Abstract
PURPOSE:
UGT2B17 gene deletion (UGT2B17*2) has been reported to affect bone health as well as the pharmacokinetics of aromatase inhibitor (AI) drugs such as exemestane. The goal of this study was to assess associations between UGT2B17 gene deletion and bone health prior to and after 24 months of AI treatment in postmenopausal women with hormone receptor positive (HR+) breast cancer.
METHODS:
Bone health in women with HR+ breast cancer enrolled on the prospective randomized Exemestane and Letrozole Pharmacogenetics (ELPh) trial was determined by measuring bone turnover markers (BTM) and bone mineral density (BMD) pre-treatment and after 3 (BTM) and 24 (BMD) months of treatment with either the steroidal AI exemestane or the nonsteroidal AI letrozole. DNA samples were genotyped for UGT2B17*2.
RESULTS:
Of the 455 subjects included in the analyses, 244 (53.6%) carried at least one copy of UGT2B17*2. UGT2B17*2 was associated with lower pre-treatment BMD at the hip (P = 0.01) and spine (P = 0.0076). Letrozole treatment was associated with a greater decrease in BMD of the hip (P = 0.03) and spine (P = 0.03) than exemestane. UGT2B17 genotype was not associated with changes in BMD from 24 months of AI treatment, though in UGT2B17*2 homozygous patients, there was a trend toward greater decreases in BMD of the spine from treatment with letrozole compared with exemestane (P = 0.05).
CONCLUSION:
UGT2B17*2 may be associated with lower baseline BMD in women with HR+ breast cancer. Exemestane is less detrimental to bone health than letrozole in postmenopausal women treated with AI, and this effect may be confined to patients carrying UGT2B17*2, though this finding requires independent validation.
Keywords: Hormone receptor positive Breast Cancer, Endocrine therapy, Bone Health, UGT2B17
Introduction
Hormone receptor positive (HR+) breast cancers are the most common type of breast cancer and a leading cause of cancer death among women worldwide [1]. Aromatase inhibitors (AI) are superior to tamoxifen [2–5] and are the gold standard for the treatment of postmenopausal women with HR+ breast cancer. AIs work by inhibiting aromatase, the rate-limiting enzyme responsible for the production of estrogens from androgens in postmenopausal women [6]. There is concern that despite the potential for additional benefit from AI therapy there could be detrimental effects of long-term therapy, including negative effects on bone health [7] such as osteoporosis and fractures [8].
There are two classes of third generation AI medications, steroidal (exemestane) and nonsteroidal (anastrozole and letrozole). Preclinical evidence suggests that exemestane may have less detrimental effects on bone health compared to nonsteroidal AIs. In ovariectomized rats, exemestane but not letrozole treatment prevented bone loss [9]. Some clinical studies have similarly shown that women receiving nonsteroidal AI have significantly worse bone mineral density (BMD) than those treated with exemestane [8, 10–12]. However, not all patients have preserved BMD with exemestane or bone loss with nonsteroidal AI therapy. Therefore, the purpose of this study was to investigate a potential mechanism underlying this variability in BMD change in AI-treated patients.
UGT2B17 is an isoform of the UDP-glucuronosyltransferases (UGTs) superfamily of phase II detoxification enzymes that catalyze the glucuronidation of a variety of compounds including steroid hormones (androgens and estrogens) [13]. UGT2B17 is polymorphic, with expression controlled by one main genetic variant [UGT2B17 gene deletion or UGT2B17*2] in humans. The minor allele frequency of UGT2B17*2 is approximately 30% in the general population [14].
Our recent pharmacogenetic study conducted in healthy postmenopausal women demonstrated that UGT2B17*2 is associated with the disposition of the steroidal AI exemestane [16].We found that there were statistically significant differences in the total plasma 17-hydroexemestane concentrations and urine 17-hydroexemestane concentrations between UGT2B17 genotype groups [15]. Specifically, we observed an almost 8-fold difference in the AUC and Cmax of conjugated 17-hydroexemestane between UGT2B17 genotype groups (UGT2B17*1/*1 vs UGT2B17*2/*2) [15]. To our knowledge, associations between UGT2B17*2 and bone health in women with HR+ breast cancer receiving steroidal versus nonsteroidal AI have not been investigated. The goal of this study was to assess associations between UGT2B17*2 and bone health in postmenopausal women with HR+ breast cancer prior to and during administration of the steroidal AI exemestane versus the nonsteroidal AI letrozole.
