Abstract
Complex regional pain syndrome (CRPS) is a pain condition that usually affects a single limb, often following an injury. The underlying pathophysiology seems to be complex and probably varies between patients. Clinical diagnosis is based on internationally agreed-upon criteria, which consider the reported symptoms, presence of signs and exclusion of alternative causes. Research into CRPS biomarkers to support patient stratification and improve diagnostic certainty is an important scientific focus, and recent progress in this area provides an opportunity for an up-to-date topical review of measurable disease-predictive, diagnostic and prognostic parameters. Clinical and biochemical attributes of CRPS that may aid diagnosis and determination of appropriate treatment are delineated. Findings that predict the development of CRPS and support the diagnosis include trauma-related factors, neurocognitive peculiarities, psychological markers, and local and systemic changes that indicate activation of the immune system. Analysis of signatures of non-coding microRNAs that could predict the treatment response represents a new line of research. Results from the past 5 years of CRPS research indicate that a single marker for CRPS will probably never be found; however, a range of biomarkers might assist in clinical diagnosis and guide prognosis and treatment.
Professor Roberto Perez (1968–2017) passed away shortly after this manuscript was initially submitted. The remaining authors dedicate this Review to him as a tribute to a good friend, brilliant scientist, outstanding teacher and true pioneer of CRPS research.
Complex regional pain syndrome (CRPS) is a persistent pain condition that often results from an injury, and is characterized by a variable combination of pain and hyperalgesia, as well as vegetative, sensory, motor and trophic symptoms, in the affected limb. By definition, these symptoms can no longer be explained by the initial event. In chronic CRPS, signs of CNS reorganization such as dystonia, body perception disturbances or sensory deficits that follow a central pattern are evident1.
Chronic pain in general is a diagnostic and therapeutic challenge, and the management of CRPS is particularly demanding. As in phantom limb pain, treatment must address both the loss of limb function and highintensity pain. If treatment of one or both of these aspects fails, lifelong suffering and disability can result. However, the outcomes of treatment can be good if CRPS is recognized early2, underlining the necessity to search for biomarkers or phenotypic characteristics that facilitate prevention, early diagnosis or treatment response. Substantial scientific progress in recent years has| identified posttraumatic inflammation as a major component of acute CRPS3, which might enable targeted treatment with antiinflammatory agents.
Here, we provide an uptodate narrative Review of the latest scientific achievements in CRPS research, focusing on biomarkers for diagnosis, treatment and clinical course prediction. Considering the stage of biomarker research in CRPS, and using a broad definition of what can be considered a ‘biomarker’ or a ‘phenotypic characteristic’ in both a clinical and a biological sense, we thought that an integrative approach was warranted, allowing the inclusion of studies with less powerful designs than are usually included in systematic reviews or metaanalyses. A true systematic approach was deemed unfeasible because emerging evidence from exploratory studies would receive insufficient attention (BOX 1).
Box 1 |. Limitations of this Review.
A major limitation of this article is that it does not fulfil the formal criteria for a systematic review. Although we made every effort to retrieve all relevant studies published between 2011 and 2016, this Review must be regarded as reflecting the opinions of experts in the field. Our decision not to undertake a systematic comprehensive review was based on the following thoughts: first, biomarker research in complex regional pain syndrome (CRPS) is in its infancy, and the available studies are too small to obtain high-level evidence; second, most of the included studies were either cohort or exploratory studies; third, the quality of reporting methods in pathophysiological studies varies considerably; and fourth, we wished to report the latest scientific achievements and to inspire future CRPS research rather than reporting on studies that are lacking and describing the shortcomings of existing studies. The third point regarding the quality of reporting methods in existing studies leads us to another limitation. The analytical methods and sample preparation protocols that were used in the various studies were often laboratory-specific, making cross-study comparisons difficult.
The final limitation is CRPS itself as a topic for a systematic review. The results of CRPS research indicate that CRPS is a heterogeneous syndrome with various clinical presentations that change over time. Therefore, the emergence of a single biomarker with the necessary precision to drive CRPS risk assessment, diagnosis or treatment seems unlikely. Hopefully, this Review will inspire research groups to mount coordinated efforts to overcome these limitations.
What do we currently know about CRPS?
CRPS seems to be a human disease. Rodent models provide valuable insights into different aspects of the pathophysiology of acute posttraumatic pain and inflammation, but these models do not reproduce all the symptoms that patients experience, especially in cases of CRPS that last for months or years. Although we have made considerable progress in understanding CRPS, the disease is still defined clinically and is diagnosed using clinical criteria.
The criteria currently endorsed by the International Association for the Study of Pain, which have been validated against several neuropathic pain conditions, encompass the reported symptoms and the presence of signs in four different clinical categories, in the absence of any other explanation for the complaints4. These clinical categories are as follows: sensory symptoms; vasomotor differences between affected and unaffected limbs; sudomotor differences or the presence of limb oedema; and a traumarelated motor disorder that includes weakness, tremor or dystonia and/or characteristic trophic changes of skin, hair and nail growth. The diagnosis of CRPS requires a history of symptoms in at least three of the four categories and the presence of signs in two of the four categories at the time of consultation. The presence of sensory symptoms alone cannot sufficiently differentiate CRPS from other neuropathic pain disorders because pain, hyperalgesia and sensory loss are common characteristics of these conditions. The diagnostic criteria are not perfect, and the specificity is only about 70% against neuropathic pain disorders4. Furthermore, variable stringency in the application of the clinical diagnostic criteria might partially explain apparent disease variability: estimates of CRPS incidence after limb trauma depend on how rigorously the diagnostic criteria are applied5.
CRPS has traditionally been differentiated into two clinical subtypes. CRPS that develops after major nerve damage is labelled CRPS type II, and the remaining cases are termed CRPS type I. The latter subtype is much more common than the former4. A recent study suggested two further subtypes: ‘warm’ CRPS and ‘cold’ CRPS. The warm subtype is characterized by a warm, red, oedematous and sweaty extremity, whereas the cold type is typically indicated by a cold, blue, less oedematous extremity6.
The pathophysiology of CRPS is still controversial, but the latest research suggests the following scenario. Most often, the initial event is peripheral limb trauma. Posttraumatic inflammation is a normal physiological response, but the exaggerated inflammation in CRPS leads to persistent oedema, vasodilation, temperature changes and probably hyperhidrosis via release of neuropeptides. Simultaneously, inflammatory mediators (for example, cytokines), growth factors, catecholamines and autoantibodies contribute to trophic changes (for example, activation of keratinocytes, fibroblasts or osteocytes) and sensitize peripheral nociceptors, inducing phenomena such as movementrelated pain and heat hyperalgesia. Some of these mediators (in particular, growth factors, cytokines and centrally released neuropeptides from primary afferents) also sensitize secondorder neurons in the spinal cord, which are responsible for both skin and deeptissue hyperalgesia and allodynia. Longterm unresolved nociceptive activity and inflammation, probably leading to peripheral nerve damage, cause not only loss of function but also brain changes in susceptible patients. Related symptoms include nondermatomal sensory deficits, disturbances of body perception, neglectlike phenomena and motor symptoms. The motor symptoms might also have a spinal pathophysiology, such as aberrant force feedback regulation from Golgi tendon organs involving inhibitory interneurons7. This CRPS model is speculative but is supported by results from animal experiments8 and investigations of patient tissue and sensorimotor function. The pathophysiology of CRPS is reviewed more extensively elsewhere9.
