Abstract
Objectives:
Electrocochleography (ECochG) obtained through a cochlear implant (CI) is increasingly being tested as an intraoperative monitor during implantation with the goal of reducing surgical trauma. Reducing trauma should aid in preserving residual hearing and improve speech perception overall. The purpose of this study was to characterize intracochlear ECochG responses throughout insertion in a range of array types and, when applicable, relate these measures to hearing preservation. The ECochG signal in CI subjects is complex, consisting of hair cell and neural generators with differing distributions depending on the etiology and history of hearing loss. Consequently, a focus was to observe and characterize response changes as an electrode advances.
Design:
In 36 human subjects, responses to 90 dB nHL tone bursts were recorded both at the round window (RW) and then through the apical contact of the CI as the array advanced into the cochlea. The specific setup used a sterile clip in the surgical field, attached to the ground of the implant with a software-controlled short to the apical contact. The end of the clip was then connected to standard audiometric recording equipment. The stimuli were 500 Hz tone bursts at 90 dB nHL. Audiometry for cases with intended hearing preservation (12/36 subjects) were correlated with intraoperative recordings.
Results:
Successful intracochlear recordings were obtained in 28 subjects. For the eight unsuccessful cases, the clip introduced excessive line noise which saturated the amplifier. Among the successful subjects, the initial intracochlear response was a median 5.8 dB larger than the response at the RW. Throughout insertion, modiolar arrays showed median response drops after stylet removal while in lateral wall arrays the maximal median response magnitude was typically at the deepest insertion depth. Four main patterns of response magnitude were seen: increases >5 dB (12/28), steady responses within 5 dB (4/28), drops >5 dB (from the initial response) at shallow insertion depths (< 15 mm deep, 7/28), or drops >5 dB occurring at deeper depths (5/28). Hearing preservation, defined as <80 dB threshold at 250 Hz, was successful in 9/12 subjects. In these subjects, an intracochlear loss of response magnitude afforded a prediction model with poor sensitivity and specificity, which improved when phase, latency and proportion of neural components was considered. The change in hearing thresholds across cases was significantly correlated with various measures of the absolute magnitudes of response, including RW response, starting response, maximal response, and final responses (p’s<0.05, min of 0.0001 for the maximal response, r’s > 0.57, max of 0.80 for the maximal response).
Conclusions:
Monitoring the cochlea with intracochlear ECochG during CI is feasible and patterns of response vary by device type. Changes in magnitude alone did not account for hearing preservation rates, but considerations of phase, latency and neural contribution can help to interpret the changes seen and improve sensitivity and specificity. The correlation between the absolute magnitude obtained either prior to or during insertion of the ECochG and the hearing threshold changes suggest that cochlear health, which varies by subject, plays an important role.
Introduction
Minimizing cochlear trauma during insertion of a cochlear implant array could improve speech perception outcomes and help preserve residual hearing when it is present. Surgical techniques have been implemented to minimize intra-insertion trauma including the use of shorter, lateral wall arrays and round window (RW) insertions rather than drilling cochleostomies (Adunka, Pillsbury et al. 2009). Despite these attempts, greater than 50% of subjects lose at least 10 dB in hearing across frequencies, presumably due to intraoperative trauma to the basilar membrane and/or postoperative fibrosis (Jurawitz, Büchner et al. 2014, Santa Maria, Gluth et al. 2014, Kamakura and Nadol 2016, O’Connell, Holder et al. 2017). Objective measures to detect and ideally avoid insertion trauma are an area of current exploration, but one technique showing promise is electrocochleography (ECochG). As a surrogate for basilar membrane (BM) integrity, a working hypothesis by our group and others is that a reduction in physiological responses from the cochlea in response to sound during CI insertion could signify acute trauma leading to loss of residual hearing and/or poor speech perception outcomes (Dalbert, Sim et al. 2014, Adunka, Giardina et al. 2015, Dalbert, Sim et al. 2015, Dalbert, Huber et al. 2016). ECochG responses can be acquired throughout CI insertion at either an extracochlear site (Mandala, Colletti et al. 2012, Radeloff, Shehata-Dieler et al. 2012, Dalbert, Pfiffner et al. 2018, Giardina, Khan et al. 2018) or from an intracochlear site through the apical array contact as it is inserted (Calloway, Fitzpatrick et al. 2014, Campbell, Kaicer et al. 2015, Acharya, Tavora-Vieira et al. 2016, Campbell, Kaicer et al. 2016, Campbell, Bester et al. 2017, Harris, Riggs et al. 2017, Harris, Riggs et al. 2017, O’Connell, Holder et al. 2017). We recently compared and evaluated extracochlear recording sites for this purpose (Giardina, Khan et al. 2018) and in this current study are considering the strengths and weakness of intracochlear recordings. In particular, we describe the response patterns for three different array types, and comment on a variety of intraoperative metrics that could be useful to account for hearing preservation or loss.
Studies in animals support the possibility that ECochG can be used to detect acute trauma during insertion. Using a rigid electrode designed to penetrate the basilar membrane, intraoperative drops in the response magnitudes to tones were associated with post-operative histologic trauma (Adunka, Mlot et al. 2010, Choudhury, Adunka et al. 2011, Ahmad, Choudhury et al. 2012). However, when a flexible electrode was used, drops in ECochG response magnitude could be reversible when the electrode was withdrawn, and in these cases histological examination verified that basilar membrane integrity was maintained (Demason, Choudhury et al. 2012). The most sensitive metric was a drop in the ongoing response magnitude to a tone, containing the cochlear microphonic (CM) and auditory nerve neurophonic (ANN), rather than changes in the compound action potential (CAP). In a study using animals with high-pass noise induced hearing loss, intended to mimic the condition of many CI subjects who have a high-frequency sloping hearing loss, trauma in the basal regions was found to affect the magnitude of a response to low frequency tones whose responses elements are located far apically (Choudhury, Adunka et al. 2011). Thus, the results of these animal studies indicated that 1) the CM was a more sensitive detector of trauma than the CAP, 2) responses from all parts of the cochlea could be obtained even if the generators, i.e. those neural and hair cell elements responsive to sound, were restricted to the apical region, and 3) reductions in response magnitudes to intense sound were a sensitive measure of interactions between an inserted array and cochlear tissues. Recent studies with normal-hearing animals demonstrate responses to higher frequency stimuli can also help elucidate basilar membrane trauma (Lo, Bester et al. 2017). Additionally, in human CI subjects with large degrees of high frequency hearing loss, markers such as CAP amplitude are highly variable and of limited utility in predicting speech outcomes with the implant (Scott, Giardina et al. 2016). As such, the ongoing response magnitude to a loud, low-frequency (500 Hz) tone is the metric most studied with regard to basilar membrane trauma in CI subjects.
There are currently two approaches to intracochlear recording. Both approaches use array contacts as the recording electrodes, but they differ in the means of outside connection to the contact and subsequent amplification and digitization. In one approach, recordings are digitized by the CI itself, and data are reported directly through the device’s telemetry (Acharya, Tavora-Vieira et al. 2016, Campbell, Kaicer et al. 2016, Harris, Riggs et al. 2017, O’Connell, Holder et al. 2017). While familiar to set up, since it uses the same connection to the magnet used for intraoperative impedance testing, data acquisition is limited in terms of available gain, sampling rates and time windows for recording, which affect both the sensitivity and the time required for recordings. The second approach is to use standard audiologic recording equipment that connects directly to the CI, using a clip in the surgical field attached to the device’s ground, and software through the CI that creates a connection between the ground and the most-apical contact on the array (Harris, Riggs et al. 2017). For this study we used the clip approach to maximize sensitivity and speed of the recordings.
With either recording approach various response patterns are observed as the array advances deeper into the cochlea. When recording from a modified MED-EL lateral wall array with a direct connection (no processor) to an exposed wire connected to the apical contact during a temporary insertion, response magnitudes tended to increase with depth, although this increase did not happen in all cases (Calloway, Fitzpatrick et al. 2014). With the Advanced Bionics MidScalar arrays (Harris, Riggs et al. 2017, O’Connell, Holder et al. 2017), responses remained steady, increased, or increased and then decreased with depth. With Cochlear Corporation’s Slim Straight (CI422/522) arrays, responses grew immediately, and a late drop in CM amplitude was associated with subsequent hearing loss (Campbell, Kaicer et al. 2016, Campbell, Bester et al. 2017).
The ECochG is a complex signal, and major hurdles remain in determining which parts of the signals and what types of changes indicate that trauma is occurring, and whether these changes in response are array-specific. The purpose of this study was to characterize intracochlear ECochG responses throughout CI insertion in a variety of array types and, when applicable, relate these intraoperative metrics to postoperative hearing preservation.
Materials and Methods
Subjects and Inclusion Criteria
A primary goal was to assess intracochlear responses across a broad range of recipients and device types, so inclusion criteria allowed CI recipients of any age, deafness etiology, and audiometric hearing status to participate. To this end, 36 subjects receiving CIs were enrolled, which included 5 adults, 18 children aged 2–18 years old, and 13 children under 2 years of age (Table I). Twelve subjects had some degree of pre-operative hearing, and 11 of these subjects received lateral wall arrays while one subject opted for a midscalar array. Software to use the clip recording system was available for subjects receiving Cochlear Corporation or Advanced Bionics arrays. Patients were excluded if they required an English interpreter, had anatomic malformations or if the procedure was a revision/replacement. All research was approved by the institution IRB (UNC IRB Protocol No. 05–2616). Consent was obtained for subjects over the age of 18, parental permission was required for subjects under the age of 18, and children aged 7 to 18 were also asked for assent with age-appropriate forms.
