Abstract
Background:
The development of novel synthetic psychoactive substances continues to accelerate. There are little or no data on the pharmacological mechanisms, behavioral effects, or abuse liability of many of the newer compounds, despite increasing reports of severe adverse effects in recreational users.
Methods:
The current study investigated the discriminative stimulus and locomotor stimulant effects of a group of synthetic cathinone analogs: N-ethylpentylone, dimethylone, dibutylone, clephedrone, 3’,4’-tetramethylene-α-pyrrolidinovalerophenone (TH-PVP). Locomotor activity was assessed in an open-field assay using Swiss-Webster mice. Discriminative stimulus effects were assessed in Sprague-Dawley rats trained to discriminate either cocaine, methamphetamine or MDMA from vehicle.
Results:
N-ethylpentylone, dimethylone, dibutylone and clephedrone increased locomotor activity. Maximal effects were similar among the test compounds. Relative potencies were: methamphetamine>N-ethylpentylone>clephedrone> dimethylone>MDMA> cocaine>dibutylone. TH-PVP dose-dependently depressed locomotor activity. N-ethylpentylone, dimethylone, dibutylone and clephedrone substituted fully for the discriminative stimulus effects of methamphetamine. N-ethylpentylone, dibutylone and clephedrone fully substituted for cocaine, whereas dimethylone produced a maximum of 67% drug-appropriate responding. Dimethylone, dibutylone and clephedrone fully substituted for MDMA, whereas N-ethylpentylone produced only 50% drug-appropriate responding. TH-PVP produced a maximum of 38% methamphetamine-appropriate responding, 50% cocaine-appropriate responding, and less than 1% MDMA-appropriate responding.
Conclusions:
These data provide initial evidence that the novel psychoactive substances N-ethylpentylone, dimethylone, dibutylone, and clephedrone demonstrate potential for abuse as psychostimulants and/or club drugs, given their ability to stimulate locomotor activity and their substitution for the discriminative stimulus effects of methamphetamine, cocaine and/or MDMA. TH-PVP has minimal activity in the assays tested and may have little or no abuse liability.
Keywords: Cathinones, Psychostimulants, Entactogens, Drug Discrimination, Abuse Liability
1. Introduction
Novel, synthetic analogs of abused drugs continue to be developed, making control of these compounds increasingly difficult. Novel compounds had been released at an accelerating rate from 2003 until a peak in 2014–2015. Since then the number of new compounds flagged by the EU Early Warning system has been decreasing, yet more than 50 new compounds were identified in 2017 (EMCDDA, 2018), indicating that synthetic analogs are still a threat to public health. Of the synthetic analogs, cathinones comprise one of the most commonly used types of recreational compounds (EMCDDA, 2018; UNODC, 2017). Unfortunately, the more recent generations of the synthetic “designer drugs” have been associated with severe adverse effects.
Clinical case studies and poison control center reports indicate cathinone compounds can acutely produce extremely high blood pressure, confusion, psychotic-like and/or aggressive behaviors (John et al., 2014; Penders et al., 2012) as well as more life-threatening effects such as convulsions, myocardial infarction, arrhythmias, and cardiac arrest, metabolic acidosis and prolonged rhabdomyolysis even after few doses (Forrrester 2012; Spiller et al., 2011; Paillet-Loilier 2014; Sivagnanam, 2013). These are very similar to self-reported adverse effects found on user forums, such as anxiety, hallucinations, nervousness, paranoia, angina, myocardial infarction, tachycardia, and difficulty urinating (Assi et al., 2017).
Five compounds have been identified by the US Drug Enforcement Agency as being of special concern, N-ethylpentylone (also known as, β-keto-ethylbenzodioxolylpentanamine, βkethyl-K, βk-EBDP, ephylone, N-ethylnorpentylone), dimethylone HCl (bk-MDDMA), dibutylone HCl (β-keto-dimethylbenzodioxolylbutanamine, βk-DMBDB), clephedrone HCl (4-chloromethcathinone, 4-CMC), and TH-PVP HCl (3’,4’-tetramethylene-α-pyrrolidinovalerophenone). To date, only N-ethylpentylone has been temporarily designated as a Schedule I compound in the US (DEA, 2018).
N-Ethylpentylone produces agitation, hallucinations, tachycardia, coma and death (Costa et al., 2018). In one case, N-ethylpentylone caused death following extremely high body temperature, hepatic and renal complications, respiratory failure and cardiac arrest, despite medical intervention (Thirakul et al., 2017). A public service group in New Zealand that tracks drug information and offers onsite chemical testing published a warning about severe adverse effects following N-ethylpentylone use, including that 13 people were hospitalized following its use (https://knowyourstuff.nz, 2018). Dibutylone, clephedrone and TH-PVP have been identified in seizures of illicit drugs (Grifell et al., 2017; Russell and Brogun 2011), and samples from postmortem examinations and testing for driving under intoxication (Krotulski et al., 2018a, 2018b; Tomczak et al., 2018; Wille et al., 2018).
Limited mechanistic data for dimethylone, dibutylone, and N-ethylpentylone exist; however, data for their mono- and methyl-substituted counterparts are abundant. Dimethylone acted as an uptake inhibitor at both the dopamine transporter (DAT) and serotonin transporter (SERT) in one study (Solis, 2017), but only at DAT in another (Eshleman et al., 2018), and did not produce monoamine release (Eshleman et al., 2018). Dibutylone was 32-fold selective at the DAT over the norepinephrine transporter (NET) for blocking uptake, produced no uptake effects at the SERT, and did not produce monoamine release (Eshleman et al., 2018). In contrast, methylone is widely considered to be a nonselective transporter substrate for DAT and SERT (Dolan et al., 2018; Eshleman et al., 2013; Simmler et al., 2013), whereas butylone has been described as either a “hybrid” compound inhibiting dopamine uptake and promoting serotonin release (Eshleman et al., 2013; Simmler et al., 2013; Saha et al., 2018) or a substrate for both transporters with weaker effects at DAT (Dolan et al., 2018).