Selecting risk factors and biomarkers
As CRPS can evolve over time (for example, acute CRPS developing into chronic CRPS, or warm CRPS turning into cold CRPS), the existence of a single biomarker that is exclusive to this condition is unlikely. Therefore, we must look for risk factors that can predict the development of CRPS after trauma and for biomarkers that characterize the early and late stages of CRPS, enabling prediction of treatment outcome. As biomarker research in chronic pain in general is still in its infancy, we use the term ‘biomarker’ in a broad sense, in accordance with the following definition proposed by the Biomarkers Definitions Working Group10: “any measurement reflecting an interaction between a biological system and a potential hazard, which may be chemical, physical, or biological. The measured response may be functional and physiological, biochemical at the cellular level, or a molecular interaction.”
For the purposes of this Review, our definition of risk factors and biomarkers encompasses characteristics that have been measured longitudinally and are predictive of CRPS onset or treatment response, as well as features that differentiate between affected and unaffected limbs and/or between CRPS and adequate pain control groups. Comparisons of patient parameters with healthy individuals alone were excluded because the results might not be sufficiently specific for CRPS.
One exception to these rules is our decision to discuss microRNAs (miRNAs) in the blood. Efforts are underway by international consortia, such as ncRNAPain, to make miRNA data available for a large number of patients with CRPS. These miRNA studies have the potential to identify posttranslational factors that might be used in the future to determine whether a given incident of trauma will cause CRPS and to predict which cases of CRPS will be cured and which will become chronic. However, use of the current technology to compare miRNA profiles between CRPS and other chronic pain disorders is not yet a viable approach because of the substantial overlap in pain signalling pathways, as well as the costs of the tests. We designate these molecular signatures for CRPS as ‘emerging biomarkers’ because of the strong promise that they hold.
Risk factors for CRPS after trauma
Demographic and trauma-related risk factors.
Evidence regarding demographic and traumarelated risk factors came from a prospective inception cohort involving 596 patients, each of whom had a single fracture5. Analysis of these factors showed that ankle fracture, fracture dislocation and an intraarticular fracture contributed significantly to the development of CRPS type I (sensitivity 62% and specificity 70% for the regression model). In addition, the presence of rheumatoid arthritis or musculoskeletal comorbidities (back pain and arthrosis) was associated with the development of CRPS.
In a prospective cohort study of 477 patients, which evaluated CRPS development after distal radius surgery, factors that were associated with the development of CRPS type I included highenergy injuries (OR 3.3), severe fractures (OR 3.1) and female sex (OR 2.2)11. In a Japanese cohort study, the occurrence of CRPS was reported to be especially high following fractures of the distal forearm (OR 2.8)12. Contrary to other reports, this study found a low incidence of CRPS following fractures of the leg, with no specific sex preference. In addition, in this study, a longer duration of anaesthesia (except for regional anaesthesia) was reported to be significantly associated with an elevated incidence of CRPS.
A longitudinal study evaluated the influence of pain during the week after fracture on the development of CRPS in 1,549 patients with fracture of carpal bones, the distal radius or ulna or both, who were treated conservatively13. Overall, the authors observed a 3.8% incidence of CRPS. In patients with average pain scores of ≥5 over 2 days on an 11-step rating scale 5–7 days after the fracture (n = 113), the incidence of CRPS was 46% after 4 months. A smaller consecutive study of 90 patients with distal radius fracture who were treated with closed reduction and casting indicated that women who reported severe pain and reduced physical quality of life had an increased likelihood of developing CRPS 4 weeks after fracture14. Unfortunately, this study found an unusually high incidence of CRPS (>30%), probably owing to the use of diagnostic criteria that were not validated for CRPS, which weakens the conclusions.
The role of female sex as a risk factor for CRPS is questionable. Women in general have lower pain thresholds than men15, and most chronic pain disorders, including migraine, fibromyalgia and low back pain, have a strong female predominance. Furthermore, the incidence of limb fracture is significantly higher in middleaged or older women than in men16,17.
Taking into account the contradicting results regarding trauma type, affected limb and sex, we regard an unusually high level of pain during the week after the trauma as the most robust risk factor for CRPS development.
Psychological risk factors.
With respect to psychological risk factors for CRPS, the most robust evidence has come from prospective cohort studies. In the aforementioned prospective multicentre cohort study, which investigated the development of CRPS type I in 596 adults with single fractures5, none of the psychological factors that were analysed — namely, agoraphobia, depression, somatization, insufficiency, interpersonal sensitivity, insomnia and life events — predicted the development of CRPS type I. In addition, scores on the Symptom Checklist90 fell well within the range of the general population. These negative findings do not, however, rule out a role for other psychological risk factors that were not tested in this study in the development of CRPS.
As a secondbest approach, CRPS cohorts have been compared with patients with other pain disorders. Unfortunately, such an approach can only provide associations and cannot prove causality. In one such investigation, researchers examined the link between CRPS and alexithymia, which is the inability (as a stable personality trait) to identify and describe feelings18. People with alexithymia have difficulty distinguishing between emotions and bodily sensations. The initial evidence suggested that alexithymia ratings on the Toronto Alexithymia Scale were significantly higher in patients with CRPS than in patients with low back pain. Pain severity in patients with CRPS correlated significantly with high levels of alexithymia (Toronto Alexithymia Scale score 67 compared with a score of 49 in patients with low back pain). By contrast, patients with CRPS and patients with low back pain were similar in terms of levels of pain, disability, depression, anxiety and kinesiophobia19.
In a recent investigation20, 38% of patients with CRPS (n = 152) had positive scores on the PostTraumatic Stress Diagnostic Scale compared with only 10% of nonCRPS controls with limb pain (n = 55). The severity score on this rating scale was significantly associated with CRPS (sensitivity 75% and specificity 72%). In most of the patients, posttraumatic stress symptoms (related to violence, rape, sexual abuse, severe accidents or diseases) started long before the CRPSrelated trauma occurred.
The results of these cohort studies await confirmation in prospective investigations. Such investigations should concentrate on individuals who experienced high pain levels shortly after the trauma.
Diagnostic features and biomarkers
Neuropsychological biomarkers.
Structural MRI — specifically, voxelbased morphometry — has revealed positive and negative correlations between pain intensity and cortical thickness in the dorsal insula, left orbitofrontal cortex and cingulate cortex21. In paediatric CRPS, restingstate functional connectivity on functional MRI (fMRI) — in particular, the connections with forebrain areas, which are related to motor, affective, cognitive and pain inhibitory and modulatory processes — seems to be reduced22. These fascinating findings might aid our understanding of CRPS pathophysiology. However, they cannot serve as biomarkers because their specificity for CRPS has not been shown owing to a lack of adequate pain control groups. Our literature search identified only one study that included different pain groups23. This study found substantial overlaps in default mode network characteristics between CRPS, chronic low back pain and osteoarthritis.
Another investigation used MRI to compare the size of the bloodoxygenleveldependent (BOLD) response in the primary somatosensory cortex after vibration stimuli on the affected versus unaffected hand in patients with CRPS24. The cortical representation area was larger on the unaffected side, probably demonstrating compensatory use of the healthy, painless hand. Whether this phenomenon is specific for CRPS or applies to all kinds of hand immobilization is currently unknown. For more information about functional imaging in CRPS, we refer the reader to reviews that concentrate on this field of research25,26.
A growing body of evidence suggests that disturbances of body representation and body perception are key features of the CRPS phenotype. Neglectlike phenomena, distorted body image, body dysmorphic features, spatial deficits and impairment of executive function have all been reported in CRPS27. Impaired spatial perception has been shown to modulate limb temperature28, tactile processing, spontaneous pain and sense of ownership of the hands in patients with this condition. Experiments in which perceived midline shifts of the hand were altered by the use of prism glasses showed that in patients with CRPS, the temperature of the affected hand was modulated by the perceived location of the hands as opposed to their actual location or anatomical alignment28. On the basis of clinical tests, these features have been linked to dysfunction of the parietal lobe29. However, another investigation demonstrated that these body perception disturbances occurred regularly after fracture, independently of CRPS30.
