Abstract
Purpose
Triple-negative breast cancer (TNBC) lacks the receptor targets estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2, and thus it does not respond to receptor-targeted treatments. TNBC has higher recurrence, metastasis, and mortality rates than other subtypes of breast cancer. Mounting data suggest that the MAPK (also known as RAS-RAF-MEK-ERK) pathway is an important therapeutic target in TNBC.
Methods
To evaluate anti-tumor and anti-metastasis efficacy of E6201, we used cell proliferation assay, soft agar assay, cell cycle assay, Annexin V staining assay, immunoblotting analysis, immunohistochemistry, migration assay, invasion assay, mammary fat pad xenograft, and experimental and spontaneous metastasis xenograft models. We also evaluated the anti-tumor efficacy of E6201 plus CDK4/6 inhibitor, mTOR inhibitor, or ATR inhibitor.
Results
E6201 inhibited TNBC cell colony formation, migration, and invasion in a dose-dependent manner. E6201 induced G1 cell cycle arrest and apoptosis. E6201 inhibited TNBC xenograft growth and inhibited TNBC lung metastasis and improved mouse survival in experimental metastasis and spontaneous metastasis assays. Immunohistochemical staining demonstrated that E6201 decreased the metastatic burden in the lung and decreased phosphorylated ERK expression in a dose-dependent manner. Combination of E6201 with CDK4/6 inhibitor or mTOR inhibitor enhanced E6201’s in vitro anti-tumor efficacy.
Conclusion
These results indicate that E6201 exhibits anti-tumor efficacy against TNBC in vitro and antimetastasis efficacy against TNBC in vivo. These results provide a rationale for further clinical development of E6201 as a MAPK-pathway-targeted therapy for TNBC.
Keywords: E6201, MEK inhibitor, MAPK pathway, Triple-negative breast cancer, Metastasis
Introduction
Triple-negative breast cancer (TNBC) is estrogen receptor negative, is progesterone receptor negative, and lacks over-expression of HER2 receptor. Thus, it does not respond to hormonal therapy or trastuzumab, and chemotherapy is the mainstay of treatment [1]. TNBC accounts for 15% to 20% of breast cancer cases but 30% to 40% of U.S. breast cancer deaths [2]. TNBC has a distinctively worse clinical outcome than other breast cancers types, with a higher recurrence rate, a higher metastasis rate, and worse overall survival after recurrence [3]. Thus, development of better therapeutic agents for TNBC constitutes a critical unmet need.
The mitogen-activated protein kinase (MAPK; also known as RAS-RAF-MEK-ERK) signaling pathway has long been viewed as a promising target in the development of novel anticancer therapies [4]. Drugs directly targeting Ras, a critical upstream regulator in the MAPK pathway, have been sought for decades, but no effective RAS inhibitor has been developed. However, small molecules targeting RAF and MEK, other important molecules in the MAPK pathway, have been developed [5–7]. Three MEK inhibitors (trametinib, cobimetinib, and binimetinib) have been approved by the U.S. Food and Drug Administration for BRAF V600E-mutated melanoma, non-small cell lung cancer, and/or anaplastic thyroid cancer.
Using preclinical models, we previously found that the MEK1/2 inhibitor selumetinib (AZD6244) inhibited TNBC cell growth, epithelial-mesenchymal transition, and lung metastasis in a TNBC xenograft model [8] and that the MEK1/2 inhibitor pimasertib (AS703026) exhibited enhanced anti-tumor efficacy when combined with a histone deacetylase inhibitor [9]. These findings suggest that the MAPK pathway is critical for TNBC progression and is a potentially important therapeutic target in this disease.
E6201, a synthetic analog of a natural product, is an ATP-competitive dual kinase inhibitor of MEK1 and FLT3 [10,11]. Preclinical studies showed that E6201 may be useful for treatment of cancers associated with elevation of MEK1 kinase activity, including melanoma and acute myeloid leukemia [12–15]. E6201 has been evaluated in a phase I clinical trial in advanced solid tumors and melanoma (trial registration ID: NCT00794781).
The purpose of this study was to determine the in vitro anti-tumor efficacy and in vivo anti-metastasis efficacy of MEK1 inhibitor E6201 in TNBC. In the present study, we evaluated the anti-tumor and anti-metastasis efficacy of E6201 in TNBC. We showed that E6201 inhibited the growth of TNBC cells, reduced metastasis, and prolonged the survival of TNBC xenograft mice. Furthermore, we found that CDK4/6 and mTOR inhibitors are potential candidates for combination treatment with E6201 targeting TNBC.
Materials and methods
Cell lines
Human TNBC cell lines BT20, HCC70, MDA-MB-231, HCC1806, and HCC1937 were purchased from American Type Culture Collection (Manassas, VA), and human TNBC cell lines SUM149 and SUM159 were purchased from Asterand Bioscience, Inc. (Detroit, MI). MDA-MB-231 lung metastasis subclone (MDA-MB-231-LM2) was obtained from Dr. Joan Massague at Memorial Sloan Kettering Cancer Center. All cell lines were authenticated by genotyping through the Characterized Cell Line Core Facility at The University of Texas MD Anderson Cancer Center and routinely tested for mycoplasma contamination using MycoAlert (Lonza, Allendale, NJ).
Reagents and antibodies
E6201 was provided by Spirita Oncology, LLC. We obtained anti-ERK and anti-pERK from Cell Signaling Technology (Danvers, MA); anti-vimentin, anti-fibronectin, anti-Ki-67, anti-ZEBl, and phalloidin-FITC from Thermo Fisher (Waltham, MA); pMEK from Santa Cruz Biotechnology (Dallas, TX); and anti-horseradish peroxidase-conjugated antibodies from Sigma-Aldrich (St. Louis, MO).
In vitro cell proliferation assay
To investigate the anti-proliferative effect of E6201 in TNBC cell lines, Cell Titer-Blue cell viability (Promega, Madison, WI) and sulforhodamine B staining assays was performed according to the manufacturer’s instructions. In brief, 1×103 to 5×103 cells were added into a 96-well plate and treated with drug for 5 days. The GraphPad Prism program and the CalcuSyn program were used to evaluate 50% inhibitory concentration (IC50).
Cell-cycle distribution and apoptosis analysis
Cells (2×105 cells/well) were plated in a 6-well plate, cultured overnight, and then treated or left untreated with E6201 for 48 hours. Cells were then harvested, fixed with ethanol, and resuspended with PI solution. The cell-cycle distribution was analyzed using flow cytometry. Apoptosis was measured with a PE Annexin V/7AAD Apoptosis Detection Kit I (BD Biosciences, San Jose, CA), which detects the loss of membrane integrity. The assay was performed according to the manufacturer’s instructions.
Soft agar assay
TNBC cells (1×103 to 10×103 cells/well) were resuspended in 2 mL of 0.4% agarose solution in complete medium and overlaid onto the bottom agar layer (0.8%) in 12-well plates. The plates were incubated for 2 to 4 weeks with or without E6201, and colonies were stained with 200 μL of MTT solution (2 mg/mL) for 2 hours. The stained colonies greater than 80 μm in diameter were counted using the GelCount colony-counting system (Oxford Optronix, UK) according to the manufacturer’s instructions.
