Abstract
Background
Beta-blockers can reduce recurrence, metastasis, and mortality in various cancers. In this study, we investigated the effect of propranolol, a non-selective beta-blocker on overall survival (OS) in unresectable/metastatic hepatocellular carcinoma (HCC) and on recurrence-free survival (RFS) in resectable, curable HCC.
Methods
Data were retrieved from the Taiwan National Health Insurance Research Database between January 2000 and December 2013. Propranolol users (for >1 year) and non-propranolol users were matched using a 1:2 propensity score in both cohorts.
Results
The unresectable/metastatic HCC cohort comprised 1,560 propranolol users and 3,120 non-propranolol users (control group). On multivariate Cox regression analysis of HCC mortality, propranolol significantly reduced the mortality risk by 22% (hazard ratio [HR] = 0.78, 95% confidence interval [CI] 0.72–0.84, P <0.001). On stratified Cox regression analysis, propranolol also reduced the mortality risk in HCC patients with hepatitis B (HR = 0.92, 95% CI 0.85–0.99, P = 0.045), hepatitis C (HR = 0.85, 95% CI = 0.78–0.92, P = 0.001), liver cirrhosis (HR = 0.78, 95% CI = 0.72–0.85, P <0.001), and diabetes mellitus (HR = 0.87, 95% CI = 0.81–0.94, P = 0.008). The resectable, curable HCC cohort comprised 289 propranolol users and 578 non-propranolol users (control group), but there was no significant difference in RFS (P = 0.762) between propranolol and non-propranolol users.
Conclusion
This study revealed that propranolol could improve OS in unresectable/metastatic HCC.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer mortality worldwide.[1] Approximately 80% of HCC cases occur in eastern Asia and the sub-Saharan Africa, with chronic hepatitis B virus (HBV) infection being the most predominant risk factor.[2] In Western countries, hepatitis C virus (HCV) infection and excessive alcohol consumption are important risk factors.[2, 3] The treatment strategies for HCC include surgery (liver resection [LR] and liver transplantation [LT]), locoregional treatment (radiofrequency ablation [RFA] and transarterial chemoembolization [TACE]), and use of multikinase inhibitors (sorafenib and regorafenib).[4] LR has been the most effective treatment for HCC, and the 5-year survival is 38–61% with different stages.[5] However, <30% of HCC patients are eligible for surgery.[6] Furthermore, recurrence after LR occurs in up to 80% of patients at 5 years.[5, 7] Meanwhile, TACE is used for patients with preserved liver function who are ineligible for LR (with asymptomatic multifocal or bulky tumor).[8] For advanced-stage HCC, sorafenib has been approved as the standard first-line treatment and regorafenib as second-line treatment.[9, 10] However, the treatment outcomes remain unsatisfactory.
The beta adrenergic receptor (ADRB) pathway is essential for normal physiologic functions. Beta-2 adrenergic receptor (ADRB2) signaling regulates multiple signaling cascades involved in cancer progression and metastasis, including proliferation and invasion of cancer cells and angiogenesis, through activation of the cyclic adenosine monophosphate/protein kinase A pathway.[11–13] Propranolol, a nonselective beta-blocker (NSBB), has shown anticancer effects in preclinical studies.[14, 15] Beta-blockers have been demonstrated to reduce metastasis, recurrence, and mortality in various types of cancer.[16–19] Several retrospective studies have shown that beta-blockers can reduce cancer risk.[20, 21] Furthermore, ADRB2 expression has been reported to be upregulated in HCC,[22] which may help explain the capability of propranolol to reduce HCC risk in patients with cirrhosis.[23, 24]
Using a large data set available in Taiwan, we conducted this nationwide population-based cohort study to clarify the effect of propranolol on the prognosis of unresectable/metastatic and resectable, curable HCC.
Materials and methods
Data source
Data were extracted from the National Health Insurance Research Database (NHIRD) of Taiwan. The Taiwan National Health Insurance (NHI) program covers >99% of the 23 million residents of Taiwan. The data in this study were specifically obtained from the Longitudinal Health Insurance Database (LHID), a subset of the NHIRD. The LHID, which consists of comprehensive information such as demographic data, dates of clinical visits, and disease diagnoses for 1 million enrollees, is derived from the medical claims records of the NHI program. The diagnostic codes are based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The Registry for Catastrophic Illness Patient Database (RCIPD) is also a subset of the NHIRD and includes data from insured residents with severe diseases defined by the NHI program, such as malignancies. The patients included in the RCIPD comprised the case group in this study. The Institutional Review Board of the Tri-Service General Hospital (TSGHIRB no. 2-105-05-082) approved the study and waived the requirement of written informed consent All methods were performed in accordance with the relevant guidelines and regulations.
Study population and definition of propranolol exposure
Between January 1, 2000, and December 31, 2013, we extracted data from the LHID and RCIPD for patients aged ≥20 years with complete age and sex information and with a history of HCC (ICD-9-CM codes 155.0 and 155.2). Patients with a diagnosis of malignancies (ICD-9-CM codes 140–209) other than HCC before study enrollment were excluded.
To investigate the role of propranolol in unresectable/metastatic HCC, we selected patients who used propranolol, and the index date was the initial date of palliative treatment with TACE, radiotherapy, chemotherapy, or sorafenib. Those who expired before enrollment were excluded. We also studied the effect of propranolol on resectable, curable HCC, and the index date was the initial date of curative hepatic surgery. Patients who had undergone a hepatic operation such as lobectomy (ICD-9-CM code 50.3), segmentectomy (ICD-9-CM code 50.22), hepatectomy (partial, ICD-9-CM code 50.4; total, ICD-9-CM code 50.22), or LT (ICD-9-CM code 50.59) before enrollment were also excluded. Moreover, patients were excluded if they received any treatment before the curative operation, including TACE (ICD-9-CM codes 99.25 + 88.47), RFA (ICD-9-CM codes 50.29 + 88.76), radiotherapy (ICD-9-CM codes 92.3x), chemotherapy (including doxorubicin, fluorouracil, gemcitabine, oxaliplatin, or cisplatin), and sorafenib.
