Abstract
A fluorescent sensor for catecholamines, NS510 is presented. The sensor is based on a quinolone fluorophore incorporating a boronic acid recognition element that gives it high affinity for catecholamines and a turn-on response to norepinephrine. The sensor results in punctate staining of norepinephrine-enriched chromaffin cells visualized using confocal microscopy indicating that it stains the norepinephrine in secretory vesicles. Amperometry in conjunction with TIRF microscopy demonstrates that the sensor can be used to observe destaining of individual chromaffin granules upon exocytosis. NS510 is the highest affinity fluorescent norepinephrine sensor currently available and can be used for measuring catecholamines in live-cell assays.
Graphical Abstract
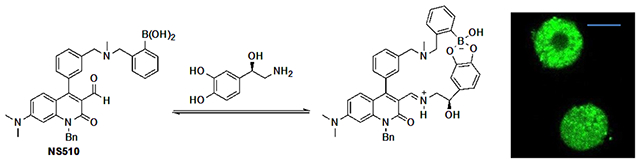
NS510 binds to norepinephrine with high affinity by both forming iminium ion and boronate ester. The sensor was used to stain chromaffin cells and observe exocytosis.
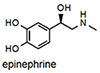
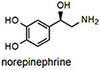
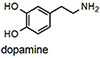
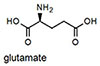
Understanding the function of the brain is a grand challenge of modern science.1 As part of this work, measurements of the uptake, storage and release of neurotransmitters are of central importance. Norepinephrine, epinephrine, dopamine, and glutamate, are the major neurotransmitters in the sympathetic nervous system,2 and are involved with many functions, including memory, attention, learning, emotion, sleep and movement.3 Aberrant levels of neurotransmitters are implicated in various disease states, thus measuring these simple organic compounds has become an important area of research. Fluorescence imaging of neurotransmitters has distinct advantages over methods such as capillary electrophoresis and microelectrochemistry because it is less invasive and has better spatial resolution.4,5 For this reason, Sames and Sulzer have developed a series of fluorescent false neurotransmitters, which are taken up into synaptic vesicles because they mimic the structure of genuine neurotransmitter, and can therefore be used to image vesicle accumulation at neuronal release sites and the loss of fluorescence that accompanies transmitter release.5b,6
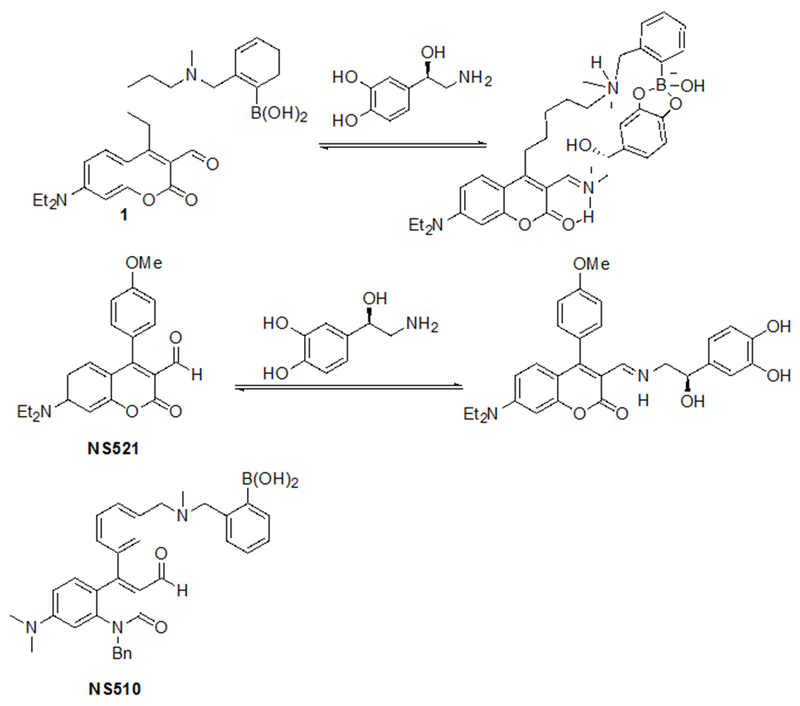
Our group is developing fluorescent sensors that label transmitter via direct binding. Sensor 1 (Figure 1) was prepared some time ago as a selective catecholamine sensor with a boronic acid moiety to bind the catechol group and an aldehyde that forms an iminium ion upon binding to primary amines.4d,7 While the sensor binds catecholamines with excellent affinity and appreciable selectivity, the fluorescence is strongly quenched by the electron-rich catechol. More recently, we developed NS521, which lacks the boronic acid group and thus has lower selectivity for catecholamine compared to sensor 1.8 However, NS521 has the advantage of having a strong turn-on fluorescence response to catecholamines in part because of electronic effect of the methoxyphenyl substituent on the fluorophore as well as the fact that the neutral catechol is not as strong of a quencher as the boronate formed when sensor 1 reacts with catecholamine.
