Abstract
BACKGROUND:
Studies using continuous access drug self-administration showed that cocaine seeking increases during abstinence (incubation of cocaine craving). Recently, studies using intermittent access self-administration showed increased motivation to self-administer and seek cocaine. We examined whether intermittent cocaine self-administration would potentiate incubation of craving in male and female rats, and the estrous cycle’s role in this incubation.
METHODS:
In Experiment 1, male and female rats self-administered cocaine either continuously (8-h/d) or intermittently (5-min ON, 25-min OFF × 16) for 12 days, followed by relapse tests after 2 or 29 days. In Experiments 2–3, female rats self-administered cocaine intermittently for 6, 12, or 18 sessions. In Experiment 4, female rats self-administered cocaine continuously followed by relapse tests after 2 or 29 days. In Experiments 3–4, the estrous cycle was measured using a vaginal smear test.
RESULTS:
Incubation of cocaine craving was observed in both sexes after either intermittent or continuous drug self-administration. Independent of access condition and abstinence day, cocaine seeking was higher in females than in males. In both sexes, cocaine seeking on both abstinence days was higher after intermittent drug access than after continuous drug access. In females, incubation of craving after either intermittent or continuous drug access was significantly higher during estrus than during non-estrus; for intermittent drug access, this effect was independent of the training duration.
CONCLUSIONS:
In both sexes, intermittent cocaine access caused time-independent increases in drug seeking during abstinence. In females, the time-dependent increase in drug seeking (incubation) is critically dependent on the estrous cycle phase.
Keywords: intermittent access, cocaine self-administration, incubation of craving, sex differences, estrous cycle, relapse
Introduction
Drug addiction is characterized by high relapse rates during abstinence (1, 2). In humans, relapse can be precipitated by exposure to cues associated with drug use (3, 4). Gawin and Kleber proposed that cue-induced cocaine craving progressively increases during abstinence (5). In rats, a similar phenomenon called “incubation of craving” was observed after forced abstinence from cocaine self-administration (6, 7), and other addictive drugs (8–10). Incubation of drug craving during the 6 months of abstinence was recently demonstrated in human cocaine users (11).
In previous incubation studies, cocaine was continuously available for the duration of the daily sessions (9, 12, 13). However, human cocaine users self-administer cocaine intermittently with large drug doses are separated by long intervals between intoxicating events (14), a drug-taking practice that induces spikes of cocaine brain concentrations. Based on these clinical observations, Zimmer et al. (15) developed an intermittent access cocaine self-administration procedure in rats that causes binge-like self-administration behavior and spiking brain cocaine levels (15). In this procedure, rats have 5 min of cocaine access followed by 25 min of timeout during daily sessions (typically 6-h/d) (15–22). Several studies have shown that rats trained under the intermittent access procedure showed strong motivation to take and seek cocaine, as assessed in progressive ratio, resistance to punishment, economic demand, and extinction-reinstatement procedures (15, 18–20, 22–26). Most recently, James et al. (27) showed that in a direct comparison between intermittent and continuous cocaine access, the motivation to take and seek cocaine in these procedures is higher in the intermittent access condition. These authors also showed that cue-induced reinstatement after short or prolonged home-cage forced abstinence was higher in the intermittent access condition but the relevance of these data to incubation of cocaine craving is unknown because there was no evidence for time-dependent increases in cue-induced reinstatement after extinction in either access condition. Thus, it is unknown whether intermittent access cocaine self-administration would potentiate incubation of cocaine craving.
Another factor that can affect incubation of cocaine craving, and more generally relapse to cocaine seeking, is sex (28, 29). Human correlational studies suggest that periods of cocaine abstinence are shorter in women (30–33) and women report stronger craving to cues associated with cocaine than men (33–37). There is also evidence for sex differences in cocaine self-administration and relapse/reinstatement in preclinical studies. Females rats acquire cocaine self-administration at a faster rate than males (38, 39), exhibit a higher cocaine-primed reinstatement response than males (40, 41), and show stronger incubation of cocaine craving (29).
Sex differences in cocaine relapse may be influenced by the estrous cycle and ovarian hormones. In human studies, women have a decrease in desire (craving) to smoke cocaine during the luteal phase (42, 43). The luteal phase is characterized by a high level of progesterone, which decreases cocaine’s subjective effects (42, 44), suggesting a protective effect of progesterone. Preclinical studies in rats report similar effects. Female rats in estrus show higher cocaine seeking after periods of abstinence (29, 45), extinction (40), and cocaine-induced reinstatement (46, 47) than females in non-estrus or males. Suppression of endogenous hormones (estrogen and progesterone) by ovariectomy (OVX) decreases cocaine-induced reinstatement compared to sham rats, while chronic estradiol treatment in OVX rats restores cocaine reinstatement to levels similar to that of sham rats (48, 49). Moreover, the role of sex and ovarian hormones in cocaine’s behavioral effects have been demonstrated using conditioned place preference (CPP) and locomotor sensitization procedures. Female rats show higher locomotor activity for acute and repeated cocaine administration than males (50–53) and exhibit a CPP at a low cocaine dose that does not cause CPP in males (54). In OVX female rats, estradiol treatment increases the magnitude of cocaine CPP and locomotor sensitization (54–57). Finally, in choice self-administration procedures (cocaine vs. food), females are more likely to choose cocaine than males (6, 58, 59) and estradiol treatment increases cocaine choice in both gonadectomized male and female rats (60, 61). Together, the data suggest that in both human and rodent models, the estrous cycle contributes to cocaine taking and seeking.
In Experiment 1 we found that intermittent cocaine access caused stronger incubation of craving than continuous cocaine access, an effect that was more pronounced in female rats. However, in Experiment 2 we did not observe the incubation effect in female rats trained under the intermittent access condition, suggesting that we overlooked key factors regulating incubated cocaine craving in females. Therefore, in Experiment 3 we investigated two possible factors: estrous cycle phase and training duration. We found that after intermittent access cocaine self-administration, incubation of cocaine craving is critically dependent on the estrous cycle’s phase but not on training duration. Therefore, in Experiment 4 we determined the generality of this effect to female rats trained under the continuous cocaine access condition.