N-ethylpentylone was 8 to 10-fold selective for DAT uptake over SERT uptake and did not produce monoamine release (Costa et al. 2018; Eshleman et al. 2018). Pentylone, conversely, has been described as an uptake inhibitor 5 to 8-fold selective for DAT over SERT (Costa et al. 2018; Eshleman et al. 2018), a nonselective uptake inhibitor (Eshleman et al., 2017), a “hybrid” compound (Simmler et al., 2014; Saha et al., 2018), or a weak DAT/SERT substrate (Dolan et al., 2018). Previous analyses of structure-activity relations among synthetic cathinones have demonstrated that tertiary amine substitution (as in dimethylone and dibutylone) or substitution with larger alkyl groups (as in ethylone or N-ethylpentylone) confers transporter-blocking properties to compounds (Kolanos et al., 2013; Solis, 2017; Glennon and Dukat, 2017), so the unstudied compounds currently under investigation potentially have more-prominent transporter-blockade effects relative to their mono- and methyl-substituted counterparts.
Clephedrone (4-Chloromethcathinone; 4-CMC) is a monoamine transporter substrate (releaser) similar potency for SERT and DAT (Blough et al., 2018; Eshleman et al., 2013; 2017), although one study reported that clephedrone was 3-fold more potent at releasing dopamine than serotonin, with equal efficacy (Bonano et al., 2015). However, when looking at time course, it is interesting to note that whereas clephedrone produced a large increase in DA levels, the onset was slow (Suyama et al., 2016). In contrast, in the same study, clephedrone produced even larger increases in serotonin levels with a fast onset. TH-PVP was a low potency uptake inhibitor with selectivity SERT>DAT≥NET and did not produce monoamine release (Eshleman et al., 2018).
Assessment of abuse liability testing involves chemical structure, pharmacological mechanism and behavioral effects. Compounds with chemical structures similar to those of abused compounds are much more likely to be abused. Similarly, compounds that bind to, or activate, pharmacological receptors that mediate the effects of abused drugs are much more likely to be abused, although not all compounds with similar mechanisms are abused. Finally, compounds that produce subjective effects similar to abused drugs and produce reward/reinforcement are highly likely to themselves be abused by human users.
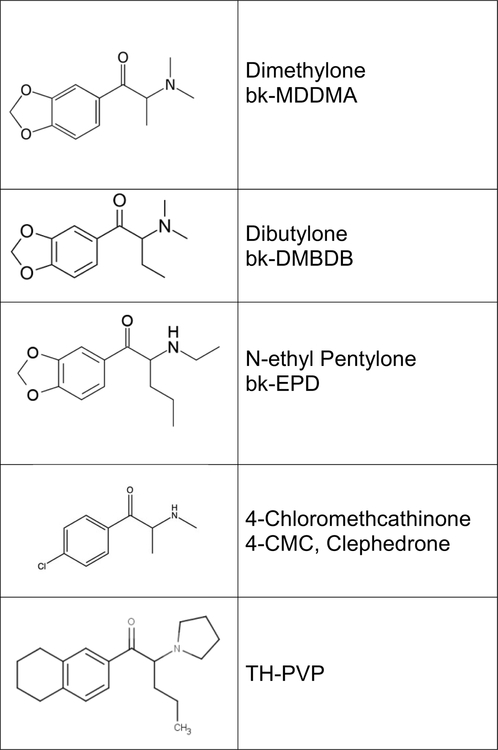
In terms of chemical structure, the test compounds in the present study are all substituted cathinones (Fig 1). Cathinone compounds, as previously mentioned, are increasingly being used by recreational users as substitutes for psychostimulants like cocaine and methamphetamine and/or as substitutes for methylenedioxymethamphetamine (MDMA) in ecstasy. In terms of pharmacological mechanism, only N-ethylpentylone and clephedrone have been tested to date. Both compounds have mechanisms similar to those of psychostimulants, acting at monoamine transporters (Bonano et al., 2015; Costa et al., 2018). In terms of behavioral testing, locomotor activity testing is used to establish the dose range and time course for compounds with little or no prior information on their behavioral effects. Potency at stimulating locomotor activity correlates well with potency in the drug discrimination assay (e.g., Katz et al., 2001; Gatch et al., 2017), and provide useful predictors of the duration of action of subjective and reinforcing effects in animal models and human users. Subjects are not habituated to the chambers before testing, which allows for observation of decreases in baseline locomotor activity. Drug discrimination is a robust animal model of the subjective effects of psychoactive compounds and is an excellent predictor of abuse liability (Balster, 1991; Carter and Griffiths, 2009; Horton et al., 2013). In the present study, the effects of N-ethylpentylone, dimethylone, dibutylone, clephedrone, and TH-PVP in the locomotor activity assay was tested, followed by assessing their ability to substitute for the discriminative stimulus effects of methamphetamine, cocaine, and MDMA.
Figure 1.
Chemical structures of the synthetic cathinones tested in the present study.
2. Methods
2.1. Subjects
Male Swiss–Webster mice were obtained from Envigo (Indianapolis, IN) at approximately 8 weeks of age and tested at approximately 10 weeks of age. Mice were group housed (4 per cage) on a 12:12-h light/dark cycle and were allowed free access to food and water. Male Sprague-Dawley rats were obtained from Envigo. All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 15 g/day which included the food received during operant sessions. Water was readily available. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
2.2. Locomotor Activity
The study was conducted using 40 Digiscan (model RXYZCM, Omnitech Electronics, Columbus, OH) locomotor activity testing chambers (40.5 X 40.5 X 30.5 cm) housed within sound-attenuating chambers in sets of two. A panel of infrared beams (16 beams) and corresponding photodetectors were located in the horizontal direction along the sides of each activity chamber. A 7.5-W incandescent light above each chamber provided dim illumination and fans provided an 80-dB ambient noise level within the chamber.
Separate groups of 8 mice were injected with either vehicle (0.9% saline), or a dose of methamphetamine (0.25, 0.5, 1, 2, 4 mg/kg), cocaine (5, 10, 20, 40 mg/kg), MDMA (2.5, 5, 10, 25, 50 mg/kg), N-ethylpentylone (0.25, 0.5, 1, 2.5, 5, 10, 25 mg/kg), dimethylone (2.5, 5, 10, 25,50 mg/kg), dibutylone (5, 10, 25, 50 mg/kg), clephedrone (1, 2.5, 5, 10 mg/kg), TH-PVP (10, 25, 50, 100 mg/kg) immediately prior to locomotor activity testing. Separate vehicle controls were tested for each test compound. In all studies, horizontal activity (interruption of photocell beams) was measured for 8 hours within 10-min periods, in order to establish a time-course of locomotor effects, beginning at 0800 h (1 h after lights on). Studies typically began with 1 mg/kg, after which higher and/or lower doses were tested from no effect, defined as not statistically different from vehicle, to full effect, defined as less than 50% of vehicle control for depressants. Stimulants typically produce an inverted-U shaped dose-effect curve for the locomotor stimulant effects. Increasing doses are tested until a peak has been reached and at least one dose on the descending limb of the dose-effect curve has been tested.