Figure 1.
Binding of two catecholamine sensors to norepinephrine
Here we report development of a next-generation sensor Neurosensor 510 (NS510, Figure 1) with stronger affinity and reduced quenching of fluorescence upon catecholamine binding. We utilize a quinolone fluorophore since it is much more electron rich than coumarin,9 and therefore is quenched less upon binding the electron-rich catechol.10 NS510 possesses the base quinolone fluorophore with a boronic acid appended to an aromatic substituent to confer significantly enhanced affinity and therefore selectivity for catecholamine binding, similar to sensor 1.
NS510 was prepared in 4 steps (Scheme 1). A hydroxymethylphenyl group was coupled to the tosylate of compound 2 to furnish compound 3. It was most efficient to chlorinate compound 3 prior to formylation to give intermediate 5. Alkylation of 5 produced the target sensor NS510 in moderate yield.
Scheme 1.
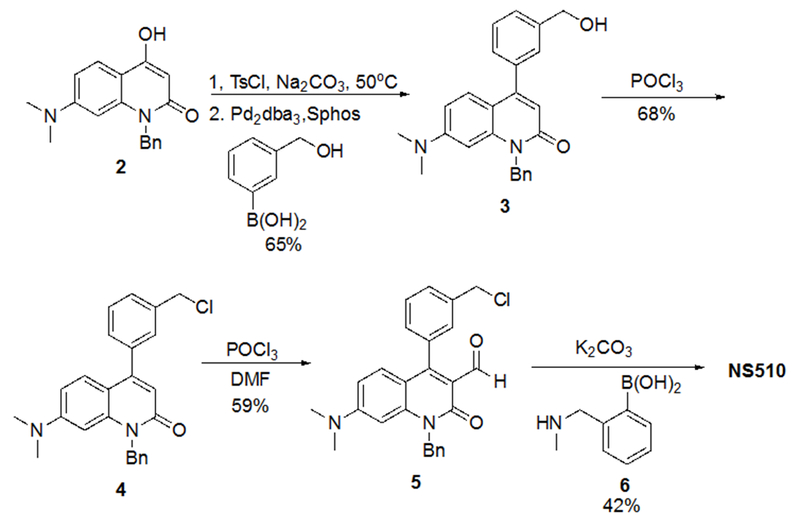
Synthesis of NS510
NS510 was screened with various amines by UV/visible spectroscopy and fluorescence spectroscopy. Table 1 summarizes the binding and spectroscopic data of the interaction of NS510 with relevant amines. NS510 showed a red shift in absorption from 428 nm to 473 nm upon addition of norepinephrine (Figure 2). The fluorescence at 510 nm roughly doubles upon binding and the binding constant was substantially higher compared to NS521 (Figure 1), which indicates that the boronic acid is participating in binding. Dopamine binds equally well to NS510 (Table 1), though the fluorescence is slightly quenched upon binding since dopamine is a more quenching analyte.12 Thus NS510 is selective for norepinephrine over dopamine based on fluorescence enhancement. To our best knowledge, this is the first sensor that can differentiate norepinephrine from dopamine.
Table 1.
Binding and Spectroscopic Properties of NS510 with Various Amines
Ka measured by fluorescence spectroscopy (see Figure 2 for conditions). Error in Ka values are ±10% based on triplicate titrations.
Isat = fluorescence intensity at saturation taken from the theoretical fit to the binding isotherm.
Ka measured by UV/vis spectroscopy.
Figure 2.