Table I:
Patient Demographics. Thirty-six subjects were enrolled in the study, with slightly more women than men. The age at implantation ranged from 9 months to 72 years of age. Sensorineural hearing loss etiologies include auditory neuropathy spectrum disorder (ANSD), genetic causes, and trauma, but most subjects had an unknown etiology. Implant sides were represented roughly equally and devices used include the AB MidScala, and Cochlear Corporation’s CI512, and CI422/522 arrays. ANSD = auditory neuropathy spectrum disorder, CMV = cytomegalovirus, EVA = enlarged vestibular aqueducts, SNHL = sensorineural hearing loss.
| Characteristic | Count | % Total |
|---|---|---|
| Sex | ||
| Female | 21 | 58% |
| Male | 15 | 42% |
| Age at Implantation | ||
| 0 ≤ 2 yr old | 13 | 36% |
| 2 ≤ 5 yr old | 9 | 25% |
| 6 ≤ 18 yr old | 9 | 25% |
| 18 ≤ 72 yr old | 5 | 14% |
| SNHL Etiology | ||
| ANSD | 8 | 22% |
| Autoimmune | 1 | 3% |
| CLDN14 mutation | 2 | 6% |
| CMV | 2 | 6% |
| Connexin 26 mutation | 2 | 6% |
| EVA | 2 | 6% |
| TECTA mutation | 2 | 6% |
| Trauma | 1 | 3% |
| Unknown | 16 | 44% |
| Implanted Side | ||
| Left | 17 | 47% |
| Right | 19 | 53% |
| Device | ||
| Advanced Bionics MidScala | 5 | 14% |
| Cochlear 512 | 20 | 56% |
| Cochlear 422/522 | 11 | 31% |
Surgical Approach and Recording Setup at the Round Window
Anesthesia was induced and a foam in-ear insert connected to an Etymotic ER-3b speaker was placed in the ipsilateral external canal for delivery of acoustic stimulation. For recording, reference and common electrodes were adhesive surface electrodes placed on the contralateral mastoid and forehead, respectively. A transmastoid facial-recess approach was then performed by surgeons to expose the round window niche. Acoustic stimuli were delivered and evoked responses recorded with the Bio-logic Navigator Pro (Natus Medical Inc., San Carlos, CA). For preliminary recordings before implantation, a Neurosign monopolar electrode (Neurosign Surgical Inc. Part 3602–00, Carmarthenshire, UK) was placed within the RW niche and served as the active recording input. Acoustic stimuli were alternating polarity tone bursts (250 Hz, 500 Hz, 750 Hz, 1 kHz, 2 kHz, 4 kHz) at 90 dB nHL. Tone bursts were shaped by a Blackman window and had a rise/fall length of one cycle or 1 ms, whichever was longer, and plateau lengths of 20 cycles or 20 ms, whichever was longer. Stimuli were presented at 17.3 Hz and digitized at a sample rate of 16 kHz except for the 4 kHz stimuli, which was presented at 23.3 Hz and digitized at a sample rate of 48 kHz. The amplifier gain was 50,000x and the bandpass filter was set from 10 Hz to 5 kHz for all stimuli except 4 kHz, which had a bandpass upper limit of 10 kHz.
Recording through the CI with the clip during Insertion
Once the RW recordings were completed, the monopolar ground electrode was removed from the field and the CI processor was seated under the temporalis muscle. With a sterile ultrasound drape, a telemetry magnet was placed over the skin above the processor and a laptop established a connection with the implanted device (Fig. 1). Software provided allowed the array’s apical contact to be shorted to the extracochlear cylindrical ground rod (Cochlear Corporation ECE1 contact) or ground ring (Advanced Bionics IE1 contact). For both implant types (Cochlear Corporation and Advanced Bionics), the software ultimately created a direct electrical connection between the deepest (apical) array contact and the BioLogic recording device through the clip connection which was electrically isolated from the surgical field. For the CI512 device, the array was inserted through a cochleostomy whereas the CI522 and AB MidScala devices were advanced through the RW.
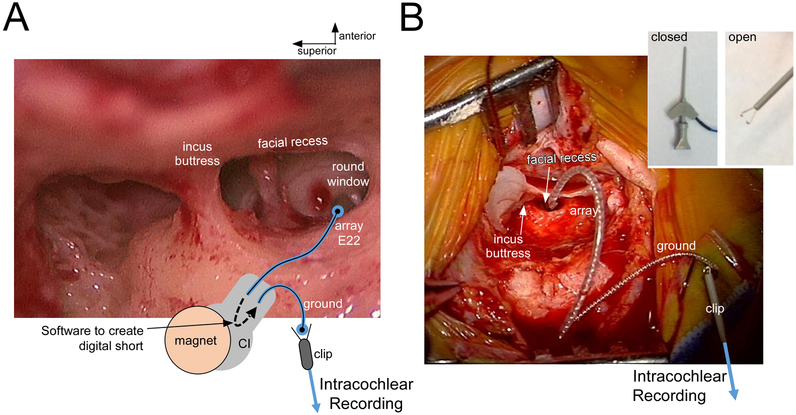
Figure 1.
Intraoperative setup for recording intracochlear ECochG with a Cochlear Corporation array. (A) View through surgical microscope with superimposed schematic diagram. The array’s deepest contact (E22) is digitally shorted to the ground rod contact (ECE1) when the processor is connected and software delivered through the telemetry is used. With this connection made, a clip electrode is connected to the ground rod and fed to the BioLogic recording device. (B) Photograph of the intraoperative recording setup, after the array was fully inserted. The ground and clip are floating above the surgical field (isolated electrically from the patient). Inset: Open vs Closed positioning of the clip electrode.
Using the set-up described above, responses to a 500 Hz tone at 90 dB nHL were collected throughout all stages of CI insertion, and the associated insertion depth for each recording was reported orally by the surgeons. In this way, response ‘tracks’, or magnitude changes throughout insertion, could be plotted as a function of insertion depth. The tones were delivered at 500 Hz and 90 dB nHL, with a rise/fall time of 1 ms, presented at a 17.3 Hz stimulus rate. During recordings with the clip systems there was line-noise contamination greater than that seen with the RW recordings. Consequently, the gain was lowered to 20,000 and the high pass filter setting was increased to 300 Hz.
ECochG Signal Analysis
Recordings at the RW and through the intracochlear clip system were exported to MATLAB (MathWorks, Natick, MA) and analyzed postoperatively. Responses were windowed with a Blackman function to isolate the ongoing portion, typically from 7 to 23 ms, and the response magnitude of the averaged waveform was calculated as the sum of spectral peaks at the fundamental stimulus frequency and the next two harmonics with significant peaks. A response peak was considered significant when its amplitude was at least 3 standard deviations above the noise floor, which was computed from the average FFT magnitude of 6 frequency bins (62 Hz wide, 3 above and 3 below the stimulus frequency). As described previously, the ongoing portion to a low frequency (like 500 Hz) can contains both the cochlear microphonic (CM) and auditory-nerve neurophonic (ANN) (Forgues, Koehn et al. 2014, McClellan, Formeister et al. 2014, Fontenot, Giardina et al. 2017) so distortions producing multiple, large spectral peaks in the responses to single tones were common. In addition to the spectrum of the ongoing response, the ‘average cycle,’ or average across all condensation and rarefaction (shifted in time to match the condensation) phase cycles in the ongoing response, was examined for evidence of the ANN. Animal studies using neurotoxins have identified distortions due to the ANN in the average cycle that are not always detectable in the spectrum (Fontenot, Giardina et al. 2017, Riggs, Roche et al. 2017). At the RW prior to insertion, the ‘Total Response’ (TR) is the sum of individual response magnitudes to 250 Hz, 500 Hz, 750 Hz, 1 kHz, 2 kHz, and 4 kHz at 90 dB nHL (Fitzpatrick, Campbell et al. 2014, McClellan, Formeister et al. 2014). Throughout CI insertion, response tracks are sequential responses to a 500 Hz, 90 dB nHL tone. Depending on signal to noise, 50 to 500 averages were collected per evoked response.
Audiometry
As standard of care, all subjects had unaided audiometric thresholds evaluated prior to implantation to determine residual hearing status and aid in device selection. Most subjects (n=24/36) were conventional implant candidates where preserving residual hearing was not a goal and mainly received the modiolar CI512 array to maximize electric hearing (n=20/24), the AB MidScala array (n=3/24), and in one case the lateral wall CI522 (n=1/24). The 12 subjects where preoperative hearing was sufficient to preserve (typically HL at 250 Hz ≤ 80 dB HL) received CI422/522 arrays (n=10/12) or AB MidScala arrays (n=2/12). In these subjects, post-operative audiometry was performed at the time of activation (median 1 month after surgery); in 3 cases the first audiometric evaluation was at around 3–6 months, and in 3 cases hearing thresholds improved around the 3 month mark (compared to thresholds at activation) so these thresholds were used as their post-operative HL instead. Hearing was considered preserved if post-operative HL at 250 Hz was < 80 dB HL and the low frequency pure-tone average (LF-PTA, average of 125 Hz, 250 Hz, 500 Hz, and 1 kHz) was also < 80 dB HL. If there were no audible precepts at a given frequency, the HL was assigned to be 5 dB larger than the maximum speaker output for that frequency.
Results
Thirty-six subjects were enrolled in the current study (Table I). The study included both children (n= 31) and adults (n=5) with a variety of etiologies. Most of the devices were from Cochlear Corporation (n=31) with the others from Advanced Bionics (n=5).