We must stress that differences in a value between patients with CRPS and limb pain controls are not sufficient to formally identify a biomarker, although such differences increase the specificity of the finding for CRPS and can demonstrate phenotypic characteristics. Three studies31–33 that compared patients with CRPS with limb pain controls indicated that the findings described above hold some specificity for CRPS or are at least more pronounced in CRPS. In the dark, righthanded patients with CRPS had a larger leftward shift of their subjective visual midline than did righthanded individuals with limb pain and healthy controls, regardless of which hand was involved31. This finding was interpreted as augmentation of right hemispheric dominance for spatial tasks in patients with CRPS, which leads to exaggerated physiological right hemispatial pseudoneglect. Interestingly, reporting of socalled neglectlike phenomena, which have a high incidence in CRPS, does not correlate with pain intensity in CRPS as it does in other types of limb pain but instead correlates with anxiety, depression, somatization and depersonalization32. Viewing bistable images exacerbated pain and sensory disturbances in CRPS, including changes in perception of the temperature and weight of the affected limb; this phenomenon was not observed in pain controls with rheumatological diseases33.
Psychophysical biomarkers.
Quantitative sensory testing has revealed substantial sensory disturbances in patients with CRPS. A study that evaluated patterns of sensory signs in upperlimb CRPS types I and II (n = 344) showed that patients with CRPS type I had more sensory gain (heat and pressure pain) and less sensory loss (thermal and mechanical detection as well as hypoalgesia to heat or pinprick) than did patients with peripheral nerve injury. Patients with CRPS types I and II had almost identical somatosensory profiles34. A reduced pressurepain threshold — that is, increased pain in response to blunt pressure on muscles of the affected limb — seems to be a distinguishing feature of CRPS.
A separate study indicated that pressurepain sensitivity was even higher over the joints of the affected extremity35. This finding had high sensitivity and specificity for distinguishing patients with CRPS from those with nonCRPS pain and healthy controls. A patient with CRPS could be identified with a sensitivity of 82% and a specificity of 94% using a cutoff value of 102 kPa for pressurepain thresholds at the proximal interphalangeal joints (mean value from five joints) on the affected hand. Thus, notable pressurepain hyperalgesia in the affected limb is a consistent finding in CRPS and seems to separate this condition from other types of limb pain.
Disturbances of central sensory processing in CRPS are indicated by bilateral impaired pairedpulse suppression of somatosensory evoked potentials in patients with this condition compared with pain controls36. However, researchers found a considerable overlap between the two groups.
A finding that has long been regarded as typical for CRPS is an impaired twopoint discrimination threshold. A recent review clarified that this phenomenon also occurs in various nonneuropathic pain conditions, including arthritic pain and chronic low back pain and, therefore, is not specific for CRPS37.
Blood and serum biomarkers.
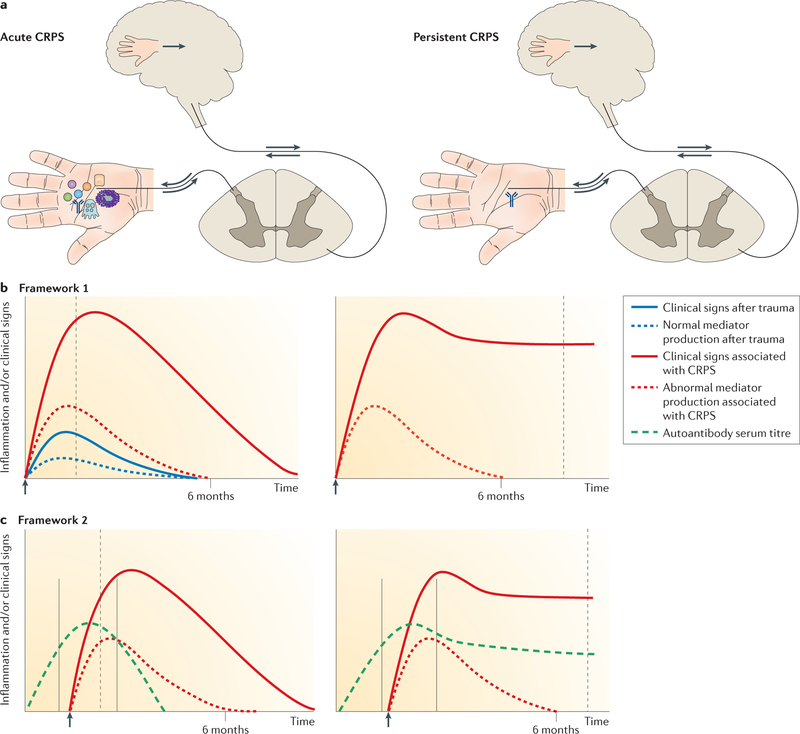
Almost all of the potential CRPS serum biomarkers that we discuss in this section indicate immune activation. The pathophysiological relevance of these markers can be considered within two broad conceptual frameworks, as outlined in FIG. 1. Both frameworks are consistent with the suggestion that early antiinflammatory treatment or limb mobilization (which can alleviate inflammation) can reduce the risk of CRPS, whereas inhibition of the cleavage of inflammatory mediators (for example, by angiotensinconverting enzyme inhibitors or limb immobilization) puts patients at higher risk (reviewed in REFS 9,38). Current evidence from suction blister investigations and skin biopsies (see next section), as well as older bone scintigraphy investigations, indicates that the early posttraumatic inflammatory immune activation in CRPS normalizes by about 6 months. Antiautonomic immunoglobulin G (IgG) serum autoantibodies are prevalent in both early and late CRPS (FIG. 1).
Figure 1. Hypothetical conceptual frameworks for CRPS.
a | Schematic representations of acute and persistent complex regional pain syndrome (CRPS). Proximally pointing arrows indicate signalling in response to distal excitation, and distally pointing arrows indicate descending signals leading to neuropeptide secretion and neurogenic inflammation. The depicted events correspond to the time points indicated by dashed grey lines in the graphs. b | Framework 1: augmented (neuro) immune activation. After injury (arrow), inflammatory mediators are produced by mast cells, sensory nerves, keratinocytes and osteocytes (represented by symbols in part a). In acute CRPS, mediator production is temporarily increased, triggering augmented post-traumatic clinical signs and primary afferent sensitization and leading to segmental spinal cord dorsal horn sensitization and sometimes a shift in the cortical representation of the affected limb with abnormal limb perception. Mediators and clinical signs normalize by about 6 months. In persistent CRPS, local production of inflammatory mediators at the affected limb normalizes and CNS changes now drive the clinical picture. In this framework, CRPS-associated autoantibodies might contribute to some clinical signs, such as sweating, but do not explain the main symptoms of pain and hypersensitivity. c | Framework 2: autoantibodies. If distal limb trauma is sustained during a time window of vulnerability characterized by high autoantibody production (solid grey lines), an enhanced trauma-induced inflammatory response renders these antibodies locally pathogenic, resulting in augmented post-traumatic signs. Autoantibodies might bind to neurons, causing afferent sensitization through changes in the transduction or transmission properties of these cells, or to perineural cells, which then release pronociceptive molecules (not shown). These autoantibodies are noninflammatory; that is, they neither activate complement nor attract immune cells. Acute CRPS resolves after cessation of autoantibody production. A small group of patients develops persistent CRPS, in which autoantibody production continues at a reduced rate that is still sufficient to sustain the clinical phenotype.
