Abstract
Granular hydrogels are emerging as a versatile and effective platform for tissue engineered constructs in regenerative medicine. The hydrogel microparticles (HMPs) that compose these materials exhibit particle jamming above a minimum packing fraction, which results in a bulk, yet dynamic, granular hydrogel scaffold. These injectable, microporous scaffolds possess self-assembling, shear-thinning, and self-healing properties. Recently, they have been utilized as cell cultures platforms and extracellular matrix mimics with remarkable success in promoting cellular infiltration and subsequent tissue remodeling in vivo. Furthermore, the modular nature of granular hydrogels accommodates heterogeneous HMP assembly, where varying HMPs have been fabricated to target distinct biological processes or deliver unique cargo. Such multifunctional materials offer enormous potential for capturing the structural and biofunctional complexity observed in native human tissue.
Graphical abstract
INTRODUCTION
Conventional viscoelastic hydrogels, which comprise a gelatinous matrix of tightly-woven polymers that absorbs and retains large amounts of water [1], have been used in a wide range of cell culture platforms and tissue repair strategies. While these hydrogels can mimic certain aspects of tissue found in the human body, they fail to adequately reproduce the extended cell responsivity and the overall structural and biofunctional heterogeneity observed in human tissue. Granular hydrogels, created by amassing many hydrogel microparticles (HMPs), are emerging as a versatile alternative to meet the complex demands of tissue repair. The HMPs of granular hydrogels can either be found in the jammed state or as free-floating, non-jammed particles in solution. Jammed microparticles are dynamic structures that possess unique physical properties, such as self-assembly, shear-thinning, and self-healing [2–5], making them an appealing candidate for tissue engineering applications. Our discussion will primarily focus on viscoelastic, jammed mediums and will specifically highlight formulations developed within the last five years. We will not address two-phase materials in which HMPs are embedded in another medium [6], nor will we cover the concept of stress-relaxing hydrogels [7]. However, we highlight the benefits of heterogeneous HMP compositions within granular hydrogels and discuss the pros and cons of different HMP manufacturing approaches. This overview should convince the reader of the enormous potential for granular hydrogels to promote tissue repair and regeneration in a highly tunable and elegant way.
JAMMING BEHAVIOR OF HYDROGEL MICROPARTICLES
Granular hydrogels are materials comprising a collection of distinct, viscoelastic HMPs that generally have a diameter greater than 10 μm. Above this size, HMPs experience stronger gravitational forces relative to thermal forces. Additionally, the van der Waals force between adjacent HMPs is nominal relative to friction. The relatively larger particle size, lack of thermal motion, and existence of friction distinguishes granular hydrogels from other particulate matter, such as colloidal gels (Fig. 1A). These features also explain why granular materials with a particle-volume fraction above ~ 0.58 (known as “random loose packing”), under sufficient conditions of stress and temperature, are often found in the jammed state, a phenomenon in which the microscopically disordered material has transitioned from “liquid-like” to “solid-like” (Fig. 1B) When HMPs are concentrated above random loose packing, especially closer to a particle-volume fraction of 0.64 (known as “random close packing”), they can collectively be perceived as a bulk entity (i.e., a granular hydrogel scaffold) possessing conventional hydrogel properties. This bulk gel, however, is dynamic in nature where minimal external force can reorganize and displace particles [5,8].
Figure 1.
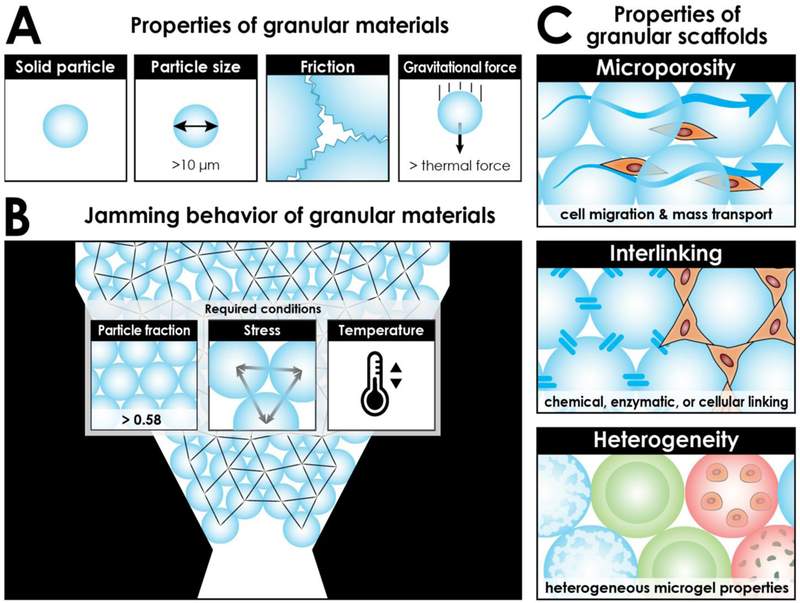
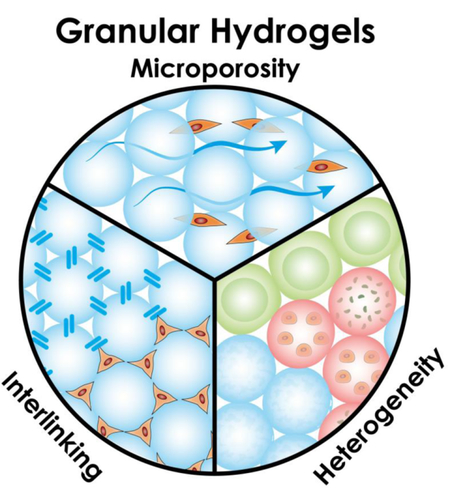
Overview of granular hydrogels. A) Defining characteristics of granular materials. These contrast other particulate matter, such as colloids (size < 10 μm; thermal forces > gravitational forces), foams (particles of air), and emulsions (particles of water). B) Particle jamming occurs when particles are packed above a minimum particle-volume fraction (and under suitable conditions of stress and temperature). Particles configured here are closer to random close packing with a particle-volume fraction of 0.64. The network of contact forces between neighboring particles is indicated by black lines. C) Inherent and tunable properties of granular hydrogel scaffolds. Microporosity: Micron-sized particles result in micron-sized pores that are similar to cell size. Interlinking: Particles can be interlinked in a variety of ways to form a stable structure. Heterogeneity: Granular scaffolds can incorporate a heterogeneous mix of particle species, such as porous particles, layered particles, particles encapsulating cells or small molecules, or particles of varying shapes.