Methods and Materials
Subjects:
see supplemental online material
Experiment 1: Effect of intermittent access cocaine self-administration on incubation of cocaine craving in male and female rats
The goal of Experiment 1 was to determine whether intermittent cocaine access will increase incubation of cocaine craving and whether there are sex differences in this effect.
Cocaine self-administration
Rats were trained to self-administer cocaine in operant conditioning chambers equipped with two levers, two cue lights above the levers, a tone, and a houselight. Each session started by inserting the two levers and illuminating the houselight. Pressing on the active lever delivered a cocaine infusion (0.1 ml/3.5 s; 0.75 mg/kg body weight/infusion) and a compound tone-light cue for 3.5 s, followed by a 3.5-s timeout during which lever pressing was not reinforced. Pressing on the inactive lever had no programmed consequence. The rats were first trained to self-administer cocaine on a fixed-ratio 1 (FR1) schedule over 4 days for 2-h/d (max infusions=20). Next, the rats self-administered cocaine either continuously or intermittently for 8-h/d for 12 days. In the continuous access condition, the rats had free access to the drug during the daily sessions. In the intermittent access condition, the rats had access to cocaine during 16 5-min ON periods that were separated by 25-min OFF periods (15), corresponding to 80-min of cocaine access during the 8-h daily session. At the onset of each 5-min ON period the lever extended and the houselight was turned on; at the end of the 5-min access period the levers retracted and the houselight was turned off.
Abstinence phase
During this phase, the rats were housed in the animal facility and were handled 2 times per week.
Relapse test
The rats tested on abstinence day 2 or 29 were matched for their cocaine intake during training (intermittent access: n=25 males/n=24 females; continuous access: n=27 males/n=23 females). On test day, the rats were placed in the same chambers where they previously self-administered cocaine. The relapse test was conducted under extinction conditions in the presence of the drug-associated cues during a single 3-h session. The session began with the illumination of the houselight and the extension of the active and inactive levers. Active lever presses resulted in contingent presentations of the tone/light cue for 3.5-s, but no cocaine, while inactive lever presses had no programmed consequence. The number of active lever presses is the operational measure of drug seeking in incubation studies (8, 10, 62).
Experiment 2: Independent replication
The original goal of Experiment 2 was to replicate the data in Experiment 1 and to characterize Fos expression in different brain areas during relapse tests performed 2 or 29 days after intermittent access cocaine self-administration in female rats. The procedures for cocaine self-administration, abstinence, and relapse tests were as in Experiment 1, except that the relapse tests lasted 90 min, an optimal time for detecting Fos protein expression in the brain.
Experiment 3: Effect of duration of self-administration training and estrous cycle on incubation of craving in female rats after intermittent access cocaine self-administration
Experiment 2 did not replicate the strong incubation effect in female rats after intermittent cocaine access observed in Experiment 1. Therefore, in Experiment 3 we investigated two possible factors: estrous cycle phase and training duration. We manipulated the amount of cocaine intake by training the female rats for 6, 12, or 18 sessions.
Cocaine self-administration and abstinence phase
Female rats went through the same procedures of self-administration, abstinence, and relapse tests (3 h) as described in Experiment 1, except that the number of intermittent self-administration sessions varied, and the estrous cycle was monitored after the relapse tests by vaginal swab. The female rats self-administered cocaine for either 6 (n=16), 12 (n=14), or 18 sessions (n=16) and tested for relapse on either abstinence days 2 or 29. These rats received vaginal cytological tests (see Supplemental online material).
Experiment 4: Effect of the estrous cycle on incubation of craving in female rats after continuous access cocaine self-administration
The goal of Experiment 4 was to examine whether the estrous cycle contributes to incubation of craving after continuous access cocaine self-administration.
Cocaine self-administration and abstinence phase
Female rats (n=46) went through the same procedures as described in Experiment 1 for continuous access cocaine self-administration, abstinence, and relapse tests (3 h). Additionally, these rats received vaginal cytological tests (see Supplemental online material).
Statistical analyses:
Results
Experiment 1: Effect of intermittent cocaine self-administration on incubation of cocaine craving in male and female rats
Cocaine self-administration
During the acquisition phase in sessions 1–4 (2 h/d continuous access) there were no sex differences in the number of infusions, the frequency of infusions (infusions/min), and active and inactive lever presses (Figure 1B–E). Results showed that escalation of cocaine intake of days was observed under both access conditions, that total daily drug intake and the number of active lever presses were higher in the continuous access condition, that infusion rate per min and the number of inactive lever presses were higher in the intermittent access condition, and that there were no sex differences in drug self-administration under either access condition. The mixed ANOVA analysis for total drug intake, which included the between-subjects factors of Access condition (intermittent, continuous) and Sex (male, female), and the within-subjects factor of Session showed significant effects of Access Condition (F1,95=153.8, p<0.001) and Session (F11.1045=80.7, p<0.001) but no effect of Sex (p>0.1). There was also a significant Access Condition × Session interaction (F11,1045=5.9, p<0.001) due to stronger escalation in the continuous access condition. The analysis of infusion rate showed significant effects of Access Condition (F1,95=434.4, p<0.001) and Session (F11,1045=48.2, p<0.001) but no effects of Sex (p>0.1). The statistical results of active and inactive lever presses are described in Table S1.