In both cohorts, patients were divided into two groups according to propranolol exposure: a propranolol group, consisting of patients who underwent propranolol therapy for at least 1 year before enrollment and had no prescription for another beta-blocker during that time, and a non-propranolol group, consisting of patients who did not receive propranolol therapy.
We used the defined daily dose (DDD), which is recommended by the World Health Organization (http://www.whocc.no/atc_ddd_index/), to measure the prescribed drug amount. DDD is the assumed average maintenance daily dose of a drug consumed for its main indication in adults. The recommended DDD of propranolol in this study was based on the treatment of mild-moderate hypertension. To examine the dose-response relationship, we categorized the propranolol cohorts into three groups (<545, 545–730, and >730 cumulative DDD).
Propensity score matching and comorbidities
To reduce selection bias, the propranolol and non-propranolol groups were selected according to twofold propensity score matching in studies of advanced/metastatic and surgically curable HCC. The propensity score was calculated using logistic regression to estimate the probability of treatment assignment according to baseline variables, including age, sex, Charlson comorbidity index score, diabetes mellitus (DM; ICD-9-CM code 250), HBV (ICD-9-CM codes V02.61, 070.20, 070.22, 070.30, and 070.32), HCV (ICD-9-CM codes V02.62, 070.41, 070.44, 070.51, 070.54, and 070.7), alcoholic liver disease (ICD-9-CM codes A213, A215, A347, 291, 303, 305, 571.0, 571.1, 571.2, and 571.3), liver cirrhosis (ICD-9-CM codes 571.2, 571.5, 571.6, 572.2, 572.3, 572.4, 572.8, and 573.0), renal failure (ICD-9-CM codes 584–586), hypertension (ICD-9-CM codes 401–405), coronary artery disease (ICD-9-CM codes 410–414), and medications of metformin, statins, aspirin, thiazolidinediones (TZDs), fibrates, and angiotensin-converting enzyme inhibitors (ACEIs).
Study outcomes
In the unresectable/metastatic HCC cohort, the study outcome was overall survival (OS), whereas in the resectable, curable HCC cohort, the study outcome was recurrence-free survival (RFS). HCC recurrence was defined as a repeat of any treatment including LR, LT, TACE, or RFA for HCC during the observation period. All subjects were followed from the index date until the occurrence of events or the end of 2013.
Statistical analysis
Continuous data are shown as the mean ± standard deviation (SD) and categorical data are shown as the frequency and percentage (%) of each baseline characteristic. Chi-squared test is used to evaluate the distributions of demographic features and common comorbidities between propranolol and non-propranolol groups. The mean ages of the cohorts were compared using a Mann-Whitney U-test. The Kaplan-Meier method was employed to plot the cumulative curves of disease recurrence and mortality, and the log-rank test was performed to examine the difference between the curves. A Cox regression model with stratification was used to evaluate the relationships between propranolol use and potential risk factors in HCC patients. Furthermore, hazard ratios (HRs) and 95% confidence intervals (CIs) for the outcomes were calculated between propranolol and non-propranolol users after adjusting for variables. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses. A P value of <0.05 was considered statistically significant.
Results
Propranolol therapy was associated with better OS in unresectable/metastatic HCC
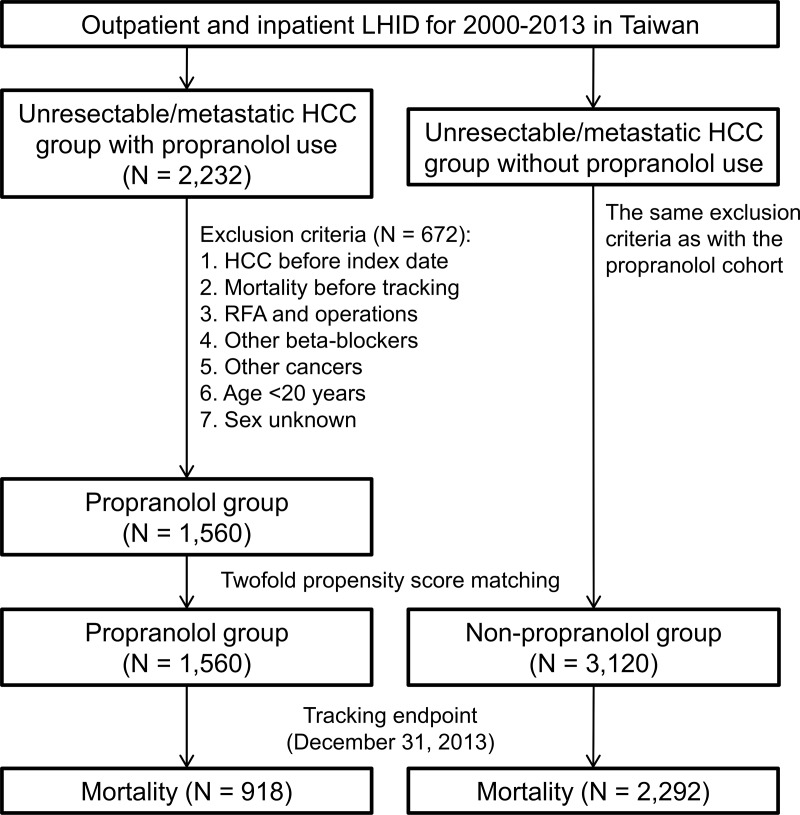
We identified 2,232 patients with unresectable/metastatic HCC who used propranolol for at least 1 year before enrollment. Among them, 672 patients were excluded after the inclusion and exclusion criteria were applied. We also randomly selected 3,120 HCC patients (matched with twofold propensity score matching) who did not use propranolol as control (Fig 1). The baseline clinical characteristics between the propranolol and non-propranolol groups showed no significant difference (Table 1).