In conventional hydrogels, liquid gel-precursor solutions can be injected into tissue and allowed to gel in situ, presenting a minimally invasive approach for biomedical applications [9]. In a similar manner, preformed HMPs exhibiting particle-jamming behave as a liquid under high injection strains, filling a three-dimensional cavity, then return to a viscoelastic solid in situ [3,10••]. This shear-thinning-like behavior enables granular hydrogels to take immediate effect as a bulk material upon injection without the need to wait for gelation. Such properties remove the need to consider toxicity effects of certain polymerization chemistries, such as ultraviolet light, radicals, and reactive nucleophiles, and are valuable given the lengthy wait times for slow-reacting crosslinking (e.g., enzymatic), which can take hours [9]. Granular hydrogels will additionally display self-healing when the attractive physical or chemical interactions among HMPs are disrupted, adding to the stability and practicality of these materials that make them particularly useful in active tissue (e.g., muscle or cardiac). Recently, the injectable characteristics of granular hydrogels have been exploited in three-dimensional printing techniques, where bio-inks comprise various HMPs [11]. Such innovations have applications in tissue construction and organ transplantation.
GRANULAR HYDROGEL SCAFFOLD POROSITY
Conventional precursor solutions of viscoelastic hydrogels contain polymers that will interlock upon gelation to form a dense matrix of entangled polymer chains, i.e., a scaffold. The scaffold’s mesh size, which is the void space between neighboring polymers, is on the length-scale of the original polymer – typically, nanoscale – which limits the rate of molecular diffusion and convectional fluid flow of nutrients and soluble signaling mediators through the hydrogel [12]. Additionally, the nanosized pores created by the void space are too small for direct cell infiltration, so cells must degrade the scaffold to tunnel through the hydrogel, which slows infiltration rates and reduces material degradation and overall tissue replacement of the bulk material [3,10••,13]. In the past, scientists have accepted these drawbacks, in part, for the benefit of injectability. In recent years, methods have been developed to create pre-formed hydrogel scaffolds with micron-sized pores that can be compressed for injection; however, they cannot conform to the shape of a wound, and additionally, they generate an expansion force upon release from the syringe, which may not be suitable in confined spaces [14].
The building-block composition of granular hydrogels makes it a powerful material design in terms of scaffold pore-microstructure. When HMPs are in a jammed state, the interstitial space among the packed particles typically forms a three-dimensional, inter-connected, porous network through which cells may freely migrate and mass transport occurs (Fig. 2A). A key structural aspect of granular hydrogel scaffolds is that the size-scale of their pores is proportional to the size-scale of the HMPs from which they are formed. Therefore, a micron-sized particle assembly produces micron-sized pores, which is optimal considering the micron size of most cells (Fig. 1C). Cells will generally spread farther and faster in the presence of a microporous hydrogel compared to a nanoporous (or “nonporous”) hydrogel [15], and additionally, the curved surfaces along the void space of packed microspheres have been shown to elicit favorable behaviors, such as osteogenic differentiation of human mesenchymal stem cells [16]. Of note, the porous network may be near-absent if HMPs are flat-faced particles in perfectly-aligned stacking, e.g. “stacked bricks” configuration [17]. Additionally, if HMPs are soft, deformable, and compressed, then the interstitial space between the particles might conceivably collapse into potential space; however, it is theoretically possible for cells to squeeze through the collapsed space given their deformability. By judiciously choosing the size distribution of uniform HMPs, material scientists can predictably control the average pore size over a range of microns, which will modulate cell infiltration rates and subsequent material integration with native tissue [3,18].
Figure 2.