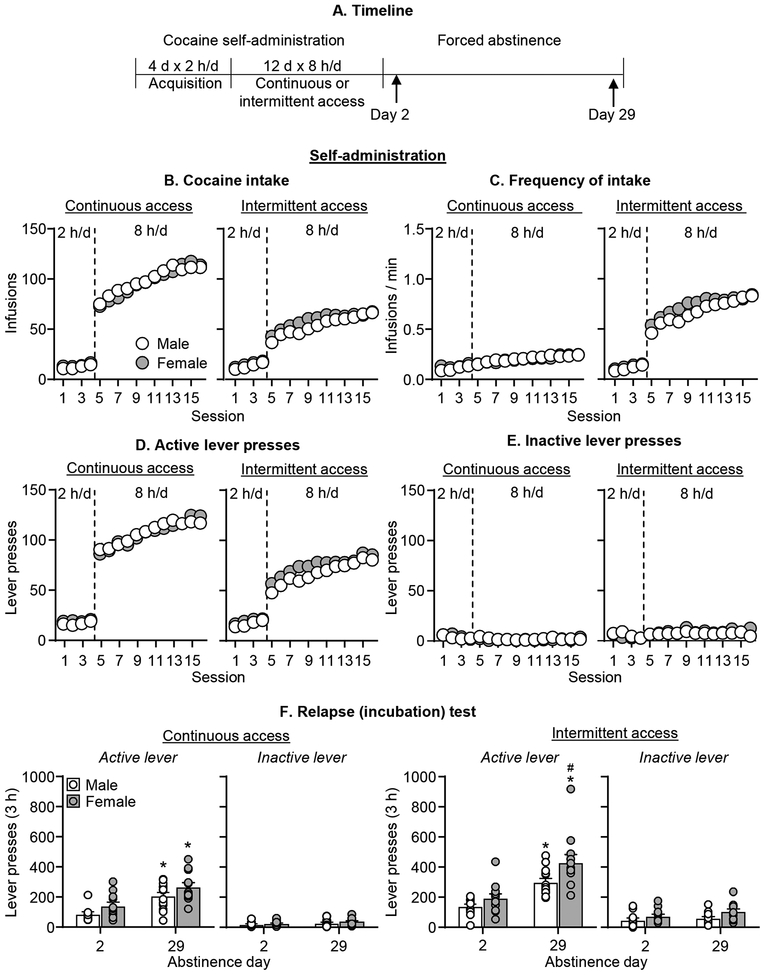
Figure 1. Continuous and intermittent cocaine self-administration and cocaine seeking for male and female rats.
(A) Timeline of the experiment. (B) Total cocaine intake. Mean ± SEM number of infusions per session (Continuous access: male, n=27; female, n=23. Intermittent access: male, n = 25; female, n = 24). (C) Frequency of intake. Mean ± SEM number of infusions per minute of cocaine access per session. Left in all graphs (sessions 1–4): 2-h continuous access self-administration acquisition sessions; right in all graphs (sessions 6–14): 8-h self-administration sessions. (D) Active lever presses. Mean ± SEM number of active lever presses per session of self-administration. (E) Inactive lever presses. Mean ± SEM number of inactive lever presses per session of self-administration. (F) Relapse (incubation) test. Mean ± SEM number of active and inactive lever presses per session (Continuous access: male: n=13 for Day 2 and n=14 for Day 29; female: n=11 for Day 2 and n=12 for Day 29. Intermittent access: male: n=12 for Day 2 and n=13 for Day 29; female: n=12 for Day 2 and Day 29). * Different from day 2 within each sex, p< .05; # Different from males on day 29 of abstinence, p=0.05.
Relapse tests
Independent of the abstinence day and sex, cocaine seeking was higher after intermittent drug access than after continuous drug access (Figure 1F). Additionally, independent of the access condition and abstinence day, cocaine seeking was higher in females than in males. Finally, independent of the access condition and sex, cocaine seeking was higher on abstinence day 29 than on day 2 (incubation of craving). The mixed ANOVA analysis of lever presses, which included the between-subjects factors of Access Condition, Sex, Abstinence Day (2,29), and the within-subjects factor of Lever (active, inactive) showed significant effects of Access Condition (F1,91=33.5, p<0.001), Sex (F1,91=16.4, p<0.001), Abstinence Day (F1,91=56.4, p<0.001), Lever (F1,91=304.3, p<0.001), but no significant interactions between Access Condition, Sex, and Abstinence Day.
Experiment 2: Independent replication
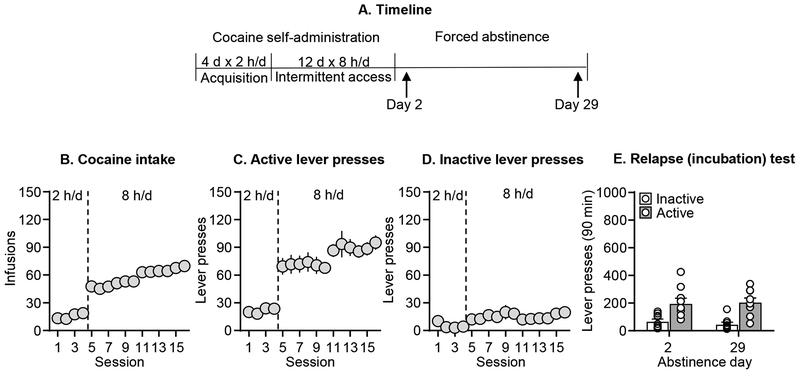
We attempted to replicate the finding of strong incubation of cocaine craving in female rats after intermittent access cocaine self-administration. As in Experiment 1, the female rats escalated their daily cocaine intake and active lever presses under the intermittent access training condition (Figure 2, see Table S1 for statistical results). Unexpectedly, we observed no evidence for incubation of cocaine craving with similar number of active lever presses during the different test days (Figure 2E). The statistical analysis, which included the between-subjects factor of Abstinence Day and the within-subjects factor of Lever, showed a significant effect of Lever (F1,16=60.8, p<0.001) but not Abstinence Day or an interaction between the two factors (p values>0.05).
Figure 2. Lack of effect of incubation of cocaine craving in female rats after intermittent access cocaine self-administration.
