious bias was suspected, yet in most studies outcome assessors were not blinded to exposure status, and no studies used power calculations to justify sample size. Risk of bias evaluations of case reports and case series are included in Table C in S3 Appendix. The included case reports/series were generally well described with sufficient details, yet none examined a dose-response relationship or made a re-challenge with 6TG, which would have been preferable.
Meta-biases
Manual searches yielded additionally two reports eligible for inclusion in the review. Six conference abstracts without a published original article were found, but the authors did not respond to our enquiries. Risk of bias was not evaluated for conference abstracts without an associated article. Protocols were only available for two RCTs [2,3] and one pharmacokinetic study, [35] in which no inconsistencies were found. Outcomes reported in the methods and results sections of the remaining reports were compared, and no serious inconsistencies were found.
Confidence in cumulative evidence
All three outcomes were evaluated in RCTs, although the largest body of evidence came from observational studies. The overall quality of the primary outcome ‘incidence of hepatotoxicity’ was graded as moderate, and the first secondary outcome ‘use of diagnostic methods’ was graded as moderate, primarily because of inconsistencies in reporting. The overall quality of the second secondary outcome ‘dose reduction or truncation of 6TG’ was graded as high. The overall quality estimate was evaluated taking the following four elements into account 1) study design, 2) study quality, 3) consistency, 4) directness (Table 1).
Table 1. Overview of GRADE evaluation.
| Outcomes | Study design, n | Study quality | Consistency | Directness | Overall quality |
|---|---|---|---|---|---|
| Incidence of hepatotoxicity | Randomised clinical trials, 4 Observational studies, 15 |
No serious limitations | Important inconsistency in the definition of hepatotoxicity and how it is described | No uncertainty about directness | Moderate |
| Diagnostic methods | Randomised clinical trials, 2 Observational studies, 8 Case reports, 5 |
No serious limitations | Important inconsistency in the use of diagnostic modalities across studies | No uncertainty about directness | Moderate |
| Reduction or truncation of 6TG | Randomised clinical trials, 3 Observational studies, 10 Case reports, 9 |
No serious limitations | No serious inconsistency | No uncertainty about directness | High |
Results of individual studies
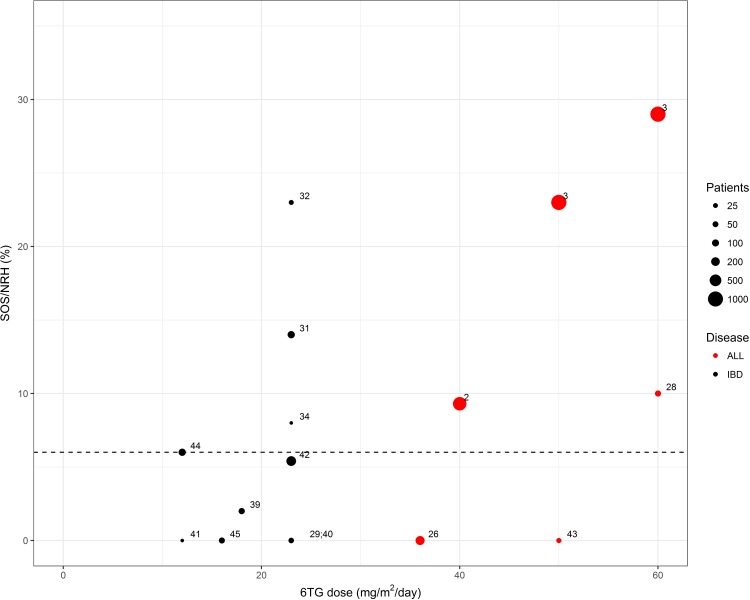
Fig 3 represents the incidence of SOS or NRH compared to 6TG dose in the included studies.
Fig 3. Incidence of sinusoidal obstruction syndrome (SOS) or nodular regenerative hyperplasia (NRH) compared to 6-thioguanine (6TG) dose in the included studies.
Studies with reported mean or median 6TG dose and incidence of hepatotoxicity defined as either SOS or NRH are depicted. Doses in mg/m2 were calculated with the assumption that an adult is 1.73 m2, and that 30 kg correspond to one m2. The dotted line refers to an incidence of 6%, corresponding to the suggested background incidence.
A detailed overview of the results of the individual studies is presented in S3 Appendix.
Synthesis of results
We included data from a total of 42 studies, encompassing four RCTs including 3,993 patients, 20 observational studies including 796 patients, and 18 case reports including 60 patients.