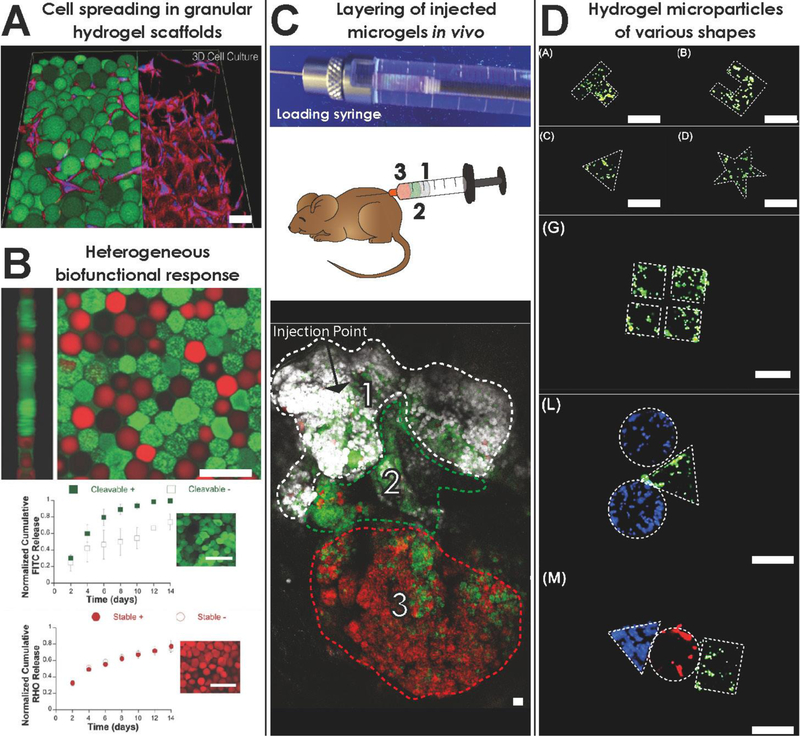
Experimental applications of granular hydrogels. A) Three-dimensional rendering of cellular infiltration and spreading in a granular hydrogel scaffold. Source: dapted from [15]. B) (Top) Heterogenous mixture of two microparticle species: (green) cleavable hydrogel loaded with FITC-BSA, (red) stable hydrogel loaded with rhodamine-dextran; (Bottom) Payload release study in the presence (+) or not in the presence (−) of collagenase. Source: Adapted from [4••]. C) Three different hydrogel microparticle species sequentially-loaded in a syringe and injected subcutaneously, retaining spatial distribution in vivo. Source: Adapted from [38•]. D) Cell-laden hydrogel microparticles with controlled, three-dimensional geometric shapes generated by flow lithography. Source: Adapted from [59•]. Scale bar = 100 μm for all figures.
COMPUTATIONAL ANALYSIS OF PORE-N TWORK PROPERTIES
Generally, the size, shape, and random orientation of HMPs that constitute a granular hydrogel will govern the microarchitecture of the local pore spaces, and computational tools are emerging to study these relationships. Mathematicians and engineers have explored ways to analyze the void space among packed particles using both computer-generated HMPs and microscopy images of experimentally-generated scaffolds. For local geometrical analyses, the void space is often segmented according to cavity regions using a variety of methods frequently based on either Euclidean distance transforms [18,19] or Voronoi tessellations [20]. Many researchers are interested in measuring the size of these pore subunits, which can be represented in a number of ways, including as a direct volume [18,21], approximation to an ellipsoid [19,22], and more interestingly, as a mean thickness based on a “volume-weighted” local thickness distribution [23]. Such data is useful considering the influence of confinement and local topography on cell behavior [16,24]. Interconnectivity of a scaffold refers to either a) connections between pore subunits, as measured by the size of connections or the number of connections per pore, or b) particle-particle contact points, which can be measured per particle. Both frameworks provide information about network properties. The former is often studied on sphere-templated scaffolds, i.e. the complement of granular scaffolds, and multiple studies have found, perhaps not surprisingly, that increased inter-pore connectivity increases cell infiltration and migration [21,25,26]. Interconnectivity in the context of particle-particle contact points is used to investigate stress-strain relationships and force transmission upon compression and shear [27–30] and contributes to an understanding of scaffold mechanics. Information about scaffold anisotropy at the macroscopic level can be obtained by analyzing the orientation of pore subunits at the microscopic level, which has been measured using principal component analysis and orientation tensors [18,28,29].
There is enormous potential for expansion in the field of computational void space analysis, not only in terms of investigating higher-order descriptors, such as curvature and tortuosity, but largely in terms of applications to cell behavior studies in the setting of granular hydrogel scaffolds. Analyzing pore space geometry is important for material characterization as well as for understanding how the shape of particles comprising a structure influences the internal microarchitecture. Such analysis in correlation with cell behavior data can help guide efficient and optimal engineering of granular scaffolds that aims to influence cell behavior and fluid flow. Researchers may also consider computer simulation studies of particle packing in concert with analytical methods as a predictive tool to save time and laboratory resources prior to material fabrication [18].