(A) Timeline of the experiment. (B) Total cocaine intake. Mean ± SEM number of infusions per session. Left in the graph (sessions 1–4): 2-h continuous access self-administration acquisition sessions; right in the graph (sessions 6–14): 8-h self-administration sessions (n=18). (C) Active lever presses. Mean ± SEM number of active lever presses per session of self-administration. (D) Inactive lever presses. Mean ± SEM number of inactive lever presses per session of self-administration. (E) Relapse (incubation) test: Mean ± SEM number of active and inactive lever presses per session (n=9 Day 2 and 29).
Experiment 3: Effect of duration of cocaine self-administration training and the estrous cycle on incubation of craving in female rats
To understand the lack of incubation effect in Experiment 2, we examined two experimental factors. First, we investigated possible role of the duration of intermittent access cocaine self-administration training. The 12 sessions of intermittent access may have been a threshold number of cocaine exposure, resulting in lack of incubation in Experiment 1. Second, we tested whether the estrous cycle regulates cocaine seeking during the relapse tests, because previous studies found significant effects of estrous cycle phases on cocaine-seeking behavior (29, 40, 45). Accordingly, by chance, during the relapse tests many rats in Experiment 1 may have been in the critical estrous cycle phase, most likely estrus, while those in Experiment 2 may have been in non-estrus.
Cocaine self-administration
As in Experiments 1–2, the female rats escalated their daily cocaine intake under the intermittent access training condition. This escalation had occurred independent of the number of training sessions, and no significant effect of the number of training sessions were observed on the active and inactive levers (Figure 3B–D; Table S1 for statistical results).
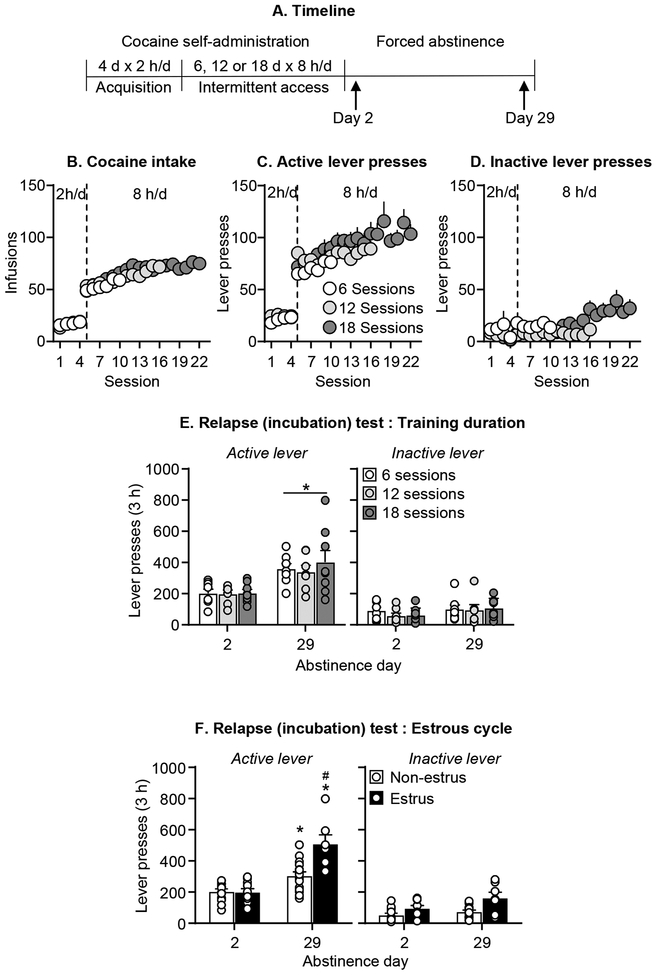
Figure 3. Effect of duration of intermittent access cocaine self-administration training and estrous cycle on incubation of craving in female rats.
(A) Timeline of the experiment. (B) Total cocaine intake. Mean ± SEM number of infusions per session. (C) Active lever presses. Mean ± SEM number of active lever presses per session of self-administration. (D) Inactive lever presses. Mean ± SEM number of inactive lever presses per session of self-administration. (E) Relapse (incubation) test: Training duration. Mean ± SEM number of active and inactive lever presses per session (6 sessions n=8, 12 sessions n=7, 16 sessions n=8 for day 2 and day 29). (F) Relapse (incubation) test: Estrous cycle. Mean ± SEM number of active and inactive lever presses per session (Non-estrus n=13–16 (Diestrus n=6–10, Proestrus n=7–6) and Estrus n=10–7 for Day 2 and 29 respectively). * Different from day 2 within each training condition or estrous phase, p< .05. # Different from Non-estrus at day 29 of abstinence, p< .05.
Relapse tests
Training duration:
The training duration had no effect on the emergence of incubation of cocaine craving (Figure 3E). The statistical analysis, which included the between-subjects factor of Training Duration (6, 12, 18 sessions) and Abstinence Day, and the within-subjects factor of Lever showed significant effects of Abstinence Day (F1,40=18.2, p<0.001) and Lever (F1,40=157.8, p<0.001), and an interaction between these two factors (F1,40=18.2, p<0.001), but no effect on Training Duration or interactions between this factor and the other factors (p values>0.05).
Estrous cycle:
We found no differences between diestrus or proestrus (data not shown), confirming results from previous studies (29, 47). Thus, we combined the diestrus and proestrus data (non-estrus) for data analysis. Across the three groups assigned to the different number of training sessions, the vaginal cytological analysis identified 13 rats in non-estrus and 10 in estrus on abstinence day 2, and 16 and 7 rats on abstinence day 29, respectively. Because there were no differences in lever presses during the relapse tests between the three training-duration groups, we combined them for the statistical analysis of the effect of estrous cycle on incubation of cocaine craving. The estrous cycle phase had no effect on non-incubated cocaine seeking on abstinence day 2 but had a strong effect on the higher or incubated cocaine seeking on abstinence day 29 (Figure 3F). The statistical analysis, which included the between-subjects factors of Abstinence Day, Estrus Phase (estrus, non-estrus), and the within-subjects factor of Lever, showed significant effects of Abstinence Day (F1,42=48.9, p<0.001), Estrus Phase (F1,42=21.4, p<0.001), and Lever (F1,42=194.9, p<0.001), and a significant interactions between Estrus Phase × Abstinence Day (F1,42=12.5 p=0.001), and Estrus Phase × Abstinence Day × Lever (F1,42=7.6, p=0.009). Finally, mean daily cocaine intake in the estrus and non-estrus groups was not associated with lever presses during the relapse tests (data not shown).