Immunoblotting analysis
TNBC cells were treated with DMSO or E6201, and total protein extracts were prepared using cold M-PER mammalian protein extraction reagent (Thermo Fisher) including phosphatase/protease inhibitors. A total of 15 μg of each sample was resolved by NuPAGE 4–12% Bis-Tris Plus gel (Thermo Fisher) and then transferred onto PVDF membrane (Bio-Rad, Hercules, CA). Membranes were incubated with target antibodies overnight. Signals were detected using an Odyssey IR imaging system (LI-COR Biosciences, Lincoln, NE) or chemiluminescent substrates (Thermo Fisher).
Immunohistochemistry
TNBC xenograft tumor tissues were fixed in neutral-buffered formalin and embedded in paraffin. Sections (5 μm thick) were deparaffinized in xylene, rehydrated in graded alcohols, and washed in distilled water. Antigens were retrieved by boiling the sections in citric acid–based antigen unmasking solution (Vector, Burlingame, CA) for 30 min. Immunohistochemistry (IHC) staining was performed using the Lab Vision automated system (Thermo Fisher) through the Division of Surgery Histology Core. Immunostained slides were scanned by using an Aperio AT2 slide scanner, and images were captured using the Aperio Image Scope V12 (Leica, Buffalo Grove, IL).
Immunofluorescence
SUM149 or MDA-MB-231 cells (1×104 cells/well) were plated in an eight-well μSlide (Ibidi USA, Fitchburg, WI), cultured overnight, and then treated or left untreated with E6201 for overnight. Cells were fixed in 4% paraformaldehyde in PBS for 10 min and permeabilized with 0.1% Triton X-100 in PBS for 5 min. Cells were then washed three times with PBS for 10 min, blocked for 30 min with 5% BSA/0.3% Triton X-100 in PBS, and incubated overnight with target antibodies in an antibody dilution buffer (Cell Signaling). Immunofluorescence staining was visualized using Alexa Fluor 488 (green), Alexa Fluor 594 (red), Alexa Fluor 647 (yellow), and DAPI (Thermo Fisher). Images were captured at a magnification of 20× and stored using a BZ-X fluorescence microscope (Keyence, Itasca, IL) according to the manufacturer’s instructions.
Migration assay and invasion assay
Migration and invasion assays were performed in triplicate using a 24-well Falcon cell culture insert with 8-μm pore size and BioCoat Matrigel invasion chamber, respectively (Thermo Fisher). SUM149 and MDA-MB-231 cells were pre-treated with E6201 for 2 hours. Cells were collected and resuspended in 0.1% BSA DMEM/F12 or F12 medium to make 5×104 cells/mL cell density. Cell suspensions (1×105 cells/200 μL) were added into the insert, and 750 μL of 5% FBS culture medium was added into the lower chamber as an attractant. Cells were incubated for 8 hours for migration and 24 hours for invasion, and then cells were fixed and stained using Diff-Quick solution (Electron Microscopy Science, Hatfield, PA) according to manufacturer’s instructions. The invaded and migrated cells were scanned and quantified using the ImageJ program (National Institutes of Health, Bethesda, MD).
Mice
Animal studies were approved by the Animal Care and Use Committee of MD Anderson Cancer Center. Female Nod.Scid gamma mice, age 4 to 6 weeks old (Jackson Laboratories, ME), were used for all of the in vivo studies. Mice were housed under pathogen-free conditions and treated in accordance with NIH guidelines.
Effect of E6201 on growth of xenograft tumors in vivo
To establish breast cancer xenografts, MDA-MB-231-LM2 cell suspensions (2×106 cells/100 μL) were injected into one site in the abdominal mammary fat pad of each mouse. E6201 or vehicle control was administered via tail vein injection three times per week starting when the tumors were approximately 100 to 150 mm3. Tumor volume [V = 0.5 × (L × W2)] was measured by caliper and body weight was measured twice weekly. Tumor samples were collected at the end of the experiment, and sections were preserved by paraffin block embedding for IHC staining.
Effect of E6201 on experimental and spontaneous metastasis
An experimental metastasis assay was performed using SUM149 cells. Cell suspensions (4×106 cells/100 μL) were injected via tail vein using a 28-gauge needle. Starting the next day, E6201 or vehicle control was delivered via intravenous injection three times per week. Mouse body weight was measured once weekly. After 6 weeks of treatment, mice were killed, and lungs were preserved in paraffin blocks. To measure metastases in lungs, formalin-fixed paraffin-embedded mouse lung sections were stained with hematoxylin and eosin and scanned using an Aperio ScanScope (Aperio, CA), and then metastatic tumor burden was quantified using the ImageJ program.
A spontaneous metastasis assay was performed using MDA-MB-231-LM2 cells. Cell suspensions (2×106 cells/100 μL) were injected into one site in the abdominal mammary fat pad of each mouse. Tumor volume [V= 0.5 × (L × W2)] was measured by caliper and body weight was measured twice weekly. Starting when tumors were approximately 100 mm3, E6201 or vehicle control was delivered via tail vein injection three times per week. When tumors were approximately 400 mm3, all tumors were removed by survival surgery. Drug treatment was then continued for 2 more weeks, at the end of which mice were killed, lungs were preserved in paraffin blocks, and metastases in lung were measured as described in the preceding paragraph.
Effect of E6201 on survival of mice with xenograft tumors
For the xenograft survival assay, MDA-MB-231-LM2 cell suspensions (2×106 cells/100 μL) were injected into one site in the abdominal mammary fat pad of each mouse. Tumor volume [V = 0.5 × (L × W2)] was measured by caliper and body weight was measured twice weekly. When tumors reached 200 mm3, mice were divided into two groups (n=14 each) and then E6201 or vehicle control was delivered via tail vein injection three times per week. When tumors were approximately 400 mm3, all tumors were removed by survival surgery. Injections of E6201 or vehicle control were then continued, and body weight was measured twice weekly. Animal survival was counted from the first day of treatment until the mice showed morbidity.
Statistical analysis
For experimental outcomes, descriptive statistics (mean and standard error of the mean) were summarized for each group. Statistical analyses were performed with Prism version 6 (GraphPad Software, La Jolla, CA). In vitro experiments were performed using an unpaired t test between control and treatment group. In vivo tumorigenicity data was compared using an analysis of variance model. Survival data were compared using log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests. P values of less than 0.05 were considered statistically significant.
Results
E6201 significantly inhibited TNBC cell proliferation and anchorage-independent colony formation in a dose-dependent manner
We first evaluated the anti-proliferation efficacy of E6201 in TNBC cell lines. TNBC cells were treated with different concentrations of E6201 for 5 days, and cell viability was measured by CellTiter-Blue and sulforhodamine-B assays. We observed dose-dependent growth inhibition in TNBC cell lines by E6201 treatment (Fig. 1a). We compared these findings from three other MEK inhibitors, selumetinib, trametinib, and pimasertib, in TNBC cell lines and found that E6201 was more effective or similar to the other inhibitors in terms of growth inhibition (Supplementary Fig. 1). Next, we determined the in vitro anti-tumor effect of E6201 by an anchorage-independent growth (soft-agar) assay. We observed that the number of colonies was significantly reduced in a dose-dependent manner (Fig. 1b). Next, because a previous study showed that E6201 inhibits acute myeloid leukemia cell proliferation through FLT3 targeting, we tested the effect of E6201 on FLT3 expression in TNBC cells and the effect of the FLT3 inhibitor quizartinib on TNBC cell viability. E6201 reduced FLT3 expression in some cell lines and increased FLT3 expression in others (Supplementary Fig. 2a). We observed weak inhibition of proliferation by quizartinib, and the inhibitory effect did not correlate with FLT3 expression in tested cell lines (Supplementary Fig. 2b). These results suggested that inhibition of TNBC cell proliferation by E6201 was not due to suppression of FLT3.
