Pediatric acute pancreatitis (AP) has increased over the last two decades (1) with the most recent incidence being 12.3/100,000 persons per year (2); and inpatient costs alone exceeds $100 million/year (2–5). Data on best practices in children are limited and practice varies widely across the US and even within the same pediatric institution (6). To bring uniformity to the diagnosis and treatment of pediatric AP, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and European Pancreas Club/Hungarian Pancreatic Study Group (EPC/HPSG) published pediatric AP management recommendations (7, 8). Given the effectiveness of evidence-based clinical guidelines to improve clinical care (9), several pediatric hospitals have independently developed center-specific pediatric AP-focused treatment algorithms and admission order sets.
We analyzed the AP treatment algorithms and admission order sets at four tertiary/quaternary care children’s hospitals in the U.S. (Cincinnati Children’s Hospital Medical Center, Lucile Packard Children’s Hospital at Stanford, Seattle Children’s Hospital, University of Iowa Stead Family Children’s Hospital) to reach a consensus for delivering consistent and evidence-based care in pediatric AP. Each institution had previously developed their own products, with Cincinnati being the first in 2013 (10). All institutions had admission order sets, while Seattle and Stanford also developed treatment algorithms. Treatment algorithms provide practical guidance to physicians on how to implement clinical guidelines in a user-friendly manner (11). All protocols focused on initial diagnosis and assessment of clinical status, frequency of vitals checks, “early aggressive” intravenous fluids, early nutrition (enteral vs. intravenous), and pain (non-opioid and opioid) management. Overall, there were minor differences between protocols, for example: types of fluids chosen, presence or absence of fluid bolus as standard management (vs. as needed), and specific opiates used for pain. Most products included teaching points for provider education. Admission order sets and treatment algorithms from the four institutions were harmonized with current NASPGHAN and EPC/HPSG recommendations (7, 8), and where applicable, the American Gastroenterological Association (AGA) AP guidelines (12). For broader consensus these were sent to all authors of the NASPGHAN Clinical Report on management of pediatric AP (7). There was broad excitement and consensus with the major tenets of the algorithm and order set, with no objections or major concerns from any of the authors. Minor comments were incorporated, as appropriate.
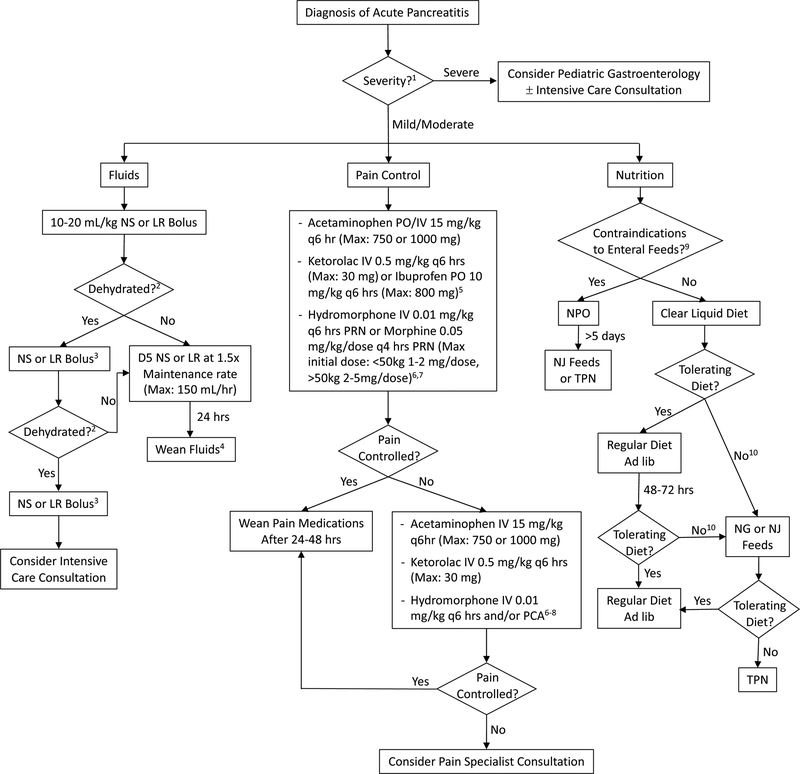
In summary, we generated a standardized and unified pediatric AP admission order set (Supplemental Digital Content) and treatment algorithm (Figure 1) that are in-line with the current NASPGHAN, EPC/HPSG and AGA AP guidelines. While these products were reviewed and approved by other pediatric pancreatologists, it should be noted that these are based on minimal evidence and expert opinion, given the paucity of relevant pediatric-specific data. We recognize that there may be institution-specific variation and accommodations made based on patient-specific circumstances, however, we hope that these resources will further standardize the treatment of pediatric AP, which in turn will improve outcomes and generate pediatric-specific data on best clinical practices for AP.
Figure 1. Treatment algorithm for pediatric AP.
