Abstract
2018 was a banner year for all thoracic oncology, but especially for early-stage non-small cell lung cancer (NSCLC). Three seminal events occurred in the approximately 18 months from mid-2017 to the end of 2018: in June 2017 at the American Society of Clinical Oncology Annual Meeting a small, relatively unheralded study from Max Diehn’s group at Stanford University reported on the use of a novel ‘cancer personalized profiling by deep sequencing’ circulating tumor-DNA technology to identify minimal residual disease in patients after curative-intent radiation or surgery for NSCLC; in April 2018 at the American Association for Cancer Research Annual Meeting, Drew Pardoll presented a small pilot study of 21 patients who had received 2 doses of preoperative Nivolumab; in September 2018, at the 19th World Conference on Lung Cancer, Harry J. De Koning presented the long-awaited results of the Dutch-Belgian Lung Cancer Screening Trial (NELSON). These three seminal studies, along with others which are reviewed in this paper, promise to accelerate our progress towards a world in which lung cancer is identified early, more patients undergo curative-intent treatment that achieves the promised cure, and those at risk for failure after treatment are identified early, when the cancer remains most vulnerable. The day is round the corner when lung cancer is de-fanged and no longer the worldwide terror it currently is. We herein present an overview of the most recent body of work that moves us inexorably towards that day.
Introduction.
Although lung cancer remains the oncologic public health challenge of our age, with a worldwide estimate of 2.1 million new diagnoses and 1.8 million deaths annually,1 exciting developments over the past year promise to transform the stage distribution more towards the curative treatment end, increase the effectiveness of curative treatment options, while minimizing the morbidity of treatment and improving the patient experience. In 2018, at the 19th World Conference on Lung Cancer, the exciting results of the Dutch-Belgian lung cancer screening trial, NELSON, were presented.2 This long-awaited trial corroborated the findings of the United States (US) National Lung Screening Trial and will stimulate widespread engagement of the opportunity represented by the challenge of implementing national lung cancer screening programs.3
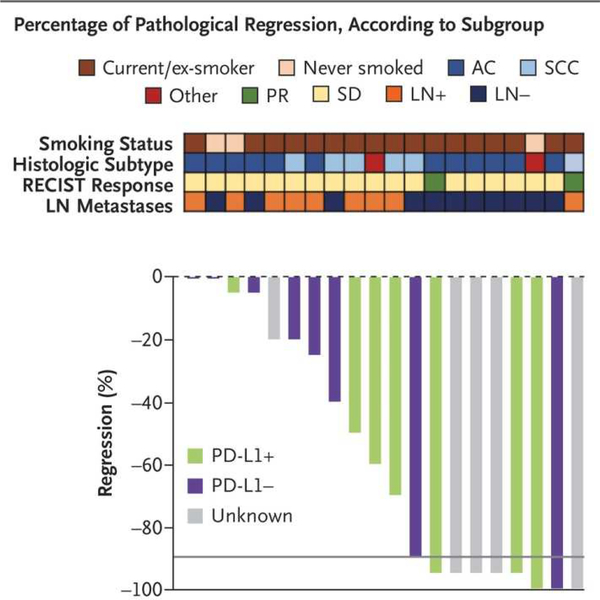
The 2018 Nobel Prize for medicine or physiology was awarded to James P. Allison and Tasuku Honjo for their seminal work leading to the development of immunotherapy.4 How fitting then that one of the most exciting developments in lung cancer in 2018 was emerging evidence of the powerful role adjuvant and neoadjuvant immunotherapy can play in increasing the success of curative-intent surgery and radiation therapy.5,6 If two doses of Nivolumab administered preoperatively can induce major pathologic response in 9 of 20 non-small-cell lung cancer (NSCLC) patients, we have much to be excited about (Fig 1)! Advances in pre-surgical care, surgical techniques and immediate postoperative care are decreasing treatment-related morbidity, thereby expanding the role of surgery where once deemed unsafe. Concurrently, the role of curative-intent nonsurgical options such as stereotactic body radiation therapy (SBRT), continues to be defined.
Figure 1.
Pathologic regression of tumor in 20 non-small cell lung cancer resection specimens following neoadjuvant blockade of Programmed Death 1 (PD-1) with 2 doses of Nivolumab. The gray horizontal line indicates the threshold for major pathologic response (90% regression). AC= adenocarcinoma, LN= lymph node, PD-L1= Programmed Death Ligand 1, PR= partial response, RECIST= Response Evaluation Criteria in Solid Tumors, SCC= squamous cell carcinoma, SD= stable disease.5
Although surgery provides the pathway to cure for most long-term survivors, ongoing efforts to increase the quality of surgical resection, improve pathologic nodal staging and, importantly, improve our ability to accurately predict failure of curative-intent treatment, early, when the opportunity for salvage is most likely, continue apace. The potential for circulating tumor DNA (ct-DNA) analysis to predict disease recurrence or progression shortly after curative-intent treatment is an exciting new possibility.7
These developments, and more, are covered in this update by an international team of clinician scientists, experts who are also the drivers of some of these advances. Our objective was to highlight and contextualize the main emerging developments in early-stage NSCLC over the 18 months from mid-2017 to the end of 2018. We have culled information from recent publications, as well as abstracts presented at major academic meetings such as the World Conference on Lung Cancer, the American Association for Cancer Research, American Society of Clinical Oncology, and European Society of Medical Oncology Annual Meetings.
Lung cancer screening and prevention.
Recent developments in lung cancer prevention promise to be game-changers. Large epidemiological studies indicate that high serum interleukin-6 and C-reactive protein are associated with increased lung cancer risk.8 In a recent large double-blind randomized trial, the treatment groups received the monoclonal antibody Canakinumab to reduce interleukin-1beta activity. In the group receiving 300 mg, incidence (HR 0.33, 95%CI 0.18–0.59) and mortality (HR 0.23, 95%CI 0.10–0.54) were significantly reduced (p=0.0002),9,10 suggesting that Canakinumab should be further investigated, most obviously in targeted prevention trials nested within screening programs.
In Europe, the scientific community has only recently pronounced in favor of mass screening for lung cancer,11 notwithstanding the 20% mortality reduction demonstrated by the National Lung Screening Trial.3 The change coincides with 10-year mortality results for the NELSON trial, Europe’s largest: the low-dose CT (LDCT)-screened arm had a 26% (95%CI 9–41%) reduction in mortality in men and a 39–61% reduction in women.2 Europe is now poised to introduce screening. However high-risk populations need to be better defined. The INTEGRAL consortium recently showed that circulating proteins can improve lung cancer risk assessment and may be useful for defining screening eligibility.12 A combined blood test assessing the levels of circulating proteins and mutations in cell-free DNA was shown to detect eight common cancer types, including lung cancer, with good sensitivity and very high specificity.13
Furthermore, since the population eligible for screening is large, screening has enormous potential for the primary prevention of cardiovascular events (CVEs). A recent systematic review concluded that LDCT-evaluated coronary artery calcification score has the potential to predict CVEs, reduce cardiovascular morbidity and mortality, and hence increase the cost-effectiveness of screening.14 European cost-effectiveness analyses revealed LDCT screening was associated with very favorable Incremental Cost-Effectiveness Ratio (the yearly incremental costs of saving a patient’s life), likely acceptable to the relevant health authorities: £6325 in the United Kingdom;15 €2943 in Italy.16 Thus screening can be implemented at relatively low cost.