Methods
Study design and patient selection
The details of this study’s design and conduct including a detailed CONSORT diagram have previously been published [7]. Briefly, postmenopausal women with HR+ breast cancer who were starting AI therapy were recruited between 2005 and 2009 and enrolled in the Exemestane and Letrozole Pharmacogenetics (ELPh) trial ( NCT00228956 (https://register.clinicaltrials.gov/). Surgery, chemotherapy, and radiation therapy were completed before enrollment. Following enrollment, women were randomly assigned to endocrine therapy with the steroidal AI exemestane (25 mg) or nonsteroidal AI letrozole (2.5 mg) daily for two years. Randomization was stratified based on prior adjuvant tamoxifen (yes/no), prior chemotherapy (yes/no), and current bisphosphonate therapy (yes/no). Vitamin D and calcium intake either in the diet or as a supplement was advised per current clinical practice, but use was not recorded. Patients taking bisphosphonate therapy were included in this analysis. The protocol was approved by the Institutional Review Boards of all participating study sites, and all enrolled patients provided written informed consent.
Bone mineral density (BMD) and bone turnover marker (BTM) Data Collection
BMD was measured in the left hip and lumbar spine at baseline and 24 months after AI treatment initiation using dual-energy x-ray absorptiometry (DXA) and converted to T-scores as described previously [7]. Fasting blood and urine specimens were collected at baseline and after three months of AI treatment for measurement of biochemical BTMs including serum bone-specific alkaline phosphatase (BAP) and urinary type I cross-linked N telopeptides (NTx). BAP and NTx were analyzed using enzyme-linked immunoassay (ELISA) kits (Quidel® Corporation, San Diego, CA) as previously described [7]. NTX concentrations were corrected for urine dilution by adjusting for the corresponding urine creatinine concentrations.
UGT2B17 Genotyping
Whole blood was collected at baseline for DNA which was extracted from whole blood using QIAamp DNA Blood Maxi Kit (Qiagen, Inc., Valencia, CA), as previously described [15, 23]. Samples were quantified and the purity was assessed using a NanoDrop ® 1000 spectrophotometer (Thermo Fisher, Waltham, MA). DNA samples were diluted to 10ng/μl for genotyping. UGT2B17*2 (gene deletion) genotyping was carried out as described previously using real-time polymerase chain reaction (RT-PCR) with allelic discrimination [15]. Each reaction well included two primers and one FAM-labeled probe to amplify exon 1 of UGT2B17, and two primers and one 6-JOE-labeled probe (spanning the deletion cut site) that amplify only in the presence of UGT2B17*2. Allelic discrimination was accomplished by RTPCR amplification performed with the Roche LightCycler 480 detection system (Indianapolis, IN, USA) with the following conditions: 50°C for 2 min, then 95°C for 15 min, followed by 50 cycles of amplification at 95°C for 1 min and 60°C for 90 sec. Data were analyzed with the LightCycler 480 software version 1.5 (Indianapolis, IN, USA). UGT2B17*1/*1 (NA11993), UGT2B17*1/*2 (NA10861), and UGT2B17*2/*2 (NA12057) provided by Coriell were used as positive quality controls [15]. The genotyping call rate was 100% and distribution of UGT2B17 genotypes were consistent with Hardy–Weinberg equilibrium.
Statistical Analysis
Our a priori defined primary hypothesis was that UGT2B17*2 will be associated with a lesser decrease in BMD during treatment, using an additive genetic model. All analyses were conducted including patients taking bisphosphonates at baseline, followed by a sensitivity analysis excluding these patients. Analyses of associations with baseline BMD and BTM, analyses of associations with change in BTM, or analyses of changes in BMD and BTM within AI-treated subsets were hypothesis-generating. BMD and BTM measures are described with means and standard deviations at baseline and 24 months, and baseline and 3 months, respectively. The influence of baseline demographic and clinical characteristics of study participants, namely, race, prior chemotherapy use, prior tamoxifen use, randomization to letrozole or exemestane, and UGT2B17 genotype on baseline BMD and BTM were assessed using t tests or Kruskal-Wallis tests, as appropriate. The influence of treatment arm (letrozole or exemestane) and UGT2B17 genotype on raw change in BMD and BTM were analyzed using Kruskal-Wallis tests. The comparison between UGT2B17*2/*2 homozygous patients treated with exemestane vs. letrozole was analyzed using t-tests. In the boxplots, Q1 is the first quartile, Q3 is the third quartile, IQR is the middle 50% (interquartile range) and the end of the whiskers are at (Q1 − 1.5*IQR) and (Q3 + 1.5*IQR). Data analyses were performed in SAS v 9.3, Python 3.7.0 and R.0.2 using the SNPassoc and ggplot2 packages.
Results
Study Participants
Four hundred fifty-five subjects were included in this retrospective pharmacogenetic analysis of the prospective observational ELPh clinical trial (Supplementary Fig. 1.). Baseline characteristics of study participants are reported in Table 1. Study participants had an average age of 59, the majority were white (88.3%), their average BMI was 29.9 kg/m2 and 83% were taking bisphosphonates. Prior to enrollment, 45.3% and 36.2% of study participants received tamoxifen and chemotherapy, respectively. At baseline, the mean BMD T-score was 0.16 at the hip and 0.12 at the spine, and the mean BTM values were BAP=23.06 and NTx=347.28. Two hundred twenty-nine patients received letrozole and 226 received exemestane. Out of the 455 subjects, 211 (46.4%) were carriers of UGT2B17*1/*1 genotype, 199 (43.7%) were carriers of UGT2B17*1/*2 genotype, and 45 (9.9%) were homozygous for UGT2B17*2 (Table 1).