2.3. Discrimination Procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA, Model E10–10) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). Response levers were positioned to the left and right of the food hopper. A house light was centered over the hopper close to the ceiling and was illuminated only when the levers were active. The computers were programmed in Med-PC for Windows, version IV (Med Associates) for the operation of the chambers and collection of data.
Using a two-lever choice methodology, a pool of rats was trained to discriminate either methamphetamine (1 mg/kg), cocaine (10 mg/kg), or MDMA (1.5 mg/kg) from saline. Rats received an injection of either saline or drug and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses on a designated injection-appropriate lever. The pretreatment time was 10 min for cocaine and methamphetamine and 15 min for MDMA. Each training session lasted a maximum of 10 min, and the rats could earn up to a maximum of 20 food pellets. The rats received approximately 60 of these sessions before they were used in tests for substitution of the experimental compounds. Rats were used in testing once they had achieved 9 of 10 sessions at 85% injection-appropriate responding for both the first reinforcer and total session. The training sessions occurred on separate days in a double alternating fashion (drug-drug-saline-saline-drug; etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one saline and one drug session occurred between each test (drug-saline-test-saline-drug-test-drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
N-Ethylpentylone, dimethylone, dibutylone, clephedrone, and TH-PVP were tested for substitution in methamphetamine-, cocaine-, and MDMA-trained rats. Test sessions lasted for a maximum of 20 min. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until all 20 reinforcers were obtained, or for a maximum of 20 min. Each compound was tested in groups of six rats. A repeated-measures design was used, such that each rat was tested at all doses of a given drug, including vehicle and training-drug controls. The dose effect of each compound was tested from no effect to full effect or rate suppression (<20% of vehicle control) or adverse effects. Starting doses and pretreatment times were inferred from the locomotor activity testing. For dose-effect experiments, intraperitoneal (i.p.) injections (1 ml/kg) of vehicle or test compound were administered with a pretreatment of 15 min for each compound, except clephedrone, which had a pretreatment time of 60 min in methamphetamine-trained rats and 120 min in cocaine- and MDMA-trained rats. Rats that failed to complete the first fixed ratio were excluded from the analysis of drug-appropriate responding, but were used for analysis of response rate.
2.4. Drugs
(+)-Methamphetamine hydrochloride, (−)-cocaine hydrochloride, (±)-3,4-methylenedioxymethamphetamine hydrochloride (MDMA), N-ethylpentylone HCl (1-(1,3-Benzodioxole-5-yl)-2-(ethylamino)-1-pentanone), dimethylone HCl (1-(1,3-Benzodioxol-5-yl)-2-(dimethylamino)propan-1-one), dibutylone HCl (1-(1,3-Benzodioxol-5-yl)-2-(dimethylamino)butan-1-one), clephedrone HCl (1-(4-Chlorophenyl)-2-(methylamino)propan-1-one), and TH-PVP HCl (2-(methylamino)-1-(2-methylphenyl)-1-propanone (1b)) were all supplied by the National Institute on Drug Abuse Drug Supply Program. Optically active cathinones were provided as racemates. All compounds except TH-PVP were dissolved in 0.9% saline and administered i.p. in a volume of 1 ml/kg in rats and 10 ml/kg in mice. For the locomotor activity study, TH-PVP was dissolved in deionized water. At the concentrations necessary for the drug discrimination studies, TH-PVP was not soluble in saline, deionized water, or dilute acid. For the methamphetamine drug-discrimination study, TH-PVP was suspended in 2% methylcellulose. For the cocaine and MDMA discrimination studies, TH-PVP was suspended in ethanol/Cremophor EL/0.9% saline (1:1:18).
2.5. Data Analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane (ambulation counts) during each 10-min period of testing. A 30-min period, beginning when maximal stimulation of locomotor activity first appeared at the lowest effective dose, was used for analysis of dose-response data. A two-way repeated measures analysis of variance was conducted on horizontal activity counts/10 min interval. A one-way analysis of variance was conducted on horizontal activity counts for the 30-min period of maximal effect (defined as the earliest time period in which a peak effect was observed), and planned comparisons were conducted for each dose against saline control using single degree-of-freedom F tests. A oneway ANOVA was conducted on peak ambulation adjusted for control (peak effects = locomotor activity drug - locomotor activity vehicle) for the five test compounds. The cut-off for statistical significance was set at p<0.05.
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses occurring in each test period. Graphs for percent drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Percent drug-appropriate responding was shown only if at least 3 rats completed the first fixed ratio. Full substitution was defined as ≥80% drug-appropriate responding and not statistically different from the training drug. Rates of responding were expressed as a function of the number of responses made divided by the total session time (sec). Response rate data were analyzed by one-way repeated measures analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts.
The potencies (ED50 and 95%-confidence intervals) of the test compounds in both assays were calculated by fitting straight lines to the linear portion of the dose-response data for each compound by means of OriginGraph (OriginLab Corporation, Northampton, MA). A one-way ANOVA was conducted on the ED50 values.