Figure 1. E6201 inhibited TNBC cell proliferation and anchorage-independent colony formation in a dose-dependent manner.
a Cell proliferation. TNBC cells were treated with E6201 for 5 days, and viability was measured by using CellTiter-Blue and sulforhodamine-B assays. IC50 μM) values of other MEK inhibitors in TNBC cell lines are shown in the table. Data shown are representative of three experiments with similar results. b Colony formation. TNBC cells were treated with E6201 and allowed to grow in an anchorage-independent environment for 2-3 weeks. Colonies were stained with MTT solution (2 mg/mL) for 2 h and counted using the GelCount system according to the manufacturer’s instructions. Statistical significance was evaluated by t test using GraphPad Prism software. Each bar represents the mean of three independent experiments; error bars indicate standard deviation. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001 for E6201 treatment compared with control.
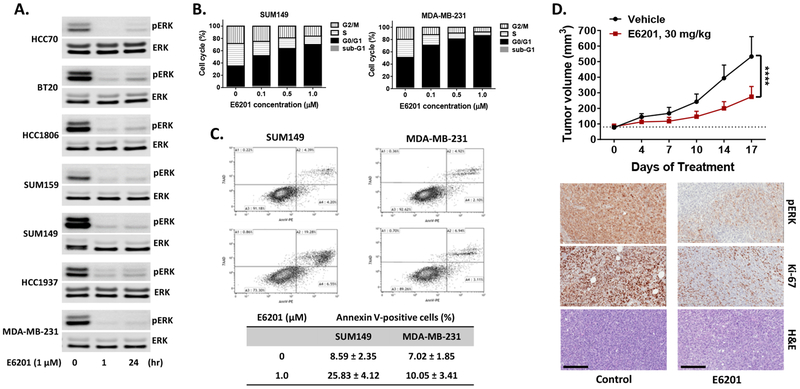
E6201 inhibited expression of phospho-ERK and induced G1 phase cell cycle arrest, apoptosis, and suppression of tumor growth in TNBC
To confirm that E6201 treatment inhibited the MAPK pathway in TNBC cells, we performed a Western blot assay with anti-phospho-ERK since ERK is the molecule immediately downstream of MEK. pERK expression level showed a rapid (apparent by 1 hour) and sustained (still apparent at 24 hours) decrease in the tested TNBC cell lines following treatment with E6201 (1 μM) (Fig. 2a). To discern the effects of E6201 on cell cycle and apoptosis, SUM149 and MDA-MB-231 cells were treated with E6201 for 48 hours, and then cell cycle and apoptosis were analyzed by using fluorescence-activated cell sorting. We observed that E6201 at a concentration of 1 μM significantly increased the proportion of cells in G0/G1 phase arrest (SUM149: 34.3% to 67.8%, MDA-MB-23 1: 49.7% to 85%) and significantly decreased the proportions of cells in S phase (SUM149: 36.5% to 13.6%, MDA-MB-231: 29.4% to 5.7%) and G2/M phase (SUM149: 28.3% to 16.3%, MDA-MB-231: 19.7% to 7.7%) (Fig. 2b). The Annexin V staining assay showed that E6201 increased the proportion of apoptotic cells (SUM149: 8.6% to 25.8%, MDA-MB-231: 7.0% to 10.1%) (Fig. 2c). We also observed that MDA-MB-231 cells needed an incubation time of more than 96 hours to reach the same level of apoptosis as seen in SUM 149 cells (data not shown).
Figure 2. E6201 inhibited the MAPK pathway, induced cell cycle arrest and apoptosis of TNBC cells, and inhibited TNBC xenograft tumor growth.
a pERK expression. TNBC cells were treated with E6201 for 1 hr or 24 hr, and then immunoblotting was performed with pERK and ERK antibodies. Data shown are representative of three independent experiments with similar results. b and c Cell cycle and apoptosis. TNBC cells were treated with E6201 for 48 hr, and then cell cycle. (b) and apoptosis (c) were analyzed by FACS. Each bar represents the mean of three independent experiments. d Xenograft tumor growth. MDA-MB-231-LM2 cells (2×106/100 μl) were injected into one mammary fat pad area. Starting when tumors reached approximately 100 mm3, E6201 or vehicle control (n=14 each) was administered via tail vein injection three times per week. Top, Change in tumor volume over time. Error bars indicate standard error of the mean. **** P<0.0001 for E6201 treatment compared with control. Bottom, Results of IHC staining of tumor specimens. IHC staining was performed with anti-pERK and anti-Ki-67 antibody. H&E, hematoxylin-eosin. Magnification, × 20; scale bars, 200 μm. Data shown are representative of three IHC staining experiments from each treatment group with similar results.
We next tested the in vivo anti-tumor effect of E6201 using the MDA-MB-231-LM2 xenograft model. Compared with mice treated with vehicle control (n=10), the E6201-treated mice (n=10) showed 60% tumor growth suppression (P <0.0001) (Fig. 2d). IHC analysis data showed that E6201 strongly inhibited pERK and Ki-67 expression in xenograft tumor tissues (Fig. 2d).
These results suggested that E6201 as a single agent suppressed TNBC cell growth in vitro and inhibited TNBC xenograft growth in vivo.
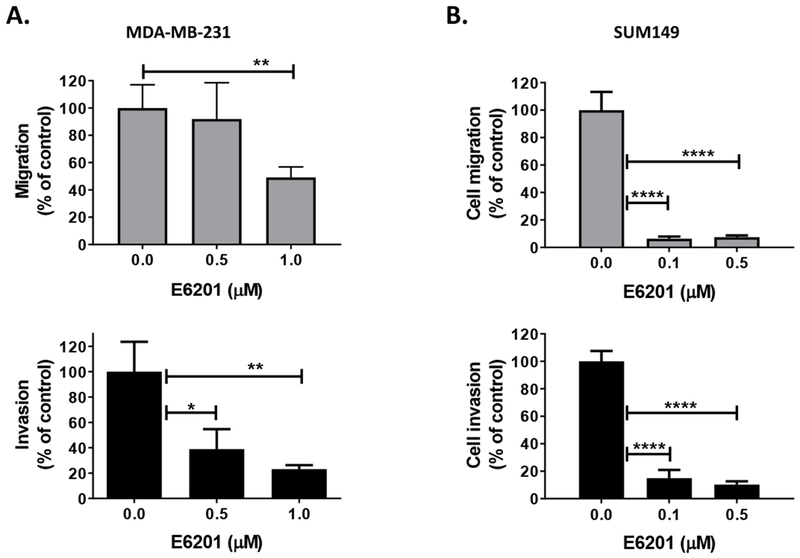
E6201 inhibits TNBC cell migration, invasion, and morphological changes
ERK has been reported to contribute to migration and invasion by modulating focal adhesion and actin dynamics [16], and we previously reported that the MEK1/2 inhibitor selumetinib significantly suppressed TNBC cell migration and invasion [8]. Thus, we hypothesized that MEK1 inhibitor E6201 can inhibit TNBC cell migration and invasion by suppression of the MAPK pathway. To test this hypothesis, we analyzed the effect of E6201 on cell migration and invasion at a clinically relevant dose (≤ 1 μmol/L). The data showed that E6201 significantly inhibited both MDA-MB-231 and SUM149 cell migration and invasion in a dose-dependent manner (Fig. 3a and 3b). To confirm that E6201 inhibited migration and invasion through MEK1 inhibition and not FLT3, we treated MDA-MB-231 and SUM149 cells using the FLT3 inhibitor quizartinib and performed migration and invasion assays. The data showed that quizartinib did not inhibit migration or invasion of TNBC cells (Supplementary Fig. 2c and 2d). These results suggested that E6201 inhibits TNBC cell migration and invasion through inhibition of the MAPK pathway.