In two of three RCTs on 6TG versus 6MP at doses 6TG of 40–60 mg/m2/day for maintenance treatment in childhood ALL, hepatotoxicity was reported in the form of SOS in up to 25% of patients in the 6TG-arm. [2,3] The incidence of SOS was highest in the CCG-1952 trial, which used the highest target dose. Moreover, in this trial the incidence of SOS was reduced to 20% when the 6TG target dose was changed from 60 to 50 mg/m2. [3] Evidence of persisting hepatotoxicity including NRH and complications of portal hypertension were reported in 2.5% of patients receiving 6TG. No hepatotoxicity was reported during 6MP treatment. [2,3] One of the RCTs on 6TG versus 6MP did not report SOS, but found evidence of hepatotoxicity by means of an increased risk of discordant thrombocytopenia during 6TG treatment. [26]
Starting doses of 6TG of approximately 23 mg/m2/day were used in five observational studies of adults with IBD, which were associated with elevated liver enzymes in 8–25% of patients and histological evidence of NRH in 14–23% of patients. Seven studies used a lower 6TG dose of approximately 12 mg/m2/day, and NRH was reported in 0–6% of patients. [44]
In regard to the included case reports, the 6TG dosage was higher in the ones that reported hepatotoxicity (14–125 mg/m2/day) [48–54,56,58–60,63] compared to the ones that did not report hepatotoxicity (0.8–21 mg/m2/day). [42,55,57,62]
Hepatotoxicity documented by blood samples, various imaging modalities, or liver biopsy was not persistently associated with higher ery-TGN levels. [31,64,65]
The included RCTs were generally well executed with low risk of bias, except in the areas of blinding participants and outcome assessment, which may lead to performance and detection bias. Risk of bias assessments in the included observational studies did not result in any suspicion of specific biases. Evaluation of risk of bias in case reports and case series did not give rise to concern for specific bias–bearing in mind that case reports and case series are always associated with an intrinsic risk of bias. The confidence in the cumulative estimate of the incidence of hepatotoxicity was graded as moderate, and the use of diagnostic methods as moderate, primarily because of the inconsistencies in reporting within these subjects. The confidence in the cumulative estimate of dose reduction or truncation of 6TG was graded as high.
Discussion
The findings of the present systematic review indicate that 6TG-induced hepatotoxicity in the form of SOS or NRH is highly dose-dependent, and that it rarely occurs at daily doses of less than 12 mg/m2/day. Furthermore, 6TG-induced hepatotoxicity appears to be largely reversible, except at high doses exceeding 40 mg/m2/day.
The three RCTs of childhood ALL, the COALL-92 trial, the CCG-1952 trial, and the UK MRC ALL97/99 trial, respectively, have previously been included in a meta-analysis, which estimated the increase in SOS between treatment arms to be a factor 7.16 (OR, 95% CI 5.66–9.06). [12] SOS was highlighted as a dose-related toxicity with a multifactorial aetiology. No SOS was reported in the COALL-92 trial, albeit a high frequency of discordant thrombocytopenia, which may reflect a minor degree of hepatotoxicity. In that trial, vincristine and corticosteroids were not co-administered with 6TG–as was the case in the other two RCTs. [26] This difference in the co-medication has been proposed to be an explanation for the differing incidence of SOS between the three RCTs. [2]
A balance between activation and inactivation pathways, which are affected by genetic variations, determines the toxicity of thiopurines. The enzyme thiopurine methyltransferase (TPMT) inactivates 6MP by methylation. A heterozygous TPMT genotype (5–10%) in the Caucasian background population) [66] is associated with around 50% enzyme activity leading to increased intracellular concentrations of thioguanine nucleotides (compared to the TPMT wildtype genotype); thus entailing an increased risk of myelotoxicity with standard-dose thiopurine therapy. [67] It has been hypothesised that germline genetic variations in TPMT may be associated with an increased risk for 6TG-related SOS. However, 6TG is a much poorer substrate for TPMT compared to 6MP, so this is presumably of less influence. [68] NUDT15 is another gene in which genetic polymorphism may lead to a higher risk of myelotoxicity due to higher intracellular thioguanine nucleotide levels. [69] The frequency of TPMT mutations is lower in Chinese and Southwest Asian populations compared to Caucasians.[70] Conversely, NUDT15 polymorphisms are over-represented in Asians. [69] Since TPMT genotyping and/or phenotyping are now routinely performed before thiopurine administration in many centres, this limits the impact of ethnicity.
In 351 patients enrolled in the 6TG arm of the CCG-1952 trial, polymorphisms of TPMT were not associated with the risk of SOS. [46] The CCG-1952 trial did not report any association between age and SOS incidence. [3] In the UK MRC ALL97/99 trial, TPMT activity was lower in 95 children who developed SOS, mean TPMT acitivity 13.4 (range, 5.6–23) U/mL erythrocytes, compared to 161 control patients, mean 15.2 (range, 5.3–27) U/mL erythrocytes. [71] Although this difference was significant, the groups greatly overlap. There was no association between SOS and age or gender. [71] Although overall gender differences in hepatotoxicity did not appear in the UK MRC ALL97/99 trial, [2] a cohort of 99 consecutive children treated in one centre found male sex to be a risk factor for SOS. [72] This excess of male children may indicate gender differences in the metabolism of thioguanine, but may also be explained by the longer treatment duration for males in this protocol.
Overall, this review finds no clear evidence for an association between SOS risk and known genetic polymorphisms, sex, age, or ethnicity.