Experiment 4: Effect of the estrous cycle on incubation of craving in female rats after continuous cocaine self-administration
Cocaine self-administration
The female rats escalated their daily cocaine intake under the continuous access training condition. (Figure 4B; Table S1 for statistical results).
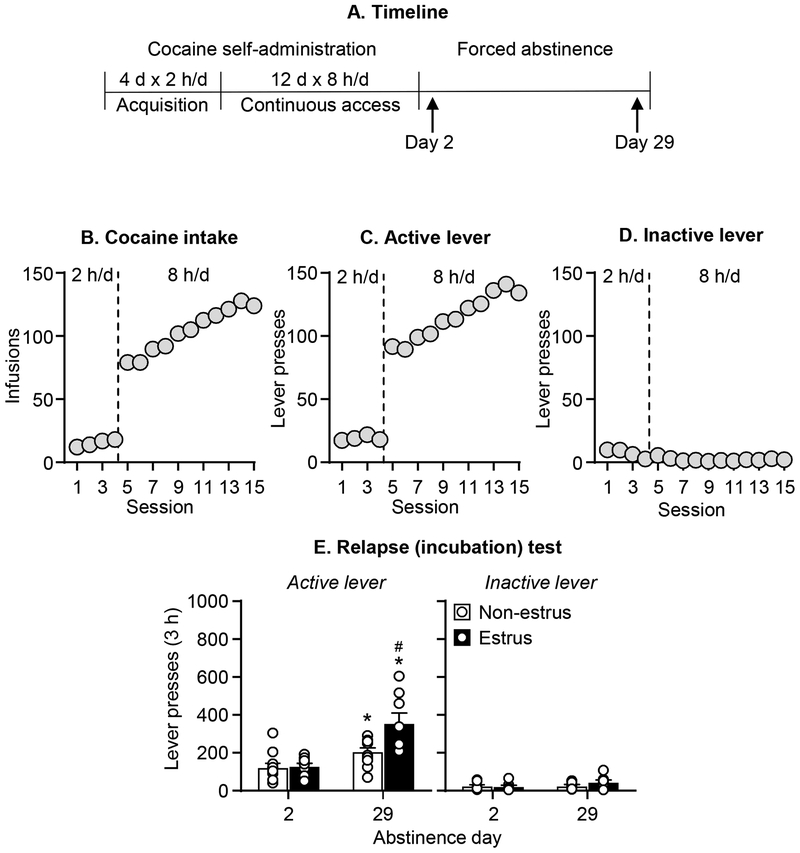
Figure 4. Effect of estrous cycle on incubation of craving after continuous access cocaine self-administration in female rats.
(A) Timeline of the experiment. (B) Total cocaine intake. Mean ± SEM number of infusions per session. (C) Active lever presses. Mean ± SEM number of active lever presses per session of self-administration. (D) Inactive lever presses. Mean ± SEM number of inactive lever presses per session of self-administration. (E) Relapse (incubation) test: Mean ± SEM number of active and inactive lever presses per session. Non-estrus n=12 (Diestrus n=6, Proestrus n=6) for day 2 and n=12 (Diestrus n=7, Proestrus n=4) for day 29; Estrus n=9 for day 2 and n = 8 for day 29. * Different from day 2 within estrus phase, p<0.05. # Different from Non-estrus at day 29 of abstinence, p< .05
Relapse tests
As in Experiment 3, the estrous cycle phase had no effect on non-incubated cocaine seeking on abstinence day 2 but had a significant effect on incubated cocaine seeking on day 29 (Figure 4E). The mixed ANOVA, which included the between-subjects factors of Abstinence Day and Estrus Phase (estrus, nonestrus), and the within-subjects factor of Lever, showed significant effects of Abstinence Day (F1,37=28.9, p<0.001), Estrus Phase (F1,37=7.9, p=0.008), and Lever (F1,37=171.8, p<0.001), and a significant interactions between Estrus Phase × Abstinence Day (F1,37=7.2 p=0.011), and Estrus Phase × Abstinence Day × Lever (F1,37=5.3, p=0.027).
Discussion
We examined the effect of intermittent cocaine self-administration on incubation of cocaine craving in male and female rats. There are four main findings in our study. First, incubation of cocaine craving during abstinence was observed after intermittent access drug self-administration, extending previous reports using the classical continuous access training procedure (9, 13, 63). Second, in both male and female rats and independent of the abstinence day, intermittent access cocaine self-administration increased relapse to cocaine seeking. Intermittent cocaine access also increased inactive lever presses during testing; the reasons for this effect are unknown. Third, independent of the abstinence day and training conditions, lever responding in the relapse tests was higher in female rats than in male rats, extending previous results on increased relapse vulnerability in female rats in rat models (29, 31, 40). Most important, independent of the cocaine access condition, incubation of cocaine craving was more pronounced during estrus than nonestrus. This finding suggests a critical role of ovarian hormones in incubation of craving.
Sex differences in cocaine self-administration and seeking
In Experiment 1, we found that both male and female rats increased cocaine intake over the sessions under either continuous or intermittent access training conditions. These results extend previous findings on escalation of cocaine intake in male and female rats under continuous access conditions (49, 64) and male rats under intermittent access conditions (24–26). Under both access conditions, we found no sex differences in cocaine self-administration. These results are not consistent with previous reports on higher cocaine intake in female than in male rats trained under continuous access conditions (39, 65, 66). The reasons for these different results are unknown and may be due to differences in the parameters of cocaine self-administration training in our studies versus these previous studies (e.g., unit dose, duration of daily session, number of training days). However, our negative results are consistent with recent studies showing lack of sex differences in extended access methamphetamine self-administration (67, 68).