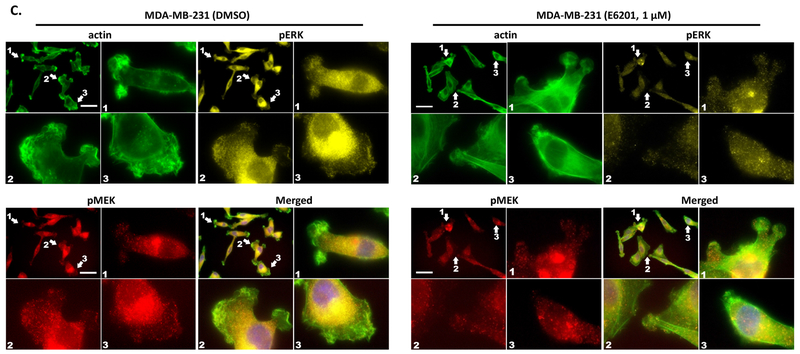
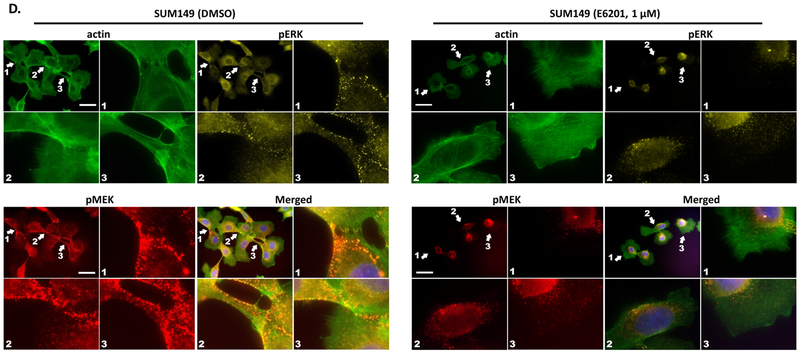
Figure 3. E6201 inhibited TNBC cell migration, invasion, and morphological changes.
a and b Migration and invasion. TNBC cells (1×105/well) were added into trans-wells and incubated with or without E6201 for 6 hr (migration, upper panels) or 24 hr (invasion, lower panels). Migration and invasion were evaluated by using ImageJ software. Error bars indicate standard deviation. * P<0.05, ** P<0.01, **** P<0.0001 for E6201 treatment compared with control. c and d actin polymerization, lamellipodia or filopodia formations. TNBC cells were treated with E6201 overnight, and then immunofluorescence assay was performed with phalloidin-FITC (green), anti-pMEK (red), anti-pERK (yellow), and DAPI (blue). Arrows indicate actin polymerization, lamellipodia or filopodia. Scale bars, 50 μm. Data shown are representative of three migration, invasion, and immunofluorescence experiments with similar results.
Next, we tested the effect of E6201 on cellular movement and morphological changes. We treated MDA-MB-231 and SUM 149 cells with E6201 and stained them with phalloidin, anti-pMEK, and anti-pERK. We observed that E6201 prevented actin polymerization, lamellipodia and filopodia formations in MDA-MB-231 (Fig. 3c), and prevented actin polymerization and filopodia formations in SUM149 (Fig. 3d). In control cells, pMEK and pERK were co-localized in the morphological change area, and treatment with E6201 inhibited co-localization of pMEK, and pERK in these area.
E6201 inhibits experimental and spontaneous lung metastasis and increases survival in a spontaneous lung metastasis model
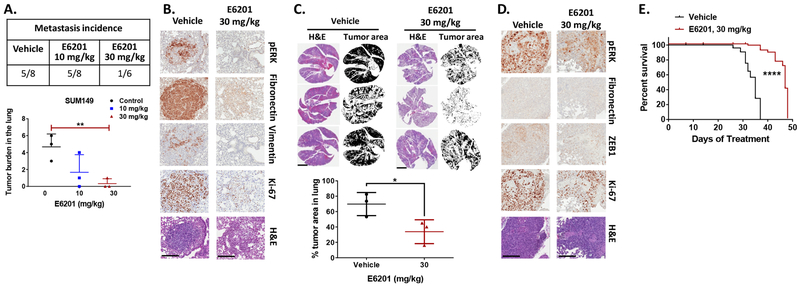
After confirming that E6201 reduced cell proliferation, migration, and invasion in vitro, we tested the ability of E6201 to reduce experimental metastasis in a SUM149 xenograft model. In this model, E6201 significantly reduced the incidence of SUM149 lung metastasis and lung tumor burden compared to the vehicle control (P < 0.0101) (Fig. 4a). We next analyzed the effect of E6201 on the MAPK pathway (pERK), mesenchymal markers (vimentin and fibronectin), and proliferation (Ki-67) in metastatic tumors in the lung. Compared to tumors in the mice treated with vehicle control, tumors in the E6201-treated mice exhibited reduced pERK, vimentin, fibronectin, and Ki-67 expression levels (Fig. 4b).
Figure 4. E6201 inhibited lung metastasis and extended mouse survival in TNBC metastasis models.
a SUM149 cells (4×106/200 μl) were injected into mice via the tail vein. Starting the next day, E6201 or vehicle control was delivered via intravenous injection three times per week. After 6 weeks of treatment, mice were killed, and incidence of lung metastasis and tumor burden in the lung were measured. Data shown are representative of three mice from each treatment group. b IHC staining was performed with anti-pERK, anti-vimentin, anti-fibronectin, or anti-Ki-67 antibody in tumors from the mice described in a. Data shown are representative of three IHC experiments from each treatment group with similar results. Magnification, × 20; scale bars, 200 μm. c MDA-MB-231-LM2 spontaneous metastasis model. MDA-MB-231-LM2 cells (2×106/100 μl) were injected into one mammary fat pad area. Starting when tumors were approximately 100 mm3, E6201 or vehicle control was administered via tail vein injection three times per week. When tumors were approximately 400 mm3, all tumors were removed by survival surgery. Drug treatment was then continued for 2 more weeks, at the end of which mice were killed. Top, Photomicrographs of hematoxylin-eosin (H&E)-stained sections showing metastatic tumor growth in the lung. Bottom, Percentage of lung occupied by metastatic tumors as visualized by using ImageJ software. Data shown are representative of three mice from each treatment group. Magnification, × 0.5; scale bars, 5 mm. d IHC staining was performed with anti-pERK, anti-fibronectin, anti-ZEB1, or anti-Ki-67 antibody in tumors from the mice described in c. Data shown are representative of three IHC staining experiments from each treatment group with similar results. Magnification, × 20; scale bars, 200 μm. e Kaplan-Meier survival curves of MDA-MB-231-LM2 spontaneous metastasis model. MDA-MB-231-LM2 cells (2×106 cells/100 μL) were injected into one mammary fat pad area. Starting when tumors were approximately 200 mm3, E6201 or vehicle control was administered via tail vein injection three times per week. When tumors were approximately 400 mm3, all tumors were removed by survival surgery. Drug treatment was then continued. Error bars indicate standard deviation. * P<0.05, ** P<0.01, **** P<0.0001 for E6201 treatment compared with vehicle control.