The theory that NRH is 6TG dose or ery-TGN level-dependent was first presented in 2005. [11] This is supported by a mouse model, in which SOS arose from high peak concentrations of 6TG, but did not occur with low daily doses of 6TG. [10] Most of the included studies did not find an association between ery-TGN-levels and hepatotoxicity, thus the findings do not support the use of ery-TGN for assessing the risk of 6TG-induced hepatotoxicity.
Several factors in the included observational studies investigating IBD may have led to an overestimation of hepatotoxicity. The risk of NRH development is associated with the use of immunosuppressive drugs often applied in IBD such as azathioprine, 6MP, ciclosporin, and steroids. [73] Thus, hepatotoxicity has been recognised in as many as 20% of IBD patients treated with azathioprine, 6MP, or methotrexate. [74] Azathioprine is the thiopurine most often associated with NRH, [75] and since most of the 6TG-treated IBD patients have received azathioprine previously, signs of NRH could have been present beforehand. As baseline liver biopsies prior to 6TG treatment were not obtained in any of the studies, some of the reported cases of NRH may likely have been triggered by other factors than 6TG. Furthermore, NRH or sinusoidal dilatation has previously been found in 6% and 34% of liver biopsies, respectively, from thiopurine-naïve IBD patients, [76] and the prevalence of NRH is probably as high as 5% in the background population, as suggested by autopsy studies of otherwise healthy individuals. [73,77] Thus, the incidence of NRH around 6% in the studies using 6TG at doses of approximately 12 mg/m2/day may not differ notably from the background incidence. Additionally, selection bias arising from only taking a biopsy in patients with suspected hepatotoxicity is supposedly present in most of the studies included in this review. Only one study performed liver biopsies in all patients in the cohort, avoiding selection bias, and found NRH in 6% (N = 7). [44]
The patient populations of the observational IBD studies had all failed conventional immunosuppressive treatment because of clinical unresponsiveness or adverse events, and undergone substantial surgery. Thus, the results cannot be generalised to the broader group of IBD patients. Moreover, 6TG undergoes extensive first-pass metabolism in the intestines and the liver; yielding bioavailability between 5–37%. [30] How IBD per se affects absorption and pharmacokinetics of 6TG is unknown. [35] The true incidence of hepatotoxicity in IBD patients treated with 6TG therefore remains unknown. However, a consensus paper by the European 6TG working party from 2006 recommends that 6TG can be used in a clinical research setting, in doses not exceeding 25 mg/day, corresponding to 14 mg/m2/day, which is considered a safe dose. [78] This is supported by the results of this systematic review.
While liver biopsy with reticulin stain remains the gold standard for diagnosis of hepatotoxicity, [75] non-invasive techniques such as magnetic resonance imaging/angiography (MRI/MRA) and Doppler ultrasound examination are frequently used as screening tools. A study comparing MRI to liver biopsy results found a sensitivity of 77% and a specificity of 72% in detecting NRH. [79] Furthermore, MRI is useful for detection of NRH-associated complications, including splenomegaly or ascites. [79] The European 6TG working party suggests screening by complete blood count, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, bilirubin, and C-reactive protein at baseline as well as one, two, four, eight, and 12 weeks followed by 3-month intervals. [78] When using low-dose 6TG, a liver biopsy is no longer routine practice but is reserved for patients with symptoms of portal hypertension or persisting liver test abnormalities. [44]
Determination of hepatic venous pressure gradient (HVPG) may be useful in the evaluation of the risk of complications, since varices only develop, when HVPG exceeds 10 mmHg. However, being an invasive technique it is rarely used. [32] The features of hepatotoxicity that have the highest sensitivity include discordant thrombocytopenia and splenomegaly. The latter is easily detected by clinical and ultrasound examinations. [80]
NRH is asymptomatic in most cases; leading to laboratory abnormalities without clinical signs, or is complicated by portal hypertension. [81] In a study evaluating 1,886 liver transplant patients, NRH was the reason for transplant in less than 1% of the cases. [82] In the studies included in this review, two patients required liver transplantation. [2,3] This indicates that NRH rarely leads to hepatic failure.
The present review emphasises the need for international standardised definitions of 6TG-related hepatotoxicity, clinical trials evaluating the use of non-invasive diagnostic methods in regard to detection of hepatotoxicity, and studies investigating the underlying mechanisms of 6TG-related hepatotoxicity. The included RCTs solely evaluated high doses of 6TG; hence, RCTs on low-dose 6TG are warranted. A RCT evaluating very low and individually titrated doses of 6TG (2.5–12.5 mg/m2/day) with concurrent conventional 6MP/methotrexate maintenance therapy in childhood ALL is currently being undertaken (ClinicalTrials.gov identifier:NCT02912676).