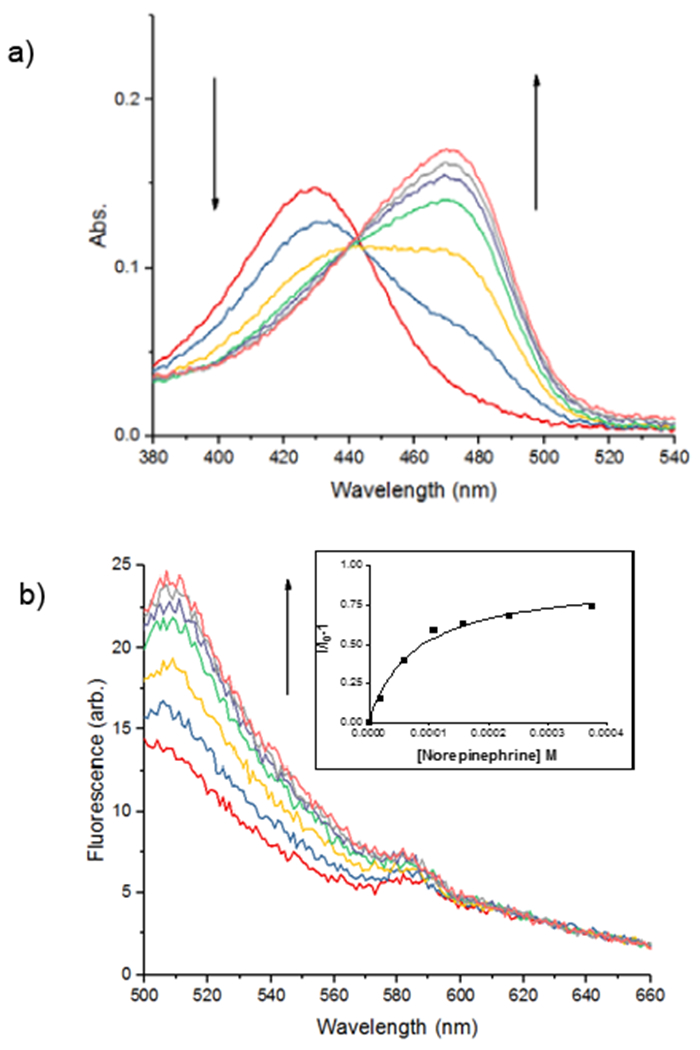
(a) UV/vis titration and (b) fluorescence titration of the sensor NS510 (10 µM in 25 mM HEPES, 50 mM Na2S2O3, pH = 7.4) with norepinephrine (10 mM in buffer, λex = 488 nm). Inset in (b) is a plot of emission intensity at 510 nm versus concentration with a fit to a one-site binding isotherm.
Epinephrine does not produce a shift in absorption because it does not form an iminium ion with its secondary amine, and binds only through the catechol group. NMR experiments demonstrated that saturating concentrations of epinephrine left the aldehyde intact (see supporting information). Because epinephrine does not form an iminum ion, it is not surprising that binding produces a fluorescence decrease. Most importantly, the binding affinity of NS510 for catecholamines is much larger than that for other amines such as glutamate (over 400 fold). In particular, amino-sugars such as glucosamine do not bind well to NS510, indicating that the binding pocket of the sensor does not match the more compact sugar structure.
Compared to NS521, the quantum yield of sensor NS510 was greatly improved. NS521 has a rather low quantum yield (ϕ=0.0053) which drops even further when norepinephrine binds (ϕ=0.0033). NS521 produces a turn-on response only because the iminium ion can be selectively excited due to the red-shift in absorbance. The sensor-analyte complex of NS521 is actually less fluorescent than the parent sensor. In contrast, NS510 starts with a higher quantum yield due to the quinolone core (ϕ=0.025). More importantly, the quantum yield of the sensor increases upon biding norepinephrine (ϕ=0.031) producing good fluorescence turn-on and overall high fluorescence in the bound state. Thus, the quinolone-based sensor is brighter than NS521.
We utilized bovine adrenal chromaffin cells as a model cell to test the fluorescent catecholamine sensor NS510. Chromaffin cells store norepinephrine and epinephrine in acidic vesicles and release them via exocytosis much like neurons. Subpopulations of chromaffin cells enriched in norepinephrine versus epinephrine can be isolated by centrifugation on a Percoll gradient.13 Live norepinephrine-enriched chromaffin cells were stained and imaged with an Olympus FluoView FV1000 confocal laser scanning inverted microscope. Cells were treated with 0.5 µM NS510 for 45 min at 37 °C and rinsed twice.