Round Window responses and Intracochlear Recordings through the Clip
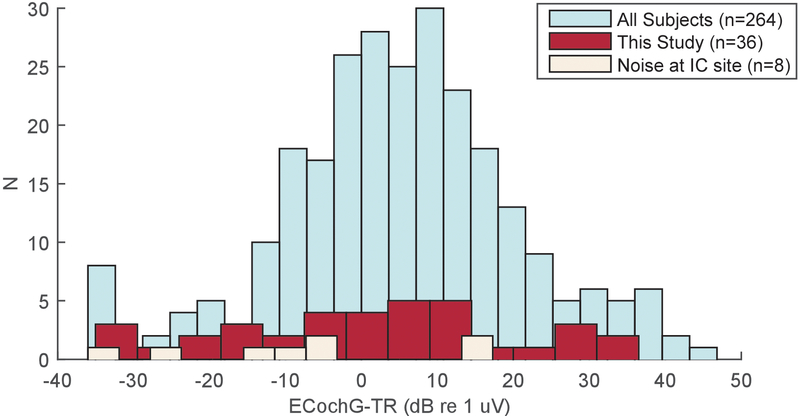
The first experimental measurements were responses recorded at the RW using the Neurosign electrode and responses were above the noise floor in almost all subjects (n=35/36). Recording through the CI using the clip method was then attempted in all subjects. These intracochlear recordings were successful in 28/36 cases (25 Cochlear Corporation and 3 Advanced Bionics). In 8 cases, AC power-associated noise (60 Hz in the USA) that saturated the recording amplifier precluding successful recordings (6 Cochlear Corporation and 2 Advanced Bionics). Three of these were early cases where the recording amplifier settings were the same as at the RW, which prompted us to lower the gain and narrow the filter settings to better deal with the noise through the clip. To investigate whether there was a RW signal cutoff which would predict whether intracochlear signals could be measured, the ECochG-TR for subjects with significant intracochlear responses were compared with those subjects without intracochlear responses, and also to the overall distribution of subjects in our database (Fig. 2). The cases where intracochlear responses failed to reach significance were on the lower end of the ECochG-TR distribution, but there was no clear magnitude cutoff where a low RW response would preclude the possibility of obtaining successful intracochlear recordings. The TR of the cases successfully recorded (red) spanned the range of our University of North Carolina population database, and the means of the distributions were not significantly different (t-test, 5.1 ± 15.5 dB vs. 0.5 ± 18.9 dB, t=1.42, df=42, p=0.17).
Figure 2.
Distribution of Total Response (ECochG-TR, see methods) from round window electrocochleography just prior to insertion. The distribution of TRs across subjects in this study (red) was not significantly different from the distribution in our larger database (teal). Noise when recording at the intracochlear site precluded recordings in 8 subjects, and while these subjects had smaller RW responses (beige), there wasn’t a clear RW magnitude cutoff which would predict whether intracochlear recordings would be feasible.
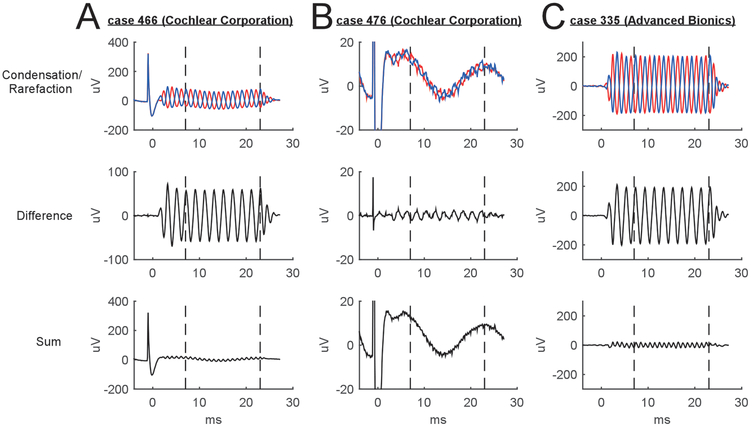
Examples of intracochlear response waveforms collected in 3 subjects are displayed in Fig. 3. With Cochlear Corporation’s software, a trigger artifact is seen prior to sound onset (0 ms) and recovery from the artifact extends into the early response to the sound (Fig 3A and 3B, top rows). Because this trigger pulse was always of the same polarity, it was largely eliminated in the ECochG difference waveform (Fig. 3A and 3B, middle row) but present in the summed response (Fig. 3A and 3B, bottom row). In addition to the onset pulse, the clip set-up was also sensitive to AC noise, and evoked responses could be seen riding on top of slower 60 Hz waves in some cases (Fig. 3B, top and bottom rows) which in the USA have a characteristic signal period of 16.7 ms. No trigger artifact occurs with the Advanced Bionics software (Fig 3C, top row).
Figure 3.
Example waveforms when recording through the intracochlear clip system. (A) A trigger artifact can be seen when using Cochlear Corporation’s software. (B) For smaller responses, the clip system is also sensitive to line noise (typically 60 Hz). (C) In Advanced Bionics arrays, there is no trigger artifact but 60 Hz noise can be seen (though not in this case).
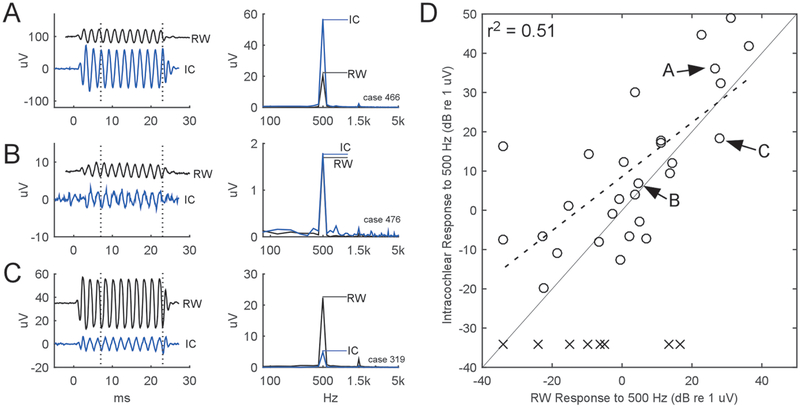
The differences between the RW and intracochlear responses, taken at the initial entrance of the electrode into the cochlea, are shown in Fig. 4. This initial recording location was typically 2.7 mm inside the RW but could be as far as 7 mm. This distance was largely device dependent: in the case of the CI512 it was difficult to get multiple response points before it came off the stylet, as a smooth motion is critical for proper modiolar placement. In most cases (n=18/28) the intracochlear response magnitude was at least 2 dB larger than the response at the RW (example case in Fig. 4A), but the two responses could also be of similar magnitude (n=3, example case in Fig. 4B) or even demonstrate a 2 dB smaller response inside the cochlea than the response previously recorded at the RW (n=7, example case in Fig. 4C). Across all the 28 subjects with successful recordings (Fig. 4D, circles), the initial intracochlear responses were a median 5.8 dB larger than those at the RW, similar to previous reports (Calloway, Fitzpatrick et al. 2014), and the values were positively correlated with those obtained from the RW response (r2 = 0.51, Fig. 4D, dashed line).
Figure 4.
Comparison of intracochlear response magnitude to those at the RW. Intracochlear responses could be (A) larger than the RW, (B) of similar magnitude to the RW, or (C) smaller than the RW. A ‘response’ was defined as sum of the FFT peaks to the first and second harmonics. (D)Across all 36 subjects, 28 intracochlear responses were above the noise floor among these, the median intracochlear response was 5.8 dB larger than the RW, and correlated positively (r2 = 0.51). Labels within (D) refer to the subjects illustrated in (A) to (C), while the ‘x’ symbols refer to cases with intracochlear noise which precluded subsequent analysis.
Changes in Response during CI Insertion
In cases where detectable responses were seen through the clip system, recording continued throughout all stages of CI insertion. In an example case with a CI522 array (Fig 5) response waveforms were taken at 5 points throughout a slow, continuous insertion (Fig. 5A, demonstrating the difference curves), and the associated response magnitudes at each insertion depth are plotted as a ‘track’ (Fig. 5B). The ‘0 mm insertion depth’ marks the first response just inside the cochlea. In this case, the response began around 12 dB (re 1 uV) and grew to a maximum 29.6 dB around an insertion depth of 24 mm, and further advancement of the array to its final insertion depth of 25 mm was associated with a 0.5 dB drop in response magnitude.
Figure 5.
Intracochlear Response Track. (A) Response waveforms to a 90 dB, 500 Hz tone in one subject were assessed at 5 stages during CI insertion. (B) Plotting the response magnitude of the ongoing response as a function of insertion depth reveals a “Response Track”.
Response tracks for all subjects are overlaid by array type in Fig. 6. In the top row, response change (in dB) from the interpolated response at 1 mm is plotted for each track. The bottom row shows the median change (in linear units) with semi interquartile ranges (line and grey bars, respectively) for all responses. For the 14 cases with the modiolar hugging CI512 array (Fig. 6A), a few showed large increases early in the insertion (top row) while some showed only small or no increases in response. On average, the median response (Fig. 6A, bottom) grew as the array was inserted through the cochleostomy, reached a maximum (white arrow) near 14.2 mm, and then dropped prior to the end. This depth corresponds to when the array was advanced off the stylet and began coiling towards the modiolus. In the 11 subjects who received CI422/522 lateral wall arrays (Fig 6B), the responses either grew or stayed relatively steady; there were no large drops (top row). This array does not have a stylet and is not precoiled, and the median response (bottom row) was steady until a rise at the end, so that the maximal median response was at the full insertion depth of 25 mm (white arrow). The depth of maximal median response differed significantly between these two array types (Wilcoxon rank-sum test, U=17.3, p=0.001), but the magnitude achieved at these depths did not differ (Wilcoxon rank-sum test, U=64, p=0.49). In the 3 subjects that received Advanced Bionics MidScala devices with successful insertion tracks (Fig. 6C), one increased early in the insertion, then dropped but recovered, another stayed relatively steady during the insertion, and the third showed a response drop early that then remained steady (Fig. 6C, top row). The median track showed a decline, but with only 3 cases the effect of each case was large, and this decline on average was caused by just one case (Fig 6C, bottom row). Taken as a whole, the individual tracks and the trends in median response demonstrate that there is substantial variability in intracochlear response patterns by both device type (modioloar vs lateral wall) and manufacturer.
Figure 6.
Response Tracks vary by device type. (A) In the Cochlear Corporation CI512 array, responses in dB scale (top row) typically demonstrate early growth, and response magnitude when normalized in uV (bottom row) demonstrates the median response was greatest at an insertion depth of 14.2 mm (white arrow). (B) In the Cochlear Corporation 422/522 arrays, responses in dB could dip (n=3, black arrow) but most growths were steady and in uV/uV scale (bottom row) the maximal median response was achieved at the deepest insertion depth (white arrow). (C) in Advanced Bionics MidScala devices, sample size was limited but responses were steady and in one case dropped with depth.