Table 1.
Baseline characteristics of study participants
| n | Mean(std) or % | ||
|---|---|---|---|
| Age | Years | 455 | 59.2 (8.79) |
| Race | White | 402 | 88.35% |
| Black | 41 | 9.01% | |
| Other | 12 | 2.64% | |
| BMI | kg/m2 | 454 | 29.95(6.48) |
| Chemotherapy | Yes | 206 | 45.27% |
| No | 249 | 54.73% | |
| Tamoxifen | Yes | 164 | 36.20% |
| No | 289 | 63.80% | |
| Aromatase Inhibitor | Letrozole | 229 | 50.33% |
| Exemestane | 226 | 49.67% | |
| Bisphosphonate Use | Yes | 78 | 17.14% |
| No | 377 | 82.86% | |
| UGT2B17 Genotype | *1/*1 | 211 | 46.37% |
| *l/*2 | 199 | 43.74% | |
| *2/*2 | 45 | 9.89% | |
| Bone mineral density | Hip (T-score) | 452 | 0.16 (6.83) |
| Spine (T-score) | 454 | 0.12 (6.76) | |
| Bone Turnover Markers | BAP (U/L) | 416 | 23.06 (10.41) |
| NTx (nM/mM) | 301 | 347.28 (298.02) |
Effects of UGT2B17 and Patient Characteristics on Baseline BMD
At baseline, UGT2B17*2 was associated with lower BMD of the hip (mean T-score UGT2B17*1/*1: 0.75, UGT2B17*1/*2: −0.37, UGT2B17*2/*2: −0.17, P = 0.01) and spine (UGT2B17*1/*1: 0.80, UGT2B17*1/*2: −0.35, UGT2B17*2/*2: −0.85, p = 0.0076, Table 2, Fig. 1.). None of the other clinical variables (race, prior chemotherapy or tamoxifen, treatment arm) were significantly associated with BMD at baseline (all p>0.05, Table 2).
Table 2.
Effects of Demographic and Clinical Characteristics (including UGT2B17) on Baseline BMD and BTM
| S | Bone Mineral Density (T-score) | Bone Turnover Markers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hip | Spine | Serum BAP | Urine NTx | ||||||
| mean (std) | p-value | mean (std) | p-value | mean (std) | p-value | mean (std) | p-value | ||
| Race | White | 0.19 (7.20) | 0.97 | 0.25 (7.21) | 0.08 | 22.81 (10.53) | 0.18 | 349.51 (305.99) | 0.29 |
| Black | −0.33 (0.66) | −0.62 (1.26) | 24.64 (10.28) | 356.72 (267.16) | |||||
| Other | 1.37 (7.43) | −1.11 (1.25) | 25.55 (8.05) | 281.61 (152.73) | |||||
| Chemo | Yes | 0.27 (7.20) | 0.54 | 0.16 (7.10) | 0.73 | 24.17 (11.11) | 0.06 | 363.83 (278.39) | 0.12 |
| No | 0.10 (6.58) | 0.11 (6.54) | 22.09 (9.80) | 334.87 (315.44) | |||||
| Tamoxifen | Yes | 0.31 (7.84) | 0.4 | 0.26 (7.91) | 0.88 | 21.46 (9.37) | 0.04 | 320.43 (289.54) | 0.12 |
| No | 0.10 (6.28) | 0.07 (6.11) | 23.80 (10.75) | 361.89 (303.65) | |||||
| Drug | Letrozole | 0.29 (6.98) | 0.59 | 0.19 (6.80) | 0.7 | 23.41 (11.29) | 0.96 | 379.27 (324.50) | 0.08 |
| Exemestane | 0.06 (6.76) | 0.07 (6.80) | 22.67 (9.55) | 317.73 (269.43) | |||||
| UGT2B17 Genotype | *1/*1 | 0.75 (9.79) | 0.01 | 0.80 (9.80) | 0.0076 | 22.05 (9.39) | 0.35 | 330.34 (271.60) | 0.66 |
| *1/*2 | −0.37 (0.99) | −0.35 (1.56) | 23.73 (11.40) | 370.37 (340.39) | |||||
| *2/*2 | −0.17 (4.05) | −0.85 (1.15) | 24.54 (10.52) | 331.66 (222.16) | |||||
Bold denotes associations significant at the p<0.05 level.
Fig. 1.