Fig 1. Flowchart for matching patients with unresectable/metastatic HCC according to propranolol exposure.
LHID, Longitudinal Health Insurance Database; HCC, hepatocellular carcinoma; RFA, radiofrequency ablation. Operations include lobectomy, segmentectomy, hepatectomy, and liver transplantation.
Table 1. Demographic characteristics and comorbidities of patients with unresectable/metastatic HCC according to propranolol use after propensity score matching.
| Variable | Propranolol | Non-propranolol | P | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 1,560 | 33.3 | 3,120 | 66.7 | |
| Sex | |||||
| Male | 1,151 | 73.8 | 2,302 | 73.8 | 1.00 |
| Female | 409 | 26.2 | 818 | 26.2 | |
| Age, years, (mean ±SD) | 63.0 ± 12.7 | 62.5 ± 12.6 | 0.65 | ||
| 20–49 | 250 | 16.0 | 500 | 16.0 | 1.00 |
| 50–64 | 559 | 35.8 | 1,118 | 35.8 | |
| ≥65 | 751 | 48.1 | 1,502 | 48.1 | |
| Comorbidity | |||||
| HBV | 440 | 28.3 | 910 | 29.3 | 0.49 |
| HCV | 352 | 22.6 | 720 | 23.1 | 0.71 |
| Alcoholic liver disease | 93 | 6.0 | 201 | 6.4 | 0.57 |
| Liver cirrhosis | 954 | 61.2 | 1,923 | 61.6 | 0.75 |
| Renal failure | 65 | 4.2 | 129 | 4.1 | 0.96 |
| DM | 278 | 17.8 | 525 | 16.8 | 0.41 |
| HTN | 249 | 16.0 | 459 | 14.7 | 0.26 |
| CAD | 39 | 2.5 | 71 | 2.3 | 0.68 |
| CCI_R, (mean ±SD) | 0.5 ± 1.1 | 0.6 ± 1.2 | 0.12 | ||
| Medication | |||||
| Aspirin | 292 | 18.7 | 581 | 18.6 | 0.94 |
| Statins | 19 | 1.2 | 40 | 1.3 | 0.89 |
| Metformin | 142 | 9.1 | 282 | 9.0 | 0.96 |
| Lipid-lowering drugs | 190 | 12.2 | 377 | 12.1 | 0.92 |
| TZDs | 186 | 11.9 | 365 | 11.7 | 0.85 |
| ACEIs | 50 | 3.2 | 103 | 3.3 | 0.93 |
| Treatment | |||||
| Sorafenib | 711 | 45.6 | 1,419 | 45.5 | 0.95 |
| TACE | 765 | 49.0 | 1,533 | 49.1 | 0.95 |
| Radiotherapy | 1,249 | 80.1 | 1,556 | 81.9 | 0.12 |
HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; DM, diabetes mellitus; HTN, hypertension; CAD, coronary artery disease; CCI_R, Charlson comorbidity index after removal of the above mentioned comorbidities; TZDs, thiazolidinediones; ACEIs, angiotensin-converting enzyme inhibitors; TACE, transarterial chemoembolization
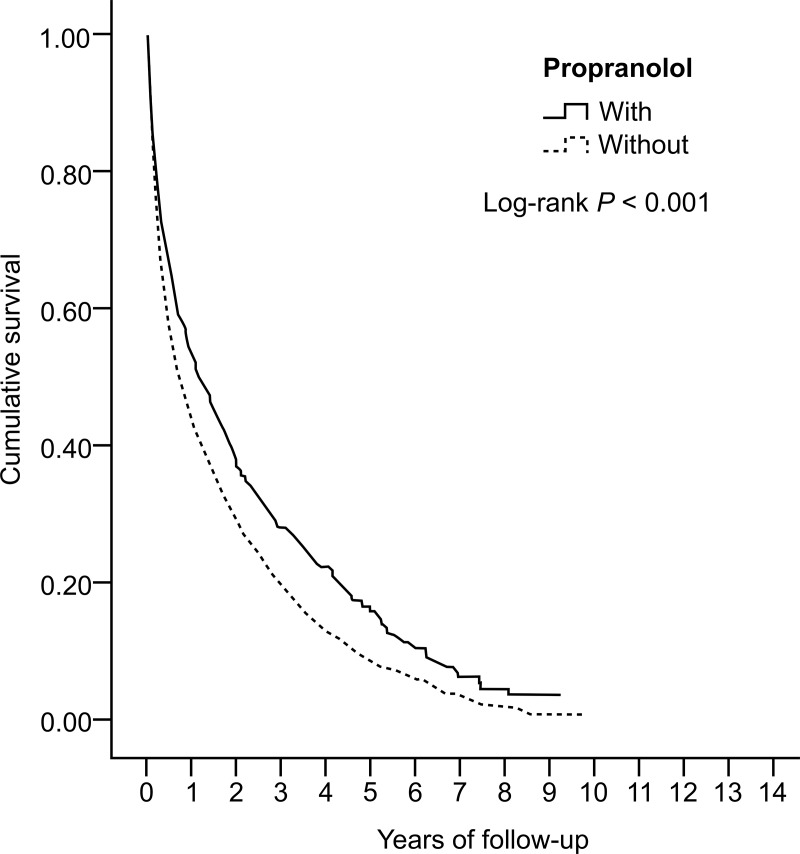
In the Kaplan-Meier analysis for OS, the propranolol group had higher survival than the non-propranolol group at the end of follow-up (41.2% vs. 26.5%, P < 0.001, Fig 2). In the Cox regression multivariate analysis of HCC mortality, propranolol significantly reduced the mortality risk by 22% (HR = 0.78, 95% CI = 0.72–0.84, P < 0.001). Males had a higher risk of mortality than females (HR = 1.14, 95% CI = 1.05–1.24, P < 0.001). Mortality risk decreased significantly with longer duration of propranolol use. Moreover, patients with increasing age showed a higher risk of mortality. HBV and HCV infection also increased the mortality risk (HR = 1.08 [95% CI = 1.01–1.18, P = 0.04) and 1.36 [95% CI = 1.17–1.50], respectively, P < 0.001). Meanwhile, the use of aspirin, statins, metformin, fibrates, TZDs, and ACEIs did not influence the mortality risk. These findings are shown in Table 2.