It is reasonable to assume that a response drop indicates some mechanical interaction between the electrode and responding elements that might indicate burgeoning cochlear trauma. However, an example (Fig. 7) indicates that a response drop can also be caused by interactions between different sources that produce the ongoing responses. In this case, the response magnitude dropped by nearly 10 dB and then recovered to within 3 dB of the starting value (Fig. 7A). Because the CI’s apical contact was moving deeper into the cochlea during this process, this shifting recording site was biased by immediately-adjacent generators. Hair cell and neural sources responding to the same stimulus frequency can constructively or destructively interfere depending on the relative strength and phase difference between them (Forgues, Koehn et al. 2014, McClellan, Formeister et al. 2014, Fontenot, Giardina et al. 2017). Evidence that this process is occurring can be seen through changes in phase, spectrum and latency. The phase changes are shown in the ‘average cycles’ Figs. 7B–D, left, see METHODS). The response (solid lines) and the best-fit sinusoids simulating the stimulus (dashed lines) show phase shifts, which are greater than 1 cycle across these points. The spectrum of the ongoing response also changed, with more second harmonic in the bottom spectrum panel (Fig. 7D, arrow). This increase of the second harmonic could be interpreted as an increase in neural activity in the form of the ANN, but an examination of the average cycle reveals distortions that are consistent with neural activity in all three curves (Riggs, Roche et al. 2017). In addition, since the ANN is periodic with the low frequency stimulus, some energy, usually most, will appear in the first harmonic (500 Hz). The first harmonic is largest in the top panel, but instead of indicating little nerve it is more likely that most of the ANN is appearing in the first harmonic in this waveform configuration. Thus, both hair cell and neural sources can overlap differently at different recording positions within the cochlea, producing changing patterns in both amplitude and phase as the array advances. Finally, the entire response shows a latency shift of 2.57 ms over this time frame (Fig. 7E), consistent with the phase change seen in the average cycle, and giving a direction to them. The latency change was measured as the time difference between the last phase-locked waveform peaks in the steady part of the response. We found this to be a more definable position than the onset of response which could be difficult to identify with precision. Thus, changes in the way different generators of the cochlea are interacting at different locations is the most likely source of the observed drop in magnitude, rather than trauma.
Figure 7.
Response magnitude could drop because of differences in phase relationship of generators rather than trauma. (A) A Response Track for one subject shows a large drop (10 dB) which recovered to within 3 dB by the end of insertion. (B) Average cycles from the ongoing response (left) at the starting point demonstrates a large response at the fundamental frequency evident in the FFT (right). (C) Mid-insertion, the phase inverts. (D) By the end of insertion, the phase again reverts and the response contains more distortions (arrow), indicating the array is likely recording from a different population of generators than those at the first intracochlear location. (E) This phase change is due to a latency shift beyond a cycle.
Patterns of Response Tracks During Insertion and Rates of Hearing Preservation
Demographics and hearing outcomes for the 12 subjects where hearing preservation was an intended goal are listed in Table II. With the definition of hearing preservation as threshold <80 dB at 250 Hz, hearing was preserved in 9/12 subjects, while in 3 subjects hearing loss was immediately apparent at the first post-operative visit.
Table II:
Hearing Preservation Rates. Candidates for hearing preservation arrays and surgical consideration were when behavioral thresholds at 250 Hz were <80 dB HL, and the low-frequency pure tone average (LF PTA), at 125 Hz, 250 Hz, 500 Hz, and 1 kHz, was also <80 dB HL. Post-operative hearing was taken within the first 3 months of implant activation and hearing was categorized as preserved if unaided thresholds remained <80 dB for both 250 Hz and the LF-PTA. ANSD = auditory neuropathy spectrum disorder, EVA = enlarged vestibular aqueducts.
| HL at 250 Hz (dB HL) | HL at 500 Hz (dB HL) | LF PTA (dB HL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | ID | Age/Sex | Etiology | Device | Pre-operative | Post-operative | Pre-operative | Post-operative | Pre-operative | Post-operative | HP Category |
| 1 | 270 | 62 F | Unknown | CI 422 | 45.0 | 80.0 | 55.0 | 90.0 | 52.5 | 83.7 | Loss |
| 2 | 297 | 4 M | ANSD | CI 422 | 10.0 | 20.0 | 55.0 | 55.0 | 53.3 | 53.6 | Preserved |
| 3 | 335 | 1 F | ANSD | AB MidScala | N/A | N/A | 80.0 | 80.0 | 81.7 | 79.8 | Preserved |
| 4 | 368 | 5 F | CLDN14 | CI 522 | 25.0 | 30.0 | 50.0 | 60.0 | 56.7 | 52.6 | Preserved |
| 5 | 379 | 13 M | Unknown | CI 522 | 15.0 | 25.0 | 25.0 | 45.0 | 36.7 | 48.3 | Preserved |
| 6 | 391 | 2 M | ANSD | CI 522 | 60.0 | 60.0 | 60.0 | 60.0 | 70.0 | 68.8 | Preserved |
| 7 | 414 | 14 M | EVA | CI 522 | 55.0 | 80.0 | 60.0 | 80.0 | 62.5 | 81.8 | Loss |
| 8 | 416 | 14 F | Unknown | CI 522 | 30.0 | 90.0 | 50.0 | 105.0 | 47.5 | 90.4 | Loss |
| 9 | 447 | 5 F | CLDN14 | CI 522 | 15.0 | 20.0 | 45.0 | 70.0 | 42.5 | 61.8 | Preserved |
| 10 | 466 | 3 M | ANSD | CI 522 | 55.0 | 55.0 | 55.0 | 50.0 | 67.5 | 66.7 | Preserved |
| 11 | 471 | 14 F | Unknown | CI 522 | 15.0 | 15.0 | 50.0 | 60.0 | 31.3 | 43.4 | Preserved |
| 12 | 476 | 11 F | Unknown | CI 522 | 60.0 | 70.0 | 75.0 | 80.0 | 67.5 | 77.5 | Preserved |
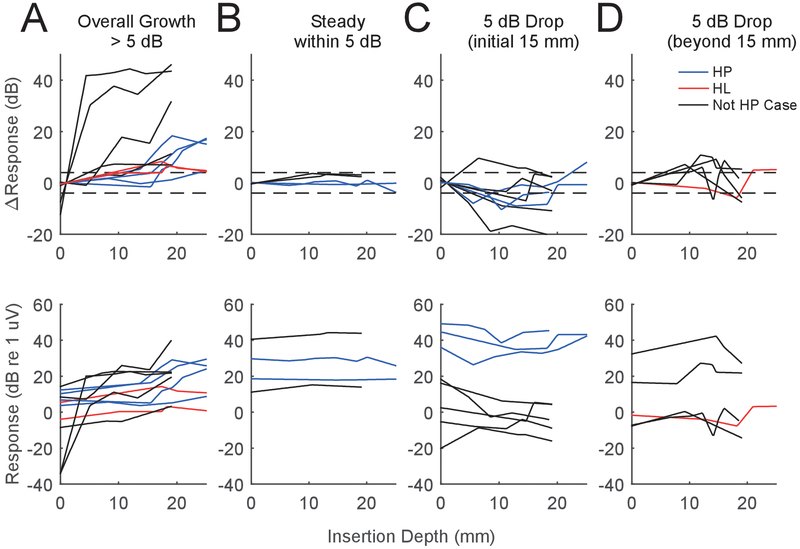
A goal of this study was to analyze tracks and observe features that might be related to atraumatic vs. traumatic insertions. To this end, we first hypothesized that a response track with an increase or stable level throughout insertion likely meant no or minimal trauma, whereas a drop in response could mean trauma. We first used a 5 dB cutoff to categorize response tracks into one of four groups- an overall growth in response, a steady response, an early drop in response, or a late drop in response (Fig. 8). We separated the early and late drops because we recently found that a drop in extracochlear response which occurred early in the insertion process (< 15 mm) was likely to demonstrate some level of response recovery, whereas a drop which occurred deeper than this was likely to be permanent (Giardina, Khan et al. 2018). Specifically, the track categories were defined by an overall (start to finish) response increase >5 dB during the insertion (n=12, Fig. 8A), steady responses within 5 dB throughout insertion (n=4, Fig. 8B), tracks with an early drop (>5 dB from the starting value before 15 mm) in response during insertion (n=7, Fig. 8C), and those with a late drop (after 15 mm) in response during insertion (n=5, Fig. 8D). Tracks are colored blue if hearing was preserved, red if hearing was lost, and black for subjects where hearing preservation was not intended and therefore not measured.
Figure 8.
Response Tracks by pattern. Categories were (A) Overall growth of 5 dB by the end of insertion, (B), a response which remained +/− 5 dB throughout insertion, (C) an early drop of >5 dB during insertion, and (D) a late drop in response during insertion. The top row demonstrates change in response (dB) whereas the bottom row shows dB re 1 uV. Cases in blue are cases where hearing was preserved, red demonstrate hearing was lost, and black are for subjects where preservation was not a goal. As is evident, there isn’t a clear pattern category which contains the hearing preserved vs lost subjects, implying trauma can occur with or without a characteristic response pattern. Note: in the top row of panel A there are two red tracks which mostly overlap, but are more distinct in absolute scale on the bottom row.
We expected that tracks in the “growth” and “steady” categories (n=16/28) would indicate atraumatic insertions, and that the latter “early drop” and ‘late drop” categories (n=12/28) could indicate trauma. Six of the 9 cases with hearing preserved resided in the growth/stable categories (Fig. 8A,B), but 3 of the 9 were in the early drop category (Fig. 8C). In the 3 subjects who lost hearing, 2 showed overall gains in response (Fig. 8A), while one showed a late drop (Fig. 8D). In summary, the increase/steady ECochG categories included 66% of the hearing preserved cases but also 66% of the hearing lost cases, and the two ECochG response loss categories included 33% of the hearing preserved cases and 33% of the hearing loss cases. These results imply that an overall change in ECochG magnitude, on its own, does not detect all trauma that occurs, and that response drops >5 dB can occur without a profound loss in hearing.