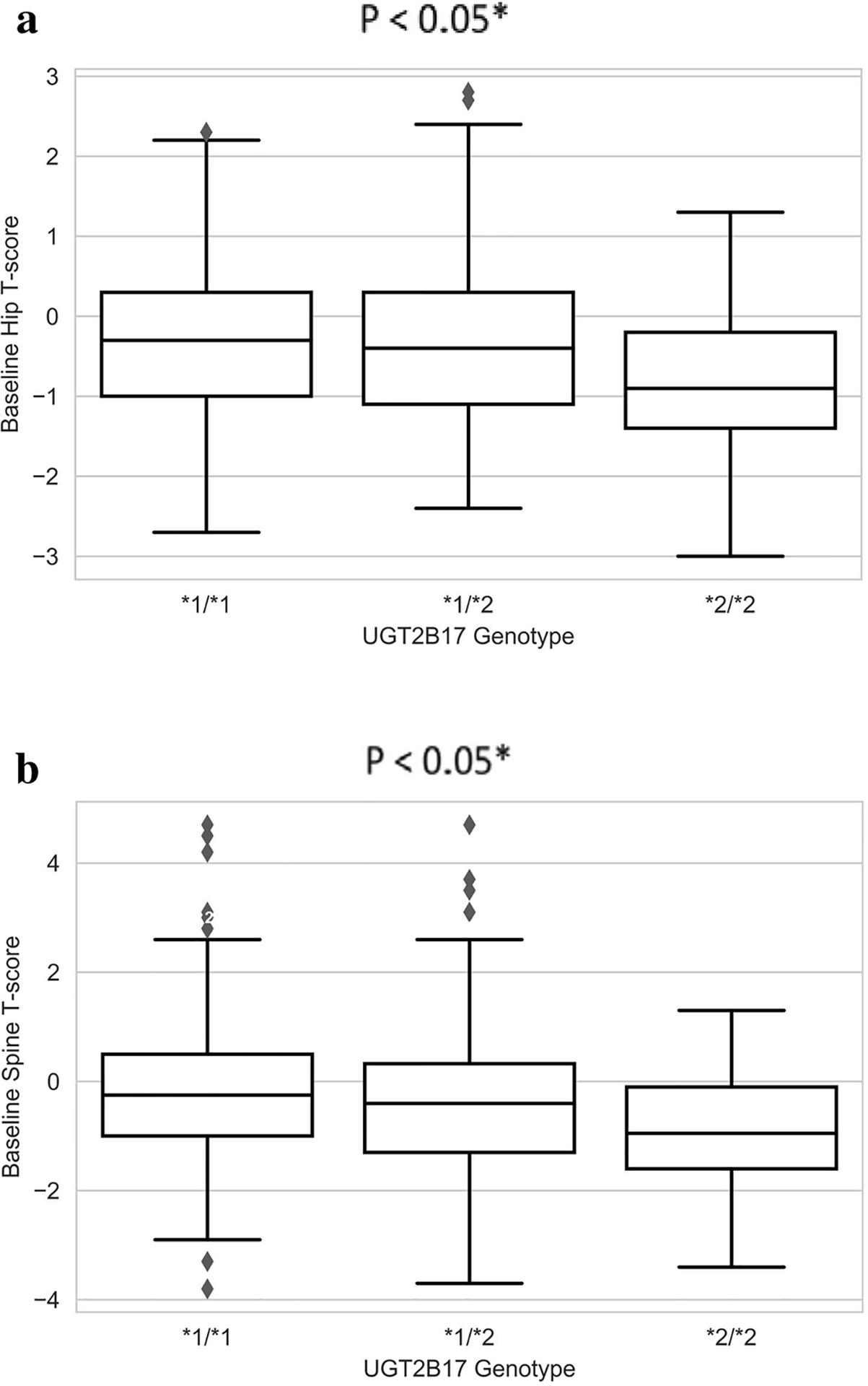
Baseline BMD stratified by UGT2B17 genotype. Box and whisker plots of baseline BMD in hip (A) and spine (B) stratified by UGT2B17 genotype.
Effects of AI Treatment and UGT2B17 genotype on Change in BMD
Letrozole caused a greater decrease than exemestane in BMD of the hip (mean T-score change letrozole: −0.27, exemestane: −0.17, p=0.03) and spine (letrozole: −0.68, exemestane: −0.17, p=0.03, Table 3). There were no significant differences in change in BMD from AI treatment between UGT2B17 genotype groups (hip: p=0.24, spine: p=0.10).
Table 3.
Effects of UG2B17 and AI treatment on Changes in BMD and BTM
| Change in Bone Mineral Density between baseline and 24 months (T-score) | Change in Bone Turnover Markers between baseline and 3 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hip | Spine | Serum BAP | Urine NTx | ||||||
| mean (std) | p-value | mean (std) | p-value | mean (std) | p-value | mean (std) | p-value | ||
| Aromatase Inhibitor | Letrozole | −0.68 (2.85) | 0.03 | −0.27 (0.49 | 0.03 | 0.10 (5.62) | 0.72 | −6.62 (345.11) | 0.02 |
| Exemestane | −0.17 (1.12) | −0.17 (0.53) | −0.05 (4.82) | 75.90 (350.39) | |||||
| UGT2B17 Genotype | *1/*1 | −0.43 (2.18) | 0.24 | −0.20 (0.56) | 0.10 | 0.35 (4.25) | 0.21 | 47.90 (319.30) | 0.25 |
| *1/*2 | −0.27 (0.40) | −0.16 (0.46) | −0.32 (6.30) | 37.62 (395.48) | |||||
| *2/*2 | −1.32 (5.30) | −0.30 (0.50) | 0.12 (3.79) | −24.52 (270.96) | |||||
Bold denotes associations significant at the p<0.05 level.