3. Results
3.1. Locomotor Activity
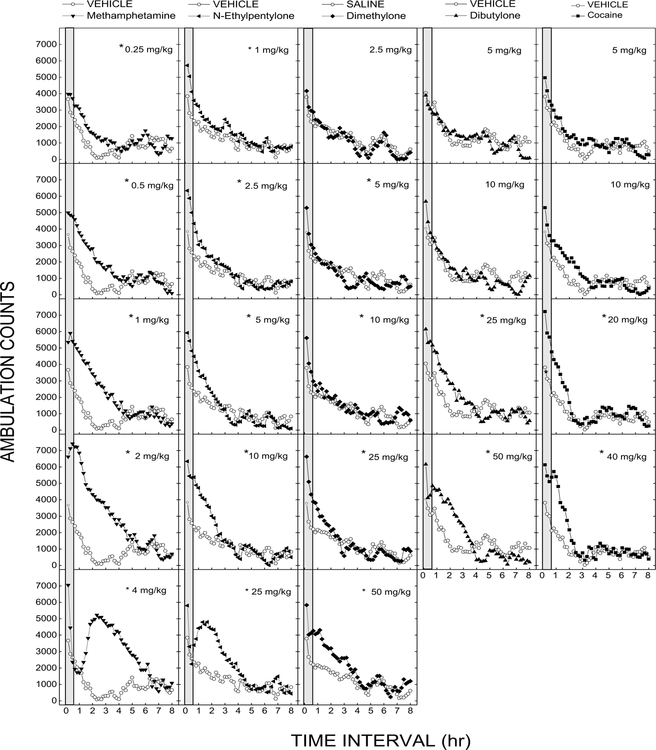
Figures 2 and 3 show the time course data for the test compounds in comparison to methamphetamine and cocaine (Fig 2) or MDMA (Fig 3). Comparison of the peak effects showed an overall effect [F(7,184)=41.62, p<0.001]. Rank order of potency was methamphetamine>N-ethylpentylone>clephedrone> dimethylone>MDMA>cocaine> dibutylone>>TH-PVP. Methamphetamine (ED50 = 0.50±0.05 mg/kg) produced dose- and time-dependent locomotor stimulation [Dose F(5,42)=25.7, p<.001; Time F(47,1974)=87.9, p<.001, and Dose x Time interaction F(235,1974)=9.9, p<.001]. Peak effect (7296±586 counts) was observed following 2 mg/kg. The peak effect was significantly different from cocaine, MDMA and all of the test compounds (p<0.05). The effects lasted three to four hours up to a dose of 2 mg/kg. A higher dose (4 mg/kg) resulted in a sharp drop-off of motor activity, followed by a delayed peak.
Figure 2.
Locomotor activity of N-ethylpentylone, dimethylone, and dibutylone compared to that of methamphetamine and cocaine. Average horizontal activity counts/10 min as a function of time (10 min bins) and dose. Data are from independent groups of 8 mice per dose. Asterisks indicate doses significantly different from vehicle during the time of peak effect (p<0.05).
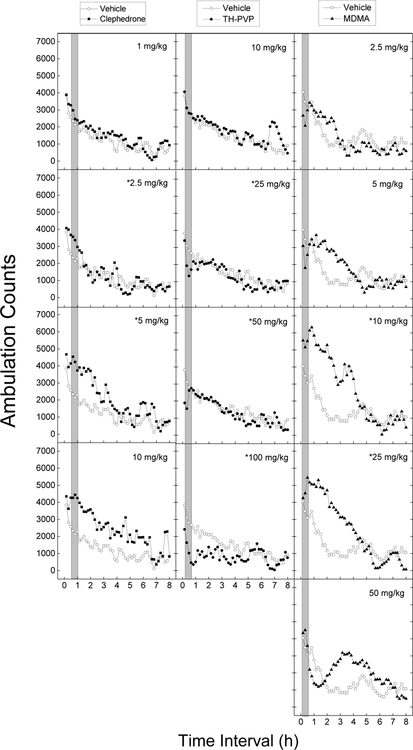
Figure 3.
Locomotor activity of clephedrone and TH-PVP compared to that of MDMA. Average horizontal activity counts/10 min as a function of time (10 min bins) and dose. Data are from independent groups of 8 mice per dose. Asterisks indicate doses significantly different from vehicle during the time of peak effect (p<0.05).
Cocaine also shows a dose- and time-dependent increase in activity [Dose F(4,35)=3.1, p<.001; Time F(47,1645)=77.0, p<.001; and Dose x Time interaction F(188,1645)=3.1, p<.001]. Peak effect (6264±463 counts) was observed following 20 mg/kg. The peak effect was significantly different from methamphetamine and TH-PVP (p<0.05). In contrast to methamphetamine, the effects lasted two to three hours. Increasing the dose to 40 mg/kg resulted in a lower level of locomotor activity without the delayed peak seen following methamphetamine.
Finally, MDMA produces significant changes in locomotor activity [Dose F(6,49)=7.58, p<.001, Time F(47,2303)=49.82, p<.001, and Dose x Time interaction F(282,2303)=4.26, p<.001]. Depressant effects were seen at low doses within the first 30 min, followed by dose-dependent increases in peak locomotor stimulation and duration up to a dose of 25 mg/kg (5820±921 counts). A higher dose resulted in the loss of rapid locomotor stimulation and a delayed peak.
N-Ethylpentylone (ED50 = 0.73±0.06 mg/kg) produced dose and time-dependent increases in locomotor activity from 0.25 to 10 mg/kg [Dose F(7,56)=4.4, p=.001; Time F(47,2632)=134.2, p<.001; Dose x Time interaction F(329,2632)=4.1, p<.001]. Figure 2 shows the 1 to 25 mg/kg dose range. A higher dose, 25 mg/kg, resulted in decreased locomotor activity during the first 30 min, followed by a delayed peak 2–3 h after administration. N-Ethylpentylone had a rapid onset (withinthe first 10 min), and its effects lasted 3–4 h depending on dose. Peak effects ranged from 5400–5700 counts at doses from 2.5 to 10 mg/kg, and a drop in locomotor activity to baseline level was seen following 25 mg/kg. Peak effects were significantly different from methamphetamine and TH-PVP (p<0.05).
Dimethylone (ED50 = 4.9 mg/kg) produced dose and time-dependent increases in locomotor activity from 2.5 to 25 mg/kg [Dose F(5,42)=5.02, p=.001; Time F(47,1974)=81.16, p<.001; Dose x Time Interaction F(235,1974)=2.44, p<.001]. Peak effect (5352±154 counts) was observed following 25 mg/kg. The peak effect was significantly different from methamphetamine and TH-PVP (p<0.05). Effects were seen within 10 min and lasted 1–2 h. The 50 mg/kg produced less locomotor stimulation at the time of overall peak effect, but the stimulation lasted 3–4 h.
Dibutylone (ED50 = 11.18 mg/kg) produced dose and time-dependent increases in locomotor activity from 5 to 25 mg/kg. Analysis of variance did not indicate a significant effect of Dose [F(4,35)=1.92, p=.129], but indicated a significant effect of Time [F(47,1645)=66.56, p<.001] and a significant Dose x Time interaction [F(188,1645)=3.58, p<.001]. Effects were seen within 10 min and lasted 2–3 h. The 50 mg/kg produced less locomotor stimulation at the time of overall peak effect. Peak effect (5620±546 counts) was observed following 25 mg/kg. Peak effects were significantly different from methamphetamine and TH-PVP (p<0.05).