Norepinephrine-enriched chromaffin cells were brightly stained by NS510 (Figure 3). Punctate staining of the chromaffin cells suggests that the sensor is labeling the secretory vesicles found throughout the cell where the norepinephrine is present in high concentrations (~ 0.5 M). Next, we imaged labeled chromaffin cells using TIRF microscopy. TIRF imaging selectively illuminates the bottom surface of the cell with the evanescent field that penetrates only ~100 nm past the glass/cell interface. It therefore can better resolve the catecholamine-containing vesicles adsorbed to the bottom surface of the cell without contamination of fluorescence from other regions of the cell. Through-objective TIRF images were obtained using an Olympus IX-80 inverted microscope fitted with an Olympus UAPO 150X oil N.A 1.45 TIRF objective. Images were acquired using a cooled CCD camera (Hamamatsu EM-CCD Digital camera C9100, Japan) controlled by Slidebook software. TIRF imaging revealed distinct fluorescent puncta in norepinephrine-enriched chromaffin cells that are consistent with labeling of granules (Figure S6; see the Supporting Information for movies from which these images were taken). Abrupt, punctate loss of fluorescence were observed upon stimulation with high-K+ solution that are likely to reflect exocytosis of a vesicle containing norepinephrine labeled with 10 µM NS510. In order to validate that punctate drops in NS510 correspond to release of catecholamine from individual vesicles, experiments were performed using an amperometric ring microelectrode patterned underneath the cell being imaged.14 Amperometric microelectrodes immediately adjacent to release sites on the cell surface measure pA-magnitude spikes of current as the catecholamine released from an individual vesicle is oxidized on the surface of the electrode.14–16 Figure 4 presents three examples of amperometric spikes recorded synchronously in time with punctate changes in NS510 fluorescence indicated by the regions of interest. In Example A, note that two release events were recorded, each with a drop in fluorescence in the region of interest presumably due to two nearby vesicles sequentially undergoing exocytosis. Note in Examples B and C that an increase in fluorescence is observed for one frame before a drop, which may be due to dye brightening as it enters the stronger evanescent field on the surface of the coverslip before it diffuses away, as previously observed.17 These data demonstrate that destaining events correspond to exocytotic events, thus NS510 can be used to monitor exocytosis in live cells.
Figure 3.
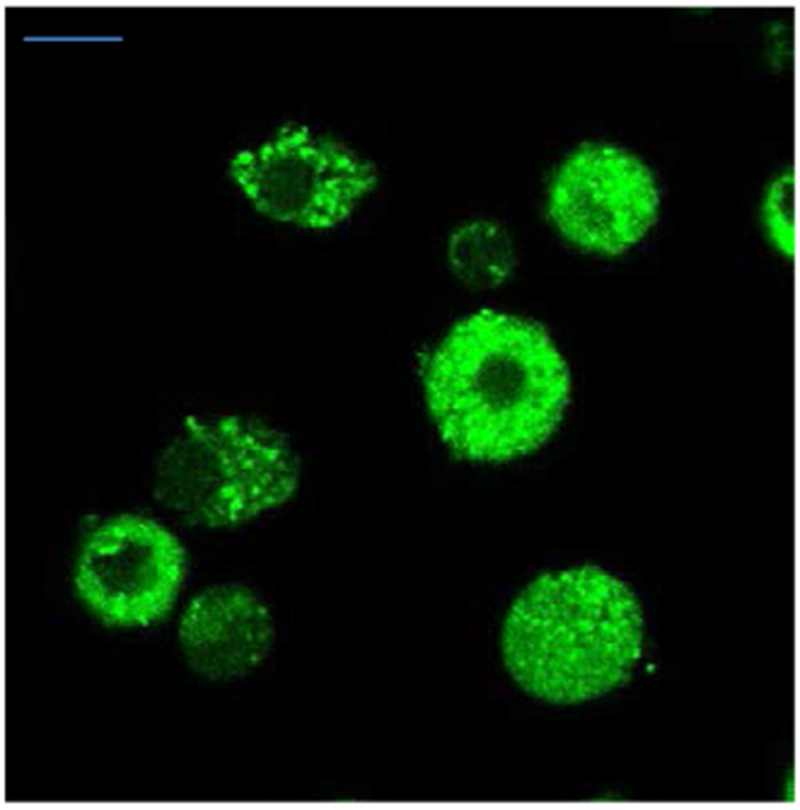
Confocal image of norepinephrine-enriched cells incubated with NS510 (0.5 µM) (λex = 488 nm). (Scale bar = 10 µm)
Figure 4.