Fig 2. Kaplan-Meier plot for the probability of overall survival in patients with unresectable/metastatic hepatocellular carcinoma according to propranolol exposure.
Table 2. Univariate and multivariate Cox regression analyses of mortality in patients with unresectable/metastatic HCC.
| Variable | Crude HR (95% CI) | P | Adjusted HR (95% CI) | P |
|---|---|---|---|---|
| Propranolol | ||||
| With | 0.77 (0.72–0.83) | <0.001* | 0.78 (0.72–0.84) | <0.001* |
| Without | Reference 1.00 | Reference 1.00 | ||
| Propranolol | ||||
| <545 days | 0.81 (0.73–0.99) | 0.04* | 0.81 (0.74–0.99) | 0.045* |
| 545–730 days | 0.77 (0.70–0.83) | 0.001* | 0.78 (0.71–0.84) | 0.002* |
| >730 days | 0.69 (0.58–0.79) | <0.001* | 0.70 (0.59–0.80) | <0.001* |
| Without | Reference 1.00 | Reference 1.00 | ||
| Sex | ||||
| Male | 1.20 (1.10–1.29) | <0.001* | 1.14 (1.05–1.24) | <0.001* |
| Female | Reference 1.00 | Reference 1.00 | ||
| Age group (years) | ||||
| ≥65 | 1.41 (1.27–1.56) | <0.001* | 1.13 (1.06–1.88) | <0.001* |
| 50–64 | 1.24 (1.15–1.34) | <0.001* | 1.09 (1.01–1.29) | 0.03* |
| 20–49 | Reference 1.00 | Reference 1.00 | ||
| HBV infection | ||||
| With | 1.09 (1.01–1.20) | 0.04* | 1.08 (1.01–1.18) | 0.04* |
| Without | Reference 1.00 | Reference 1.00 | ||
| HCV infection | ||||
| With | 1.45 (1.29–1.63) | <0.001* | 1.36 (1.17–1.50) | <0.001* |
| Without | Reference 1.00 | Reference 1.00 | ||
| Alcoholic liver disease | ||||
| With | 1.04 (0.91–1.20) | 0.56 | 1.08 (0.76–1.12) | 0.10 |
| Without | Reference 1.00 | Reference 1.00 | ||
| Liver cirrhosis | ||||
| With | 1.07 (1.01–1.15) | 0.045* | 1.06 (0.99–1.15) | 0.12 |
| Without | Reference 1.00 | Reference 1.00 | ||
| Renal failure | ||||
| With | 1.12 (0.94–1.33) | 0.21 | 1.11 (0.94–1.32) | 0.23 |
| Without | Reference 1.00 | Reference 1.00 | ||
| DM | ||||
| With | 1.01 (0.83–1.00) | 0.05 | 1.07 (0.89–1.08) | 0.46 |
| Without | Reference 1.00 | Reference 1.00 | ||
| HTN | ||||
| With | 1.81 (1.74–1.90) | <0.001* | 1.01 (0.82–1.01) | 0.08 |
| Without | Reference 1.00 | Reference 1.00 | ||
| CAD | ||||
| With | 1.00 (0.79–1.25) | 0.95 | 1.07 (0.86–1.37) | 0.49 |
| Without | Reference 1.00 | Reference 1.00 | ||
| CCI_R | 1.05 (1.02–1.09) | 0.001* | 1.03 (0.99–1.06) | 0.12 |
| Aspirin | ||||
| With | 1.27 (0.64–1.91) | 0.45 | 1.25 (0.66–1.97) | 0.45 |
| Without | Reference 1.00 | Reference 1.00 | ||
| Statins | ||||
| With | 1.14 (0.88–1.80) | 0.29 | 1.11 (0.83–1.86) | 0.30 |
| Without | Reference 1.00 | Reference 1.00 | ||
| Metformin | ||||
| With | 1.10 (0.75–1.39) | 0.34 | 1.10 (0.77–1.34) | 0.34 |
| Without | Reference 1.00 | Reference 1.00 | ||
| Fibrates | ||||
| With | 1.45 (0.67–2.19) | 0.39 | 1.35 (0.67–2.03) | 0.40 |
| Without | Reference 1.00 | Reference 1.00 | ||
| TZDs | ||||
| With | 1.20 (0.52–1.60) | 0.48 | 1.12 (0.50–1.60) | 0.48 |
| Without | Reference 1.00 | Reference 1.00 | ||
| ACEIs | ||||
| With | 1.10 (0.83–1.56) | 0.67 | 1.08 (0.85–1.46) | 0.60 |
| Without | Reference 1.00 | Reference 1.00 | ||
*Significantly correlated with outcome, P-value < 0.05. HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; DM, diabetes mellitus; HTN, hypertension; CAD, coronary artery disease; CCI_R, Charlson comorbidity index after removal of the above mentioned comorbidities; TZDs, thiazolidinediones; ACEIs, angiotensin-converting enzyme inhibitors
We used stratified Cox regression analysis to evaluate the effect of propranolol on each parameter. Propranolol reduced the mortality risk in both males and females and significantly in all age groups. Propranolol also reduced the mortality risk in HCC patients with HBV infection (HR = 0.92, 95% CI = 0.85–0.99, P = 0.045), HCV infection (HR = 0.85, 95% CI = 0.78–0.92, P = 0.001), liver cirrhosis (HR = 0.78, 95% CI = 0.72–0.85, P < 0.001), and DM (HR = 0.87, 95% CI = 0.81–0.94, P = 0.008). Propranolol significantly lowered the mortality risk in patients who used and did not use aspirin, metformin, fibrates, and ACEIs (S1 Table).