Clephedrone (ED50 = 1.37 mg/kg) produced dose and time-dependent increases in locomotor activity from 1 to 10 mg/kg. Analysis of variance did not indicate a significant effect of Dose [F(4,35)=2.2, p=.09], but did indicate a significant effect of Time [F(47,1645)=28.9, p<.001] and the Dose x Time Interaction [F(188,1645)=1.43, p<.001]. Effects were delayed, peaking from 30 – 60 min after administration. Duration was also dose-dependent, increasing to 6 h at 10 mg/kg. Peak effect (4201±366) was observed following 5 mg/kg and was significantly different from methamphetamine and TH-PVP (p<0.05).
TH-PVP (ED50 = 76.00 mg/kg) produced dose and time-dependent decreases in locomotor activity from 10 to 100 mg/kg [Dose F(4,35)=7.04, p<.001; Time F(47,1645)=17.3, p<.001; and Dose x Time interaction F(188,1645)=2.1, p<.001]. Effects were observed within 20 min and lasted 30 min, but were extended at 100 mg/kg, lasting 2.5 to 3 h. Maximal depressant effects were 1034±252.0 counts following 100 mg/kg.
3.2. Drug Discrimination
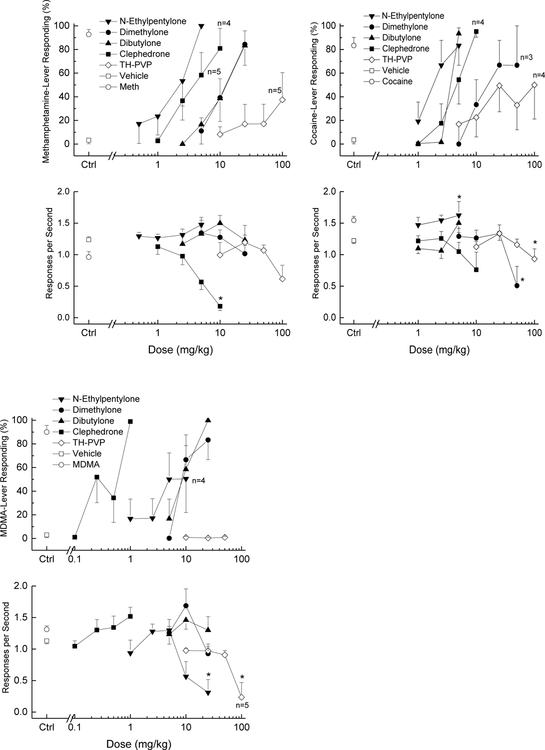
N-ethylpentylone (ED50=1.7±0.1 mg/kg), dimethylone (n=9) (ED50=12.1±0.1 mg/kg), dibutylone (ED50=11.4±0.1 mg/kg) and clephedrone (ED50=3.9±0.1 mg/kg) all fully substituted for the discriminative stimulus effects of methamphetamine at doses that did not alter response rate (Fig. 4). Rank order of potency was N-ethylpentylone>clephedrone>dibutylone= dimethylone. Response rate was decreased following 5 and 10 mg/kg clephedrone [F(4,20)=34.41, p<.001]. TH-PVP produced a peak of 38% methamphetamine-appropriate responding following 100 mg/kg. Response rate was not affected.
Figure 4.
Substitution for the discriminative stimulus effects of methamphetamine (Meth, left panels), cocaine (center panels), and MDMA (right panels): Top panels show percentage of total responses made on the drug-appropriate lever. Bottom panels show rate of responding in responses per second (r/s). n=6 unless otherwise shown, except dimethylone in methamphetamine-trained rats n=9. Vehicle and training drug controls are pooled averages (n=30). Ctrl indicates vehicle and training drug control values. * indicates response rate different from vehicle control (p < 0.05).
N-ethylpentylone (ED50=2.0±0.1 mg/kg), dibutylone (ED50=3.6±0.01 mg/kg) and clephedrone (ED50=4.3±0.1 mg/kg) all fully substituted for the discriminative stimulus effects of cocaine. Rank order of potency was N-ethylpentylone>clephedrone= dibutylone. Response rate was increased following 2.5 and 5 mg/kg N-ethylpentylone [F(5,25)=2.96, p=.031]. Dimethylone produced a peak of 67% cocaine-appropriate responding following 25 and 50 mg/kg. Response rate was decreased following 50 mg/kg dimethylone such that 3 of 6 rats failed to complete the first fixed-ratio [F(5,25)=6.29, p=.001]. TH-PVP produced a peak of 50% cocaine-appropriate responding following 100 mg/kg. Response rate was decreased following 100 mg/kg TH-PVP such that 2 of 6 rats failed to complete the first fixed-ratio [F(5,25)=3.358, p=.019].
Dimethylone (ED50=10.8±0.1 mg/kg), dibutylone (ED50=9.2±0.1 mg/kg) and clephedrone (ED50=0.37±0.10 mg/kg) all fully substituted for the discriminative stimulus effects of MDMA. Rank order of potency was clephedrone>>dibutylone=dimethylone. N-Ethylpentylone produced a peak of 50% MDMA-appropriate responding at 5 and 10 mg/kg. Response rate was decreased following 10 and 25 mg/kg N-ethylpentylone, such that 2 of 6 rats failed to complete the first fixed ratio following 10 mg/kg and 4 of 6 rats following 25 mg/kg [F(6,30)=4.22, p=.003]. TH-PVP produced a peak of 16% MDMA-appropriate responding at 50 mg/kg. Response rate was decreased following 100 mg/kg TH-PVP, such that 4 of 5 rats failed to complete the first fixed ratio [F(4,16)=8.697, p=.001].
4. Discussion
The present study evaluated the behavioral effects of N-ethylpentylone, dimethylone, dibutylone, clephedrone, TH-PVP. Locomotor activity was tested to determine the onset and duration of action of each compound. Drug discrimination testing determined whether the test compounds produce discriminative stimulus effects similar to those of methamphetamine, cocaine, and/or MDMA.