A more thorough analysis of the waveforms can help explain why some magnitude drops were likely not associated with trauma – i.e. why blue tracks where hearing was preserved were found in response drop categories (Fig. 8C), and also why some cases did not show a drop along the track as large as 5 dB but still lost hearing (Fig. 8A, red). Earlier in Fig. 7, a large, but reversible response drop was attributed to a changing phase relationship between hair cells and neuronal sources (CM and ANN, respectively) interfering destructively as the array advanced. This illustrated case was actually one of the three in the “early drop” category (Fig. 8C), which had preserved hearing. A second case in this category also demonstrated this phase-shifting phenomenon, and also had preserved hearing. Figure 9 shows the third case with preserved hearing from the early drop category (Fig. 9, left panels A-C), and its waveforms, alongside another case with preserved hearing that was in the steady growth category (Fig 9, right panels D-F). In the first subject of Fig. 9 (left, A-C) the waveforms reveal another apparent interaction between CM and ANN, this time without a change in phase. In this track, the response dropped by about 10 dB between 6 and 11 mm of insertion depth (Fig. 9A). Before the change, the average cycle shows a large, nearly-sinusoidal response with a peak in the best-fit sine at 0.15 cycles (Fig. 9B). At the next insertion depth (Fig. 9C), the response became more distorted (Fig 9 B,C arrows), which is a sign of a changing proportion of neural and hair cell generators (Riggs, Roche et al. 2017), but the phase of the best-fit sine only shifted to 0.12 cycles, a change of 0.03 cycles. Using an algorithm we previously developed to approximate the relative contribution of ANN and CM in each response (Fontenot, Giardina et al. 2017), it was found that the percentage of ANN/CM increased from 9 to 25%. In review, all three cases of hearing preservation which showed a large, early drop in response also demonstrated concurrent shifts in the phase or proportion of ANN/CM, implying again these drops were from shifting sources rather than overt trauma.
Figure 9.
Response drops in two subjects without a concurrent change in phase. The first subject demonstrates (A) a 10 dB drop between (B) and (C). While the phase of the best-fit sine doesn’t change between (B) and (C), the proportion of neural activity is seen by both the distortions in the average cycle (arrows) and the changing proportion of ANN/CM (see methods for this calculation). In a second subject, a response drop of 4.3 dB (D) shows no change in phase or proportion of ANN/CM.
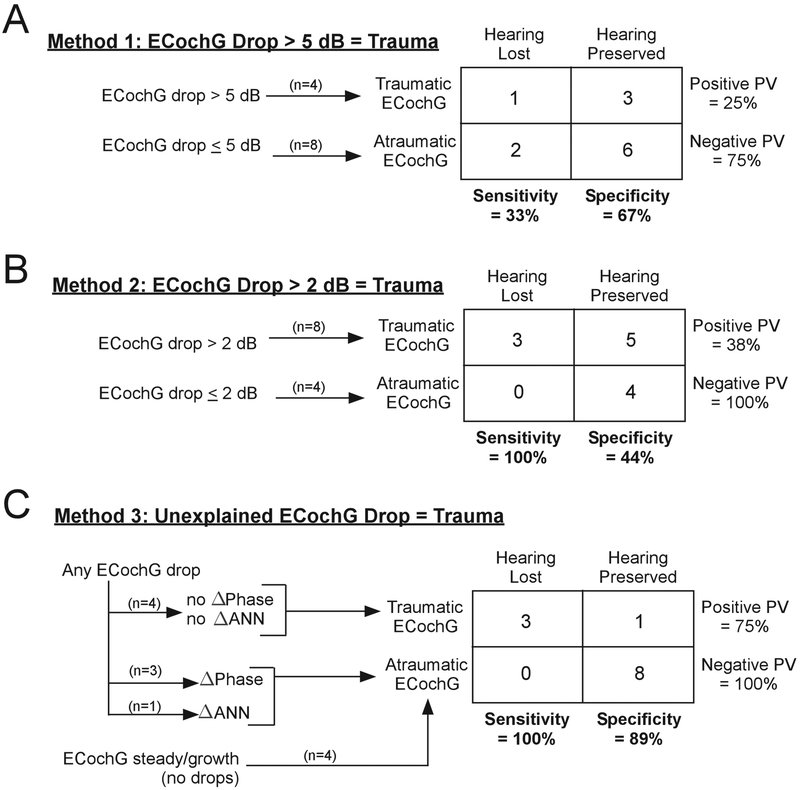
Conversely, a response drop that occurs without an associated change in phase or ANN may be a more likely indication of trauma. This result appeared to be the case for each of the three cases that lost hearing. Examination of the two cases that lost hearing in Fig. 8A (red tracks) show response drops of less than 5 dB along the track, while the case that lost hearing in Fig. 8D has a larger drop. Closer examination of the average cycles in these three cases did not reveal a change in phase, but the signal to noise ratios were low making the use of the average cycle either visually or in the model less reliable. That large drops without indications of shifting sources in the CM and ANN can occur is shown by a case in Fig. 9D–F. This case had a large increase in response from start to finish and was placed in the ‘steady growth’ category in Fig. 8A. However, there was a 4.3 dB drop at the end, which was not associated with any change in shape or phase of the average cycle, and the proportion of ANN to CM remained similar between E and F. This therefore represents a case where trauma might have been predicted, but the hearing loss was small, 0 dB at 250 Hz and only 10 dB at 500 Hz (case 471, Table I).To quantify these observations, we constructed contingency tables designed around detecting trauma using ECochG as a predictor of loss of hearing (Fig. 10). In the first model (Fig. 10A), we used the 5 dB cutoffs from Fig. 8 to categorize whether ECochG appeared traumatic. Four cases dropped below 5 dB, but only one of them lost hearing. Conversely, most tracks (8/12) appeared atraumatic and 6 had preserved hearing. In the contingency table, this 5 dB cutoff afforded a model with only 33% sensitivity and 67% specificity for trauma detection predicting hearing loss. We tested the range of cutoff dB values from 1–20, and the value with the largest average sensitivity/specificity was found when using a 2 dB cutoff (Fig. 10B). Using this new cutoff, the sensitivity jumped to 100% - all cases where hearing was not preserved had at least some drops. However, most cases with hearing preservation also had drops, so the specificity was low, only 44%. We then investigated the nature of the drops, under the assumption that drops associated with shifts in phase or composition of the response (ANN) do not necessarily indicate trauma and hearing loss, while drops without such shifts are indicative of trauma (Fig. 10C). None of the 3 cases that lost hearing showed such shifts when drops were seen, with the caveat noted above that the S/N ratio in these cases was low. Of the 5 cases with drops where hearing was preserved, four showed clear evidence of shifts in phase or ANN/CM ratio that indicated changing interactions among different sources. The one case that showed no phase or composition changes was illustrated in Fig. 9D–F. Thus, with these new criteria, ECochG as a marker to identify hearing loss had a specificity of 89% - while retaining a sensitivity of 100%. In sum, steady increases in ECochG likely indicate there is no trauma occurring, while response drops on their own are an unreliable marker, but analysis of the waveforms may be crucial in deciphering whether trauma is in fact occurring.
Figure 10.
Contingency tables for using three approaches of ECochG to identify trauma leading to hearing loss. In the first model (A), a 5 dB cutoff is used to connote trauma, which was associated with poor sensitivity and specificity. (B) The best magnitude cutoff we found, 2 dB, properly identified all cases of hearing loss but the specificity was poor. (C) Using magnitude drops and analysis of phase and ANN/CM, the sensitivity remained high and the specificity was improved. PV, predictive value.
Response Metrics and Changes in Absolute Hearing Thresholds
In addition to the bimodal metric of hearing preserved vs. lost, we further explored the relationship between ECochG magnitude and the amount of audiometric threshold increase at 500 Hz in all hearing preservation subjects (Fig. 11). When the tracks were plotted on an absolute scale (Fig 11A), the tracks with lower absolute magnitudes were more likely to be associated with hearing loss (red vs blue). To explore this trend, the initial value, maximal value, and final value from each track (in absolute scale, i.e dB 1 uV) were then compared to threshold increase. We then analyzed the relationship between track magnitudes and changes with absolute threshold shift. Because five comparisons were made from the same subjects, a Bonferroni correction was applied to the level of significance such that the necessary alpha needed to reject the null hypothesis became 0.01 instead of 0.05. We found significant or nearly significant correlations between the starting intracochlear magnitude (r= −0.72, p=0.014, Fig. 11B), the final intracochlear magnitude (r= −0.79, p=0.002, Fig. 11C), and the maximum intracochlear response measured during the insertion (r= −0.80, p=0.002, Fig. 11D) with the amount of hearing loss. These results indicate that the absolute degree of hearing loss tends to be greater with those who start with smaller cochlear responses overall. In contrast, the pattern of change in ECochG magnitude was not sufficient on its own to predict which subjects would most likely lose hearing (Fig. 11E, red vs blue) and, as expected from the previously presented results (Fig. 10A,B) there was no correlation between the overall change in response magnitude (start to finish) throughout insertion (p=0.61, Fig. 11F) or the size of the largest response drop (p=0.32, Fig. 11G) and the amount of behavioral hearing loss at 500 Hz.
Figure 11.
Response Tracks for Hearing Preservation cases and Relationship between hearing loss at 500 Hz and intraoperative track magnitudes. (A) The absolute track values demonstrate those who lost hearing (red) had smaller responses. Specifically, the starting magnitude (B), final magnitude (C), and maximal magnitude (D) all correlated significantly with amount of threshold gain. (E) The change in response does not in and of itself help predict which cases will have preserved hearing versus hearing loss. Changes in response magnitude including the overall growth (B) and the largest drop (C) were not correlated with hearing loss.
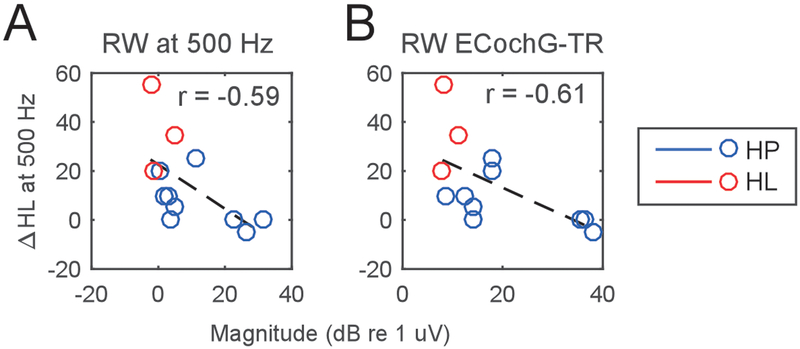
Given the response magnitude of the tracks was correlated with hearing loss, we then asked whether the response recorded at the RW prior to insertion was similarly correlated. The response magnitude at the RW to 500 Hz was correlated with the amount of hearing loss (r= −0.59, p=0.04, Fig 12A), as was the TR (r= −0.61, p=0.03, Fig. 12B). These findings demonstrate that the pre-operative health of the cochlea as assessed with RW recordings is an indicator of how much the hearing is likely to change due to surgery – with healthier cochleae incurring smaller losses.