Limitations
We included only patient populations with IBD and childhood ALL, which are the two largest groups of patients treated with 6TG. Generalisation in respect to 6TG-related hepatotoxicity of these two distinct populations must be interpreted with caution. A few separate reports exist of 6TG treatment in coeliac disease, psoriasis, autoimmune hepatitis, pilocytic astrocytoma, vertebral chloroma, and collagenous sprue. Furthermore, 6TG is used in the treatment of acute myeloid leukaemia as well as in intensive combination chemotherapy phases of many ALL protocols. These studies were not included because of short duration of 6TG administration and the extensive concomitant therapy with hepatotoxic drugs. Moreover, we found inconsistencies in the definitions of hepatotoxicity as well as in the diagnostic modalities across the included studies, which may limit the comparability between the studies. Finally, all studies investigating 6TG treatment in the IBD population were observational, without control groups, which entail higher risk of bias, particularly publication bias and confounding bias. However, observational studies are valuable for the description of rare adverse events, and provide a large body of evidence in the present review.
Conclusions
Acute and severe hepatotoxicity occur in up to 25% of patients, when using 6TG doses of 40 mg/m2/day or higher in studies on 6TG for adult IBD and childhood ALL. 6TG-related hepatotoxicity persists in the form of NRH in 3% of the patients. However, the use of 6TG doses of approximately 12 mg/m2/day or less leads to hepatotoxicity in only 6% of the adult patients, corresponding to the incidence in the background population. Chronic hepatotoxicity in the form of portal hypertension has not been observed when using 6TG doses less than 40 mg/m2/day. Thus, 6TG doses below 12 /m2/day can be considered safe.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The Danish Childhood Cancer Foundation and the Danish Cancer Society supported this work financially. The funders were not involved in the protocol development, review conduct, data analysis and interpretation, or dissemination of the systematic review.
References
- 1.Griner PF, Elbadawi A, Packman CH. Veno-occlusive disease of the liver after chemotherapy of acute leukemia. Report of two cases. Ann Intern Med. 1976;85(5):578–82. [DOI] [PubMed] [Google Scholar]
- 2.Vora A, Mitchell CD, Lennard L, Eden TOB, Kinsey SE, Lilleyman J, et al. Toxicity and efficacy of 6-thioguanine versus 6-mercaptopurine in childhood lymphoblastic leukaemia: a randomised trial. Lancet. 2006. October 14;368(9544):1339–48. 10.1016/S0140-6736(06)69558-5 [DOI] [PubMed] [Google Scholar]
- 3.Stork LC, Matloub Y, Broxson E, La M, Yanofsky R, Sather H, et al. Oral 6-mercaptopurine versus oral 6-thioguanine and veno-occlusive disease in children with standard-risk acute lymphoblastic leukemia: report of the Children’s Oncology Group CCG-1952 clinical trial. Blood. 2010. April 8;115(14):2740–8. 10.1182/blood-2009-07-230656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toksvang LN, De Pietri S, Nielsen SN, Nersting J, Albertsen BK, Wehner PS, et al. Hepatic sinusoidal obstruction syndrome during maintenance therapy of childhood acute lymphoblastic leukemia is associated with continuous asparaginase therapy and mercaptopurine metabolites. Pediatr Blood Cancer. 2017. September;64(9):e26519. [DOI] [PubMed] [Google Scholar]
- 5.Musumba CO. Review article: the association between nodular regenerative hyperplasia, inflammatory bowel disease and thiopurine therapy. Aliment Pharmacol Ther. 2013. November;38(9):1025–37. 10.1111/apt.12490 [DOI] [PubMed] [Google Scholar]
- 6.Roy Moulik N, M Taj M. Long-term risk of portal hypertension and related complications in children treated with 6-thioguanine for acute lymphoblastic leukemia: A single-center experience. Pediatr Blood Cancer. 2017. October;64(10):e26495. [DOI] [PubMed] [Google Scholar]
- 7.DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis. 2002. February;22(1):27–42. 10.1055/s-2002-23204 [DOI] [PubMed] [Google Scholar]
- 8.DeLeve LD. Vascular Liver Disease In: DeLeve LD, Garcia-Tsao G, editors. Vascular liver disease: Mechanisms and management. New York, NY: Springer New York; 2011. p. 25–40. [Google Scholar]
- 9.Ghabril M, Vuppalanchi R. Drug-induced nodular regenerative hyperplasia. Semin Liver Dis. 2014. May 31;34(2):240–5. 10.1055/s-0034-1375963 [DOI] [PubMed] [Google Scholar]