Advances in diagnostics, clinical staging, clinical prognostic markers.
Besides the exciting results of the NELSON trial which should stimulate development of a screening strategy in most countries,2 the widespread use of chest CT scans in the workup of different diseases means a growing number of solitary pulmonary nodules are being found.17,18 At present no reliable biomarker or imaging technique is available to determine whether a lesion is, or will become, malignant in an individual patient within a clinically meaningful time-frame. The implementation of volume doubling time or radiomics to determine whether a lesion is malignant remains unvalidated, pathological diagnosis based on tissue biopsy is still the gold standard in patients with undetermined nodules. The choice between radiologic surveillance for high-risk imaging features and invasive diagnostic procedures for histological or cytological confirmation of the nature of a lesion remains difficult for patients and their clinicians. The complication rates of invasive procedures and the potential inaccuracies of imaging techniques necessitate thorough discussion with the patient.
With the increasing prevalence of small malignant lesions and growing awareness of the prognostic implications of tumor size, size was more deeply incorporated in the 8th edition TNM staging system, which was implemented in Asia and Europe in 2017 and in the US in 2018. T1 was split into 3 subgroups: (T1a< =1cm, T1b>1cm to≤2cm, T1c>2cm to≤3cm); T2 into 2 subgroups (T2a>3cm to ≤4cm, T2b>4cm to ≤5cm); tumors >5 to ≤7 cm are now designated as T3; and those ˃7 cm as T4.19 Although size, distinct from lymph node metastasis, is appreciated as a risk factor for NSCLC recurrence, more exact biomarkers for predicting recurrence are needed.
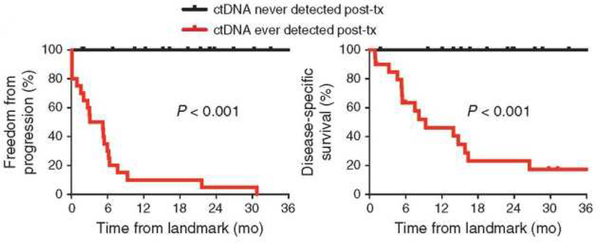
Two potential such biomarkers are ct-DNA7 and the Tumor Mutation Burden (TMB).20 In a study of 40 patients with stage I-III lung cancer who had curative-intent surgery or radiation therapy, a ‘cancer personalized profiling by deep sequencing (CAPP-seq)’ ct-DNA analysis of the first post-treatment blood sample identified 94% of patients whose disease recurred, preceding radiographic progression in 72%, by a median of 5.2 months (Fig 2).7 We may now have the technology to reliably identify minimal residual disease in lung cancer. Re-analysis of specimens from the Lung Adjuvant Cisplatin Evaluation-Bio-II study suggested that high non-synonymous TMB (>8 mutations/megabase) was favorably prognostic and adjuvant chemotherapy mostly benefited patients with low TMB (≤4 mutations/megabase).20 If corroborated, these biomarkers will guide clinicians in the choice of adjuvant treatment.
Figure 2.
Kaplan-Meier analysis for freedom-from progression (left) and disease-specific survival (right) stratified by circulating tumor DNA (ctDNA) detection status during posttreatment surveillance; ever positive (n=20) versus never positive (n=17). Landmark analysis was performed from the first post-treatment blood draw. Tx= treatment.7
Recent results of immunotherapy in advanced and early-stage NSCLC have reinvigorated the question whether the intra-tumoral immune infiltrate has prognostic or predictive value to establish indications for (neo)adjuvant treatment, and which treatment may be best. Early evidence suggests the prognostic and potential predictive capabilities of immune gene signatures in early-stage NSCLC but further prospective validation is necessary before use in clinical practice.21 After neoadjuvant treatment with the Program Death (PD)-1 checkpoint inhibitor Nivolumab, the number of major pathological responses was related to the TMB.5 With only 9 relevant patients in this study, firm conclusions must await future validation.5 In addition, technical requirements to perform the kinds of genomic tests, whole-exome sequencing and neo-antigen prediction assays, used in this study must be simplified for wide use in clinical practice. Although most focus is currently on the pretreatment composition of the tumor tissue, the effect of treatment on the tumor must be taken into account. For instance, it was recently shown that SBRT in early-stage NSCLC induces a beneficial immune activation which may also be predictive of the effect of a PD-(ligand [L])1 checkpoint inhibitor.22
Surgery for early-stage NSCLC.
Except for ‘medically-inoperable’ patients, the prevailing belief is that surgical resection offers the best chance of cure for early-stage NSCLC,23 but oncologically sound resection is imperative for optimal prognosis. Maximal reduction of the risk and trauma of surgery is also important, which is the potential advantage of minimally-invasive surgical techniques. The rising proportion of surgical resection candidates with smaller ground-glass nodules (GGNs) raises a dilemma: malignant GGNs have a more indolent nature than solid nodules.24
In a prospective multicenter trial including 795 patients with 1238 GGNs, only 1.2% pure GGNs developed into heterogeneous GGNs (with the solid component appreciated only on lung windows) and 5.4% into part-solid nodules in a mean time of 3.8 years. Among 81 heterogeneous GGNs, 20% developed into part-solid nodules after a mean of 2.1 years. All resected pure GGNs and heterogeneous GGNs were pre-invasive lesions. Even among the 49 part-solid nodules resected, only 12 turned out to be invasive adenocarcinomas.25 These results reinforce the 2017 Fleischner Society Guideline recommendations for extended follow-up.26 It was recently suggested that automated interpretation of the radiological characteristics by deep learning techniques might improve diagnostic accuracy in GGNs.27 Currently, a conservative approach remains the best strategy for such patients, to avoid over-hasty surgery upon initial detection and in the absence of significant change on CT surveillance.
Increased detection of smaller and ground-glass containing early-stage lung cancers has led to revival of sublobar resections that were once reserved for functionally compromised patients. Limited resection, especially anatomical segmentectomy, may carry similar oncological outcomes as standard lobectomy. However, even if this non-inferiority in oncological outcomes is proven by the two ongoing trials (JCOG 0802 and CALGB 140503), the benefit of segmentectomy may still be relative. Unlike wedge resection, segmentectomy is technically more demanding than lobectomy, and it may be necessary to first demonstrate its superiority in peri-operative outcomes and/or pulmonary function preservation.
Initial results of JCOG0802 showed that segmentectomy was associated with increased blood-loss and air-leak.28 A post-hoc analysis of CALGB 140503 revealed no difference in either perioperative morbidity or mortality between lobectomy and sublobectomy in good-risk patients, even though 59% of the sublobectomies were actually wedge resection.29 As to function preservation, in good-risk patients pulmonary function loss after video-assisted thoracoscopic surgery (VATS) segmentectomy was indeed significantly less than after VATS lobectomy.30 But average loss per-segment resected was almost doubled after segmentectomy, probably due to distortion of the remaining lobe after dividing the intersegmental plane.30 All these should be taken into account when selecting appropriate procedures for individual patients.