INTERLINKING HYDROGEL MICROPARTICLES IN A GRANULAR HYDROGEL SCAFFOLD
Granular hydrogel scaffolds are inherently dynamic in nature [5,8]; however, HMPs can be linked together to create a stable, inter-connected structure that requires degradation to rearrange the particle-network configuration (Fig. 1C). By interlinking particles, scaffold mechanics are strengthened and biodegradation times are lengthened, which may be desirable depending on the application [15]. HMP interlinking to form microporous annealed particle scaffolds (termed “MAP” scaffolds by [3]) has been achieved via sintering techniques ex vivo [31], covalent crosslinking in vivo [10••,24,25], and even cell-bridging through cell-particle adhesion [8,34]. By utilizing non-toxic interlinking techniques, such as biorthogonal chemical, enzymatic, or visible light crosslinking [15,35,36], that are applied in vivo, engineers may retain injectability for biomedical applications. Remarkably, polyethylene glycol-based injectable MAP scaffolds utilizing Factor XIII activation to interlink HMPs promoted not only cutaneous tissue repair in mice in terms of re-epithelialization and vasculature development but also tissue regeneration, as evidenced by regrowth of hair follicles and sebaceous glands in the wound bed – structures not present in scar tissue [3]. Promising results have also been demonstrated in stroke repair using a hyaluronic acid-based MAP hydrogel that was injected into the stroke cavity of mice. The scaffold enhanced vascularization and neural progenitor cell migration toward the injured tissue, suggesting improved tissue remodeling [10••]. A self-healing method for interlinking was developed that utilized the guest–host interactions between adamantane and cyclodextrin. Modified hyaluronic acid polymers and hyaluronic acid-based HMPs together were able to form a material that behaved as an interlinked granular hydrogel scaffold immediately upon injection, which addressed a challenge of targeting fast-moving tissue, such as cardiac muscle, that readily displaces slow-gelling precursor solutions or dynamic scaffolds [4••].
HETEROGENEOUS ASSEMBLY OF HYDROGEL MICROPARTICLES
The native tissue is a complex microenvironment with numerous structural and biochemical elements that support the interplay of cells and signaling molecules. In order to modulate it more effectively, there is a need to develop a single biomaterial that can target multiple cellular processes simultaneously. The aggregate nature of granular hydrogels makes it an optimal platform for incorporating heterogeneity into biomaterials (Fig. 1C). By mixing multiple species of HMPs with varying characteristics, tissue engineers can create an injectable, composite material that is mechanically anisotropic and functionally heterogeneous. HM s have been previously fabricated as porous particles [37], layered (i.e., coated) beads [38,39], cargo carriers [13,40–44], and with different backbone materials or surface chemistry modifications. Though heterogeneity within a microparticle has been demonstrated in these contexts, our examples focus on heterogeneity among HMPs composing the granular scaffold as a whole.
Proof of concept has already been established for certain heterogeneous combinations. A single granular hydrogel was created comprising two HMP species of modified hyaluronic acid that differed by material degradability and payload (Fig. 2B). In vivo injections into post-myocardial infarction tissue in rats showed a two-component response that exploited the elevated protease activity in the injured tissue [4••]. In another study, two HMP species, i.e., a positively-charged species containing bone morphogenetic protein-2 (BMP-2) to induce osteogenesis and a negatively-charged species containing berberine chloride for bacteriostasis were combined. Opposing particle charges contributed to scaffold self-assembly, and the potential for synergistic therapeutic effects was supported in vivo by way of faster bone formation and absence of infection [37•].1
SPATIAL HETEROGENEITY OF GRANULAR SCAFFOLDS
A well-mixed assortment of different HMP species produces a material that is uniformly distributed in space; however, the concept of syringe layering offers the potential for spatial heterogeneity within a scaffold. A syringe can be loaded with layers of different HMP formulae and, after extrusion, maintain these distinct layers [38•] (Fig. 2C). This is a direct consequence of the physical properties of granular materials and particle jamming, which enables the layered material to move as a collective unit without significant mixing. Layering techniques can produce overall heterogeneous materials that are uniquely non-homogenous in space, offering potential for injectable therapies that form into compartmentalized scaffolds.
As more HMP species are introduced into a material, developers will need to consider particle crosstalk or unfavorable species interactions, and it will become an increasing challenge to understand which species are contributing toward positive outcomes. With complexity in design comes complexity in functionality and understanding, and with so many options for HMP combinations, a deep insight into the complex biofunctionalities that could result from particle mixing will be invaluable.