- 10.Oancea I, Png CW, Das I, Lourie R, Winkler IG, Eri R, et al. A novel mouse model of veno-occlusive disease provides strategies to prevent thioguanine-induced hepatic toxicity. Gut. 2013. April;62(4):594–605. 10.1136/gutjnl-2012-302274 [DOI] [PubMed] [Google Scholar]
- 11.de Boer NK, Mulder CJJ, van Bodegraven AA. Nodular regenerative hyperplasia and thiopurines: the case for level-dependent toxicity. Liver Transplant. 2005. October;11(10):1300–1. [DOI] [PubMed] [Google Scholar]
- 12.Escherich G, Richards S, Stork LC, Vora AJ. Meta-analysis of randomised trials comparing thiopurines in childhood acute lymphoblastic leukaemia. Leukemia. 2011. June;25(6):953–9. 10.1038/leu.2011.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santiago R, Vairy S, Sinnett D, Krajinovic M, Bittencourt H. Novel therapy for childhood acute lymphoblastic leukemia. Expert Opin Pharmacother. 2017. July 24;18(11):1081–99. 10.1080/14656566.2017.1340938 [DOI] [PubMed] [Google Scholar]
- 14.Oskarsson T, Söderhäll S, Arvidson J, Forestier E, Montgomery S, Bottai M, et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: Prognostic factors, treatment and outcome. Haematologica. 2016;101(1):68–76. 10.3324/haematol.2015.131680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamson PC, Poplack DG, Balis FM. The cytotoxicity of thioguanine vs mercaptopurine in acute lymphoblastic leukemia. Leuk Res. 1994. November;18(11):805–10. [DOI] [PubMed] [Google Scholar]
- 16.de Boer NK, van Bodegraven AA, Jharap B, de Graaf P, Mulder CJ. Drug Insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nat Clin Pract Gastroenterol Hepatol. 2007. December 1;4(12):686–94. 10.1038/ncpgasthep1000 [DOI] [PubMed] [Google Scholar]
- 17.Nielsen SN, Grell K, Nersting J, Abrahamsson J, Lund B, Kanerva J, et al. DNA-thioguanine nucleotide concentration and relapse-free survival during maintenance therapy of childhood acute lymphoblastic leukaemia (NOPHO ALL2008): a prospective substudy of a phase 3 trial. Lancet Oncol. 2017. April 1;18(4):515–24. 10.1016/S1470-2045(17)30154-7 [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Internet]. Available from: www.cochrane-handbook.org [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009. July 21;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009. October;62(10):e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 21.Schmiegelow K, Attarbaschi A, Barzilai S, Escherich G, Frandsen TL, Halsey C, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016. June;17(6):e231–9. 10.1016/S1470-2045(16)30035-3 [DOI] [PubMed] [Google Scholar]
- 22.Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007. July;102(7):1518–27. 10.1111/j.1572-0241.2007.01187.x [DOI] [PubMed] [Google Scholar]
- 23.NIH, National Heart Lung and Blood Institute. The study quality assessment tools of the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) for quality assessment of Observational Cohort and Cross-Sectional Studies and Con [Internet]. [cited 2018 Oct 1]. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools
- 24.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ evidence-based Med. 2018. April;23(2):60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004. June 19;328(7454):1490 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harms DO, Gobel U, Spaar HJ, Graubner UB, Jorch N, Gutjahr P, et al. Thioguanine offers no advantage over mercaptopurine in maintenance treatment of childhood ALL: Results of the randomized trial COALL-92. Blood. 2003;102(8):2736–40. 10.1182/blood-2002-08-2372 [DOI] [PubMed] [Google Scholar]
- 27.Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005;23(31):7942–50. 10.1200/JCO.2005.01.1031 [DOI] [PubMed] [Google Scholar]
- 28.Jacobs SS, Stork LC, Bostrom BC, Hutchinson R, Holcenberg J, Reaman GH, et al. Substitution of oral and intravenous thioguanine for mercaptopurine in a treatment regimen for children with standard risk acute lymphoblastic leukemia: A collaborative Children’s Oncology Group/National Cancer Institute pilot trial (CCG-1942). Pediatr Blood Cancer. 2007;49(3):250–5. 10.1002/pbc.20964 [DOI] [PubMed] [Google Scholar]
- 29.Cheung TK, Florin THJ. 6-Thioguanine: A new old drug to procure remission in inflammatory bowel disease. Intern Med J. 2003;33:44–6. [DOI] [PubMed] [Google Scholar]
- 30.Deibert P, Dilger K, Fischer C, Hofmann U, Nauck S, Stoelben S, et al. High variation of tioguanine absorption in patients with chronic active Crohn’s disease. Aliment Pharmacol Ther. 2003;18(2):183–9. [DOI] [PubMed] [Google Scholar]