Lymph node dissection is an integral part of lung cancer surgery. The purpose of systematic lymphadenectomy is to assure accurate staging and complete disease removal. A recent metaanalysis found improved survival but also higher morbidity after lymphadenectomy than after sampling.31 This seems to contradict ACOSOG Z0030, which found only 4% nodal upstaging after dissection than after systemic sampling and no difference in post-operative complications, survival or recurrence rates in patients with clinical T1–2, N0 and non-hilar N1 tumors.32 But it is important to recall that patients were randomized after invasive mediastinal staging. Thus the ACOSOG Z0030 results may not be generalizable to patients staged radiographically or those with higher-stage tumors.
Z0030 notwithstanding, the importance of high quality intraoperative nodal staging in early-stage NSCLC continues to receive significant attention. Dissection of less than 15 nodes was associated with risk of under-staging, less than 6 nodes with worse survival.33 Thus, either rigorous preoperative mediastinal staging or systematic intraoperative dissection or sampling are still recommended. A recent multi-institutional prospective study showed that the introduction and compliance with basic quality measures for lymph node evaluation by surgeons and pathologists (examination of at least 1 N1 lymph node, at least 10 total lymph nodes, and at least 3 mediastinal nodal stations) significantly increased lymph node yield,34 improved survival, and better stratified post-operative stage-specific survival curves.35 The authors also implicated variations in the quality of intraoperative nodal staging for the intercontinental differences in post-operative survival.35
With the increasing use of sub-lobar resection for small GGNs, which seem to have a low risk of nodal involvement, the extent of required nodal evaluation is an emerging controversy. Noticeably no lymph nodes were examined in 49% and 23% of wedge and segmental resections for stage I tumors ≤ 2cm, and the number of nodes examined was revealed as an independent risk factor for survival while the extent of resection was not.36 However for GGNs, mediastinal dissection may not have significant impact on either staging or survival.37 In a propensity-matched study comparing mediastinal or hilar-only dissection for stage I NSCLC appearing as mixed GGN, mediastinal nodal involvement was found in only 9 of 329 patients (2.7%) with solid-dominant lesions but in none with part-solid lesions. The extent of nodal dissection was neither a risk-factor for survival nor predictive of nodal involvement.37
Minimally-invasive approaches.
Thoracic surgery is becoming less invasive while procedures are becoming more sophisticated.38,39 A 2018 study used spontaneous-ventilation VATS to perform tracheal and carinal resections in 18 awake patients, achieving anastomosis in 22–40 minutes, mean duration of surgery of 162 minutes, and low postoperative morbidity and mortality.39 A recent multi-national study showed that robotic surgery could be safely extended to selected stage III NSCLC cases with acceptable complication and conversion rates, satisfactory long-term survival, and low local recurrence rates.40 Similarly, a multi-institutional retrospective review of 1339 robotic lobectomies showed encouraging results particularly for N2 disease.41
Despite the success of robotic thoracic surgery, costs remain higher than for VATS,42,43 except in the study of Musgrove et al. where robotic and VATS segmentectomy had similar costs, with a trend to shorter length of stay and fewer complications in VATS cases.44 In over 1600 propensity-matched cases, conversions and postoperative complications were fewer in robotic than VATS cases after excluding learning-curve robotic cases.45 Conversion from minimally-invasive to open surgery may not raise postoperative morbidity or mortality risk.46 On the other hand, as robotic thoracic surgery is increasingly adopted, awareness of the risks inherent in this complex new technology has grown, and a group of European thoracic surgeons recently proposed a standardized training curriculum for young doctors.47 Although robotic and other innovative surgical approaches such as uniportal or subxyphoid VATS are receiving more attention, their additional functional benefit is questionable, as traditional VATS incisions cause loss of only 5% of pulmonary function.30 Although these procedures are shown to be safe and feasible, their cost-effectiveness remains to be demonstrated. Well-designed studies are needed to better assess their advantages.
Enhancing surgical outcomes.
Novel protocols aimed at increasing the efficacy and tolerability of lung cancer resections now expand beyond minimally-invasive incisions, extending throughout the peri-operative setting. Pre-operative assessment is focusing on frailty measures, which is a more significant marker of operative risk than age. Multiple investigators have demonstrated the ability to preoperatively identify frail thoracic surgery patients.48,49 A recent analysis used a combination of grip strength, gait, weight loss, and self-reported exhaustion and activity to identify 12% of thoracic surgery patients as frail and 57% as prefrail.48 Ongoing trials are evaluating the ability of “prehabilitiation” to mitigate increased rates of operative morbidity in frail patients.
Pulmonary rehabilitation is a cornerstone of chronic obstructive pulmonary disease management, but not routinely used in preparation for NSCLC resections, due to the urgency between diagnosis and resection. Traditional endurance therapy (ET) requires daily sessions over 6–12 weeks, but recent evidence suggests that high-intensity interval training (HIIT) over 2–4 weeks provides meaningful rehabilitation for high-risk frail surgical patients. HIIT increases respiratory muscle strength and aerobic capacity similar to ET,50 and significantly improves V02max, dyspnea and fatigue compared to baseline.51 Prospective studies of HIIT in NSCLC patients demonstrate measurable improvements in pulmonary function within the interval needed for cancer resections. Patients with poor pulmonary function had better short-term surgical outcomes after undergoing pre-operative HIIT.52 Standard-risk patients did not see similar reductions in peri-operative morbidity following HIIT in one study,53 but HIIT helped to prevent decline after surgery in all patients in another study.54
Similarly, Enhanced Recovery After Surgery (ERAS) protocols, are revolutionizing perioperative thoracic surgery care. The European Society of Thoracic Surgeons published ERAS guidelines that include 45 evidence-based care recommendations throughout the peri-operative experience emphasizing minimally-invasive surgery, decreased fasting, opioid-sparing analgesia, early mobilization, and increased patient education.55 The thoracic surgeons from MD Anderson Cancer Center are leaders in ERAS use in the US, and reported decreased length of stay and cardiopulmonary complications,56 and an increased compliance with adjuvant chemotherapy with implementation of their ERAS program.57
New adjuvant therapies and neo-adjuvant immunotherapy in early-stage NSCLC.
Disease recurrence remains the bane of curative-intent surgical resection.58 Therefore, novel treatments are needed beyond the current standard adjuvant (or neoadjuvant) cytotoxic chemotherapies. In the advanced-disease setting, molecular targeted therapy with tyrosine kinase inhibitors (TKIs) and immunotherapy with immune-checkpoint inhibitors have shown superior efficacy and lower toxicity compared to cytotoxic chemotherapy.59 This experience has stimulated many clinical trials using TKIs or immune checkpoint inhibitors in the neoadjuvant and adjuvant settings. Most of the phase III trials were still ongoing in 2018, but some have presented preliminary data.
Advances in adjuvant therapies in surgically resected NSCLC.