Figure 12.
Relationship between Round Window (RW) response magnitudes prior to CI insertion and subsequent hearing loss at 500 Hz. (A) The magnitude of the response to a 90 dB, 500 Hz tone correlated with the degree of hearing loss. (B) Across a broad range of stimulus frequencies (see ECochG-TR in methods), the total response (TR) also correlates with degree of hearing loss.
Discussion
The idea behind using ECochG during insertion of a cochlear implant array is that a change in the cochlear response to sound could be a metric to determine when trauma is impending or actively occurring. To this end, we used intracochlear ECochG to record patterns of response changes during insertion for several array types and used subsequent hearing loss as a metric for trauma in a subset of cases where hearing preservation was a goal. The first finding was that intracochlear response patterns can be partially explained by array type; particularly that stylet removal in modiolar-hugging arrays was associated with a drop in response which was not seen with lateral wall arrays. We then demonstrated that some ECochG response drops could be reversible and likely atraumatic, if the drops were associated with concurrent changes in phase or distortions indicative of a destructive interference between hair cell and neural generators. In the hearing preservation cases, all cases with monotonically increasing responses demonstrated preserved hearing, while those recordings with any drops included both hearing preserved and hearing lost subjects. There was no magnitude cutoff for these drops that could reliably predict which subjects would lose hearing. However, many of the drops were associated with changes in phase or in the proportion of ANN, indicating changing source relationships rather than trauma could be the cause for the drops. When these factors were taken into account the sensitivity and specificity for detecting loss of hearing was better than measures based on magnitude alone. Additionally, it was found that the absolute size of ECochG response magnitudes, rather than changes in magnitude during the track, was the best predictor of the amount of postoperative threshold shift.
Technical issues of intracochlear recording
Responses just within the cochlea were typically 5 dB larger than those at the RW, consistent with our previous study (Calloway, Fitzpatrick et al. 2014). However, failures to obtain significant intracochlear responses occurred, and these were usually due to the increased line noise when using the clip recording approach. We have not determined why this noise using the clip is present, compared either with extracochlear recordings or through telemetry from the device. It is likely to be due to a higher impedance pathway between the external amplifier and array contact as the connection is made using the clip and shorting through the device to connect to the external ground. Noise precluded recording in 8 of the 36 subjects, usually early cases in the study until we changed the hi-pass filter to 200 Hz and reduced the gain to avoid saturation of the amplifier. The only previous study with the clip was reported from 2 subjects and successful recordings were achieved both times (Harris, Riggs et al. 2017), however those recordings were made after some early experience with the clip and a passive hi-pass filter was introduced prior to amplification to reduce the noise.
Recording with the clip system has strengths and weaknesses compared to recording through the telemetry. The clip system is an extra step to setup and has the line noise problem as mentioned, but data acquisition is faster than with telemetry because there is no wireless data transfer step. Additionally, the individual recording windows with telemetry are only 3 ms with the Cochlear Corporation system (Campbell, Kaicer et al. 2015) and 1.7 ms with the MED-EL system (Adel, Rader et al. 2015), so longer responses can only be acquired by collecting separate, shorter recordings at different delays relative to the stimulus and then piecing them together. The AB system does not have this limitation (Harris, Riggs et al. 2017). Additionally, the on-board analog-to-digital converter (ADC) of the CI processor is of much lower resolution than that of standard audiometric hardware (such as the BioLogic) which are specifically designed to detect ECochGs. The telemetry approach was reverse-engineered to measure ECochGs rather than electrically-evoked neural responses. The telemetry approach is, however, simpler to setup and recordings can be made throughout the rest of the surgery and post-operatively (Dalbert, Pfiffner et al. 2015, Koka, Saoji et al. 2017), whereas the clip needs to be removed to complete the surgery.
Patterns of Response throughout CI Insertion
Recording from the apical contact throughout CI insertion provides a wealth of information regarding the interaction between the array and cochlear structures. For the cases with successful intracochlear recordings throughout insertion, response changes could be partially explained by the array type. For instance, magnitude drops were seen after stylet removal in the CI512’s perimodiolar array (Fig. 6A, white arrow), a step which allows the array to coil inwards towards the BM, causing mechanical dampening of the BM or even translocation through it – processes which are known to cause response reductions in animal models (Demason, Choudhury et al. 2012, Lo, Bester et al. 2017). Conversely, the CI522 lateral wall array demonstrated on average a relatively steady median response with insertion depth until an increase was seen near the end of insertion. Examples going into this median were a mixture, including some increasing, some flat, and some decreasing. The increase of the median magnitude at deeper insertion depths indicates more consistency across cases as the array approached apical regions. The variety of patterns seen with the CI522 (Fig. 6B, top row) differs from previous studies, where the responses in general were consistently increasing (Campbell, Kaicer et al. 2015, Campbell, Kaicer et al. 2016).
Across all arrays, four major patterns of response were seen: increases in response with insertion depth, steady responses throughout insertion, drops in magnitude early in the insertion, and late drops in magnitude during insertion (Fig. 8). We separated the drops into early and late categories and used a 5 dB cutoff because we found drops in extracochlear ECochG beyond 5 dB that occurred in the first 15 mm of insertion were more likely to show some level of response recovery (Giardina, Khan et al. 2018). In the current study, 7 of the 13 recordings with losses greater than 5 dB showed some degree of recovery. These reversible changes are important, because an ongoing hypothesis is that any response drop could indicate trauma.
A reversible change could indicate a physical interaction with the membrane, which isn’t necessarily traumatic. It is possible that a drop occurs because the array interacts with the basilar membrane in a temporary, atraumatic way, as the array slides past the first turn. Our categorizing drops as “early” versus a “late” uses a cutoff of 15 mm insertion depth, which is of anatomic significance because it marks the approximate lateral wall depth of the basal turn (Kawano, Seldon et al. 1996, Franke-Trieger and Mürbe 2015). Among the responses with early drops (n=8, Fig. 8C), 5 were at least partially reversible (63%) and among the late drops (n=5, Fig. 8D), only 2 were partially reversible (40%). This pattern where early drops are more likely to be reversible than late drops is a finding consistent with previous data collected using extracochlear ECochG (Giardina, Khan et al. 2018).
Response drops during insertion can also occur without any mechanical interaction with the BM, because of a changing recording location passing across a region of heterogeneous intracochlear generators. Responses to the 500 Hz tone from different parts of the cochlea can overlap, interfering either constructively or destructively as the array moves through the cochlea, causing changes in the magnitude and phase of the net response (Fig. 7). Thus, response decrements at any given position cannot definitively be attributed to trauma. To help overcome this problem, it is first necessary to consider how generators can be distributed at different cochlear regions, and what their effects on the net response would be at different recording locations. These overlapping responses could come from hair cells (the CM), from the auditory nerve (ANN), or from the interaction between CM and ANN. The CM makes up the bulk of the ECochG response in CI subjects (Fontenot, Giardina et al. 2017) so changes in its sources should have the largest effects. In cases with responses to frequencies higher than 500 Hz, particularly 2 and 4 kHz (as was the case in the subject shown in Fig. 7), the CM recorded will include the summed response from two cochlear regions with different properties. One region of generators is the basal segment of the cochlea with CFs higher than the 500 Hz tone, where the traveling wave will pass through quickly and responses to a wide extent of the cochlea will be in phase. The other region is the part of the cochlea near the CF, where the traveling wave slows down and responses occur with a longer latency and changing phase to allow for maximal BM displacement and tonotopic resolution (Robles and Ruggero 2001). Thus, when the electrode first enters the cochlea, it will “see” the more basal region with in-phase responses, but as the array advances apically through this high frequency region the tip electrode will get increasingly more input from the CF region, which is at a different phase due to the slowing of the traveling wave (van der Heijden 2014, van der Heijden and Versteegh 2015, Campbell, Bester et al. 2017). As the proportion of the response from the CF region increases, the two sources interact destructively, causing the net response to drop, and with even further insertion the response increases again as it becomes dominated by the single source, now located deeper in the cochlea, with a longer latency. These different possibilities indicate that it will be necessary to use as much information as is available to accurately interpret reductions in responses as either a changing phase relationship or possible trauma.
Response Patterns and Hearing Preservation
An initial hypothesis was that increasing or steady responses would indicate an atraumatic insertion, whereas drops in magnitude during insertion would likely indicate immediate insertion trauma. This is the basic metric used in most previous studies. Campbell et al. reported the response track as a binary metric – whether the ongoing response magnitude was preserved or not by the end of insertion, although the criterion for preservation was not given (Campbell, Kaicer et al. 2016). In 15 hearing-preservation subjects, they found patients with preserved ECochG (n=7) responses at the end of surgery had 15 dB better low-frequency hearing postoperatively than those who demonstrated ECochG losses (n=8) during surgery. This was an early indication that surgical trauma detectable by ECochG could affect hearing preservation. Dalbert, Pfiffner et al. (2018) recorded intracochlear tracks in 3 hearing-preservation subjects and found slowly growing responses associated with hearing preservation in 2 subjects, and a response that grew and then dropped in the third subject – who completely lost hearing. Harris et al. further stratified response tracks into 3 categories – Type A, B, and C (Harris, Riggs et al. 2017). Type A demonstrated an overall increase in amplitude from beginning to end of insertion, analogous to the “CM preserved” category used by Campbell, Kaicer et al. (2017) and our ‘overall growth’ category. Harris’s Type B had a maximal value at the beginning of insertion and drops throughout insertion, similar to our early drop category, and their Type C had a similar response magnitude at the beginning and end, but a maximal response magnitude mid-insertion, similar to the “CM not preserved” category used by Campbell, and our late drop category. While it was hypothesized that subjects in Harris’s B and C categories would have incurred trauma, only a few of the 17 subjects in their study had any meaningful pre-operative hearing so no conclusions could be drawn regarding track pattern and hearing preservation. Acharya et al. recorded intracochlear responses during CI insertion in two pediatric subjects and found the first subject, with stable intracochlear responses, had complete hearing preservation whereas the second subject, with a mild drop in response, had a small degree of hearing loss (Acharya, Tavora-Vieira et al. 2016). O’Connell et al. studied intra-insertion ECochG in 13 subjects and utilized post-operative imaging to determine the absolute scalar position (O’Connell, Holder et al. 2017), because scalar translocation is associated with hearing loss (Finley, Holden et al. 2008, O’Connell, Hunter et al. 2016). The patterns of response change in the cohort with completely-within-ST insertions were similar in both magnitude and pattern to those insertions which translocated into the SV, implying scalar displacement was not easily recognizable by shifts in magnitude alone. In short, interest in intracochlear ECochG as a predictor of hearing preservation is robust, but groups are coming to differing conclusions regarding which ECochG changes are normal, and which indicate trauma.