On the basis on CRPS studies without pain controls, inflammatory and antiinflammatory serum cytokines are potential biomarkers for CRPS. However, in two studies, serum cytokine levels in patients with CRPS (mostly acute CRPS) did not differ from those in patients with fractures or upperlimb pain of other origins39,40 (TABLE 1).
Table 1 |.
Key serum biomarker studies for CRPS
| Type of CRPS | Mean CRPS duration (range) In months | Mean pain intensity (range)a | Test target | Test type | Definition of test positivity | Controls | No. of patients/controls tested | Sensitivity and specificity | Clinical and prognostic associations |
|---|---|---|---|---|---|---|---|---|---|
| Early39 | 3.5 (0.2–15.8) | 6 (2–10) | Serum cytokines | Bead-based multiplex assay | Detection limits specified by manufacturer | Patients with upper-limb pain | 28/15 | No differences between patients and controls | None identified |
| Both early and persistent40 | 8 (2–21) | 3 (0–5) | Serum TNF | ELISA | Detection limit: <1.7 pg/ml | Patients after upper-limb fracture or with severe knee or hip osteoarthritis | 10/20 | No differences between patients with CRPS and patients with osteoarthritis; patients with fractures had lower levels than the other groups | In CRPS, temperature difference between limbs correlated with plasma TNF level (P < 0.02, ρ = 0.756) |
| Early41 | 3.0 (2.0–5.5) | 5 (IQR: 3–8) | Osteoprotegerin | ELISA | >90% CI of the control mean | Patients after limb trauma and patients undergoing three-phased bone scan for cancer staging | 23/31 | Sensitivity 74% and specificity 79%; AUC 0.8 (CI 0.68–0.91) | Carpal bone tracer uptake in three-phase bone scan correlated with serum osteoprotegerin (ρ = 0.391) |
| Early42 | 3.5 (0.5–19.0) | 4 (0–10) | β2‑Adrenergic receptor activation and M2 muscarinic receptor activation | Live-cell bioassay confirmed by ELISA | Healthy control mean + 2.5 × CI | Healthy individuals and patients with noninflammatory neuropathy or peripheral nerve lesion | 20/19 | Combined ELISA test results for the two targets: sensitivity 75% and specificity 100% versus all controls | None identified |
| Persistent43 | 17 (6–120) | 7.5 (6.0–10.0) | α1A‑Adrenergic receptor activation and muscarinic receptor activation | Live-cell bioassay | Healthy control mean ± 3 × s.d. | Healthy individuals and patients with neuropathic pain, fibromyalgia or myasthenia gravis | 18/57 | Combined test results for the two targets: sensitivity 61% and specificity 98% versus all controls | None identified |
ρ, Spearman’s rank correlation oefficient; AUC, area under the curve; CRPS, complex regional pain syndrome; ELISA, enzyme-linked immunosorbent assay; IQR, interquartile range; TNF, tumour necrosis factor.
Measured on an 11-point (0–10) pain-rating scale.
Localized osteoporosis and increased bone turnover are frequent findings in early CRPS, but serum markers for these processes have been investigated only in the past few years41. Osteoprotegerin, a cytokine receptor that is involved in the regulation of bone turnover, was found to be elevated in a study of patients with early CRPS, who were compared with patients who had normal healing after limb fracture, patients receiving threephase bone scanning for cancer staging, and a historical healthy reference group. The test showed a sensitivity of 74% and a specificity of 79%, supporting its potential for development as a diagnostic assay (TABLE 1). Interestingly, the study showed no correlation between osteoprotegerin concentration and disease duration or any of the CRPS clinical signs; however, the number of patients was small.
The recent description of autoantibodies in CRPS is promising for biomarker research. Using livecell bioassays, two groups have shown that up to 70% of patients with CRPS have antiautonomic IgG autoantibodies in their serum42,43. CRPS is associated with autonomic disturbances, and abnormal adrenergic receptor activation has previously been linked to the generation of pain, raising the possibility that these antiautonomic antibodies contribute to CRPS pathophysiology.
Specific IgG serum autoantibodies that activate β2adrenergic or M2muscarinic receptors were first identified in patients with early CRPS42. The effects of these antibodies on live cells were blocked by coapplication of synthetic peptides located on the second extracellular loops of the receptors. Enzymelinked immunosorbent assays (ELISAs) coated with these peptides recognized both autoantibodies with high specificity and sensitivity in the serum from patients with CRPS (TABLE 1). Surface binding was confirmed by flow cytometry in most preparations. These autoantibodies belonged to the IgG1–IgG3 subclasses, and no crossreactivity between them was observed44.
In another study, the effects of serum immunoglobulin derived from patients with longstanding CRPS were examined in adult rodent cardiomyocytes43. The researchers identified antibodies that activated either α1Aadrenergic or muscarinic receptors (TABLE 1); the activating antibodies bound their targets with high affinity. Subsequent flow cytometric and spectrofluorimetric analyses suggested the presence of distinct pathways of antibodyinduced α1Aadrenergic receptor activation.
The specificity of these autoantibodies for CRPS will need to be confirmed in larger, more heterogeneous CRPS and pain control populations. These additional studies are needed to clarify whether clinical CRPS phenotypes correspond to the presence of autoantibodies and to provide a better estimate of the test performance.
Additional approaches to identifying autoantibodies in CRPS, which can form the basis for future test development, include the in vivo passive transfer trauma model and in vitro studies on primary dorsal root ganglion neurons. In small studies, rodents that received injections of IgG from patients with CRPS displayed significantly increased mechanical hyperalgesia and swelling in comparison with animals that received control IgG injections, but only in the injured hindpaws45,46. This finding suggests that pathogenic autoantibodies develop their activity only in the context of injury, consistent with the post traumatic development of CRPS. A similar phenomenon was shown in an in vitro study in which incubation with CRPS IgG altered the calcium responses to potassium depolarization in primary rodent dorsal root ganglion cells, but only if these cells had been coincubated with inflammatory mediators47. The biology underpinning the observed dependence of autoantibody activity on injury and inflammation is unknown.
Abnormalities in plasma levels of various amino acids48, antioxidants49 and circulating CD14+CD16+ monocytes50 have also been described in CRPS, but unfortunately in studies that did not include appropriate pain control groups. Therefore, the utility of these factors as biomarkers for CRPS remains to be determined.
Biomarkers from skin biopsy and suction blisters.
Skin biopsies allow the investigation of the affected tissue itself. In a rodent fracture and casting CRPS model51, skin immunohistochemistry demonstrated keratinocyte proliferation and increased skin mast cell count. Increased production of inflammatory cytokines by keratinocytes was related to pain behaviour. These findings prompted an investigation of skin biopsies in patients with CRPS52. Skin biopsy samples were collected from the affected skin and the contralateral mirror site in 55 patients with CRPS and were immunostained for keratinocyte proliferation, mast cell markers, tumour necrosis factor (TNF) and IL6. In the early stages of CRPS, keratinocytes were activated in the affected skin, resulting in proliferation and epidermal thickening. Furthermore, TNF and IL6 were upregulated in about 40% of very acute cases, although these factors were only assessed qualitatively. In later CRPS (from 6 months to many years in pain), reduced keratinocyte proliferation was found, leading to epidermal thinning in the affected skin, and no differences were found in immunostaining of TNF and IL6 between the two sides of the body. Patients with acute CRPS also showed increased mast cell accumulation in the affected skin (FIGS. 2,3), a finding that is not observed in chronic CRPS52; in a separate investigation, Langerhans cell numbers were even reduced in longstanding CRPS53.