We next examined whether E6201 would inhibit spontaneous metastasis in the MDA-MB-231-LM2 xenograft model. We observed spontaneous lung metastasis in all mice in both the vehicle control and E6201 treatment groups (n=5 each); however, the lung tumor burden was significantly reduced in the mice treated with E6201 (Fig. 4c, P=0.0449). We next analyzed the effect of E6201 on the MAPK pathway (pERK), mesenchymal markers (fibronectin and ZEB1), and proliferation (Ki-67) in metastatic tumors in the lung. Compared to tumors in the mice treated with vehicle control, tumors in the E6201-treated mice exhibited reduced pERK, fibronectin, ZEB1, and Ki-67 expression levels (Fig. 4d). Our in vivo results showed that E6201 effectively inhibited TNBC tumor cell metastasis and decreased metastatic lung tumor burden.
Given the significant contribution of metastasis to breast cancer-caused mortality, we hypothesized that E6201 can prolong survival in the MDA-MB-231-LM2 spontaneous metastasis xenograft model. We observed that E6201 treatment did significantly extend mouse survival: the median survival duration was 35 days for the vehicle control group vs. 47 days for the E6201 treatment group (Fig. 4e, P<0.0001 by log-rank test).
Combination of E6201 with CDK4/6 or mTOR inhibitor increases E6201’s in vitro anti-tumor efficacy
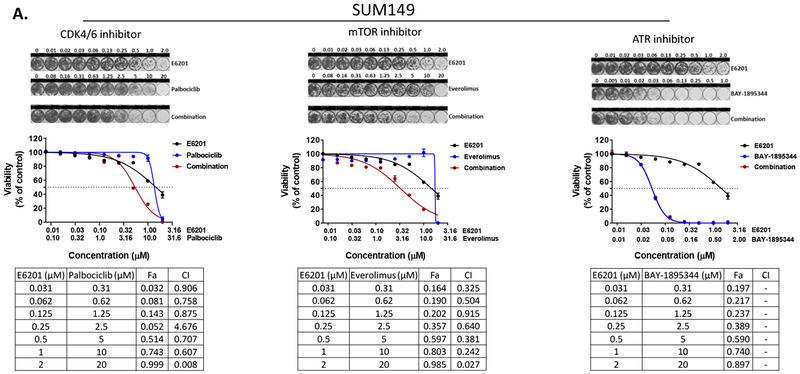
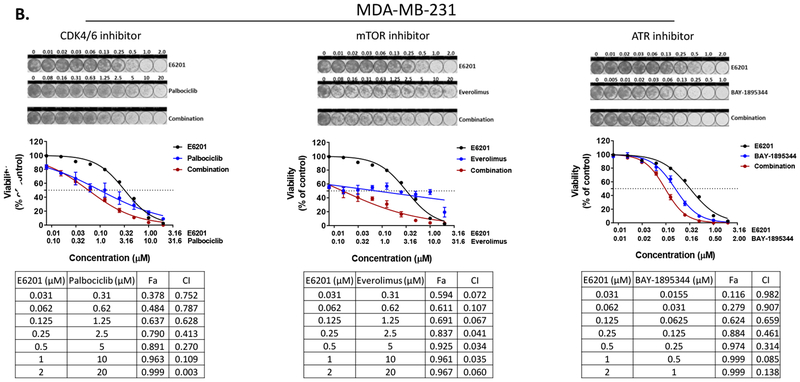
TNBC is a heterogeneous disease with a high rate of recurrence after chemotherapy, which is the standard first-line treatment. If approved by the U.S. Food and Drug Administration for treatment of TNBC, E6201 would be given as a second-line or later treatment option, in combination with chemotherapy or another kinase inhibitor. Because cumulative chemotherapy-related toxicities remain a clinical problem, we decided to evaluate the in vitro anti-tumor efficacy of combinations of E6201 with kinase inhibitors. Our previous high-throughput kinome RNAi screening with MEK inhibitor revealed that inhibitors of the cell cycle, PI3K/mTOR, and ATM/ATR pathways are potential agents for combination with MEK inhibitor in TNBC [9]. Thus, we investigated the combinational anti-tumor effect of E6201 in combination with palbociclib (CDK4/6 inhibitor), everolimus (mTOR inhibitor), and BAY-1895344 (ATR inhibitor). We observed a strong combinational anti-tumor effect when E6201 was combined with CDK4/6 inhibitor (combinational index [CI] values range, 0.01 ~ 0.9) or mTOR inhibitor (CI values range, 0.1 ~ 0.9) compared to the anti-tumor effect with single treatment in both SUM149 and MDA-MB-231 cells. However, E6201 combined with ATR inhibitor showed only a weak combinational anti-tumor effect in MDA-MB-231 cells only (Fig. 5a [SUM149] and 5b [MDA-MB-231]).
Figure 5. Combinational anti-tumor effect of E6201 in combination with CDK4/6, mTOR, or ATR inhibitor.
SUM149 (a) and MDA-MB-231 (b) cells were treated with E6201 in combination with other kinase inhibitor for 5 days, and viability was measured by using CellTiter-Blue and sulforhodamine-B assays. Data shown are representative of three independent experiments with similar results. error bars indicate standard deviation. Combinational index (CI) and fractional cell killing effect (Fa) were determined by using CalcuSyn software 2.1 (Biosoft). CI <0.1 indicates very strong synergism; 0.10-0.30, strong synergism; 0.31-0.70, synergism; 0.71-0.85, moderate synergism; 0.86-0.90, slight synergism; 0.91-1.10, nearly additive; 1.11-1.20, slight antagonism; 1.21-1.45, moderate antagonism; 1.46-3.30, antagonism; 3.31-10, strong antagonism; and >10, very strong antagonism.
Discussion
Here we showed that single-agent E6201, a MEK1 inhibitor, has anti-metastasis activity that leads to prolonged survival of mice with TNBC xenograft tumors. Our mechanistic studies showed that improved antimetastasis activity and survival were due to specific MAPK pathway inhibition. Our in vitro studies of E6201 against TNBC demonstrated similar growth inhibition compared to two other MEK inhibitors, pimasertib and trametinib; dose-dependent inhibition of TNBC cell colony formation in anchorage-independent growth conditions; and inhibition of pERK expression. In experimental and spontaneous metastasis models of TNBC, we found that E6201 inhibited lung metastasis, and this effect was associated with dose-dependent inhibition of pERK. These data demonstrate that E6201 has potential for use in treatment of TNBC that is dependent on the MAPK pathway.
Because the MAPK pathway plays a central role in regulating the growth and survival of cancer cells, this pathway has long been viewed as a promising target for anticancer therapy [17]. The MAPK pathway contributes to the survival and progression of cancers through diverse mechanisms [18–22]. MEK is a critical component of the MAPK pathway (Raf-MEK-ERK) and is a key regulator of cell proliferation, survival, differentiation, and malignant transformation. In TNBC chemotherapy resistance, the MAPK pathway regulates downstream caspase-mediated apoptosis pathways favoring cancer cell survival [23]. While a substantial body of literature is available on MEK inhibitors and their effects on other solid tumors, such as lung, colorectal, and head and neck, the effect of MEK inhibitors on breast cancer has been less well elucidated to date. Inhibitors of MEK induce cell death in TNBC, but with only partial tumor growth inhibition [24]. Inhibitors of MEK effectively inhibit the phosphorylation of ERK as well as tumor growth and metastasis but exhibit moderate activity as single agents against TNBC [24]. In cancer cells with nongenomic MAPK activation, such as the majority of breast cancers, MEK inhibitors can modulate the immune microenvironment by up-regulation of tumor antigen expression and presentation [25].