Effects of UGT2B17 genotype on BMD within AI Arms
Since letrozole and exemestane were associated with differing magnitudes of BMD change, a secondary subset analysis was conducted to assess the impact of UGT2B17*2 on BMD changes within each AI group. In the exemestane group, there was a trend toward less decrease in spine BMD for UGT2B17*2/*2 compared to the other genotype groups (mean T-score change UGT2B17*1/*1: −0.24, UGT2B17*1/*2: −0.11, UGT2B17*2/*2: −0.04, p=0.14, Table 4, Fig. 2.), although no similar trend was seen in hip BMD (p=0.58). Among UGT2B17*2/*2 patients, treatment with letrozole was associated with a trend toward greater decreases in spine BMD compared to exemestane (−0.43 vs. −0.04, p=0.05, Fig. 3.), but again no similar trend was seen in hip BMD (p=0.43).
Table 4.
Effects of UGT2B17 gene deletion on changes in BMD between baseline and 24 months within each AI Arm.
| UGT2B17 *1/*1 | UGT2B17 *l/*2 | UGT2B17 *2/*2 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | mean (SD) | n | mean (SD) | n | mean (SD) | p-value | ||
| Letrozole | Hip | 55 | −0.79 (2.69) | 70 | −0.28 (0.45) | 18 | −1.89 (6.46) | 0.49 |
| Spine | 56 | −0.31 (0.57) | 70 | −0.19 (0.45) | 19 | −0.43 (0.36) | 0.85 | |
| Exemestane | Hip | 61 | 0.10 (1.52) | 49 | −0.26 (0.33) | 9 | −0.17 (0.48) | 0.58 |
| Spine | 60 | −0.24 (0.56) | 51 | −0.11 (0.48) | 9 | −0.04 (0.66) | 0.14 | |
Fig. 2.
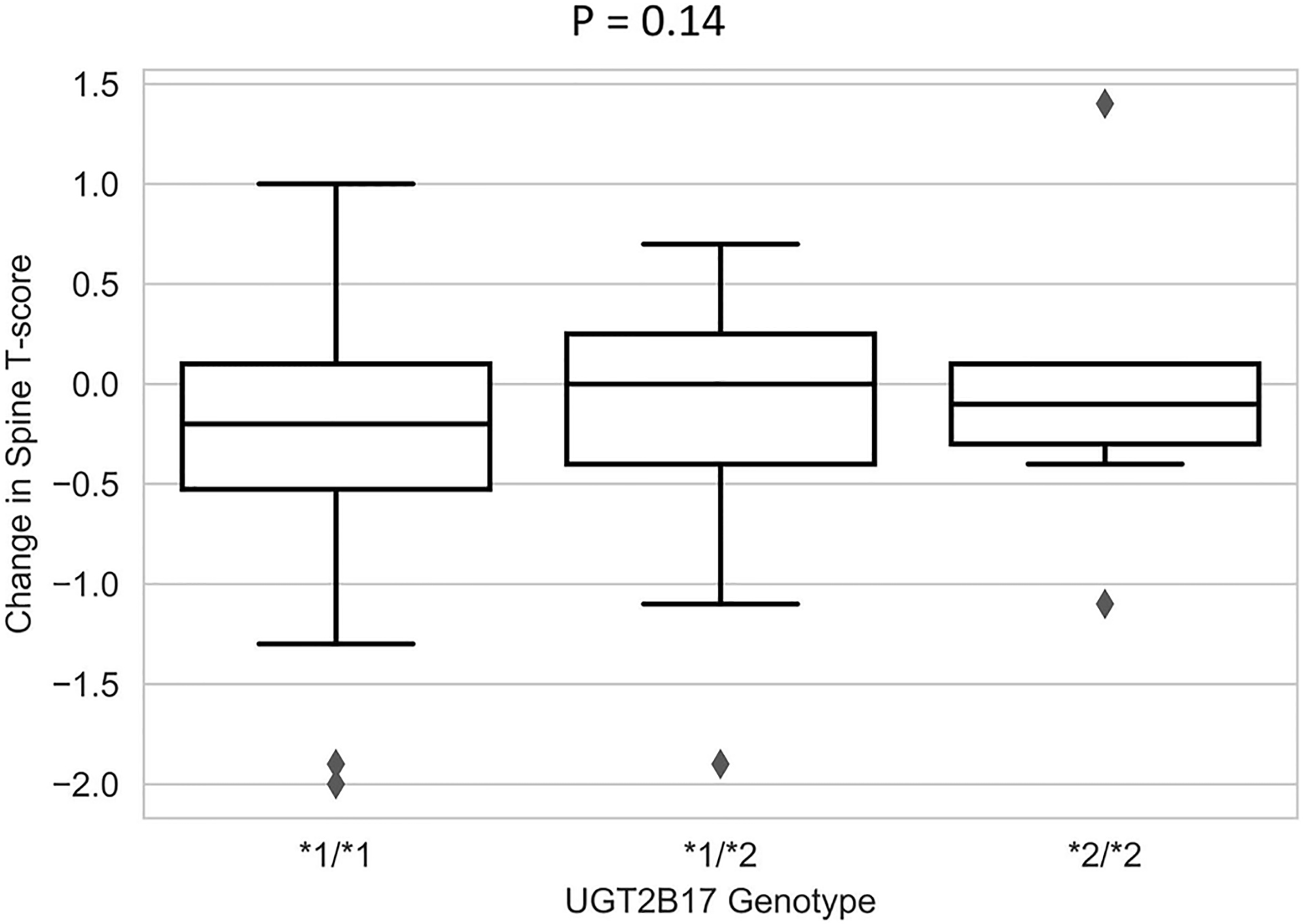
Association between UGT2B17 genotype and change in spine BMD T-score between baseline and 24 months in patients treated with exemestane. Box and whisker plots of changes in spine BMD in patients receiving exemestane stratified by UGT2B17 genotypes.