Figure 2. Mast cell accumulation in CRPS.
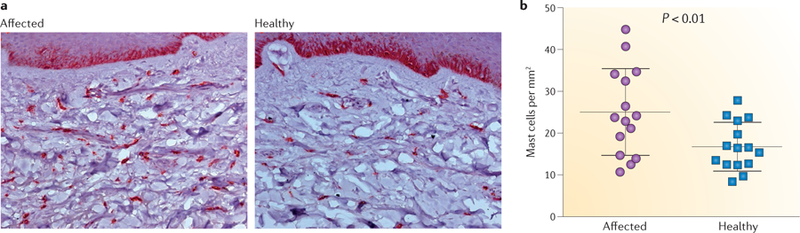
a | CD117-positive mast cells, which are stained dark red, in skin biopsy samples from the affected limb and a corresponding site on the unaffected limb of a patient with complex regional pain syndrome (CRPS). b | The number of mast cells per square millimetre in the subcutaneous tissue is significantly increased in the affected skin of patients with acute CRPS52 (≤6 months after diagnosis).
Figure 3. The possible role of keratinocytes in CRPS pathophysiology.
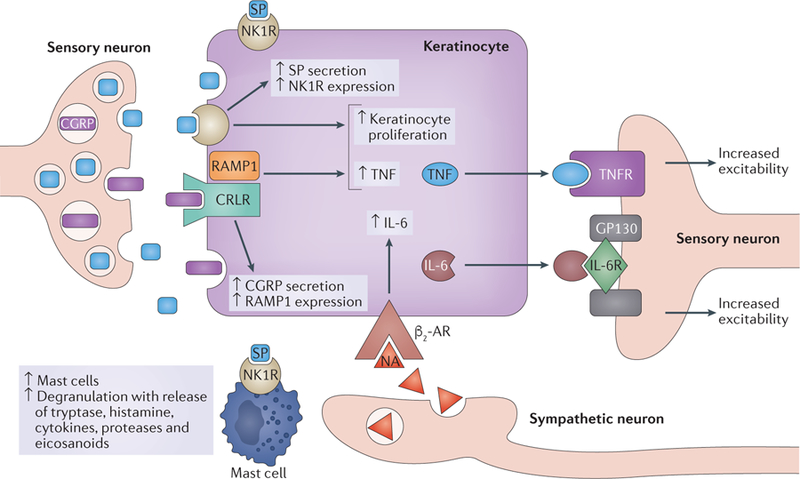
In complex regional pain syndrome (CRPS), activation of peptidergic nociceptors not only causes pain but also leads to release of neuropeptides (substance P (SP) and calcitonin gene-related peptide (CGRP)). Both of these peptides activate keratinocytes and promote their proliferation. In turn, the keratinocytes secrete cytokines, which sensitize the peptidergic nociceptors for forthcoming stimuli. The resulting facilitated release of SP from primary afferent neurons leads to activation of mast cells via specific receptors. Mast cells also release inflammatory mediators, which sensitize nociceptors and further activate keratinocytes. Pain activates the sympathetic nervous system, releasing noradrenaline (NA) that also activates keratinocytes. Consequently, a vicious circle might be established. This figure is based on the findings of a series of studies on tibia fracture models in rodents and research in patients with CRPS. β2-AR, β2-adrenergic receptor; CRLR, calcitonin receptor-like receptor; GP130, membrane glycoprotein 130; IL-6R, IL-6 receptor; NK1R, neurokinin 1 receptor (also known as SPR); RAMP1, receptor activity-modifying protein 1; TNF, tumour necrosis factor; TNFR, TNF receptor. Figure adapted with permission from REF. 52, Elsevier.
These data fit well with clinical impressions that the unaffected side is usually ‘normal’, and that changes in pathophysiology occur during the shift from acute to chronic CRPS (see FIG. 3 for the role of keratinocytes). The studies were limited in having no pain control group and — more importantly — no normalfracturehealing control group. Thus, the diagnostic value of skin biopsies is currently limited. Acute CRPS in particular might show some overlap with normal fracture healing. However, in the aforementioned study52, all patients, regardless of whether their condition was acute or chronic, met the official diagnostic criteria for CRPS, which do not differentiate between different CRPSpathologies. Therefore, the results from skin biopsies not only demonstrate objective pathophysiological differences between acute and chronic CRPS but might also aid in the choice of treatment in the future.
To further address the upregulation of TNF in the skin of patients with CRPS, researchers took punch biopsies from three different groups: patients with osteoarthritis or acute fractures who were awaiting surgery, and patients with CRPS who had a disease duration of ~6 months (biopsies were taken from the affected side)40. Quantification of TNF by ELISA showed that patients with CRPS had elevated TNF levels in the skin compared with the two control groups. These results corroborate the findings discussed above. However, substantial overlap in the values between the groups limits the use of skin TNF as a biomarker.
An alternative approach to skin biopsy is the investigation of skin suction blister fluids. Proinflammatory and antiinflammatory cytokines, including the IL1 receptor antagonist protein (IL1Ra), IL2, IL6, IL8, IL10, IL12p40, TNF and the chemokines eotaxin, monocyte chemotactic protein 1 (MCP1) and macro phage inflammatory protein 1β (MIP1β), were analysed in skin blister fluids from patients with CRPS (disease duration <1 year) and patients with upperlimb pain of other origin (nonCRPS controls)39. This analysis was repeated after 6 months of CRPS treatment. Blister fluid was collected from the affected and unaffected sides of the body. Compared with controls, patients with CRPS showed a bilateral increase in TNF and MIP1β and a decrease in antiinflammatory IL1Ra protein levels. Neither group showed differences between the two sides of the body. Again, the overlap in levels of individual cytokines between the groups was high. However, the pattern of changes (increase in proinflammatory and decrease in antiinflammatory cytokines; see also Parkitny et al.3) seems to be relatively specific for CRPS because it was not present on either side in the control group. After 6 months of CRPS treatment, levels of most cytokines in patients with CRPS reverted bilaterally to the levels seen in nonCRPS controls. These changes were not related to treatment outcome. This unexpected bilaterality could indicate a ‘trait’ or a generalized inflammatory reaction, or it might be related to the long suction period, which allows systemic substances to enter the fluid, as has been repeatedly shown in pharmacokinetic studies.
Small non-coding RNAs (microRNAs) as emerging biomarkers in CRPS.
Circulating miRNAs are providing a novel source of prognostic and diagnostic biomarkers. Encoded in the genome, miRNAs are naturally occurring, small noncoding RNA molecules of ~22 nucleotides. Complementarity of a 6 bp seed sequence facilitates the binding of a mature miRNA to its target mRNA. An miRNA can negatively regulate gene expression either by repressing protein translation or through mRNA degradation54.
Enclosure of miRNAs in membranous vesicles or their association with RNAbinding proteins is now known to confer protection from RNases, allowing circulating miRNAs to travel long distances without undergoing degradation55. Exosomes are 30–100 nm vesicles that carry miRNAs, mRNAs, proteins and lipids, and the composition varies depending on the secreting cell56. Horizontal transfer of circulating miRNAs between cells is a novel mode of intercellular communication57.
Studies on miRNAs in chronic pain, and particularly CRPS, are still in their infancy. The mode of action of miRNAs (they can regulate multiple pathways) renders them promising candidates to help explain the pathophysiology of complex diseases. In view of their potential, we include research on miRNAs in CRPS in this Review despite the lack of studies in large patient populations with adequate controls.