N-Ethylpentylone, dimethylone, dibutylone, clephedrone all increased locomotor activity at some dose range, whereas TH-PVP decreased locomotor activity. N-Ethylpentylone produced locomotor stimulant effects similar to those of methamphetamine, with large, dose-dependent increases in locomotor activity with rapid onset. Similar to methamphetamine, further increasing the dose led to a decreased stimulant effect immediately followed by drop to baseline levels for 60 min, followed by delayed stimulant effects. N-Ethylpentylone was less potent and shorter acting than methamphetamine. In contrast, dimethylone and dibutylone produced locomotor stimulant effects more similar to those of cocaine, which produces a shorter time course without the rebound in the time course of the top dose as seen following methamphetamine. The locomotor stimulant effects of clephedrone were somewhat more like those of MDMA, with a slow onset and long-acting effects. Clephedrone, however, did not produce the initial depressant effects produced by low-dose MDMA.
Overall, these findings are similar to those previously reported for cathinones and structurally related compounds with psychostimulant effects; these compounds produce dose-dependent stimulation of motor activity to a peak level, whereas higher doses produce decreases in locomotor activity (Gatch et al., 2013; 2015a;b; 2017; Naylor et al., 2015). Given that N-ethylpentylone, dimethylone and dibutylone are selective for dopamine transporters, their ability to increase locomotor activity is not surprising. Clephedrone is a non-selective DAT/SERT releaser (Blough et al., 2018; Eshleman et al., 2013; 2017), which may account for its more MDMA-like effects. Taken together, these findings suggest that N-ethylpentylone, dimethylone, dibutylone, clephedrone will have psychostimulant-like effects in human users, although clephedrone may be more of a MDMA-like club drug than a methamphetamine-like psychostimulant.
In contrast, TH-PVP dose-dependently decreased locomotor activity. Other compounds with psychostimulant-like effects also decrease locomotor activity, although most of them (MDMA, flephedrone, MDAI, and DMAA) produce rebound stimulant effects later in the time course (Dolan and Gatch, 2015; Gatch et al., 2013; 2016). 4-Fluoroamphetamine (4-FA) did produce depressant effects with little or no rebound stimulant effects (Dolan et al., 2017) similar to those produced by TH-PVP in the present study. None of these compounds are selectively dopaminergic, as MDMA and MDAI produce both serotonergically and dopaminergically mediated effects and DMAA produces its effects primarily through adrenergic receptors (Monte et al., 1993; Iversen et al., 2013). Not surprisingly, TH-PVP was reported to be a low potency uptake inhibitor with selectivity SERT>DAT≥NET and did not produce monoamine release (Eshleman et al., 2018). As a side-note, it is interesting that flephedrone (4-fluoromethcathinone) produced initial depressant effects (Gatch et al., 2013) whereas, in the present study, clephedrone (4-chloromethcathinone) did not. Given the depressant effects of both 4-FA and flephedrone, it is possible that the fluorine substitution at the 4 position contributes to the depressant effects.
In the present study, those compounds that produced locomotor stimulant effects (N-ethylpentylone, dimethylone, dibutylone, clephedrone) fully substituted for the discriminative stimulus effects of methamphetamine. These results are not surprising as the potency of locomotor stimulant effects are highly predictive of potency of substitution for the discriminative stimulus effects of psychostimulants (e.g., Katz et al., 2001; Gatch et al., 2017), and as most of the substituted cathinones previously tested have also substituted for methamphetamine (Dolan et al., 2018; Gatch et al., 2013; 2015a;b; 2017; Naylor et al., 2015). The time courses of the locomotor effects were also predictive. Most of the compounds produced locomotor stimulant effects within 10–20 min and fully substituted at 15 min after administration. The locomotor stimulant effects of clephedrone had a slow onset, and substitution in methamphetamine-trained rats was seen 60 min following administration.
Most of the compounds also fully substituted for cocaine and MDMA; however, dimethylone failed to fully substitute for cocaine, and N-ethylpentylone failed to fully substitute for MDMA. Pentylone, which is strongly dopaminergic, also failed to substitute for MDMA (Dolan et al., 2018), so it is not surprising that N-ethylpentylone, which is also more potent at DAT than at SERT failed to fully substitute for MDMA in the present study. In contrast, clephedrone was 10-fold more potent at producing MDMA-like discriminative stimulus effects than it was at producing cocaine- or methamphetamine-like discriminative stimulus effects. This finding is in line with clephedrone producing locomotor stimulant effects much more like MDMA than like cocaine or methamphetamine. Currently, the findings from functional assays provide some support that clephedrone acts like MDMA, since clephedrone showed fairly similar potency for SERT and DAT (Blough et al., 2018; Bonano et al., 2015; Eshleman et al., 2013), and produces large increases in both dopamine and serotonin levels, although to a larger degree and faster onset for serotonin (Suyama et al., 2016).
The failure of dimethylone to fully substitute for cocaine is difficult to explain as there are few reports on the receptor pharmacology of dimethylone that do not completely agree, with dimethylone acting as an uptake inhibitor at both DAT and SERT in one study (Solis, 2017), but only at DAT in another (Eshleman et al., 2018). However, alpha-PVT, 4-MePPP and 3,4-MPBP were previously reported to produce sub-maximal levels of cocaine-lever responding (Gatch et al., 2017). Mephedrone (4-methylmethcathinone) also failed to fully substitute for cocaine in rhesus monkeys (Smith et al. 2016), despite fully substituting for cocaine in rats (Gatch et al. 2013), and cocaine did not fully substitute in rats trained to discriminate 3 mg/kg mephedrone (Berquist et al., 2017; Varner et al. 2013); although cocaine did fully substitute when the training dose of mephedrone was 1 mg/kg. Likely, there are subtle differences in the receptor pharmacology of these compounds which have not yet been fully elucidated that contribute to differences in discriminative stimulus effects in methamphetamine- and cocaine-trained subjects.
TH-PVP, which was a locomotor depressant in the present study, did not fully substitute for any of the training drugs, methamphetamine, cocaine or MDMA. Other compounds, MDMA, MDAI, 4-FMC, 4-FA and dimethylamylamine produce at least some locomotor depressant effects, but still produced full substitution for cocaine, methamphetamine, and/or MDMA. TH-PVP is a low potency uptake inhibitor with selectivity SERT>DAT≥NET (Eshleman et al., 2018). It is possible that TH-PVP is pharmacologically active at other receptors and may have other psychoactive effects—hallucinogens, GABAergic depressants and cannabinoids all depress locomotor activity. Testing the pharmacological activity of TH-PVP in binding and functional assays will be necessary to determine whether it has activity at receptors that contribute to abuse liability. It is likely that TH-PVP is of very low efficacy and has minimal abuse liability.