Overall in breast cancer, the biological contribution of the MAPK pathway is less studied than the biological contribution of the PI3K-AKT-mTOR pathway. Currently, 15 ongoing studies are testing MEK inhibitors in breast cancer [26], and the majority of these studies are testing the efficacy of MEK inhibitors in combination with other agents instead of the efficacy of MEK inhibitors as single agents. Combinations of MEK inhibitor and checkpoint inhibitor are being studied in breast cancer, although the rationale for such combinations is based on data from other cancers. For example, a trial of the combination of atezolizumab, cobimetinib, and eribulin (NCT 03202316) that is looking at the effect of MAPK inhibition on the immune microenvironment of advanced breast cancer is based on data from lung and colorectal cancer. Our study of MAPK inhibitors enhances the knowledge of pathway inhibition in breast cancer and thereby provides a further direct disease site-oriented rationale for translation.
Three MEK inhibitors, trametinib, cobimetinib, and binimetinib, have been approved in combination with BRAF inhibitors for patients with BRAF-mutated metastatic melanoma, non-small cell lung cancer, and anaplastic thyroid cancer [27]. Although the rate of BRAF gene alteration in breast cancer is low (1.2%) [28] and MAP2K1 gene alteration in breast cancer is rare, aberrant RAF-MEK signaling activation by tyrosine kinases is frequently observed in breast cancer. EGFR protein expression has been found in 15% to 45% of TNBC patient samples [29], and IGF-1R protein expression has been found in 25% to 40% of TNBC patient samples [30]. Elevated c-Src tyrosine kinase activity has been found in TNBC patient samples as well [31]. Preclinical data indicated that both EGFR and IGF-1R are key activators of the MAPK signaling pathway and mediated TNBC cell proliferation and survival as well as TNBC clinical disease progression [32,33]. TNBC patients with high ERK2 expression are at higher risk of death than those with low ERK2 expression [34]. The MAPK pathway has been shown to be a marker of breast cancer metastasis [8,35,36].
MEK inhibitors, including both approved and investigational agents, have shown in vitro and in vivo preclinical anticancer activity in breast cancer cell lines, including TNBC and basal-like breast cancer. In preclinical studies, MEK inhibitors have demonstrated anticancer efficacy against TNBC cells, but the strength of the activity has differed by cell line, and MEK inhibitors as single agents have produced mostly moderate activity [37]. ATP noncompetitive MEK inhibitors selumetinib, trametinib, pimasertib, and U0126 showed TNBC cell growth inhibition and tumor growth inhibition in xenograft models [8,9,38,39]. Our previous study showed that high-pEGFR-expressing TNBC cell lines, including MDA-MB-468, MDA-MB-231, and SUM149, were more sensitive to pimasertib or trametinib treatment than were low-pEGFR-expressing TNBC cell lines [9,40]. E6201 is an ATP-competitive inhibitor; thus, we anticipate that cellular ATP concentration may affect the efficacy of E6201. In the current study, we observed a strong anti-proliferative effect of E6201, and this efficacy was not correlated with TNBC molecular subtype. ATP-competitive inhibitors must have high affinity due to competing with intracellular ATP; our data indicated that E6201 effectively inhibited the ATP binding site of MEK and had an IC50 concentration similar to that of ATP-non-competitive MEK inhibitors.
MEK’s inhibitory effect in breast cancer is of short duration because of resistance developing from multiple bypass feedback loops, including activation of RTK or PI3K/AKT/mTOR pathways [32,41,42]. Because of these dynamic resistance mechanisms in breast cancer, monotherapy with a MEK inhibitor is probably not an effective strategy, especially for treating breast cancer and specifically TNBC. MEK inhibitor dynamic resistance mechanisms most likely explain the relatively low efficacy of MEK inhibitor monotherapy seen in completed clinical trials, including those with breast cancer patients [43,44]. These trials showed no evidence of clinical response in breast cancer patients (unspecified subtypes). Consequently, to our knowledge, there are no ongoing clinical trials with MEK inhibitor monotherapy focused on breast cancer or TNBC and only MEK inhibitor combination clinical trials are currently being performed in breast cancer patients (clinicaltrials.gov). Dual inhibition strategies involving combination of MEK inhibitors with other agents are probably needed for efficacy in breast cancer, and this concept is supported by preclinical studies showing synergistic in vitro and in vivo anticancer activity with combination of MEK inhibitors with other agents in TNBC [9,32,39,45–47]. Preclinical studies showed that MEK inhibitors enhanced the anti-tumor effects of BRAF, EGFR, PI3K, mTOR, and CDK4/6 inhibitors in advanced solid tumors [48–51]. In chemotherapy-resistant TNBC, alteration of MAPK pathway proteins was noted in more than 30% of patient samples [52]. Given the up-regulation of MAPK pathway in chemotherapy-resistant TNBC, the combination of the MEK inhibitor cobimetinib and the taxane paclitaxel is being evaluated as first-line treatment in patients with advanced TNBC (COLET study) [53]. Recently, a clinical study of a MEK inhibitor in combination with a checkpoint inhibitor in advanced breast cancer was initiated [54]. Our current findings, taken together with previously reported findings, suggest that the best use of MEK inhibitors in TNBC may be in combination with other drugs or as maintenance therapy in patients with metastatic disease. We previously showed that the MEK inhibitor selumetinib had modest activity but could prevent the growth and metastasis of TNBC [8], and our current findings with E6201 were similar. Therefore, E6201 combinational treatment may be necessary to maximize anti-tumor and anti-metastasis activity against TNBC.
The results of our current study support the use of a MEK inhibitor as part of a novel strategy to keep TNBC in check and prevent metastasis. Progression-free survival is an important clinical outcome for patients with breast cancer. Often, metastasis continues even during active therapy, requiring a change of therapeutics with additional toxicity and reduced quality of life. Thus, it is important to develop a novel “anti-metastasis” strategy. In summary, we have demonstrated the efficacy of MEK1 inhibitor E6201 in suppressing the progression of tumor growth and improving survival in preclinical models of TNBC. In our models, the efficacy of E6201 against TNBC was not via inhibition of FLT3. Collectively, our data provide a rationale for clinical investigation of targeting the MAPK pathway and possible MEK1-inhibitor-based combination therapy in metastatic TNBC.
Supplementary Material
Supplemental figure 1. Anti-proliferation effect of MEK inhibitors in TNBC cell lines. TNBC cells were treated with selumetinib, pimasertib, or trametinib for 5 days, and viability was measured by using CellTiter-Blue and sulforhodamine-B assays. Data shown are representative of three experiments with similar results. Each point represents the mean of three independent experiments; error bars indicate standard deviation.
Supplemental figure 2. FLT3 inhibition does not affect TNBC cell proliferation, migration, or invasion. a Western blotting of cells treated with E6201 for the indicated times. b Proliferation assay. TNBC cell lines were treated with quizartinib for 5 days, and viability was measured by using CellTiter-Blue. c and d Migration and invasion assays. TNBC cells (1×105/well) were added into trans-wells with or without quizartinib for 6 hr (migration, c) or 24 hr (invasion, d). Migration and invasion were evaluated by using ImageJ software. Data shown are representative of three experiments with similar results; error bars indicate standard deviation.
Acknowledgments:
We thank Sunita Patterson and Stephanie Deming of the Department of Scientific Publications at MD Anderson Cancer Center for editing the manuscript.