The lack of uniformity in the pathophysiology underlying CRPS led researchers to wonder whether circulating miRNAs could aid in patient stratification. In one of the first studies, circulating miRNAs were profiled in whole blood from 41 patients with CRPS and 20 controls58. The researchers found that levels of 18 miRNAs differed significantly between patients and controls. The miRNA signatures also enabled patient stratification: on the basis of changes in levels of relevant miRNAs, a cluster analysis revealed three different groups. One group comprised 60% of the patients with CRPS and none of the controls. The remaining 40% of patients with CRPS had more heterogeneous miRNA profiles and were clustered together with the control individuals in the other two groups. The patients in the first group did not differ clinically from the patients with CRPS in the other groups, suggesting that the symptoms that patients with CRPS share, and on which diagnosis is based, do not necessarily result from identical molecular mechanisms. When the 60% of patients with CRPS were analysed as a group, additional miRNAs and inflammatory markers emerged that were not evident in the overall CRPS patient population in the study. Thus, the molecular signature of circulating miRNAs can aid identification of additional biomarkers that are specific to a subset of patients57.
Exosomes purified from the serum of six patients with CRPS and six healthy controls were used to study whether exosomal miRNAs reflect miRNA signatures in whole blood59. Interestingly, significant differences in 127 exosomal miRNAs were observed between the two groups. Additional studies are needed to investigate factors that influence the packaging of specific miRNAs into exosomes in patients with CRPS.
Circulating miRNAs could be an indicator of aberrations in cellular homeostasis that underlie disease60, and investigation of the target mRNAs of differentially expressed miRNAs might provide important insights into aberrant gene expression that contributes to the disease pathology. A 2011 study investigated the mechanistic relevance of miR939 (REF. 58), which was found to be downregulated 4.3fold in patients with CRPS. Using a variety of in vitro molecular and biochemical approaches, the researchers showed that miR939 can target several mRNAs encoding various proinflammatory mediators, including IL6, vascular endothelial growth factor, nitric oxide synthase 2 and nuclear factorκB2. This finding suggests that miR939 regulates multiple proinflammatory genes, and downregulation of this miRNA in patients with CRPS may contribute to an increase in inflammation and pain61. This epigenetic research is promising, but the associated workload and costs preclude routine testing at the moment.
These results are consistent with a genomewide RNA expression profiling study, in which blood from patients with CRPS was examined62. Eighty genes, many of which were associated with immunity and defence, were differentially expressed. Most striking was the fourfold upregulation of the matrix metalloproteinase 9 (MMP9) gene, as assessed by quantitative reverse transcription PCR.
By contrast, in another study, no differences in DNA singlenucleotide polymorphisms (SNPs) were found between patients with CRPS and unspecified controls63. A large number of SNPs (>200,000) were analysed, but no difference passed the statistical threshold after Bonferroni correction. This negative result is not unexpected, because association studies with a broad approach require much larger cohorts, and such an effort would be challenging for a rare disease such as CRPS.
Markers for progression and treatment
Clinical and psychological biomarkers and clinical characteristics.
A systematic review published in 2014 found evidence that most patients with CRPS partially recover within 6–13 months; however, a substantial number of patients with this condition experience lasting symptoms, chronic pain and disability64. This paper was followed up by a prospective cohort study that included patients with wellcharacterized disease. Focusing on important clinical parameters65, the study showed that clinically relevant reductions could be observed in the majority of signs and symptoms of CRPS at 1 year; the greatest improvements were achieved within 6 months. At 12 months, however, onequarter of the patients still met the current diagnostic criteria for CRPS, and just 5% were symptomfree. Another cohort study involving patients with postfracture CRPS reported that none of the patients were symptomfree 12 months after the trauma5.
Data from a prospective study66 suggest that in patients who receive conservative treatment, female sex and high levels of baseline pain and disability predict high CRPS severity 12 months after disease onset. Patients with low painrelated fear had lower levels of disability over the 12month period, and baseline anxiety and disability were positively associated with pain intensity over 12 months. By contrast, body perception disturbances, depression and general stress did not predict outcomes. The relevance of predictors might be treatmentspecific, as a study that evaluated the effects of two physical therapy modalities found no indication that painrelated fear mediates outcome67. Limited evidence indicates that sympathetic blocks are efficacious for treating CRPS pain, and allodynia and hypoaesthesia were reported to be negative predictors for response to this treatment after 7 days68.
To reinforce the conclusions from the aforementioned studies, replication with standardized treatment approaches are needed.
Circulating microRNAs.
Ketamine is an established treatment for CRPS in some countries, but a subset of patients responds poorly to this drug. A proofofconcept study investigated whether expression of circulating miRNAs in whole blood from patients with treatmentresistant CRPS was altered in response to ketamine therapy69. This analysis identified 14 miRNAs that were differentially expressed before and after treatment in both good and poor responders to ketamine. Comparison of miRNAs in responders relative to poor responders before treatment showed differential expression of 33 miRNAs. In the posttreatment samples, 43 miRNAs differed between responders and poor responders. These results indicate the potential feasibility of using miRNA signatures in the circulation as biomarkers to predict treatment response.
Determining the functional relevance of miRNA changes in the circulation is challenging because miRNAs can bind to multiple mRNAs through seed sequence complementarity. Although several miRNAs were altered in the aforementioned proofofconcept study69, the authors focused on miR548d for detailed target validation studies because of its potential regulatory role in ketamine metabolism according to bioinformatic predictions. Biotransformation of drugs and xenobiotics generally occurs in two stages, termed phase I and phase II. The phase I or oxidation–reduction step is primarily mediated by cytochrome P450 superfamily enzymes. The UDPglucuronosyltransferases (UDPGTs) are conjugative enzymes that mediate phase II metabolism. In phase I, ketamine is metabolized to norketamine; both ketamine and norketamine are further metabolized and eliminated by phase II enzymes, specifically UDPGTs, through glucuronidation. In vitro studies in a liver cell line confirmed that miR548d5p can bind and regulate the UDPGT gene UGT1A1. Levels of miR548d5p were 18fold lower in poor responders than in responders to ketamine. These results suggest that different levels of miR548d5p lead to differences in UDPGT activity between responders and poor responders.
In addition to miR548d5p, a second miRNA, miR34a, was investigated in the context of BMI in ketaminetreated patients with CRPS70. On average, the BMI was lower in poor responders than in responders. Proopiomelanocortin (POMC)related peptides have an important role in the regulation of body weight, appetite and energy expenditure, and reduced POMC expression is associated with increased body weight. Ketamine treatment did not alter POMC expression, but poor responders had higher levels of POMC mRNA than did responders, both before and after treatment. Corticotropinreleasing hormone (CRH; also known as corticoliberin) is a key regulator of POMC expression — an effect that is mediated by CRH receptor 1 (CRHR1). The researchers found that miR34a, which is a negative regulator of CRHR1, was markedly downregulated in poor responders. Poor responders showed higher expression of CRHR1 mRNA in whole blood than did responders, indicating a regulatory role for miR34a. Pretreatment levels of miR34a correlated positively with BMI and with response to ketamine therapy: poor responders had lower BMI and lower pretreatment levels of circulating miR34a. These findings indicate a mechanism through which miR34a can regulate the CRH– CRHR1–POMC axis and possibly influence BMI. If sufficiently powered studies can replicate these findings and define combined cutoff points for miR548d5p, miR34a and BMI, a simple score to predict pain response to prolonged infusion of ketamine seems possible, and unnecessary treatment could be avoided.
Conclusions and future directions
Substantial progress has been made in CRPS research in recent years, enabling a better understanding of this condition, which is a prerequisite for the identification of risk factors and biomarkers for prevention, diagnosis, disease course and treatment. Biomarker research in CRPS is far from perfect, however, and most conclusions that can be drawn are still tentative.