Experience with treatment of stage IV NSCLC informs adjuvant therapy trials. Epidermal growth factor receptor (EGFR)-TKIs (Gefitinib, Erlotinib, Afatinib, Dacomitinib, and Osimertinib) are the most potent drugs against NSCLC with activating mutations of EGFR. Failure of 2 years of Erlotinib therapy to produce a survival benefit over placebo in the international randomized trial, RADIANT, did not cool interest in exploring the role of adjuvant EGFR-TKI therapy because in the 16.5% of patients with known activating mutations of EGFR, disease-free survival (DFS) was 46.4 v 28.5 months (HR 0.81 (0.38 – 0.98; p= 0.039, impressive, but not significant in the hierarchical testing procedure used in this trial).60 In a single-arm phase II trial (SELECT), two years of Erlotinib after standard adjuvant chemotherapy showed an improved 2-year DFS compared with historic genotype-matched controls.61 A randomized study, ADJUVANT, compared Gefitinib (24 months) versus Vinorelbine/Cisplatin in patients with pathologic Stage II - IIIA (N1–N2) EGFR-mutated NSCLC.62 DFS, the primary endpoint of this study, was significantly longer with Gefitinib than with Vinorelbine/Cisplatin (HR 0.60, 95% CI 0.42–0.87; p=0.0054). In the safety population, the Gefitinib group had reduced toxicity and improved quality of life. However, overall survival (OS) data of ADJUVANT, together with results of other ongoing studies, including IMPACT/WJOG6410L (Gefitinib), ALCHEMIST (Erlotinib or Crizotinib based on driver mutation), ADAURA (Osimertinib), and ALINA/BO40336 (Alectinib), are essential before adjuvant TKIs (EGFR-TKIs or TKIs targeting other drivers) can become standard of care. Given the superior outcomes with Osimertinib over Gefitinib and Erlotinib therapy in patients with untreated EGFR-mutated advanced NSCLC (DFS 18.9 months v 10.2 months; HR for death, 0.63 [0.45 – 0.88], p=0.007),63 results of ADAURA are eagerly anticipated.
Patients re-challenged with Erlotinib after recurrence in SELECT experienced durable response.61 Recurrence patterns after adjuvant EGFR-TKIs may be spatially and temporally unique: Central Nervous System metastasis was frequent as the first site of recurrence in the Gefitinib group and the highest peaks were delayed in that group (e.g. the first peak of extracranial metastases appeared during 9 – 15 months and 24 – 30 months after surgery in the Vinorelbine/Cisplatin and Gefitinib groups, respectively).64 Clinical trials are also evaluating the roles of PD-1 / PD-L1 immune checkpoint inhibitors in the adjuvant setting. Within these trials, patients receive immune checkpoint inhibitors, usually for up to 1 year, after or without adjuvant chemotherapy, such as in EA5142- Adjuvant Nivolumab in Resected Lung Cancer (ANVIL).
Use of TKIs and immune checkpoint inhibitors as neoadjuvant therapy.
Neoadjuvant chemotherapy is considered equivalent in survival impact to adjuvant chemotherapy.65 Effective neoadjuvant therapy may also reduce the risk of incomplete resection or enable avoidance of pneumonectomy. In a randomized study for clinical stage IIIA (N2) EGFR-mutated NSCLC, Erlotinib administered for 42 days neoadjuvantly and 12 months adjuvantly, was associated with significantly longer PFS and lower grade 3/4 toxicity compared with Gemcitabine/Cisplatin.66 Early reports on neoadjuvant immune checkpoint inhibitor monotherapy (Nivolumab, Atezolizumab, or Pembrolizumab) or combination therapy (Atezolizumab plus chemotherapy) suggest relatively high efficacy (major pathologic responses 21 – 45 % in monotherapy and 50% in combination), acceptable toxicity, without enhanced surgical complications.4,67–69
Considering the regression bed (the area of immune-mediated tumor clearance), “Immune-Related Pathologic Response Criteria” were proposed by one of these study groups.70 Currently, several randomized trials evaluating the roles of neoadjuvant immune checkpoint inhibitors are underway and expectations for these trials are high based on the idea that earlier administration may provide better outcomes. From a translational research aspect, neoadjuvant treatment is also important because it will provide a great opportunity to explore molecular mechanisms of inherent resistance or drug tolerant states.71
Non-surgical treatment.
SBRT has become standard-of-care in patients with medically inoperable early-stage NSCLC. The phase III CHISEL trial compared SBRT with conventional radiotherapy for inoperable stage I NSCLC, showing better OS and freedom from local failure in the SBRT arm,72 with no differences in quality-of-life measures,73 further supporting SBRT. There are no well-powered prospective studies comparing SBRT with surgery in early-stage NSCLC. A pooled, albeit severely under-powered, analysis of two randomized phase III trials, STARS and ROSEL (both prematurely closed due to slow accrual), comparing SBRT with surgery in patients with operable stage I NSCLC, showed better 3-year OS for SBRT (95%) versus surgery (79%, p=0.037), with fewer grade 3–4 adverse events with SBRT.74 This analysis should provide equipoise for clinicians to support much-needed randomized trials in this setting.
Retrospective comparisons of SBRT versus surgery should be interpreted with great care, because of the risk of unmeasured confounding factors, including co-morbidities, that may influence the results. Nevertheless, a retrospective study demonstrated improved cancer-specific survival with lobectomy compared to SBRT in stage I NSCLC (HR 1.45), although no difference between sub-lobar resection and SBRT (HR 1.25).75 In a meta-analysis, OS was better after surgery compared to SBRT in stage I NSCLC, however, lung cancer–specific survival was similar, indicating comparable treatment efficacy.76
Further investigation of the effectiveness of SBRT in operable patients includes updated 4-year results from RTOG 0618 with SBRT (54Gy/3 fractions) showing a high rate of primary tumor and local control (both 96%), and DFS and OS of 57% and 56%, respectively.77 Another retrospective study found no significant differences between operable and inoperable patients in 5-year local control (93.1% vs. 96.7%), cancer-specific survival (80.6% vs. 91.0%), but a trend for worse OS (34.2% vs. 45.3%; P = .068), most likely due to more co-morbidities.78 The true pathologic complete response (pCR) rate after SBRT is unknown. The phase II trial MISSILE-NSCLC with SBRT in the neoadjuvant setting found the pCR rate after SBRT to be 60%, although this may be an underestimation because surgery was performed at 10 weeks and pCR to SBRT may take longer time.79
With the recent advances in treatment with immune checkpoint inhibitors, the combination of SBRT and immunotherapy is the focus of several ongoing clinical trials. One study of 3 single-institutional phase I/II trials concluded that combined treatment is safe in the short term, with no patients having grade ≥4 events and 9 pulmonary-specific grade 3 events (4 patients), encouraging further combination studies.80
Imaging after SBRT for early-stage NSCLC can detect recurrences, but may also be difficult to interpret. Recommendations for surveillance after SBRT were established by an international expert group, which identified a number of high-risk features for local recurrence: infiltration into adjacent structures; bulging margins; sustained, mass-like, spherical, or cranio-caudal growth; and loss of air bronchograms.81,82
SBRT of ultra-central tumors (in which the planning target volume touches or overlaps the central bronchial tree, esophagus, or pulmonary artery) may pose a higher risk of serious toxicity. Tekatli and colleagues found significant predictors for ≥grade 3 toxicity to be a planning target volume overlapping the trachea or main stem bronchus (P = .005), chronic obstructive pulmonary disease (P = .034), and the total volume receiving an ‘equivalent dose of a 2 Gy per fraction treatment of 130 Gy’ (P = .012).83 Additional studies are needed to better understand toxicity for ultra-central tumors.