Under both access conditions, lever responding in the relapse tests was higher in females than in males. These results agree with those from previous studies on increased resistance to extinction and cue- and cocaine priming-induced reinstatement in female rats (40, 41, 46, 47, 69, 70). Similar results have been extended to other drugs, showing increased cue- and drug priming- induced reinstatement of methamphetamine and nicotine seeking in female rats (71–73), but see Venniro et al. (68) for lack of sex differences in incubation of methamphetamine craving(68). Together, our results extend previous results on sex differences in relapse to cocaine seeking (29, 31, 41), and highlight the importance of sex as a biological variable in cocaine relapse studies.
Role of the estrous cycle in the incubation of cocaine craving
In Experiment 2 we failed to replicate Experiment 1 finding on incubation of craving in female rats after intermittent access self-administration training. To address this unexpected finding, we investigated the role of two factors: total drug exposure and estrous cycle phase. As for the first factor, twelve 8-h daily sessions of intermittent access cocaine self-administration corresponds to 80 min/day of cocaine access and may have been a threshold level of cocaine exposure to induce incubation of cocaine craving. In this regard, parametric studies in male rats have shown that incubation of cocaine craving, as assessed in extinction and cue-induced reinstatement tests, is less robust after 10 days of 2-h continuous access daily sessions than after 10 days of 6-h daily sessions (8). We found that 6, 12, or 18 sessions of intermittent access cocaine self-administration produced similar levels of incubation, indicating that the discrepant results between Experiments 1 and 2 are not due to the duration of the training phase or amount of drug intake during this phase. Regarding the second factor, as discussed in the Introduction, several studies have shown a role of ovarian hormones and estrous cycle in cocaine self-administration (38, 39, 49, 52, 65, 66, 74, 75) and reinstatement of cocaine seeking after continuous access drug self-administration (40, 46–48, 76, 77). We found potentiation of incubation of cocaine craving during estrus, an effect that was observed after either continuous or intermittent access cocaine self-administration. Thus, the lack of incubation in Experiment 2 may be due to a high proportion of rats being tested during non-estrus on day 29. However, it cannot be ruled out that other unknown experimental factors contributed to the negative results in Experiment 2, because in Experiments 3–4 we observed modest but statistically significant incubation in non-estrus rats. These results suggest that incubation of cocaine craving occurs during both estrus and non-estrus but this incubation is weaker and less robust during non-estrus. Another reason for the replication failure in Experiment 2 is the use of different testing durations (90 min versus 180 min in Experiment 1). However, this possibility can be ruled out, because as shown in Figure S1, reliable incubation was observed in Experiment 1 (and Experiments 3–4) when we analyzed the first 90-min data in these experiments.
Our data for the relapse tests on abstinence day 29 are consistent with previous findings showing that female rats in estrus show higher cocaine-induced reinstatement and are more resistant to extinction than female rats in non-estrus (40, 46, 47). In contrast, we did not observe any differences in cocaine seeking on abstinence day 2 between the rats in estrus and non-estrus. Previous studies found that the estrus phase is associated with increased cocaine seeking during early abstinence extinction sessions (29, 47). The reasons for these different results are unknown and it cannot be ruled out that the lack of effect of estrus phase on day 2 is due to a floor effect or to differences in the training procedures used: extended 8-h access in our study versus as 2-h limited access in the previous studies (see above). Additionally, cocaine exposure can disrupt the reproductive cycle in rodents and humans (43, 78), and in rats, the dysregulation of the estrous cycle lasts for up to 18 days after withdrawal from cocaine (79). Therefore, the negative results regarding the estrous cycle’s role in cocaine seeking during early abstinence should be interpreted with caution.
A methodological issue to consider in Experiment 1 is that we determined the estrus phase based on a single-day cytology analysis that is less reliable than multiple-day analysis. However, it is unlikely that this methodological issue confounds data interpretation, because we replicated and extended Experiment 1 results in two experiments where we determined the estrus phase based on multiple-day analysis. In Experiment 4, we showed a critical role of estrus phase in incubation after continuous access cocaine self-administration. Additionally, in an experiment where we only determined cocaine seeking 29 days after intermittent access cocaine self-administration, we found higher lever presses during estrus versus nonestrus (Figure S2).
Finally, a future research question is the mechanisms of estrus phase’s role in incubation of craving. In this regard, a potential mechanism is potentiation of activity of dopaminergic projections from ventral tegmental area (VTA) to nucleus accumbens (NAc) during estrus. This projection plays a critical role in relapse/reinstatement of cocaine seeking (80, 81). An early rat study showed potentiation of amphetamine-induced striatal dopamine release and stereotypy during estrus (82). More recently, a mouse study showed that dopamine neuron activity in the VTA-to-NAc projection and cocaine’s rewarding effects in the CPP procedure are potentiated during estrus (83).
Conclusions and clinical implications
Our results demonstrate that the phenomenon of incubation of cocaine craving generalizes to female and male rats trained under the intermittent access drug self-administration procedure (15). Additionally, in both male and female rats, intermittent access cocaine self-administration potentiates cocaine seeking during both early and late abstinence. Finally, we demonstrated a critical relationship between the phase of the estrous cycle and the magnitude of incubation of craving in female rats after both continuous and intermittent self-administration, a finding that may mediate sex differences in cocaine seeking. Thus, to the degree that results from rats models generalize to humans (84–87) our findings implicate the phase of the menstrual cycle as a risk factor for relapse in women and, therefore, should be taken into consideration in the development of relapse prevention treatments. A translational question for future research is whether incubation of cocaine craving, recently demonstrated in humans using an EEG-based measure (11), is modulated by the menstrual cycle.