To encapsulate the biomarker research of the past 5 years, as reported in this Review, we present a ‘prototypical’ patient in BOX 2. We are aware that the description of this patient is not supported by rigorous analysis of the published results, such as by a systematic review. However, even this tentative description would not have been possible a few years ago. The fact that specific ‘biomarkers’ can be linked to particular subgroups of patients with CRPS indicates divergent causes of pain and other symptoms. Thus, the discovery of specific biomarkers, which in turn can result in individualized treatment, is likely to depend on reliable subtyping. Accomplishment of this goal will require identification of new molecular signatures of CRPS or analysis of various combinations of existing signatures, including DNA, RNA and proteins, along with clinical parameters. The biomarkers must be easily accessible and testable. This research will not eliminate CRPS, but it holds the promise of reducing pain and suffering and facilitating healing in a substantial number of patients.
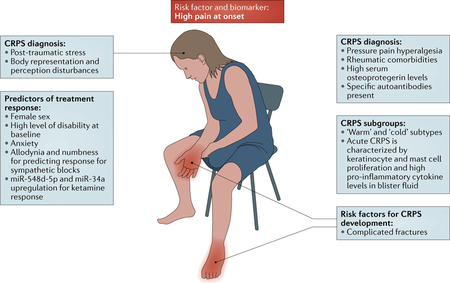
Box 2 |. A prototypical patient with CRPS.
By depicting a ‘prototypical’ patient with complex regional pain syndrome (CRPS), the figure delineates and summarizes how the biomarkers and risk factors that have been discovered in the past 5 years could help to identify patients at risk of CRPS, improve diagnosis through specification of PNS-derived and CNS-derived phenotypic characteristics and offer the possibility of individualized treatment in the future.
Given the overrepresentation of postmenopausal women among patients with CRPS, our patient is a middle-aged woman with a history of rheumatic disease. She sustains a complicated fracture, and her pain is still extreme 1 week after the fracture. A diagnosis of CRPS is likely if she fulfils the current clinical diagnostic criteria, but alternative diseases must still be excluded4. If the patient demonstrates a leftward shift of the body midline in the dark and her pain intensifies while she concentrates on bistable images, the likelihood of a CRPS diagnosis increases, particularly if the pain substantially worsens when mild pressure is applied to the joints of the affected limb distal to the injury site. Psychological investigations demonstrate high levels of alexithymia and post-traumatic stress. If blood analysis reveals high serum osteoprotegerin levels and autoantibodies against autonomic nervous system receptors, the probability of CRPS increases further. To enable bespoke treatment, skin biopsies or suction blister examinations are performed. If inflammation is present, anti-inflammatory treatment can be initiated. Intravenous ketamine for pain control is started only if levels of circulating microRNAs — in particular, miR-548d-5p and miR-34a — in the blood predict a favourable response. Owing to this patient’s high disability and anxiety at baseline, she must be closely followed and her disease-related anxiety must be addressed (for example, in graded exposure treatment71).
Key points.
Complex regional pain syndrome (CRPS) is a persistent pain condition that often results from an injury and usually affects a single limb.
An unusually high level of pain during the week after the injury seems to be the most robust risk factor for CRPS development.
Post-traumatic inflammation has been identified as a major component of acute CRPS, and growth factors, catecholamines and autoantibodies have also been implicated in CRPS pathogenesis.
A growing body of evidence indicates that disturbances of body representation and body perception are key features of the CRPS phenotype.
Small non-coding RNAs (microRNAs) are emerging as diagnostic and prognostic biomarkers for CRPS.
A single marker for CRPS is unlikely to be found; however, a range of biomarkers might assist in clinical diagnosis and guide prognosis and treatment.
Acknowledgements
F.B. acknowledges support from the Deutsche Forschungs-gemeinschaft (Germany; grant Bi579/8–1) and the Dietmar-Hopp Foundation. F.B. and C.S. acknowledge support from the European Commission (ncRNAPain, FP7 grant 602133). A.G. has received funding from the Pain Relief Foundation, Liverpool, UK. S.K.A. has received grants from the NIH (National Institute of Neurological Disorders and Stroke 1R21NS082991–01), the Rita Allen Foundation and the Drexel University Clinical and Translational Research Institute.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Neurology thanks Candida McCabe, David Clark and the other anonymous reviewer for their contribution to the peer review of this work.
FURTHER INFORMATION
ncRNAPain: http://www.ncrna-pain.eu
References
- 1.Marinus J et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol 10, 637–648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birklein F, O’Neill D & Schlereth T Complex regional pain syndrome: an optimistic perspective. Neurology 84, 89–96 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Parkitny L et al. Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology 80, 106–117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harden RN et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain 150, 268–274 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beerthuizen A et al. Demographic and medical parameters in the development of complex regional pain syndrome type 1 (CRPS1): prospective study on 596 patients with a fracture. Pain 153, 1187–1192 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Bruehl S et al. Complex regional pain syndrome: evidence for warm and cold subtypes in a large prospective clinical sample. Pain 157, 1674–1681 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Mugge W, Schouten AC, van Hilten JJ & van der Helm FC Impaired inhibitory force feedback in fixed dystonia. IEEE Trans. Neural Syst. Rehabil. Eng 24, 475–484 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Shi X et al. Facilitated spinal neuropeptide signaling and upregulated inflammatory mediator expression contribute to postfracture nociceptive sensitization. Pain 156, 1852–1863 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birklein F & Schlereth T Complex regional pain syndrome — significant progress in understanding. Pain 156 (Suppl. 1), S94–S103 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther 69, 89–95 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Roh YH et al. Factors associated with complex regional pain syndrome type I in patients with surgically treated distal radius fracture. Arch. Orthop. Trauma Surg 134, 1775–1781 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Sumitani M et al. Perioperative factors affecting the occurrence of acute complex regional pain syndrome following limb bone fracture surgery: data from the Japanese Diagnosis Procedure Combination database. Rheumatology 53, 1186–1193 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Moseley GL et al. Intense pain soon after wrist fracture strongly predicts who will develop complex regional pain syndrome: prospective cohort study. J. Pain 15, 16–23 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Jellad A, Salah S & Ben Salah Frih Z Complex regional pain syndrome type I: incidence and risk factors in patients with fracture of the distal radius. Arch. Phys. Med. Rehabil 95, 487–492 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Pfau DB et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): reference data for the trunk and application in patients with chronic postherpetic neuralgia. Pain 155, 1002–1015 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Beerekamp MS et al. Epidemiology of extremity fractures in the Netherlands. Injury 48, 1355–1362 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Somersalo A et al. Incidence of fractures requiring inpatient care. Acta Orthop 85, 525–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margalit D, Ben Har L, Brill S & Vatine JJ Complex regional pain syndrome, alexithymia, and psychological distress. J. Psychosom. Res 77, 273–277 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Bean DJ, Johnson MH & Kydd RR Relationships between psychological factors, pain, and disability in complex regional pain syndrome and low back pain. Clin. J. Pain 30, 647–653 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Speck V, Schlereth T, Birklein F & Maihofner C Increased prevalence of posttraumatic stress disorder in CRPS. Eur. J. Pain 21, 466–473 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Barad MJ, Ueno T, Younger J, Chatterjee N & Mackey S Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. J. Pain 15, 197–203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erpelding N et al. Habenula functional resting-state connectivity in pediatric CRPS. J. Neurophysiol 111, 239–247 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baliki MN, Mansour AR, Baria AT & Apkarian AV Functional reorganization of the default mode network across chronic pain conditions. PLoS ONE 9, e106133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Pietro F, Stanton TR, Moseley GL, Lotze M & McAuley JH Interhemispheric somatosensory differences in chronic pain reflect abnormality of the healthy side. Hum. Brain Mapp 36, 508–518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upadhyay