As described above, all these previous studies reported only magnitude changes during the insertion. In our hearing preservation sample (n=12), there was no obvious trend between response drop and rates of hearing preservation; drops in response occurred in some subjects with preserved hearing (Fig. 8C), and some subjects with increasing or steady responses essentially lost all hearing postoperatively (Fig. 8A). A specificity/sensitivity analysis showed little indication that a metric based on magnitude drop with an arbitrary cut-off value (5 dB in this case) would prove useful (Fig. 10A), and the best cut-off (2 dB) had 100% sensitivity but only 44% specificity (Fig. 10B). In contrast to a sole reliance on magnitudes, a more promising result was obtained when the relationship between magnitude and phase changes was considered - with the sensitivity remaining at 100% but the specificity increasing to 89% (Fig. 10C). The one outlier was the case from Fig. 8D–F, where a 4.3 dB drop in ECochG was seen, without a clear change in phase or ANN/CM ratio, but hearing was well preserved.
Although not seen in our limited data set, it is also possible that a completely atraumatic insertion is seen on ECochG during insertion, yet near-total loss of hearing occurs post-operatively (Campbell, Kaicer et al. 2016). The mechanism for this would be a foreign body reaction that occurs hours to weeks after implantation (Anderson, Rodriguez et al. 2008), eventually leading to fibrosis and loss of hearing (Jia, Wang et al. 2013). With a larger sample size, we would expect to see more cases with profound hearing loss despite an apparently atraumatic ECochG.
Implications for using ECochG as a runtime monitor of insertion trauma
In designing a system to monitor cochlear responses and detect trauma, an initial design decision is whether to monitor these responses from within the cochlea (intracochlear recordings) or from a fixed location outside of the cochlea (extracochlear recordings). Each approach has its own benefits, and they provide complementary yet distinct information regarding the state of the cochlea. For a fixed extracochlear electrode, any measured change in response must inherently be due to a change in the cochlea’s ability to transduce acoustic energy into electric responses. Models have shown a completely-within-tympani (atraumatic) insertion shouldn’t significantly affect BM propagation energy (Greene, Mattingly et al. 2015), so any extracochlear response change must be the result of a change in intracochlear fluid pressure gradients, basilar membrane displacement pattern, basilar membrane integrity, or a change in the generators themselves. The fixed recording location, often placed near the base, may be biased towards immediately-adjacent (high-CF) generators, but this stability in location minimizes other confounders such as movement artifact that may be important when analyzing intracochlear recordings.
A substantial benefit to intracochlear recordings, compared to extracochlear recordings, are the higher signal to noise, which minimizes the number of responses needed to obtain a significant response and aids in the speed of feedback to surgeons. However, the shift of recording location as the array advances, coupled with different distributions of generator sources due to individual etiologies and histories of hearing loss leading to cochlear implantation, introduces confounders. This report demonstrates that a significant drop in intracochlear response pattern can be hypothesized to be traumatic or atraumatic due to changing relationships to generators as the contact advances. While we had some post-hoc success in accounting for the responses seen in relation to hearing outcomes, the ability to account for the various possible patterns in near-real time would seem to require a priori knowledge of what might be expected in each case. However, there are possible ways to normalize for the pattern seen during a track. One way is to monitor if consecutive contacts after the apical electrode are following the same track as they pass the same region in the cochlea, i.e., that they are observing comparable responses at the same location in the cochlea in all respects (amplitude, phase and ANN/CM composition). Another way would be to monitor responses at the most basal electrode, shifting the contact number as each enters the cochlea. This way would be pseudo-extracochlear in the sense of recording from a stable location, but would gain the benefit of signal-to-noise from the intracochlear location and requires no separate hardware or software.
Cochlear Health and Hearing Preservation
We have previously shown a high correlation between ECochG magnitudes and speech perception (46% variability accounted for in adults, 36% in older children) (Fitzpatrick, Campbell et al. 2014, McClellan, Formeister et al. 2014, Formeister, McClellan et al. 2015, Fontenot, Giardina et al. 2017). In contrast, there was only 20% of variability in speech perception accounted for by auditory thresholds in the same dataset (McClellan, Formeister et al. 2014, Fontenot, Giardina et al. 2017), and this number is an outlier because most studies report no relationship with the audiogram (Gifford, Dorman et al. 2010). The information from ECochG is different from the audiogram because it is measuring hair cell as well as neural responses from the cochlea. A larger RW ECochG is associated with better preoperative thresholds, but the variability accounted for is modest (r2=0.39) (Fitzpatrick, Campbell et al. 2014). In addition, the neural responses, in the form of the CAP and ANN are highly variable, being small or absent in some subjects despite a large CM, and up to or slightly exceeding the CM in other subjects (Scott, Giardina et al. 2016, Fontenot, Giardina et al. 2017). Children with auditory spectrum disorder are a particularly good example where, to high frequency stimuli, the CM is typically large while neural responses in the form of a CAP are rare (Riggs, Roche et al. 2017). The pattern is found in some adults and older children without ANSD as well. Functional hair cells in the absence of neural responses are symptomatic of cochlear synaptopathy demonstrated in animal models and in humans from anatomical data (Makary, Shin et al. 2011, Viana, O’Malley et al. 2015). Success with electrical stimulation does not depend on the presence of complete connections between the hair cell and nerve, but does presumably depend on the neural substrate available to be stimulated, i.e., on the health of spiral ganglion cells. We think the presence of functional hair cells is an indication of the health of the cochlea that can be used as proxy for the neural substrate in cases of progressive hearing loss in older children and adults. Our finding that the overall size of the ECochG response is related to hearing preservation further suggest that cochlear health is important in resisting or recovering from the detrimental effects of the implantation. This finding is consistent with Wanna, O’Connell et al. (2018), who performed a multivariate analysis and determined lower pre-operative thresholds are predictive of better functional residual hearing status. In utilizing ECochG to predict residual hearing status, it may be necessary to use overall magnitudes, in addition to track changes, to characterize each cochlea.
Acknowledgments
Financial Disclosures/Conflicts of Interest:
This project was funded by the NIH through NIDCD (F30 DC015168). The senior author DF has or has had research projects with MED-EL, Cochlear Corporation and Advanced Bionics. CG, KB, KH, and HP declare that their involvement in research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. CB is a consultant for Advanced Bionics and Cochlear Corporation, and OA is a consultant for MED-EL and Advanced Bionics. OA and CB have equity stakes in Advanced Cochlear Diagnostics.