Fig. 3.
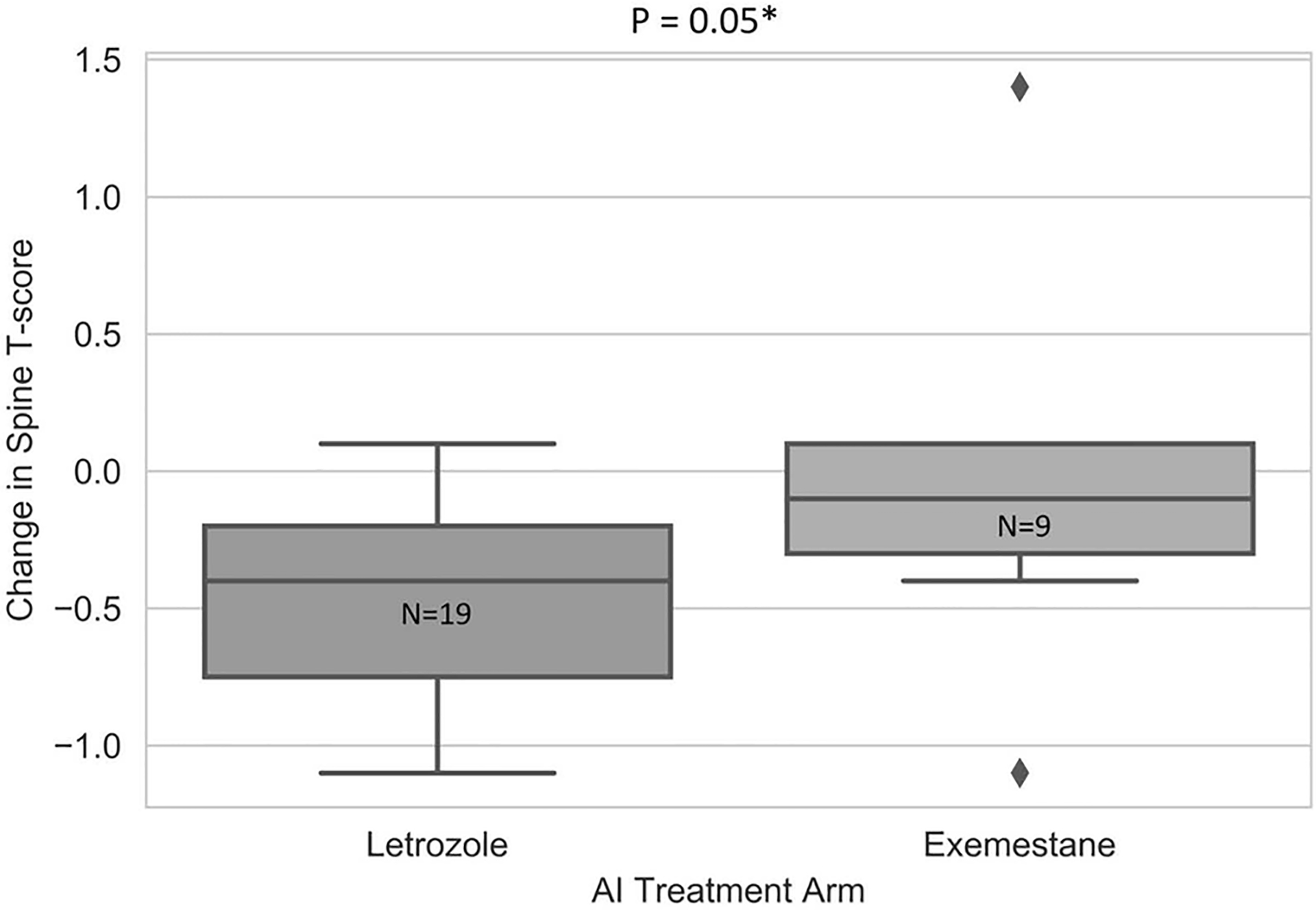
Change in spine BMD T-scores between baseline and 24 months in letrozole- and exemestane- treated patients homozygous for UGT2B17*2 gene deletion. Box and whisker plots of changes in BMD in spine in patients with UGT2B17*2/*2 stratified by AI.