Testing of the reward/reinforcing effects of these compounds in self-administration and place conditioning assays is necessary to confirm that these compounds will maintain drug-seeking and/or compulsive use. Prior studies have indicated that the cathinone compounds have a range of effects on drug-taking in the self-administration assay. Compounds that are more dopaminergic engender higher levels of drug-taking, while serotonergic compounds show lower levels of drug-taking and are less likely to produce compulsive drug taking (Dolan et al., 2018; Negus and Banks, 2017). Of the compounds tested in the present study, clephedrone did show reward-like effects in studies of ICSS (Bonano et al., 2015). Based on the findings of the present study, N-ethylpentylone, dimethylone, dibutylone are likely to be self-administered;TH-PVP is not.
In summary, N-ethylpentylone, dimethylone, dibutylone, clephedrone all increased locomotor activity and fully substituted for methamphetamine, cocaine, and/or MDMA. These findings suggest that these compounds may have substantial abuse liability. N-Ethylpentylone may be more strongly methamphetamine-like, whereas clephedrone may be more MDMA-like, but further testing will be necessary to establish the patterns of their use. In contrast, TH-PVP decreased locomotor activity and failed to fully substitute for methamphetamine, cocaine, or MDMA. This may indicate that TH-PVP has a minimal risk of abuse liability. Alternatively, TH-PVP may be active at other receptors and have psychoactive effects similar to hallucinogens, sedatives or other compounds that reduce locomotor activity. Again, further testing of the molecular and behavioral effects of TH-PVP will be necessary to assess these possibilities.
Highlights.
N-ethylpentylone, dimethylone, dibutylone, clephedrone, TH-PVP are street drugs.
TH-PVP was a locomotor depressant with little psychostimulant-like effects.
The remaining drugs produced methamphetamine-like discriminative stimulus effects.
Clephedrone was primarily MDMA-like.
All of the compounds but TH-PVP have substantial potential for abuse.
Role of Funding Source
Funding for this study was provided by NIDA contract N01DA-13–8908. NIDA ATDP authorized the study design and compounds tested. NIDA had no further role in the analysis or publication of this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared.
References
- Assi S, Gulyamova N, Kneller P, Osselton D, 2017. The effects and toxicity of cathinones from the users’ perspectives: A qualitative study. Hum. Psychopharmacol 32, e2610. [DOI] [PubMed] [Google Scholar]
- Balster RL, 1991. Drug abuse potential evaluation in animals. Br. J. Addict 86, 1549–1558. [DOI] [PubMed] [Google Scholar]
- Berquist MD 2nd, Thompson NA, Baker LE., 2017. Evaluation of training dose in male Sprague-Dawley rats trained to discriminate 4-methylmethcathinone. Psychopharmacology (Berl) 234, 3271–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough BE, Decker AM, Landavazo A, Namjoshi OA, Partilla JS, Baumann MH, Rothman RB, 2018. The dopamine, serotonin and norepinephrine releasing activities of a series of methcathinone analogs in male rat brain synaptosomes. Psychopharmacology (Berl) doi: 10.1007/s00213-018-5063-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Banks ML, Kolanos R, Sakloth F, Barnier ML, Glennon RA, Cozzi NV, artilla JS, Baumann MH, Negus SS, 2015. Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. Br. J. Pharmacol 172, 2433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, 2009. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend 105, S14–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JL, Cunha KF, Lanaro R, Cunha RL, Walther D, Baumann MH, 2018. Analytical quantification, intoxication case series, and pharmacological mechanism of action for N-ethylnorpentylone (N-ethylpentylone or ephylone). Drug Test. Anal 11, 461–471. doi: 10.1002/dta.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SB, Gatch MB, 2015. Abuse liability of the dietary supplement dimethylamylamine. Drug Alcohol Depend 146, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SB, Forster MJ, Gatch MB, 2017. Discriminative stimulus and locomotor effects of para-substituted and benzofuran analogs of amphetamine. Drug Alcohol Depend 180, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SB, Chen Z, Huang R, Gatch MB, 2018. “Ecstasy” to addiction: Mechanisms and reinforcing effects of three synthetic cathinone analogs of MDMA. Neuropharmacol 133, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, Department of Justice, 2018. Schedules of controlled substances: Temporary placement of n-ethylpentylone in schedule I. Fed. Regist 83, 444474–44478. https://www.deadiversion.usdoj.gov/fed_regs/rules/2018/fr0831_2.htm [PubMed] [Google Scholar]
- Eshleman AJ, Nagarajan S, Wolfrum KM, Reed JF, Swanson TL, Nilsen A, Janowsky A, 2018. Structure-activity relationships of bath salt components: Substituted cathinones and benzofurans at biogenic amine transporters. Psychopharmacology (Berl)doi: 10.1007/s00213-018-5059-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A, 2013. Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol 85, 1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, Janowsky A, 2017. Structure-activity relationships of substituted cathinones, with transporter binding, uptake, and release. J. Pharmacol. Exp. Ther 360, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction, 2018. European Drug Report 2018:Trends and developments Retrieved from http://www.emcdda.europa.eu/edr2018
- Forrester MB, 2012. Synthetic cathinone exposures reported to Texas poison centers. Am. J. Drug Alcohol Abuse 38, 609–15. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ, 2013. Locomotor stimulant and discriminative stimulus effects of “Bath Salt” cathinones. Behav. Pharmacol 24, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ, 2015a. Comparative behavioral pharmacology of three pyrrolidine-containing synthetic cathinone derivatives. J. Pharmacol. Exp. Ther 354, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge M, Forster MJ, 2015b. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology (Berl.) 232, 197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ, 2017. Locomotor activity and discriminative stimulus effects of a novel series of synthetic cathinone analogs in mice and rats. Psychopharmacology (Berl.) 234, 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Dukat M, 2017. Structure-activity relationships of synthetic cathinones. Curr.Top Behav. Neurosci 32, 19–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifell M, Ventura M, Carbón X, Quintana P, Galindo L, Palma Á, Fornis I, Gil C, Farre M, Torrens M, 2017. Patterns of use and toxicity of new para-halogenated substituted cathinones: 4-CMC (clephedrone), 4-CEC (4-chloroethcatinone) and 4-BMC (brephedrone). Hum Psychopharmacol 32. doi: 10.1002/hup.2621. [DOI] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN, 2013. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav. Pharmacol 24, 10–36. [DOI] [PubMed] [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL, 2013. Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol 700, 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ME, Thomas-Rozea C, Hahn D, 2014. Bath salts abuse leading to new onset psychosis and potential for violence. Clin. Schizophr. Relat. Psychoses 20, 1–14. [DOI] [PubMed] [Google Scholar]
- Katz JL, Agoston GE, Alling KL, Kline RH, Forster MJ, Woolverton WL, Kopajtic TA, Newman AH, 2001. Dopamine transporter binding without cocaine-like behavioral effects: Synthesis and evaluation of benztropine analogs alone and in combination with cocaine in rodents. Psychopharmacology (Berl.) 154, 362–374. [DOI] [PubMed] [Google Scholar]
- Know Your Stuff N. Z., 2018. This summer’s crap drug: n-ethylpentylone Retrieved from https://knowyourstuff.nz/2018/02/07/this-summers-crap-drug-n-ethylpentylone/ (Accessed 10/29/2018).