- 31.Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, et al. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 2003. August;125(2):298–303. [DOI] [PubMed] [Google Scholar]
- 32.Ferlitsch A, Teml A, Reinisch W, Ulbrich G, Wrba F, Homoncik M, et al. 6-Thioguanine associated nodular regenerative hyperplasia in patients with inflammatory bowel disease may induce portal hypertension. Am J Gastroenterol. 2007;102(11):2495–503. 10.1111/j.1572-0241.2007.01530.x [DOI] [PubMed] [Google Scholar]
- 33.Hasyagar CP, Foster EN, Gerich M, Prindiville TP. Treatment of inflammatory bowel disease with 6-thioguanine (6-TG): Retrospective case series from a tertiary care center. Am J Gastroenterol. 2007;102:S490. [Google Scholar]
- 34.Ivastinovic D, Hoegenauer C, Petritsch W, Wenzl HH, Hinterleitner TA. Efficacy of 6-thioguanine in patients with Crohn’s disease intolerant to azathioprine. Gastroenterology. 2004;126(4):A630–1. [Google Scholar]
- 35.Jharap B, de Boer NK, Vos RM, Smid K, Zwiers A, Peters GJ, et al. Biotransformation of 6-thioguanine in inflammatory bowel disease patients: A comparison of oral and intravenous administration of 6-thioguanine. Br J Pharmacol. 2011;163(4):722–31. 10.1111/j.1476-5381.2011.01265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kornbluth A, Kissous M, George J, Dubinsky MC. “Lower” 6-thioguanine (6-TG) levels may be as effective as “higher” 6-TG levels in IBD patients treated with thioguanine. Gastroenterology. 2002;122(4):A221. [Google Scholar]
- 37.Lancaster DL, Patel N, Lennard L, Lilleyman JS. 6-Thioguanine in children with acute lymphoblastic leukaemia: Influence of food on parent drug pharmacokinetics and 6-thioguanine nucleotide concentrations. Br J Clin Pharmacol. 2001;51(6):531–9. 10.1046/j.0306-5251.2001.01391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omer OS, Salehi S, Pee L, Gera A, Loganayagam A. 6-Thioguanine as an alternative therapy in inflammatory bowel disease-experience in a London district general hospital. United Eur Gastroenterol J. 2015;1:A437. [Google Scholar]
- 39.Pavlidis P, Ansari A, Duley J, Oancea I, Florin T. Splitting a therapeutic dose of thioguanine may avoid liver toxicity and be an efficacious treatment for severe inflammatory bowel disease: a 2-center observational cohort study. Inflamm Bowel Dis. 2014. December;20(12):2239–46. 10.1097/MIB.0000000000000206 [DOI] [PubMed] [Google Scholar]
- 40.Qasim A, McDonald S, Sebastian S, McLoughlin R, Buckley M, O’Connor H, et al. Efficacy and safety of 6-thioguanine in the management of inflammatory bowel disease. Scand J Gastroenterol. 2007;42(2):194–9. 10.1080/00365520600825166 [DOI] [PubMed] [Google Scholar]
- 41.Saleem R, Gera A, Tranah TH, Paolino A, Loganayagam A. To evaluate the efficacy and safety of 6-thioguanine therapy in patients with inflammatory bowel disease-a London DGH experience. United Eur Gastroenterol J. 2013;1:A526. [Google Scholar]
- 42.Teml A, Schwab M, Hommes DW, Almer S, Lukas M, Feichtenschlager T, et al. A systematic survey evaluating 6-thioguanine-related hepatotoxicity in patients with inflammatory bowel disease. Wien Klin Wochenschr. 2007. September;119(17–18):519–26. 10.1007/s00508-007-0841-0 [DOI] [PubMed] [Google Scholar]
- 43.Thomson B, Park JR, Felgenhauer J, Meshinchi S, Holcenberg J, Geyer JR, et al. Toxicity and efficacy of intensive chemotherapy for children with acute lymphoblastic leukemia (ALL) after first bone marrow or extramedullary relapse. Pediatr Blood Cancer. 2004;43(5):571–9. 10.1002/pbc.20128 [DOI] [PubMed] [Google Scholar]
- 44.van Asseldonk DP, Jharap B, Verheij J, den Hartog G, Westerveld DB, Becx MC, et al. The Prevalence of Nodular Regenerative Hyperplasia in Inflammatory Bowel Disease Patients Treated with Thioguanine Is Not Associated with Clinically Significant Liver Disease. Inflamm Bowel Dis. 2016. September;22(9):2112–20. 10.1097/MIB.0000000000000869 [DOI] [PubMed] [Google Scholar]
- 45.Ward MG, Patel K V, Kariyawasam VC, Goel R, Warner B, Elliott TR, et al. Thioguanine in inflammatory bowel disease: Long-term efficacy and safety. United Eur Gastroenterol J. 2017. June;5(4):563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wray L, Vujkovic M, McWilliams T, Cannon S, Devidas M, Stork L, et al. TPMT and MTHFR genotype is not associated with altered risk of thioguanine-related sinusoidal obstruction syndrome in pediatric acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2014. November;61(11):2086–8. 10.1002/pbc.25057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bisschop D, Germain ML, Munzer M, Trenque T. [Thioguanine, pancreatotoxicity?]. Therapie. 2001;56(1):67–9. [PubMed] [Google Scholar]