Associations with BTM at Baseline and Changes from AI Treatment
Patients who received prior tamoxifen treatment had a nominally smaller increase in serum BAP during AI treatment (23.80 vs. 21.46, p=0.04, Table 2). None of the other clinical or genetic variables were associated with BTM (all p>0.05). As previously reported, exemestane was associated with an increase in NTx but letrozole was not (exemestane 75.90, letrozole −6.62, p=0.02) [7]. There was no association of change in BAP with UGT2B17 genotype (Table 3).
Discussion
In this pharmacogenetic study using samples derived 455 postmenopausal women with HR+ early stage breast cancer treated with AI therapy on the prospective randomized ELPh trial we demonstrate statistically significant association between UGT2B17*2 gene deletion and lower BMD in the hip and spine prior to AI initiation. In addition, we found that compared to letrozole, exemestane may be less detrimental to bone health and this effect may be confined to women homozygous for UGT2B17*2.
To our knowledge, this is the first study to assess the association between UGT2B17*2 and changes in bone health (BMD and BTM) with a steroidal (exemestane) or a nonsteroidal (letrozole) AI in postmenopausal women with HR+ early stage breast cancer. We did not find evidence supporting our pre-specified primary hypothesis that patients carrying UGT2B17*2 would have a lesser decrease in BMD during AI therapy, and there was overall no association between UGT2B17*2 and changes in BMD or BTM with AI therapy. However, in an exploratory analysis, exemestane appeared to be less detrimental to bone health (BMD of the spine) than letrozole in women carrying the UGT2B17*2. If this association is validated in independent patient cohorts, these data may support the preferred use of exemestane in patients with pre-existing osteoporosis or at high risk for developing osteoporosis. Although our results were not meaningfully changed by excluding patients taking bisphosphonates at baseline, it will also be important to examine the impact of bisphosphonate therapy on the phenotype-genotype association, since bisphosphonates are commonly used to reduce bone loss and fracture risk in this patient population.
Whereas our study found that UGT2B17*2 was associated with lower baseline BMD, previous investigators reported higher BMD in these patients [16–18]. UGT2B17*2 is expected to result in decreased metabolism of steroidal hormones such as testosterone and estradiol, leading to higher hormone concentrations and increased BMD [18]. The reasons for this discrepancy are not quite clear; in a post-hoc subset analysis no relationship was found between UGT2B17 genotypes and co-medication with bisphosphonates.
Nonsteroidal and steroidal AIs result in similar reductions in disease recurrence and have similar toxicity profiles [19]. However, individual patients may tolerate one AI better than another, although the mechanism underlying this difference is not clear [20–23]. There are no predictors for which AI will be tolerated by a patient, which is important for making personalized treatment decisions, though inherited genetic variants that result in differences in drug metabolism or activity may play a role [20–23].
One key strength of this analysis is it uses samples and data collected on the prospective randomized ELPh trial, in which patients underwent rigorously performed DXA scans using standardized protocols and longitudinal cross-calibration across study sites. Bone densitometry data were analyzed in a blinded fashion at a centralized laboratory. Study limitations include the relatively small sample size for the phenotype–genotype association study. There was a high rate of missing data at the 24-month time point due to high rates of treatment discontinuation due to toxicity. In addition, patients were encouraged to consume calcium and vitamin D but intake was not recorded. Finally, participants may have consumed foods or supplements (e.g. red wine) that can inhibit UGT2B17 activity.
In summary, our study confirms that AI cause bone loss and an exploratory analysis suggests that exemestane may cause less bone loss in patients homozygous for UGT2B17*2. Additional well-powered studies are needed to confirm the impact of UGT2B17*2 gene deletion on bone-related toxicity and efficacy from nonsteroidal versus steroidal AIs. Confirmatory replication could lead to selection of endocrine therapy for postmenopausal women with HR+ breast cancer based on UGT2B17 genotype.
Supplementary Material
Supplemental Fig. 1. Consort diagram describing patient flow from trial enrollment to each analysis.
Acknowledgments
Sources of funding
This work was supported in part by the Arkansas INBRE program, supported by grant funding from the National Institute of Health (NIH) National Institute of General Medical Sciences (NIGMS) (P20GM103429), by the National Institutes of Health (grant numbers U-01GM61373 (DAF), 5T32-GM08425 (DAF, JDR), M01-RR000042 (UM), M01-RR00750 (IU), and M01-RR00052 (JHU)), the Department of Defense (grant number W81XWH-10-1-0349 (JDR), Pfizer (DFH), Novartis Pharma AG (DFH), and the Fashion Footwear Association of New York/QVC Presents Shoes on Sale™ (DFH). Study medication was provided by Pfizer, Inc. and Novartis Pharma AG. We would like to thank the participating patients with breast cancer and the research nurse coordinators at each of the clinical trial sites.