J, Geber C, Hargreaves R, Birklein F & Borsook D A critical evaluation of validity and utility of translational imaging in pain and analgesia: utilizing functional imaging to enhance the process. Neurosci. Biobehav. Rev 84, 407–423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Pietro F et al. Primary somatosensory cortex function in complex regional pain syndrome: a systematic review and meta-analysis. J. Pain 14, 1001–1018 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Torta DM, Legrain V, Rossetti Y & Mouraux A Prisms for pain. Can visuo-motor rehabilitation strategies alleviate chronic pain? Eur. J. Pain 20, 64–69 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moseley GL, Gallace A, Di Pietro F, Spence C & Iannetti GD Limb-specific autonomic dysfunction in complex regional pain syndrome modulated by wearing prism glasses. Pain 154, 2463–2468 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Cohen H et al. Clinical evidence of parietal cortex dysfunction and correlation with extent of allodynia in CRPS type 1. Eur. J. Pain 17, 527–538 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Hall J et al. Sensorimotor dysfunction after limb fracture — an exploratory study. Eur. J. Pain 20, 1402–1412 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Reinersmann A et al. Impaired spatial body representation in complex regional pain syndrome type 1 (CRPS I). Pain 153, 2174–2181 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Michal M et al. Association of neglect-like symptoms with anxiety, somatization, and depersonalization in complex regional pain syndrome. Pain Med 18, 764–772 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Hall J et al. Pain and other symptoms of CRPS can be increased by ambiguous visual stimuli — an exploratory study. Eur. J. Pain 15, 17–22 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Gierthmuhlen J et al. Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain 153, 765–774 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Mainka T et al. Comparison of muscle and joint pressure-pain thresholds in patients with complex regional pain syndrome and upper limb pain of other origin. Pain 155, 591–597 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Lenz M et al. Bilateral somatosensory cortex disinhibition in complex regional pain syndrome type I. Neurology 77, 1096–1101 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Catley MJ, O’Connell NE, Berryman C, Ayhan FF & Moseley GL Is tactile acuity altered in people with chronic pain? A systematic review and meta-analysis. J. Pain 15, 985–1000 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Guo TZ et al. Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture in rats. J. Pain 15, 1033–1045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenz M et al. Local cytokine changes in complex regional pain syndrome type I (CRPS I) resolve after 6 months. Pain 154, 2142–2149 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Kramer HH et al. TNF-α in CRPS and ‘normal’ trauma — significant differences between tissue and serum. Pain 152, 285–290 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Kramer HH et al. Osteoprotegerin: a new biomarker for impaired bone metabolism in complex regional pain syndrome? Pain 155, 889–895 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Kohr D et al. Autoimmunity against the β2 adrenergic receptor and muscarinic-2 receptor in complex regional pain syndrome. Pain 152, 2690–2700 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Dubuis E et al. Longstanding complex regional pain syndrome is associated with activating autoantibodies against alpha-1a adrenoceptors. Pain 155, 2408–2417 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Hendrickson JE et al. Complex regional pain syndrome and dysautonomia in a 14-year-old girl responsive to therapeutic plasma exchange. J. Clin. Apher 31, 368–374 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Goebel A et al. The passive transfer of immunoglobulin G serum antibodies from patients with longstanding complex regional pain syndrome. Eur. J. Pain 15, 504.e1–504.e6 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Tekus V et al. A CRPS-IgG-transfer-trauma model reproducing inflammatory and positive sensory signs associated with complex regional pain syndrome. Pain 155, 299–308 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Reilly JM et al. Effects of serum immunoglobulins from patients with complex regional pain syndrome (CRPS) on depolarisation-induced calcium transients in isolated dorsal root ganglion (DRG) neurons. Exp. Neurol 277, 96–102 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Alexander GM et al. Plasma amino acids changes in complex regional pain syndrome. Pain Res. Treat 2013, 742407 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baykal T, Seferoglu B, Karsan O, Kiziltunc A & Senel K Antioxidant profile in patients with complex regional pain syndrome type I. Int. J. Rheum. Dis 17, 156–158 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Ritz BW et al. Elevated blood levels of inflammatory monocytes (CD14+CD16+) in patients with complex regional pain syndrome. Clin. Exp. Immunol 164, 108–117 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo TZ, Wei T, Li WW, Li XQ, Clark JD & Kingery WS Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture in rats. J. Pain 15, 1033–1045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Birklein F et al. Activation of cutaneous immune responses in complex regional pain syndrome. J. Pain 15, 485–495 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osborne S et al. Cutaneous immunopathology of long-standing complex regional pain syndrome. Eur. J. Pain 19, 1516–1526 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Agarwal V, Bell GW, Nam JW & Bartel DP Predicting effective microRNA target sites in mammalian mRNAs. eLife 4, e05005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X, Liang H, Zhang J, Zen K & Zhang CY Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol 22, 125–132 (2012). [DOI] [PubMed] [Google Scholar]
- 56.El Andaloussi S, Lakhal S, Mäger I & Wood MJ Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Deliv. Rev 65, 391–397 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Kowal J, Tkach M & Thery C Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol 29, 116–125 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Orlova IA et al. MicroRNA modulation in complex regional pain syndrome. J. Transl Med 9, 195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDonald MK et al. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain 155, 1527–1539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mendell JT & Olson EN MicroRNAs in stress signaling and human disease. Cell 148, 1172–1187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald MK et al. Regulation of proinflammatory genes by the circulating microRNA hsa-miR-939. Sci. Rep 6, 30976 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin EH et al. Genome-wide expression profiling of complex regional pain syndrome. PLoS ONE 8, e79435 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janicki PK, Alexander GM, Eckert J, Postula M & Schwartzman RJ Analysis of common single nucleotide polymorphisms in complex regional pain syndrome: genome wide association study approach and pooled DNA strategy. Pain Med 17, 2344–2352 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Bean DJ, Johnson MH & Kydd RR The outcome of complex regional pain syndrome type 1: a systematic review. J. Pain 15, 677–690 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Bean DJ, Johnson MH, Heiss-Dunlop W & Kydd RR Extent of recovery in the first 12 months of complex regional pain syndrome type-1: a prospective study. Eur. J. Pain 20, 884–894 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Bean DJ, Johnson MH, Heiss-Dunlop W, Lee AC & Kydd RR Do psychological factors influence recovery from complex regional pain syndrome type 1? A prospective study. Pain 156, 2310–2318 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Barnhoorn KJ et al. Are pain-related fears mediators for reducing disability and pain in patients with complex regional pain syndrome type 1? An explorative analysis on pain exposure physical therapy. PLoS ONE 10, e0123008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Eijs F et al. Predictors of pain relieving response to sympathetic blockade in complex regional pain syndrome type 1. Anesthesiology 116, 113–121 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Douglas SR et al. Analgesic response to intravenous ketamine is linked to a circulating microRNA signature in female patients with complex regional pain syndrome. J. Pain 16, 814–824 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Shenoda BB, Alexander GM & Ajit SK Hsa-miR-34a mediated repression of corticotrophin releasing hormone receptor 1 regulates pro-opiomelanocortin expression in patients with complex regional pain syndrome. J. Transl Med 14, 64 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.den Hollander M et al. Expose or protect? A randomized controlled trial of exposure in vivo versus pain-contingent treatment as usual in patients with complex regional pain syndrome type 1. Pain 157, 2318–2329 (2016). [DOI] [PubMed] [Google Scholar]