We further analyzed the effect of palliative treatment (sorafenib, TACE, or radiotherapy) with and without propranolol use on HCC mortality. In patients with palliative treatment, those who received propranolol had a lower risk of mortality than those who did not receive propranolol (S2 Table).
Propranolol therapy did not improve RFS in resectable, curable HCC
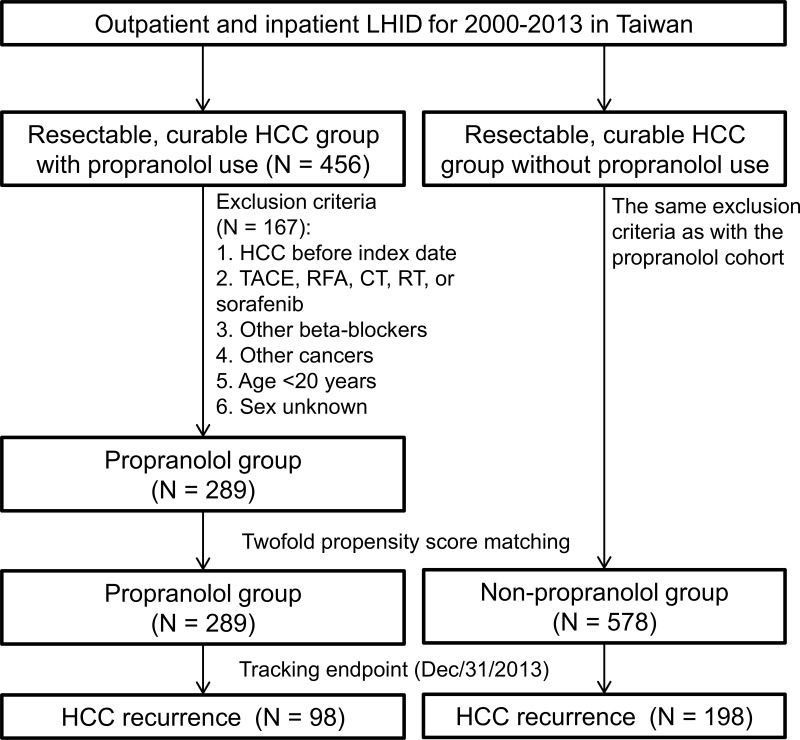
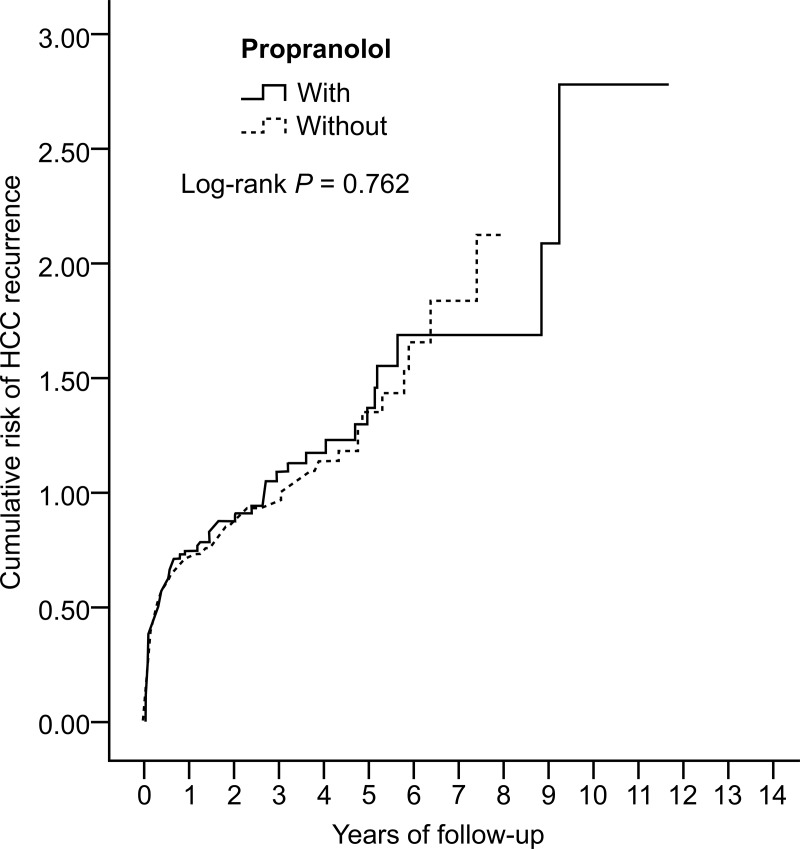
We identified 456 patients with resectable, curable HCC who received propranolol for at least 1 year before enrollment. Among them, 167 patients were excluded after the inclusion and exclusion criteria were applied. We also randomly selected 578 HCC patients (matched with twofold propensity score matching) who did not receive propranolol as control (Fig 3). RFS was not significantly different between propranolol and non-propranolol users (98/289 = 33.9% vs. 198/578 = 34.3%, P = 0.762, Fig 4). Events of early recurrence (≤2 years) (82/289 = 28.4% vs. 179/578 = 31.0%, P = 0.166) and late recurrence (>2 years) (16/289 = 5.5% vs. 19/578 = 3.3%, P = 0.780) were also not significantly different between propranolol and non-propranolol users.