Alternative non-surgical treatment methods besides SBRT include radiofrequency ablation (RFA). Previous studies have demonstrated poorer local tumor control rates and a higher risk of adverse events, including pneumothorax, with RFA compared to SBRT.84 Recently, two studies of RFA, one a retrospective analysis,85 the other a single arm prospective phase II trial,86 reported OS rates comparable to SBRT, but with considerable risk of pneumothorax in the prospective study.86 These results should be interpreted with care because of the retrospective or indirect comparisons. Validation of this invasive approach to the care of medically inoperable patients needs prospective comparative-effectiveness studies.
Future developments.
The exciting developments reviewed in the preceding sections illustrate dynamic progress in management of early-stage NSCLC, where lung cancer is a survivorship story. Accelerating that progress mandates timely completion of the many rigorous studies already ongoing or in development. Ultimately, progress must be measured by our ability to deploy the emerging array of diagnostic, staging, treatment and surveillance options within the clinical arena, where lives are saved or lost. Immunotherapy will expand the candidate pool of patients for curative-intent treatment, while transforming long-term outcomes of recipients of such treatments, whether surgery, radiation therapy or other emerging alternatives.5,6,87
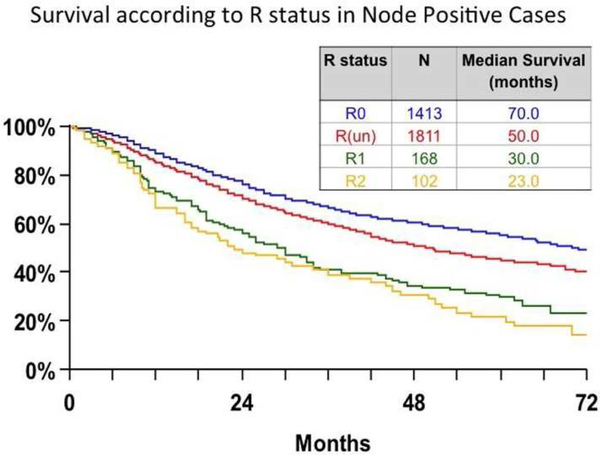
Equally exciting is the prospect of accurately gauging post-treatment risk by using biologic markers. Illustrating the need, the International Association for the Study of Lung Cancer (IASLC) Staging and Prognostic Factors Committee reported that 56% of 14,712 NSCLC resections in their database had ‘R-uncertain resections’, defined as resections with negative margins which nevertheless have high risk of residual disease (indicated by failure to perform systematic or lobe-specific nodal dissection, involvement of the highest mediastinal lymph node, carcinoma in-situ at the resection margin or positive pleural lavage cytology).88,89 A population-based cohort corroborated the IASLC finding (Fig 3)89 that such patients have significantly worse survival than those meeting the new definition of complete resection.90
Figure 3.
Survival according to residual disease (‘R’) status in the International Association for the Study of Lung Cancer international lung cancer staging database. R0= complete resection, R(un)= uncertain resections (defined as resection with microscopic negative margins but residual risk features including any combination of intraoperative lymph node evaluation less rigorous than systematic or lobe-specific nodal dissection, positive highest mediastinal lymph node, carcinoma in-situ at the bronchial margin, positive pleural lavage cytology), R1= microscopic tumor involvement at the resection margin, extracapsular extension of tumor in lymph nodes removed separately or present at the margin of the main lung specimen), R2= macroscopic evidence of residual disease including positive pleural or pericardial effusions and nodes known to be positive but not removed.83,84
In this molecular age, the possibility that ct-DNA might accurately identify patients at risk for disease recurrence with a high degree of specificity, serial measurements might identify radiologically inevident recurrence, and also accurately categorize recurrence risk in patients with radiologically ambigious lesions (such as after radiation therapy with the usual scar-like changes), is definitely something to look forward to (Fig 2).7 Admittedly not yet ready for primetime,91 this emerging technology will transform our thinking about patient selection for adjuvant treatments, increase transparency in evaluating the likelihood of success after attempted curative-intent treatment and will significantly improve our ability to conduct comparative-effectiveness trials between treatment modalities and methodologies.
Arguably, the 3 most important developments in the 18 months up to 2018 are the publication of the NELSON trial results (there can now be no doubt about the need to implement lung cancer screening programs);2 the emergence of neoadjuvant immunotherapy;5 and the potential utility of ct-DNA for evaluating residual disease status and monitoring for treatment failure.7 Successful implementation of these 3 technology-based opportunities will transform lung cancer into a routinely curable disease within our lifetimes.
Acknowledgments
Declarations: Supported by 2R01CA172253–06 (Osarogiagbon).
Disclosures:
Dr. Osarogiagbon reports grants from National Institutes of Health, during the conduct of the study; other from Eli Lilly, other from Pfizer, personal fees from Genentech/Roche, personal fees from Association of Community Cancer Centers, outside the submitted work; In addition, Dr. Osarogiagbon has a patent Lymph node specimen collection kit issued, and a patent Lymph node specimen collection kit pending.
Dr. Veronesi received personal fees outside the submitted work from Medtronic, J and J and AB medica.
Dr. Suda reports grants from Boehringer Ingelheim Japan, Inc., outside the submitted work.
Dr. Aerts reports personal fees and non-financial support from BMS, personal fees and non-financial support from MSD, personal fees from Boehringer Ingelheim, grants and personal fees from Amphera, personal fees from Takeda, personal fees and non-financial support from Eli-Lilly, personal fees and non-financial support from Roche, outside the submitted work; In addition, Dr. Aerts has a patent Kinase activity profiles for predicting NSCLC response to therapy pending, a patent SNP associated with adverse events and clinical activity in pd-1 treated NSCLC patients pending, and a patent tumor cell lysate in immunotherapy licensed to Amphera.
Dr. Donington reports personal fees and non-financial support from AstraZeneca, outside the submitted work.
Drs. Fang and Ekman have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Raymond U. Osarogiagbon, Multidisciplinary Thoracic Oncology Program, Baptist Cancer Center, Memphis, Tennessee, USA.
Giulia Veronesi, Division of Thoracic Surgery, Humanitas Hospital, Milan, Italy.
Wentao Fang, Department of Thoracic Surgery, Shanghai Chest Hospital, Shanghai, China.
Simon Ekman, Department of Oncology, Karolinska University Hospital, Stockholm, Sweden.
Kenichi Suda, Division of Thoracic Surgery, Department of Surgery, Kindai University Faculty of Medicine, Osaka-Sayama, Japan.
Joachim G. Aerts, Thoracic Oncology Department, Erasumus University Medical Center, Rotterdam, The Netherlands.
Jessica Donington, Section of Thoracic Surgery, University of Chicago, Chicago, Illinois, USA.