- 48.Broxson EH, Dole M, Wong R, Laya BF, Stork L. Portal hypertension develops in a subset of children with standard risk acute lymphoblastic leukemia treated with oral 6-thioguanine during maintenance therapy. Pediatr Blood Cancer. 2005. March;44(3):226–31. 10.1002/pbc.20202 [DOI] [PubMed] [Google Scholar]
- 49.Chojnacki C, Romanowski M, Wachowska-Kelly P. Psychosomatic complications during treatment for ulcerative colitis. Gastroenterol Rev. 2012;1(1):52–5. [Google Scholar]
- 50.Rawat D, Gillett PM, Devadason D, Wilson DC, McKiernan PJ. Long-term follow-up of children with 6-thioguanine-related chronic hepatoxicity following treatment for acute lymphoblastic leukaemia. J Pediatr Gastroenterol Nutr. 2011. November;53(5):478–9. 10.1097/MPG.0b013e31822960e9 [DOI] [PubMed] [Google Scholar]
- 51.Fritz E, Wrba F, Knoflach P. Variceal hemorrhage in a patient with ulcerative colitis treated with 6-thioguanine. Inflamm Bowel Dis. 2008;14(4):582–3. 10.1002/ibd.20323 [DOI] [PubMed] [Google Scholar]
- 52.Kane S, Cohen SM, Hart J. Acute sinusoidal obstruction syndrome after 6-thioguanine therapy for Crohn’s disease. Inflamm Bowel Dis. 2004. Sep;10(5):652–4. 10.1097/00054725-200409000-00023 [DOI] [PubMed] [Google Scholar]
- 53.Mao J, Zhao F, Song H, Yang S, Shen D, Tang Y. Thioguanine treatment-related sinusoidal obstruction syndrome in 2 children. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15(9):788–90. [PubMed] [Google Scholar]
- 54.Marasco G, Scaioli E, Renzulli M, Colecchia A, Golfieri R, Festi D, et al. MRI Patterns in a Case of 6-Thioguanine-Related Hepatic Sinusoidal Obstruction Syndrome. Am J Gastroenterol. 2016;111(6):767. [DOI] [PubMed] [Google Scholar]
- 55.Mares WG, Wong DR, Gilissen LP, Masclee AA, Hooymans PM, Engels LG. Safe 6-thioguanine therapy of a TPMT deficient Crohn’s disease patient by using therapeutic drug monitoring. J Crohn’s Colitis. 2009;3(2):128–30. [DOI] [PubMed] [Google Scholar]
- 56.Merino JM, Casanova F, Saez-Royuela F, Velasco A, Gonzalez JB. Veno-occlusive disease of the liver associated with thiopurines in a child with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2000;17(5):429–31. 10.1080/08880010050034391 [DOI] [PubMed] [Google Scholar]
- 57.Nielsen SN, Frandsen TL, Nersting J, Hjalgrim LL, Schmiegelow K. Pharmacokinetics of 6-thioguanine and 6-mercaptopurine combination maintenance therapy of childhood ALL: Hypothesis and case report. J Pediatr Hematol Oncol. 2015;37(3):e206–9. 10.1097/MPH.0000000000000246 [DOI] [PubMed] [Google Scholar]
- 58.Radys W.; Kaminska B.; Landowski P.; Brodzicki J. The case of colitis ulcerosa—Diagnostic and therapeutic difficulties. Pediatr Wspolczesna. 2006;8(2):131–3. [Google Scholar]
- 59.Rulyak SJ, Saunders MD, Lee SD. Hepatotoxicity associated with 6-thioguanine therapy for Crohn’s disease. J Clin Gastroenterol. 2003;36(3 PG-234-237):234–7. [DOI] [PubMed] [Google Scholar]
- 60.Salerno A, Vacante M, Pollina D, Stancanelli B, Martini S, David E, et al. Liver veno-occlusive disease (VOD) in a patient given 6-thioguanine for crohn’s disease. Br J Med Pract. 2014;7(2):a711. [Google Scholar]
- 61.Shastri S, Dubinsky MC, Fred Poordad F, Vasiliauskas EA, Geller SA. Early nodular hyperplasia of the liver occurring with inflammatory bowel diseases in association with thioguanine therapy. Arch Pathol Lab Med. 2004;128(1):49–53. [DOI] [PubMed] [Google Scholar]
- 62.Van den Berg SA, de Boer M, van der Meulen-de Jong AE, Jansen JM, Hoentjen F, Russel MG, et al. Safety of Tioguanine During Pregnancy in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(2):159–65. 10.1093/ecco-jcc/jjv189 [DOI] [PubMed] [Google Scholar]
- 63.Wenzl HH, Hogenauer C, Fickert P, Petritsch W. Thioguanine-induced symptomatic thrombocytopenia. Am J Gastroenterol. 2004;99(6):1195 10.1111/j.1572-0241.2004.30292.x [DOI] [PubMed] [Google Scholar]
- 64.de Boer NK, de Graaf P, Wilhelm AJ, Mulder CJJ, van Bodegraven AA. On the limitation of 6-tioguaninenucleotide monitoring during tioguanine treatment. Aliment Pharmacol Ther. 2005;22(5):447–51. 10.1111/j.1365-2036.2005.02581.x [DOI] [PubMed] [Google Scholar]
- 65.Ward MG, Kariyawasam VC, Patel K V, Elliot TR, Blaker PA, Mogan SB, et al. Thioguanine use in inflammatory bowel disease: 13 year experience in a tertiary centre. J Gastroenterol Hepatol. 2013;2:99–100. [Google Scholar]