Funding: This work was supported by the Morgan Welch Inflammatory Breast Cancer Research Program, the State of Texas Rare and Aggressive Breast Cancer Research Program, MD Anderson’s Cancer Center Support Grant (P30CA016672, used the Characterized Cell Line Core Facility and Flow Cytometry and Cellular Imaging Facility), and Spirita Oncology, LLC.
Footnotes
Compliance with Ethical Standards
Ethical approval involving animals: Animal studies and procedures were approved by The University of Texas MD Anderson Cancer Center Animal Care and Use Committee. Protocol #: 00000968-RN02 (approval date 5/1/2015, expiration date 4/22/2021)
Ethical approval involving human participants: This article does not contain any studies with human participants by any of the authors.
Disclosure of potential conflicts of interest: Naoto T. Ueno has a research agreement with Spirita Oncology, LLC. Linda J. Paradiso and Thomas Myers are employees of Spirita Oncology, LLC. All other authors declare no potential conflicts of interest.
References
- 1.Ismail-Khan R, Bui MM (2010) A review of triple-negative breast cancer. Cancer Control 17 (3):173–176. [DOI] [PubMed] [Google Scholar]
- 2.Brouckaert O, Wildiers H, Floris G, Neven P (2012) Update on triple-negative breast cancer: prognosis and management strategies. Int J Womens Health 4:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13 (15 Pt 1):4429–4434. [DOI] [PubMed] [Google Scholar]
- 4.Santarpia L, Lippman SM, El-Naggar AK (2012) Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 16 (1):103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eroglu Z, Ribas A (2016) Combination therapy with BRAF and MEK inhibitors for melanoma: latest evidence and place in therapy. Ther Adv Med Oncol 8 (1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran KA, Cheng MY, Mitra A, Ogawa H, Shi VY, Olney LP, et al. (2016) MEK inhibitors and their potential in the treatment of advanced melanoma: the advantages of combination therapy. Drug Des Devel Ther 10:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Liu YH, Sun X, Yu MW, Yang L, Cheng PY, et al. (2017) Risk of peripheral edema in cancer patients treated with MEK inhibitors: a systematic review and meta-analysis of clinical trials. Curr Med Res Opin 33 (9):1663–1675. [DOI] [PubMed] [Google Scholar]
- 8.Bartholomeusz C, Xie X, Pitner MK, Kondo K, Dadbin A, Lee J, et al. (2015) MEK Inhibitor Selumetinib (AZD6244; ARRY-142886) Prevents Lung Metastasis in a Triple-Negative Breast Cancer Xenograft Model. Mol Cancer Ther 14 (12):2773–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Galloway R, Grandjean G, Jacob J, Humphries J, Bartholomeusz C, et al. (2015) Comprehensive Two-and Three-Dimensional RNAi Screening Identifies PI3K Inhibition as a Complement to MEK Inhibitor AS703026 for Combination Treatment of Triple-Negative Breast Cancer. J Cancer 6 (12):1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muramoto K, Goto M, Inoue Y, Ishii N, Chiba K, Kuboi Y, et al. (2010) E6201, a novel kinase inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-1 and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase-1: in vivo effects on cutaneous inflammatory responses by topical administration. J Pharmacol Exp Ther 335 (1):23–31. [DOI] [PubMed] [Google Scholar]
- 11.Ikemori-Kawada M, Inoue A, Goto M, Wang YJ, Kawakami Y (2012) Docking simulation study and kinase selectivity of f152A1 and its analogs. J Chem Inf Model 52 (8):2059–2068. [DOI] [PubMed] [Google Scholar]
- 12.Byron SA, Loch DC, Wellens CL, Wortmann A, Wu J, Wang J, et al. (2012) Sensitivity to the MEK inhibitor E6201 in melanoma cells is associated with mutant BRAF and wildtype PTEN status. Mol Cancer 11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gampa G, Kim M, Cook-Rostie N, Laramy JK, Sarkaria JN, Paradiso L, et al. (2018) Brain Distribution of a Novel MEK Inhibitor E6201: Implications in the Treatment of Melanoma Brain Metastases. Drug Metab Dispos 46 (5):658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tibes R, Borad MJ, Dutcus CE, Reyderman L, Feit K, Eisen A, et al. (2018) Safety, pharmacokinetics, and preliminary efficacy of E6201 in patients with advanced solid tumours, including melanoma: results of a phase 1 study. Br J Cancer 118 (12):1580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Borthakur G, Gao C, Chen Y, Mu H, Ruvolo VR, et al. (2016) The Dual MEK/FLT3 Inhibitor E6201 Exerts Cytotoxic Activity against Acute Myeloid Leukemia Cells Harboring Resistance-Conferring FLT3 Mutations. Cancer Res 76 (6):1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carragher NO, Frame MC (2004) Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol 14 (5):241–249. [DOI] [PubMed] [Google Scholar]
- 17.Wallace EM, Lyssikatos JP, Yeh T, Winkler JD, Koch K (2005) Progress towards therapeutic small molecule MEK inhibitors for use in cancer therapy. Curr Top Med Chem 5 (2):215–229 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell C, Yacoub A, Hossein H, Martin AP, Bareford MD, Eulitt P, et al. (2010) Inhibition of MCL-1 in breast cancer cells promotes cell death in vitro and in vivo. Cancer Biol Ther 10 (9):903–917. doi: 10.4161/cbt.10.9.13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chetoui N, Sylla K, Gagnon-Houde JV, Alcaide-Loridan C, Charron D, Al-Daccak R, et al. (2008) Down-regulation of mcl-1 by small interfering RNA sensitizes resistant melanoma cells to fas-mediated apoptosis. Mol Cancer Res 6 (1):42–52. doi: 10.1158/1541-7786.MCR-07-0080 [DOI] [PubMed] [Google Scholar]
- 20.Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, et al. (2012) MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia 26 (4):778–787. doi: 10.1038/leu.2011.287 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Hermanson DL, Das SG, Li Y, Xing C (2013) Overexpression of Mcl-1 confers multidrug resistance, whereas topoisomerase IIbeta downregulation introduces mitoxantrone-specific drug resistance in acute myeloid leukemia. Mol Pharmacol 84 (2):236–243. doi: 10.1124/mol.113.086140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Schiff DS, Lin Y, Neboori HJ, Goyal S, Feng Z, et al. (2014) Ionizing radiation sensitizes breast cancer cells to Bcl-2 inhibitor, ABT-737, through regulating Mcl-1. Radiat Res 182 (6):618–625. doi: 10.1667/RR13856.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami H, Huang S, Pal K, Dutta SK, Mukhopadhyay D, Sinicrope FA (2016) Mutant BRAF Upregulates MCL-1 to Confer Apoptosis Resistance that Is Reversed by MCL-1 Antagonism and Cobimetinib in Colorectal Cancer. Mol Cancer Ther 15 (12):3015–3027. doi: 10.1158/1535-7163.mct-16-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartholomeusz C, Xie X, Pitner MK, Kondo K, Dadbin A, Lee J, et al. (2015) MEK inhibitor selumetinib (AZD6244; ARRY-142886) prevents lung metastasis in a triple-negative breast cancer xenograft model. Mol Cancer Ther. doi: 10.1158/1535-7163.mct-15-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arthur JS, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13 (9):679–692. doi: 10.1038/nri3495 [DOI] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov (2019) Search of: MEK inhibitor | Interventional Studies | breast cancer - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=breast+cancer&term=MEK+inhibitor&type=Intr&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&lupd_s=&lupd_e=&sort=.