GRANULAR HYDROGEL BUILDING BLOCK FABRICATION
HMPs have been produced in a number of different ways and from a wide variety of materials such as alginate, chitosan, gelatin, hyaluronic acid, sericin, and various synthetic materials [44–48]. Bulk production methods of HMPs allow for a high-throughput and fast fabrication of large amounts of gel particles. One straightforward approach involves fragmentation of crosslinked, bulk hydrogel scaffolds into microparticles by physical crushing, then sieving to narrow the size distribution, resulting in rugged, nonhomogeneous particles [49–51]. For more spherical particles, batch oil-in-water or water-in-oil emulsion polymerization methods [34,37•] have been widely used for the rapid production of large volumes of particles [52]. Both methods suffer from a lack of particle size and shape uniformity and reproducibility, and for more complex engineering approaches that require controlled, well-defined HMPs, these methods may be unsuitable [53]. In contrast, the emergence of various microfluidic approaches has paved the way for high-quality HMPs. In these methods, droplets are generated on a micro-scale by injecting a hydrogel precursor solution into an air, or more commonly oil, flow to form spherical droplets, which are subsequently crosslinked by chemical, enzymatic, or light-based polymerization [8]. Most commonly, capillary-based coaxial flow devices, chip-based flow focusing, or electrohydrodynamic jetting are used to generate HMPs within a narrow polydispersity [44,52,54–56]. While most batch methods lack the precision and control of HMP characteristics over microfluidic methods, they out-perform other methods in terms of their scalability. Any method for particle generation must balance both precision and reproducibility with scalability, which encompasses cost and efficiency, in accordance with specific requirements of the intended biomedical application.
PRODUCING COMPLEX HYDROGEL MICROPARTICLE SHAPES
At the forefront of particle fabrication advances are methods tailored to make microparticles with complex three-dimensional shapes and anisotropic composition (Fig. 2D). Generation of non-spherical shapes has been demonstrated with template and mold-based approaches as well as lithography techniques [26,49–52•] and micro-array chips [37,43]. Sacrificial molds can be utilized to produce detailed microgel geometries by casting the hydrogel precursor solution into a silicone mold of a specified shape and dissolving the mold after polymerization [34,60]. Higher fabrication throughput has been achieved by flow lithography methods, which generate geometrically complex HMPs by exposing a continuously-flowing photo-crosslinkable polymer solution to light through a photomask containing the intended pattern of the three-dimensional particle [52•]. Flow lithography can also be used to generate anisotropic, spatially-defined biofunctionalizations in particles using a stable co-flow of different precursor solutions. As HMP generation rates continue to increase with advancements in fabrication technology, materials comprising complex, three-dimensional HMPs with functional patterning will become increasingly more feasible to utilize for clinically translatable applications.
CONCLUSION
Granular hydrogels are a new class of biomaterials with a number of advantageous characteristics (Fig. 1) that make them promising for regenerative therapies and other biomedical applications [3,10••]. The hydrogel microparticles (HMPs) that comprise granular hydrogels have commonly been utilized as cargo carriers for protected and efficient delivery of small molecules and cells to sites of injury [35–37•,41]. However, when HMPs are packed above the minimum particle-packing fraction and behave as a jammed system, novel systemic properties arise: The resulting granular hydrogel scaffold not only comprises micron-sized pores for easier cellular infiltration, but microinvasive injectability is retained due to the aggregate nature and shear-thinning behavior of the dynamic structure. Computational void space analysis is an underutilized avenue that can help guide fabrication-optimization efforts by quantifying pore microarchitectural properties that result from HMPs of known shapes and sizes. HMPs can be modified to produce a stable scaffold of interlinked particles, termed microporous annealed particle (MAP) scaffolds by [3], which has shown promising results in vivo as a platform for tissue regeneration [3,10••]. By combining multiple HMP species with differing functionalities to compose a single granular scaffold, researchers can produce heterogeneous materials of tremendous complexity that allow for greater spatial and temporal control over the cellular response. Based on the advancements in HMP fabrication techniques and their widespread commercial availability, we expect the usage of granular materials will continue to rise, ultimately becoming a standard for three-dimensional culture platforms and in situ tissue engineering applications.
HIGHLIGHTS.
Jamming phenomenon in granular hydrogels enables microporosity and injectability
Heterogeneous combinations of microgels produce complex, multifunctional materials
In vivo interlinking of microparticles supports tissue remodeling in skin and stroke
Computational analysis of granular hydrogel porosity enables smarter material design
ACKNOWLEDGEMENTS
LR is credited with conception and writing of the manuscript. LS edited several drafts and generated the figures. TS provided valuable edits, suggestions, and feedback. This work was supported by the National Institutes of Health (grant number R01NS094599). LS is a recipient of a research fellowship granted by the German Research Foundation (DFG). The authors thank Renee Riley, PharmD for her comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study incorporated both granular and colloidal particles, where colloidal particles are characteristically less than 10 μm.