Abbreviations: NS=normal saline, LR=lactated Ringers, D5=5% Dextrose, PO=per os, IV=intravenous, PRN: pro re nata (as needed), PCA: patient controlled analgesia, NPO: nil per os (nothing by mouth), NJ=nasojejunal, NG=nasogastric, TPN=total parental nutrition. 1 To help guide management, determine severity of AP (13). 2 Need for continued boluses determined by: signs of dehydration: Urine output < 1 cc/kg/hr, tachycardia, hypotension, delayed capillary refill, and poor skin turgor. Avoiding aggressive fluids and use of goal-directed fluid therapy is essential to preventing complications such as pulmonary edema. 3 10–20 mL/kg, based on clinical status. Monitor for signs of fluid overload or third-spacing. Consider LR over NS if metabolic acidosis is present. 4 Wean based on clinical status and enteral intake. 5 Use non-steroidal anti-inflammatory drugs only if BUN and creatinine are normal. 6 Other opiates may be substituted based on patient needs and institutional preferences. 7 When using opioids, place patient on laxatives. Recommend: Polyethylene glycol 3350 1g/kg/day (divided once or twice daily) if no stools in 24–48 hrs. May increase to achieve goal of at least one soft stool daily. 8 Consult pain service when on PCA, if service available. 9 Examples of contraindications to enteral feeding include, but are not limited to: disrupted pancreatic duct, intestinal obstruction, and ileus. 10 If not tolerating adequate diet within 48–72 hrs, consider if pain and/or nausea adequately controlled. For antiemetics, recommend: IV or PO ondansetron 0.15 mg/kg/dose q6–8 hrs as needed for nausea and emesis. Maximum dose of 8 mg q8 hrs. Also consider imaging to evaluate for complications from pancreatitis (e.g. pancreatic fluid collection/necrosis or pancreatic duct stricture/stones). Recommend: IV contrast enhanced CT or MRCP if biliary/pancreatic duct abnormalities are suspected (with IV secretin if available for pancreatic duct evaluation).
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the authors of the NASPGHAN Clinic Report on management of AP (7) were provided the algorithm and order set. The following provided comments: Jose Antonio Quiros, Bradley Barth, Samuel Bitton, Alvin Jay Freeman, Tanja Gonska, Amit S. Grover, Sohail Z. Husain, Asim Maqbool, and Veronique D. Morinville.
Funding and Conflicts of Interest: No funding sources directly contributed to this work. The authors have no relevant conflicts of interest.
REFERENCES
- 1.Morinville VD, Barmada MM, Lowe ME Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: is greater awareness among physicians responsible? Pancreas 2010;39(1):5–8. [DOI] [PubMed] [Google Scholar]
- 2.Sellers ZM, MacIsaac D, Yu H, et al. Nationwide Trends in Acute and Chronic Pancreatitis Among Privately Insured Children and Non-Elderly Adults in the United States, 2007–2014. Gastroenterology 2018;155(2):469–78e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pohl JF, Uc A Paediatric pancreatitis. Curr Opin Gastroenterol 2015;31(5):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pant C, Deshpande A, Olyaee M, et al. Epidemiology of acute pancreatitis in hospitalized children in the United States from 2000–2009. PLoS One 2014;9(5):e95552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ting J, Wilson L, Schwarzenberg SJ, et al. Direct Costs of Acute Recurrent and Chronic Pancreatitis in Children in the INSPPIRE Registry. J Pediatr Gastroenterol Nutr 2016;62(3):443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-El-Haija M, Palermo JJ, Fei L, et al. Variability in Pancreatitis Care in Pediatrics: A Single Institution’s Survey Report. Pancreas 2016;45(1):40–5. [DOI] [PubMed] [Google Scholar]
- 7.Abu-El-Haija M, Kumar S, Quiros JA, et al. Management of Acute Pancreatitis in the Pediatric Population: A Clinical Report From the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee. J Pediatr Gastroenterol Nutr 2018;66(1):159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parniczky A, Abu-El-Haija M, Husain S, et al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology 2018;18(2):146–60. [DOI] [PubMed] [Google Scholar]
- 9.Lugtenberg M, Burgers JS, Westert GP Effects of evidence-based clinical practice guidelines on quality of care: a systematic review. Qual Saf Health Care 2009;18(5):385–92. [DOI] [PubMed] [Google Scholar]
- 10.Szabo FK, Fei L, Cruz LA, et al. Early Enteral Nutrition and Aggressive Fluid Resuscitation are Associated with Improved Clinical Outcomes in Acute Pancreatitis. J Pediatr 2015;167(2):397–402e1. [DOI] [PubMed] [Google Scholar]
- 11.Lavelle J, Schast A, Keren R Standardizing Care Processes and Improving Quality Using Pathways and Continuous Quality Improvement. Current Treatment Options in Pediatrics 2015;1(4):347–58. [Google Scholar]
- 12.Crockett SD, Wani S, Gardner TB, et al. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018;154(4):1096–101. [DOI] [PubMed] [Google Scholar]
- 13.Abu-El-Haija M, Kumar S, Szabo F, et al. Classification of Acute Pancreatitis in the Pediatric Population: Clinical Report From the NASPGHAN Pancreas Committee. J Pediatr Gastroenterol Nutr 2017;64(6):984–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.