Fig 3. Flowchart for matching patients with resectable, curable HCC according to propranolol exposure.
LHID, Longitudinal Health Insurance Database; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; CT, chemotherapy; RT, radiotherapy.
Fig 4. Kaplan-Meier plot for the probability of recurrence of resectable, curable hepatocellular carcinoma according to propranolol exposure.
Discussion
Our study showed that propranolol can reduce the mortality risk by 22% in unresectable/metastatic HCC. The risk was reduced in both sexes and all age groups. Propranolol also significantly reduced the mortality risk in patients with HBV and HCV infection, liver cirrhosis, and DM. There was also risk reduction with longer duration of propranolol use. Additionally, propranolol treatment could reduce the mortality risk in HCC patients regardless of aspirin, metformin, fibrate, and ACEI use. However, propranolol did not improve RFS in resectable, curable HCC.
Extensive preclinical data have firmly established the relevance of the ADRB signaling system in cancer biology, whose effects are mediated mainly through ADRB2 activation of the protein kinase A pathway.[11–13] Several retrospective studies have examined the influence of beta-blockers on cancer survival.[16–19, 25–29] However, some studies support the protective effect of beta-blockers,[16–19] while others do not.[25–29] In most of the studies, beta-blockers were not categorized according to their beta-1 or beta-2 selectivity. The similarity between ADRB1 and ADRB2 and the affinity of beta-blockers toward these receptors could explain the confusing results in such studies.[30] Furthermore, NSBBs have been shown to have more survival benefit than selective beta-blockers in ovarian cancer.[18]
Liver cirrhosis is the long-term complication of chronic hepatitis, and most HCCs develop in cirrhotic livers. Serum levels of catecholamines in patients with cirrhotic livers have been reported to be associated with severity of liver disease.[31] NSBBs are recommended in patients with liver cirrhosis and esophageal varices for bleeding prophylaxis. Long-term use of propranolol, an NSBB, has been demonstrated to reduce the risk of HCC developing in patients with HCV-associated cirrhosis.[23] A meta-analysis also showed that NSBBs may reduce HCC risk in patients with cirrhosis.[24] These findings are consistent with the findings in our study, which revealed that propranolol could reduce the mortality risk in patients with HBV and HCV infection, liver cirrhosis, and DM, and the protective effect was more substantial when the propranolol exposure was longer. Aspirin, statins, TZDs, and metformin are known for their anticancer effects,[32] while ACEIs and fibrates have equivocal effects on cancer risk and survival.[33, 34] However, in our study, propranolol was an independent prognostic factor for mortality in the Cox regression analysis, while the above candidate medications were not. Furthermore, in the stratified Cox regression analysis, propranolol could lower the mortality risk in patients who did not use these candidate medications.
In the joint analysis, we found that propranolol may have a synergistic effect with sorafenib, radiotherapy, and TACE. Sorafenib is currently the standard treatment for metastatic HCC, but the development of acquired resistance is almost inevitable with this drug. The phosphatidylinositol-3-kinase/protein kinase B pathway, autophagy, epithelial-mesenchymal transition, epigenetic regulation, and tumor environment are involved in the resistance mechanism.[35] Interestingly, ADRB2 signaling blockade by propranolol has been demonstrated to enhance the autophagic degradation of hypoxia-inducible factor 1-alpha, thereby enhancing sorafenib efficacy.[36] Moreover, beta-blocker use during definitive radiotherapy has been reported to improve survival in patients with non-small cell lung cancer.[37] ADRB2 signaling promotes angiogenesis through crosstalk between endothelial and cancer cells.[38] ADRB2s also play a critical role in endothelial cell proliferation, revascularization, and neoangiogenesis in response to ischemia.[39] Therefore, the combination of NSBBs and TACE may achieve a more effective antitumor effect.
LR and LT are some of the treatment options for HCC. However, recurrence is not uncommon after LR and is the leading cause of postoperative death.[5, 6] The early recurrence of HCC has been reported to be within 2 years after LR and is related to micrometastasis, whereas the late recurrence has been reported to occur >2 years after LR, which is attributed to the de novo tumor.[7] Clinicopathologic parameters such as tumor stage, vascular invasion, alpha-fetoprotein, liver cirrhosis, multi-nodularity, and grade of hepatitis activities are known predictors of recurrence.[7, 40, 41] Several adjuvant treatments including interferon, chemotherapy, and sorafenib have been investigated in clinical trials, but none of the studies reported successful outcomes except with interferon therapy, which prolonged the survival in specific patient groups.[42] In our study, propranolol did not improve RFS in resectable, curable HCC. There was also no significant difference in the risk of early or late recurrence between propranolol and non-propranolol users. Because the NHIRD did not provide clinicopathologic data, we could not use these parameters for further analysis. ADRB2 expression in HCC tissues is associated with several poor prognostic factors and is an independent prognostic factor for RFS and OS in surgically curable HCC.[43, 44] Hence, immunohistochemical staining of ADRB2 expression may provide useful information for considering NSBB treatment.
This study has several limitations. First, the clinicopathologic and laboratory parameters of enrolled patients were not available from the NHIRD, and we could not classify the patients according to individual prognostic factors. Propensity score matching and multivariate analysis were used to adjust for potential confounders. Second, we could not measure the actual dose of propranolol and duration of use. We presumed that all medications were taken by the patients as prescribed. The DDD was calculated based on official prescriptions and we could not investigate the dose-response effect. Finally, the incidence of HCC recurrence might have been underestimated. However, this problem would have occurred in both matched groups and might have a limited impact on our results.