Footnotes
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
VS has received research funding from Novartis and Pfizer. DFH has consulted for Pfizer and received research funding from Pfizer. NLH is the local principal investigator for two Pfizer-sponsored clinical trials. The remaining authors have no conflicts of interest to declare.
References
- 1.Parkin DM, et al. , Global cancer statistics, 2002. CA Cancer J Clin, 2005. 55(2): p. 74–108. [DOI] [PubMed] [Google Scholar]
- 2.Buzdar AU, et al. , Summary of aromatase inhibitor clinical trials in postmenopausal women with early breast cancer. Cancer, 2008. 112(3 Suppl): p. 700–9. [DOI] [PubMed] [Google Scholar]
- 3.Coombes RC, et al. , Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet, 2007. 369(9561): p. 559–70. [DOI] [PubMed] [Google Scholar]
- 4.Keating GM, Letrozole: a review of its use in the treatment of postmenopausal women with hormone-responsive early breast cancer. Drugs, 2009. 69(12): p. 1681–705. [DOI] [PubMed] [Google Scholar]
- 5.Robinson A, A review of the use of exemestane in early breast cancer. Ther Clin Risk Manag, 2009. 5(1): p. 91–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Ingle JN, Postmenopausal women with hormone receptor-positive breast cancer: balancing benefit and toxicity from aromatase inhibitors. Breast, 2013. 22 Suppl 2: p. S180–3. [DOI] [PubMed] [Google Scholar]
- 7.Oesterreich S, et al. , Associations between genetic variants and the effect of letrozole and exemestane on bone mass and bone turnover. Breast Cancer Res Treat, 2015. 154(2): p. 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien AJ and Goss PE, Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol, 2006. 24(33): p. 5305–12. [DOI] [PubMed] [Google Scholar]
- 9.Rabaglio M, et al. , Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1–98 trial. Ann Oncol, 2009. 20(9): p. 1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goss PE, et al. , The steroidal aromatase inhibitor exemestane prevents bone loss in ovariectomized rats. Bone, 2004. 34(3): p. 384–92. [DOI] [PubMed] [Google Scholar]
- 11.Cheung AM, Tomlinson G, and Goss PE, Bone loss with exemestane: Is the jury still out? J Clin Oncol, 2005. 23(36): p. 9433–4; author reply 9433–5. [DOI] [PubMed] [Google Scholar]
- 12.Goss PE, et al. , Effects of steroidal and nonsteroidal aromatase inhibitors on markers of bone turnover in healthy postmenopausal women. Breast Cancer Res, 2007. 9(4): p. R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lonning PE, et al. , Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol, 2005. 23(22): p. 5126–37. [DOI] [PubMed] [Google Scholar]
- 14.Wilson W 3rd, et al. , Characterization of a common deletion polymorphism of the UGT2B17 gene linked to UGT2B15. Genomics, 2004. 84(4): p. 707–14. [DOI] [PubMed] [Google Scholar]
- 15.Chen SM, et al. , Impact of UGT2B17 Gene Deletion on the Pharmacokinetics of 17-hydroexemestane in Healthy Volunteers. J Clin Pharmacol, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chew S, et al. , Homozygous deletion of the UGT2B17 gene is not associated with osteoporosis risk in elderly Caucasian women. Osteoporos Int, 2011. 22(6): p. 1981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giroux S, et al. , UGT2B17 gene deletion associated with an increase in bone mineral density similar to the effect of hormone replacement in postmenopausal women. Osteoporos Int, 2012. 23(3): p. 1163–70. [DOI] [PubMed] [Google Scholar]
- 18.Yang TL, et al. , Genome-wide copy-number-variation study identified a susceptibility gene, UGT2B17, for osteoporosis. Am J Hum Genet, 2008. 83(6): p. 663–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goss PE et al. , Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol, 2013. 31(11): p.1398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadakia KC et al. , Prospective assessment of patient-reported outcomes and estradiol and drug concentrations in patients experiencing toxicity from adjuvant aromatase inhibitors. Breast Cancer Res Treat, 2017. 164(2): p411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briot K et al. , Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat, 2010. 120(1): p127–34. [DOI] [PubMed] [Google Scholar]
- 22.Desta Z et al. , Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin Pharmacol Ther, 2011. 90(5): p693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertz DL et al. , Germline genetic predictors of aromatase inhibitor concentrations, estrogen suppression and drug efficacy and toxicity in breast cancer patients. Pharmacogenomics. 2017. 18(5): p481–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Consort diagram describing patient flow from trial enrollment to each analysis.