References
- Acharya AN, Tavora-Vieira D and Rajan GP (2016). “Using the Implant Electrode Array to Conduct Real-time Intraoperative Hearing Monitoring During Pediatric Cochlear Implantation: Preliminary Experiences.” Otology & Neurotology 37(2): e148–e153. [DOI] [PubMed] [Google Scholar]
- Adel Y, Rader Y, Bahmer A and Baumann U (2015). Recording Low-Frequency Acoustically Evoked Potentials using Cochlear Implants. 2015 Conference on Implantable Auditory Prostheses. [Google Scholar]
- Adunka OF, Giardina CK, Formeister EJ, Choudhury B, Buchman CA and Fitzpatrick DC (2015). “Round window electrocochleography before and after cochlear implant electrode insertion.” Laryngoscope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adunka OF, Mlot S, Suberman TA, Campbell AP, Surowitz J, Buchman CA and Fitzpatrick DC (2010). “Intracochlear recordings of electrophysiological parameters indicating cochlear damage.” Otol Neurotol 31(8): 1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adunka OF, Pillsbury H and Buchman C (2009). “Minimizing intracochlear trauma during cochlear implantation.” [DOI] [PubMed] [Google Scholar]
- Ahmad FI, Choudhury B, De Mason CE, Adunka OF, Finley CC and Fitzpatrick DC (2012). “Detection of intracochlear damage during cochlear implant electrode insertion using extracochlear measurements in the gerbil.” Laryngoscope 122(3): 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Rodriguez A and Chang DT (2008). Foreign body reaction to biomaterials Seminars in immunology, Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloway NH, Fitzpatrick DC, Campbell AP, Iseli C, Pulver S, Buchman CA and Adunka OF (2014). “Intracochlear electrocochleography during cochlear implantation.” Otol Neurotol 35(8): 1451–1457. [DOI] [PubMed] [Google Scholar]
- Campbell L, Bester C, Iseli C, Sly D, Dragovic A, Gummer AW and O’leary S (2017). “Electrophysiological evidence of the basilar-membrane travelling wave and frequency place coding of sound in cochlear implant recipients.” Audiology and Neurotology 22(3): 180–189. [DOI] [PubMed] [Google Scholar]
- Campbell L, Kaicer A, Briggs R and O’Leary S (2015). “Cochlear response telemetry: intracochlear electrocochleography via cochlear implant neural response telemetry pilot study results.” Otol Neurotol 36(3): 399–405. [DOI] [PubMed] [Google Scholar]
- Campbell L, Kaicer A, Sly D, Iseli C, Wei B, Briggs R and O’Leary S (2016). “Intraoperative Real-time Cochlear Response Telemetry Predicts Hearing Preservation in Cochlear Implantation.” Otology & Neurotology 37(4): 332–338. [DOI] [PubMed] [Google Scholar]
- Choudhury B, Adunka OF, Demason CE, Ahmad FI, Buchman CA and Fitzpatrick DC (2011). “Detection of intracochlear damage with cochlear implantation in a gerbil model of hearing loss.” Otol Neurotol 32(8): 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbert A, Huber A, Veraguth D, Roosli C and Pfiffner F (2016). “Assessment of Cochlear Trauma During Cochlear Implantation Using Electrocochleography and Cone Beam Computed Tomography.” Otology & Neurotology 37(5): 446–453. [DOI] [PubMed] [Google Scholar]
- Dalbert A, Pfiffner F, Hoesli M, Koka K, Veraguth D, Roosli C and Huber A (2018). “Assessment of Cochlear Function during Cochlear Implantation by Extra-and Intracochlear Electrocochleography.” Frontiers in neuroscience 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbert A, Pfiffner F, Roeoesli C, Thoele K, Sim JH, Gerig R and Huber AM (2015). “Extra-and Intracochlear Electrocochleography in Cochlear Implant Recipients.” Audiology and Neurotology 20(5): 339–348. [DOI] [PubMed] [Google Scholar]
- Dalbert A, Sim JH, Gerig R, Pfiffner F, Roosli C and Huber A (2015). “Correlation of Electrophysiological Properties and Hearing Preservation in Cochlear Implant Patients.” Otol Neurotol 36(7): 1172–1180. [DOI] [PubMed] [Google Scholar]
- Dalbert A, Sim JH and Huber AM (2014). “Electrophysiologic Monitoring of Residual Hearing During and After Cochlear Implantation.” Association for Research in Otolaryngology Abstracts 37: 317–318. [Google Scholar]
- Demason C, Choudhury B, Ahmad F, Fitzpatrick DC, Wang J, Buchman CA and Adunka OF (2012). “Electrophysiological properties of cochlear implantation in the gerbil using a flexible array.” Ear Hear 33(4): 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley CC, Holden TA, Holden LK, Whiting BR, Chole RA, Neely GJ, Hullar TE and Skinner MW (2008). “Role of electrode placement as a contributor to variability in cochlear implant outcomes.” Otol Neurotol 29(7): 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DC, Campbell AT, Choudhury B, Dillon MP, Forgues M, Buchman CA and Adunka OF (2014). “Round window electrocochleography just before cochlear implantation: relationship to word recognition outcomes in adults.” Otol Neurotol 35(1): 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot TE, Giardina CK and Fitzpatrick DC (2017). “A Model-Based Approach for Separating the Cochlear Microphonic from the Auditory Nerve Neurophonic in the Ongoing Response Using Electrocochleography.” Frontiers in neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot TE, Giardina CK, Teagle HF, Park LR, Adunka OF, Buchman CA, Brown KD and Fitzpatrick DC (2017). “Clinical role of electrocochleography in children with auditory neuropathy spectrum disorder.” International journal of pediatric otorhinolaryngology 99: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgues M, Koehn HA, Dunnon AK, Pulver SH, Buchman CA, Adunka OF and Fitzpatrick DC (2014). “Distinguishing hair cell from neural potentials recorded at the round window.” J Neurophysiol 111(3): 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formeister EJ, McClellan JH, Merwin WH III, Iseli CE, Calloway NH, Teagle HF, Buchman CA, Adunka OF and Fitzpatrick DC (2015). “Intraoperative round window electrocochleography and speech perception outcomes in pediatric cochlear implant recipients.” Ear and hearing 36(2): 249–260. [DOI] [PubMed] [Google Scholar]
- Franke-Trieger A and Mürbe D (2015). “Estimation of insertion depth angle based on cochlea diameter and linear insertion depth: a prediction tool for the CI422.” European Archives of Oto-Rhino-Laryngology 272(11): 3193–3199. [DOI] [PubMed] [Google Scholar]
- Giardina CK, Khan TE, Pulver SH, Adunka OF, Buchman CA, Brown KD, Pillsbury HC and Fitzpatrick DC (2018). “Response Changes During Insertion of a Cochlear Implant Using Extracochlear Electrocochleography.” Ear and Hearing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Dorman MF, Shallop JK and Sydlowski SA (2010). “Evidence for the expansion of adult cochlear implant candidacy.” Ear and hearing 31(2): 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene NT, Mattingly JK, Jenkins HA, Tollin DJ, Easter JR and Cass SP (2015). “Cochlear implant electrode effect on sound energy transfer within the cochlea during acoustic stimulation.” Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 36(9): 1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MS, Riggs WJ, Giardina CK, O’Connell BP, Holder JT, Dwyer RT, Koka K, Labadie RF, Fitzpatrick DC and Adunka OF (2017). “Patterns Seen During Electrode Insertion Using Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant.” Otology & Neurotology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MS, Riggs WJ, Koka K, Litvak LM, Malhotra P, Moberly AC, O’connell BP, Holder J, Di Lella FA and Boccio CM (2017). “Real-time intracochlear electrocochleography obtained directly through a cochlear implant.” Otology & Neurotology 38(6): e107–e113. [DOI] [PubMed] [Google Scholar]
- Jia H, Wang J, François F, Uziel A, Puel J-L and Venail F (2013). “Molecular and cellular mechanisms of loss of residual hearing after cochlear implantation.” Annals of Otology, Rhinology & Laryngology 122(1): 33–39. [DOI] [PubMed] [Google Scholar]
- Jurawitz M-C, Büchner A, Harpel T, Schüssler M, Majdani O, Lesinski-Schiedat A and Lenarz T (2014). “Hearing preservation outcomes with different cochlear implant electrodes: Nucleus® Hybrid™-L24 and Nucleus Freedom™ CI422.” Audiology and Neurotology 19(5): 293–309. [DOI] [PubMed] [Google Scholar]
- Kamakura T and Nadol JB (2016). “Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human.” Hearing research 339: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano A, Seldon HL and Clark GM (1996). “Computer-aided three-dimensional reconstruction in human cochlear maps: measurement of the lengths of organ of Corti, outer wall, inner wall, and Rosenthal’s canal.” Annals of Otology, Rhinology & Laryngology 105(9): 701–709. [DOI] [PubMed] [Google Scholar]
- Koka K, Saoji AA and Litvak LM (2017). “Electrocochleography in cochlear implant recipients with residual hearing: comparison with audiometric thresholds.” Ear and hearing 38(3): e161–e167. [DOI] [PubMed] [Google Scholar]
- Lo J, Bester C, Collins A, Newbold C, Hampson A, Chambers S, Eastwood H and O’Leary S (2017). “Intraoperative force and electrocochleography measurements in an animal model of cochlear implantation.” Hearing research. [DOI] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC and Merchant SN (2011). “Age-related primary cochlear neuronal degeneration in human temporal bones.” Journal of the Association for Research in Otolaryngology 12(6): 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala M, Colletti L, Tonoli G and Colletti V (2012). “Electrocochleography during cochlear implantation for hearing preservation.” Otolaryngol Head Neck Surg 146(5): 774–781. [DOI] [PubMed] [Google Scholar]
- McClellan JH, Formeister EJ, Merwin WH 3rd, Dillon MT, Calloway N, Iseli C, Buchman CA, Fitzpatrick DC and Adunka OF (2014). “Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: comparison with audiometric and biographical information.” Otol Neurotol 35(9): e245–252. [DOI] [PubMed] [Google Scholar]
- O’Connell BP, Holder JT, Dwyer RT, Gifford RH, Noble JH, Bennett ML, Rivas A, Wanna GB, Haynes DS and Labadie RF (2017). “Intra-and postoperative electrocochleography may be predictive of final electrode position and postoperative hearing preservation.” Frontiers in neuroscience 11: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell BP, Hunter JB and Wanna GB (2016). “The importance of electrode location in cochlear implantation.” Laryngoscope Investigative Otolaryngology 1(6): 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeloff A, Shehata-Dieler W, Scherzed A, Rak K, Harnisch W, Hagen R and Mlynski R (2012). “Intraoperative monitoring using cochlear microphonics in cochlear implant patients with residual hearing.” Otol Neurotol 33(3): 348–354. [DOI] [PubMed] [Google Scholar]
- Riggs WJ, Roche JP, Giardina CK, Harris MS, Bastian ZJ, Fontenot TE, Buchman CA, Brown KD, Adunka OF and Fitzpatrick DC (2017). “Intraoperative electrocochleographic characteristics of auditory neuropathy spectrum disorder in cochlear implant subjects.” Frontiers in neuroscience 11: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles L and Ruggero MA (2001). “Mechanics of the mammalian cochlea.” Physiological reviews 81(3): 1305–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Maria PL, Gluth MB, Yuan Y, Atlas MD and Blevins NH (2014). “Hearing preservation surgery for cochlear implantation: a meta-analysis.” Otology & Neurotology 35(10): e256–e269. [DOI] [PubMed] [Google Scholar]
- Scott WC, Giardina CK, Pappa AK, Fontenot TE, Anderson ML, Dillon MT, Brown KD, Pillsbury HC, Adunka OF and Buchman CA (2016). “The Compound Action Potential in Subjects Receiving a Cochlear Implant.” Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 37(10): 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden M (2014). “Frequency selectivity without resonance in a fluid waveguide.” Proceedings of the National Academy of Sciences 111(40): 14548–14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden M and Versteegh CP (2015). “Energy flux in the cochlea: Evidence against power amplification of the traveling wave.” Journal of the Association for Research in Otolaryngology 16(5): 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CA, Santos F, Merchant SN, Liberman LD and Liberman MC (2015). “Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue.” Hearing research 327: 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanna GB, O’Connell BP, Francis DO, Gifford RH, Hunter JB, Holder JT, Bennett ML, Rivas A, Labadie RF and Haynes DS (2018). “Predictive factors for short‐and long‐term hearing preservation in cochlear implantation with conventional‐length electrodes.” The Laryngoscope 128(2): 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]