- Kolanos R, Solis E, Sakloth F, De Felice LJ, Glennon RA, 2013. “Deconstruction” of the abuse synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of the effects at the human dopamine transporter. ACS Chemical Neurosci 4, 1524–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotulski AJ, Mohr ALA, Papsun DM, Logan BK, 2018a. Dibutylone (bk-DM.B.DB):Intoxications, quantitative confirmations and metabolism in authentic biological specimens. J. Anal. Toxicol 42, 437–445. doi: 10.1093/jat/bky022. [DOI] [PubMed] [Google Scholar]
- Krotulski AJ, Papsun DM, De Martinis BS, Mohr ALA, Logan BK, 2018b. N-Ethyl pentylone (Ephylone) intoxications: Quantitative confirmation and metabolite identification in authentic human biological specimens. J. Anal. Toxicol 42, 467–475. [DOI] [PubMed] [Google Scholar]
- Monte AP, Marona-Lewicka D, Cozzi NV, Nichols DE, 1993. Synthesis and pharmacological examination of benzofuran, Indian, and tetralin analogues of 3,4-(methylenedioxy) amphetamine. J. Med. Chem 36, 3700–3706. [DOI] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the care and use of laboratory animals, eighth ed.National Academies Press, Washington, D.C. [Google Scholar]
- Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL, 2015. Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend 149, 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Banks ML, 2017. Decoding the structure of abuse potential for new psychoactive substances: Structure-activity relationships for abuse-related effects of 4-substituted methcathinone analogs. Curr. Top. Behav. Neurosci 32, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillet-Loilier M, Cesbron A, Le Boisselier R, Bourgine J, Debruyne D, 2014. Emerging drugs of abuse: Current perspectives on substituted cathinones. Subst. Abuse Rehabil 26, 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders TM, Gestring RE, Vilensky DA, 2012. Excited delirium following use of synthetic cathinones (bath salts). Gen. Hosp. Psychiatry 34, 647–650. [DOI] [PubMed] [Google Scholar]
- Russell MJ, Bogun B, 2011. New “party pill” components in New Zealand: The synthesis and analysis of some β-ketone analogues of 3,4-methylenedioxymethamphetamine (MDMA) including βk-DM.B.DB (β-ketone-N, N-dimethyl-1-(1,3-benzodioxol-5-yl)-2-butanamine). Forensic Sci. Int 210, 174–181. doi: 10.1016/j.forsciint.2011.03.005 [DOI] [PubMed] [Google Scholar]
- Saha K, Li Y, Holy M, Lehner KR, Bukhari MO, Partilla JS, Sandtner W, Sitte HH,Baumann MH, 2018. The synthetic cathinones, butylone and pentylone, are stimulants that act as dopamine transporter blockers but 5-HT transporter substrates. Psychopharmacology (Berl.) doi: 10.1007/s00213-018-5075-5 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S,Hoener MC, Liechti ME, 2013. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol 168, 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME, 2014. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 79, 152–160. [DOI] [PubMed] [Google Scholar]
- Sivagnanam K, Chaudari D, Lopez P, Sutherland ME, Ramu VK, 2013. “Bath salts” induced severe reversible cardiomyopathy. Am. J. Case Rep 14, 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Blough BE, Banks ML, 2017. Cocaine-like discriminative stimulus effects of amphetamine, cathinone, methamphetamine, and their 3,4-methylenedioxy analogs in male rhesus monkeys. Psychopharmacology (Berl.) 234, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E, 2017. Electrophysiological actions of synthetic cathinones on monoamine transporters. Curr. Top Behav. Neurosci 32, 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J, 2011. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin. Toxicol 49, 499–505. [DOI] [PubMed] [Google Scholar]
- Suyama JA, Sakloth F, Kolanos R, Glennon RA, Lazenka MF, Negus SS, Banks ML, 2016. Abuse-related neurochemical effects of para-substituted methcathinone analogs in rats: Microdialysis studies of nucleus accumbens dopamine and serotonin. J. Pharmacol. Exp. Ther 356, 182–190. doi: 10.1124/jpet.115.229559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirakul P, S. Hair L, L. Bergen K, M. Pearson J, 2017. Clinical presentation, autopsy results and toxicology findings in an acute n-ethylpentylone fatality. J. Anal. Toxicol 41, 342–346. doi: 10.1093/jat/bkx004. [DOI] [PubMed] [Google Scholar]
- Tomczak E, Woźniak MK, Kata M, Wiergowsk IM, Szpiech B, Biziuk M, 2018. Blood concentrations of a new psychoactive substance 4-chloromethcathinone (4-CMC) determined in 15 forensic cases. Forensic Toxicol 36, 476–485.doi: 10.1007/s11419-018-0427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC), 2017. World Drug Report https://www.unodc.org/wdr2017/index.html
- Varner KJ, Daigle K, Weed PF, Lewis PB, Mahne SE, Sankaranarayanan A, Winsauer PJ, 2013. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology (Berl) 225, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille SMR, Richeval C, Nachon-Phanithavong M, Gaulier JM, Di Fazio V, Humbert L, Samyn N, Allorge D, 2018. Prevalence of new psychoactive substances and prescription drugs in the Belgian driving under the influence of drugs population. Drug Test Anal 10, 539–547. [DOI] [PubMed] [Google Scholar]