Supplementary Material
Acknowledgments
The research was supported by the Intramural Research Program of NIDA (SI and YS) and a fellowship from the NIDA‐INSERM program (CN). CN, TIR, AP, SM, AH, and ZBY carried out the experiments; CN and TIR performed data analysis. CN, MMC, AH, YS, and SI designed the study. CN, YS, and SI wrote the manuscript. All authors critically reviewed the content and approved the final version before submission. We thank Brendan Tunstall for inspiring and helpful comments on the project and Marco Venniro for helpful suggestions on the manuscript and figures. We thank Hannah Korah for assisting in running the experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Hunt WA, Barnett LW, Branch LG (1971): Relapse rates in addiction programs. J Clin Psychol. 27:455–456. [DOI] [PubMed] [Google Scholar]
- 2.Sinha R (2011): New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 13:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien CP, Childress AR, McLellan AT, Ehrman R (1992): Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 654:400–415. [DOI] [PubMed] [Google Scholar]
- 4.Wikler A (1973): Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 28:611–616. [DOI] [PubMed] [Google Scholar]
- 5.Gawin FH, Kleber HD (1986): Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 43:107–113. [DOI] [PubMed] [Google Scholar]
- 6.Grimm JW, Hope BT, Wise RA, Shaham Y (2001): Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 412:141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL (1998): Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 19:48–59. [DOI] [PubMed] [Google Scholar]
- 8.Lu L, Grimm JW, Hope BT, Shaham Y (2004): Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 47 Suppl 1:214–226. [DOI] [PubMed] [Google Scholar]
- 9.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011): Neurobiology of the incubation of drug craving. Trends Neurosci. 34:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venniro M, Caprioli D, Shaham Y (2016): Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 224:25–52. [DOI] [PubMed] [Google Scholar]
- 11.Parvaz MA, Moeller SJ, Goldstein RZ (2016): Incubation of Cue-Induced Craving in Adults Addicted to Cocaine Measured by Electroencephalography. JAMA Psychiatry. 73:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szumlinski KK, Shin CB (2018): Kinase interest you in treating incubated cocaine-craving? A hypothetical model for treatment intervention during protracted withdrawal from cocaine. Genes Brain Behav. 17:e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf E, Kuhn M, Normann C, Mainberger F, Maier JG, Maywald S, et al. (2016): Synaptic plasticity model of therapeutic sleep deprivation in major depression. Sleep Med Rev. 30:53–62. [DOI] [PubMed] [Google Scholar]
- 14.Allain F, Minogianis EA, Roberts DC, Samaha AN (2015): How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev. 56:166–179. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer BA, Dobrin CV, Roberts DC (2011): Brain-cocaine concentrations determine the dose self-administered by rats on a novel behaviorally dependent dosing schedule. Neuropsychopharmacology. 36:2741–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allain F, Roberts DCS, Levesque D, Samaha AN (2017): Intermittent intake of rapid cocaine injections promotes robust psychomotor sensitization, increased incentive motivation for the drug and mGlu2/3 receptor dysregulation. Neuropharmacology. 117:227–237. [DOI] [PubMed] [Google Scholar]
- 17.Calipari ES, Ferris MJ, Siciliano CA, Zimmer BA, Jones SR (2014): Intermittent cocaine self-administration produces sensitization of stimulant effects at the dopamine transporter. J Pharmacol Exp Ther. 349:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR (2013): Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology. 38:2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calipari ES, Jones SR (2014): Sensitized nucleus accumbens dopamine terminal responses to methylphenidate and dopamine transporter releasers after intermittent-access self-administration. Neuropharmacology. 82:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calipari ES, Siciliano CA, Zimmer BA, Jones SR (2015): Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology. 40:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts DC, Gabriele A, Zimmer BA (2013): Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations. Neurosci Biobehav Rev. 37:2026–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmer BA, Oleson EB, Roberts DC (2012): The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology. 37:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allain F, Bouayad-Gervais K, Samaha AN (2018): High and escalating levels of cocaine intake are dissociable from subsequent incentive motivation for the drug in rats. Psychopharmacology (Berl). 235:317–328. [DOI] [PubMed] [Google Scholar]
- 24.Allain F, Samaha AN (2018): Revisiting long-access versus short-access cocaine self-administration in rats: intermittent intake promotes addiction symptoms independent of session length. Addict Biol. [DOI] [PubMed] [Google Scholar]
- 25.Kawa AB, Bentzley BS, Robinson TE (2016): Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl). 233:3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer BF, Fadanelli M, Kawa AB, Robinson TE (2018): Are Cocaine-Seeking “Habits” Necessary for the Development of Addiction-Like Behavior in Rats? J Neurosci. 38:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G (2018): Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll ME, Lynch WJ (2016): How to study sex differences in addiction using animal models. Addict Biol. 21:1007–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE (2008): Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl). 198:63–75. [DOI] [PubMed] [Google Scholar]
- 30.Becker JB, Perry AN, Westenbroek C (2012): Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch WJ, Roth ME, Carroll ME (2002): Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl). 164:121–137. [DOI] [PubMed] [Google Scholar]
- 32.McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI (1996): Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 184:616–622. [DOI] [PubMed] [Google Scholar]
- 33.Robbins SJ, Ehrman RN, Childress AR, O’Brien CP (1999): Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 53:223–230. [DOI] [PubMed] [Google Scholar]
- 34.Fox H, Sinha R (2014): The role of guanfacine as a therapeutic agent to address stress-related pathophysiology in cocaine-dependent individuals. Adv Pharmacol. 69:217–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R (2013): The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 38:1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li CS, Kemp K, Milivojevic V, Sinha R (2005): Neuroimaging study of sex differences in the neuropathology of cocaine abuse. Gend Med. 2:174–182. [DOI] [PubMed] [Google Scholar]
- 37.Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R (2012): Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 169:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu M, Crombag HS, Robinson TE, Becker JB (2004): Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 29:81–85. [DOI] [PubMed] [Google Scholar]
- 39.Lynch WJ, Carroll ME (1999): Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl). 144:77–82. [DOI] [PubMed] [Google Scholar]
- 40.Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, et al. (2005): Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl). 182:245–252. [DOI] [PubMed] [Google Scholar]