In conclusion, this study revealed that propranolol could improve OS in patients with unresectable/metastatic HCC. Propranolol could not only lower the risk of variceal bleeding and HCC development in patients with cirrhotic livers but also lower the mortality risk in those with advanced HCC. Future prospective studies based on ADRB2 expression in HCC patients are warranted to identify who would benefit most from propranolol treatment.
Supporting information
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability
The data underlying this study is from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).
Funding Statement
This study was supported by grants from Tri-Service General Hospital Research Foundation (TSGH-C108-003 to W-CC), and the sponsor has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127(12):2893–917. Epub 2011/02/26. 10.1002/ijc.25516 . [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–73.e1. Epub 2012/04/28. 10.1053/j.gastro.2011.12.061 ; PubMed Central PMCID: PMCPmc3338949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S87–96. Epub 2004/10/28. . [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London, England). 2018;391(10127):1301–14. Epub 2018/01/09. 10.1016/s0140-6736(18)30010-2 . [DOI] [PubMed] [Google Scholar]
- 5.Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Annals of surgery. 2013;257(5):929–37. Epub 2013/02/22. 10.1097/SLA.0b013e31828329b8 . [DOI] [PubMed] [Google Scholar]
- 6.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Seminars in liver disease. 2010;30(1):61–74. Epub 2010/02/23. 10.1055/s-0030-1247133 . [DOI] [PubMed] [Google Scholar]
- 7.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. Journal of hepatology. 2003;38(2):200–7. Epub 2003/01/28. . [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology (Baltimore, Md). 2003;37(2):429–42. Epub 2003/01/24. 10.1053/jhep.2003.50047 . [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359(4):378–90. Epub 2008/07/25. 10.1056/NEJMoa0708857 . [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England). 2017;389(10064):56–66. Epub 2016/12/10. 10.1016/s0140-6736(16)32453-9 . [DOI] [PubMed] [Google Scholar]
- 11.Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9(12):4514–21. Epub 2003/10/14. . [PubMed] [Google Scholar]
- 12.Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(2):369–75. Epub 2006/01/24. 10.1158/1078-0432.ccr-05-1698 ; PubMed Central PMCID: PMCPmc3141061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature medicine. 2006;12(8):939–44. Epub 2006/07/25. 10.1038/nm1447 . [DOI] [PubMed] [Google Scholar]
- 14.Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D, et al. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncology reports. 2009;22(4):825–30. Epub 2009/09/03. . [DOI] [PubMed] [Google Scholar]
- 15.Palm D, Lang K, Niggemann B, Drell TLt, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. International journal of cancer. 2006;118(11):2744–9. Epub 2005/12/29. 10.1002/ijc.21723 . [DOI] [PubMed] [Google Scholar]
- 16.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(19):2635–44. Epub 2011/06/03. 10.1200/jco.2010.33.5422 . [DOI] [PubMed] [Google Scholar]
- 17.Grytli HH, Fagerland MW, Fossa SD, Tasken KA. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. European urology. 2014;65(3):635–41. Epub 2013/01/29. 10.1016/j.eururo.2013.01.007 . [DOI] [PubMed] [Google Scholar]
- 18.Watkins JL, Thaker PH, Nick AM, Ramondetta LM, Kumar S, Urbauer DL, et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015;121(19):3444–51. Epub 2015/08/25. 10.1002/cncr.29392 ; PubMed Central PMCID: PMCPmc4575637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemeshow S, Sorensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, et al. beta-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(10):2273–9. Epub 2011/09/22. 10.1158/1055-9965.epi-11-0249 ; PubMed Central PMCID: PMCPmc3652234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monami M, Filippi L, Ungar A, Sgrilli F, Antenore A, Dicembrini I, et al. Further data on beta-blockers and cancer risk: observational study and meta-analysis of randomized clinical trials. Current medical research and opinion. 2013;29(4):369–78. Epub 2013/02/02. 10.1185/03007995.2013.772505 . [DOI] [PubMed] [Google Scholar]
- 21.Chang PY, Huang WY, Lin CL, Huang TC, Wu YY, Chen JH, et al. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine. 2015;94(27):e1097 Epub 2015/07/15. 10.1097/MD.0000000000001097 ; PubMed Central PMCID: PMCPmc4504645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kassahun WT, Guenl B, Ungemach FR, Jonas S, Abraham G. Expression and functional coupling of liver beta2—adrenoceptors in the human hepatocellular carcinoma. Pharmacology. 2012;89(5–6):313–20. Epub 2012/05/16. 10.1159/000337381 . [DOI] [PubMed] [Google Scholar]
- 23.Nkontchou G, Aout M, Mahmoudi A, Roulot D, Bourcier V, Grando-Lemaire V, et al. Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer prevention research (Philadelphia, Pa). 2012;5(8):1007–14. Epub 2012/04/25. 10.1158/1940-6207.capr-11-0450 . [DOI] [PubMed] [Google Scholar]
- 24.Thiele M, Albillos A, Abazi R, Wiest R, Gluud LL, Krag A. Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: a meta-analysis of randomized trials. Liver international: official journal of the International Association for the Study of the Liver. 2015;35(8):2009–16. Epub 2015/01/13. 10.1111/liv.12782 . [DOI] [PubMed] [Google Scholar]