- 66.Relling M V, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui C-H, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin Pharmacol Ther. 2018. November 17; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanderson J, Ansari A, Marinaki T, Duley J. Thiopurine methyltransferase: should it be measured before commencing thiopurine drug therapy? Ann Clin Biochem. 2004. July 1;41(4):294–302. [DOI] [PubMed] [Google Scholar]
- 68.Karim H, Ghalali A, Lafolie P, Vitols S, Fotoohi AK. Differential role of thiopurine methyltransferase in the cytotoxic effects of 6-mercaptopurine and 6-thioguanine on human leukemia cells. Biochem Biophys Res Commun. 2013;437:280–6. 10.1016/j.bbrc.2013.06.067 [DOI] [PubMed] [Google Scholar]
- 69.Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016. April;48(4):367–73. 10.1038/ng.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engen R, Marsh S, Van Booven D, McLeod H. Ethnic Differences in Pharmacogenetically Relevant Genes. Curr Drug Targets. 2006;7(12):1641–8. [DOI] [PubMed] [Google Scholar]
- 71.Lennard L, Richards S, Cartwright CS, Mitchell C, Lilleyman JS, Vora A. The thiopurine methyltransferase genetic polymorphism is associated with thioguanine-related veno-occlusive disease of the liver in children with acute lymphoblastic leukemia. Clin Pharmacol Ther. 2006. October;80(4):375–83. 10.1016/j.clpt.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 72.Stoneham S, Lennard L, Coen P, Lilleyman J, Saha V. Veno-occlusive disease in patients receiving thiopurines during maintenance therapy for childhood acute lymphoblastic leukaemia. Br J Haematol. 2003. October;123(1):100–2. [DOI] [PubMed] [Google Scholar]
- 73.Gilissen LPL, Derijks LJJ, Driessen A, Bos LP, Hooymans PM, Stockbrügger RW, et al. Toxicity of 6-thioguanine: no hepatotoxicity in a series of IBD patients treated with long-term, low dose 6-thioguanine. Some evidence for dose or metabolite level dependent effects? Dig Liver Dis. 2007. February;39(2):156–9. 10.1016/j.dld.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 74.Meijer B, van Everdingen CK, Ramsoekh D, Stedman C, Frampton CMA, Mulder CJJ, et al. Transient elastography to assess liver stiffness in patients with inflammatory bowel disease. Dig Liver Dis. 2018;50(1):48–53. 10.1016/j.dld.2017.09.128 [DOI] [PubMed] [Google Scholar]
- 75.Geller SA, Dubinsky MC, Poordad FF, Vasiliauskas EA, Cohen AH, Abreu MT, et al. Early hepatic nodular hyperplasia and submicroscopic fibrosis associated with 6-thioguanine therapy in inflammatory bowel disease. Am J Surg Pathol. 2004. September;28(9):1204–11. [DOI] [PubMed] [Google Scholar]
- 76.de Boer NK, Tuynman H, Bloemena E, Westerga J, Van der Peet DL, Mulder CJ, et al. Histopathology of liver biopsies from a thiopurine-naïve inflammatory bowel disease cohort: prevalence of nodular regenerative hyperplasia. Scand J Gastroenterol. 2008. January 8;43(5):604–8. 10.1080/00365520701800266 [DOI] [PubMed] [Google Scholar]
- 77.Wanless IR. Micronodular transpormation (nodular regenerative hyperplasia) of the liver: A report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990. May 1;11(5):787–97. [DOI] [PubMed] [Google Scholar]
- 78.de Boer NK, Reinisch W, Teml A, van Bodegraven AA, Schwab M, Lukas M, et al. 6-Thioguanine treatment in inflammatory bowel disease: a critical appraisal by a European 6-TG working party. Digestion. 2006;73(1):25–31. 10.1159/000091662 [DOI] [PubMed] [Google Scholar]
- 79.Seiderer J, Zech CJ, Reinisch W, Lukas M, Diebold J, Wrba F, et al. A multicenter assessment of liver toxicity by MRI and biopsy in IBD patients on 6-thioguanine. J Hepatol. 2005. August;43(2):303–9. 10.1016/j.jhep.2005.02.051 [DOI] [PubMed] [Google Scholar]
- 80.Ravikumara M, Hill FGH, Wilson DC, Gillett PM, Thomas A, Brown R, et al. 6-Thioguanine-related chronic hepatotoxicity and variceal haemorrhage in children treated for acute lymphoblastic leukaemia—A dual-centre experience. J Pediatr Gastroenterol Nutr. 2006;42(5):535–8. 10.1097/01.mpg.0000221901.76404.07 [DOI] [PubMed] [Google Scholar]
- 81.Hartleb M, Gutkowski K, Milkiewicz P. Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. World J Gastroenterol. 2011. March 21;17(11):1400–9. 10.3748/wjg.v17.i11.1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meijer B, Simsek M, Blokzijl H, de Man RA, Coenraad MJ, Dijkstra G, et al. Nodular regenerative hyperplasia rarely leads to liver transplantation: A 20-year cohort study in all Dutch liver transplant units. United Eur Gastroenterol J. 2017. August 16;5(5):658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.