- 41.Lynch WJ, Carroll ME (2000): Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl). 148:196–200. [DOI] [PubMed] [Google Scholar]
- 42.Evans SM, Haney M, Foltin RW (2002): The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl). 159:397–406. [DOI] [PubMed] [Google Scholar]
- 43.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK (1999): Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 7:274–283. [DOI] [PubMed] [Google Scholar]
- 44.Sofuoglu M, Mitchell E, Kosten TR (2004): Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 78:699–705. [DOI] [PubMed] [Google Scholar]
- 45.Carroll ME, Anker JJ (2010): Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 58:44–56. [DOI] [PubMed] [Google Scholar]
- 46.Feltenstein MW, Byrd EA, Henderson AR, See RE (2009): Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 34:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feltenstein MW, See RE (2007): Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 89:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anker JJ, Larson EB, Gliddon LA, Carroll ME (2007): Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 15:472–480. [DOI] [PubMed] [Google Scholar]
- 49.Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME (2007): Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 15:461–471. [DOI] [PubMed] [Google Scholar]
- 50.Craft RM, Stratmann JA (1996): Discriminative stimulus effects of cocaine in female versus male rats. Drug Alcohol Depend. 42:27–37. [DOI] [PubMed] [Google Scholar]
- 51.Glick SD, Hinds PA (1984): Sex differences in sensitization to cocaine-induced rotation. Eur J Pharmacol. 99:119–121. [DOI] [PubMed] [Google Scholar]
- 52.Hu M, Becker JB (2008): Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend. 94:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Haaren F, Meyer ME (1991): Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 39:923–927. [DOI] [PubMed] [Google Scholar]
- 54.Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, et al. (2003): Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 120:523–533. [DOI] [PubMed] [Google Scholar]
- 55.Cummings JA, Jagannathan L, Jackson LR, Becker JB (2014): Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 135:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perrotti LI, Russo SJ, Fletcher H, Chin J, Webb T, Jenab S, et al. (2001): Ovarian hormones modulate cocaine-induced locomotor and stereotypic activity. Ann N Y Acad Sci. 937:202–216. [DOI] [PubMed] [Google Scholar]
- 57.Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V (2003): Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 970:214–220. [DOI] [PubMed] [Google Scholar]
- 58.Perry AN, Westenbroek C, Becker JB (2013): The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS One. 8:e79465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perry AN, Westenbroek C, Jagannathan L, Becker JB (2015): The Roles of Dopamine and alpha1-Adrenergic Receptors in Cocaine Preferences in Female and Male Rats. Neuropsychopharmacology. 40:2696–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagley JR, Adams J, Bozadjian RV, Bubalo L, Ploense KL, Kippin TE (2017): Estradiol increases choice of cocaine over food in male rats. Physiol Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE (2012): Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. 37:2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Caprioli D, Marchant NJ (2015): Recent updates on incubation of drug craving: a mini-review. Addict Biol. 20:872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong Y, Taylor JR, Wolf ME, Shaham Y (2017): Circuit and Synaptic Plasticity Mechanisms of Drug Relapse. J Neurosci. 37:10867–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed SH, Koob GF (1998): Transition from moderate to excessive drug intake: change in hedonic set point. Science. 282:298–300. [DOI] [PubMed] [Google Scholar]
- 65.Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK (2002): Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl). 161:304–313. [DOI] [PubMed] [Google Scholar]
- 66.Roth ME, Carroll ME (2004): Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 78:199–207. [DOI] [PubMed] [Google Scholar]
- 67.Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. (2018): Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 21:1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venniro M, Zhang M, Shaham Y, Caprioli D (2017): Incubation of Methamphetamine but not Heroin Craving After Voluntary Abstinence in Male and Female Rats. Neuropsychopharmacology. 42:1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Becker JB (2016): Sex differences in addiction. Dialogues Clin Neurosci. 18:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feltenstein MW, Henderson AR, See RE (2011): Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl). 216:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cox BM, Young AB, See RE, Reichel CM (2013): Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 38:2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feltenstein MW, Ghee SM, See RE (2012): Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 121:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holtz NA, Lozama A, Prisinzano TE, Carroll ME (2012): Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend. 120:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grimm JW, See RE (1997): Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav. 61:755–761. [DOI] [PubMed] [Google Scholar]
- 75.Jackson LR, Robinson TE, Becker JB (2006): Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 31:129–138. [DOI] [PubMed] [Google Scholar]
- 76.Doncheck EM, Urbanik LA, DeBaker MC, Barron LM, Liddiard GT, Tuscher JJ, et al. (2018): 17beta-Estradiol Potentiates the Reinstatement of Cocaine Seeking in Female Rats: Role of the Prelimbic Prefrontal Cortex and Cannabinoid Type-1 Receptors. Neuropsychopharmacology. 43:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larson EB, Roth ME, Anker JJ, Carroll ME (2005): Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 82:98–108. [DOI] [PubMed] [Google Scholar]
- 78.Chen CJ, Vandenbergh JG (1994): Effect of chronic cocaine on reproduction in female house mice. Pharmacol Biochem Behav. 48:909–913. [DOI] [PubMed] [Google Scholar]
- 79.King TS, Canez MS, Gaskill S, Javors MA, Schenken RS (1993): Chronic cocaine disruption of estrous cyclicity in the rat: dose-dependent effects. J Pharmacol Exp Ther. 264:29–34. [PubMed] [Google Scholar]
- 80.Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC (2005): Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 526:65–76. [DOI] [PubMed] [Google Scholar]
- 81.Shalev U, Grimm JW, Shaham Y (2002): Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 54:1–42. [DOI] [PubMed] [Google Scholar]
- 82.Becker JB, Cha JH (1989): Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 35:117–125. [DOI] [PubMed] [Google Scholar]
- 83.Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, et al. (2017): Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 8:13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Epstein DH, Preston KL, Stewart J, Shaham Y (2006): Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl). 189:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heilig M, Epstein DH, Nader MA, Shaham Y (2016): Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci. 17:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y (2018): Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sinha R, Shaham Y, Heilig M (2011): Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl). 218:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.