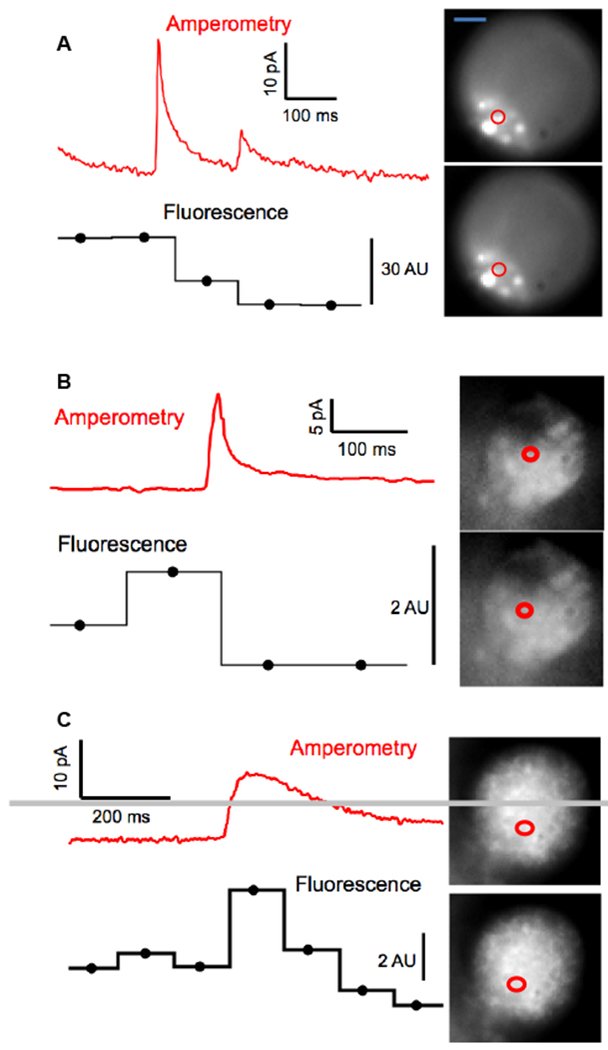
Combination of amperometry with TIRF imaging demonstrates that NS510 can resolve catecholamine release from an individual vesicle. Three recordings from three different bovine adrenal chromaffin cells on an Au ring electrode demonstrate punctate changes in NS510 fluorescence synchronous with amperometric spikes resulting from oxidation of catecholamine release from a single vesicle. Circles depict the regions of interest where changes in fluorescence were observed as quantified in Arbitrary Units (AU) in the horizontal lines below the amperometric traces. (Scale bar = 5 µm)
In conclusion, a fluorescent sensor was designed and synthesized for high affinity for catecholamines and visualizing norepinephrine in live cells. NS510 demonstrated that the quinolone was brighter than the corresponding coumarin and gave punctate staining of secretory vesicles using confocal microscopy. More importantly the boronic acid group of NS510 gave it excellent affinity and selectivity for catecholamines over other amines. Although the senor was somewhat quenched by the catechol group, it could be used to image quantal norepinephrine release in live cells. These data indicate that NS510 is the most selective and highest affinity catecholamine sensor for use in live cells.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (R01 EB020415).
Footnotes
Supporting information
UV/vis and fluorescence titrations of all amines tested.
Synthetic procedures and NMR spectra of all new compounds.
Detailed procedures for the cells studies.