- 27.Cheng Y, Tian H (2017) Current Development Status of MEK Inhibitors. Molecules 22 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albanell J, Elvin JA, Ali SM, Schrock AB, Chung J, Vergilio J-A, et al. (2017) BRAF: An emerging target for triple-negative breast cancer. Journal of Clinical Oncology 35 (15_suppl):1099–1099. [Google Scholar]
- 29.Rimawi MF, Shetty PB, Weiss HL, Schiff R, Osborne CK, Chamness GC, et al. (2010) Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer 116 (5):1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, et al. (2008) Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res 68 (24):10238–10246. [DOI] [PubMed] [Google Scholar]
- 31.Verbeek BS, Vroom TM, Adriaansen-Slot SS, Ottenhoff-Kalff AE, Geertzema JG, Hennipman A, et al. (1996) c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J Pathol 180 (4):383–388. [DOI] [PubMed] [Google Scholar]
- 32.Maiello MR, D’Alessio A, Bevilacqua S, Gallo M, Normanno N, De Luca A (2015) EGFR and MEK Blockade in Triple Negative Breast Cancer Cells. J Cell Biochem 116 (12):2778–2785. [DOI] [PubMed] [Google Scholar]
- 33.Nakai K, Hung MC, Yamaguchi H (2016) A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res 6 (8):1609–1623 [PMC free article] [PubMed] [Google Scholar]
- 34.Bartholomeusz C, Oishi T, Saso H, Akar U, Liu P, Kondo K, et al. (2012) MEK1/2 inhibitor selumetinib (AZD6244) inhibits growth of ovarian clear cell carcinoma in a PEA-15-dependent manner in a mouse xenograft model. Mol Cancer Ther 11 (2):360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Zheng Z, Chen L, Zheng H (2017) MAPK, NFkappaB, and VEGF signaling pathways regulate breast cancer liver metastasis. Oncotarget 8 (60):101452–101460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao GL, Wang MC, Fan XL, Zhong L, Ji SF, Sang G, et al. (2018) Correlation Between Raf/MEK/ERK Signaling Pathway and Clinicopathological Features and Prognosis for Patients With Breast Cancer Having Axillary Lymph Node Metastasis. Technol Cancer Res Treat 17:1533034617754024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres-Adorno AM, Lee J, Kogawa T, Ordentlich P, Tripathy D, Lim B, et al. (2017) Histone Deacetylase Inhibitor Enhances the Efficacy of MEK Inhibitor through NOXA-Mediated MCL1 Degradation in Triple-Negative and Inflammatory Breast Cancer. Clin Cancer Res 23 (16):4780–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jing J, Greshock J, Holbrook JD, Gilmartin A, Zhang X, McNeil E, et al. (2012) Comprehensive predictive biomarker analysis for MEK inhibitor GSK1120212. Mol Cancer Ther 11 (3):720–729. [DOI] [PubMed] [Google Scholar]
- 39.Nagaria TS, Shi C, Leduc C, Hoskin V, Sikdar S, Sangrar W, et al. (2017) Combined targeting of Raf and Mek synergistically inhibits tumorigenesis in triple negative breast cancer model systems. Oncotarget 8 (46):80804–80819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato N, Wakabayashi M, Nakatsuji M, Kashiwagura H, Shimoji N, Sakamoto S, et al. (2017) MEK and PI3K catalytic activity as predictor of the response to molecularly targeted agents in triple-negative breast cancer. Biochem Biophys Res Commun 489 (4):484–489. [DOI] [PubMed] [Google Scholar]
- 41.Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, et al. (2012) Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell 149 (2):307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mirzoeva OK, Das D, Heiser LM, Bhattacharya S, Siwak D, Gendelman R, et al. (2009) Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res 69 (2):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 26 (13):2139–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, et al. (2004) Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol 22 (22):4456–4462. [DOI] [PubMed] [Google Scholar]
- 45.Hoeflich KP, O’Brien C, Boyd Z, Cavet G, Guerrero S, Jung K, et al. (2009) In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res 15 (14):4649–4664. [DOI] [PubMed] [Google Scholar]
- 46.Leung EY, Kim JE, Askarian-Amiri M, Rewcastle GW, Finlay GJ, Baguley BC (2014) Relationships between signaling pathway usage and sensitivity to a pathway inhibitor: examination of trametinib responses in cultured breast cancer lines. PLoS One 9 (8):e105792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Swearingen AED, Sambade MJ, Siegel MB, Sud S, McNeill RS, Bevill SM, et al. (2017) Combined kinase inhibitors of MEK1/2 and either PI3K or PDGFR are efficacious in intracranial triple-negative breast cancer. Neuro Oncol 19 (11):1481–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corcoran RB, Andre T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. (2018) Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAF(V600E)-Mutant Colorectal Cancer. Cancer Discov 8 (4):428–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaudio E, Tarantelli C, Kwee I, Barassi C, Bernasconi E, Rinaldi A, et al. (2016) Combination of the MEK inhibitor pimasertib with BTK or PI3K-delta inhibitors is active in preclinical models of aggressive lymphomas. Ann Oncol 27 (6):1123–1128. [DOI] [PubMed] [Google Scholar]
- 50.Lee MS, Helms TL, Feng N, Gay J, Chang QE, Tian F, et al. (2016) Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models. Oncotarget 7 (26):39595–39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao H, Cui K, Nie F, Wang L, Brandi MB, Jin G, et al. (2012) The effect of mTOR inhibition alone or combined with MEK inhibitors on brain metastasis: an in vivo analysis in triple-negative breast cancer models. Breast Cancer Res Treat 131 (2):425–436. [DOI] [PubMed] [Google Scholar]
- 52.Balko JM, Giltnane JM, Wang K, Schwarz LJ, Young CD, Cook RS, et al. (2014) Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov 4 (2):232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brufsky A, Miles D, Zvirbule Z, Eniu A, Lopez-Miranda E, Seo JH, et al. (2018) Abstract P5-21-01: Cobimetinib combined with paclitaxel as first-line treatment for patients with advanced triple-negative breast cancer (COLET study): Primary analysis of cohort I. Cancer Research. SABCS17-P5-21-01 [Google Scholar]
- 54.Chumsri S, Polley M-Y, Anderson SL, O’Sullivan CCM, Colon-Otero G, Knutson KL, et al. (2018) Phase I/II trial of pembrolizumab in combination with binimetinib in unresectable locally advanced or metastatic triple negative breast cancer. Journal of Clinical Oncology 36 (5_suppl):TPS17–TPS17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Anti-proliferation effect of MEK inhibitors in TNBC cell lines. TNBC cells were treated with selumetinib, pimasertib, or trametinib for 5 days, and viability was measured by using CellTiter-Blue and sulforhodamine-B assays. Data shown are representative of three experiments with similar results. Each point represents the mean of three independent experiments; error bars indicate standard deviation.
Supplemental figure 2. FLT3 inhibition does not affect TNBC cell proliferation, migration, or invasion. a Western blotting of cells treated with E6201 for the indicated times. b Proliferation assay. TNBC cell lines were treated with quizartinib for 5 days, and viability was measured by using CellTiter-Blue. c and d Migration and invasion assays. TNBC cells (1×105/well) were added into trans-wells with or without quizartinib for 6 hr (migration, c) or 24 hr (invasion, d). Migration and invasion were evaluated by using ImageJ software. Data shown are representative of three experiments with similar results; error bars indicate standard deviation.