References:
- 1.World Health Organization. Cancer Fact Sheet, 2018. Accessed January 6, 2019 at: http://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf
- 2.“NELSON study shows CT screening for nodule volume management reduces lung cancer mortality by 26 percent in men.” 2018. WCLC Press Program Press Release De Koning 9.25 FINAL.pdf https://wclc2018.iaslc.org/ [Google Scholar]
- 3.National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. https://www.nobelprize.org/prizes/medicine/2018/press-release/
- 5.Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. Forde PM, Chaft JE, Smith KN, Anagnostou V, et al. N Engl J Med. 2018. May 24;378(21):1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonia SJ, Villegas A, Daniel D, Vicente D, et al. ; PACIFIC Investigators. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018. September 25. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhury AA, Chabon JJ, Lovejoy AF, Newman AM, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 2017;7(12):1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young RP, Hopkins RJ. Update on the potential role of statins in chronic obstructive pulmonary disease and its co-morbidities. Expert Rev Respir Med. 2013;7(5):533–44. doi: 10.1586/17476348.2013.838018. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, MacFayden JG, Thuren T, Everett BM, Libby P, Glynn RJ, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins BJ. Potential efficacy of interleukin-1β inhibition in lung cancer. Lancet. 2017;390(10105):1813–1814. doi: 10.1016/S0140-6736(17)32289-4. [DOI] [PubMed] [Google Scholar]
- 11.Oudkerk M, Devaraj A, Vliegenthart R, Henzler T, Prosch H, Heussel CP et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18(12):e754–e766. doi: 10.1016/S1470-2045(17)30861-6. [DOI] [PubMed] [Google Scholar]
- 12.Integrative Analysis of Lung Cancer Etiology and Risk (INTEGRAL) Consortium for Early Detection of Lung Cancer. Assessment of lung cancer risk on the basis of a biomarker panel of circulating proteins. JAMA Oncol. 2018;4(10):e182078. doi: 10.1001/jamaoncol.2018.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen Joshua D., Li Lu, Wang Yuxuan, Thoburn Christopher, Afsari Bahman, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science, 2018; 359, 926–930. DOI: 10.1126/science.aar3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events. A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi: 10.1097/MD.0000000000010461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veronesi G, Ghislandi S, Vanni E, Dieci E, Gallus S, Toschi L, et al. Analysis indicates low incremental cost-effectiveness ratio for implementation of lung cancer screening in Italy. International Association for the Study of Lung Cancer: 19th World Conference on Lung Cancer, Toronto, Canada 2018. [Google Scholar]
- 16.Field JK, Duffy SW, Baldwin DR, Brain KE, Devaraj A, Eisen T, et al. The UK Lung Cancer Screening Trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol Assess 2016;20(40). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Transthoracic Needle Biopsies: It’s More than Just Hitting the Bull’s-eye. Aerts JG. Clin Cancer Res. 2016. January 15;22(2):273–4. [DOI] [PubMed] [Google Scholar]
- 18.Smeltzer M, Faris N, Kethireddy J, Meadows M, et al. Comparing Lung Cancer Diagnosed by Low Dose CT (LDCT), Incidental Lung Nodule Program (ILNP), and Non-Program-Based Detection. J Thorac Oncol October 2018;13(10):S569. [Google Scholar]
- 19.The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forth- coming eighth edition of the TNM classification for lung cancer. Rami-Porta R, Bolejack V, Crowley J et al. J Thor Oncol 2015; 10: 990–1003. [DOI] [PubMed] [Google Scholar]
- 20.Devarakonda S, Rotolo F, Tsao M, et al. Tumor Mutation Burden as a Biomarker in Resected Non-Small-Cell Lung Cancer. J Clin Oncol 2018; 36:2995–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Development and Validation of an Individualized Immune Prognostic Signature in Early-Stage Nonsquamous Non-Small Cell Lung Cancer. Li B, Cui Y, Diehn M, Li R. JAMA Oncol. 2017:1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stereotactic Ablative Radiotherapy Induces Peripheral T-Cell Activation in Patients with Early-Stage Lung Cancer. de Goeje PL, Smit EF, Waasdorp C, Schram MTB, Kaijen-Lambers MEH, Bezemer K, de Mol M,Hartemink KJ, Nuyttens JJME, Maat APWM, Hegmans JPJJ, Hendriks RW, Senan S, Aerts JG. Am J Respir Crit Care Med. 2017. 196(9):1224–1227 [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 2.2019). Available online: https://www.nccn.org/professionals/physician_gls/
- 24.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakinuma R, Noguchi M, Ashizawa K, et al. Natural history of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol2016;11:1012–28. [DOI] [PubMed] [Google Scholar]
- 26.Macmahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology, 2017:284:228–43. [DOI] [PubMed] [Google Scholar]
- 27.Mei X, Wang R, Yang W, et al. Predicting malignancy of pulmonary ground-glass nodules and their invasiveness by random forest. J Thorac Dis 2018;10:458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki K, Saji H, Watanabe S, et al. Comparison of morbidity of pulmonary segmentectomy and lobectomy: initial results of a phase III randomized trial of lobectomy versus segmentectomy for small (2 cm or Less) peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). AATS abstract
- 29.Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Oncol 10.1016/S2213-2600(18)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu Z, Wang H, Mao T, et al. Pulmonary function changes after different extent of pulmonary resection under video-assisted thoracic surgery. J Thorac Dis 2018;10:2331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mokhles S, Macbeth F, Treasure T, et al. Systematic lymphadenectomy versus sampling of ipsilateral mediastinal lymph-nodes during lobectomy for non-small-cell lung cancer: a systematic review of randomized trials and a meta-analysis. Eur J Cardio-ThoracSurg 2017;51:1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versuscomplete lymphadenectomy during pulmonary resection in thepatient with N0 or N1 (less than hilar) non–small cell carcinoma:Results of the American College of Surgery Oncology Group Z0030Trial. J ThoracCardiovascSurg 2011;141:662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krantz SB, Lutfi W, Kuchta K, Wang CH, Kim KW, and Howington JA. Improved lymph node staging in early-stage lung cancer in the National Cancer Database. Ann Thorac Surg 2017;104:1805–14. [DOI] [PubMed] [Google Scholar]