OF INTEREST
OF SPECIAL INTEREST
REFERENCES
- 1.Kamata H, Li X, Chung U Il, Sakai T: Design of Hydrogels for Biomedical Applications. Adv Healthc Mater 2015, 4:2360–2374. [DOI] [PubMed] [Google Scholar]
- 2.Van Tomme SR, van Nostrum CF, Dijkstra M, De Smedt SC, Hennink WE: Effect of particle size and charge on the network properties of microsphere-based hydrogels. Eur J Pharm Biopharm 2008, 70:522–530. [DOI] [PubMed] [Google Scholar]
- 3.Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T: Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater 2015, 14:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mealy JE, Chung JJ, Jeong HH, Issadore D, Lee D, Atluri P, Burdick JA: Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications. Adv Mater 2018, 30:1–7. [DOI] [PubMed] [Google Scholar]; Mealy, et al. (Advanced Materials, 2018) Injectable Granular Hydrogels with Multifunctional Properties for The authors generate a self-healing granular hydrogel comprising modified hyaluronic acid-based hydrogel microparticles (HMPs) with a guest–host interlinking chemistry. They combine two HMP species with different degradation characteristics and demonstrate the heterogeneous material-tissue responsivity of their granular hydrogel in an in vivo myocardial infarction model
- 5.Weeks ER: Soft jammed materials. In Statistical Physics of Complex Fluids. Edited by Maruyam S, Tokuyama M. Tohoku University Press; 2007:2–1-- 2–87. [Google Scholar]
- 6.Rose JC, Cámara-Torres M, Rahimi K, Köhler J,möller m, De Laporte L: Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance. Nano Lett 2017, 17:3782–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H, Lippens E, Duda GN, et al. : Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 2016, 15:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz DMG, Ivirico JLE, Gomes MM, Ribelles JLG, Sanchez MS, Reis RL, Mano JF: Chitosan microparticles as injectable scaffolds for tissue engineering. J Tissue Eng Regen Med 2008, 2:378–380. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Rodrigues J, Tomás H: Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem Soc Rev 2012, 41:2193–2221. [DOI] [PubMed] [Google Scholar]
- 10.Nih LR, Sideris E, Carmichael ST, Segura T: Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Adv Mater 2017, 29:1606471. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nih, et al. (Advanced Materials, 2017) Injection of Microporous Annealing Particle (MAP) Hydrogels in the Stroke Cavity Reduces Gliosis and Inflammation and Promotes NPC Migration to the Lesion. Hyaluronic acid-based hydrogel microparticles were interlinked in situ via a biocompatible, enzyme-mediated annealing process utilizing a transglutaminase reaction. Their material was injected into a stroke cavity induced in mice, and the porous material supported stroke recovery by allowing neural progenitor cells to easily infiltrate and migrate freely through the scaffold.
- 11.Highley CB, Song KH, Daly AC, Burdick JA: Jammed Microgel Inks for 3D Printing Applications. Press 2018, doi:10.1002/)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J: Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng 2000, 2:9–29. [DOI] [PubMed] [Google Scholar]
- 13.Li F, Truong VX, Fisch P, Levinson C, Glattauer V, Zenobi-Wong M, Thissen H, Forsythe JS, Frith JE: Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater 2018, doi: 10.1016/j.actbio.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Bencherif SA, Warren Sands R, Ali OA, Li WA, Lewin SA, Braschler TM, Shih T-Y, Verbeke CS, Bhatta D, Dranoff G, et al. : Injectable cryogel-based whole-cell cancer vaccines. Nat Commun 2015, 6:7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sideris E, Griffin DR, Ding Y, Li S, Weaver WM, Di Carlo D, Hsiai T, Segura T: Particle Hydrogels Based on Hyaluronic Acid Building Blocks. ACS Biomater Sci Eng 2016, 2:2034–2041. [DOI] [PubMed] [Google Scholar]
- 16.Werner M, Blanquer SBG, Haimi SP, Korus G, Dunlop JWC, Duda GN, Grijpma DW, Petersen A: Surface Curvature Differentially Regulates Stem Cell Migration and Differentiation via Altered Attachment Morphology and Nuclear Deformation. Adv Sci 2017, 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le LV, Mohindra P, Fang Q, Sievers RE, Mkrtschjan MA, Solis C, Safranek CW, Russell B, Lee RJ, Desai TA: Injectable hyaluronic acid based microrods provide local micromechanical and biochemical cues to attenuate cardiac fibrosis after myocardial infarction. Biomaterials 2018, 169:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roozbahani M, Borela R, Frost J: Pore Size Distribution in Granular Material Microstructure. Materials (Basel) 2017, 10:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones AC, Arns CH, Hutmacher DW, Milthorpe BK, Sheppard AP, Knackstedt MA: The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30:1440–1451. [DOI] [PubMed] [Google Scholar]
- 20.Schaller FM, Kapfer SC, Hilton JE, Cleary PW, Mecke K, De Michele C, Schilling T, Saadatfar M, Schröter M, Delaney GW, et al. : Non-universal Voronoi cell shapes in amorphous ellipsoid packs. Epl 2015, 111:0–6. [Google Scholar]
- 21.Malafaya PB, Santos TC, van Griensven M, Reis RL: Morphology, mechanical characterization and in vivo neo-vascularization of chitosan particle aggregated scaffoldsarchitectures. Biomaterials 2008,29:3914–3926. [DOI] [PubMed] [Google Scholar]
- 22.Jones JR, Atwood RC, Poologasundarampillai G, Yue S, Lee PD: Quantifying the 3D macrostructure of tissue scaffolds. J Mater Sci Mater Med 2009, 20:463–471. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrand T, Ruegsegger P: A new method for the model independent assessment of thickness in three dimensional images. J Microsc 1997, 185:67–75. [Google Scholar]