Movies of destaining norepinephrine enriched vesicles
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Akil H, Martone ME, Van Essen DC, Science 2011, 331, 708–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Marc DT, Ailts JW, Ailts Campeau DC, Bull MJ, Olson KJ, Neurosci. Biobehav. Rev 2011, 35, 635–644. [DOI] [PubMed] [Google Scholar]; (b) Chen Y, Zhang K, Wen G, Rao F, Sanchez AP, Wang L, Rodriguez-Flores JL, Mahata M, Mahata SK, Waalen J, Ziegler MG, Hamilton BA and O’Connor DT, Am. J. Hypertens 2011, 24, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Omiatek DM, Dong, Heien ML and Ewing AG, ACS Chem. Neurosci 2010, 1, 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Boyd BW, Witowski SR and Kennedy RT, Anal. Chem 2000, 72, 865–871. [DOI] [PubMed] [Google Scholar]; (b) Deng Y-H, Wang H and Zhang H-S, J. Sep. Sci 2008, 31, 3088–3097. [DOI] [PubMed] [Google Scholar]
- 4.(a) Liu Y, Li M, Zhang F, Zhu A and Shi G, Anal. Chem 2015, 87, 5531–5538. [DOI] [PubMed] [Google Scholar]; (b) Jin S, Li M, Zhu C, Tran V and Wang B, ChemBioChem 2008, 9, 1431–1438. [DOI] [PubMed] [Google Scholar]; (c) Orwar O, Jardemark K, Jacobsen I, Moscho A, Fishman HA, Scheller RH, Zare RN, Science 1996, 272, 1779–1782. [DOI] [PubMed] [Google Scholar]; (d) Jang YJ, Jun JH, Swamy KMK, Nakamura K, Koh HS, Yoon YJ, Yoon J, Bull. Korean Chem. Soc 2005, 26, 2041–2043. [Google Scholar]
- 5.(a) Ge S, Koseoglu S, Haynes CL, Anal. Bioanal. Chem 2010, 397, 3281–3304. [DOI] [PubMed] [Google Scholar]; (b) Gubernator NG, Zhang H, Staal RGW, Mosharov EV, Pereira DB, Yue M, Balsanek V, Vadola PA, Mukherjee B, Edwards RH, Sulzer D, Sames D, Science 2009, 324, 1441–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Rodriguez PC, Pereira DB, Borgkvist A, Wong MY, Barnard C, Sonders MS, Zhang H, Sames D and Sulzer D, Proc. Natl. Acad. Sci 2012, 110, 870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lee M, Gubernator NG, Sulzer D and Sames D, J. Am. Chem. Soc 2010, 132, 8828–8830. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Pereira DB, Schmitz Y, Mészáros J, Merchant P, Hu G, Li S, Henke A, Lizardi-Ortiz JE, Karpowicz RJ Jr, Morgenstein TJ, Sonders MS, Kanter E, Rodriguez PC, Mosharov EV, Sames D and Sulzer D, Nat. Neurosci 2016, 19, 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Secor KE, and Glass TE, Org. Lett 2004, 6, 3727–3730. [DOI] [PubMed] [Google Scholar]; (b) Maue M and Schrader T, Angew. Chem. Int. Ed 2005, 44, 2265–2270. [DOI] [PubMed] [Google Scholar]
- 8.Hettie KS, Liu X, Gillis KD, Glass TE, ACS Chem. Neurosci 2013, 4, 918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gӧrner H, Wolff T, Photochem. Photobiol 2008, 84, 1224–1230. [DOI] [PubMed] [Google Scholar]
- 10.Secor K, Plante J, Avetta C, Glass TE, J. Mater. Chem 2005, 15, 4073–4077. [Google Scholar]
- 11.Hettie KS, Glass TE, ACS Chem. Neurosci 2016, 7, 21–25. [DOI] [PubMed] [Google Scholar]
- 12.Hettie KS, Glass TE, Chem. Eur. J 2014, 20, 17488–17499. [DOI] [PubMed] [Google Scholar]
- 13.(a) Yang Y, Craig TJ, Chen X, Ciufo LF, Takahashi M, Morgan A, Gillis KD, J. Gen. Physiol 2007, 129, 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Moro MA, Leṕez MG, Gandía L, Michelena P; García G. Anal. Biochem 1990, 185, 243–248. [DOI] [PubMed] [Google Scholar]
- 14.Gillis KD, Liu XA, Marcantoni A, Carabelli V, Pflugers Arch. – Eur. J. Physiol 2018, 470, 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DL, Near JA, Diliberto EJ Jr. O. H. Viveros, Proc Natl Acad Sci USA 1991, 88 (23), 10754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou X, Savy A, Maurin S, Grimaud L, Darchen F, Quynton D, Labbé E, Buriez O, Delacotte J, Lemaître F, Guille-Collingnon Angew M. Chem. Int. Ed 2017, 56, 2366–23. [DOI] [PubMed] [Google Scholar]
- 17.Burchfield JG, Lopez JA, Mele K, Vallotton P and Hughes WE Traffic 2010, 11, 429–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.