- 34.Ray MA, Faris NR, Smeltzer MP, et al. Effectiveness of Implemented Interventions on Pathologic Nodal Staging of Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;106:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smeltzer MP, Faris NR, Ray MA, Osarogiagbon RU. Association of Pathologic Nodal Staging Quality With Survival Among Patients With Non-Small Cell Lung Cancer After Resection With Curative Intent. JAMA Oncol 2018;4:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yendamuri S, Dhillon SS, Groman A, et al. Effect of the number of lymph nodes examined on the survival of patients with stage I non–small cell lung cancer who undergo sublobar resection. J Thorac Cardiovasc Surg2018;156:394–402. [DOI] [PubMed] [Google Scholar]
- 37.Hattori A, Matsunaga T, Takamochi K, Oh S, and Suzuki K. Significance of lymphadenectomy in part-solid lung adenocarcinoma: propensity score matched analysis. Ann Thorac Surg 2018;106:989–97. [DOI] [PubMed] [Google Scholar]
- 38.Hennon MW, Kumar A, Devisetty H, D’Amico T, Demmy TL, Groman A, Yendamuri S. Minimally invasive approaches do not compromise outcomes for pneumonectomy: a comparison using the National Cancer Database J ThoracOncol. 2018; pii: S1556–0864(18)33176–9. doi: 10.1016/j.jtho.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Jiang L, Liu J, Gonzalez-Rivas D, Shargall Y, Kolb M, Shao W, Dong Q, Liang L, He J. Thoracoscopic surgery for tracheal and carinal resection and reconstruction under spontaneous ventilation. J ThoracCardiovasc Surg. 2018. June;155(6):2746–2754. doi: 10.1016/j.jtcvs.2017.12.153. [DOI] [PubMed] [Google Scholar]
- 40.Veronesi G, Park B, Cerfolio R, Dylewski M, Toker A, Fontaine JP et al. Robotic resection of stage III lung cancer: an international retrospective study. Eur J CardiothoracSurg 2018;54:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerfolio RJ, Ghanim AF, Dylewski M, Veronesi G, Spaggiari L, Park BJ. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J ThoracCardiovasc Surg. 2018;155(2):778–786. doi: 10.1016/j.jtcvs.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novellis P, Bottoni E, Voulaz E, Cariboni U, Testori A, BertolacciniL, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10(2):790–798. doi: 10.21037/jtd.2018.01.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur MN, Xie F, Shiwcharan A, Patterson L, Shargall Y, Finley C, et al. Robotic versus video-assisted thoracoscopic lung resection during early program development. Ann Thorac Surg. 2018;105(4):1050–1057. doi: 10.1016/j.athoracsur.2017.11.013.. [DOI] [PubMed] [Google Scholar]
- 44.Musgrove KA, Hayanga JA, Holmes SD, Leung A, Abbas G. Robotic versus video-assisted thoracoscopic surgery pulmonary segmentectomy: A cost analysis. Innovations (Phila). 2018;13(5):338–343. doi: 10.1097/IMI.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 45.Reddy RM, Gorrepati ML, Oh DS, Mehendale S, Reed MF. Robotic-assisted versus thoracoscopic lobectomy outcomes from high-volume thoracic surgeons. Ann Thorac Surg. 2018;106(3):902–908. doi: 10.1016/j.athoracsur.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 46.Fourdrain A, De Dominicis F, Iquille J, Lafitte S, Merlusca G, Witte-Pfister A et al. Intraoperative conversion during video-assisted thoracoscopy does not constitute a treatment failure. Eur J CardiothoracSurg 2018; doi: 10.1093/ejcts/ezy343. [DOI] [PubMed] [Google Scholar]
- 47.Veronesi G, Dorn P, Dunning J, Cardillo G, Schmid RA, Collins J, et al. Outcomes from the Delphi process of the Thoracic Robotic Curriculum Development Committee. Eur J Cardiothorac Surg. 2018. 1;53(6):1173–1179. doi: 10.1093/ejcts/ezx466. [DOI] [PubMed] [Google Scholar]
- 48.Beckert AK, Huisingh-Scheetz M, Thompson K, et al. Screening for Frailty in Thoracic Surgical Patients. Ann Thorac Surg 2017;103:956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirpara DH, Kidane B, Rogalla P, et al. Frailty assessment prior to thoracic surgery for lung or esophageal cancer: a feasibility study. Support Care Cancer 2018. [DOI] [PubMed] [Google Scholar]
- 50.Dunham C, Harms CA. Effects of high-intensity interval training on pulmonary function. Eur J Appl Physiol 2012;112:3061–8. [DOI] [PubMed] [Google Scholar]
- 51.Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer 2012;20:3169–77. [DOI] [PubMed] [Google Scholar]
- 52.Boujibar F, Bonnevie T, Debeaumont D, et al. Impact of prehabilitation on morbidity and mortality after pulmonary lobectomy by minimally invasive surgery: a cohort study. J Thorac Dis 2018;10:2240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Licker M, Karenovics W, Diaper J, et al. Short-Term Preoperative High-Intensity Interval Training in Patients Awaiting Lung Cancer Surgery: A Randomized Controlled Trial. J Thorac Oncol 2017;12:323–33. [DOI] [PubMed] [Google Scholar]
- 54.Sebio Garcia R, Yanez-Brage MI, Gimenez Moolhuyzen E, Salorio Riobo M, Lista Paz A, Borro Mate JM. Preoperative exercise training prevents functional decline after lung resection surgery: a randomized, single-blind controlled trial. Clin Rehabil 2017;31:1057–67. [DOI] [PubMed] [Google Scholar]
- 55.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS(R)) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2018. [DOI] [PubMed] [Google Scholar]
- 56.Van Haren RM, Mehran RJ, Mena GE, et al. Enhanced Recovery Decreases Pulmonary and Cardiac Complications After Thoracotomy for Lung Cancer. Ann Thorac Surg 2018;106:272–9. [DOI] [PubMed] [Google Scholar]
- 57.Nelson D, Mehran RJ, Mitchell K, et al. Enhanced Recovery After Thoracic Surgery Facilitates Adjuvant Chemotherapy for Non-Small Cell Lung Cancer. AATS Internatioanl Thoracic Surgical Oncology Summit New York, NY 2018. [Google Scholar]
- 58.Suda K, Sato K, Mizuuchi H, Kobayashi Y, Shimoji M, Tomizawa K, et al. Recent evidence, advances, and current practices in surgical treatment of lung cancer. Respir Investig. 2014. November;52(6):322–9. [DOI] [PubMed] [Google Scholar]
- 59.Hirsch FR, Suda K, Wiens J, Bunn PA, Jr. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016. September 3;388(10048):1012–24. [DOI] [PubMed] [Google Scholar]
- 60.Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2015;33:4007–14. [DOI] [PubMed] [Google Scholar]
- 61.Pennell NA, Neal JW, Chaft JE, Azzoli CG, Janne PA, Govindan R, et al. SELECT: A Phase II Trial of Adjuvant Erlotinib in Patients With Resected Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J Clin Oncol. 2018. November 16:JCO1800131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Shen Y, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. The Lancer Oncol. 2018. January;19(1):139–48. [DOI] [PubMed] [Google Scholar]
- 63.Soria JC, Ohe Y, Vansteenkiste J, et al. ; FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 64.Xu ST, Xi JJ, Zhong WZ, Mao WM, Wu L, Shen Y, et al. The unique spatial-temporal treatment failure patterns of adjuvant gefitinib therapy: A post-hoc analysis of the ADJUVANT trial (CTONG 1104). J Thorac Oncol. 2018. December 3, in press. [DOI] [PubMed] [Google Scholar]