- 24.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF: Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci 2013, 110:17253–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X, Wang W, Liu D, Zhang H, Gao P, Geng L, Yuan Y, Lu J, Wang Z: The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci Rep 2015, 5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somo SI, Akar B, Bayrak ES, Larson JC, Appel AA, Mehdizadeh H, Cinar A, Brey EM: Pore Interconnectivity Influences Growth Factor-Mediated Vascularization in Sphere-Templated Hydrogels. Tissue Eng Part Methods 2015, 21:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodu N, Dijksman JA, Behringer RP: Spanning the scales of granular materials through microscopic force imaging. Nat Commun 2015, 6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Li X-S: Micro-Macro Quantification of the Internal Structure of Granular Materials. J Eng Mech 2009, 135:641–656. [Google Scholar]
- 29.Zhao S, Evans TM, Zhou X: Three-dimensional Voronoi analysis of monodisperse ellipsoids during triaxial shear. Powder Technol 2018, 323:323–336. [Google Scholar]
- 30.Walker D M, Tordesillas A, Brodu N, Dijksman JA, Behringer RP, Froyland G: Self-assembly in a near-frictionless granular material: conformational structures and transitions in uniaxial cyclic compression of hydrogel spheres. Soft Matter 2015, 11:2157–2173. [DOI] [PubMed] [Google Scholar]
- 31.Borden M, Attawia M, Khan Y, l-Amin SF, Laurencin CT: Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. J Bone Joint Surg Br 2004, 86:1200–8. [DOI] [PubMed] [Google Scholar]
- 32.Jivan F, Yegappan R, Pearce H, Carrow JK, McShane M, Gaharwar AK, Alge DL: Sequential Thiol–Ene and Tetrazine Click Reactions for the olymerization and Functionalization of Hydrogel Microparticles. Biomacromolecules 2016, 17:3516–3523. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Zhang J, Liu Z, Lin Q, Liu X, Bao C, Wang Y, Zhu L: Tissue-Integratable and Biocompatible Photogelation by the Imine Crosslinking Reaction. Adv Mater 2016, 28:2724–2730. [DOI] [PubMed] [Google Scholar]
- 34.Leferink A, Schipper D, Arts E, Vrij E, Rivron N, Karperien M, Mittmann K, Van Blitterswijk C, Moroni L, Truckenmüller R: Engineered micro-objects as scaffolding elements in cellular building blocks for bottom-up tissue engineering approaches. Adv Mater 2014, 26:2592–2599. [DOI] [PubMed] [Google Scholar]
- 35.Caldwell AS, Campbell GT, Shekiro KMT, Anseth KS: Clickable Microgel Scaffolds as Platforms for 3D Cell Encapsulation. Adv Healthc Mater 2017, 6:1700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa SA, Simon JR, Amiram M, Tang L, Zauscher S, Brustad EM, Isaacs FJ, Chilkoti A: Photo-Crosslinkable Unnatural Amino Acids Enable Facile Synthesis of Thermoresponsive Nano- to Microgels of Intrinsically Disordered Polypeptides. Adv Mater 2018, 30:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng Y, Zhu L, Han Q, Liu W, Mao X, Li Y, Yu N, Feng S, Fu Q, Wang X, et al. : Preformed gelatin microcryogels as injectable cell carriers for enhanced skin wound healing. Acta Biomater 2015, 25:291–303. [DOI] [PubMed] [Google Scholar]
- 38.Thompson KL, Williams M, Armes SP: Colloidosomes: Synthesis, properties and applications. J Colloid Interface Sci 2014, 447:217–228. [DOI] [PubMed] [Google Scholar]
- 39.Alessandri K, Feyeux M, Gurchenkov B, Delgado C, Trushko A, Krause KH, Vignjević D, Nassoy P, Roux A: A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC). Lab Chip 2016, 16:1593–1604. [DOI] [PubMed] [Google Scholar]
- 40.Annamalai RT, Tapan N, Haley P, Andrew PJ, Jan SP: Biofabrication of injectable fibrin microtissues for minimally-invasive therapies: Application of surfactants. Biomed Mater 2018, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou Y, Xie W, Achazi K, Cuellar-Camacho JL, Melzig MF, Chen W, Haag R: Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. Acta Biomater 2018, doi: 10.1016/J.ACTBIO.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Hu Z, Ma C, Rong X, Zou S, Liu X: Immunomodulatory ECM-like Microspheres for Accelerated Bone Regeneration in Diabetes Mellitus. ACS Appl Mater Interfaces 2018, 10:2377–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi C, Li Y, Badger P, Yu H, You Z, Yan X, Liu W, Shi Y, Xia T, Dong J, et al. : Pathology-targeted cell delivery via injectable micro-scaffold capsule mediated by endogenous TGase. Biomaterials 2017, 126:1–9. [DOI] [PubMed] [Google Scholar]
- 44.Kim P-H, Yim H-G, Choi Y-J, Kang B-J, Kim J, Kwon S-M,Kim B-S,Hwang NS,Cho J-Y:Injectablemultifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J Control Release 2014, 187:1–13. [DOI] [PubMed] [Google Scholar]
- 45.Cai B, Zou Q, Zuo Y, Mei Q, Ma J, Lin L, Chen L, Li Y: An injectable gel constructs with regenerative and anti-infective dual effects based on assembled chitosan microspheres. ACS Appl Mater Interfaces 2018, 10:25099–25112. [DOI] [PubMed] [Google Scholar]; Cai, et al. (ACS Applied Materials and Interfaces, 2018) An injectable gel constructs with regenerative and anti-infective dual effects based on assembled chitosan microspheres Osteogenic and bacteriostatic properties were integrated into a single granular material by mixing positively- and negatively-charged hydrogel microparticles (HMPs) loaded with bone morphogenic protein and berberine, respectively. The charge interaction among the HMPs allowed for shear-shinning and self-assembling behavior of the heterogeneous granular material, and results were suggestive of a dual biofunctionality within their material.