- 25.Cardwell CR, Coleman HG, Murray LJ, Entschladen F, Powe DG. Beta-blocker usage and breast cancer survival: a nested case-control study within a UK clinical practice research datalink cohort. International journal of epidemiology. 2013;42(6):1852–61. Epub 2014/02/19. 10.1093/ije/dyt196 . [DOI] [PubMed] [Google Scholar]
- 26.Cardwell CR, Coleman HG, Murray LJ, O'Sullivan JM, Powe DG. Beta-blocker usage and prostate cancer survival: a nested case-control study in the UK Clinical Practice Research Datalink cohort. Cancer epidemiology. 2014;38(3):279–85. Epub 2014/05/03. 10.1016/j.canep.2014.03.011 . [DOI] [PubMed] [Google Scholar]
- 27.McCourt C, Coleman HG, Murray LJ, Cantwell MM, Dolan O, Powe DG, et al. Beta-blocker usage after malignant melanoma diagnosis and survival: a population-based nested case-control study. The British journal of dermatology. 2014;170(4):930–8. Epub 2014/03/07. 10.1111/bjd.12894 . [DOI] [PubMed] [Google Scholar]
- 28.Hicks BM, Murray LJ, Powe DG, Hughes CM, Cardwell CR. beta-Blocker usage and colorectal cancer mortality: a nested case-control study in the UK Clinical Practice Research Datalink cohort. Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24(12):3100–6. Epub 2013/09/21. 10.1093/annonc/mdt381 . [DOI] [PubMed] [Google Scholar]
- 29.Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. British journal of clinical pharmacology. 2011;72(1):157–61. Epub 2011/04/02. 10.1111/j.1365-2125.2011.03980.x ; PubMed Central PMCID: PMCPmc3141198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith C, Teitler M. Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors. Cardiovascular drugs and therapy. 1999;13(2):123–6. Epub 1999/06/18. . [DOI] [PubMed] [Google Scholar]
- 31.Braillon A, Gaudin C, Poo JL, Moreau R, Debaene B, Lebrec D. Plasma catecholamine concentrations are a reliable index of sympathetic vascular tone in patients with cirrhosis. Hepatology (Baltimore, Md). 1992;15(1):58–62. Epub 1992/01/01. . [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nature reviews Gastroenterology & hepatology. 2014;11(1):45–54. Epub 2013/08/14. 10.1038/nrgastro.2013.143 ; PubMed Central PMCID: PMCPmc4334449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen J, Huang YM, Wang M, Hong XZ, Song XN, Zou X, et al. Renin-angiotensin system blockade for the risk of cancer and death. Journal of the renin-angiotensin-aldosterone system: JRAAS. 2016;17(3). Epub 2016/07/13. 10.1177/1470320316656679 ; PubMed Central PMCID: PMCPmc5843874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonovas S, Nikolopoulos GK, Bagos PG. Use of fibrates and cancer risk: a systematic review and meta-analysis of 17 long-term randomized placebo-controlled trials. PloS one. 2012;7(9):e45259 Epub 2012/10/03. 10.1371/journal.pone.0045259 ; PubMed Central PMCID: PMCPmc3446944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu YJ, Zheng B, Wang HY, Chen L. New knowledge of the mechanisms of sorafenib resistance in liver cancer. Acta pharmacologica Sinica. 2017;38(5):614–22. Epub 2017/03/28. 10.1038/aps.2017.5 ; PubMed Central PMCID: PMCPmc5457690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu FQ, Fang T, Yu LX, Lv GS, Lv HW, Liang D, et al. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1alpha. Journal of hepatology. 2016;65(2):314–24. Epub 2016/05/08. 10.1016/j.jhep.2016.04.019 . [DOI] [PubMed] [Google Scholar]
- 37.Wang HM, Liao ZX, Komaki R, Welsh JW, O'Reilly MS, Chang JY, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Annals of oncology: official journal of the European Society for Medical Oncology. 2013;24(5):1312–9. Epub 2013/01/10. 10.1093/annonc/mds616 ; PubMed Central PMCID: PMCPmc3629895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Liu D, Yang Z, Sun L, Deng Q, Yang S, et al. Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocrine-related cancer. 2014;21(5):783–95. Epub 2014/09/03. 10.1530/ERC-14-0236 . [DOI] [PubMed] [Google Scholar]
- 39.Iaccarino G, Ciccarelli M, Sorriento D, Galasso G, Campanile A, Santulli G, et al. Ischemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic system. Circulation research. 2005;97(11):1182–9. Epub 2005/10/22. 10.1161/01.RES.0000191541.06788.bb . [DOI] [PubMed] [Google Scholar]
- 40.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89(3):500–7. Epub 2000/08/10. . [PubMed] [Google Scholar]
- 41.Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. Journal of hepatology. 2009;51(5):890–7. Epub 2009/09/15. 10.1016/j.jhep.2009.07.009 . [DOI] [PubMed] [Google Scholar]
- 42.Lu LC, Cheng AL, Poon RT. Recent advances in the prevention of hepatocellular carcinoma recurrence. Seminars in liver disease. 2014;34(4):427–34. Epub 2014/11/05. 10.1055/s-0034-1394141 . [DOI] [PubMed] [Google Scholar]
- 43.Chen D, Xing W, Hong J, Wang M, Huang Y, Zhu C, et al. The beta2-adrenergic receptor is a potential prognostic biomarker for human hepatocellular carcinoma after curative resection. Annals of surgical oncology. 2012;19(11):3556–65. Epub 2012/05/17. 10.1245/s10434-012-2396-1 . [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZF, Feng XS, Chen H, Duan ZJ, Wang LX, Yang D, et al. Prognostic significance of synergistic hexokinase-2 and beta2-adrenergic receptor expression in human hepatocelluar carcinoma after curative resection. BMC gastroenterology. 2016;16(1):57 Epub 2016/06/04. 10.1186/s12876-016-0474-8 ; PubMed Central PMCID: PMCPmc4891884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
The data underlying this study is from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).