- 65.Blumenthal GM, Bunn PA Jr., Chaft JE, McCoach CE, Perez EA, Scagliotti GV, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol. 2018. December;13(12):1818–31. [DOI] [PubMed] [Google Scholar]
- 66.Zhong WZ, Wu YL, Chen KN, Et a. LBA48_PR CTONG 1103: Erlotinib versus Gemcitabine plus Cisplatin as Neo-adjuvant Treatment for Stage IIIA-N2 EGFR-mutation NOn-small-cell lung cancer (EMERGING): a Randomised Stugy. Ann Oncol. 2018;29(Supplement 8):mdy424.058. [Google Scholar]
- 67.Rusch VW, Chaft JE, Johnson B, Et a. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Initial results from a multicenter study (LCMC3). J Clin Oncol. 2018;36(suppl 15):8541. [Google Scholar]
- 68.Shu CA, Grigg C, C C, Et a. Neoadjuvant atezolizumab + chemotherapy in resectable non-small cell lung cancer (NSCLC). J Clin Oncol. 2018;36(suppl 15):8532. [Google Scholar]
- 69.Ben Num A, Golan N, Ofek E, Et a. 1360P: Neoadjuvant pembrolizumab (Pembro) for early stage non-small cell lung cancer (NSCLC): Initial report of a phase I study, MK3475–223. Ann Oncol. 2018;29(suppl_8):mdy290.011. [Google Scholar]
- 70.Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol. 2018. August 1;29(8):1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suda K, Bunn PA Jr., Rivard CJ, Mitsudomi T, Hirsch FR. Primary Double-Strike Therapy for Cancers to Overcome EGFR Kinase Inhibitor Resistance: Proposal from the Bench. J Thorac Oncol. 2017. January;12(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ball D, Mai T, Vinod S, et al. MA 13.07 A Randomized Trial of SABR vs Conventional Radiotherapy for Inoperable Stage I Non-Small Cell Lung Cancer: TROG09.02 (CHISEL). Journal of Thoracic Oncology 2017;12(11):S1853 doi: 10.1016/j.jtho.2017.09.565[published Online First: Epub Date]|. [DOI] [Google Scholar]
- 73.Ball D, Mai GT, Vinod S, et al. Quality of life in the CHISEL randomized trial of stereotactic ablative radiotherapy (SABR) versus standard radiotherapy for stage I non-small cell lung cancer (Trans Tasman Radiation Oncology Group 09.02). Annals of Oncology (2018) 29 (suppl_9): ix139–ix142. 10.1093/annonc/mdy445. [DOI] [Google Scholar]
- 74.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015. June;16(6):630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bryant AK, Mundt RC, Sandhu AP, et al. Stereotactic Body Radiation Therapy Versus Surgery for Early Lung Cancer Among US Veterans. Ann Thorac Surg 2018;105(2):425–31 doi: 10.1016/j.athoracsur.2017.07.048[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 76.Chen H, Laba JM, Boldt RG, et al. Stereotactic Ablative Radiation Therapy Versus Surgery in Early Lung Cancer: A Meta-analysis of Propensity Score Studies. International Journal of Radiation Oncology • Biology • Physics 2018;101(1):186–94 doi: 10.1016/j.ijrobp.2018.01.064[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 77.Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol 2018;4(9):1263–66 doi: 10.1001/jamaoncol.2018.1251[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schonewolf CA, Heskel M, Doucette A, et al. Five-year Long-term Outcomes of Stereotactic Body Radiation Therapy for Operable Versus Medically Inoperable Stage I Non-small-cell Lung Cancer: Analysis by Operability, Fractionation Regimen, Tumor Size, and Tumor Location. Clin Lung Cancer 2018. doi: 10.1016/j.cllc.2018.09.004[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 79.David Palma AL, Malthaner Richard, Fortin Dalilah, Rodrigues George, Yaremko Brian, Laba Joanna M, Kwan Keith, Gaede Stewart, Lee Ting, Ward Aaron, Warner Andrew, Inculet Richard. MISSILE-NSCLC: A Phase II Trial Measuring the Integration of Stereotactic Radiotherapy Plus Surgery in Early-Stage Non-Small Cell Lung Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verma V, Cushman TR, Selek U, Tang C, Welsh JW. Safety of Combined Immunotherapy and Thoracic Radiation Therapy: Analysis of 3 Single-Institutional Phase I/II Trials. Int J Radiat Oncol Biol Phys 2018;101(5):1141–48 doi: 10.1016/j.ijrobp.2018.04.054[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen TK, Senan S, Bradley JD, et al. Optimal imaging surveillance after stereotactic ablative radiation therapy for early-stage non-small cell lung cancer: Findings of an International Delphi Consensus Study. Pract Radiat Oncol 2018;8(2):e71–e78 doi: 10.1016/j.prro.2017.10.008[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 82.Ronden MI, Palma D, Slotman BJ, Senan S, Cancer ARTCotIAftSoL. Brief Report on Radiological Changes following Stereotactic Ablative Radiotherapy (SABR) for Early-Stage Lung Tumors: A Pictorial Essay. J Thorac Oncol 2018;13(6):855–62 doi: 10.1016/j.jtho.2018.02.023[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 83.Tekatli H, Duijm M, Oomen-de Hoop E, et al. Normal Tissue Complication Probability Modeling of Pulmonary Toxicity After Stereotactic and Hypofractionated Radiation Therapy for Central Lung Tumors. Int J Radiat Oncol Biol Phys 2018;100(3):738–47 doi: 10.1016/j.ijrobp.2017.11.022[published Online First: Epub Date]. [DOI] [PubMed] [Google Scholar]
- 84.Bi N, Shedden K, Zheng X, Kong FS. Comparison of the Effectiveness of Radiofrequency Ablation With Stereotactic Body Radiation Therapy in Inoperable Stage I Non-Small Cell Lung Cancer: A Systemic Review and Pooled Analysis. Int J Radiat Oncol Biol Phys. 2016. August 1;95(5):1378–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lam A, Yoshida EJ, Bui K, Fernando D, Nelson K, Abi-Jaoudeh N. A National Cancer Database Analysis of Radiofrequency Ablation versus Stereotactic Body Radiotherapy in Early-Stage Non-Small Cell Lung Cancer. J Vasc Interv Radiol 2018;29(9):1211–17.e1 doi: 10.1016/j.jvir.2018.04.029[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 86.Palussière J, Chomy F, Savina M, et al. Radiofrequency ablation of stage IA non–small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. Journal of Cardiothoracic Surgery 2018;13(1):91 doi: 10.1186/s13019-018-0773-y[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gajewski TF. Fast forward- neoadjuvant cancer immunotherapy. N Engl J Med 2018;378:2034–35 [DOI] [PubMed] [Google Scholar]
- 88.Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25–33. [DOI] [PubMed] [Google Scholar]
- 89.Edwards J, Chansky K, Shemanski L, Van Schil P, Asamura H, Rami-Porta R. The IASLC Lung Cancer Staging Project: Analysis of Resection Margin Status and Proposals for R Status Descriptors for Non-Small Cell Lung Cancer. J Thorac Oncol November 2017;12(11):S1605. [DOI] [PubMed] [Google Scholar]
- 90.Smeltzer M, Faris N, Ray M, Fehnel C, et al. A Population-Based Validation Study of the Proposed ‘R-Factor’ Classification in a Lung Cancer-Endemic Region of the US. J Thorac Oncol October 2018; 13(10):S646 [Google Scholar]
- 91.Merker JD, Oxnard GR, Compton C, Diehn M, et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol. 2018. June 1;36(16):1631–1641. [DOI] [PubMed] [Google Scholar]