- 46.Darling NJ, Sideris E, Hamada N, Carmichael ST, Segura T: Injectable and spatially patterned microporous annealed particle (MAP) hydrogels for tissue repair applications. Adv Sci 2018, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; Darling et al. (Advanced Science, 2018) Injectable and spatially patterned microporous annealed particle (MAP) hydrogels for tissue repair applications Sequential loading of three distinct species of hydrogel microparticles into a syringe resulted in a spatially-patterned, layered granular scaffold in situ after injection. Successful layering of material in a dermal biopsy and stroke cavity demonstrated the feasibility of this approach for biomedical applications where minimally-invasive, spatially-controlled heterogeneity is required.
- 47.Nayak S, Dey S, Kundu SC: Silk sericin–alginate–chitosan microcapsules: Hepatocytes encapsulation for enhanced cellular functions. Int J Biol Macromol 2014, 65:258–266. [DOI] [PubMed] [Google Scholar]
- 48.Xin S, Wyman OM, Alge DL: Assembly of PEG Microgels into Porous Cell-Instructive 3D Scaffolds via Thiol-Ene Click Chemistry. Adv Healthc Mater 2018, 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seib FP, Tsurkan M, Freudenberg U, Kaplan DL, Werner C: Heparin-Modified Polyethylene Glycol Microparticle Aggregates for Focal Cancer Chemotherapy. ACS Biomater Sci Eng 2016, 2:2287–2293. [DOI] [PubMed] [Google Scholar]
- 50.Tezel A, Fredrickson GH: The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther 2008, 10:35–42. [DOI] [PubMed] [Google Scholar]
- 51.Öhrlund JÅ, Edsman KLM: The Myth of the “Biphasic” Hyaluronic Acid Filler. Dermatol Surg 2015, 41:S358–S364. [DOI] [PubMed] [Google Scholar]
- 52.Skop NB, Calderon F, Levison SW, Gandhi CD, Cho CH: Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomater 2013, 9:6834–6843. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Li Y, Wang X, Wang J, Tian H, Zhao P, Tian Y, Gu Y, Wang L, Wang C, et al. : Droplet Microfluidics for the Production of Microparticles and Nanoparticles . icromachines 2017, 8:22. [Google Scholar]
- 54.Bock N, Woodruff MA, Hutmacher DW, Dargaville TR: Electrospraying, a reproducible method for production of polymeric microspheres for biomedical applications. Polymers (Basel) 2011, 3:131–149. [Google Scholar]
- 55.Ma M, Chiu A, Sahay G, Doloff JC, Dholakia N, Thakrar R, Cohen J, Vegas A, Chen D, Bratlie KM, et al. : Core-Shell Hydrogel Microcapsules for Improved Islets Encapsulation. Adv Healthc Mater 2013, 2:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeyhani M, Mak SY, Sammut S, Shum HC, Hwang DK, Tsai SSH: Controlled Electrospray Generation of Nonspherical Alginate Microparticles. ChemPhysChem 2018, 19:2113–2118. [DOI] [PubMed] [Google Scholar]
- 57.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS: Continuous-flow lithography for high-throughput microparticle synthesis. Nat Mater 2006, 5:365–369. [DOI] [PubMed] [Google Scholar]
- 58.Dendukuri D, Gu SS, Pregibon DC, Hatton TA, Doyle PS: Stop-flow lithography in a microfluidic device. Lab Chip 2007, 7:818–828. [DOI] [PubMed] [Google Scholar]
- 59.Yang W, Yu H, Li G, Wang Y, Liu L: High-Throughput Fabrication and Modular Assembly of 3D Heterogeneous Microscale Tissues. Small 2017, 13:1602769. [DOI] [PubMed] [Google Scholar]; Yang et al. (Small, 2017) High-Throughput Fabrication and Modular Assembly of 3D Heterogeneous Microscale Tissues Cell-laden hydrogel microparticles (HMPs) of non standard -standard shapes were generated in a flow lithography approach. Complex, heterogenous microtissues were created by assembling HMPs of different cell types through optically-induced dielectrophoresis. he authors demonstrate high-throughput fabrication methods for HMPs with complex geometries and utilize their particles to form spatially-controlled, heterogenous microenvironments.
- 60.Tang MD, Golden AP, Tien J: Molding of Three-Dimensional Microstructures of Gels. J Am Chem Soc 2003, 125:12988–12989. [DOI] [PubMed] [Google Scholar]